Abstract
Background
The COVID-19 pandemic presents challenges in participant recruitment strategies for clinical research involving people with opioid use disorders recently engaged in treatment. We describe challenges to participant recruitment in a trial comparing virtual buprenorphine treatment platform to office-based buprenorphine treatment.
Methods
The parent study was a cohort trial of telehealth delivered buprenorphine treatment compared to office-based buprenorphine treatment, however, due to the pandemic potential participant recruitment for both arms became virtual. Between 9/27/2021 and 7/11/2022, telephone, email, flyers, and word-of-mouth were used to recruit study participants from each treatment setting. Recruitment tracking documents recorded the primary outcomes: number of outreach attempts and most effective contact methods.
Results
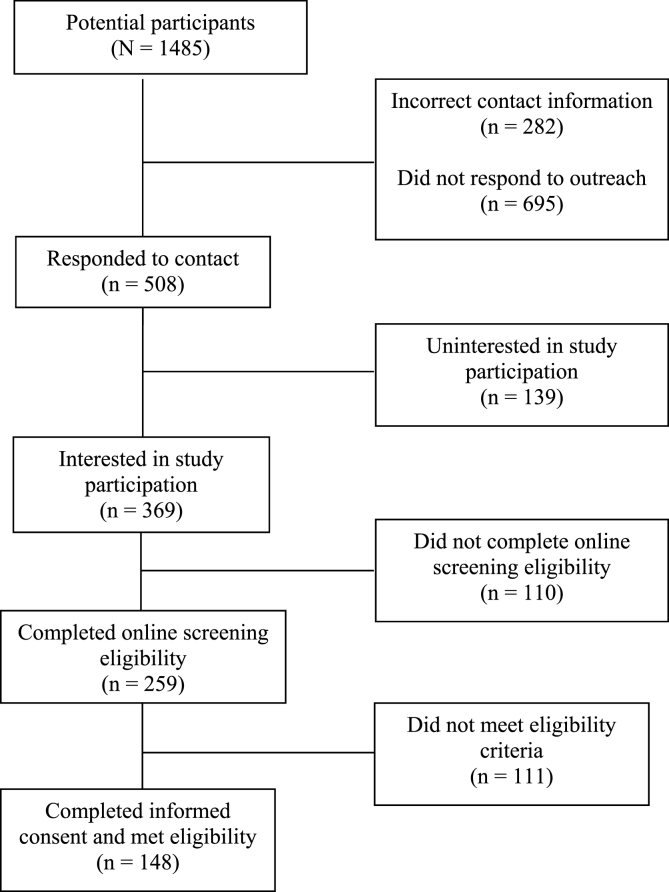
Treatment settings provided contact information for 1485 potential study participants. Information was incorrect or disconnected for 282 (19%) individuals, 695 (47%) did not respond to outreach, and 508 (34%) responded to outreach. Of these responders, 369 were interested in study participation, 259 completed the online informed consent and screening assessment, and 148 met eligibility criteria and enrolled in the study. A total of 3804 virtual outreach attempts across 1485 potential participants were made, resulting in an average of 2.7 attempts per contact and a mean of 25.7 attempts per enrolled participant (n = 148).
Conclusion
Conducting research during the COVID-19 pandemic required shifting from in-person to virtual recruitment strategies to contact and engage potential study participants. Virtual recruitment for this population during a pandemic appears to be less efficient and hindered efforts to meet recruitment goals.
Keywords: Recruitment challenges, Virtual recruitment, COVID-19, Telemedicine, Buprenorphine, Opioid Use Disorder
Highlights
-
•
Recruitment during the pandemic was challenging and led to low recruitment outcomes.
-
•
Findings are important for future study design given the potential budgetary impact.
-
•
Required an average of 25.7 recruitment outreach attempts per enrolled participant.
Abbreviations
- OUD
Opioid Use Disorder
- NIH
National Institutes of Health
- OHSU
Oregon Health & Science University
- NIDA
National Institute on Drug Abuse
Funding
This work was supported by the National Institute on Drug Abuse [NIDA 4 R44 DA050345].
Role of funding source
This project was funded by the National Institute on Drug Abuse Small Business Innovation Research (SBIR) grant; therefore, by design, has academics and industry working together. The funder did not participate in the design of the study, nor in the collection, analysis, interpretation of data, or writing of the manuscript.
1. Background
During the COVID-19 pandemic, up to 80% of non-COVID-19 trials were interrupted or terminated [1]. Clinic closures, staffing issues, and restrictions on clinical research activities at many sites impeded recruitment [2]. For studies that continued, recruitment strategies focused on online and telephone interactions [3]. In a pre-pandemic meta-analysis comparing online recruitment versus traditional in-person strategies, online recruitment strategies were more effective at recruiting eligible participants but offline/in-person recruitment showed a higher rate of enrolling participants [4]. Barriers to pandemic study recruitment should be addressed early to avoid delays in recruitment and mitigate threats to internal and external study validity [[5], [6], [7]].
Opioid use disorder (OUD) treatment studies typically recruited participants in treatment settings where they had one or more in-person visits [3]. Telehealth makes it easier to connect with potential participants who have difficulty meeting in-person due to the logistical barriers people with OUD often experience [8]. Online engagement, however, can be limited by participants’ access to and comfort with technology [8]. People with substance use disorders often lack access to a phone, wireless connection, or data plan, making online engagement more difficult [9].
The Buprenorphine Evaluation and Telehealth Study (CTN 05529225) awarded prior to the pandemic planned to recruit from two arms: 1) adults receiving buprenorphine for OUD through telehealth only with virtual recruitment and 2) adults receiving buprenorphine for OUD through office-based treatment with in-clinic, in-person recruitment. However, due to pandemic constraints, recruitment for both arms became virtual. We analyzed recruitment data to identify recruitment challenges.
2. Methods
The parent study was a cohort trial of telehealth delivered buprenorphine treatment compared to office-based buprenorphine treatment, however, due to the pandemic, potential participant recruitment for both arms became virtual. Between September 27, 2021, and July 11, 2022, eligible participants – adults 18 years of age or older, within 45 days of a new buprenorphine prescription for OUD treatment, and not pending court appearances or incarceration – were recruited from electronic health records (EHR) and provider lists in Oregon and Washington. Eligibility screening, informed consent and baseline assessments were completed online. Participants received compensation for participation via a reloadable gift card at each study visit. The Oregon Health & Science University IRB approved all study procedures.
2.1. Description of contact and recruitment methods
2.1.1. Phone
Potential participants were contacted using a toll-free phone number from RingCentral [10] or by Doximity [11] (HIPAA-compliant software that allows medical professionals to use medical office phone numbers from personal telephones). RingCentral displayed a recognized phone number of the telehealth clinic to potential telehealth-only participants. Doximity displayed a recognized phone number of an office-based clinic to potential office-based participants.
2.1.2. Email
Two email domains (.edu or.care) were used to recruit and follow study participants.
2.1.3. Fliers
With permission from individual clinics who were still operating in-person, paper fliers were posted for patients obtaining in-person treatment.
2.1.4. Word of mouth
Participants shared information about the study with friends and family.
2.2. Outcomes
We tracked every attempted contact, noting the methods and outcomes for each. The primary outcomes were the number of outreach attempts and most effective contact methods overall, and by each method of recruitment.
2.3. Analysis
Recruitment tracking documents were analyzed by each recruitment method using total counts, percentage, and the mean response rate overall. We analyzed the outcomes to understand recruitment rates related to the method of recruitment.
3. Results
We received contact information for 1485 potential participants. Nearly half (47%; n = 695) did not respond to repeated contact attempts. Information was incorrect or not in service for 282 (19%) individuals. One in three potential participants (n = 508; 34%) responded to a contact attempt. Among the 508 individuals who responded, 369 (73%) were interested in study participation, 259 (51%) completed the online screening assessment, and 148 (29%) completed informed consent, met eligibility criteria and enrolled in the study (Fig. 1). Overall, of 1485 potential participants, 148 (10%) enrolled in the study.
Fig. 1.
Enrollment of potential participant pool.
Among 369 individuals who responded to outreach efforts and were interested in study participation, 53% responded to email, 45% were contacted by phone, 1% were contacted by word of mouth, and 1% contacted us after viewing a flier. Of 148 eligible participants, 69% were recruited through email, 36% were recruited through phone, 2% were referred by word of mouth, and 1% through flier advertisement. Study personnel made 3804 outreach attempts across 1485 potential participants, yielding an average of 2.7 attempts per contact and a mean of 25.7 attempts per enrolled participant (n = 148).
The study planned to recruit 100 participants in each arm. We recruited 100 in the telehealth arm between September 2021 and April 2022 and 34 in the planned office-based arm between November 2021 and April 2022.
4. Conclusion
Conducting research during the COVID-19 pandemic required adaptation of study recruitment methods and a shift from in-person to telephone and email strategies to contact and engage potential participants. Recruitment during a pandemic was challenging and the shift to virtual methods led to lower-than-expected recruitment in the planned office-based arm. Traditional pre-pandemic recruitment protocols need to be improved and adapted to support virtual recruitment strategies.
Our study differed from similar previous studies [[4], [5], [6], [7]] as we planned to recruit telehealth participants using online strategies and office-based participants in-person in treatment centers. However, access to in-person settings was restricted due to COVID-19. Virtual recruitment differed from in-person recruitment due to the lack of face-to-face communication and the computer literacy needed to access and engage with the study's online survey platforms.
Online recruitment was not as effective for enrolling participants receiving planned office-based buprenorphine compared to participants receiving telehealth only. A possible explanation is that telehealth participants were comfortable with engaging virtually and may have had more regular access to cell phones, computers, or tablets with internet access compared to participants receiving planned office-based treatment. Participants with virtual healthcare experience might also be more trusting of phone or internet recruitment. Additionally, many clinics providing what would have been office-based buprenorphine were safety-net clinics serving a more medically complex population who might not have the resources to engage online.
4.1. The study team identified six challenges
4.1.1. COVID-19 related delays
We were unable to start office-based recruitment on time due to clinic closures, staffing issues, restrictions on all clinical research activities, and lack of identification of a point person at each clinic to obtain participant contact information.
4.1.2. IRB restrictions
We experienced IRB delays during the study start-up phase due to IRB staffing issues. We received IRB approval to start recruitment in September 2021.
During the study it became clear that participants preferred text messaging. The IRB initially did not approve text messaging due to confidentiality concerns, however, a compromise was reached where texting, via the secure RingCentral app, was limited to consented participants who agreed to texting. This restriction adversely impacted the number of people reached during the recruitment phase.
4.1.3. Electronic health record and provider lists
Electronic health record and provider lists often contained incorrect or missing contact information and we did not know the proportion of the list that would have been eligible to participate in this study. This added to the challenges brought on by COVID-19 and reflects how contact data and diagnoses are stored in the EHR, the data we were able to pull, and how we received the lists. We adjusted the EHR data pull variables frequently throughout recruitment to refine the list of potential participants but gained few additional successful recruitments from this.
4.1.4. Relationships with clinics
COVID-19 research restrictions made it difficult to establish working relationships with office-based study sites. The availability of potential participant contact information from clinics was irregular. A clinic providing occasional in-person care was reluctant to participate because of the pandemic and concerns regarding additional staff burden. Study staff met with clinic leadership and staff to facilitate research participation. In contrast, the telehealth clinic was better suited for online recruitment because the pandemic's impact on their care model was minimal. An established relationship with the clinic was essential to recruitment success and to bring the study to completion. In the absence of these partnerships, recruitment would have been more difficult.
In-Person Recruitment Less Effective.
Because office-based buprenorphine clinics were operating virtually, the fliers were rarely seen, and word of mouth was less effective than expected.
4.1.5. Unrecognized phone numbers and emails
Study staff made persistent efforts to contact potential study participants and called at various times of day and days of the week to accommodate their needs. We learned from potential participants that they ignored unrecognized telephone numbers. RingCentral and Doximity were used to mitigate this and display a recognizable telephone number to potential participants in each arm. Additionally, the domain of the email used to recruit mattered. Based on participant feedback, we learned non “.edu” emails ended up in spam or were deleted without opening.
The cumulative impact of these challenges during the funded study period was an inability to recruit the planned 100 office-based buprenorphine participants. The recruitment and contact obstacles are important for designing future studies given the time and budgetary impact of delayed recruitment.
Author contributions
Kellie Pertl: Writing - Original Draft, Formal analysis, Methodology, Investigation, Data Curation, Visualization Ritwika Petluri: Writing - Original Draft, Formal analysis, Methodology, Investigation, Data Curation, Visualization Katharina Wiest: Conceptualization, Methodology, Writing - Review & Editing Kim Hoffman: Writing - Review & Editing Dennis McCarty: Writing - Review & Editing Ximena A. Levander: Writing - Review & Editing, Resources Brian Chan: Writing - Review & Editing, Resources Stephen A. Martin: Funding acquisition, Writing - Review & Editing, Resources P. Todd Korthuis: Supervision, Writing - Review & Editing, Resources.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Ms. Pertl reported being an employee of Boulder Care. Ms. Petluri reported being an employee of Boulder Care. Dr. Wiest reported being a consultant of Boulder Care. Dr. Martin reported being an employee of Boulder Care. The other authors declare they have no competing interests. To reduce potential conflict of interest, OHSU researchers served as final manuscript reviewers and corresponding author.
Data availability
The data that has been used is confidential.
References
- 1.van Dorn A. COVID-19 and readjusting clinical trials. Lancet Lond. Engl. 2020;396(10250):523–524. doi: 10.1016/S0140-6736(20)31787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID19-Response8.0_Clinical-Trials_2020824_v1.pdf. https://www.medidata.com/wp-content/uploads/2020/08/COVID19-Response8.0_Clinical-Trials_2020824_v1.pdf
- 3.Steinhubl S.R., Wolff-Hughes D.L., Nilsen W., Iturriaga E., Califf R.M. Digital clinical trials: creating a vision for the future. NPJ Digit Med. 2019;2:126. doi: 10.1038/s41746-019-0203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brøgger-Mikkelsen M., Ali Z., Zibert J.R., Andersen A.D., Thomsen S.F. Online patient recruitment in clinical trials: systematic Review and meta-analysis. J. Med. Internet Res. 2020;22(11) doi: 10.2196/22179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan-Bolyai S., Bova C., Deatrick J.A., et al. Barriers and strategies for recruiting study participants in clinical settings. West. J. Nurs. Res. 2007;29(4):486–500. doi: 10.1177/0193945907299658. [DOI] [PubMed] [Google Scholar]
- 6.Hoeflich C.C., Wang A., Otufowora A., Cottler L.B., Striley C.W. Virtual recruitment and participant engagement for substance use research during a pandemic. Curr. Opin. Psychiatr. 2022;35(4):252–258. doi: 10.1097/YCO.0000000000000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gul R.B., Ali P.A. Clinical trials: the challenge of recruitment and retention of participants. J. Clin. Nurs. 2010;19(1–2):227–233. doi: 10.1111/j.1365-2702.2009.03041. [DOI] [PubMed] [Google Scholar]
- 8.Aronowitz S.V., Engel-Rebitzer E., Dolan A., et al. Telehealth for opioid use disorder treatment in low-barrier clinic settings: an exploration of clinician and staff perspectives. Harm Reduct. J. 2021;18:119. doi: 10.1186/s12954-021-00572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchheit B.M., Wheelock H., Lee A., Brandt K., Gregg J. Low-barrier buprenorphine during the COVID-19 pandemic: a rapid transition to on-demand telemedicine with wide-ranging effects. J. Subst. Abuse Treat. 2021;131:108444. doi: 10.1016/j.jsat.2021.108444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Video Phone | RingCentral Message. https://www.ringcentral.com/
- 11.Doximity https://www.doximity.com
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.