Abstract
As a type of regulated cell death (RCD) mode, pyroptosis plays an important role in several kinds of cancers. Pyroptosis is induced by different stimuli, whose pathways are divided into the canonical pathway and the noncanonical pathway depending on the formation of the inflammasomes. The canonical pathway is triggered by the assembly of inflammasomes, and the activation of caspase-1 and then the cleavage of effector protein gasdermin D (GSDMD) are promoted. While in the noncanonical pathway, the caspase-4/5/11 (caspase 4/5 in humans and caspase 11 in mice) directly cleave GSDMD without the assembly of inflammasomes. Pyroptosis is involved in various cancers, such as lung cancer, gastric cancer, hepatic carcinoma, breast cancer, and colorectal carcinoma. Pyroptosis in gastric cancer, hepatic carcinoma, breast cancer, and colorectal carcinoma is related to the canonical pathway, while both the canonical and noncanonical pathway participate in lung cancer. Moreover, simvastatin, metformin, and curcumin have effect on these cancers and simultaneously promote the pyroptosis of cancer cells. Accordingly, pyroptosis may be an important therapeutic target for cancer.
1. Introduction
Cancer seriously threatens human health worldwide. Based on the most recent data compiled by the International Agency for Research on Cancer (IARC), 19.3 million new cancer cases were diagnosed in 2020 [1]. In 2022, 1,918,030 new cancer cases and 609,360 cancer deaths are predicted to occur in the United States [2]. Lung, stomach, liver, breast, and colon cancer are the top five primary causes of cancer-related death [1]. At present, cancer treatment methods are limited and ineffective, which can only be performed by surgical resection, radiotherapy, or chemotherapy [3]. In addition, the high cost of cancer treatment and the large amount of medical investment also cause a great deal of economic burden to the individual and society [4, 5]. Accordingly, it is crucial to search more efficient and cost-effective ways to treat cancer.
In normal mammalian cells, cells undergo death and renewal as a result of cell aging, infection, or damage, which present homeostasis of cells. Currently, several types of cell death are found, including apoptosis, autophagy, necrosis, necrotic apoptosis, and pyroptosis [6]. Apoptosis and autophagy are important targets of anticancer defense and have been widely studied. Apoptosis is the most common programmed cell death, which is a physiological process involving multiple factors, including the immune response, gene regulation, and signal transduction [7]. Abnormal apoptosis leads to a series of pathological effects, such as tumors, while inducing apoptosis in cancer cells may become a viable therapy for treating tumors [8, 9]. Autophagy is a lysosome-based catabolic process that maintains homeostasis, and the defense capabilities of autophagy are degrading endogenous and foreign substances which are held in vesicles [10]. Autophagy suppresses the development of tumors by eliminating damaged proteins and organelles and avoiding genome damage [11]. Most traditional chemotherapy strategies for cancer are inducing apoptosis or autophagy of tumor cells with erlotinib, paclitaxel, gefitinib, crizotinib, or cisplatin [12–15]. However, studies show that cancer cells undergo infinite proliferation, and cancer cells with a epidermal growth factor receptor (EGFR) wild-type is resistant to chemotherapy drugs [16]; thus, there are few cancer cells which execute apoptosis or autophagy. It indicates that under some circumstances, chemotherapy drugs can not give rise to the apoptosis or autophagy of cancer cells, thus resulting in drug resistance of chemotherapy [17]. Consequently, in order to improve the treatment of cancer, it is crucial to induce another type of cell death. Pyroptosis is a new type of inflammatory cell death which is triggered by the assembly of inflammasomes. The activated caspase-1 results in the cleavage of gasdermin D (GSDMD), the secretion of interleukin-1β (IL-1β) and interleukin-18 (IL-18), and consequent death of cells [18]. Accordingly, pyroptosis is considered as an important target to treat cancer. This article aims to review the morphological characteristics, signaling pathways of pyroptosis, as well as the relationship between pyroptosis and cancer.
2. Characteristics of Pyroptosis
In 1992, a programmed cell death of host macrophages caused by Shigella flexneri was mistaken for apoptosis at that time, but this programmed cell death is actually pyroptosis [19]. Cookson and Brennan first proposed the use of “pyroptosis” to describe this new mode of programmed cell death, where “Pyro” refers to the release of proinflammatory cytokines in 2001 [20]. The Cell Death Nomenclature Committee (CDNC) classified cell death into 13 types based on morphological characteristics, among which pyroptosis was listed in 2009 [21], and in 2018, the definition of pyroptosis was further clarified as “pyroptosis is a kind of regulated cell death (RCD) which minutely depends upon the formation of cell membrane pores by gasdermins and often is caused by inflammatory caspase activation” [6].
The morphological characteristics of pyroptosis include cell enlargement, small amount of DNA damage, and chromatin concentration, but the nucleus remains intact [22]. In the early stage of pyroptosis, there is a very specific DNA damage which is different from that of apoptosis. Compared with apoptosis, the intensity of DNA damage in pyroptotic cells is lower, and the nucleus is intact [23, 24]. The pores which are consisted of gasdermin oligomerization appear on the cell membrane and the cell expands when the pyroptosis occurs [25]. In addition, the proinflammatory cytokines such as the IL-1β and IL-18 are released through those cell membrane pores; consequently, the pyroptosis continues. Accordingly, pyroptosis is different from apoptosis whose morphological characteristics are manifested in cell shrinkage, DNA degradation, nuclear membrane rupture, and cell membrane integrity [6]. It is generally believed that apoptosis is a regular form of cell death, while pyroptosis is induced by intracellular or extracellular stimulation, such as viral, bacterial, toxin, and chemotherapy drugs [26].
3. Signal Pathways of Pyroptosis
The main pathways of pyroptosis are divided into the canonical pathway and the noncanonical pathway according to the upstream signal transducing mechanism [27]. In the canonical pathway, the upstream stimuli lead to the NOD-like receptors (NLRs) which are the members of pattern-recognition receptors (PRRs) that assemble into inflammasomes and then trigger the maturation of pro-caspase-1 to cleave the GSDMD, the pro-IL-1β, and the pro-IL-18 [28, 29]. While in the noncanonical pathway, upstream stimuli directly trigger the cleavage of GSDMD by caspase-4/5/11 (caspase 4/5 in humans and caspase 11 in mice) rather than the assembly of the inflammasomes [30].
3.1. Canonical Pyroptosis Pathway
The pattern-recognition receptor (PRR) is a vital part of our natural immune system [31]. PRRs recognize pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), degrading pathogens and endogenous substances by assembling into inflammasomes [32–34]. In pyroptosis, it is the NLRs which are the PRRs that assemble into inflammasomes [35]. Except for NLRP1, NLRs contain three usual domains: C-terminalleucine-rich repeat (LRR) domain, central nucleotide-binding and oligomerization (NACHT) domain, and N-terminal pyrin domain (PYD) or caspase activation and recruitment domain (CARD) [23, 36]. The LRR domain has the function of ligand recognition as well as automatic inhibition, the NACHT domain activates signal complexes with the help of ATP, and the PYD domain or CARD domain mediates isotypic protein-protein reciprocities [28, 37]. When immune stimulation occurs, the PYD domain binds NLR to apoptosis-associatedspeck-like protein (ASC) which also incorporates a PYD domain through PYD-PYD interaction [38]. The binding reaction triggers the formation of ASC focal points, which recruit pro-caspase-1 and assemble into inflammasome through CARD-CARD interaction [38, 39]. Subsequently, the assembly of inflammasome results in the transformation of pro-caspase-1 to catalytically activated P10 and P20 subunits, which boosts the activation and maturation of pro-IL-1β and pro-IL-18 [40–42].
Activated caspase-1 also facilitates the cleavage of gasdermins besides the pro-IL-1β and pro-IL-18 [43]. The gasdermins are proteins which assemble membrane pores by polymerization, thus causing the outflow of cell contents [44]. Most of gasdermins such as gasdermin A (GSDMA), gasdermin B (GSDMB), gasdermin C (GSDMC), gasdermin D (GSDMD), and gasdermin E (GSDME) except DFNB59 have similar structures and functions of forming pores on the cell membrane [44]. When gasdermin is unactivated, the inhibitory C-terminal domain and the functional N-terminal domain connect together to form complete gasdermin, which cannot assemble membrane pores. However, when gasdermin is activated, the N-terminal domain breaks away from the C-terminal domain so that gasdermin assembles membrane pores and triggers further reactions [45]. Increasing evidence suggest that the GSDMD is more important in membrane pores formation compared with other gasdermins [46, 47]. However, the cellular functions and activation mechanisms of gasdermins remain unclear [48].
The upstream signaling of canonical pyroptosis pathway stimulates the assembly of inflammasome whose PRR is generally NLRP3 [49]. The NLRP3 inflammasome transforms pro-caspase-1 into activatory caspase-1, which promotes the activation of pro-IL-1β and pro-IL-18 [28, 37, 50]. At the same time, caspase-1 causes the cleavage of GSDMD and activates the GSDMD [29, 51]. The activated GSDMD forms the membrane pores, which make cell contents, such as IL-1β and IL-18, to be released [52, 53]. Consequently, the inflammatory response occurs. Undoubtedly, caspase-1 and GSDMD take up irreplaceable roles in the canonical pyroptosis pathway, and the activation of caspase-1 is mainly sparked by the assembly of NLRP3 inflammasome, so the NLRP3 inflammasome ought to occupy another important role in pyroptosis. It is reported that the reactive oxygen species (ROS)/nuclear factor kappa B (NF-κB) signaling pathway takes part in the activation of NLRP3 inflammasome [54]. ROS promotes the release and activation of proinflammatory transcription factors such as NF-κB, which mainly regulates the NLRP3 inflammasome [55, 56] (Figure 1).
Figure 1.
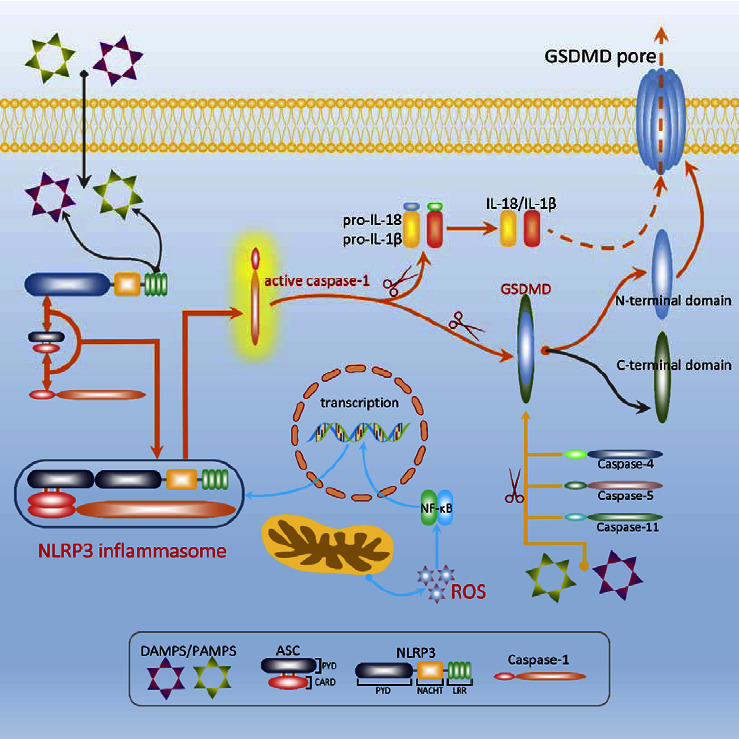
In the canonical pathway of pyroptosis, NLRP3 recognizes stimulus (DAMPS/PAMPS) and binds to the ACS through PYD-PYD interaction and then occurs the formation of ASC focal points which triggers the recruitment of pro-caspase-1 to assemble into NLRP3 inflammasome. In the inflammasome, pro-caspase-1 is cleaved into the active forms, which catalyzes the cleavage of pro-IL-1β, pro-IL-18, and GSDMD. Mature GSDMD forms pores on cell membrane, leading to the outflow of cell contents such as IL-1β and IL-18, thus exhibiting an inflammatory response. It is noteworthy that the transcription of NLRP3 genes is regulated by the ROS/NF-κB signaling pathway. In the noncanonical pathway of pyroptosis, caspase-4/5/11 directly recognizes stimulus and gets activated, then causing cleaving GSDMD to promote pyroptosis.
3.2. Noncanonical Pathway of Pyroptosis
The downstream of noncanonical pyroptosis pathway is the same as that of canonical pyroptosis pathway and presents GSDMD as the effector protein which causes the formation of cell membrane pores [30, 47]. In addition, the morphological characteristics of the noncanonical pyroptosis pathway are basically the same as that of the canonical pyroptosis pathway. However, the upstream of noncanonical pyroptosis pathway is substantially different from that of the canonical pathway. In the canonical pathway, the assembly of the inflammasomes promotes the maturation of caspase-1, which not only boosts the proteolytic maturation of pro-IL-1β and pro-IL-18 but also promotes the cleavage of GSDMD to form cell membrane pores. While in the noncanonical pyroptosis pathway, the caspase-4/5/11 directly receives stimulation, binding to stimulating protein which mainly is the lipopolysaccharides (LPS) of Gram-negative bacteria and then promote the cleavage of GSDMD to form pores rather than assemble into inflammasomes [29, 30, 43, 57, 58]. At the same time, the amino-terminal fragments which are produced in the process of caspase-11 cleaving GSDMD promote the NLRP3 inflammasome and the caspase-1 to be activated, which suggest that the noncanonical pathway crosstalks with the canonical pathway [30] (Figure 1).
4. Pyroptosis and Cancer
4.1. Pyroptosis and Lung Cancer
Lung cancer (LC) seriously threatens human health worldwide. The survival rate for a period of 5 years is less than 15% [59, 60]. Lung cancer is included into two subtypes which are small-cell lung cancer (SCLC) and nonsmall-cell lung cancer (NSCLC), and the NSCLC accounts for about 85% of lung cancer cases [61]. Chemotherapy is one of the conventional treatment methods of LC [62]. However, chemotherapy is less sensitive and less effective in the therapy of LC [63] because cancer cells have multiple strategies to circumvent or limit apoptosis which is a normal mechanism to protect cells [64]. Accordingly, it is very important for LC to propose new therapeutic strategies.
SCLC accounts for approximately 15% of all lung cancers and is classified as a high-grade neuroendocrine (NE) tumor which has a high death rate and poor prognosis [65]. However, there are only a few studies to explore the relationship between SCLC and pyroptosis. It is reported that chemosensitivity is related to the pyroptosis which is connected with the expression of yes-associated protein (YAP) and GSDME, and the activation of YAP suppresses GSDME expression to enhance the chemoresistance in SCLC cells, while the inactivation of YAP in SCLC tumor cells switches cell death from apoptosis to pyroptosis [66].
In pyroptosis, it is clear that the assembly of NLRP3 inflammasome is closely related to the activation of caspase-1, which is involved in the cleavage and maturation of the GSDMD, and the GSDMD is the executor of pyroptosis. It indicates that NLRP3 inflammasome, caspase-1, and GSDMD are crucial factors in the process of pyroptosis. Accordingly, inducing pyroptosis of NSCLC cells through the NLRP3/caspase-1/GSDMD pathway may be potential targets for inhibiting the tumor progression of NSCLC [54]. Wang et al. demonstrated that caspase-1 was downregulated in NSCLC tumor tissues and found that simvastatin (SIM), an anti-hyperlipidaemia drug, inhibits the growth of NSCLC by activating caspase-1-dependent pyroptosis in xenograft mouse models and in A549 and H1299 lung cancer cells [67]. Additionally, it is suggested that SIM also induces apoptosis by downregulating the cyclin-dependent kinases (CDKs) and matrix metalloproteinases-9 (MMP-9) levels or by inhibiting the activity of proteasome and upregulating p21 and p53 [68, 69]. Further research confirms that SIM induces ROS generation and accumulation in mitochondria and cytosol, thus leading to apoptosis of NSCLC cells [70–72]. The polyphyllin VI (PPVI), a chief saponin extracted from trillium tschonoskii maxim (TTM), induces caspase-1-dependent pyroptosis through the ROS/NF-κB/NLRP3/GSDMD signal axis and inhibits the progression of NSCLC [54]. In addition, PPVI promotes the accumulation of ROS and the cleavage of caspase-3, downregulates the B-celllymphoma-2 (Bcl-2) expression, upregulates the Bcl-2-associated X (Bax) and p53 expression, and arrests the cell cycle in G2/M; thus, apoptosis of NSCLC cells is triggered [73, 74]. Meanwhile, PPVI also exerts the anti-NSCLC effect by inducing apoptosis through the phosphatidylinositol-3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway [75]. The cucurbitacin B (CuB), a compound extracted from muskmelon pedicel, inhibits NSCLC by bounding to toll-like receptor 4 (TLR4) to activate the NLRP3 inflammasome and triggering GSDMD dependent pyroptosis [76]. Also, CuB enhances the mitochondrial ROS to trigger pyroptosis of NSCLC cell [76]. Moreover, CuB induces the apoptosis of NSCLC cells by inhibiting the long noncoding RNA Xinactive-specific transcript (lncRNA-XIST)/miR-let-7c/IL-6/signal transducer and activator of transcription 3 (STAT3) axis and suppressing the mitogen-activated protein kinases (MAPK) and PI3K pathways [77, 78]. Additionally, CuB arrests the cell cycle of NSCLC cells at the G2/M phase and downregulates the level of Bcl-2, thus inducing apoptosis via the STAT3 pathway [79, 80]. In addition, CuB induces apoptosis by interfering with EGFR activation and its downstream signal path which includes Akt and extracellular-signal-regulated kinases (ERK) [81, 82]. It is presented that dasatinib (DAS), a multikinase inhibitor, promotes the cleavage and secretion of the GSDMD and GSDME which induce the pyroptosis of A549 cells, which thus inhibits the progress of NSCLC [83]. DAS also induces the apoptosis of lung cancer cells by upregulating the ROS level or downregulating the Bcl-2 family member Bcl-xL [84, 85]. In addition, CD8 (+) T cells require GSDMD for an immune response to NSCLC, while GSDMD deficiency results in the cytolytic capacity of CD8 (+) T cells [86].
The ROS/NF-κB pathway is involved in the expression of NLRP3 inflammasome which indicates that both the ROS and the NF-κB act vital roles in pyroptosis [56, 87, 88]. Likewise, both ROS and NF-κB are targeted to induce pyroptosis in NSCLC therapy [54]. Chalcone, a natural structure, induces pyroptosis by the upregulation of ROS and inhibits the progress of A549 and H1975 cells [89]. Meanwhile, chalcone upregulates the caspase-3, caspase-8, Bax, and ROS and inhibits the cell cycle at the G2/M phase ultimately resulting in apoptosis of A549 cell [90, 91]. Metformin (MET), a biguanide drug, induces pyroptosis of tumors by the adenosine monophosphate (AMP)-activated protein kinase (AMPK)/Sirtuin-1 (SIRT1)/NF-κB/caspase-3/GSDME pathway [92]. The mechanisms are that MET upregulates the AMPK/SIRT1 pathway and increases the expression of NF-κB, activating the cleavage of GSDME by caspase-3 [92]. Moreover, MET induces caspase-3-dependent apoptosis through regulating SIRT1 and activating the c-junN-terminal kinase (JNK)/p38 MAPK pathway [93, 94]. Piperlongumine (PL) analogue L50377, a natural product with less toxicity, is applied to induce pyroptosis of NSCLC through upregulating the level of ROS and activating the expression of NF-κB [95]. It is shown that PL also induces apoptosis and autophagy of NSCLC cells through activating the PI3K/Akt/mTOR pathway, upregulating the microRNA-34b-3p, and downregulating the transforming growth factor beta type I receptor (TGFBR1) [96, 97]. It is reported that 13 d (a modified EF24 with low toxicity) or L61H10 (a thiopyran derivative) may mediate the apoptosis-pyroptosis switch in NSCLC through the NF-κB signaling pathway [98, 99]. Furthermore, EF24 analogues promote ROS generation and accumulation, resulting in apoptosis of NSCLC cells [100, 101].
Apurinic endonuclease 1 (APE1) acts as a key factor in base excision repair (BER) and exerts the function of apurinic sites excision [102]. It is reported that the poor prognosis of NSCLC links with high level of APE1 [103–105]. The NO.0449-0145 (a small molecule compound) improves the condition of NSCLC by inhibiting the expression of APE1 and inducing pyroptosis [106]. Furthermore, Zhu et al. suggested that inhibiting the activation of APE1 leads to elevation of the p53 protein level and increase of the NSCLC apoptosis [107, 108].
Maternal embryonic leucine zipper kinase (MELK) is a carcinogenic kinase and is essential in NSCLC mitotic progression, metastasis by regulating the process of cell death [109]. It is reported that MEIK has overexpression in cancer cells [110]. Tang et al. demonstrated that OTSSP167, as a potent inhibitor for MELK, blocks the G2/M phase cycle of lung adenocarcinoma (LUAD) cells by inhibiting MELK to trigger the pyroptosis [111]. The inhibition of MELK also decreases its downstream forkhead box protein M1 (FOXM1) activation and Akt expression in lung cancer cells, leading to apoptosis of NSCLC cells [112].
Other member of the gasdermins such as GSDME serves as special targets to induce pyroptosis in NSCLC therapy [113]. It is reported that paclitaxel and cisplatin inhibit A549 lung cancer cells by inducing pyroptosis through the caspase-3/GSDME pathway [114]. DAS induces the pyroptosis of A549 cells by upregulating the level of GSDME [83].
Intracellular LPS induces the pyroptosis through the noncanonical pathway [115, 116]. In addition, LPS may directly lead to regression of some tumor; however, the underlying mechanism remains unclear [117]. Currently, it is suggested that the secretoglobin (SCGB) 3A2, as a multifunctional secreted protein, eliminates human lung adenocarcinoma cells through noncanonical inflammasome pathway mediated by LPS [118]. Human NSCLC cells with SCGB3A2-sensitivity express caspase-4 which is a crucial molecule of the noncanonical inflammasome pathway [118].
It is proposed that the pyroptosis is induced by inhibiting the lncRNA-XIST through the Mir-335/SOD2/ROS signaling pathway, and then the NSCLC is inhibited [119]. In addition, the knockout of lncRNA-XIST gene induces the pyroptosis of tumor cells, which suppresses the growth of NSCLC cells and promotes the chemotherapy sensitivity of cisplatin [120]. Moreover, the upregulation of the p53 inhibits tumor growth by promoting pyroptosis in NSCLC [121]. In addition, 4-hydroxybenzoic acid (4-HBA) leads to the activation of pyroptosis by accelerating the transcription of caspase-1, IL-1β, and IL-18 genes in A549 cells [122] (Table 1).
Table 1.
The mechanism of different medicines to induce pyroptosis or apoptosis in different cancers.
| Pyroptosis | Apoptosis | |
|---|---|---|
| Lung cancer | ||
| Simvastatin | Activates caspase-1-dependent pyroptosis [67] | Downregulates the CDKs and MMP-9 levels; inhibits the activity of proteasome; and upregulates p21, p53, and ROS [68–72] |
| Polyphyllin VI | Activates the ROS/NF-κB/NLRP3/caspase-1/GSDMD signal axis [54] | Upregulates ROS, caspase-3, Bax, and p53; downregulates the Bcl-2; and regulates the PI3K/Akt/mTOR pathway [73–75] |
| Cucurbitacin B | Upregulates the NLRP3 inflammasome, GSDMD, and ROS levels [76] | Inhibits the lncRNA-XIST/miR-let-7c/IL-6/STAT3 axis; suppresses the MAPK and PI3K pathways; and interferes with EGFR activation [77–82] |
| Dasatinib | Promotes the cleavage and secretion of the GSDMD and GSDME [83] | Upregulates ROS level and downregulates Bcl-2 family member Bcl-xL [84, 85] |
| Chalcone | Upregulates the level of ROS [89] | Upregulates the capspase-3, caspase-8, Bax, and ROS and inhibits cell cycle at the G2/M phase [90, 91] |
| Metformin | Activates AMPK/SIRT1/NF-κB/caspase3/GSDME pathway [92] | Regulates SIRT1 and activates the JNK/p38 MAPK pathway [93, 94] |
| Piperlongumine | Upregulates the level of ROS and activates the expression of NF-κB [95] | Activates the PI3K/Akt/mTOR pathway; upregulates the microRNA-34b-3p; and downregulates the TGFBR1 [96, 97] |
| EF24 | Mediates the apoptosis-pyroptosis switch through the NF-κB signaling pathway [98] | Promotes ROS generation and accumulation [100, 101] |
| L61H10 | Mediates the apoptosis-pyroptosis switch through the NF-κB signaling pathway [99] | |
| NO.0449-0145 | Inhibits the expression of APE1 [106] | |
| OTSSP167 | Blocks the G2/M phase cycle by inhibiting MELK [111] | |
| secretoglobin3A2 | Activates the noncanonical inflammasome pathway mediated by LPS [118] | |
| 4-Hydroxybenzoic acid | Accelerates the transcription of caspase-1, IL-1β, and IL-18 genes [122] | |
|
| ||
| Gastric cancer | ||
| Simvastatin | Activates caspase-3/GSDME expression [136] | Suppresses the expression of β-catenin and inhibits the activation of YAP and NF-κB [137, 138] |
| Icariin | Activates the NLRP3 inflammasomes [139] | Regulates the hsa_circ_0003159/eIF4A3/bcl-2 axis [140] |
| Diosbulbin-B | Activates NLRP3-mediated pyroptosis by downregulating PD-L1 [141] | Downregulates the level of CDR1 [142] |
|
| ||
| Hepatic carcinoma | ||
| Crizotinib | Accumulates the ROS in cancer cells [159] | Inhibits the activation of ALK, Akt, and ERK [160] |
| Cannabidiol | Regulates the caspase-3/GSDME pathway [161] | Arrests the G0/G1 phase in the cell cycle and induces mitochondrial-dependent apoptosis [162] |
| Metformin | Promots FOXO3 expression and activates NLRP3 transcription [163] | Regulates AMPK/p53/p38/miR-23a/FOXA1 pathway, regulates PI3K/Akt/mTOR pathway, and downregulates Bcl-2 [164–167] |
| Curcumin | Increases the generation and accumulation of ROS [168] | Promotes the P53-dependent apoptosis and inhibits the PI3K/Akt/GSK-3β signaling pathway [169–171] |
| 17β-estradiol | Induces the activation of NLRP3 inflammasome [173] | Increases FOXO3 phosphorylation, induces oxidative stress, and downregulates IL-6/STAT3 signaling [174, 175] |
| Berberine | Induces caspase-1-dependent pyroptosis [176] | Regulates NF-κB/p65 pathway and induces adenosine AMPK-mediatedcaspase-dependent apoptosis [177, 178] |
| Euxanthone | Promotes pyroptosis in a caspase-dependent manner [156] | |
| Alpinumisoflavone | Induces NLRP3 inflammasome-mediated pyroptosis [157] | |
|
| ||
| Breast cancer | ||
| Polydatin | Downregulates the JAK2 and STAT3 levels [184] | Suppresses the ROS/PI3K/Akt pathway [185] |
| Cisplatin | Activates the NLRP3/caspase-1/GSDMD pathway [186] | Downregulates the PI3K/Akt/mTOR signaling pathway [187] |
| Dihydroartemisinin | Activates the AIM2/caspase-3/GSDME axis [188] | Upregulates the expression of caspase-8/9 and downregulates the level of Bcl-2 [189] |
| Nobiletin | Regulates the microRNA-200b/JAZF1/NF-κB [190] | Decreases the Bcl-2 and Bcl-xL; inhibits Akt/mTOR pathway; and increases the Bax, p53, and caspase-3 [191, 192] |
| Tetraarsenic hexoxide | Activates the ROS/caspase-3/GSDME aixs [193] | |
| Triclabendazole | Induces GSDME-dependent pyroptosis by activating caspase-3 [194] | |
|
| ||
| Colorectal carcinoma | ||
| Arsenic trioxide | Upregulates the expression of caspase-1 and promotes the formation of inflammasomes [195] | Inhibits the activation of telomerase and induces caspase-3-dependent apoptosis [196] |
| Decitabine | Upregulates the expression of inflammasomes [197] | Increases the expression of miR-133b [198] |
However, the high expression of some key molecules of pyroptosis may not lead to amelioration but results in poor prognosis and deterioration of NSCLC in some specific cases. It is suggested that GSDMC is overexpressed in LUAD patients who have poor prognosis [123]. In addition, the high level of GSDMD do not induce pyroptosis but is associated with aggressive characteristics, such as more advanced tumor-lymph node metastasis (TNM) phase, larger tumor size, and poorer prognosis in NSCLC [124]. Zou et al. suggested that NLRP3 promotes the cell proliferation and the migration of NSCLC [125]. Accordingly, more researches are needed to confirm the role of pyroptosis in NSCLC.
4.2. Pyroptosis and Gastric Cancer
Gastric cancer (GC) is one of the most common cancers which is seriously harmful to human health [2, 126]. Accordingly, it is very crucial to search new effective methods to treat GC. Here, we discuss some strategies for the treatment of GC by inducing pyroptosis and apoptosis. The pyroptosis-related risk signals and the pyroptosis-related genes (PRGs) in GC may potentially predict the treatment benefit, the prognosis, the survival of individuals, and their response to immunotherapy [127–131]. Moreover, the pyroptosis-related protein GSDMD may inhibit the cell proliferation of GC, and when GSDME is knocked down, the growth of GC cells is affected [132, 133]. It is demonstrated that the release of ROS by sonodynamic therapy (SDT) treatment induces the pyroptosis of GC cells and plays the antitumor function [134]. It is reported that treating GC cells with famotidine triggers the activation of NLPR3 inflammasomes and leads to the mature and secretion of GSDME and IL-18, resulting in the pyroptosis of GC cells [135]. It is presented that SIM activates caspase-3/GSDME expression and thereby induces pyroptosis of GC [136]. In addition, SIM treatment suppresses the expression of β-catenin, inhibits the activation of YAP and NF-κB, and thus promotes the apoptosis in GC cells [137, 138]. Icariin (ICA), an active component from TCM epimedium grandiflorum, inhibits the progression of GC cells by activating the NLRP3 inflammasomes and inducing pyroptosis [139]. Meanwhile, ICA could effectively induce apoptosis via hsa_circ_0003159/eIF4A3/bcl-2 axis to reduce the GC cell activity [140]. It is confirmed that diosbulbin-B (DB) is effective to activate NLRP3-mediated pyroptosis in GC by downregulating programmed death ligand-1(PD-L1) [141]. Furthermore, DB inhibits the proliferation of GC cells by knocking-down cerebellar degeneration-related protein 1 (CDR1) (a type of circular RNA) to promote apoptosis [142] (Table 1).
However, the cytotoxin-related gene A (CagA) protein, an important pathogenic factor of Helicobacter pylori (H. pylori) [143], promotes the invasion and migration of GC cells by activating NLRP3 inflammasome, while the suppression of H. pylori-triggered inflammatory response and the depression of pyroptosis via the ROS/NLRP3/caspase-1/IL-1β pathway may suppress the progression of GC [144]. These results indicate that there is a close and complex relationship between GC and pyroptosis, while more researches are necessary in future.
4.3. Pyroptosis and Hepatic Carcinoma
Hepatic carcinoma (HCC) is a common kind of cancers which seriously hazards human health [2]. However, increasing researches demonstrate that HCC cells present multiple strategies to achieve drug resistance [145, 146]. Accordingly, it is necessary to search effective strategy to treat HCC. It is confirmed that PRGs such as pyroptosis-related lncRNA may serve as a promising biomarker for HCC patients to predict the prognosis and guide precision drug treatment and immunotherapy [147–151]. Meanwhile, pyroptosis-related proteins especially the GSDMD and the GSDME have the potential to become crucial biomarkers for the diagnosis and prognosis of HCC, which provide a new insight for the development of therapeutic targets [152, 153]. NIMA-related kinase 7 (NEK7) is a serine/threonine kinase which progresses the eukaryotic cell cycle [154]. Knocking-down of NEK7 in HCC cells significantly upregulates the expression of NLRP3, caspase-1, and GSDMD to induce pyroptosis and inhibit the migration of HCC cells [155]. It is revealed that euxanthone promotes pyroptosis in a caspase-dependent manner in HCC cells, and alpinumisoflavone inhibits the growth of HCC cells by inducing NLRP3 inflammasome-mediated pyroptosis [156, 157]. Miltirone, a derivative of phenanthrene-quinone isolated from the root of Salvia miltiorrhiza Bunge, promotes the accumulation of intracellular ROS and induces the GSDME-dependent pyroptosis of HCC [158]. Likewise, crizotinib (CRIZO) increases ROS in HL-7702 cells to promote pyroptosis and inhibit HCC [159]. Furthermore, CRIZO induces apoptosis and suppresses the proliferation of HCC cells by inhibiting the phosphorylation of the anaplastic lymphoma kinase (ALK), Akt, and ERK [160]. In addition, Cannabidiol (CBD), a cannabis sativa constituent, may induce pyroptosis via caspase-3/GSDME pathway to inhibit the growth of HCC cells in vivo and in vitro [161]. Moreover, CBD arrests the G0/G1 phase in the cell cycle and induces mitochondrial-dependent apoptosis in HCC cell lines [162]. It is confirmed that MET induces the pyroptosis by promoting forkhead box protein O3 (FOXO3) expression and activating NLRP3 transcription to suppress the progression of HCC cells [163]. Meanwhile, MET induces apoptosis in HCC through the AMPK/p53/p38/miR-23a/FOXA1 pathway or PI3K/Akt/mTOR pathway [164–166]. Furthermore, MET induces the downregulation of Bcl-2 in HCC cells to enhance apoptosis [167]. It is demonstrated that curcumin (CUR) induces pyroptosis in HspG2 cells by increasing ROS [168]. In addition, CUR may inhibit the growth of HepG2 cells by promoting the P53-dependent apoptosis [169]. Moreover, CUR triggers mitochondrial apoptosis in HCC cells by inhibiting the PI3K/Akt/glycogen synthase kinase-3β (GSK-3β) signaling pathway [170, 171]. 17β-estradiol (E2) is a kind of hormonally active compounds [172]. It is suggested that E2-induced activation of the NLRP3 inflammasome may serve as a suppressor in HCC progression [173]. In addition, E2 may promote apoptosis in HepG2 cells by increasing FOXO3 phosphorylation and inducing oxidative stress [174]. Furthermore, E2 inhibits the proliferation of HCC cells through downregulation of IL-6/STAT3 signaling and arresting cell cycle at the G2/M phase [175]. It is confirmed that berberine, a kind of isoquinoline alkaloids, inhibits the progression of HepG2 cells by inducing caspase-1-dependent pyroptosis both in vitro and in vivo or promoting apoptosis through the NF-κB/p65 pathway [176, 177]. Additionally, berberine effectively inhibits the growth of HHC cells by inducing adenosine AMPK-mediatedcaspase-dependent apoptosis [178]. These researches highlight the possibilities of inducing pyroptosis or apoptosis for treating HCC and indicate that more studies are needed to clarify the mechanism of pyroptosis in HCC (Table 1).
4.4. Pyroptosis and Breast Cancer
Breast cancer (BC) does great harm to women health which ranks second among cancer-related death in women [2]. It is critical to seek a valid treatment strategy for BC. It is confirmed that PRGs may serve as an important prognostic predictor and a chemotherapy target for the treatment of BC [179–183]. In addition, polydatin (PD) downregulates the janus kinase (JAK) 2 and STAT3 levels thus induces pyroptosis, which play an anticancer role in triple-negative BC (TNBC) [184]. Moreover, PD induces apoptosis by suppressing the ROS/PI3K/Akt pathway to inhibit cell proliferation, migration, and invasion of BC cells [185]. It is discovered that cisplatin (DDP) activates the NLRP3/caspase-1/GSDMD pathway to induce pyroptosis of BC cells to exert antitumor effects [186]. Furthermore, DDP induces apoptosis which is connected with downregulating the PI3K/Akt/mTOR signaling pathway in BC cells [187]. It is reported that pyroptosis of BC cells is induced with the AIM2/caspase-3/GSDME axis being activated when BC cells are administrated by dihydroartemisinin (DHA) [188]. Meanwhile, administration of DHA dramatically upregulates the expression of caspase-8/9 and downregulates the level of Bcl-2 and thus results in apoptosis and G0/G1 cell cycle arrest of BC cells [189]. It is discovered that Nobiletin induces the pyroptosis of BC cells by regulating the MicroRNA-200b/zinc finger gene 1 (JAZF1)/NF-κB pathway [190]. In addition, Nobiletin decreases the expression of Bcl-2, Bcl-xL, increases the expression of Bax, p53, and caspase-3, and inhibits the Akt/mTOR pathway to induce apoptosis of BC cells and suppress the progression of BC [191, 192]. Tetraarsenic hexoxide induces the pyroptotic cell death through the ROS/caspase-3/GSDME axis to suppress the progression of TNBC cells [193]. Likewise, Triclabendazole induces GSDME-dependent pyroptosis by activating caspase-3 in BC cells [194]. Accordingly, pyroptosis provides a new therapeutic approach for patients with BC (Table 1).
4.5. Pyroptosis and Colorectal Carcinoma
Colorectal carcinoma (CRC) is the third most common form of cancer in adults which has a poor prognosis and significantly damages the patient's daily life and mental health [2]. Effective therapeutic strategies are urgently needed to achieve better prognosis and therapeutic outcomes of CRC. It is demonstrated that arsenic trioxide (ATO) and ascorbic acid (AA) corporately upregulates the expression of caspase-1 and promotes the formation of inflammasomes to induce pyroptosis in CRC [195]. Meanwhile, ATO inhibits CRC cells growth by inhibiting the activation of telomerase and inducing caspase-3-dependent apoptosis [196]. It is presented that the expression of inflammasomes is increased both in vitro and in vivo after treating CRC cells with decitabine (DAC), which suggests that DAC suppresses the growth of colon cancer by inducing pyroptosis [197]. In addition, DAC increases the expression of miR-133b and triggers the apoptosis in CRC cells [198]. Thus, pyroptosis may be a target of ATO and DAC on CRC (Table 1).
5. Conclusion
As a type of RCD mode, pyroptosis plays an important role in several kinds of cancers, whose pathways are divided into the canonical and noncanonical pathway depending on whether formation of the inflammasomes. The canonical pathway is triggered by the assembly of inflammasomes and mainly regulated by the activation of caspase-1. Activated caspase-1 not only promotes the cleavage of effector protein GSDMD but also promotes the proteolytic maturation of proinflammatory cytokines IL-1β and IL-18, resulting in the morphological characteristics of pyroptosis. While in the noncanonical pathway, the caspase-4/5/11 directly cleave GSDMD, resulting in the pyroptosis. In addition, pyroptosis is affected by the ROS and NF-κB which influence the upstream pathway of pyroptosis.
Pyroptosis in gastric cancer, hepatic carcinoma, breast cancer, and colorectal carcinoma is related to the canonical pathway, while both the canonical and noncanonical pathway participate in lung cancer. Moreover, simvastatin, metformin, and curcumin have effect on these cancers and simultaneously promote the pyroptosis of cancer cells. Accordingly, pyroptosis may be an important therapeutic target to cancer though the relationship between pyroptosis and a few cancers such as CRC and SCLC remain unclear, and more researches on pyroptosis in these cancers are needed in future.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 82160007) and the Yunnan Provincial Science and Technology Department (No. 2019FE001 (-058)).
Data Availability
No data were used to support this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Cao W., Chen H. D., Yu Y. W., Li N., Chen W. Q. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chinese Medical Journal . 2021;134(7):783–791. doi: 10.1097/cm9.0000000000001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R. L., Miller K. D., Fuchs H. E., Jemal A. Cancer statistics, 2022. CA: A Cancer Journal for Clinicians . 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Roy P. S., Saikia B. J. Cancer and cure: a critical analysis. Indian Journal of Cancer . 2016;53(3):441–442. doi: 10.4103/0019-509X.200658. [DOI] [PubMed] [Google Scholar]
- 4.Carrera P. M., Kantarjian H. M., Blinder V. S. The financial burden and distress of patients with cancer: understanding and stepping-up action on the financial toxicity of cancer treatment. CA: A Cancer Journal for Clinicians . 2018;68(2):153–165. doi: 10.3322/caac.21443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richard P., Patel N., Lu Y. C., Walker R., Younis M. The financial burden of cancer on families in the United States. International Journal of Environmental Research and Public Health . 2021;18(7):p. 3790. doi: 10.3390/ijerph18073790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galluzzi L., Vitale I., Aaronson S. A., et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ . 2018;25(3):486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerr J. F. R., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. British Journal of Cancer . 1972;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhirnov O. P., Konakova T. E., Wolff T., Klenk H. D. NS1 protein of influenza A virus down-regulates apoptosis. Journal of Virology . 2002;76(4):1617–1625. doi: 10.1128/jvi.76.4.1617-1625.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn G. P., Bruce A. T., Ikeda H., Old L. J., Schreiber R. D. Cancer immunoediting: from immunosurveillance to tumor escape. Nature Immunology . 2002;3(11):991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 10.Mizushima N., Levine B., Cuervo A. M., Klionsky D. J. Autophagy fights disease through cellular self-digestion. Nature . 2008;451(7182):1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giansanti V., Torriglia A., Scovassi A. I. Conversation between apoptosis and autophagy: “Is it your turn or mine?”. Apoptosis . 2011;16(4):321–333. doi: 10.1007/s10495-011-0589-x. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Z. Q., Yu Z. Y., Li J., Ouyang X. N. Gefitinib induces lung cancer cell autophagy and apoptosis via blockade of the PI3K/AKT/mTOR pathway. Oncology Letters . 2016;12(1):63–68. doi: 10.3892/ol.2016.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaushal G. P., Kaushal V., Herzog C., Yang C. Autophagy delays apoptosis in renal tubular epithelial cells in cisplatin cytotoxicity. Autophagy . 2008;4(5):710–712. doi: 10.4161/auto.6309. [DOI] [PubMed] [Google Scholar]
- 14.Ajabnoor G. M. A., Crook T., Coley H. M. Paclitaxel resistance is associated with switch from apoptotic to autophagic cell death in MCF-7 breast cancer cells. Cell Death & Disease . 2012;3(1) doi: 10.1038/cddis.2011.139.e260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo L. X., Li Y., Liu Z. Q., et al. Honokiol induces apoptosis, G1 arrest, and autophagy in KRAS mutant lung cancer cells. Frontiers in Pharmacology . 2017;8:p. 199. doi: 10.3389/fphar.2017.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiang M., Jiang H. G., Shu Y., et al. Bisdemethoxycurcumin enhances the sensitivity of non-small cell lung cancer cells to icotinib via dual induction of autophagy and apoptosis. International Journal of Biological Sciences . 2020;16(9):1536–1550. doi: 10.7150/ijbs.40042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu W. J., Du Y., Wen R., Yang M., Xu J. Drug resistance to targeted therapeutic strategies in non-small cell lung cancer. Pharmacology & Therapeutics . 2020;206 doi: 10.1016/j.pharmthera.2019.107438.107438 [DOI] [PubMed] [Google Scholar]
- 18.Fang Y., Tian S., Pan Y., et al. Pyroptosis: a new Frontier in cancer. Biomedicine & Pharmacotherapy . 2020;121 doi: 10.1016/j.biopha.2019.109595.109595 [DOI] [PubMed] [Google Scholar]
- 19.Zychlinsky A., Prevost M. C., Sansonetti P. J. Shigella flexneri induces apoptosis in infected macrophages. Nature . 1992;358(6382):167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 20.Cookson B. T., Brennan M. A. Pro-inflammatory programmed cell death. Trends in Microbiology . 2001;9(3):113–114. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 21.Kroemer G., Galluzzi L., Vandenabeele P., et al. Classification of cell death: recommendations of the nomenclature committee on cell death 2009. Cell Death Differ . 2009;16(1):3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergsbaken T., Fink S. L., Cookson B. T. Pyroptosis: host cell death and inflammation. Nature Reviews Microbiology . 2009;7(2):99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Y. J., Zheng L., Hu Y. W., Wang Q. Pyroptosis and its relationship to atherosclerosis. Clinica Chimica Acta . 2018;476:28–37. doi: 10.1016/j.cca.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Jorgensen I., Miao E. A. Pyroptotic cell death defends against intracellular pathogens. Immunological Reviews . 2015;265(1):130–142. doi: 10.1111/imr.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Man S. M., Karki R., Kanneganti T. D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunological Reviews . 2017;277(1):61–75. doi: 10.1111/imr.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang R., Xu J., Zhang B., et al. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. Journal of Hematology & Oncology . 2020;13(1):p. 110. doi: 10.1186/s13045-020-00946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kayagaki N., Warming S., Lamkanfi M., et al. Non-canonical inflammasome activation targets caspase-11. Nature . 2011;479(7371):117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 28.Franchi L., Warner N., Viani K., Nuñez G. Function of Nod-like receptors in microbial recognition and host defense. Immunological Reviews . 2009;227(1):106–128. doi: 10.1111/j.1600-065x.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi J., Zhao Y., Wang K., et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature . 2015;526(7575):660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 30.Kayagaki N., Stowe I. B., Lee B. L., et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature . 2015;526(7575):666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 31.Mogensen T. H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clinical Microbiology Reviews . 2009;22(2):240–273. doi: 10.1128/cmr.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeuchi O., Akira S. Pattern recognition receptors and inflammation. Cell . 2010;140(6):805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 33.Latz E., Xiao T. S., Stutz A. Activation and regulation of the inflammasomes. Nature Reviews Immunology . 2013;13(6):397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Man S. M., Kanneganti T. D. Regulation of inflammasome activation. Immunological Reviews . 2015;265(1):6–21. doi: 10.1111/imr.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malik A., Kanneganti T. D. Inflammasome activation and assembly at a glance. Journal of Cell Science . 2017;130(23):3955–3963. doi: 10.1242/jcs.207365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carriere J., Dorfleutner A., Stehlik C. NLRP7: from inflammasome regulation to human disease. Immunology . 2021;163(4):363–376. doi: 10.1111/imm.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinon F., Burns K., Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Molecular Cell . 2002;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 38.Ge X., Li W., Huang S., et al. The pathological role of NLRs and AIM2 inflammasome-mediated pyroptosis in damaged blood-brain barrier after traumatic brain injury. Brain Research . 2018;1697:10–20. doi: 10.1016/j.brainres.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Broz P., Dixit V. M. Inflammasomes: mechanism of assembly, regulation and signalling. Nature Reviews Immunology . 2016;16(7):407–420. doi: 10.1038/nri.2016.58. [DOI] [PubMed] [Google Scholar]
- 40.Moore K. J., Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell . 2011;145(3):341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajamäki K., Lappalainen J., Oörni K., et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One . 2010;5(7) doi: 10.1371/journal.pone.0011765.e11765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duewell P., Kono H., Rayner K. J., et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature . 2010;464(7293):1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Z., Wang C., Yang J., et al. Caspase-1 engages full-length gasdermin D through two distinct interfaces that mediate caspase recruitment and substrate cleavage. Immunity . 2020;53(1):106–114.e5. doi: 10.1016/j.immuni.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Broz P., Pelegrín P., Shao F. The gasdermins, a protein family executing cell death and inflammation. Nature Reviews Immunology . 2020;20(3):143–157. doi: 10.1038/s41577-019-0228-2. [DOI] [PubMed] [Google Scholar]
- 45.Broz P. Immunology: caspase target drives pyroptosis. Nature . 2015;526(7575):642–643. doi: 10.1038/nature15632. [DOI] [PubMed] [Google Scholar]
- 46.Kovacs S. B., Miao E. A. Gasdermins: effectors of pyroptosis. Trends in Cell Biology . 2017;27(9):673–684. doi: 10.1016/j.tcb.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He W. T., Wan H., Hu L., et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Research . 2015;25(12):1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi J., Gao W., Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends in Biochemical Sciences . 2017;42(4):245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 49.Haque M. E., Akther M., Jakaria M., Kim I. S., Azam S., Choi D. K. Targeting the microglial NLRP3 inflammasome and its role in Parkinson’s disease. Movement Disorders . 2020;35(1):20–33. doi: 10.1002/mds.27874. [DOI] [PubMed] [Google Scholar]
- 50.Fernandes-Alnemri T., Wu J., Yu J. W., et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ . 2007;14(9):1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fink S. L., Cookson B. T. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cellular Microbiology . 2006;8(11):1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 52.Sborgi L., Rühl S., Mulvihill E., et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO Journal . 2016;35(16):1766–1778. doi: 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ding J., Wang K., Liu W., et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature . 2016;535(7610):111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 54.Teng J. F., Mei Q. B., Zhou X. G., et al. Polyphyllin VI induces caspase-1-mediated pyroptosis via the induction of ROS/NF-κB/NLRP3/GSDMD signal Axis in non-small cell lung cancer. Cancers . 2020;12(1):p. 193. doi: 10.3390/cancers12010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haddad J. J. Oxygen-sensitivepro-inflammatory cytokines, apoptosis signaling and redox-responsive transcription factors in development and pathophysiology. Cytokines, Cellular & Molecular Therapy . 2002;7:1–14. doi: 10.1080/13684730216401. [DOI] [PubMed] [Google Scholar]
- 56.Zheng Y., Lilo S., Brodsky I. E., et al. A Yersinia effector with enhanced inhibitory activity on the NF-κB pathway activates the NLRP3/ASC/caspase-1 inflammasome in macrophages. PLoS Pathogens . 2011;7(4) doi: 10.1371/journal.ppat.1002026.e1002026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aglietti R. A., Estevez A., Gupta A., et al. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proceedings of the National Academy of Sciences of the United States of America . 2016;113(28):7858–7863. doi: 10.1073/pnas.1607769113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rathinam V. A. K., Zhao Y., Shao F. Innate immunity to intracellular LPS. Nature Immunology . 2019;20(5):527–533. doi: 10.1038/s41590-019-0368-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suwinski R., Giglok M., Galwas-Kliber K., et al. Blood serum proteins as biomarkers for prediction of survival, locoregional control and distant metastasis rate in radiotherapy and radio-chemotherapy for non-small cell lung cancer. BMC Cancer . 2019;19(1):p. 427. doi: 10.1186/s12885-019-5617-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwartz A. G., Cote M. L. Epidemiology of lung cancer. Advances in Experimental Medicine and Biology . 2016;893:21–41. doi: 10.1007/978-3-319-24223-1_2. [DOI] [PubMed] [Google Scholar]
- 61.Fan T. W. M., Zhang X., Wang C., et al. Exosomal lipids for classifying early and late stage non-small cell lung cancer. Analytica Chimica Acta . 2018;1037:256–264. doi: 10.1016/j.aca.2018.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang P. T., Yu M. W., Yang Z. Y. [Comparative study on the methods of Chinese medicine and Western medicine therapeutic evaluation for advanced non-small cell lung cancer] Zhongguo Zhong Xi Yi Jie He Za Zhi . 2010;30(7):702–705. [PubMed] [Google Scholar]
- 63.West H., McCleod M., Hussein M., et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. The Lancet Oncology . 2019;20(7):924–937. doi: 10.1016/s1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 64.Hanahan D., Weinberg R. A. Hallmarks of cancer: the next generation. Cell . 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 65.Gay C. M., Stewart C. A., Park E. M., et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell . 2021;39(3):346–360.e7. doi: 10.1016/j.ccell.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu Q., Guo J., Liu Y., et al. YAP drives fate conversion and chemoresistance of small cell lung cancer. Science Advances . 2021;7(40) doi: 10.1126/sciadv.abg1850.eabg1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang F., Liu W., Ning J., et al. Simvastatin suppresses proliferation and migration in non-small cell lung cancer via pyroptosis. International Journal of Biological Sciences . 2018;14(4):406–417. doi: 10.7150/ijbs.23542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu X., Pan Y., Ma H., Li W. Simvastatin inhibits proliferation and induces apoptosis in human lung cancer cells. Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics . 2013;20(8):351–357. doi: 10.3727/096504013x13657689382897. [DOI] [PubMed] [Google Scholar]
- 69.Park I. H., Kim J. Y., Choi J. Y., Han J. Y. Simvastatin enhances irinotecan-induced apoptosis in human non-small cell lung cancer cells by inhibition of proteasome activity. Investigational New Drugs . 2011;29(5):883–890. doi: 10.1007/s10637-010-9439-x. [DOI] [PubMed] [Google Scholar]
- 70.Hwang K. E., Park C., Kwon S. J., et al. Synergistic induction of apoptosis by sulindac and simvastatin in A549 human lung cancer cells via reactive oxygen species-dependent mitochondrial dysfunction. International Journal of Oncology . 2013;43(1):262–270. doi: 10.3892/ijo.2013.1933. [DOI] [PubMed] [Google Scholar]
- 71.Hwang K. E., Kim Y. S., Hwang Y. R., et al. Enhanced apoptosis by pemetrexed and simvastatin in malignant mesothelioma and lung cancer cells by reactive oxygen species-dependent mitochondrial dysfunction and Bim induction. International Journal of Oncology . 2014;45(4):1769–1777. doi: 10.3892/ijo.2014.2584. [DOI] [PubMed] [Google Scholar]
- 72.Hwang K. E., Kim Y. S., Jung J. W., et al. Inhibition of autophagy potentiates pemetrexed and simvastatin-induced apoptotic cell death in malignant mesothelioma and non-small cell lung cancer cells. Oncotarget . 2015;6(30):29482–29496. doi: 10.18632/oncotarget.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.You L. J., Geng H., Yang X. F., et al. The comparison analysis of polyphyllin I and its analogues induced apoptosis of colon and lung cancer cells via mitochondrial dysfunction. Basic and Clinical Pharmacology and Toxicology . 2021;129(1):15–25. doi: 10.1111/bcpt.13596. [DOI] [PubMed] [Google Scholar]
- 74.Lin Z., Liu Y., Li F., et al. Anti-lung cancer effects of polyphyllin VI and VII potentially correlate with apoptosis in vitro and in vivo. Phytotherapy Research . 2015;29(10):1568–1576. doi: 10.1002/ptr.5430. [DOI] [PubMed] [Google Scholar]
- 75.Teng J. F., Qin D. L., Mei Q. B., et al. Polyphyllin VI, a saponin from Trillium tschonoskii Maxim. induces apoptotic and autophagic cell death via the ROS triggered mTOR signaling pathway in non-small cell lung cancer. Pharmacological Research . 2019;147 doi: 10.1016/j.phrs.2019.104396.104396 [DOI] [PubMed] [Google Scholar]
- 76.Yuan R., Zhao W., Wang Q. Q., et al. Cucurbitacin B inhibits non-small cell lung cancer in vivo and in vitro by triggering TLR4/NLRP3/GSDMD-dependent pyroptosis. Pharmacological Research . 2021;170 doi: 10.1016/j.phrs.2021.105748.105748 [DOI] [PubMed] [Google Scholar]
- 77.Liu J. H., Li C., Cao L., Zhang C. H., Zhang Z. H. Cucurbitacin B regulates lung cancer cell proliferation and apoptosis via inhibiting the IL-6/STAT3 pathway through the lncRNA XIST/miR-let-7c axis. Pharmaceutical Biology . 2022;60(1):154–162. doi: 10.1080/13880209.2021.2016866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Silva I. T., Geller F. C., Persich L., et al. Cytotoxic effects of natural and semisynthetic cucurbitacins on lung cancer cell line A549. Investigational New Drugs . 2016;34(2):139–148. doi: 10.1007/s10637-015-0317-4. [DOI] [PubMed] [Google Scholar]
- 79.Kausar H., Munagala R., Bansal S. S., Aqil F., Vadhanam M. V., Gupta R. C. Cucurbitacin B potently suppresses non-small-cell lung cancer growth: identification of intracellular thiols as critical targets. Cancer Letters . 2013;332(1):35–45. doi: 10.1016/j.canlet.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 80.Zhang M., Bian Z. G., Zhang Y., et al. Cucurbitacin B inhibits proliferation and induces apoptosis via STAT3 pathway inhibition in A549 lung cancer cells. Molecular Medicine Reports . 2014;10(6):2905–2911. doi: 10.3892/mmr.2014.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Silva I. T., Carvalho A., Lang K. L., et al. In vitro and in vivo antitumor activity of a novel semisynthetic derivative of cucurbitacin B. PLoS One . 2015;10(2) doi: 10.1371/journal.pone.0117794.e0117794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu P., Xiang Y., Liu X., et al. Cucurbitacin B induces the lysosomal degradation of EGFR and suppresses the CIP2A/PP2A/akt signaling Axis in gefitinib-resistantnon-small cell lung cancer. Molecules . 2019;24(3):p. 647. doi: 10.3390/molecules24030647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang J., Chen Y., He Q. Distinct characteristics of dasatinib-induced pyroptosis in gasdermin E-expressing human lung cancer A549 cells and neuroblastoma SH-SY5Y cells. Oncology Letters . 2020;20(1):145–154. doi: 10.3892/ol.2020.11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang H., Sun B., Xu K., et al. Pharmaco-transcriptomic correlation analysis reveals novel responsive signatures to HDAC inhibitors and identifies Dasatinib as a synergistic interactor in small-cell lung cancer. EBioMedicine . 2021;69 doi: 10.1016/j.ebiom.2021.103457.103457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Watanabe S., Yoshida T., Kawakami H., et al. T790M-Selective EGFR-TKI combined with dasatinib as an optimal strategy for overcoming EGFR-TKI resistance in t790m-positivenon-small cell lung cancer. Molecular Cancer Therapeutics . 2017;16(11):2563–2571. doi: 10.1158/1535-7163.mct-17-0351. [DOI] [PubMed] [Google Scholar]
- 86.Xi G., Gao J., Wan B., et al. GSDMD is required for effector CD8(+) T cell responses to lung cancer cells. International Immunopharmacology . 2019;74 doi: 10.1016/j.intimp.2019.105713.105713 [DOI] [PubMed] [Google Scholar]
- 87.An Y., Zhang H., Wang C., et al. Activation of ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis. The FASEB Journal . 2019;33(11):12515–12527. doi: 10.1096/fj.201802805rr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu X., Lan P., Hou X., et al. HBV inhibits LPS-induced NLRP3 inflammasome activation and IL-1β production via suppressing the NF-κB pathway and ROS production. Journal of Hepatology . 2017;66(4):693–702. doi: 10.1016/j.jhep.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 89.Zhu M., Wang J., Xie J., et al. Design, synthesis, and evaluation of chalcone analogues incorporate α, β-Unsaturated ketone functionality as anti-lung cancer agents via evoking ROS to induce pyroptosis. European Journal of Medicinal Chemistry . 2018;157:1395–1405. doi: 10.1016/j.ejmech.2018.08.072. [DOI] [PubMed] [Google Scholar]
- 90.Yang J., Mu W. W., Liu G. Y. Synthesis and evaluation of the anticancer activity of bischalcone analogs in human lung carcinoma (A549) cell line. European Journal of Pharmacology . 2020;888 doi: 10.1016/j.ejphar.2020.173396.173396 [DOI] [PubMed] [Google Scholar]
- 91.Dong N., Liu X., Zhao T., et al. Apoptosis-inducing effects and growth inhibitory of a novel chalcone, in human hepatic cancer cells and lung cancer cells. Biomedicine & Pharmacotherapy . 2018;105:195–203. doi: 10.1016/j.biopha.2018.05.126. [DOI] [PubMed] [Google Scholar]
- 92.Zheng Z., Bian Y., Zhang Y., Ren G., Li G. Metformin activates AMPK/SIRT1/NF-κB pathway and induces mitochondrial dysfunction to drive caspase3/GSDME-mediated cancer cell pyroptosis. Cell Cycle . 2020;19(10):1089–1104. doi: 10.1080/15384101.2020.1743911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu N., Gu C., Gu H., Hu H., Han Y., Li Q. Metformin induces apoptosis of lung cancer cells through activating JNK/p38 MAPK pathway and GADD153. Neoplasma . 2011;58(6):482–490. doi: 10.4149/neo_2011_06_482. [DOI] [PubMed] [Google Scholar]
- 94.Lee B. B., Kim Y., Kim D., et al. Metformin and tenovin-6 synergistically induces apoptosis through LKB1-independent SIRT1 down-regulation in non-small cell lung cancer cells. Journal of Cellular and Molecular Medicine . 2019;23(4):2872–2889. doi: 10.1111/jcmm.14194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li Q., Chen L., Dong Z., et al. Piperlongumine analogue L50377 induces pyroptosis via ROS mediated NF-κB suppression in non-small-cell lung cancer. Chemico-Biological Interactions . 2019;313 doi: 10.1016/j.cbi.2019.108820.108820 [DOI] [PubMed] [Google Scholar]
- 96.Wang F., Mao Y., You Q., Hua D., Cai D. Piperlongumine induces apoptosis and autophagy in human lung cancer cells through inhibition of PI3K/Akt/mTOR pathway. International Journal of Immunopathology & Pharmacology . 2015;28(3):362–373. doi: 10.1177/0394632015598849. [DOI] [PubMed] [Google Scholar]
- 97.Lu X., Xu C., Xu Z., et al. Piperlongumine inhibits the growth of non-small cell lung cancer cells via the miR-34b-3p/TGFBR1 pathway. BMC Complement Med Ther . 2021;21(1):p. 15. doi: 10.1186/s12906-020-03123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen L., Li Q., Zheng Z., et al. Design and optimize N-substituted EF24 as effective and low toxicity NF-κB inhibitor for lung cancer therapy via apoptosis-to-pyroptosis switch. Chemical Biology & Drug Design . 2019;94(1):1368–1377. doi: 10.1111/cbdd.13514. [DOI] [PubMed] [Google Scholar]
- 99.Chen L., Weng B., Li H., et al. A thiopyran derivative with low murine toxicity with therapeutic potential on lung cancer acting through a NF-κB mediated apoptosis-to-pyroptosis switch. Apoptosis . 2019;24(1-2):74–82. doi: 10.1007/s10495-018-1499-y. [DOI] [PubMed] [Google Scholar]
- 100.Wu J., Wu S., Shi L., et al. Design, synthesis, and evaluation of asymmetric EF24 analogues as potential anti-cancer agents for lung cancer. European Journal of Medicinal Chemistry . 2017;125:1321–1331. doi: 10.1016/j.ejmech.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 101.Chang M., Shang M., Yuan F., Guo W., Wang C. EF24 exerts cytotoxicity against NSCLC via inducing ROS accumulation. Cancer Cell International . 2021;21(1):p. 531. doi: 10.1186/s12935-021-02240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bhakat K. K., Mantha A. K., Mitra S. Transcriptional regulatory functions of mammalian AP-endonuclease (APE1/Ref-1), an essential multifunctional protein. Antioxidants and Redox Signaling . 2009;11(3):621–637. doi: 10.1089/ars.2008.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu X., Roth J. A., Zhao H., et al. Cell cycle checkpoints, DNA damage/repair, and lung cancer risk. Cancer Research . 2005;65(1):349–357. doi: 10.1158/0008-5472.349.65.1. [DOI] [PubMed] [Google Scholar]
- 104.Orlow I., Park B. J., Mujumdar U., et al. DNA damage and repair capacity in patients with lung cancer: prediction of multiple primary tumors. Journal of Clinical Oncology . 2008;26(21):3560–3566. doi: 10.1200/jco.2007.13.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang D., Xiang D. B., Yang X. Q., et al. APE1 overexpression is associated with cisplatin resistance in non-small cell lung cancer and targeted inhibition of APE1 enhances the activity of cisplatin in A549 cells. Lung Cancer . 2009;66(3):298–304. doi: 10.1016/j.lungcan.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 106.Long K., Gu L., Li L., et al. Small-molecule inhibition of APE1 induces apoptosis, pyroptosis, and necroptosis in non-small cell lung cancer. Cell Death & Disease . 2021;12(6):p. 503. doi: 10.1038/s41419-021-03804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhu J., Zhang C., Qing Y., et al. Genistein induces apoptosis by stabilizing intracellular p53 protein through an APE1-mediated pathway. Free Radical Biology and Medicine . 2015;86:209–218. doi: 10.1016/j.freeradbiomed.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 108.Wang Z., Xu W., Lin Z., et al. Reduced apurinic/apyrimidinic endonuclease activity enhances the antitumor activity of oxymatrine in lung cancer cells. International Journal of Oncology . 2016;49(6):2331–2340. doi: 10.3892/ijo.2016.3734. [DOI] [PubMed] [Google Scholar]
- 109.Jiang P., Zhang D. Maternal embryonic leucine zipper kinase (MELK): a novel regulator in cell cycle control, embryonic development, and cancer. International Journal of Molecular Sciences . 2013;14(11):21551–21560. doi: 10.3390/ijms141121551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kuner R., Fälth M., Pressinotti N. C., et al. The maternal embryonic leucine zipper kinase (MELK) is upregulated in high-grade prostate cancer. Journal of Molecular Medicine (Berlin) . 2013;91(2):237–248. doi: 10.1007/s00109-012-0949-1. [DOI] [PubMed] [Google Scholar]
- 111.Tang Q., Li W., Zheng X., et al. MELK is an oncogenic kinase essential for metastasis, mitotic progression, and programmed death in lung carcinoma. Signal Transduction and Targeted Therapy . 2020;5(1):p. 279. doi: 10.1038/s41392-020-00288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Inoue H., Kato T., Olugbile S., et al. Effective growth-suppressive activity of maternal embryonic leucine-zipper kinase (MELK) inhibitor against small cell lung cancer. Oncotarget . 2016;7(12):13621–13633. doi: 10.18632/oncotarget.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lu H., Zhang S., Wu J., et al. Molecular targeted therapies elicit concurrent apoptotic and GSDME-dependent pyroptotic tumor cell death. Clinical Cancer Research . 2018;24(23):6066–6077. doi: 10.1158/1078-0432.ccr-18-1478. [DOI] [PubMed] [Google Scholar]
- 114.Zhang C. C., Li C. G., Wang Y. F., et al. Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation. Apoptosis . 2019;24(3-4):312–325. doi: 10.1007/s10495-019-01515-1. [DOI] [PubMed] [Google Scholar]
- 115.Arioz B. I., Tastan B., Tarakcioglu E., et al. Melatonin attenuates LPS-induced acute depressive-like behaviors and microglial NLRP3 inflammasome activation through the SIRT1/nrf2 pathway. Frontiers in Immunology . 2019;10:p. 1511. doi: 10.3389/fimmu.2019.01511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Qiu Z., He Y., Ming H., Lei S., Leng Y., Xia Z. Y. Lipopolysaccharide (LPS) aggravates high glucose- and hypoxia/reoxygenation-induced injury through activating ROS-dependent NLRP3 inflammasome-mediated pyroptosis in H9C2 cardiomyocytes. Journal of Diabetes Research . 2019;2019:12. doi: 10.1155/2019/8151836.8151836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yokoyama S., Cai Y., Murata M., et al. A novel pathway of LPS uptake through syndecan-1 leading to pyroptotic cell death. Elife . 2018;7 doi: 10.7554/elife.37854.e37854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yokoyama S., Nakayama S., Xu L., Pilon A. L., Kimura S. Secretoglobin 3A2 eliminates human cancer cells through pyroptosis. Cell Death & Disease . 2021;7(1):p. 12. doi: 10.1038/s41420-020-00385-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu J., Yao L., Zhang M., Jiang J., Yang M., Wang Y. Downregulation of LncRNA-XIST inhibited development of non-small cell lung cancer by activating miR-335/SOD2/ROS signal pathway mediated pyroptotic cell death. Aging (Albany NY) . 2019;11(18):7830–7846. doi: 10.18632/aging.102291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xu X., Zhou X., Chen Z., Gao C., Zhao L., Cui Y. Silencing of lncRNA XIST inhibits non-small cell lung cancer growth and promotes chemosensitivity to cisplatin. Aging (Albany NY) . 2020;12(6):4711–4726. doi: 10.18632/aging.102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang T., Li Y., Zhu R., et al. Transcription factor p53 suppresses tumor growth by prompting pyroptosis in non-small-cell lung cancer. Oxidative Medicine and Cellular Longevity . 2019;2019:9. doi: 10.1155/2019/8746895.8746895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sannino F., Sansone C., Galasso C., et al. Pseudoalteromonas haloplanktis TAC125 produces 4-hydroxybenzoic acid that induces pyroptosis in human A459 lung adenocarcinoma cells. Scientific Reports . 2018;8(1):p. 1190. doi: 10.1038/s41598-018-19536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wei J., Xu Z., Chen X., et al. Overexpression of GSDMC is a prognostic factor for predicting a poor outcome in lung adenocarcinoma. Molecular Medicine Reports . 2020;21(1):360–370. doi: 10.3892/mmr.2019.10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gao J., Qiu X., Xi G., et al. Downregulation of GSDMD attenuates tumor proliferation via the intrinsic mitochondrial apoptotic pathway and inhibition of EGFR/Akt signaling and predicts a good prognosis in non-small cell lung cancer. Oncology Reports . 2018;40(4):1971–1984. doi: 10.3892/or.2018.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zou J., Yang Y., Yang Y., Liu X. Polydatin suppresses proliferation and metastasis of non-small cell lung cancer cells by inhibiting NLRP3 inflammasome activation via NF-κB pathway. Biomedicine & Pharmacotherapy . 2018;108:130–136. doi: 10.1016/j.biopha.2018.09.051. [DOI] [PubMed] [Google Scholar]
- 126.Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians . 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 127.Liang C., Fan J., Liang C., Guo J. Identification and validation of a pyroptosis-related prognostic model for gastric cancer. Frontiers in Genetics . 2021;12 doi: 10.3389/fgene.2021.699503.699503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang W., Niu L., Zhao X., et al. Pyroptosis impacts the prognosis and treatment response in gastric cancer via immune system modulation. Am J Cancer Res . 2022;12(4):1511–1534. [PMC free article] [PubMed] [Google Scholar]
- 129.Cao F., Hu J., Yuan H., Cao P., Cheng Y., Wang Y. Identification of pyroptosis-related subtypes, development of a prognostic model, and characterization of tumour microenvironment infiltration in gastric cancer. Frontiers in Genetics . 2022;13 doi: 10.3389/fgene.2022.963565.963565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhou J., Nie R. C., Yin Y. X., et al. Genomic analysis uncovers the prognostic and immunogenetic feature of pyroptosis in gastric carcinoma: indication for immunotherapy. Frontiers in Cell and Developmental Biology . 2022;10 doi: 10.3389/fcell.2022.906759.906759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xu T., Gu H., Zhang C., Zhang W., Liang X., Cheng X. A novel risk model identified based on pyroptosis-related lncRNA predicts overall survival and associates with the immune landscape of GC patients. Frontiers in Genetics . 2022;13 doi: 10.3389/fgene.2022.843538.843538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang W. J., Chen D., Jiang M. Z., et al. Downregulation of gasdermin D promotes gastric cancer proliferation by regulating cell cycle-related proteins. Journal of Digestive Diseases . 2018;19(2):74–83. doi: 10.1111/1751-2980.12576. [DOI] [PubMed] [Google Scholar]
- 133.Yin J., Che G., Wang W., Chen S., Liu J. Investigating the prognostic significance of pyroptosis-related genes in gastric cancer and their impact on cells’ biological functions. Frontiers Oncology . 2022;12 doi: 10.3389/fonc.2022.861284.861284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yu Z., Cao W., Han C., et al. Biomimetic metal-organic framework nanoparticles for synergistic combining of SDT-chemotherapy induce pyroptosis in gastric cancer. Frontiers in Bioengineering and Biotechnology . 2022;10 doi: 10.3389/fbioe.2022.796820.796820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huang J., Fan P., Liu M., et al. Famotidine promotes inflammation by triggering cell pyroptosis in gastric cancer cells. BMC Pharmacol Toxicol . 2021;22(1):p. 62. doi: 10.1186/s40360-021-00533-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xia Y., Jin Y., Cui D., et al. Antitumor effect of simvastatin in combination with DNA methyltransferase inhibitor on gastric cancer via GSDME-mediated pyroptosis. Frontiers in Pharmacology . 2022;13 doi: 10.3389/fphar.2022.860546.860546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu Q., Xia H., Zhou S., et al. <p>Simvastatin inhibits the malignant behaviors of gastric cancer cells by simultaneously suppressing YAP and β-catenin signaling</p>. OncoTargets and Therapy . 2020;13:2057–2066. doi: 10.2147/ott.s237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Manu K. A., Shanmugam M. K., Li F., et al. Simvastatin sensitizes human gastric cancer xenograft in nude mice to capecitabine by suppressing nuclear factor-kappaB-regulated gene products. Journal of Molecular Medicine (Berlin) . 2014;92(3):267–276. doi: 10.1007/s00109-013-1095-0. [DOI] [PubMed] [Google Scholar]
- 139.Zhang F., Yin Y., Xu W., et al. Icariin inhibits gastric cancer cell growth by regulating the hsa_circ_0003159/miR-223-3p/NLRP3 signaling axis. Human & Experimental Toxicology . 2022;41 doi: 10.1177/09603271221097363.096032712210973 [DOI] [PubMed] [Google Scholar]
- 140.Yin Y., Xu W., Song Y., Zhou Z., Sun X., Zhang F. Icariin regulates the hsa_circ_0003159/eIF4A3/bcl-2 Axis to promote gastric cancer cell apoptosis. Evidence-based Complementary and Alternative Medicine . 2022;2022:1955101–11. doi: 10.1155/2022/1955101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li C., Qiu J., Xue Y. Low-doseDiosbulbin-B (DB) activates tumor-intrinsic PD-L1/NLRP3 signaling pathway mediated pyroptotic cell death to increase cisplatin-sensitivity in gastric cancer (GC) Cell & Bioscience . 2021;11(1):p. 38. doi: 10.1186/s13578-021-00548-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li C., Li M., Xue Y. Downregulation of CircRNA CDR1as specifically triggered low-doseDiosbulbin-B induced gastric cancer cell death by regulating miR-7-5p/REGγ axis. Biomedicine & Pharmacotherapy . 2019;120 doi: 10.1016/j.biopha.2019.109462.109462 [DOI] [PubMed] [Google Scholar]
- 143.Zhang X., Li C., Chen D., et al. H. pylori CagA activates the NLRP3 inflammasome to promote gastric cancer cell migration and invasion. Inflammation Research . 2022;71(1):141–155. doi: 10.1007/s00011-021-01522-6. [DOI] [PubMed] [Google Scholar]
- 144.Li L., Bao B., Chai X., et al. The anti-inflammatory effect of callicarpa nudiflora extract on H. Pylori-infected GES-1 cells through the inhibition of ROS/NLRP3/Caspase-1/IL-1β signaling Axis. The Canadian Journal of Infectious Diseases & Medical Microbiology . 2022;2022:8. doi: 10.1155/2022/5469236.5469236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wei C. Y., Zhu M. X., Zhang P. F., et al. PKCα/ZFP64/CSF1 axis resets the tumor microenvironment and fuels anti-PD1 resistance in hepatocellular carcinoma. Journal of Hepatology . 2022;77(1):163–176. doi: 10.1016/j.jhep.2022.02.019. [DOI] [PubMed] [Google Scholar]
- 146.Zhang T., Guan G., Zhang J., et al. E2F1-mediated AUF1 upregulation promotes HCC development and enhances drug resistance via stabilization of AKR1B10. Cancer Science . 2022;113(4):1154–1167. doi: 10.1111/cas.15272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang T., Yang Y., Sun T., et al. The pyroptosis-related long noncoding RNA signature predicts prognosis and indicates immunotherapeutic efficiency in hepatocellular carcinoma. Frontiers in Cell and Developmental Biology . 2022;10 doi: 10.3389/fcell.2022.779269.779269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Qu G., Wang D., Xu W., Guo W. Comprehensive analysis of the correlation between pyroptosis-related LncRNAs and tumor microenvironment, prognosis, and immune infiltration in hepatocellular carcinoma. Frontiers in Genetics . 2022;13 doi: 10.3389/fgene.2022.867627.867627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang L., Zhang X., Zhao W., et al. NLRP6-Dependentpyroptosis-related lncRNAs predict the prognosis of hepatocellular carcinoma. Frontiers of Medicine . 2022;9 doi: 10.3389/fmed.2022.760722.760722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu Z. K., Wu K. F., Zhang R. Y., et al. Pyroptosis-related LncRNA signature predicts prognosis and is associated with immune infiltration in hepatocellular carcinoma. Frontiers Oncology . 2022;12 doi: 10.3389/fonc.2022.794034.794034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang Z., Xia F., Xu Z., et al. Identification and validation of a novel pyroptosis-related lncRNAs signature associated with prognosis and immune regulation of hepatocellular carcinoma. Scientific Reports . 2022;12(1):p. 8886. doi: 10.1038/s41598-022-13046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hu K., Xu Z., Yao L., Yan Y., Zhou L., Li J. Integrated analysis of expression, prognostic value and immune infiltration of GSDMs in hepatocellular carcinoma. Aging (Albany NY) . 2021;13(21):24117–24135. doi: 10.18632/aging.203669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Qiu S., Hu Y., Dong S. Pan-cancer analysis reveals the expression, genetic alteration and prognosis of pyroptosis key gene GSDMD. International Immunopharmacology . 2021;101 doi: 10.1016/j.intimp.2021.108270.108270 [DOI] [PubMed] [Google Scholar]
- 154.Li Q., Feng H., Wang H., et al. Licochalcone B specifically inhibits the NLRP3 inflammasome by disrupting NEK7-NLRP3 interaction. EMBO Reports . 2022;23(2) doi: 10.15252/embr.202153499.e53499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yan Z., Da Q., Li Z., et al. Inhibition of NEK7 suppressed hepatocellular carcinoma progression by mediating cancer cell pyroptosis. Frontiers Oncology . 2022;12 doi: 10.3389/fonc.2022.812655.812655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen Y. F., Qi H. Y., Wu F. L. Euxanthone exhibits anti-proliferative and anti-invasive activities in hepatocellular carcinoma by inducing pyroptosis: preliminary results. European Review for Medical and Pharmacological Sciences . 2018;22(23):8186–8196. doi: 10.26355/eurrev_201812_16511. [DOI] [PubMed] [Google Scholar]
- 157.Zhang Y., Yang H., Sun M., et al. Alpinumisoflavone suppresses hepatocellular carcinoma cell growth and metastasis via NLRP3 inflammasome-mediated pyroptosis. Pharmacological Reports . 2020;72(5):1370–1382. doi: 10.1007/s43440-020-00064-8. [DOI] [PubMed] [Google Scholar]
- 158.Zhang X., Zhang P., An L., et al. Miltirone induces cell death in hepatocellular carcinoma cell through GSDME-dependent pyroptosis. Acta Pharmaceutica Sinica B . 2020;10(8):1397–1413. doi: 10.1016/j.apsb.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li M., Wang C., Yu Z., et al. MgIG exerts therapeutic effects on crizotinib-induced hepatotoxicity by limiting ROS-mediated autophagy and pyroptosis. Journal of Cellular and Molecular Medicine . 2022;26(16):4492–4505. doi: 10.1111/jcmm.17474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yu Z., Zhao R. Inhibition of anaplastic lymphoma kinase promotes apoptosis and suppresses proliferation in human hepatocellular carcinoma. Anti-Cancer Drugs . 2018;29(6):513–519. doi: 10.1097/cad.0000000000000616. [DOI] [PubMed] [Google Scholar]
- 161.Shangguan F., Zhou H., Ma N., et al. A novel mechanism of Cannabidiol in suppressing hepatocellular carcinoma by inducing GSDME dependent pyroptosis. Frontiers in Cell and Developmental Biology . 2021;9 doi: 10.3389/fcell.2021.697832.697832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tajik T., Baghaei K., Moghadam V. E., Farrokhi N., Salami S. A. Extracellular vesicles of cannabis with high CBD content induce anticancer signaling in human hepatocellular carcinoma. Biomedicine & Pharmacotherapy . 2022;152 doi: 10.1016/j.biopha.2022.113209.113209 [DOI] [PubMed] [Google Scholar]
- 163.Shen Z., Zhou H., Li A., et al. Metformin inhibits hepatocellular carcinoma development by inducing apoptosis and pyroptosis through regulating FOXO3. Aging (Albany NY) . 2021;13(18):22120–22133. doi: 10.18632/aging.203464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang Q., Sun Y., Tao C., et al. Metformin induces apoptosis of human hepatocellular carcinoma HepG2 cells by activating an AMPK/p53/miR-23a/FOXA1 pathway. OncoTargets and Therapy . 2016;9:2845–2853. doi: 10.2147/ott.s99770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Park D. B. Metformin promotes apoptosis but suppresses autophagy in glucose-deprived H4IIE hepatocellular carcinoma cells. Diabetes & Metabolism J . 2015;39(6):518–527. doi: 10.4093/dmj.2015.39.6.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sun R., Zhai R., Ma C., Miao W. Combination of aloin and metformin enhances the antitumor effect by inhibiting the growth and invasion and inducing apoptosis and autophagy in hepatocellular carcinoma through PI3K/AKT/mTOR pathway. Cancer Medicine . 2020;9(3):1141–1151. doi: 10.1002/cam4.2723. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 167.Yang X., Sun D., Tian Y., Ling S., Wang L. Metformin sensitizes hepatocellular carcinoma to arsenic trioxide-induced apoptosis by downregulating Bcl2 expression. Tumor Biology . 2015;36(4):2957–2964. doi: 10.1007/s13277-014-2926-5. [DOI] [PubMed] [Google Scholar]
- 168.Liang W. F., Gong Y. X., Li H. F., et al. Curcumin activates ROS signaling to promote pyroptosis in hepatocellular carcinoma HepG2 cells. In Vivo . 2021;35(1):249–257. doi: 10.21873/invivo.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chen Y., Li Q., Ren S., et al. Investigation and experimental validation of curcumin-related mechanisms against hepatocellular carcinoma based on network pharmacology. Journal of Zhejiang University - Science B . 2022;23(8):682–698. doi: 10.1631/jzus.b2200038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bai C., Zhao J., Su J., et al. Curcumin induces mitochondrial apoptosis in human hepatoma cells through BCLAF1-mediated modulation of PI3K/AKT/GSK-3β signaling. Life Sciences . 2022;306 doi: 10.1016/j.lfs.2022.120804.120804 [DOI] [PubMed] [Google Scholar]
- 171.Yu P., Cao W., Zhao L., et al. Design, synthesis, and antitumor evaluation of novel mono-carbonyl curcumin analogs in hepatocellular carcinoma cell. Pharmaceuticals . 2022;15(8):p. 950. doi: 10.3390/ph15080950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hariri L., Rehman A. Estradiol. StatPearls. Treasure Island (FL): StatPearls PublishingCopyright © 2022 . StatPearls Publishing LLC; 2022. [Google Scholar]
- 173.Wei Q., Zhu R., Zhu J., Zhao R., Li M. E2-Induced activation of the NLRP3 inflammasome triggers pyroptosis and inhibits autophagy in HCC cells. Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics . 2019;27(7):827–834. doi: 10.3727/096504018x15462920753012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Guo Y., Cai X., Lu H., et al. 17β-Estradiol promotes apoptosis of HepG2 cells caused by oxidative stress by increasing Foxo3a phosphorylation. Frontiers in Pharmacology . 2021;12 doi: 10.3389/fphar.2021.607379.607379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lee S., Lee M., Kim J. B., et al. 17β-estradiol exerts anticancer effects in anoikis-resistant hepatocellular carcinoma cell lines by targeting IL-6/STAT3 signaling. Biochemical and Biophysical Research Communications . 2016;473(4):1247–1254. doi: 10.1016/j.bbrc.2016.04.049. [DOI] [PubMed] [Google Scholar]
- 176.Chu Q., Jiang Y., Zhang W., et al. Pyroptosis is involved in the pathogenesis of human hepatocellular carcinoma. Oncotarget . 2016;7(51):84658–84665. doi: 10.18632/oncotarget.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Li M., Zhang M., Zhang Z. L., et al. Induction of apoptosis by berberine in hepatocellular carcinoma HepG2 cells via downregulation of NF-κB. Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics . 2017;25(2):233–239. doi: 10.3727/096504016x14742891049073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yang X., Huang N. Berberine induces selective apoptosis through the AMPK-mediated mitochondrial/caspase pathway in hepatocellular carcinoma. Molecular Medicine Reports . 2013;8(2):505–510. doi: 10.3892/mmr.2013.1506. [DOI] [PubMed] [Google Scholar]
- 179.Luo J., Lai J. Pyroptosis-related molecular classification and immune microenvironment infiltration in breast cancer: a novel therapeutic target. Journal of Cellular and Molecular Medicine . 2022;26(8):2259–2272. doi: 10.1111/jcmm.17247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Huang G., Zhou J., Chen J., Liu G. Identification of pyroptosis related subtypes and tumor microenvironment infiltration characteristics in breast cancer. Scientific Reports . 2022;12(1) doi: 10.1038/s41598-022-14897-1.10640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zheng Y., Wang K., Li N., Zhang Q., Chen F., Li M. Prognostic and immune implications of a novel pyroptosis-relatedfive-gene signature in breast cancer. Front Surg . 2022;9 doi: 10.3389/fsurg.2022.837848.837848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tan M., Huang G., Chen J., et al. Construction and validation of an eight pyroptosis-related lncRNA risk model for breast cancer. American Journal of Translational Research . 2022;14(5):2779–2800. [PMC free article] [PubMed] [Google Scholar]
- 183.Yang X., Weng X., Yang Y., Jiang Z. Pyroptosis-related lncRNAs predict the prognosis and immune response in patients with breast cancer. Frontiers in Genetics . 2021;12 doi: 10.3389/fgene.2021.792106.792106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu M., Li Y., Kong B., Zhang G., Zhang Q. Polydatin down-regulates the phosphorylation level of STAT3 and induces pyroptosis in triple-negative breast cancer mice with a high-fat diet. Annals of Translational Medicine . 2022;10(4):p. 173. doi: 10.21037/atm-22-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhang T., Zhu X., Wu H., et al. Targeting the ROS/PI3K/AKT/HIF-1α/HK2 axis of breast cancer cells: combined administration of Polydatin and 2-Deoxy-d-glucose. Journal of Cellular and Molecular Medicine . 2019;23(5):3711–3723. doi: 10.1111/jcmm.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yan H., Luo B., Wu X., et al. Cisplatin induces pyroptosis via activation of MEG3/NLRP3/caspase-1/GSDMD pathway in triple-negative breast cancer. International Journal of Biological Sciences . 2021;17(10):2606–2621. doi: 10.7150/ijbs.60292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Huang J., Liu C., Duan S., et al. Gigantol inhibits proliferation and enhances DDP-induced apoptosis in breast-cancer cells by downregulating the PI3K/Akt/mTOR signaling pathway. Life Sciences . 2021;274 doi: 10.1016/j.lfs.2021.119354.119354 [DOI] [PubMed] [Google Scholar]
- 188.Li Y., Wang W., Li A., et al. Dihydroartemisinin induces pyroptosis by promoting the AIM2/caspase-3/DFNA5 axis in breast cancer cells. Chemico-Biological Interactions . 2021;340 doi: 10.1016/j.cbi.2021.109434.109434 [DOI] [PubMed] [Google Scholar]
- 189.Mao H., Gu H., Qu X., et al. Involvement of the mitochondrial pathway and Bim/Bcl-2 balance in dihydroartemisinin-induced apoptosis in human breast cancer in vitro. International Journal of Molecular Medicine . 2013;31(1):213–218. doi: 10.3892/ijmm.2012.1176. [DOI] [PubMed] [Google Scholar]
- 190.Wang J. G., Jian W. J., Li Y., Zhang J. Nobiletin promotes the pyroptosis of breast cancer via regulation of miR-200b/JAZF1 axis. The Kaohsiung Journal of Medical Sciences . 2021;37(7):572–582. doi: 10.1002/kjm2.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Liu J., Wang S., Tian S., et al. Nobiletin inhibits breast cancer via p38 mitogen-activated protein kinase, nuclear transcription factor-κB, and nuclear factor erythroid 2-related factor 2 pathways in MCF-7 cells. Food & Nutrition Research . 2018;62(0) doi: 10.29219/fnr.v62.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chen C., Ono M., Takeshima M., Nakano S. Antiproliferative and apoptosis-inducing activity of nobiletin against three subtypes of human breast cancer cell lines. Anticancer Research . 2014;34(4):1785–1792. [PubMed] [Google Scholar]
- 193.An H., Heo J. S., Kim P., et al. Tetraarsenic hexoxide enhances generation of mitochondrial ROS to promote pyroptosis by inducing the activation of caspase-3/GSDME in triple-negative breast cancer cells. Cell Death & Disease . 2021;12(2):p. 159. doi: 10.1038/s41419-021-03454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yan L., Liu Y., Ma X. F., et al. Triclabendazole induces pyroptosis by activating caspase-3 to cleave GSDME in breast cancer cells. Frontiers in Pharmacology . 2021;12 doi: 10.3389/fphar.2021.670081.670081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tian W., Wang Z., Tang N. N., et al. Ascorbic acid sensitizes colorectal carcinoma to the cytotoxicity of arsenic trioxide via promoting reactive oxygen species-dependent apoptosis and pyroptosis. Frontiers in Pharmacology . 2020;11:p. 123. doi: 10.3389/fphar.2020.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang X., Wang G., Dong D., Fu S., Yang B. Inhibition on LS-174T cell growth and activity of telomerase in vitro and in vivo by arsenic trioxide. Experimental & Toxicologic Pathology . 2008;60(6):481–488. doi: 10.1016/j.etp.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 197.Chen C., Wang B., Sun J., et al. DAC can restore expression of NALP1 to suppress tumor growth in colon cancer. Cell Death & Disease . 2015;6(1) doi: 10.1038/cddis.2014.532.e1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lv L., Zhou J., Lin C., et al. DNA methylation is involved in the aberrant expression of miR-133b in colorectal cancer cells. Oncology Letters . 2015;10(2):907–912. doi: 10.3892/ol.2015.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used to support this study.


