Abstract
It is challenging to diagnose metastatic tumors whose cellular morphology is different from the primary. We characterized canine primary pulmonary adenocarcinoma (PAC) and its xenografted tumors by histological and immunohistochemical analyses for critical diagnostic and cancer stem cell (CSC) markers. To generate a tumor xenograft model, we subsequently transplanted the tissue pieces from the PAC into athymic nude mice. Immunohistochemical examination was performed for diagnostic (TTF-1, Napsin A, and SP-A) and CSC markers (CD44 and CD133). The use of CSC markers together with diagnostic markers can improve the detection and diagnosis of canine primary and metastatic adenocarcinomas.
Keywords: Canine pulmonary adenocarcinoma, diagnostic markers, immunohistochemistry, stem cell markers, tumor xenograft model
INTRODUCTION
It is clinically challenging to diagnose the metastatic tumors because they are generally different from the primary ones in cellular and histological morphology. Canine pulmonary adenocarcinoma (PAC) is in similar situation, although several markers were suggested for diagnosis of originality and malignancy [1]. In addition, the sites of metastasis from lung tumors are rarely recorded in animals because they are often euthanized before metastatic tumors fully develop [2]. The histological patterns of PACs are subclassified as lepidic, papillary, micropapillary, acinar, and solid [3]. To examine cancer, patient-derived xenograft models are still utilized in the study of biomarker development and metastasis. Metastasis models are modified for evaluation and prediction of cancer progression [4,5,6].
As discrimination and differential diagnosis may not always be possible based on morphology alone [1], immunohistochemistry is the most important method in lung cancer diagnosis and classification, which can help confirm the presence of metastasis [3]. For diagnostic markers, Thyroid transcription factor-1 (TTF-1) is a commonly used marker for diagnosis of PAC and prediction of prognosis [7,8]. Novel aspartic proteinase of the pepsin family A (Napsin A) has emerged as an alternative marker to TTF-1 for the diagnosis of PAC and is rarely seen in adenocarcinomas arising outside the lung or kidney [8]. Pulmonary surfactant protein A (SP-A) is a mixture of phospholipids and proteins, which is thought to function by reducing surface tension at the air/fluid interface lining the alveolar space [9]. In normal lung tissue, SP-A immunoreactivity is observed on the apical surface of alveolar cells, within the cytoplasm and [7,10] other subtypes of PAC [11]. Lung cancer stem cells (CSCs) have self-renewal and differentiation potential as well as the potential to initiate new cancer formation, progression, and metastasis. CSCs of lung cancer are well recognized by their specific markers, such as CD44 and CD133 [12,13]. The proliferation index is estimated by immunohistochemistry for Ki-67 which is generally associated with tumor cell proliferation and growth. The prognosis generally tends to be poor in cases of PAC with a high proliferation index [14].
In this study, we characterized the histological and immunohistochemical phenotypes of both primary and xenografted PAC and identified suitable markers for diagnosis of canine PAC and its metastatic-like tumors.
MATERIALS AND METHODS
Animals and live tissue bank xenograft establishment
Xenografted canine pulmonary tumor models were established from a 14-year-old spayed female Shih Tzu dog. The tumor mass was surgically excised and stored in cold Dulbecco’s modified Eagle Medium and transplanted into the inguinal region of an athymic nude mouse (F1 tumor). Then it was subsequently transplanted into another mouse (F2). During transplantation from F1 to F2, some pieces of F1 tumor tissue were stored in liquid nitrogen. The frozen tumor tissue specimens were thawed 3 months later and transplanted into another mouse (F2(C)). The success of the live tissue bank was verified by confirming the growth of the tumor. When it had grown sufficiently, it was examined to compare its molecular phenotypes with those of the non-frozen tumors.
Histopathology and immunohistochemistry
The primary and xenografted tumors were histopathologically characterized on hematoxylin and eosin-stained sections and immunohistochemical characterization was performed for six specific antigens (Supplementary Table 1).
Immunoreactivity scoring
Immunoreactivities for diagnostic and CSC markers were scored semiquantitatively based on the distribution [1] and intensity of staining [10]. Immunoreactivity was compared between the glandular and solid structures by evaluating the staining intensity of positive cells. The proportion of the Ki-67 positive neoplastic cells was calculated according to the method of Griffey et al. [15].
RESULTS
Histopathological examination
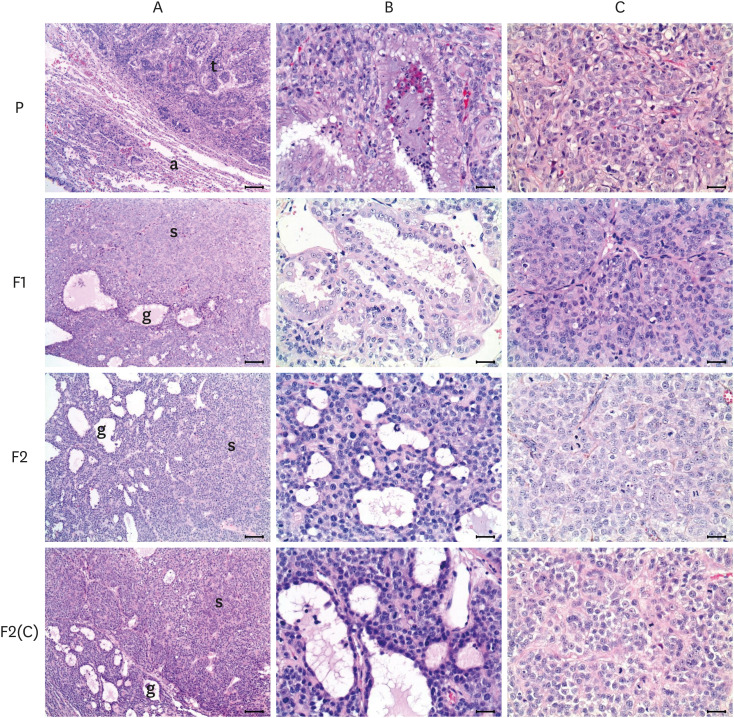
Histologically, the PAC was an invasive adenocarcinoma with a predominantly acinar pattern admixed with other regions of differentiated lepidic, papillary, and solid growth. These differentiated patterns were classified into 2 groups as the glandular and solid structures in this study. Glandular structures were mainly composed of stratified cuboidal or columnar neoplastic cells with clear cell boundaries, light cytoplasm. On the other hand, solid structures were defined as neoplastic cells or areas consisting of solid sheets, forming solid clusters of tumor cells lacking a recognizable lumen. The neoplastic cells had basophilic cytoplasm and heterochromatic nuclei with a prominent nucleolus and increased nucleus-to-cytoplasm ratio (N:C ratio) (Fig. 1).
Fig. 1. Histological features of primary (A) and xenografted pulmonary adenocarcinomas (F1, F2 and F2(C)). The primary tumor (t) was composed of glandular and solid patterns, compressing adjacent normal parenchyma consisting of alveoli (a). The areas of glandular structures (P-B), which was the major component, were characterized by formation of irregularly shaped glands with a central lumen, lined by cuboidal or columnar epithelial cells, while solid structures (P-C) showed growth of polygonal tumor cells and lacked recognizable patterns. The histological characteristics of the primary tumor maintained in the F1, F2, and F(C) xenografted tumors, but the portion of solid structures was notably increased in the xenografted tumors, compared with that in the primary. ‘B’ and ‘C’ figures represent the magnified glandular and solid structures, respectively. Hematoxylin and eosin stain. Bars = 250 μm for ‘A’ and 25 μm for ‘B’ and ‘C’.
In the F1 and other xenografted tumors, the proportion of glandular structures gradually decreased, while the solid pattern became more dominant with the serial passage. F2 and F2(C) were poorly differentiated tumors with a similar growth rate after transplantation (Supplementary Fig. 1), microscopically seen to have some obscure glandular structures consisting of cuboidal to slightly flattened epithelial cells without a distinct stroma (Fig. 1).
Immunohistochemical characterization and scoring
All markers were expressed in normal alveolar/bronchiolar epithelial cells and neoplastic cells in both primary and F1 tissue. Immunoreactivity with all diagnostic and CSC antibodies varied among histological patterns, scored on the basis of expression distribution and intensity.
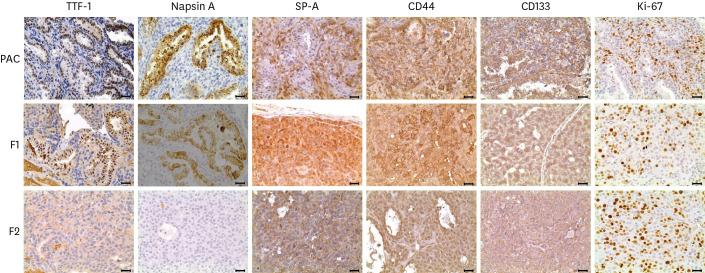
In the normal lung and glandular structures in the primary and F1 tumor, TTF-1 and Napsin A immunoreactivity were seen in the cells (Table 1, Fig. 2). Conversely, they were not detected in neoplastic cells in solid structures. While the neoplastic cells forming glandular structures did not show SP-A immunostaining but the surrounding solid-patterned cells showed moderate to intense labeling (Table 1, Fig. 2). In addition, the localization of SP-A positivity corresponded to the expression of Ki-67 in this study.
Table 1. The distribution and the intensity of each marker at the different morphological structures.
| Marker | TTF-1 | Napsin A | SP-A | CD44 | CD133 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Distribution of each marker | |||||||||||
| Score | DS | IE | DS | IE | DS | IE | DS | IE | DS | IE | |
| Primary | 2 | +++ | 2 | +++ | 1 | +++ | 3 | +++ | 2 | ++ | |
| F1 | 1 | +++ | 1 | +++ | 1 | ++ | 2 | ++ | 1 | + | |
| F2 | 0 | - | 0 | - | 1 | + | 1 | + | 1 | + | |
| F2(C) | 0 | - | 0 | - | 1 | + | 2 | + | 1 | + | |
| Intensity of each marker in the glandular and solid structure | |||||||||||
| Structurea | G | S | G | S | G | S | G | S | G | S | |
| Primary | +++ | - | +++ | - | - | +++ | +++ | +++ | +++ | ++ | |
| F1 | +++ | - | +++ | - | - | +++ | +++ | ++ | ++ | + | |
| F2 | - | - | - | - | - | + | + | + | + | + | |
| F2(C) | - | - | - | - | - | + | ++ | ++ | + | + | |
DS (distribution of staining) was scored according to the following criteria: 0 (tumor cells were negative), 1 (5%–25% positive tumor cells), 2 (25%–60% positive tumor cells) and 3 (more than 60% positive cells); IE (intensity expression score) was based on the followings: (−) negative; (+) weak; (++) moderate; (+++) strong.
aG, glandular structure; S, solid structure.
Fig. 2. Immunohistochemistry of primary pulmonary adenocarcimoma and first xenografted tumor (F1). In the glandular structure, strong TTF-1, Napsin A and CD44 and moderate CD133 reactivity were observed in more than 50% of cells, whereas SP-A was weakly expressed in some tumor cells of glandular structures but its expression was notable in the solid-patterned neoplastic cells, and particularly intruding neoplastic cells close to the surrounding connective tissue. Ki-67 labeling index was increased in the xenografted tumors, compared to that of the primary. ABC method. Bars = 25 μm.
The intensities of CD44 and CD133 staining were strong in both glandular and solid structures in the primary tumor but the proportion of CD133-positive cells was somewhat lower (Table 1). In addition, their immunoreactivity declined in the xenografted tumors, as the neoplastic cells composing the tumors became mostly undifferentiated.
Tumor proliferation
The overall proportion of Ki-67-positive neoplastic cells was 27.3% in the primary tumor, mainly labeled in the solid-patterned neoplastic cells (Fig. 2), which increased markedly to above 50% in F1 and F2 (Supplementary Fig. 2). Thus, the proliferation of tumor cells was accelerated by transplantation.
DISCUSSION
The pulmonary tumor from a Shih Tzu dog in this study was classified as an invasive adenocarcinoma with predominant acinar pattern. The PAC was characterized by a high proportion of glandular structures, while the xenografted tumors (F2 and F2(C)) showed significantly decreased areas of glandular structures and increased areas of solid structures lacking recognizable adenocarcinoma patterns, indicating that they were less differentiated. The increase in solid appearance may reflect poor differentiation, thus predicting more aggressive behavior [1]. The increased malignancy in the xenografted tumors also corresponded to enhanced cell proliferation.
Immunohistochemical analyses showed that differences in immunoreactivity for the diagnostic and CSC markers were related to the cell differentiation status and tumor category. The PAC and F1 PAC showed immunoreactivity for all of the markers examined, and expressed recognizable histological features mostly resembling normal bronchiolar and alveolar structures, which implies good prognosis. The results showed that the F1 xenograft model retained some characteristics of the primary tumor tissue. Although some tumor histological subtypes were preserved in the F1 xenografted tumor, specific morphological details changed during serial passage. The overall morphologies of F2 and F2(C) tumors clearly indicated poorly differentiated cancers that were suspected to have greater malignancy. On the other hand, neoplastic cells in the undifferentiated or solid areas, which were common in the xenograft tumors, were negative for TTF-1 and Napsin A, but mostly positive for SP-A and the stem cell markers, CD44 and CD133. In humans, TTF-1, Napsin A, and SP-A expression have been used in combination to diagnose lung tumors and differentiate primary lung cancers from metastatic lesions [10]. Our results are consistent with previous studies in that TTF-1 and Napsin A were generally expressed in the primary and F1 adenocarcinomas [1] and were negative or expressed at low levels in the relatively poorly differentiated F2 and F2(C) tumors [3,10]. However, in the present study, SP-A expression was absent in bronchiole-like structures and notable in invading neoplastic cells adjacent to the encapsulating fibrous connective tissue. SP-A expression has been postulated to be related to prognosis, its role in progression of lung cancer is unknown [5]. In addition, the pattern of SP-A expression was coincident with that of Ki-67 expression, both of which were highly expressed in the neoplastic cells along the marginal area of the tumor. The results of Ki-67 expression in this study support a previous report that predominantly solid areas showed the highest degree of proliferation followed by acinar and other patterns [14]. Due to these discrepancies in the results, the significance of SP-A in canine lung cancer remains unclear.
CSC markers were detected in both glandular and poorly differentiated areas of both the primary and xenografted canine lung tumors. However, the percentage and intensity of CSC marker-positive cells decreased slightly with serial passage. These findings are discordant with the assumption that poorly differentiated neoplastic cells would express CSC markers. Previous studies have shown that CD44 and CD133 have poor diagnostic value in lung cancer [8]. As CD44 was a distinct marker of PAC in this study, with expression in neoplastic cells of areas of both glandular and solid patterns in all generations, it may be a reliable marker for use in combination with other markers. Our xenograft model provides an increased understanding of cancer diagnosis and characteristics.
In conclusion, our results may be beneficial for effective differential diagnosis of canine PAC and evaluation of its malignancy. In addition, the successfully established live tissue bank of canine PAC could be used for various cancer studies, including anticancer drug development and basic mechanistic and therapeutic studies.
ACKNOWLEDGEMENTS
The first author, Warisraporn Tangchang, would like to express her gratitude for the financial support of the NVI Thailand and Naresuan University in Thailand.
Footnotes
Funding: This study was supported by the National Vaccine Institute (NVI) in Thailand and Kangwon National University in the Republic of Korea.
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Yoon B, Oh YI, Kim HW.
- Formal analysis: Tangchang W, Kim YH, Oh YI.
- Funding acquisition: Yoon B.
- Methodology: Tangchang W, Kim YH, Oh YI.
- Supervision: Yoon B, Kim HW.
- Writing - original draft: Tangchang W, Kim YH.
- Writing - review & editing: Yoon B.
SUPPLEMENTARY MATERIALS
Antibodies used in immunohistochemistry staining
The growth curves of the xenografted canine pulmonary adenocarcinoma, F2 and F2(C).
The labeling indices of Ki-67 positive neoplastic cells in the primary and xenografted canine pulmonary adenocarcinoma.
References
- 1.Bettini G, Marconato L, Morini M, Ferrari F. Thyroid transcription factor-1 immunohistochemistry: diagnostic tool and malignancy marker in canine malignant lung tumours. Vet Comp Oncol. 2009;7(1):28–37. doi: 10.1111/j.1476-5829.2008.00166.x. [DOI] [PubMed] [Google Scholar]
- 2.Moulton JE, von Tscharner C, Schneider R. Classification of lung carcinomas in the dog and cat. Vet Pathol. 1981;18(4):513–528. doi: 10.1177/030098588101800409. [DOI] [PubMed] [Google Scholar]
- 3.Wilson DW. In: Tumors in Domestic Animals. 5th ed. Donald JM, editor. Wiley Online Library; 2016. Tumors of the respiratory tract; pp. 467–498. [Google Scholar]
- 4.Jung J. Human tumor xenograft models for preclinical assessment of anticancer drug development. Toxicol Res. 2014;30(1):1–5. doi: 10.5487/TR.2014.30.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hidalgo M, Amant F, Biankin AV, Budinská E, Byrne AT, Caldas C, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4(9):998–1013. doi: 10.1158/2159-8290.CD-14-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai Y, Wei X, Lin S, Qin L, Cheng L, Li P. Current status and perspectives of patient-derived xenograft models in cancer research. J Hematol Oncol. 2017;10(1):106. doi: 10.1186/s13045-017-0470-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Costa S, Yoon BI, Kim DY, Motsinger-Reif AA, Williams M, Kim Y. Morphologic and molecular analysis of 39 spontaneous feline pulmonary carcinomas. Vet Pathol. 2012;49(6):971–978. doi: 10.1177/0300985811419529. [DOI] [PubMed] [Google Scholar]
- 8.Wu J, Chu PG, Jiang Z, Lau SK. Napsin A expression in primary mucin-producing adenocarcinomas of the lung: an immunohistochemical study. Am J Clin Pathol. 2013;139(2):160–166. doi: 10.1309/AJCP62WJUAMSZCOM. [DOI] [PubMed] [Google Scholar]
- 9.Grossman DA, Hiti AL, McNiel EA, Ye Y, Alpaugh ML, Barsky SH. Comparative oncological studies of feline bronchioloalveolar lung carcinoma, its derived cell line and xenograft. Cancer Res. 2002;62(13):3826–3833. [PubMed] [Google Scholar]
- 10.Beck J, Miller MA, Frank C, DuSold D, Ramos-Vara JA. Surfactant protein A and Napsin A in the immunohistochemical characterization of canine pulmonary carcinomas: comparison with thyroid transcription factor-1. Vet Pathol. 2017;54(5):767–774. doi: 10.1177/0300985817712559. [DOI] [PubMed] [Google Scholar]
- 11.Uzaslan E, Stuempel T, Ebsen M, Freudenberg N, Nakamura S, Costabel U, et al. Surfactant protein A detection in primary pulmonary adenocarcinoma without bronchioloalveolar pattern. Respiration. 2005;72(3):249–253. doi: 10.1159/000085365. [DOI] [PubMed] [Google Scholar]
- 12.Hardavella G, George R, Sethi T. Lung cancer stem cells-characteristics, phenotype. Transl Lung Cancer Res. 2016;5(3):272–279. doi: 10.21037/tlcr.2016.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeaki M, Takashi Y, Tetsuya S, Tsunehiro O, Hidetaka U, Toshihiro O, et al. Cancer stem cell markers in lung cancer. Personal Med Univ. 2015;4:40–45. [Google Scholar]
- 14.Warth A, Cortis J, Soltermann A, Meister M, Budczies J, Stenzinger A, et al. Tumour cell proliferation (Ki-67) in non-small cell lung cancer: a critical reappraisal of its prognostic role. Br J Cancer. 2014;111(6):1222–1229. doi: 10.1038/bjc.2014.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffey SM, Kraegel SA, Madewell BR. Proliferation indices in spontaneous canine lung cancer: proliferating cell nuclear antigen (PCNA), Ki-67 (MIB1) and mitotic counts. J Comp Pathol. 1999;120(4):321–332. doi: 10.1053/jcpa.1998.0281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Antibodies used in immunohistochemistry staining
The growth curves of the xenografted canine pulmonary adenocarcinoma, F2 and F2(C).
The labeling indices of Ki-67 positive neoplastic cells in the primary and xenografted canine pulmonary adenocarcinoma.