Abstract
Women are at twice the risk for anxiety and depression disorders as men are, although the underlying biological factors and mechanisms are largely unknown. In this review, we address this sex disparity at both the etiological and mechanistic level. We dissect the role of fluctuating sex hormones as a critical biological factor contributing to the increased depression and anxiety risk in women. We provide parallel evidence in humans and rodents that brain structure and function vary with naturally-cycling ovarian hormones. This female-unique brain plasticity and associated vulnerability are primarily driven by estrogen level changes. For the first time, we provide a sex hormone-driven molecular mechanism, namely chromatin organizational changes, that regulates neuronal gene expression and brain plasticity but may also prime the (epi)genome for psychopathology. Finally, we map out future directions including experimental and clinical studies that will facilitate novel sex- and gender-informed approaches to treat depression and anxiety disorders.
Keywords: anxiety disorders, depression, estrous cycle, menstrual cycle, estrogen, epigenetics, estrogen receptors, brain structure, hippocampus, chromatin
Introduction
Depression and anxiety disorders are highly prevalent, debilitating, and often co-morbid mental disorders, with reported life-time prevalence rates of 20.6% (Hasin et al., 2018) and up to 33.7% (Bandelow and Michaelis, 2015), respectively. Depression disorders, of which major depressive disorder (MDD) is the most common, are characterized by depressed mood, diminished interests or an inability to feel pleasure, impaired cognitive function, and vegetative symptoms such as loss of energy and disturbed sleep or appetite (Otte et al., 2016). Anxiety disorders, including social anxiety disorder, panic disorder, generalized anxiety disorder, and specific phobias, are characterized by excessive and enduring fear, anxiety or avoidance of perceived threats, and can also include panic attacks (Craske et al., 2017).
Both genetic and environmental factors, particularly stressful life events, are strongly implicated in the etiology of depression and anxiety disorders and have been extensively investigated in both clinical and animal studies. Another very well established risk factor is the individual’s sex or gender; women are at twice the risk for developing anxiety and depression than men are (Kuehner, 2017; Li and Graham, 2017). Symptom profile can also vary with sex, as females with MDD are, in general, more likely than males to experience disturbances of sleep, appetite, and energy and to have comorbid anxiety disorder (Altemus et al., 2014). There are also reports of greater symptom severity, slightly longer episodes, and more chronic course of depression in women (Essau et al., 2010; Marcus et al., 2008). However, surprisingly, little is still known about factors and mechanisms that underlie sex disparity in these disorders.
While socioeconomic factors, difference in trauma exposure, and reporting biases are all likely contributors (Li and Graham, 2017), a strong body of evidence implicates sex hormone fluctuation in women as the major biological factor driving sex differences in anxiety and depression risk (Altemus et al., 2014; Deecher et al., 2008; Soares and Zitek, 2008; Steiner et al., 2003). We believe that this biological risk factor is common to all menstruating individuals across the genders (including cis women, non-binary individuals, and transgender men) but, as we review currently available literature, we will be focusing on presumably cis women and female animals to discuss the impact of physiological changes in ovarian hormones on the brain and behavior.
In this review, we first cover clinical evidence and animal behavioral studies supporting the role of fluctuating sex hormones in increased female vulnerability to depression and anxiety disorders. We further compare and contrast studies in humans and rodents which implicate brain structural and functional changes across the ovarian cycle in dynamic emotion regulation and vulnerability of the female brain. Finally, we review the most recent studies implicating epigenetic mechanisms in dynamic regulation of female brain plasticity and behavior and propose a novel molecular framework to uncover the female-specific susceptibility to depression and anxiety disorders. We end our discussion with future directions including the experimental and clinical studies that can lead us to novel sex- and gender-informed approaches to treat sex-biased brain disorders such as anxiety disorders and depression.
1. Evidence for the role of ovarian hormones in anxiety and depression risk
During the mammalian reproductive period, from puberty to menopause, the female brain is exposed to rhythmic changes in sex hormone levels over the cycles known as menstrual (in humans) or estrous (in rodents) (Figure 1). In humans, each menstrual cycle typically lasts 28–35 days and includes the follicular (high estrogen-low progesterone) phase and the luteal (low estrogen-high progesterone) phase (Figure 1). Women of reproductive age represent close to 50% of the female population and close to 25% of the total worldwide population. Among these women, close to 58% are naturally cycling (Frank et al., 2010), undergoing monthly physiological estradiol and progesterone fluctuations across the menstrual cycle (Figure 1) (Dubol et al., 2021). While the rodent estrous cycle is shorter (typically 4–5 days) and has four hormonally-distinct phases (proestrus, estrus, metestrus, and diestrus), the overall hormonal profiles are very similar to the menstrual cycle. The human follicular and luteal phases are mimicked by the rodent proestrus (high estrogen-low progesterone) and early diestrus (low estrogen-high progesterone) phases, respectively (Figure 1). While being indispensable for reproductive function, fluctuating hormone levels dynamically impact female brain morphology (Dubol et al., 2021; Woolley et al., 1990), function (Albert et al., 2015; Marrocco and McEwen, 2016; Sundstrom Poromaa and Gingnell, 2014), and neurochemistry (Barth et al., 2015), and are likely contributors to female-specific risks for neuropsychiatric conditions such as depression and anxiety disorders (Altemus et al., 2014; Deecher et al., 2008; Steiner et al., 2003).
Figure 1. Sex hormone profiles across the menstrual (human) and estrous (mouse) cycle.
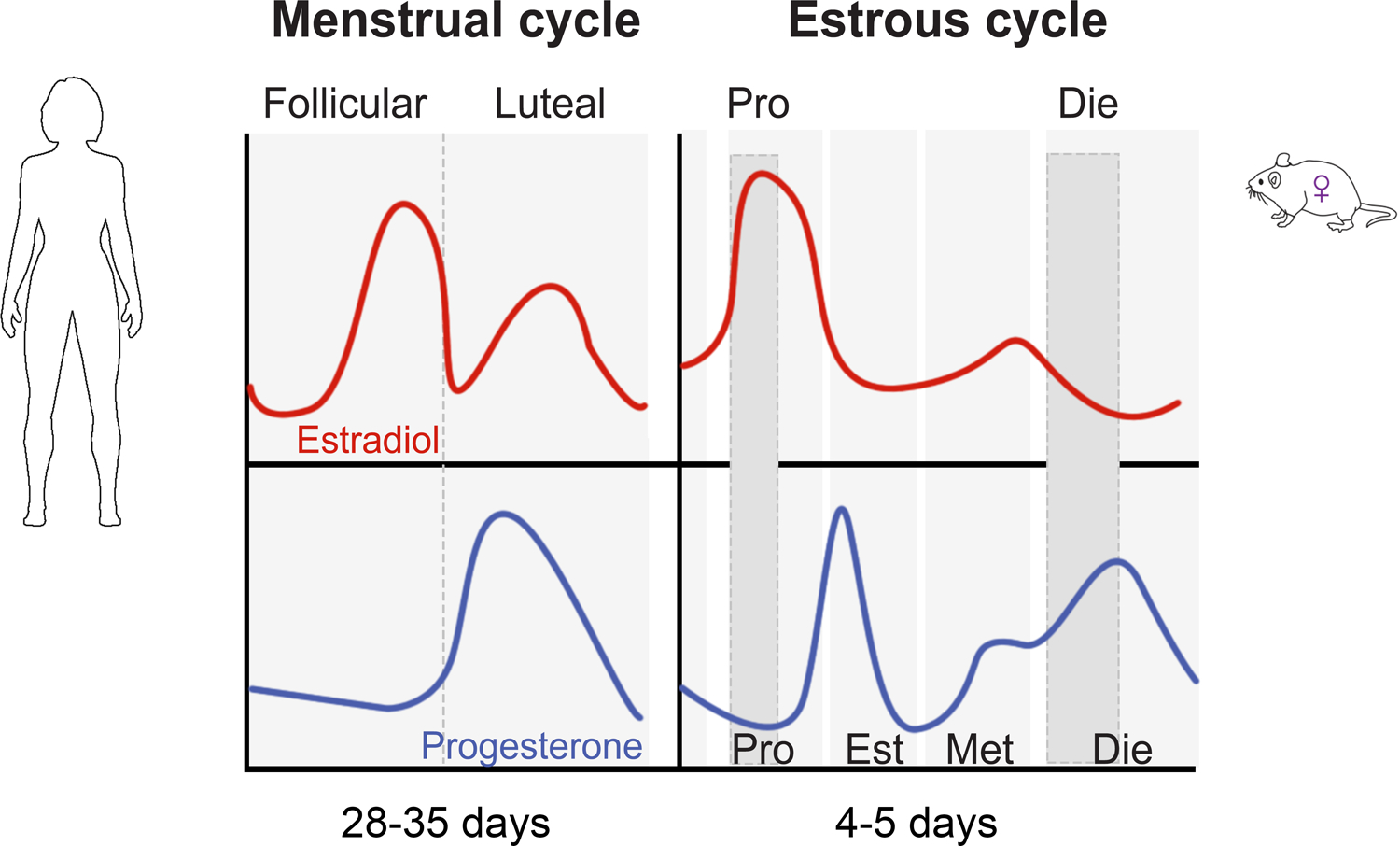
Pro, proestrus; Die, diestrus; Est, estrus; Met, metestrus.
Ovarian hormones, estrogen and progesterone, have potent neuromodulatory effects and have been shown in both human and animal studies to shape female emotionality (Albert et al., 2015; Marrocco and McEwen, 2016; Young and Becker, 2009). In fact, many studies indicate that estrogen has protective anxiolytic- and antidepressant-like effects, which seems at odds with an increased risk for depression and anxiety disorders in women. However, the gene encoding the estrogen receptor beta (ESR2) has been implicated in depression in the genetic studies of this disorder (Howard et al., 2019). Here, we present the evidence that estrogen is not a simple risk or resilience factor for these disorders but that its role depends on the context including age, reproductive window, and temporal dynamics. Particularly, we highlight the role of sex hormone fluctuations and, more specifically, estrogen withdrawal in increased female vulnerability to depression and anxiety disorders.
1.1. Clinical studies
In discussing sex differences in depression risk, an often overlooked fact is that the gap in depression rates between men and women is apparent during the reproductive period only (Figure 2). Before puberty, the risk of depression is very similar or may even be higher in boys than in girls (Deecher et al., 2008) (Figure 2). The female bias in depression rates is first noted with the onset of the menarche in girls, when sex hormones start fluctuating across the menstrual cycles. Female risk then stays elevated during the entire reproductive period, from puberty to menopause, which is characterized by cyclical hormone levels (Figure 1). This risk increases further during perimenopause, which is characterized by the most extreme hormone fluctuations (Gordon et al., 2015). Studies have shown that depressive episodes cluster in the later stages of the menopause transition, with 2 to 5-fold increased risk for MDD during perimenopause versus late premenopause, as well as in the first-year postmenopause (Bromberger and Epperson, 2018; Steinberg et al., 2008), suggesting a role for erratic hormone fluctuation and estradiol withdrawal in the development of depression during this period (Steinberg et al., 2008). Importantly, depression risk in women finally drops and becomes more similar to age-matched males when stable, low estradiol levels are established at postmenopause (Steiner et al., 2003) (Figure 2). Overall, these findings indicate that, rather than low or high sex hormone levels, the fluctuation in hormones and particularly estrogen withdrawal is a risk factor for depression in women.
Figure 2. Sex difference in depression risk and sex hormone levels in females across the life span.
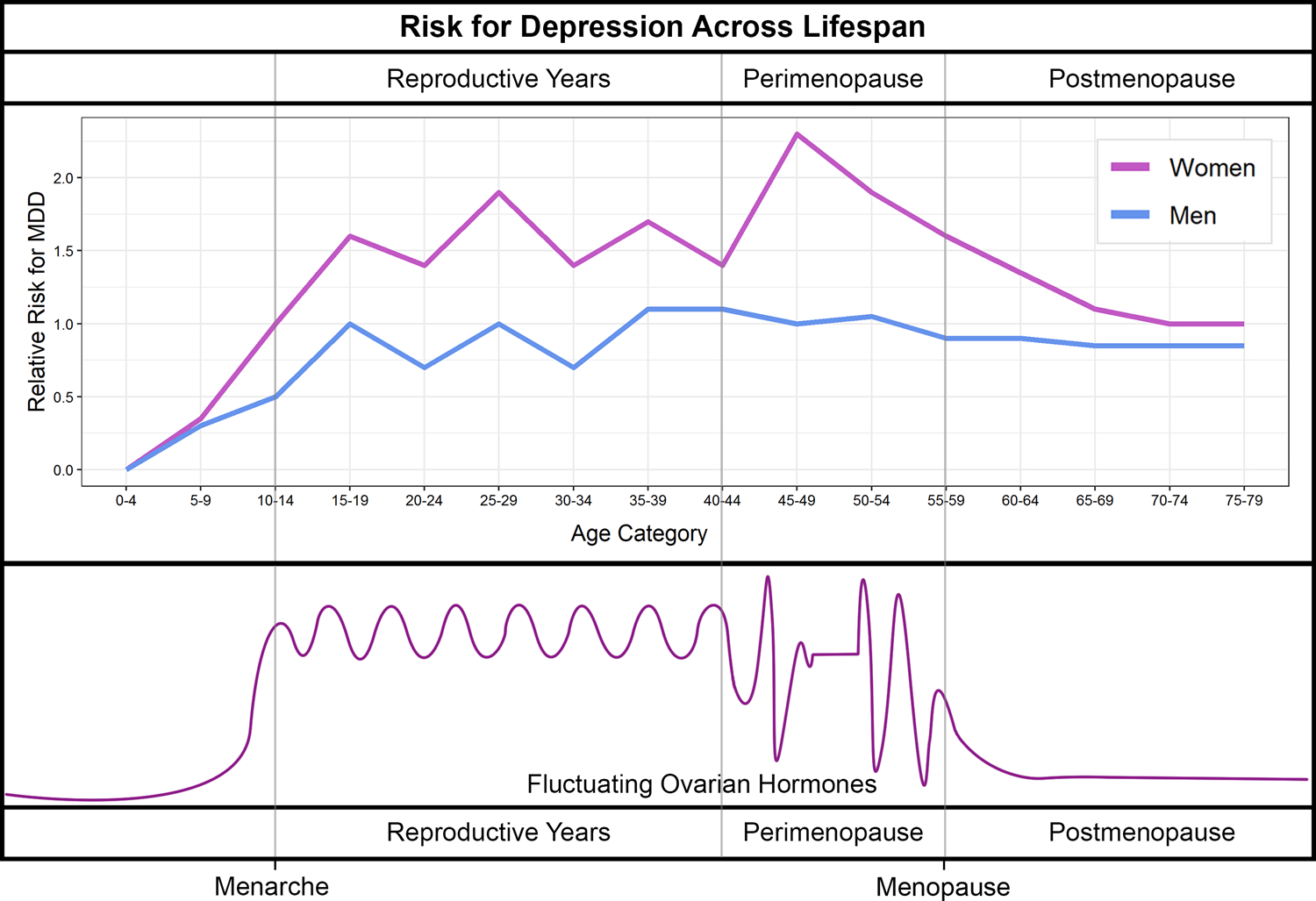
(adapted from Deecher et al, 2008).
Indeed, studies have shown that, in more than 50% of menstruating individuals with mood disorders, symptoms fluctuate across the menstrual cycle (Kuehner and Nayman, 2021). For instance, women with major depression, even those under antidepressant treatment, often experience worsening of their symptoms in the premenstrual phase of the cycle which is associated with a drop in sex hormones and particularly low estrogen (Altemus et al., 2014; Kornstein et al., 2005). This phenomenon is known as premenstrual exacerbation (PME) and is similarly found in women with anxiety disorders who report premenstrual worsening of anxiety symptoms (Li and Graham, 2017). Consistent with these reports in patients, in healthy women, a pharmacologically induced sex hormone fluctuation using a gonadotropin-releasing hormone (GnRH) agonist induces subclinical depressive symptoms relative to placebo (Frokjaer et al., 2015).
There are additional clinical observations that support the role of natural hormonal shifts in depression and anxiety risk. For instance, one of the most extreme examples is postpartum depression (PPD), which is associated with severe drops in estrogen (100-fold) and progesterone (10- fold) levels post-partum, and is diagnosed in up to 19% of individuals during the first year following child birth (Hahn-Holbrook et al., 2017; O’hara and Swain, 1996; Wang et al., 2021). Moreover, a subset (5–8%) of women suffer from premenstrual dysphoric disorder (PMDD), which is separate from PME and is characterized by severely impaired mood during the premenstrual phase of the cycle only, requiring intermittent antidepressant treatment (Yonkers et al., 2008). Interestingly, patients with PMDD do not have lower sex hormone levels but seem to be particularly sensitive to physiological hormonal changes during the menstrual cycle.
Together, these clinical findings indicate that sex hormone fluctuations can lead to both increased vulnerability to the development of depression and anxiety disorders and periodic symptom exacerbation serving to maintain depressive and anxiety symptoms.
1.2. Behavioral studies in rodents
Depression and anxiety disorders are complex syndromes in humans and this complexity makes them particularly challenging to model in rodents. However, due to very similar reproductive cycles (Figure 1) and comparable effects of hormonal changes on behavioral indices, rodent models may in fact have a good translational value for studying the depression and anxiety risk associated with hormonal fluctuations. Certainly, the major advantage to studying rodents is the ability to control for genetic and environmental factors as well as to perform molecular analyses in the brain and mechanistic studies that can address the underlying mechanisms and discover new molecular targets for treatments.
Comparable to human studies, multiple rodent studies have shown that female emotionality varies with naturally cycling sex hormone levels (Jaric et al., 2019b; Maeng and Milad, 2015) and that behavioral effects of antidepressant drugs vary with the estrous cycle (Carrier and Kabbaj, 2013; Kokras et al., 2015). Estrogen is implicated in emotional behavior in rodents, particularly in naturally occurring trait anxiety (Donner and Lowry, 2013; Walf and Frye, 2006). During the estrous cycle in rats, it was found that proestrus (high-estrogenic) females exhibit lower anxiety measures compared to low- estrogenic females (Frye et al., 2000; Gouveia et al., 2004; Marcondes et al., 2001; Mora et al., 1996). More recently, we were able to reproduce these findings across different anxiety tests in mice (open field, light dark box, and elevated plus maze), providing a comparison with age-matched males as well (Figure 3). Diestrus female mice consistently exhibit increased anxiety indices compared to proestrus females and males (Jaric et al., 2019b) (Figure 3), implying that a physiological drop in estrogen increases the risk for anxiety in females, consistent with previous findings in rats (Mora et al., 1996) and in humans (Albert et al., 2015). Importantly these findings were specific to anxiety indices while no significant change in overall activity in mice has been noted across the estrous cycle or sex (Jaric et al., 2019b).
Figure 3. Anxiety-related behavior varies across the estrous cycle and sex in mice.
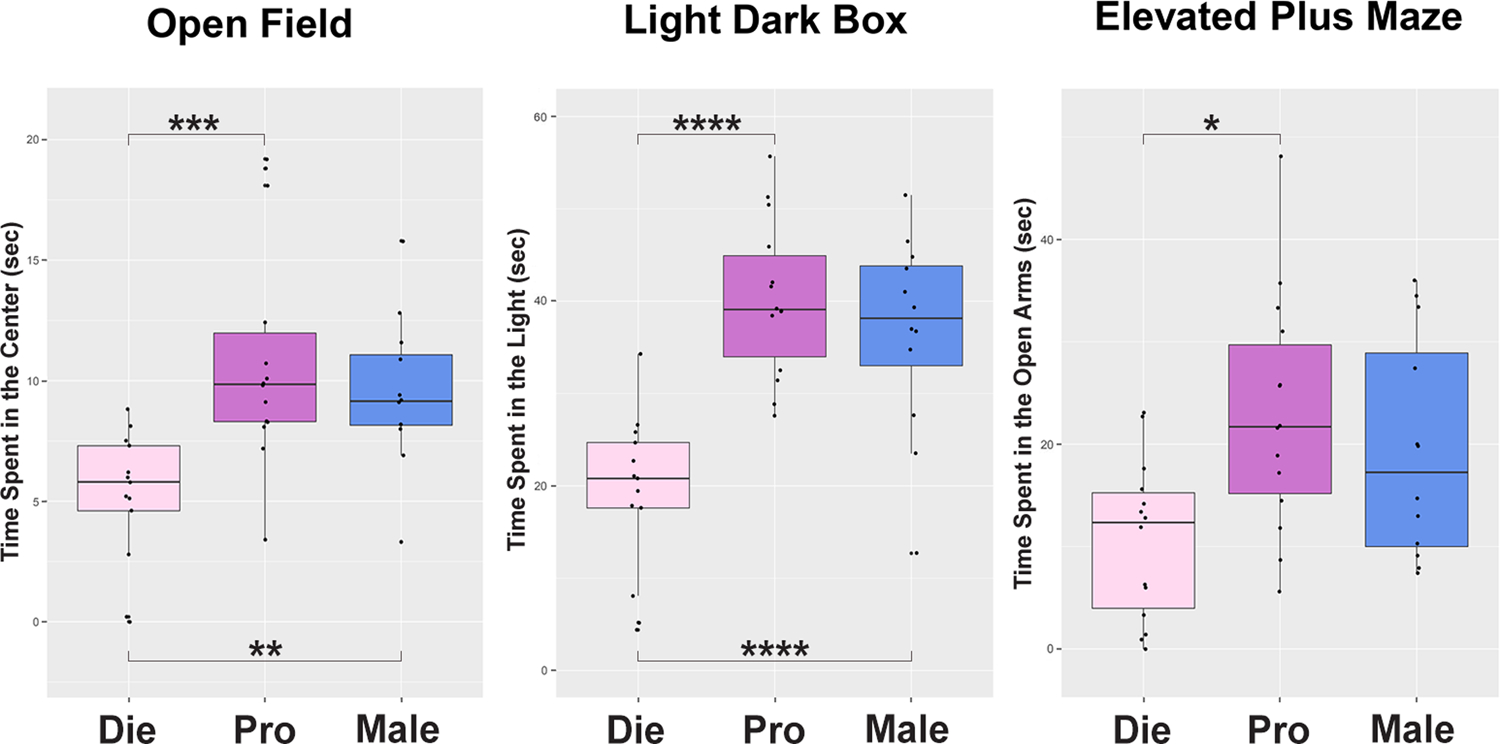
Die, diestrus (pink); Pro, proestrus (purple); Male, males (blue). (adapted from Jaric et al., 2019b)
Similar findings were reported in fear extinction experiments that, unlike the above mentioned anxiety tests, include a training or learning component (Maeng and Milad, 2015). Female rats that underwent extinction learning in the metestrus phase (low ovarian hormones) exhibit more freezing behavior during recall compared to females that were extinguished in the proestrus, high-estrogenic phase as well as compared to male rats (Milad et al., 2009). The decreased fear expression following extinction learning in proestrus is consistent with the reported anxiolytic effects of estrogen.
The role of the estrous cycle was also examined in depression-related behavioral phenotypes in rodents. In the forced swim test (FST), meant to model learned helplessness, an aspect of depression, we found that high-estrogenic female mice have significantly shorter immobility time than low estrogenic females, implying an antidepressant-like effect of estrogen (Jaric et al., 2019a). In rats, the results were mixed and while some studies reported lower immobility time in the high-estrogenic proestrus phase compared to metestrus and diestrus (Estrada-Camarena et al., 2011; Frye and Walf, 2002; Schneider and Popik, 2007), others did not find that the estrous cycle stage affects baseline performance in the FST (Kokras et al., 2015). However, even when there was no effect observed at the baseline level, response to an antidepressant drug sertraline (a selective serotonin reuptake inhibitor, SSRI) in the FST test was found to be significantly affected by the estrous cycle in female rats (Kokras et al., 2015). Consistent with this finding, the antidepressant-like effects of low-dose ketamine treatment was abolished with ovariectomy and restored upon estrogen and progesterone replacement (Carrier and Kabbaj, 2013), suggesting a critical role for ovarian hormones in enhancing the antidepressant-like effects of ketamine in female rats.
While the hormonal profiles of the estrous cycle are complex (Figure 1), the critical role of estrogen and its withdrawal in anxiety- and depression-related behavior in rodents have been confirmed in both cycling and ovariectomized (OVX) animals using different paradigms. For instance, it has been shown that systemic or intra-hippocampal administration of estradiol decreases anxiety- and depression-related behaviors in low-estrogenic naturally cycling (Marcondes et al., 2001; Rocks et al., 2022) or OVX (Walf and Frye, 2006) female rodents. Estrogen administration in metestrus female rats before or within 4 hours after extinction training, increased fear extinction memory and reduced freezing during recall (Maeng and Milad, 2015; Zeidan et al., 2011). In a model of postpartum depression in rats, in which OVX rats underwent hormone-stimulated pregnancy, estrogen withdrawal led to increased depression-like behaviors in both forced swim test (Galea et al., 2001) and sucrose preference test (Green et al., 2009), a model of anhedonia. These behaviors were also attenuated by estradiol treatment (Galea et al., 2001; Green et al., 2009).
In summary, while animal models for anxiety and depression are too simple to account for the full complexity of these syndromes in humans, there are important parallels that are observed in how sex hormone fluctuations affect anxiety and depression indices in humans and mice. Specifically, estrogen withdrawal in rodents, both physiological or induced by pharmacological or surgical manipulations, leads to increased depression and anxiety indices that can be attenuated by estrogen treatment, similar to what is observed in healthy humans or humans suffering from MDD, PMDD, PPD, or perimenopausal depression. As such, the parallel examination of human and animal studies can lead us to a better understanding of hormone-driven cellular and molecular mechanisms underlying increased female risk for anxiety and depression.
2. Structural basis for increased female vulnerability
The relationship between brain structure and function, and the structural basis of psychiatric disorders in particular, are complicated. The activities in multiple brain areas contribute to even the simplest animal behaviors. However, there is good evidence that several limbic and cortical brain regions including the hippocampus, the amygdala, the cingulate cortex, and the prefrontal cortex (PFC) are involved in emotion regulation and are implicated in anxiety and depression related phenotypes in mice and humans. Here we will focus on the hippocampus for several reasons. First, there is a large body of evidence that implicates decreased hippocampal volume in the pathophysiology of depression and posttraumatic stress disorder (PTSD) (Bromis et al., 2018; Gurvits et al., 1996; MacQueen and Frodl, 2011; Villarreal et al., 2002). Second, stress (a major risk factor for anxiety and depression) has been associated with shrinkage of the hippocampus in human subjects (Opel et al., 2014) and hippocampal dendritic atrophy and neuronal cell loss in animals (Magariños and McEwen, 1995; McEwen and Magarinos, 1997). Third, interventions such as antidepressant drug treatments (Arnone et al., 2013; Duman et al., 2001; Malberg et al., 2000), exercise (Erickson et al., 2011; Redila and Christie, 2006; van Praag et al., 1999), and mindfulness training (Hölzel et al., 2011), all of which are associated with lower depression scores and decreased stress response, have been shown to increase hippocampal gray matter and hippocampal neurogenesis in humans or rodents. Finally, the importance of neuroplasticity in the hippocampus for antidepressant action has been highlighted even more recently as the newer and faster antidepressant compounds such as ketamine and other psychedelics have been shown to have neuroplastic activity (Ly et al., 2018; Olson, 2018). In this section, we will look into changes in the hippocampus across the menstrual and estrous cycle and discuss estrogen’s neuroplastic action as well as ovarian cycle-driven variability in hippocampal structure as a vulnerability factor for anxiety disorders and depression.
2.1. Studies in humans
A growing body of literature shows that the structure and functional connectivity in the human female brain are dynamic and vary with fluctuating sex hormone levels (Barth et al., 2016; Hagemann et al., 2011; Lisofsky et al., 2015; Protopopescu et al., 2008). For the most comprehensive review of this topic we refer readers to the recent review article by Dubol et al. that summarizes the results and estimates the confidence level of 77 neuroimaging studies that examined the effects of the menstrual cycle on brain structure and function, performed on a total of 1,304 naturally cycling women (Dubol et al., 2021). While, in general, the results show significant modulatory effects of ovarian hormone fluctuations on the activity and structure of various cortico-limbic brain regions, many studies focused on the hippocampus (Barth et al., 2016; Lisofsky et al., 2015; Protopopescu et al., 2008). Structural studies consistently reported an increase in gray matter volume in the hippocampus during the high-estrogenic, late follicular phase compared to the early follicular and mid-luteal phases, both of which are characterized by lower estradiol levels (Figures 1 and 4) (Lisofsky et al., 2015). In addition, a positive correlation was found between estradiol levels and both hippocampal gray matter volume and fractional anisotropy measures associated with hippocampal white matter structure (Figure 4A) (Barth et al., 2016). To examine these relationships more closely, Barth et al. performed longitudinal neuroimaging coupled with rigorous menstrual cycle monitoring and hormone measurements, every second or third day across two full menstrual cycles (Figure 4A) (Barth et al., 2016). This study shows that hippocampal changes are rapid and closely parallel fluctuations in endogenous ovarian hormones levels, and particularly the estrogen pattern, across the menstrual cycle.
Figure 4. Comparable changes in hippocampal brain structure across the menstrual cycle in humans and the estrous cycle in mice.
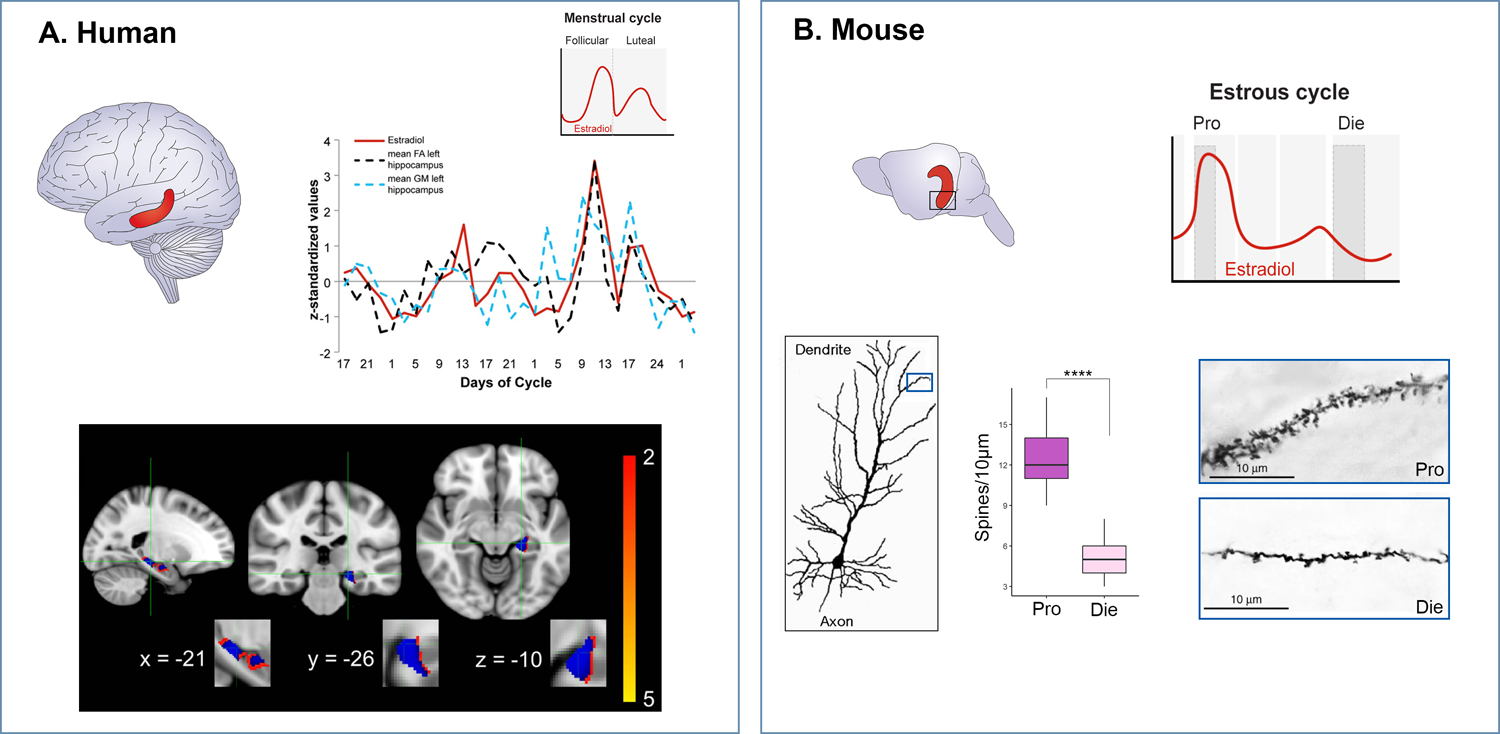
A. In the human hippocampus (red), bilateral fractional anisotropy and left grey matter values are plotted against estrogen levels (in red) across the two menstrual cycles assessed (upper panel; adapted from Barth et al, 2016). In the lower panel, grey matter changes in left hippocampus are displayed with blue voxels corresponding to statistically significant results. B. In the mouse hippocampus (red), we showed structural changes in the ventral hippocampus (black square) as estradiol levels vary across the estrous cycle. In the lower panel, dendritic spine density is shown in the high-estrogenic (Pro, proestrus) phase versus low-estrogenic (Die, diestrus) phase (adapted from Jaric et al, 2019b).
At the functional level, task-based fMRI studies consistently showed enhanced brain reactivity during affective processing in the hippocampus during the late follicular and the mid-luteal phases compared to the early follicular and the late luteal phase (Albert et al., 2015; Andreano and Cahill, 2010; Bayer et al., 2014; Goldstein et al., 2005). Again, when integrated with hormonal data, the functional studies showed positive correlations between estradiol concentrations and the hippocampal gray matter volume, white matter integrity, and activity during affective processing (Albert et al., 2015; Barth et al., 2016; Dubol et al., 2021; Lisofsky et al., 2015).
Other life stages that involve dramatic hormonal shifts and increased depression risk in women such as pregnancy (Hoekzema et al., 2017) and the (peri)menopausal transition (Mosconi et al., 2021) are also associated with reduced hippocampal volume that seems to recover within two years postpartum and in postmenopause, respectively. Together, these studies strongly suggest that natural fluctuations in ovarian hormones are able to change brain structure and function, and that these changes may represent a neural substrate for the increased female vulnerability associated with sex hormone changes and, particularly, with estrogen withdrawal.
2.2. Studies in rodents
While studies in humans show dynamic hormone-driven structural and functional changes in the limbic brain areas, the molecular and cellular substrate of this is difficult to study in the human brain. However, there are structural parallels that can be studied in the rodent brain and these include changes in synaptic plasticity reflected in varying dendritic spine density in specific brain regions (Figure 4). In fact, studies in Bruce McEwen’s lab in the early ‘90s showed that the spine density in the rat hippocampus fluctuates with the estrous cycle (Woolley et al., 1990) and further demonstrated that these changes are driven by fluctuating estrogen levels (Woolley and McEwen, 1992). Indeed, the highest dendritic spine density was found in proestrus and the density was significantly reduced upon estrogen’s drop in the estrus phase (Woolley et al., 1990). In addition, other aspects of hippocampal physiology, such as long-term potentiation (Koss and Frick, 2017; Warren et al., 1995) and dentate gyrus neurogenesis (Sheppard et al., 2019; Tanapat et al., 1999) also show cyclic patterns and are enhanced during the high-estrogenic phase of the cycle. These early studies were largely focused on the dorsal hippocampus as the region critically important for memory formation in rodents. In fact, the rodent hippocampus is a complex brain region containing functionally distinct sub-regions. The ventral hippocampus (vHIP) is essential for emotion regulation, whereas the dorsal part is primarily involved in spatial memory (Bannerman et al., 2004; Bannerman et al., 2014; Fanselow and Dong, 2010) (Fig. 4). It is well-established that selective cytotoxic lesions of vHIP (Bannerman et al., 2003) or the optogenetic activation of vHIP neurons (Kheirbek et al., 2013) specifically affect anxiety-related behavior (but not spatial memory) in mice. For this reason, we recently analyzed dendritic spine density in the vHIP across the estrous cycle in mice and were able to confirm that the proestrus (high-estrogenic) phase is associated with higher dendritic spine density while the number of spines drops dramatically in the diestrus (low-estrogenic) stage, again indicating that this neuroplastic effect is primarily driven by estrogen (Jaric et al., 2019b) (Figure 4b). Importantly, in the vHIP, the varying dendritic spine density as a function of fluctuating estrogen levels may be important for anxiety- and depression-related phenotypes rather than for memory.
It is important to note that although changes in dendritic spine density have been linked to depression-related behaviors and antidepressant-like activity (Duman and Duman, 2015), the majority of these past studies were performed in male animals only, thus failing to address how this cyclical, female-unique synaptic plasticity could factor into sex-specific depression risk. It has been shown that stress, as a major risk factor for depression, induces reduced dendritic spine density and dendritic atrophy in the hippocampus, the amygdala, and the PFC (Duman and Duman, 2015; McEwen and Magarinos, 1997). Furthermore, studies show the correlation between loss of spines, synaptic function, and depression-related behaviors (Li et al., 2011; Qiao et al., 2014). Antidepressants, on the contrary, are able to reverse changes in dendritic spines and depression-related behavior induced by stress (Duman and Duman, 2015); it is important to note that this effect requires chronic treatment with traditional, slow-acting antidepressants (Hajszan et al., 2009) while ketamine induces the changes quickly in line with its fast antidepressant action (Li et al., 2010; Zhang et al., 2019). In fact, it was proposed that rapidly-induced neuroplasticity may be a shared mechanism for the action of newer compounds with fast-acting properties such as ketamine and serotonergic psychedelics (Kadriu et al., 2021; Olson, 2018).
Based on the presented evidence, we propose that reduced dendritic spine density and decreased brain volume associated with estrogen withdrawals are likely to represent, in part, brain substrates contributing to the increased female vulnerability to anxiety and depression. The estrogen withdrawals and brain structure changes are the most extreme during the periods of highest depression and anxiety risks such as pre-menstrually, post-partum, and peri-menopausally, and have been shown to respond to estrogen treatments. Considering that estrogen has a quick neuroplastic action, it may be one of the best anxiolytic and antidepressant compounds for women if given at the right time and dose. However, unfortunately, estrogen has diverse effects on multiple tissues and is associated with potentially serious side effects such as cardiovascular effects and increased risk for reproductive tumors (Grimes and Lobo, 2002; Nelson et al., 2002). Therefore, learning more about molecular mechanisms and mediators through which estrogen and other ovarian cycle-specific factors induce beneficial effects on mood and anxiety levels could lead us to better candidates for sex-tailored treatments. The estrogen effect is, at least in part, mediated via changes in the expression of genes involved in synaptic plasticity (e.g. BDNF) and serotonergic transmission, which have been shown to be modulated by estrogen levels (Barth et al., 2015; Scharfman and MacLusky, 2006). Before reviewing estrogen receptor signalling and gene regulation, we will first cover the changes in neurotransmitter systems in the brain that are likely to contribute to sex hormone-driven plasticity and disease risk.
3. Neurochemical basis for increased female vulnerability
Estrogen is a potent neuromodulator that has been shown to affect multiple neurotransmitter systems in the brain including serotonergic, noradrenergic, GABAergic, dopaminergic, and glutamatergic systems and thus is able to affect mood, emotion and reward processing, and cognition, among other brain functions. These effects of sex hormones were described in more detail in a recent review by Julia Sacher and colleagues (Barth et al., 2015). Here, we focus on the serotonergic system as a system that is strongly implicated in depression and anxiety disorders in humans and in related behavioral phenotypes in mice (Thibaut, 2017). The monoaminergic hypothesis of depression, which implicates the deficit of monoamine neurotransmitters (including serotonin) in this disorder, while oversimplified, is still one of the major hypotheses of this disorder. In fact, selective serotonin reuptake inhibitors (SSRIs), the drugs that increase serotonin levels in the synapse, are the first-line treatment for both depression and the majority of anxiety disorders (Altemus et al., 2014; Barth et al., 2015). Some faster-acting drugs such as ketamine may have newer, non-monoaminergic mechanisms of action including the effect on the NMDA receptors and glutamatergic system with a rapid induction of BDNF and dendritic spine density (Deyama and Duman, 2020), which are likely to be shared, in part, with estrogen (Barth et al., 2015), as we discuss more later. However, it is important to note that the majority of psychedelic drugs that have been studied as promising new treatments for depression and anxiety disorders are also serotonergic drugs, primarily acting as agonists of 5-HT2A receptors (Kadriu et al., 2021; Ly et al., 2018). Therefore, the interaction between estrogen and serotonin is an extremely important point of consideration when thinking of both estrogen’s beneficial effects in anxiety and mood regulation as well as the vulnerability that comes with estrogen’s withdrawal during natural hormonal shifts (Amin et al., 2005; Barth et al., 2015). We will summarize important human and animal data that show sex hormone-induced changes in serotonergic function and suggest their relevance for hormone-related changes in mood and anxiety levels.
3.1. Studies in humans
There are several lines of evidence indicating that “withdrawal” or drop in estrogen during natural hormonal shifts (within the menstrual cycle, postpartum, and during the menopause transition) increases anxiety and depression risk because it requires periodic neurochemical adaptations, specifically adaptation in serotonergic function important for the regulation of mood and anxiety levels. In a PET imaging study, it has been shown that individuals with PMDD exhibit increases in serotonin transporter (SERT) binding across the menstrual cycle (Sacher et al., 2020), which coincides with their increased sensitivity to hormonal changes. Importantly, unlike patients with major depressive disorder, patients suffering from PMDD respond to SSRIs quickly and, in general, can be treated with SSRIs intermittently as their symptoms occur within the cycle (Steinberg et al., 2012). SSRIs are also much more efficacious than tricyclic antidepressants in PMDD patients, again highlighting the role of the serotonergic system in this hormone fluctuation-induced disorder (Freeman et al., 1999). Another PET study used a GnRH agonist to model a biphasic ovarian sex hormone fluctuation and showed that this manipulation triggered subclinical depressive symptoms which were associated with decreases in estradiol levels and increased SERT binding in the brain (Frokjaer et al., 2015). Interestingly, in general, women seem to be more responsive to SSRIs than men are, indicating that the serotonin reuptake inhibition may attenuate the anxiogenic and mood-destabilizing effect of ovarian hormone fluctuation (Altemus et al, 2014).
3.2. Studies in rodents
Studies in rodents have shown that estrogen enhances serotonergic and noradrenergic transmission, which is consistent with estrogen’s antidepressant- and anxiolytic-like activity. The enhancing effect of estrogen on serotonergic neurotransmission seems to involve multiple mechanisms, from modulating the level of serotonin synthesis and degradation to changing the density and binding of serotonin (5-HT) receptors (Deecher et al., 2008). Specifically, estrogen has been shown to change the expression of serotonergic genes including the serotonin synthesizing enzyme tryptophan hydroxylase; the 5-HT2A, 5-HT1B, and 5-HT1A serotonin receptors; the serotonin degrading enzyme monoamine oxidase; and the serotonin transporter SERT mRNA (Barth et al., 2015). We have also shown that the expression of serotonergic genes in vHIP neurons vary with the estrous cycle (Jaric et al., 2019b). In the next section we will discuss sex hormone receptor pathways and the molecular mechanisms through which expression of genes underlying serotonergic transmission and brain plasticity may change with fluctuating sex hormone levels and contribute to female vulnerability to anxiety and depression risk.
4. Epigenetic basis for hormone driven brain plasticity and disease risk
So far, we have presented the well-established evidence that sex hormone fluctuations play an important role in brain plasticity and the increased risk for depression and anxiety disorders in women. However, we have just started to discover the molecular mechanisms underlying this sex hormone-induced, dynamic nature of the female brain. As previously indicated, hormonal profiles of the ovarian cycle stages are complex (Figure 1), and it is likely that different hormones including estrogen, progesterone, and gonadotropins may all contribute to brain and behavioral plasticity that we and others have observed. Here we largely focused on estrogen due to its established role in structural (Woolley and McEwen, 1992) and behavioral (Maeng and Milad, 2015; Rocks et al., 2022) changes across the estrous and menstrual cycle while we expect that other cyclic hormones are also involved and may contribute through similar or converging mechanisms.
4.1. Sex hormone receptor mechanisms and cyclical gene regulation in the brain
Estrogen can act through either nuclear or membrane estrogen receptors (ERs), which include classic ERs α and β and G-protein-coupled ER (GPER), widely distributed throughout the brain (Figure 5) (Galea et al., 2017; Kundakovic et al., 2013; Marrocco and McEwen, 2016). All ERs are present in the hippocampus and expressed in neurons and glia cells although at different levels (Cui et al., 2013). Estrogens can influence hippocampal function through changes in gene expression or through rapid membrane signaling via kinase-induced protein phosphorylation (Figure 5).
Figure 5. Working model of ER signaling mediating chromatin and gene expression changes in vHIP neurons across the estrous cycle.
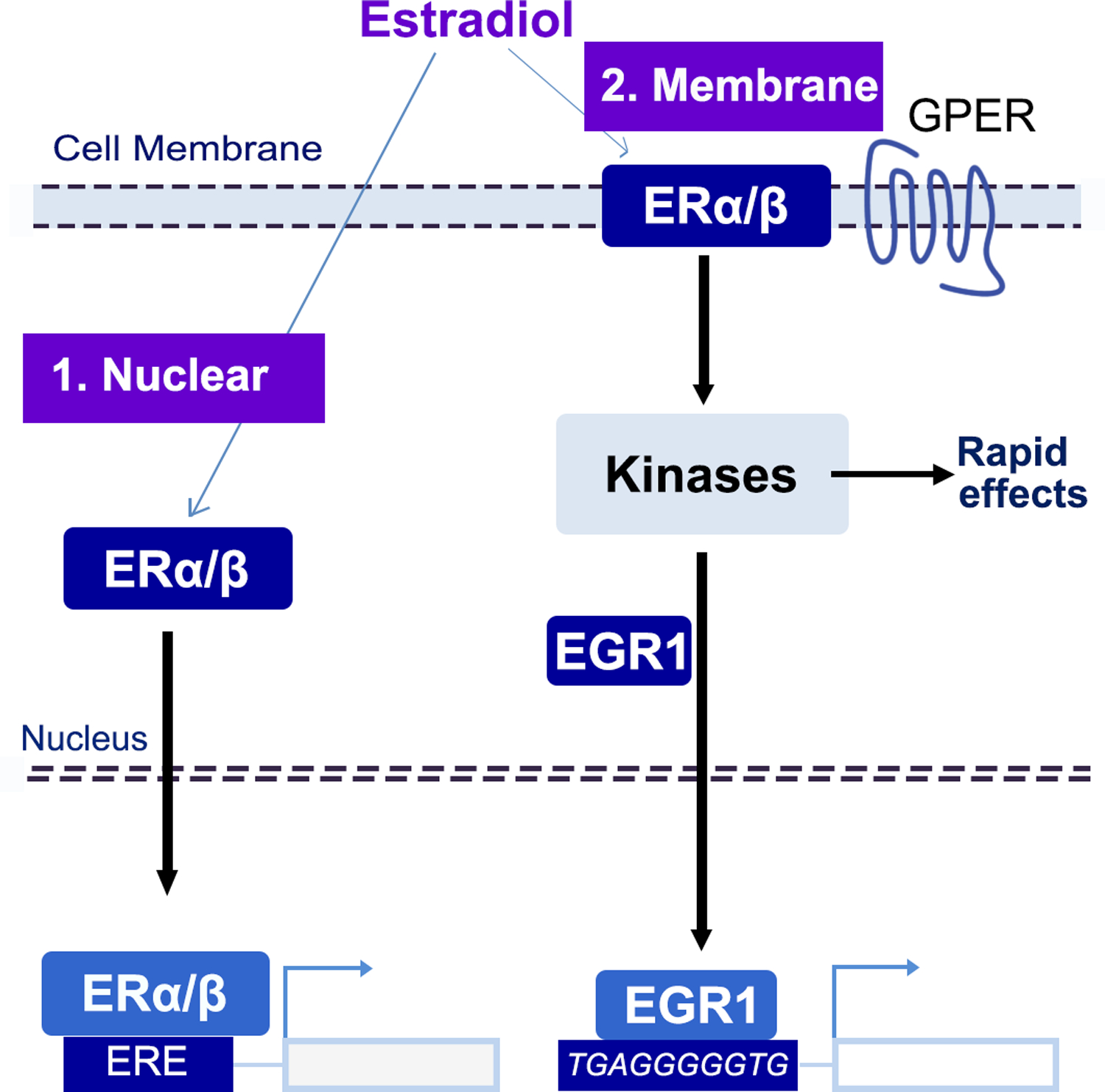
Estradiol could act through: 1. Nuclear ER that activates transcription by binding to estrogen response elements (ERE); 2. Membrane-bound ER that regulates transcription indirectly, via kinase-dependent activation of Egr1 and other estrogen-dependent transcription factors.
The classic estrogen signaling includes the activation of nuclear ERα and ERβ; upon activation by estradiol, these receptors act as transcription factors, bind estrogen response elements (EREs) in the DNA, and modulate expression of estrogen-target genes (Figure 5) (Beato, 1989). ERα and ERβ are also localized in the cell membrane and mediate rapid estrogen effects (Sellers et al., 2015). The membrane ER signaling has been associated with the activation of protein kinase cascades, including MAPK/ERK, PI3K/AKT, PKA, and tyrosine kinase pathways. GPER is a membrane estrogen receptor independent of ERα and ERβ; although it is not specific to estrogen, it can trigger rapid, estrogen-responsive kinase signaling pathways. While the membrane estrogen receptors have a prominent role in the fast estrogen signalling in the hippocampus (Srivastava et al., 2011), they are likely to also include indirect genomic effects through downstream effects of kinase pathways on transcriptional regulators other than ERs such as Egr1 (Figure 5). In addition, there is also evidence of an interaction of estrogen’s non-genomic and genomic mechanisms (Vasudevan and Pfaff, 2008).
While estrogen mainly acts by changing gene expression, which typically involves epigenetic mechanisms such as chromatin remodeling (Magnani and Lupien, 2014), these mechanisms have been largely described in cancer cell lines, and remained poorly understood in the brain until recently. We and others have demonstrated significant gene expression changes across the estrous cycle in the hippocampus (DiCarlo et al., 2017; Iqbal et al., 2020; Jaric et al., 2019b), the prefrontal cortex (Duclot and Kabbaj, 2015), and the hypothalamus (Vastagh and Liposits, 2017). In the prefrontal cortex, the estrous cycle-driven gene expression differences were found to surpass the transcriptional differences among the sexes (Duclot and Kabbaj, 2015), although these findings appear to be brain region-specific. As noted earlier, we were specifically interested in examining the effect of the estrous cycle in vHIP neurons that show plastic changes across the cycle (Figure 4) and are known to drive anxiety-related behaviors (Fanselow and Dong, 2010; Jaric et al., 2019b) (Figure 3). Our findings provide a dynamic model of cyclical gene regulation underlying both the plasticity and the vulnerability of the female brain (Figure 6).
Figure 6. Model for epigenetic regulation of anxiety-related behavior by the estrous cycle.
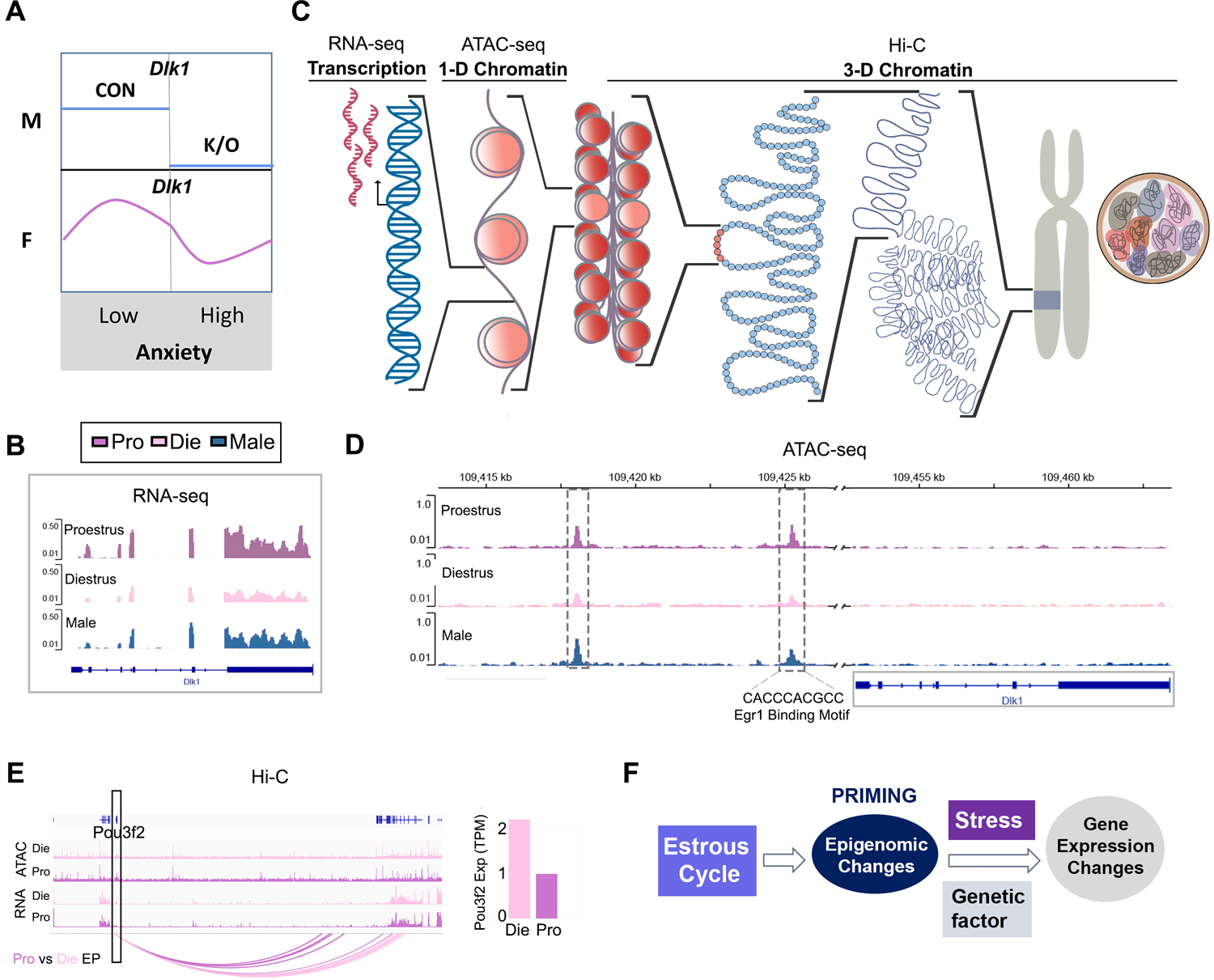
Dlk1 represents an example of a gene whose genetic deletion (knockout, k/o) leads to increased anxiety indices in males (M) but whose expression naturally varies with the estrous cycle in females (F) (A-B). Gene expression is regulated by the accessibility of the regulatory DNA which is packaged into chromatin at the nucleosome level (1D chromatin) or at higher order chromatin organization (3D chromatin) (C). Dlk1 gene changes chromatin organization within putative regulatory regions (dashed rectangles) during the estrous cycle. One of these regions contains an Egr1 binding motif. (D). Other genes such as Pou3f2 (marked by rectangle) show changes in 3D chromatin interactions. Shown are arcs representing differential enhancer-promoter interactions between proestrus and diestrus (left) which are associated with differential gene expression found by RNA-seq (right; TPM, normalized read counts as transcripts per million). All genomic data are derived from triplicates and are depicted here as data merged per group for simplicity (E). Epigenomic changes across the estrous cycle exceed changes in gene expression indicating that they may prime the genome while larger gene expression changes may require another stimulus such as a genetic risk factor or stress exposure (data derived from and figures adapted from Jaric et al, 2019b and Rocks et al, 2022). Die, diestrus (pink); Pro, proestrus (purple); Male, males (blue).
First, it is important to discuss how cyclical changes in gene expression could contribute to the variation in anxiety-related behavior in females. We see more than a hundred genes changing the expression in vHIP neurons across the estrous cycle including genes implicated in the regulation of fear and anxiety behavior (Jaric et al., 2019b). With the Dlk1 gene as an example, the following simplified model explains how sex hormone-driven, dynamic gene regulation can contribute to the sex-specific control of anxiety-related behavior (Fig. 6A). Dlk1 (Delta Like Non-Canonical Notch Ligand 1) is an imprinted gene whose expression in the adult brain is largely limited to the reward system (Ferron et al., 2011; Garcia-Gutierrez et al., 2018) and the ventral hippocampus (Dong et al., 2009). Dlk1 deletion in male mice is associated with increased anxiety indices in the elevated plus maze, open-field, and light-dark box tests (Garcia-Gutierrez et al., 2018) (Figure 6A). However, while in males Dlk1 confers vulnerability to anxiety-related behaviors upon genetic deletion, the expression of this same gene naturally cycles in females, reaching low levels during diestrus, when endogenous estrogen levels drop (Fig. 6A–B). It is striking that low-estrogenic diestrus females show the same anxiety-related phenotype as Dlk1−/− males (Garcia-Gutierrez et al., 2018; Jaric et al., 2019b) (Fig. 2, Fig. 6A), exemplifying how fluctuating hormone levels may mediate female-specific vulnerability to anxiety through cyclic changes in gene expression.
4.2. Chromatin dynamics and gene regulation in the brain across the estrous cycle
Based on earlier studies in cancer cell lines, it was known that estradiol can induce gene expression changes via chromatin remodeling and other epigenetic alterations in the proximity of its target genes (Le Dily and Beato, 2018; Le Dily et al., 2019; Magnani and Lupien, 2014). Regulation of chromatin organization is a major regulatory mechanism controlling gene expression (Bell et al., 2011). In the nucleus, DNA is wrapped around histone proteins into several levels of organization including nucleosomes or “beads-on-a-string” as the first level of chromatin organization (or 1D chromatin) followed by higher order (3D) chromatin organization including loops, compartments, and chromosome territories (Bonev and Cavalli, 2016; Rowley and Corces, 2018) (Figure 6C). At the nucleosome level, chromatin opening promotes transcriptional initiation by making gene regulatory elements, promoters and enhancers, accessible for the binding of transcriptional regulators. In neurons, activity-dependent changes in chromatin accessibility allow for dynamic gene regulation critical for brain function (Gallegos et al., 2018; Su et al., 2017). The higher level chromatin organization, also known as “3D genome”, enables interactions of genes with their distant cis-regulatory elements and is also thought to play an important role in dynamic transcriptional regulation in the brain (Beagan et al., 2020; Fernandez-Albert et al., 2019; Harabula and Pombo, 2021; Marco et al., 2020) and has been implicated in psychiatric disorders (Girdhar et al., 2022; Hu et al., 2021). Typically, though, studies of chromatin regulation in the brain were either focused on the male brain or did not explore sex differences and sex hormone-mediated influences on this regulation.
We showed for the first time that sex hormones are able to dynamically regulate chromatin in postmitotic vHIP neurons across the estrous cycle, thus providing a molecular mechanism for cycle-driven gene expression changes in the female brain (Jaric et al., 2019b; Rocks et al., 2022). Interestingly, our data support the hypothesis that both nuclear- and membrane-bound receptor signalling may be involved in this dynamic gene regulation (Figure 5).
Indeed, by profiling chromatin accessibility (the first organization level of chromatin, Fig. 6C) using the ATAC-seq method in purified vHIP neurons, we found that around 30% of the profiled genomic regions in vHIP neurons change chromatin organization, either by chromatin opening or closing, across the estrous cycle (Jaric et al., 2019b). We found that the regions that are specifically open during the high estrogenic (proestrus) phase do not contain EREs, but are enriched for binding sites of several other transcription factors including Egr1, thus implicating membrane-bound estrogen receptors in chromatin and gene expression changes across the estrous cycle (Fig. 5). Intriguingly, Egr1 is an estrogen-responsive gene, as its expression, too, changes across the cycle, becoming more highly expressed as estrogen levels rise (Jaric et al., 2019b). This gene is also an immediate early gene and, as such, it is an excellent candidate to direct chromatin opening in neurons, as proposed by previous studies (Su et al., 2017; Vierbuchen et al., 2017). Finally, Egr1 has also been implicated in estrous cycle-driven gene regulation in the PFC (Duclot and Kabbaj, 2015), thus it may be a shared estrogen-sensitive chromatin remodeler across the brain regions. Taking the expression changes of the Dlk1 gene across the estrous cycle as an example (Fig. 6A–B), our findings indicate that these changes are mediated by chromatin re-organization. We see more open chromatin in proestrus and closed chromatin in diestrus within two putative regulatory regions located upstream of the Dlk1 transcription start site (TSS) (Jaric et al., 2019b) (Fig. 6D). Importantly, Dlk1 has an Egr1 binding motif within the region showing differential chromatin accessibility (−27 kb, Fig. 6D), as well as in the vicinity of the TSS (Jaric et al., 2019b), implying that Dlk1 is likely activated by Egr1 binding after Egr1’s induction by estrogen in proestrus (Fig. 5).
However, we also found that sex hormones can dynamically change higher order chromatin organization in the brain (Fig. 6C) including chromosomal compartments, CTCF loops, and enhancer-promoter interactions (Rocks et al., 2022), all of which have been implicated in gene regulation (Rowley and Corces, 2018). Again, estrogen was previously shown to induce 3D genome changes in dividing, breast cancer cell lines (Le Dily et al., 2019) but we now know that this is also a dynamic process in post-mitotic vHIP neurons that is likely to contribute to behavioral plasticity (Rocks et al., 2022). The estrous cycle-driven 3D genome changes at all levels of organization are associated with changes in expression of relevant genes. As an example, we found very different sets of long-range, enhancer-promoter interactions to control the expression of the neuron-specific gene Pou3f2 in proestrus vs. diestrus (Fig. 6E). In addition, we found differential enhancer-promoter interactions to be enriched for genes implicated in brain disorders, specifically in the serotonin synapse and anxiety, further linking chromatin changes and anxiety-related behavior across the estrous cycle. Interestingly, though, for the thousands of 3D interactions that we found to differ between proestrus and diestrus, only a small fraction of differential 3D interactions translated into changes in gene expression (Rocks et al., 2022). As will be further explained in the next section, we hypothesize that fluctuating hormones induce epigenetic priming (Fig. 6F) where the majority of cycle-driven epigenomic changes are dormant under basal conditions. However, if another stimulus such as stress is present, it could convert epigenomic changes into more widespread changes in gene expression leading to adaptive behavioral changes in animals (and possibly precipitating psychopathology in humans). This type of “epigenetic priming” was previously observed in the learning and memory field where it was shown that epigenetically-primed genes may not undergo expression changes under basal conditions but are affected in a stimulus- and activity-dependent way (Bevington et al., 2016; Marco et al., 2020; Sams et al., 2016). Our findings, in fact, suggest that these “non-functional” 3D organizational changes, therefore, could be part of the cellular memory associated with cycling events.
Intriguingly, estrous cycle-dependent 3D interactions were consistently enriched for EREs (Rocks et al., 2022), indicating that dynamic 3D genome organization in vHIP neurons in females is driven by nuclear ERα, consistent with the involvement of nuclear ER signaling in vHIP neurons (Figure 5). Since both estrogen levels and ERα expression vary with the estrous cycle (Jaric et al., 2019b; Mitterling et al., 2010), the cycle-dependent ERα binding to the genome provides a likely mechanism for changing 3D chromatin organization with varying sex hormone levels. Consistent with our findings, studies in breast cancer cells found that the ER binding has an instructive role in chromatin looping (Le Dily and Beato, 2018; Le Dily et al., 2019) and, more specifically, can mediate 3D genome remodeling during the development of endocrine resistance (Achinger-Kawecka et al., 2020), further linking ERα with 3D genome organization. We hypothesize that ERα and Egr1 may have different but complementary roles in neuronal chromatin organization, allowing for the interaction between the membrane-bound and nuclear-bound ER pathways in gene regulation across the estrous cycle (Figure 5).
We and others have also performed studies in OVX animals to address the role of ovarian hormones and ERα in chromatin and gene regulation in the female brain (Gegenhuber et al., 2022; Knoedler et al., 2022; Rocks et al., 2022). Knoedler et al. simulated the estrus phase with estrogen and progesterone treatments in OVX female mice and found significant sex hormone-induced changes in gene expression in ERα positive cells of the four sexually dimorphic brain regions involved in social behavior, namely the bed nucleus of the stria terminalis, medial amygdala, and two hypothalamic subregions (Knoedler et al., 2022). In related brain regions, acute 4-hour estrogen treatment induced ERα binding to the genome which was associated with extensive chromatin opening, predominantly in EREs, and changes in gene expression in OVX animals (Gegenhuber et al., 2022). Both of these studies are consistent with our findings that neuronal chromatin is very responsive to sex hormone changes in the female brain and is involved in hormone-mediated dynamic control of gene expression.
To specifically address changes in higher chromatin organization during high-estrogenic proestrus phase of the cycle, we performed acute 4-hour estrogen treatment and found changes across all levels of 3D genome organization in vHIP neurons of OVX female mice (Rocks et al., 2022). However, while the degree of 3D genome changes found following estrogen treatment in OVX animals was similar to that seen across the estrous cycle, we found only partial (15–25%) overlap between the two comparisons. These results are unsurprising considering that the removal of ovaries leads to extensive brain adaptations, on almost every level, from alteration in sex hormone receptor expression to changes in neurotransmitter levels, dendritic spine density, behavior and response to sex hormones (Khayum et al., 2020; Sárvári et al., 2016; Wallace et al., 2006). And, indeed, while we were able to reproduce proestrus-like reduction in anxiety-related behavior following acute estrogen treatment in low-estrogenic, cycling female mice, this was not the case in OVX mice (Rocks et al., 2022). Therefore, our results strongly indicate that understanding the effect of fluctuating hormones on chromatin and behavior requires studying the female brain in its naturalistic context, across the physiological estrous cycle.
In summary, there is increasing evidence that sex hormone fluctuations during the estrous cycle induce dynamic changes in chromatin organization which lead to changes in gene expression, synaptic plasticity, and anxiety-related behavior (Jaric et al., 2019b; Rocks et al., 2022). In addition, these epigenetic changes may prime the genome for future changes in gene expression in the presence of another stimulus (Rocks et al., 2022).
5. The interaction between hormone status, stress, and genetics: the hormonal and molecular priming hypothesis for increased female risk for anxiety and depression.
While sex hormone fluctuations increase the risk for anxiety and depression, it is obvious that they are not sufficient to induce these disorders in the majority of women and menstruating individuals. We propose that an interaction of sex hormone status and other risk factors such as stress and genetic background are responsible for the increased female risk for these disorders (Figure 7). There are several lines of evidence at the behavioral, structural, and molecular level that support this hypothesis.
Figure 7. Novel model for the sex-biased development of anxiety and depression disorders.
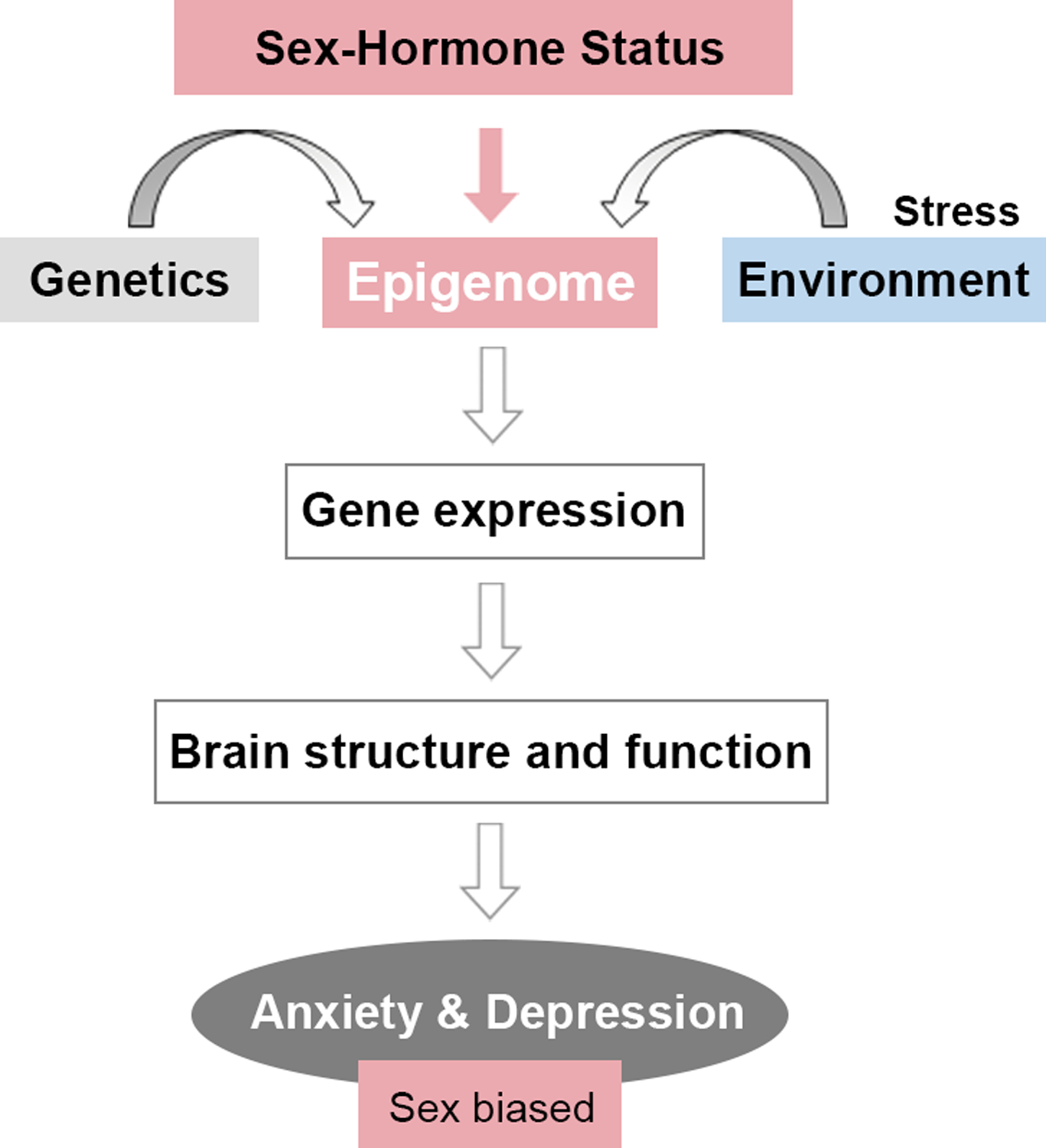
Sex-hormone status in women is added as a third risk factor in addition to the individual’s genetic background and stress exposure. Hormone-driven changes in the epigenome may translate into changes in gene expression, brain structure, and function and contribute to the symptomatology of anxiety and depression disorders and their increased vulnerability in women.
5.1. Sex hormone-stress interaction
First, we showed that female mice that experienced early life stress in the form of maternal separation exhibit increased anxiety-like behavior in adulthood compared to control females; this effect is primarily driven by increased anxiety indices during the high-estrogenic stage of the cycle in maternally separated females (Jaric et al., 2019a). These data indicate that the protective, anxiolytic-like effect of estrogen is lost following early life stress and this may be due to either disrupted ovarian function (lower estrogen levels) or lost sensitivity to estrogen induced by early life stress. Either way, these findings are consistent with the idea that stress and hormone status may interact to induce increased female vulnerability (Doherty and Roth, 2018). A similar interaction has also been reported at the structural level in response to acute stress. The study by Shors et al. that used the acute restraint and tail shock stressor in rats showed the opposite effect of acute stress on dendritic spine density in males and females; acute stress led to an increase in dendritic spine density in males and a decrease in spine density in females, specifically in proestrus (Shors et al., 2001). As discussed previously, estrogen was shown to be a neuroplastic agent (Woolley and McEwen, 1992), capable of increasing dendritic spine density in the matter of days if not hours. The study of Shors et al. indicates that this protective neuroplastic effect of estrogen seems to be inhibited by stress exposure, again revealing the assumed interaction between stress and estrogen. Interestingly, this effect was specific to the hippocampus and was not seen in the somatosensory cortex in this study (Shors et al., 2001).
Yet, different findings were reported in male and female rats in response to repeated restraint stress over a 21-day period (Galea et al., 1997). This chronic stress exposure induced apical dendritic atrophy (a decrease in the number of apical branch points and dendritic length) of the hippocampal CA3 pyramidal neurons in male rats. In contrast, female rats did not show significant dendritic atrophy in the apical field but showed a decrease in the number of branch points in the basal dendritic tree in response to chronic stress exposure. While baseline and stress levels of plasma corticosterone were higher in female rats compared to male rats, females also exhibited lower estradiol levels in response to chronic stress (Galea et al., 1997). Together, all these studies highlight the idea that regardless of the type of stress (early life, acute adult, or chronic adult stress), stress exposure may interact with sex hormone status in female animals and lead to female-specific structural and behavioral adaptation and possibly psychopathology in humans (Figure 7).
The interaction of stress and the estrous cycle stage was also reported in the studies of gene expression in the hippocampus. In response to acute stress, Marrocco et al. found that acute stress leads to larger changes in hippocampal gene expression in females than in males, and that close to 2,000 genes (or ~30% of differentially expressed genes) in females show varied response to stress dependent on whether the animals were in the high-estrogenic (proestrus) or low-estrogenic (metestrus or diestrus) phase of the cycle (Marrocco et al., 2017). These data are consistent with our priming hypothesis and invite further investigation into the genes and gene pathways involved in this sex hormone-stress interaction.
5.2. Sex hormone-gene interaction
Genetic factors have also been proposed and empirically shown to interact with sex hormone status in female mice and humans. Longitudinal epidemiological studies have identified Tanner Stage III, the start of ovarian cycling, as the onset of increased rates of major depression in girls (Angold et al., 1998). The increased depression risk was evident in girls with a family history of depression, suggesting that hormonal fluctuations at the onset of puberty may activate genetic vulnerability for this disorder in women.
Consistent with the gene × hormone interaction model, it was found that the BDNF genotype and ovarian hormones interact to control hippocampal function and anxiety-related behavior in cycling female mice (Bath et al., 2012; Spencer et al., 2010). The female transgenic mice used in these studies harbored the uniquely human BDNF Val66Met variant, with approximately 30% of the Caucasian population carrying the Met allele and 4% being homozygous for this allele (Petryshen et al., 2010). The Met allele is associated with a reduction in the activity-dependent release of BDNF and is linked to subtle changes in memory function (Egan et al., 2003) as well as with neuropsychiatric disorders including anxiety and depressive disorders (Chen et al., 2006; Rybakowski, 2008). The BDNF gene is of particular interest for exploring gene-sex hormone interaction because estradiol induces BDNF expression, which is part of the mechanism through which this hormone affects hippocampal function (Scharfman and MacLusky, 2006). Enhancing effects on BDNF expression is also consistent with the antidepressant-like activity of estradiol (Castrén and Monteggia, 2021; Deyama and Duman, 2020).
When female mice homozygous for the BDNF Val66Met mutation (Met/Met) were compared to wild-type animals, it was observed that the Met/Met genotype led to an increase in anxiety indices specifically in the estrus phase of the estrous cycle, in both the elevated plus maze and open field tests (Bath et al., 2012). Importantly, estrogen, and consequently, BDNF levels rapidly decline during the estrus stage, thus this effect in Met/Met mutants is consistent with the role of estrogen withdrawal in female-specific risk for anxiety and depression. In addition, these results suggested that women carrying the BDNF Met allele may be more sensitive to the potential impact of reproductive hormones on anxiety. Consistent with this hypothesis, a human study showed that the BDNF Val66Met genotype and ovarian steroids interactively modulate hippocampal function in women (Wei et al., 2018). The study included women who were either Val homozygotes or Met carriers, and who underwent hormonal manipulation that included three different hormonal states: 1) hormonal suppression using pharmacological manipulation with the GnRH agonist Lupron (low levels of estradiol and progesterone); 2) estradiol add-back (estradiol reaching the mid-follicular range); and 3) progesterone add-back (progesterone reaching the mid-luteal phase levels). Combining the hormonal manipulation protocol with a working memory paradigm and neuroimaging, the researchers were able to show a hippocampal deactivation during working memory in all three hormonal states in Val homozygotes and in all hormonal states except estradiol add-back in the Met carriers. This study demonstrates, again, that hippocampal function in women is modulated by estradiol but in a genotypically specific manner, thus, further providing support for the importance of gene-hormone interactions for brain function in women.
5.3. New model for hormonally-induced, sex-biased development of anxiety and depression disorders
Overall, we would like to propose a new model for the development of anxiety and depression disorders that addresses the increased female risk at both the etiological and mechanistic level (Figure 7). Based on clinical and preclinical evidence, we propose to include a third factor in the etiology of depression and anxiety so that, in addition to genetic and environmental (particularly stress) factors, we include sex-hormone status in women. By interacting with genetic make-up and stress exposure history, fluctuating hormones in women are able to affect brain structure and function and contribute to, at least in part, symptomatology of anxiety and depression that more frequently affects women. Based on our findings, we propose that the effects of fluctuating sex hormones are mediated via dynamic changes in the epigenome, namely chromatin remodeling, which lead to changes in gene expression (Figure 7). We found that sex hormones induce larger changes in chromatin than gene expression in the brain, which is consistent with our hypothesis of molecular or epigenetic priming by hormones (Figure 6F). We propose that hormonal fluctuations induce changes in the epigenome that only partially translate into changes in gene expression, brain structure, and function. Particularly with estrogen withdrawal, we see indications for reduced structural plasticity and increased anxiety indices that may reflect increased female vulnerability for anxiety and depression at the structural and behavioral level. However, as hormonal changes are associated with normal reproductive function in women, they are typically physiological and may lead to the development of psychopathology if environmental and genetic risk factors converge on hormonally-induced risk (Figure 7). At the molecular level, we believe that this is reflected in hormonal priming of the epigenome through extensive chromatin reorganization, which may be translated into gene expression changes of brain disorder-relevant genes in a particular genetic context or upon stress exposure (Figure 6F).
6. Future Directions
The ultimate goal of this research is to clearly define sex-specific risk factors and molecular drivers that could be targeted for gender- and sex-specific therapies for depression and anxiety disorders. To us, it seems a missed opportunity that, so far, so little has been done to understand the biological basis of this sex disparity. We propose several approaches to find suitable molecular targets for sex-specific and sex-informed therapy for anxiety and depression disorders.
1) In animals, physiological studies of the estrous cycle are necessary to better understand the molecular basis of the intrinsic, hormonally-driven female vulnerability to anxiety and depression disorders. Estradiol is a natural anxiolytic and antidepressant but, as a therapeutic, its effects may be complicated, dose- and time-dependent, and associated with increased risk for reproductive cancers and cardiovascular disorders. We propose that finding an appropriate, brain-specific downstream target of estradiol may reveal a promising candidate for sex- and gender-specific treatment of anxiety and depression. In fact, this approach may also benefit people of all genders if the therapeutic effect may be dissociated from the endocrine effect.
2) It will be critical to include study designs in animals that will incorporate sex hormone-stress or sex hormone-gene interactions. As discussed previously, the interactions of risk factors are likely to lead to psychopathology and this should be modeled in animals and understood at the molecular level so that further molecular targets can be found and explored therapeutically.
3) While animal studies are indispensable for understanding the mechanistic basis of sex hormone-induced risk for psychopathology, complementary studies in humans will be critical for this research to reach its translational potential and contribute to novel therapeutics. As we discussed, similarities that we see in humans and mice, at both structural and behavioral levels in response to cycling sex hormones, confirm that rodent models are likely to have a good translational value for studying depression and anxiety risk associated with hormonal fluctuations. Our possibilities to study this in humans are much more limited, though. Brain tissue is inaccessible in living humans while postmortem brain tissue is likely to be from postmenopausal women or not to have associated information about the menstrual cycle phase. Until this information becomes more readily available, we will search for the indicators or proxies that can link sex hormone status to chromatin and gene expression state in the female human postmortem brain. In addition, we should explore human brain organoids and peripheral blood cells to study the effect of estrogen and the menstrual cycle on chromatin dynamics and its relation to the genetic risk architecture of anxiety and depression. By combining the information gained from animal studies and human studies, we expect to find the most suitable candidates for novel therapeutics for anxiety and depression. This new approach is going to be informed by sex-specific risk factors and molecular mechanisms and may transform the treatment of these debilitating disorders that are the leading causes of disability worldwide, overwhelmingly affecting women.
7. Gender, sex hormones, and risk for anxiety and depression – note on inclusivity.
In this manuscript, we used the term sex as a binary phenomenon referring to women (females) and men (males). Considering that there is limited data in clinical studies beyond the binary gender, the clinical evidence presented in this manuscript is likely to largely include cis women and men. However, we would like to emphasize that the findings and theoretical frameworks presented here are likely to be applicable to all menstruating individuals that experience fluctuations in ovarian hormones including cis women, non-binary individuals, and transgender men that are menstruating. It is our intention to carry out the research and formulate hypotheses that will be inclusive and benefit all the members of our society across and beyond the gender. As clinical studies collect more information on gender non-conforming and transgender individuals, we aim to adjust our hypotheses so that new treatments for anxiety and depression that we have in mind are going to be sex- and gender-informed, contributing to the improved health of women and other menstruating individuals.
Funding:
This work was supported by the National Institute of Mental Health under Award Number R01MH123523 (to M.K.)
Glossary
- ATAC-seq
A method which profiles open chromatin regions across the genome
- Binding motif
A short DNA sequence recognized by a transcription factor, allowing the transcription factor to regulate the expression of specific genes
- Chromatin
A dynamic structure composed of DNA and bound proteins, mostly histones, which serve to package the DNA in the nucleus and regulate gene expression
- Chromatin accessibility
Chromatin can occupy either an open (accessible) or closed conformation, which determines the availability of a DNA sequence to transcriptional machinery
- Chromosome territories
Chromosomes occupy distinct regions of the nucleus, evidenced by intrachromosomal interactions far outnumbering interchromosomal interactions
- Compartments
analysis of Hi-C data categorizes genomic regions into A and B compartments, with A compartments corresponding to open chromatin and sites of active genes and B compartments corresponding to repressed chromatin located near the nuclear lamina
- CTCF Loops
A DNA loop whose formation is mediated by the interaction of CTCF and cohesin proteins. These loops are important for gene regulation
- Enhancer-promoter interactions
A DNA loop that brings the promoter region of a gene in close physical proximity to a distal enhancer region, thereby facilitating gene expression
- Hi-C
A method which profiles 3D genome interactions across the genome
- RNA-Seq
A method which allows for quantification and comparison of gene expression profiles
- TSS
Transcription start site, the region of a gene near the promoter where transcription by RNA polymerase begins
Footnotes
Declarations of interest: none
References
- Achinger-Kawecka J, et al. , 2020. Epigenetic reprogramming at estrogen-receptor binding sites alters 3D chromatin landscape in endocrine-resistant breast cancer. Nat Commun. 11, 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert K, Pruessner J, Newhouse P, 2015. Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology. 59, 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altemus M, Sarvaiya N, Neill Epperson C, 2014. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol. 35, 320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin Z, Canli T, Epperson CN, 2005. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev. 4, 43–58. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill LJN, 2010. Menstrual cycle modulation of medial temporal activity evoked by negative emotion. Neuroimage 53, 1286–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D, et al. , 2013. State-dependent changes in hippocampal grey matter in depression. Mol Psychiatry. 18, 1265–72. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Michaelis S, 2015. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci. 17, 327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman D, et al. , 2004. Regional dissociations within the hippocampus—memory and anxiety. Neuroscience & Biobehavioral Reviews. 28, 273–283. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, et al. , 2003. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 139, 197–213. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, et al. , 2014. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat Rev Neurosci. 15, 181–92. [DOI] [PubMed] [Google Scholar]
- Barth C, Villringer A, Sacher J, 2015. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci. 9, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth C, et al. , 2016. In-vivo Dynamics of the Human Hippocampus across the Menstrual Cycle. Sci Rep. 6, 32833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, et al. , 2012. Variant brain-derived neurotrophic factor (Valine66Methionine) polymorphism contributes to developmental and estrous stage-specific expression of anxiety-like behavior in female mice. Biol Psychiatry. 72, 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer J, et al. , 2014. Menstrual-cycle dependent fluctuations in ovarian hormones affect emotional memory. Neurobiol. Learn. Mem 110, 55–63. [DOI] [PubMed] [Google Scholar]
- Beagan JA, et al. , 2020. Three-dimensional genome restructuring across timescales of activity-induced neuronal gene expression. Nat Neurosci. 23, 707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M, 1989. Gene regulation by steroid hormones. Cell. 56, 335–44. [DOI] [PubMed] [Google Scholar]
- Bell O, et al. , 2011. Determinants and dynamics of genome accessibility. Nat Rev Genet. 12, 554–64. [DOI] [PubMed] [Google Scholar]
- Bevington SL, et al. , 2016. Inducible chromatin priming is associated with the establishment of immunological memory in T cells. EMBO J. 35, 515–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonev B, Cavalli G, 2016. Organization and function of the 3D genome. Nature Reviews Genetics. 17, 661–678. [DOI] [PubMed] [Google Scholar]
- Bromberger JT, Epperson CN, 2018. Depression During and After the Perimenopause: Impact of Hormones, Genetics, and Environmental Determinants of Disease. Obstet Gynecol Clin North Am. 45, 663–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromis K, et al. , 2018. Meta-Analysis of 89 Structural MRI Studies in Posttraumatic Stress Disorder and Comparison With Major Depressive Disorder. Am J Psychiatry. 175, 989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M, 2013. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology. 70, 27–34. [DOI] [PubMed] [Google Scholar]
- Castrén E, Monteggia LM, 2021. Brain-Derived Neurotrophic Factor Signaling in Depression and Antidepressant Action. Biol Psychiatry. 90, 128–136. [DOI] [PubMed] [Google Scholar]
- Chen ZY, et al. , 2006. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 314, 140–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, et al. , 2017. Anxiety disorders. Nat Rev Dis Primers. 3, 17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Shen Y, Li R, 2013. Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol Med. 19, 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deecher D, et al. , 2008. From menarche to menopause: exploring the underlying biology of depression in women experiencing hormonal changes. Psychoneuroendocrinology. 33, 3–17. [DOI] [PubMed] [Google Scholar]
- Deyama S, Duman RS, 2020. Neurotrophic mechanisms underlying the rapid and sustained antidepressant actions of ketamine. Pharmacol Biochem Behav. 188, 172837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo LM, Vied C, Nowakowski RS, 2017. The stability of the transcriptome during the estrous cycle in four regions of the mouse brain. J. Comp. Neurol 525, 3360–3387. [DOI] [PubMed] [Google Scholar]
- Doherty TS, Roth TL, 2018. Epigenetic Landscapes of the Adversity-Exposed Brain. Prog Mol Biol Transl Sci. 157, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H-W, et al. , 2009. Genomic–anatomic evidence for distinct functional domains in hippocampal field CA1. Proceedings of the National Academy of Sciences. 106, 11794–11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner NC, Lowry CA, 2013. Sex differences in anxiety and emotional behavior. Pflugers Arch. 465, 601–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubol M, et al. , 2021. Neuroimaging the menstrual cycle: A multimodal systematic review. Front Neuroendocrinol. 60, 100878. [DOI] [PubMed] [Google Scholar]
- Duclot F, Kabbaj M, 2015. The estrous cycle surpasses sex differences in regulating the transcriptome in the rat medial prefrontal cortex and reveals an underlying role of early growth response 1. Genome Biology. 16, 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman CH, Duman RS, 2015. Spine synapse remodeling in the pathophysiology and treatment of depression. Neurosci Lett. 601, 20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Nakagawa S, Malberg J, 2001. Regulation of adult neurogenesis by antidepressant treatment. Neuropsychopharmacology. 25, 836–44. [DOI] [PubMed] [Google Scholar]
- Egan MF, et al. , 2003. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 112, 257–69. [DOI] [PubMed] [Google Scholar]
- Erickson KI, et al. , 2011. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 108, 3017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essau CA, et al. , 2010. Gender differences in the developmental course of depression. J Affect Disord. 127, 185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Camarena E, et al. , 2011. Long-term ovariectomy modulates the antidepressant-like action of estrogens, but not of antidepressants. J Psychopharmacol. 25, 1365–77. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW, 2010. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 65, 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Albert J, et al. , 2019. Immediate and deferred epigenomic signatures of in vivo neuronal activation in mouse hippocampus. Nat Neurosci. 22, 1718–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron SR, et al. , 2011. Postnatal loss of Dlk1 imprinting in stem cells and niche astrocytes regulates neurogenesis. Nature. 475, 381–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank TC, et al. , 2010. Effect of menstrual cycle phase on corticolimbic brain activation by visual food cues. Brain Res. 1363, 81–92. [DOI] [PubMed] [Google Scholar]
- Freeman EW, et al. , 1999. Differential Response to Antidepressants in Women With Premenstrual Syndrome/Premenstrual Dysphoric Disorder: A Randomized Controlled Trial. Archives of General Psychiatry. 56, 932–939. [DOI] [PubMed] [Google Scholar]
- Frokjaer VG, et al. , 2015. Role of Serotonin Transporter Changes in Depressive Responses to Sex-Steroid Hormone Manipulation: A Positron Emission Tomography Study. Biol Psychiatry. 78, 534–43. [DOI] [PubMed] [Google Scholar]
- Frye CA, Petralia SM, Rhodes ME, 2000. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3alpha, 5alpha-THP. Pharmacol Biochem Behav. 67, 587–96. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA, 2002. Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav. 41, 306–15. [DOI] [PubMed] [Google Scholar]
- Galea LA, et al. , 1997. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 81, 689–97. [DOI] [PubMed] [Google Scholar]
- Galea LA, Wide JK, Barr AM, 2001. Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behav Brain Res. 122, 1–9. [DOI] [PubMed] [Google Scholar]
- Galea LAM, et al. , 2017. Why estrogens matter for behavior and brain health. Neurosci Biobehav Rev. 76, 363–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos DA, et al. , 2018. Chromatin Regulation of Neuronal Maturation and Plasticity. Trends Neurosci. 41, 311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gutierrez MS, et al. , 2018. Deletion of Dlk1 increases the vulnerability to developing anxiety-like behaviors and ethanol consumption in mice. Biochem Pharmacol. 158, 37–44. [DOI] [PubMed] [Google Scholar]
- Gegenhuber B, et al. , 2022. Gene regulation by gonadal hormone receptors underlies brain sex differences. Nature 606, 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdhar K, et al. , 2022. Chromatin domain alterations linked to 3D genome organization in a large cohort of schizophrenia and bipolar disorder brains. Nat Neurosci. 25, 474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, et al. , 2005. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J. Neurosci 25, 9309–9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JL, et al. , 2015. Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: a novel heuristic model. Am J Psychiatry. 172, 227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia A Jr., et al. , 2004. Influence of the estrous cycle on the behavior of rats in the elevated T-maze. Behav Processes. 67, 167–71. [DOI] [PubMed] [Google Scholar]
- Green AD, Barr AM, Galea LA, 2009. Role of estradiol withdrawal in ‘anhedonic’ sucrose consumption: a model of postpartum depression. Physiol Behav. 97, 259–65. [DOI] [PubMed] [Google Scholar]
- Grimes DA, Lobo RA, 2002. Perspectives on the Women’s Health Initiative trial of hormone replacement therapy. Obstet Gynecol. 100, 1344–53. [DOI] [PubMed] [Google Scholar]
- Gurvits TV, et al. , 1996. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol. Psychiatry 40, 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann G, et al. , 2011. Changes in brain size during the menstrual cycle. PLoS One. 6, e14655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn-Holbrook J, Cornwell-Hinrichs T, Anaya I, 2017. Economic and Health Predictors of National Postpartum Depression Prevalence: A Systematic Review, Meta-analysis, and Meta-Regression of 291 Studies from 56 Countries. Front Psychiatry. 8, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, et al. , 2009. Remodeling of hippocampal spine synapses in the rat learned helplessness model of depression. Biol. Psychiatry 65, 392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harabula I, Pombo A, 2021. The dynamics of chromatin architecture in brain development and function. Curr Opin Genet Dev. 67, 84–93. [DOI] [PubMed] [Google Scholar]
- Hasin DS, et al. , 2018. Epidemiology of Adult DSM-5 Major Depressive Disorder and Its Specifiers in the United States. JAMA Psychiatry. 75, 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekzema E, et al. , 2017. Pregnancy leads to long-lasting changes in human brain structure. Nat Neurosci. 20, 287–296. [DOI] [PubMed] [Google Scholar]
- Hölzel BK, et al. , 2011. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. 191, 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard DM, et al. , 2019. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 22, 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, et al. , 2021. Neuronal and glial 3D chromatin architecture informs the cellular etiology of brain disorders. Nat Commun. 12, 3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J, et al. , 2020. Estradiol Alters Hippocampal Gene Expression during the Estrous Cycle. Endocrine Research. 45, 84–101. [DOI] [PubMed] [Google Scholar]
- Jaric I, et al. , 2019a. Sex and Estrous Cycle Effects on Anxiety- and Depression-Related Phenotypes in a Two-Hit Developmental Stress Model. Front Mol Neurosci. 12, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaric I, et al. , 2019b. Chromatin organization in the female mouse brain fluctuates across the oestrous cycle. Nat Commun. 10, 2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadriu B, et al. , 2021. Ketamine and Serotonergic Psychedelics: Common Mechanisms Underlying the Effects of Rapid-Acting Antidepressants. Int J Neuropsychopharmacol. 24, 8–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayum MA, et al. , 2020. Ovariectomy-induced depressive-like behavior and brain glucose metabolism changes in female rats are not affected by chronic mild stress. Psychoneuroendocrinology. 115, 104610. [DOI] [PubMed] [Google Scholar]
- Kheirbek MA, et al. , 2013. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron. 77, 955–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoedler JR, et al. , 2022. A functional cellular framework for sex and estrous cycle-dependent gene expression and behavior. Cell 185, 654–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokras N, et al. , 2015. Forced swim test: What about females? Neuropharmacology. 99, 408–21. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, et al. , 2005. Self-reported premenstrual exacerbation of depressive symptoms in patients seeking treatment for major depression. Psychol Med. 35, 683–92. [DOI] [PubMed] [Google Scholar]
- Koss WA, Frick KM, 2017. Sex differences in hippocampal function. J Neurosci Res. 95, 539–562. [DOI] [PubMed] [Google Scholar]
- Kuehner C, 2017. Why is depression more common among women than among men? The Lancet Psychiatry. 4, 146–158. [DOI] [PubMed] [Google Scholar]
- Kuehner C, Nayman S, 2021. Premenstrual Exacerbations of Mood Disorders: Findings and Knowledge Gaps. Curr Psychiatry Rep. 23, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundakovic M, et al. , 2013. Sex-specific and strain-dependent effects of early life adversity on behavioral and epigenetic outcomes. Front Psychiatry. 4, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Dily F, Beato M, 2018. Signaling by Steroid Hormones in the 3D Nuclear Space. Int. J. Mol. Sci 19, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Dily F, et al. , 2019. Hormone-control regions mediate steroid receptor-dependent genome organization. Genome Res. 29, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, et al. , 2010. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 329, 959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, et al. , 2011. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 69, 754–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SH, Graham BM, 2017. Why are women so vulnerable to anxiety, trauma-related and stress-related disorders? The potential role of sex hormones. The Lancet Psychiatry. 4, 73–82. [DOI] [PubMed] [Google Scholar]
- Lisofsky N, et al. , 2015. Hippocampal volume and functional connectivity changes during the female menstrual cycle. Neuroimage. 118, 154–62. [DOI] [PubMed] [Google Scholar]
- Ly C, et al. , 2018. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 23, 3170–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen G, Frodl T, 2011. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry. 16, 252–64. [DOI] [PubMed] [Google Scholar]
- Maeng LY, Milad MR, 2015. Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Horm Behav. 76, 106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magariños AM, McEwen BS, 1995. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience. 69, 83–8. [DOI] [PubMed] [Google Scholar]
- Magnani L, Lupien M, 2014. Chromatin and epigenetic determinants of estrogen receptor alpha (ESR1) signaling. Mol Cell Endocrinol. 382, 633–41. [DOI] [PubMed] [Google Scholar]
- Malberg JE, et al. , 2000. Chronic Antidepressant Treatment Increases Neurogenesis in Adult Rat Hippocampus. J. Neurosci 20, 9104–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco A, et al. , 2020. Mapping the epigenomic and transcriptomic interplay during memory formation and recall in the hippocampal engram ensemble. Nat Neurosci. 23, 1606–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes FK, et al. , 2001. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav. 74, 435–40. [DOI] [PubMed] [Google Scholar]
- Marcus SM, et al. , 2008. Sex differences in depression symptoms in treatment-seeking adults: confirmatory analyses from the Sequenced Treatment Alternatives to Relieve Depression study. Compr Psychiatry. 49, 238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrocco J, McEwen BS, 2016. Sex in the brain: hormones and sex differences. Dialogues Clin Neurosci. 18, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrocco J, et al. , 2017. A sexually dimorphic pre-stressed translational signature in CA3 pyramidal neurons of BDNF Val66Met mice. Nat Commun. 8, 808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Magarinos AM, 1997. Stress effects on morphology and function of the hippocampus. Ann N Y Acad Sci. 821, 271–84. [DOI] [PubMed] [Google Scholar]
- Milad MR, et al. , 2009. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 164, 887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitterling KL, et al. , 2010. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J Comp Neurol. 518, 2729–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora S, Dussaubat N, Diaz-Veliz G, 1996. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 21, 609–20. [DOI] [PubMed] [Google Scholar]
- Mosconi L, et al. , 2021. Menopause impacts human brain structure, connectivity, energy metabolism, and amyloid-beta deposition. Scientific Reports. 11, 10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HD, et al. , 2002. Postmenopausal hormone replacement therapy: scientific review. JAMA. 288, 872–881. [DOI] [PubMed] [Google Scholar]
- O’hara MW, Swain AM, 1996. Rates and risk of postpartum depression—a meta-analysis. Int. Rev. Psychiatry 8, 37–54. [Google Scholar]
- Olson DE, 2018. Psychoplastogens: A Promising Class of Plasticity-Promoting Neurotherapeutics. J Exp Neurosci. 12, 1179069518800508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opel N, et al. , 2014. Hippocampal atrophy in major depression: a function of childhood maltreatment rather than diagnosis? Neuropsychopharmacology. 39, 2723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte C, et al. , 2016. Major depressive disorder. Nat Rev Dis Primers. 2, 16065. [DOI] [PubMed] [Google Scholar]
- Petryshen TL, et al. , 2010. Population genetic study of the brain-derived neurotrophic factor (BDNF) gene. Mol Psychiatry. 15, 810–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopescu X, et al. , 2008. Hippocampal structural changes across the menstrual cycle. Hippocampus. 18, 985–8. [DOI] [PubMed] [Google Scholar]
- Qiao H, et al. , 2014. Progressive alterations of hippocampal CA3-CA1 synapses in an animal model of depression. Behav Brain Res. 275, 191–200. [DOI] [PubMed] [Google Scholar]
- Redila VA, Christie BR, 2006. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 137, 1299–307. [DOI] [PubMed] [Google Scholar]
- Rocks D, et al. , 2022. Sex-specific multi-level 3D genome dynamics in the mouse brain. Nat. Commun 13, 3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley MJ, Corces VG, 2018. Organizational principles of 3D genome architecture. Nat Rev Genet. 19, 789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybakowski JK, 2008. BDNF gene: functional Val66Met polymorphism in mood disorders and schizophrenia. Pharmacogenomics. 9, 1589–93. [DOI] [PubMed] [Google Scholar]
- Sacher J, et al. , 2020. Increase in Serotonin Transporter Binding Across the Menstrual Cycle in Patients With Premenstrual Dysphoric Disorder: A Case-Control Longitudinal Positron Emission Tomography (PET) Imaging Study. In: NEUROPSYCHOPHARMACOLOGY. Vol. 45, ed.êds. SPRINGERNATURE CAMPUS, 4 CRINAN ST, LONDON, N1 9XW, ENGLAND, pp. 106–106. [Google Scholar]
- Sams DS, et al. , 2016. Neuronal CTCF Is Necessary for Basal and Experience-Dependent Gene Regulation, Memory Formation, and Genomic Structure of BDNF and Arc. Cell Rep. 17, 2418–2430. [DOI] [PubMed] [Google Scholar]
- Sárvári M, et al. , 2016. Ovariectomy Alters Gene Expression of the Hippocampal Formation in Middle-Aged Rats. Endocrinology. 158, 69–83. [DOI] [PubMed] [Google Scholar]
- Scharfman HE, MacLusky NJ, 2006. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: complexity of steroid hormone-growth factor interactions in the adult CNS. Front Neuroendocrinol. 27, 415–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T, Popik P, 2007. Increased depressive-like traits in an animal model of premenstrual irritability. Horm Behav. 51, 142–8. [DOI] [PubMed] [Google Scholar]
- Sellers K, Raval P, Srivastava DP, 2015. Molecular signature of rapid estrogen regulation of synaptic connectivity and cognition. Front Neuroendocrinol. 36, 72–89. [DOI] [PubMed] [Google Scholar]
- Sheppard PAS, Choleris E, Galea LAM, 2019. Structural plasticity of the hippocampus in response to estrogens in female rodents. Mol Brain. 12, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J, 2001. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 21, 6292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares CN, Zitek B, 2008. Reproductive hormone sensitivity and risk for depression across the female life cycle: a continuum of vulnerability? J Psychiatry Neurosci. 33, 331–43. [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, et al. , 2010. BDNF variant Val66Met interacts with estrous cycle in the control of hippocampal function. Proc. Natl. Acad. Sci 107, 4395–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, et al. , 2011. Rapid estrogen signaling in the brain: implications for the fine-tuning of neuronal circuitry. J Neurosci. 31, 16056–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg EM, et al. , 2008. A cross-sectional evaluation of perimenopausal depression. J Clin Psychiatry. 69, 973–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg EM, et al. , 2012. Rapid response to fluoxetine in women with premenstrual dysphoric disorder. Depress Anxiety. 29, 531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner M, Dunn E, Born L, 2003. Hormones and mood: from menarche to menopause and beyond. Journal of affective disorders. 74, 67–83. [DOI] [PubMed] [Google Scholar]
- Su Y, et al. , 2017. Neuronal activity modifies the chromatin accessibility landscape in the adult brain. Nat Neurosci. 20, 476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom Poromaa I, Gingnell M, 2014. Menstrual cycle influence on cognitive function and emotion processing-from a reproductive perspective. Front Neurosci. 8, 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, et al. , 1999. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 19, 5792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaut F, 2017. Anxiety disorders: a review of current literature. Dialogues Clin Neurosci. 19, 87–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH, 1999. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 2, 266–70. [DOI] [PubMed] [Google Scholar]
- Vastagh C, Liposits Z, 2017. Impact of proestrus on gene expression in the medial preoptic area of mice. Front. Cell Neurosci 11, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW, 2008. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 29, 238–257. [DOI] [PubMed] [Google Scholar]
- Vierbuchen T, et al. , 2017. AP-1 Transcription Factors and the BAF Complex Mediate Signal-Dependent Enhancer Selection. Mol Cell. 68, 1067–1082 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal G, et al. , 2002. Reduced hippocampal volume and total white matter volume in posttraumatic stress disorder. Biol. Psychiatry 52, 119–125. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA, 2006. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 31, 1097–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M, et al. , 2006. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res. 1126, 176–82. [DOI] [PubMed] [Google Scholar]
- Wang Z, et al. , 2021. Mapping global prevalence of depression among postpartum women. Transl Psychiatry. 11, 543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SG, et al. , 1995. LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain Res. 703, 26–30. [DOI] [PubMed] [Google Scholar]
- Wei SM, et al. , 2018. Brain-derived neurotrophic factor Val(66)Met genotype and ovarian steroids interactively modulate working memory-related hippocampal function in women: a multimodal neuroimaging study. Mol Psychiatry. 23, 1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, et al. , 1990. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 10, 4035–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS, 1992. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 12, 2549–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers KA, O’Brien PS, Eriksson E, 2008. Premenstrual syndrome. The Lancet. 371, 1200–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Becker JB, 2009. Perspective: sex matters: gonadal steroids and the brain. Neuropsychopharmacology. 34, 537–8. [DOI] [PubMed] [Google Scholar]
- Zeidan MA, et al. , 2011. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiatry. 70, 920–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, et al. , 2019. (R)-Ketamine Rapidly Ameliorates the Decreased Spine Density in the Medial Prefrontal Cortex and Hippocampus of Susceptible Mice After Chronic Social Defeat Stress. Int J Neuropsychopharmacol. 22, 675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]


