1. Introduction
A worldwide COVID-19 mass vaccination campaign targeting adults was launched in late December 2020. Subsequently, the Comirnaty (BNT162b2) vaccine was recommended for children aged 12–15 years in May 2021 [1]. In Norway, only one dose of the Comirnaty vaccine was recommended to children aged 12–15 years. Vaccination was not recommended for children who had been infected with SARS-CoV-2. In line with findings in older age groups, the most prevalent adverse events after vaccination that have been reported in 12- to 15-year-old adolescents are injection site pain (in 79 to 86 % of participants), fatigue (in 60 to 66 %), and headache (in 55 to 65 %) [2]. Adolescents aged 12–17 years have been found to have a moderately higher risk of adverse reactions than adults [3].
For new vaccines, clinical trials typically collect data on commonly recognized adverse events and safety profiles. However, questions about the menstrual cycle have not been included in clinical studies. A significant number of reports on menstrual disturbances after COVID-19 vaccination have been registered in spontaneous adverse events surveillance systems in several countries (USA, UK, Norway, the Netherlands) [4], [5], [6], [7]. Since June 2021, the Norwegian Medicines Agency has received such reports through their routine surveillance system for adverse effects of medications and vaccines [4]. Upon media attention, an increasing number of reports were received during the summer of 2021. The Netherlands Pharmacovigilance Centre Lareb reported a signal for COVID-19 vaccine-related menstrual disturbances and for postmenopausal bleeding after COVID-19 vaccination [7]. In October 2022, The European Medicines Agency (EMA) also recommended to add heavy menstrual bleeding to the product information as a possible side effect of the mRNA COVID-19 vaccines Comirnaty and Spikevax [8]. Since unverified claims of adverse effects may give rise to vaccine hesitancy or refusal, accurate scientific investigation of these phenomena is imperative for public health, also in young teenage girls.
In a recent study, we showed that women aged 18–30 years experienced menstrual cycle changes after COVID-19 vaccination [9], but importantly, such changes were also common prior to vaccination. The aim of the current study was to estimate the association between COVID-19 vaccination and menstrual disturbances in girls aged 12–15 years using maternal questionnaire responses in a large population-based cohort.
2. Methods
2.1. Study population
The Norwegian Mother, Father and Child Cohort Study (MoBa) is an ongoing population-based pregnancy cohort study established by the Norwegian Institute of Public Health. Participants were recruited during pregnancy from all over Norway during 1999–2008 [10]. The participation rate was 41 % among all invited women. The cohort now includes approximately 114 000 children and their parents, who are followed up with regular questionnaires and registry linkages. MoBa was established with the overall aim to understand causes of diseases.
Since March 2020, all active adult cohort participants (about 149 000) have also been invited to answer short electronic questionnaires every 14 days regarding symptoms related to COVID-19 disease and vaccination, life situation during the pandemic, and more. The response rate over the first 50 survey rounds during the pandemic was 46–80 %, with an average (mean) response rate of 66 %. Participation has been stable throughout the pandemic. For instance, the average participation rate was 71 % for the first ten rounds (March to August 2020), and 68 % for rounds 31–40 (May to September 2021).
In the 41st questionnaire round in autumn 2021, a subsample of participating MoBa mothers with one child aged 12–15 years (n = 29 959) were invited to answer an electronic questionnaire on behalf of their child. The questionnaire included questions about (in chronological order) COVID-19 symptoms, testing, and adverse events after vaccination. The questionnaire was distributed via text message to the mothers’ mobile phones on October 13, 2021. The response rate was 64 %. Using each participant’s (mother’s and child’s) unique national identification number, the questionnaire data and information from MoBa were linked to the Norwegian Immunization Registry (SYSVAK) and the National Surveillance System for Communicable Diseases (MSIS) [11]. It is mandatory for health care workers providing the vaccine to report vaccinations against COVID-19 electronically to SYSVAK, and for laboratories to report polymerase chain reaction (PCR)-confirmed SARS-CoV-2 infection to MSIS. During the fall of 2021, when children went back to school after the summer holidays in Norway, extensive testing and screening against SARS-CoV-2 was performed, due to a surge in circulation of the Delta variant.
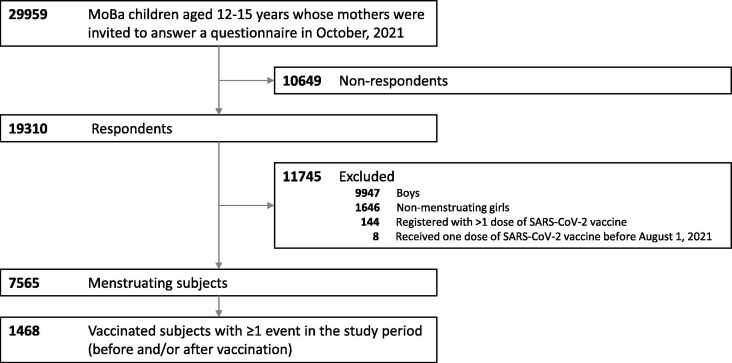
From the group of 19 310 questionnaire respondents, we excluded 9947 boys and 1646 non-menstruating girls (Fig. 1 ). We also excluded 152 individuals who had received two doses of COVID-19 vaccine or were vaccinated before August 1, 2021, under the assumption that they likely belonged to a risk group due to underlying disease. Thus, the study sample consisted of 7565 girls who had started to menstruate (Fig. 1).
Fig. 1.
Inclusion of individuals in the study.
2.2. Exposure
Date of vaccination for each girl was obtained from the records in the national vaccine registry SYSVAK. We categorized subjects into two main groups: Vaccinated subjects included girls who were vaccinated between August 1st and the date of questionnaire completion, while unvaccinated subjects were girls who were not vaccinated upon completion of the questionnaire.
2.3. Outcomes
Questions about menstrual disturbances were posed to all mothers of girls who had started to menstruate (Supplementary Table 1). For vaccinated girls, the mothers were asked whether their daughters had experienced any of the following disturbances in their last menstruation before the first vaccine dose: 1) heavier bleeding than usual, 2) prolonged menstruation, 3) shorter interval between menstruations than usual, 4) longer interval between menstruations than usual, 5) spot bleedings between menstruations, 6) stronger pain during menstruation, 7) period pain without bleeding, and 8) any other symptoms from the pelvic region. Subsequently, they were asked the same list of questions for their first menstrual cycle after the first vaccine dose (Supplementary Table 1). Mothers of girls who had not yet had a menstrual cycle after vaccination were recommended to answer “don’t know” for any of the post-vaccination disturbances. Mothers of unvaccinated girls were asked to answer the same list of questions for their daughter’s last menstruation.
2.4. Other variables
Other variables included PCR-confirmed SARS-CoV-2 infection (MSIS), the mother’s vaccination status (obtained from SYSVAK) and her education level, which was self-reported in August 2021. For those with missing information on education from 2021 (about 20 %), we used the most recent available self-reported record of education level from the existing MoBa database. The girl’s birth year was obtained from The Medical Birth Registry of Norway. From a questionnaire subsequently distributed to mothers in April 2022, we obtained information about use of a period tracker app (or similar). The question was asked as follows: “Is she using an app/calendar/diary/other method to track her menstruation?” (No/Yes/Not sure/Not relevant) and if Yes, “For how long has she been using this?” (<1 year, 1–2 years, >2 years, Not sure). Those answering “Yes” and duration of use “1–2 years” or “>2 years” were defined as menstrual app users to ensure that the girls had been using the app for several months prior to vaccination.
2.5. Statistical analyses
We used a self-controlled case series (SCCS) analysis to estimate associations between COVID-19 vaccination and menstrual disturbances [12]. In this design, only vaccinated cases with the outcome in question (i.e., a menstrual disturbance event before and/or after vaccination) were included. Cases who reported “don’t know” or with no answer (missing) before and/or after vaccination were excluded. The cases were their own control in the sense that we compared the girl’s risk of the outcome within a specified exposure window against the risk in a non-exposed window. We used the first cycle after vaccination as the window of exposure and the last cycle prior to vaccination as the non-exposed window. We chose the SCCS analysis and not a comparison between vaccinated and unvaccinated subjects because we considered the SCCS design less prone to selection and information bias in this setting. Log-binomial regression was used to estimate risk ratios (RRs) and 95 % confidence intervals (CIs). The model was fitted with generalized estimating equations to account for the within-individual dependencies. We also performed the SCCS analysis stratified by age (12–13 and 14–15 years) and on a subsample (n = 1006) who had been using an app, calendar, diary or other method to track their menstrual cycle prospectively. In a sensitivity analysis, we excluded n = 22 subjects with a previous SARS-CoV-2 infection from the SCCS analysis.
The median time between vaccination and completion of the questionnaire was 27 days (interquartile range 22–30, 95th percentile 37 days). Between 19.7 % and 22.3 % of mothers of vaccinated girls answered “don’t know” or did not answer questions about irregularities for their first period after vaccination. Absolute and relative risks were therefore estimated after excluding these subjects, based on the assumption that a large proportion of these girls had not yet experienced a menstrual period after vaccination.
Statistical analyses were done in Stata/SE 16.0 (Stat Corp) and R [13], version 4.1.0 [14].
2.6. Ethics
The establishment of MoBa and initial data collection was based on a license from the Norwegian Data Protection Agency and approval from The Regional Committees for Medical and Health Research Ethics. The MoBa cohort is now based on regulations related to the Norwegian Health Registry Act. The current sub-study was approved by The Regional Committee for Medical and Health Research Ethics, South East Norway C, no. 127708.
3. Results
About four out of five (6196 out of 7565, 81.9 %) menstruating girls had received one dose of a COVID-19 vaccine at the time of questionnaire completion (Table 1). Nearly all vaccinated girls (99.9 %) were registered with the Comirnaty vaccine, while only nine girls were registered with Vaxzevria or Spikevax as the first dose. Almost all mothers of vaccinated girls were themselves vaccinated (99.6 %). Among mothers of unvaccinated girls, about 90 % were vaccinated (Table 1 ). Only 1.5 % of the vaccinated girls had previously had a laboratory confirmed SARS-CoV-2 infection, compared to 28.0 % of the unvaccinated girls. In mother-daughter pairs where both were unvaccinated (n = 144 pairs), the mother tended to be younger and have a lower level of education than in pairs where both were vaccinated (n = 6173 pairs), Supplementary Table 2.
Table 1.
Background characteristics of the study population, including 7 565 girls aged 12–15 years.
| Vaccinated with 1 dose of COVID-19 vaccinea (n = 6196) |
Unvaccinated (n = 1369) |
|||
|---|---|---|---|---|
| n | % | n | % | |
| Birth year | ||||
| 2006 | 2393 | 38.6 | 451 | 32.9 |
| 2007 | 2198 | 35.5 | 484 | 35.4 |
| 2008 | 1368 | 22.1 | 354 | 25.9 |
| 2009 | 223 | 3.6 | 79 | 5.8 |
| Missing | 14 | 0.2 | 1 | 0.1 |
| Maternal level of education | ||||
| < High school | 251 | 4.1 | 79 | 5.8 |
| High school | 1349 | 21.8 | 367 | 26.8 |
| College ≤ 4 years | 2700 | 43.6 | 569 | 41.6 |
| >4 years college | 1758 | 28.4 | 320 | 23.4 |
| Missing | 138 | 2.2 | 34 | 2.5 |
| Maternal COVID-19 vaccination status | ||||
| Vaccinated | 6173 | 99.6 | 1225 | 89.5 |
| Unvaccinated | 23 | 0.4 | 144 | 10.5 |
| History of laboratory confirmed SARS-CoV-2 infection | ||||
| Yes | 94 | 1.5 | 384 | 28.0 |
| No | 6102 | 98.5 | 985 | 72.0 |
Nearly all (99.9%) were vaccinated with the mRNA Comirnaty (BNT162b2) vaccine, which was recommended for children with no history of SARS-CoV-2 infection.
Notably, menstrual irregularities were relatively common in this sample of 12–15-year-old girls, independent of vaccination and infection status (Table 2). The proportion of vaccinated subjects who reported one or more menstrual irregularities in their last period prior to vaccination was 22.6 %. In comparison, 25.1 % of this group reported at least one event for the first cycle after vaccination. Of these, one single menstrual irregularity (for instance, unusually heavy bleeding) was reported by 14.2 %, two different irregularities were reported by 6.1 %, and three irregularities by 2.8 %. Only 2.0 % reported four irregularities or more in the first cycle after vaccination (data not shown). In a subsample using an app or similar method to track their menstruation, the proportions reporting irregularities were slightly higher, both before and after vaccination (Supplementary Table 3). Unvaccinated girls with a history of SARS-CoV-2 infection reported more menstrual disturbances compared to unvaccinated girls with no reported infection (Table 2). Vaccinated girls had more than twofold increased risk of reporting heavy menstrual bleeding and prolonged bleeding after vaccination compared to the last cycle for unvaccinated subjects (Table 2).
Table 2.
Number of menstrual disturbance events reported for menstruating girls aged 12–15 years, according to COVID-19 vaccination (single dose) and SARS-CoV-2 infection status.
| Vaccinateda (n = 6196) |
Unvaccinated, no SARS-CoV-2 infection (n = 985) |
Unvaccinated, history of SARS-CoV-2 infection (n = 384) |
||||||
|---|---|---|---|---|---|---|---|---|
| Event registered for last cycle before vaccine |
Event registered for first cycle after vaccineb |
Event registered for last cycle |
Event registered for last cycle |
|||||
| n | % c | n | % c | n | % c | n | % c | |
| Any menstrual disturbance eventd | ||||||||
| Yes | 1054 | 22.6 | 1091 | 25.1 | 165 | 22.3 | 95 | 33.0 |
| No | 3619 | 3251 | 576 | 193 | ||||
| Don't know | 1385 | 1733 | 217 | 90 | ||||
| Missinge | 138 | 121 | 27 | 6 | ||||
| Heavier bleeding | ||||||||
| Yes | 235 | 4.7 | 333 | 7.3 | 22 | 2.8 | 15 | 5.1 |
| No | 4757 | 4198 | 773 | 280 | ||||
| Don't know | 1147 | 1635 | 178 | 86 | ||||
| Missing | 57 | 30 | 12 | 3 | ||||
| Prolonged bleeding | ||||||||
| Yes | 199 | 3.9 | 245 | 5.4 | 16 | 2.0 | 12 | 4.0 |
| No | 4844 | 4302 | 789 | 291 | ||||
| Don't know | 1086 | 1617 | 165 | 78 | ||||
| Missing | 67 | 32 | 15 | 3 | ||||
| Shorter interval | ||||||||
| Yes | 269 | 5.5 | 285 | 6.3 | 39 | 4.9 | 21 | 7.0 |
| No | 4658 | 4227 | 755 | 278 | ||||
| Don't know | 1194 | 1643 | 176 | 81 | ||||
| Missing | 75 | 41 | 15 | 4 | ||||
| Longer interval | ||||||||
| Yes | 376 | 7.7 | 339 | 7.5 | 65 | 8.2 | 51 | 16.7 |
| No | 4516 | 4160 | 725 | 255 | ||||
| Don't know | 1238 | 1650 | 178 | 75 | ||||
| Missing | 66 | 47 | 17 | 3 | ||||
| Spot bleeding | ||||||||
| Yes | 153 | 3.1 | 142 | 3.1 | 24 | 3.0 | 9 | 3.0 |
| No | 4836 | 4470 | 785 | 289 | ||||
| Don't know | 1139 | 1534 | 162 | 82 | ||||
| Missing | 68 | 50 | 14 | 4 | ||||
| Stronger period pains | ||||||||
| Yes | 333 | 6.4 | 326 | 6.9 | 59 | 6.9 | 23 | 7.3 |
| No | 4879 | 4381 | 795 | 294 | ||||
| Don't know | 918 | 1449 | 118 | 64 | ||||
| Missing | 66 | 40 | 13 | 3 | ||||
| Period pains without bleeding | ||||||||
| Yes | 292 | 5.8 | 244 | 5.2 | 42 | 5.1 | 25 | 8.3 |
| No | 4741 | 4411 | 782 | 277 | ||||
| Don't know | 1096 | 1500 | 148 | 79 | ||||
| Missing | 67 | 41 | 13 | 3 | ||||
| Other symptoms from the pelvic region | ||||||||
| Yes | 42 | 0.8 | 38 | 0.8 | 8 | 1.0 | 4 | 1.3 |
| No | 4998 | 4645 | 804 | 294 | ||||
| Don't know | 1096 | 1470 | 160 | 82 | ||||
| Missing | 60 | 43 | 13 | 4 | ||||
Vaccinated subjects had received one dose of COVID-19 vaccine.
Median time between vaccination and completion of the questionnaire was 27 days (interquartile range 22–30).
Proportions were calculated based on valid answers (“yes” and “no”), excluding “don’t know” and missing answers.
“Yes” includes subjects with at least one event reported. “No” includes subjects answering “no” for all events.
“Missing” includes subjects with at least one missing answer across all events.
In the SCCS analyses, we found that the risk of heavier menstrual bleeding and prolonged bleeding was higher in the menstrual cycle after vaccination than in the cycle before vaccination, RRs 1.61 (95 % CI 1.43 to 1.81) and 1.40 (95 % CI 1.23 to 1.60), respectively (Table 3). Vaccination was also associated with increased risk of shorter interval, longer interval, and stronger period pains (RRs 1.14 to 1.19). The effect sizes were of similar magnitude among girls aged 12–13 years and girls aged 14–15 years, and among those who were prospectively (with regard to vaccination) tracking their menstruation using an app or other method. There was no association between vaccination and spot bleeding, period pains without bleeding, or other symptoms from the pelvic region (Table 3). The risks were similar after excluding n = 22 subjects with a history of SARS-CoV-2 from the analysis sample (Supplementary Table 4).
Table 3.
Risk of menstrual disturbances during the first cycle after vaccination compared to the last cycle prior to vaccination among n = 1468 vaccinated girls aged 12–15 years and in a subsample who reported use of a menstruation app (or similar) at least 6 months prior to vaccinationa. Relative risks (RR) were estimated using a self-controlled case series analysis, for girls who experienced any menstrual changes prior to and/or after vaccination.
| Age group (years) | No. of subjects | No. of events |
RR (95 % CI) | ||
|---|---|---|---|---|---|
| Prior to vaccination | After vaccination | ||||
| Heavier bleeding | |||||
| 12–15b | 369 | 197 | 316 | 1.60 (1.43 to 1.80) | |
| 12–13c | 87 | 46 | 76 | 1.65 (1.30 to 2.10) | |
| 14–15 d | 282 | 151 | 240 | 1.59 (1.39 to 1.82) | |
| 12–15, app users | 74 | 43 | 68 | 1.58 (1.27 to 1.97) | |
| Prolonged bleeding | |||||
| 12–15 | 279 | 163 | 227 | 1.39 (1.22 to 1.59) | |
| 12–13 | 73 | 46 | 61 | 1.33 (1.05 to 1.67) | |
| 14–15 | 205 | 116 | 166 | 1.43 (1.22 to 1.68) | |
| 12–15, app users | 51 | 32 | 45 | 1.41 (1.09 to 1.82) | |
| Shorter interval | |||||
| 12–15 | 338 | 228 | 272 | 1.19 (1.07 to 1.32) | |
| 12–13 | 87 | 57 | 70 | 1.23 (0.99 to 1.52) | |
| 14–15 | 250 | 170 | 201 | 1.18 (1.05 to 1.33) | |
| 12–15, app users | 74 | 49 | 59 | 1.20 (0.96 to 1.51) | |
| Longer interval | |||||
| 12–15 | 423 | 284 | 328 | 1.15 (1.05 to 1.27) | |
| 12–13 | 120 | 79 | 88 | 1.11 (0.91 to 1.36) | |
| 14–15 | 302 | 204 | 239 | 1.17 (1.05 to 1.31) | |
| 12–15, app users | 81 | 54 | 65 | 1.20 (0.97 to 1.50) | |
| Spot bleeding | |||||
| 12–15 | 176 | 125 | 133 | 1.06 (0.92 to 1.23) | |
| 12–13 | 41 | 27 | 31 | 1.15 (0.82 to 1.60) | |
| 14–15 | 133 | 96 | 101 | 1.05 (0.89 to 1.24) | |
| 12–15, app users | 38 | 24 | 29 | 1.21 (0.85 to 1.72) | |
| Stronger period pains | |||||
| 12–15 | 388 | 271 | 310 | 1.14 (1.04 to 1.26) | |
| 12–13 | 71 | 51 | 60 | 1.18 (0.97 to 1.43) | |
| 14–15 | 316 | 219 | 249 | 1.14 (1.02 to 1.27) | |
| 12–15, app users | 67 | 48 | 55 | 1.15 (0.93 to 1.42) | |
| Period pains without bleeding | |||||
| 12–15 | 315 | 240 | 240 | 1.00 (0.90 to 1.11) | |
| 12–13 | 80 | 56 | 61 | 1.09 (0.87 to 1.36) | |
| 14–15 | 235 | 184 | 179 | 0.97 (0.87 to 1.09) | |
| 12–15, app users | 59 | 42 | 46 | 1.10 (0.86 to 1.40) | |
| Other symptoms from the pelvic region | |||||
| 12–15 | 49 | 38 | 37 | 0.97 (0.76 to 1.25) | |
| 12–13 | 18 | 12 | 14 | 1.17 (0.72 to 1.88) | |
| 14–15 | 31 | 26 | 23 | 0.88 (0.66 to 1.18) | |
| 12–15, app users | 4 | 3 | 3 | 1.00 (0.40 to 2.52) | |
Among n = 7565 menstruating subjects in the study sample, n = 4455 (59 %) had answered a questionnaire in April 2022 (i.e., approximately 6 months after collection of menstrual data), which included questions about menstruation registration. The question asked was “Is she using an app/calendar/diary/other method to log her menstruation?” and if Yes, “For how long has she been using this?”. Subjects who reported use of a menstruation registration method for at least 1 year were included in this subsample, referred to as “app users”.
Birth year 2006–2009.
Birth year 2008–2009.
Birth year 2006–2007.
4. Discussion
In this study of adolescent girls, we found increased risks of menstrual disturbances in the first cycle after receiving one dose of COVID-19 vaccine. The risks of heavier or more prolonged bleeding than usual were significantly higher in the first menstrual cycle after vaccination than in the last cycle prior to vaccination. We also found increased risks of shorter interval, longer interval and more pain during periods following vaccination. Notably, menstrual irregularities were common also prior to vaccination and among unvaccinated subjects. For most girls, no changes were reported following COVID-19 vaccination.
Our study design has several strengths. We use a large population-based cohort, with recruitment many years prior to the exposures. The representativeness of MoBa has previously been studied, indicating a somewhat higher socio-economic status among participants than in the general Norwegian population [15], [16]. In the SCCS analyses, differences in time invariant factors, such as genetics, socio-economic status, or underlying diseases, are cancelled out, which reduces bias [12]. This design should therefore provide more valid risk estimates than a design comparing different subgroups (i.e., vaccinated vs unvaccinated subjects). Another strength of the study is that information on both vaccination and SARS-CoV-2 infection status was obtained from nationwide registries with mandatory reporting. Although self-reported data collection could be subjected to bias, using digital surveys is a safe way to perform research during the pandemic and valuable in a situation where many are vaccinated in a short period. Moreover, responding mothers have participated in MoBa for more than a decade and are accustomed to answering questionnaires, also on behalf of their child. The questionnaire used in the current study was not solely focused on vaccination and menstruation but covered other aspects of the pandemic, which may have reduced selection bias for menstruation-related questions.
This study also has some limitations that need to be addressed. First, mothers were reporting on behalf of their daughters, which may have introduced misclassification, likely underreporting, of the outcomes. Still, the questions covered a range of symptoms potentially related to both infection and vaccination (such as respiratory symptoms, headache, dizziness, unwellness, skin rash, and more), which warranted close communication between mother and daughter as a basis for answering the questionnaire. Second, the occurrence of menstrual disturbances both before and after vaccination was reported by mothers at the same timepoint. Therefore, we cannot rule out that the mother’s report of irregularities before vaccination is biased by the outcome after vaccination. Still, our findings seem relatively robust to recall bias, as similar relative risks were estimated for a subsample who used a menstruation app or similar method to track their menstruation. Third, the media’s emphasis on a potential link between menstrual cycle irregularities and COVID-19 vaccination may have increased the awareness among vaccinated participants, thus increasing the likelihood of them reporting such events after vaccination [17]. Indeed, in this study, some menstrual disturbances were reported more often for vaccinated girls compared to unvaccinated girls even before they were vaccinated. This may indicate that mothers of vaccinated girls, or the vaccinated girls themselves, are more aware about menstrual irregularities and the potential adverse events after COVID-19 vaccination. The increased awareness may potentially have introduced some information bias to our analysis but probably to a limited extent for the estimated relative risks since our subjects act as their own controls. Also, the lack of association between vaccination and spot bleeding, period pains without bleeding, or other symptoms from the pelvic region, may support that bias does not solely explain the associations seen for the other outcomes. Lastly, the interval between vaccination and the date of responding to the questionnaire may have been too short to allow detection of the outcomes in all girls, especially the outcome “longer interval”, and some girls probably did not have time to experience a menstruation after vaccination. We cannot rule out that this outcome may be prone to information bias due to this limitation. Also, the exclusion of subjects answering “don’t know” in the SCCS analysis may potentially introduce bias if the occurrence of menstrual disturbances is different in this group before and after vaccination.
The reports concerning irregularities in the menstrual cycle after receiving mRNA COVID-19 vaccines among women have been widely discussed [18], [19]. Menstrual disturbances are more common after COVID-19 vaccines than after non-COVID-19 vaccines in VAERS reports [19]. A study from the US reported that COVID-19 vaccination was associated with a small change in cycle length but not menses length [20]. In an online questionnaire with participants from Saudi-Arabia, one per cent of female participants (broad age groups) who had received the Comirnaty vaccine reported abnormal menstrual cycle as a post-vaccinal short term adverse event, in an open field (i.e., the outcome was not listed as a response alternative) [21]. In data collected in the UK prior to the widespread media attention to menstrual disturbances following COVID-19 vaccination, one study found that among menstruating, pre-menopausal, vaccinated individuals, 20 % reported changes to their menstrual cycles up to 4 months after receiving their first vaccine dose [22]. A prospectively recruited cohort with 79 individuals showed that COVID-19 vaccine is associated with a delay to the subsequent period, and interestingly detected no association between menstrual changes and other commonly-reported side effects [23]. In a large survey from the US (median age 33 years), increased bleeding appeared to be the most common post-vaccination adverse events [24]. Moreover, a recent large study from our group observed an increased risk of menstrual disturbances after vaccination in women aged 18–30 years, both after the first and after the second vaccine dose [9]. However, another study from the UK using a retrospective recruitment did not find any association between COVID-19 vaccination and menstrual disturbances [25]. The results in our current study therefore lie within the (wide) range of estimates reported in the existing, though limited, data on adults. Given the lack of data for adolescents in particular, our findings need to be confirmed in other studies.
There have also been indications that infection with SARS-CoV-2 may cause changes in the menstruation cycle [18], [26], [27]. It has been proposed that since ACE2 receptors are present on ovarian and endometrial tissue, SARS-CoV-2 infection may exert a direct impact on the female reproductive system [26], [27]. In the current study, we observed a higher occurrence of menstrual disturbances among unvaccinated girls with a previous SARS-CoV-2 infection compared to uninfected girls in the unvaccinated group. However, our study was not designed to evaluate menstrual disturbances after SARS-CoV-2 infection and this observation needs to be confirmed in other studies. Also, whether menstrual disturbances are equally common after SARS-CoV-2 infection and vaccination should be elucidated. COVID-19 vaccination and infection may potentially influence the menstruation cycle through a similar mechanism involving ACE2 receptors. The vaccine activates the immune system, possibly attacking immune cells and inflammatory molecules in the uterus [28]. Thus, some studies suggest that vaccination is less likely to affect menstruation via ovarian hormone pathways, and more likely along the inflammatory pathways [20], [24], [29]. Still, the pathophysiological mechanisms are yet unknown.
Assessment of the safety aspects are important when deciding on whether to vaccinate children or not since children seldom get seriously ill with SARS-CoV-2-infection [2]. After the rapid development and emergency authorization of SARS-CoV-2 vaccines, serious adverse reactions have been reported more frequently for the SARS-CoV-2 vaccines in comparison to other vaccines, such as influenza vaccines [6]. Careful evaluation of the short- and long-term effects of both the infection itself, as well as the vaccine used for prevention, should always be performed when evaluating new vaccines. This is highly actualized during the COVID-19 pandemic where long-term consequences of SARS-CoV-2 infection are becoming evident and mass vaccination campaigns with vaccines based on new technologies have been rolled out quickly and in the similar time frame. Although the COVID-19 vaccine has become available to children, not all countries have recommended a second dose or have delayed the recommendation for safety issues. Understanding the risk of adverse reactions associated with the vaccine will assist parents in making an informed decision on whether their child should get vaccinated or not, or how many doses. The safety risk must be weighed against the risk of disease from the virus and may change depending on the circulating viral variant. Despite many reports of altered menstrual bleeding patterns after vaccination, it is still not possible to exclude the possibility that these might reflect normal variation amongst the millions of individuals that have had SARS-CoV-2 infections and received COVID-19 vaccine.
To our knowledge, this is the first study to estimate the risk of menstrual disturbances after COVID-19 vaccination in adolescents. More data collected in real-time is needed to confirm our findings and to assess the risk of recurrence after a second dose in this age group. Also, studies exploring potential mechanisms are warranted. Studies that assess the direct effect of vaccination on the menstrual cycle are few and far between. Therefore, a continuous monitoring of COVID-19 vaccines is essential to enhance the reassurance and acceptance of COVID-19 vaccinations in all age groups.
5. Conclusions
In this population-based study conducted in Norway, vaccination against COVID-19 for adolescent girls was associated with increased risk of experiencing menstrual disturbances in the first cycle after vaccination. However, most young girls reported no changes to their menstrual cycle following COVID-19 vaccination. Nearly all adolescent girls in Norway received one dose of the Comirnaty vaccine, which should be considered in the interpretation of our findings.
Contributors
IHC and LKJ are co-first authors and contributed equally to this study. LT and PM designed the study and collected the data. All authors contributed to the study conceptualization and methods. IHC, LKJ, BF, and IL had full access to all the data and performed statistical analyses. All authors interpreted the data. IHC, LKJ and LT prepared the first draft of the manuscript. All authors revised the manuscript, approved the final version. Co-first authors IHC and LKJ had final responsibility for the decision to submit for publication.
Data statement
Data from the cohort is available for analysis after approval from a Norwegian ethics committee and application to the Norwegian Institute of Public Health.
Funding
This work was supported by The Norwegian Research Council’s Centres of Excellence Funding Scheme (no. 262700) and the Norwegian Institute of Public Health (NIPH).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The Norwegian Mother, Father and Child Cohort Study is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research. We are grateful to all the participating families in Norway who take part in this on-going cohort study. We also thank all NIPH staff involved in collection and preparation of data and follow up of cohort participants.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.11.068.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data from the cohort is available for analysis after approval from a Norwegian ethics committee and application to the Norwegian Institute of Public Health.
References
- 1.First COVID-19 vaccine approved for children aged 12 to 15 in EU. https://www.ema.europa.eu/en/news/first-covid-19-vaccine-approved-children-aged-12-15-eu. 2021.
- 2.Frenck R.W., Klein N.P., Kitchin N., Gurtman A., Absalon J., Lockhart S., et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 Covid-19 Vaccine in Adolescents. N Engl J Med. 2021;385:239–250. doi: 10.1056/NEJMoa2107456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan E.W.W., Leung M.T.Y., Lau L.K.W., Leung J., Lum D., Wong R.S., et al. Comparing self-reported reactogenicity between adolescents and adults following the use of BNT162b2 (Pfizer-BioNTech) messenger RNA Covid-19 vaccine: a prospective cohort study. Int J Infect Dis. 2021 doi: 10.1016/j.ijid.2021.12.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Norwegian Medicines Agency, Reported suspected adverse reactions of covid-19 vaccines. https://legemiddelverket.no/english/covid-19-and-medicines/vaccines-against-covid-19/reported-suspected-adverse-reactions-of-covid-19-vaccines
- 5.Medicine and Healthcare Products Regulatory Agency. Coronavirus vaccine—weekly summary of Yellow Card reporting. 13 Jan 2022. https://www.gov.uk/government/publications/coronaviruscovid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting#annex-1-vaccine-analysis-print. 2022.
- 6.Montano D. Frequency and Associations of Adverse Reactions of COVID-19 Vaccines Reported to Pharmacovigilance Systems in the European Union and the United States. Front. Public Health. 2021 doi: 10.3389/fpubh.2021.756633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Signal for COVID-19 vaccine-related menstrual disorders. Reactions Weekly. 2022;1887:5. doi: 10.1007/s40278-022-07670-3. [DOI] [Google Scholar]
- 8.EMA. Meeting highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 24 - 27 October 2022. https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-24-27-october-2022
- 9.Trogstad L. Increased Occurrence of Menstrual Disturbances in 18- to 30-Year-Old Women after COVID-19 Vaccination (January 1, 2022). Available at SSRN: https://ssrn.com/abstract=3998180 or http://dx.doi.org/10.2139/ssrn.3998180. 2022.
- 10.Magnus P., Birke C., Vejrup K., Haugan A., Alsaker E., Daltveit A.K., et al. Cohort Profile Update: The Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2016;45:382–388. doi: 10.1093/ije/dyw029. [DOI] [PubMed] [Google Scholar]
- 11.Trogstad L., Ung G., Hagerup-Jenssen M., Cappelen I., Haugen I.L., Feiring B. The Norwegian immunisation register–SYSVAK. Euro Surveill. 2012;17 [PubMed] [Google Scholar]
- 12.Whitaker H.J., Farrington C.P., Spiessens B., Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med. 2006;25:1768–1797. doi: 10.1002/sim.2302. [DOI] [PubMed] [Google Scholar]
- 13.R core team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. 2021.
- 14.Wickham H, Averick M, Bryan J, Chang W, McGowan LDA. Welcome to the tidyverse. Journal of Open Source Software, 4(43), 1686, https://doi.org/10.21105/joss.01686. 2019.
- 15.Nilsen R.M., Vollset S.E., Gjessing H.K., Skjaerven R., Melve K.K., Schreuder P., et al. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23:597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 16.Biele G., Gustavson K., Czajkowski N.O., Nilsen R.M., Reichborn-Kjennerud T., Magnus P.M., et al. Bias from self selection and loss to follow-up in prospective cohort studies. Eur J Epidemiol. 2019;34:927–938. doi: 10.1007/s10654-019-00550-1. [DOI] [PubMed] [Google Scholar]
- 17.Katz A, Tepper Y, Birk O, Eran A. Web and social media searches highlight menstrual irregularities as a global concern in COVID-19 vaccinations. Sci Rep. 2022 Oct 21;12(1):17657. doi: 10.1038/s41598-022-20844-x. PMID: 36271079; PMCID: PMC9587257. [DOI] [PMC free article] [PubMed]
- 18.Male V. Menstrual changes after covid-19 vaccination. BMJ. 2021;374 doi: 10.1136/bmj.n2211. [DOI] [PubMed] [Google Scholar]
- 19.Zhang B., Yu X., Liu J., Liu P. COVID-19 vaccine and Menstrual conditions in female: data analysis of the Vaccine Adverse Event Reporting System. BMC Womens Health. 2022;5(22(1)):403. doi: 10.1186/s12905-022-01934-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edelman A, Boniface ER, Benhar E, Han L, Matteson KA, Favaro C, et al. Association Between Menstrual Cycle Length and Coronavirus Disease 2019 (COVID-19) Vaccination: A U.S. Cohort. Obstetrics & Gynecology. 2022:10.1097/AOG.0000000000004695. [DOI] [PMC free article] [PubMed]
- 21.Alghamdi A.N., Alotaibi M.I., Alqahtani A.S., Al Aboud D., Abdel-Moneim A.S. BNT162b2 and ChAdOx1 SARS-CoV-2 Post-vaccination Side-Effects Among Saudi Vaccinees. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.760047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alvergne A., Kountourides G., Argentieri A., Agyen L., Rogers N., Knight D., et al. medRxiv; 2021. COVID-19 vaccination and menstrual cycle changes: A United Kingdom (UK) retrospective case-control study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Von Woon E., Male V. medRxiv; 2022. Effect of COVID-19 vaccination on menstrual periods in a prospectively recruited cohort. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee KM, Junkins EJ, Fatima UA, Cox ML, Clancy KB. Characterizing menstrual bleeding changes occurring after SARS-CoV-2 vaccination. medRxiv. 2021:2021.10.11.21264863.
- 25.Male V. Effect of COVID-19 vaccination on menstrual periods in a retrospectively recruited cohort. medRxiv. 2021:2021.11.15.21266317.
- 26.Phelan N., Behan L.A., Owens L. The Impact of the COVID-19 Pandemic on Women's Reproductive Health. Front Endocrinol (Lausanne) 2021;12 doi: 10.3389/fendo.2021.642755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharp G.C., Fraser A., Sawyer G., Kountourides G., Easey K.E., Ford G., et al. The COVID-19 pandemic and the menstrual cycle: research gaps and opportunities. Int J Epidemiol. 2021 doi: 10.1093/ije/dyab239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sağsöz N. Does COVID-19 Infection Affect Female Reproductive System? International Journal of Women’s Health and Reproduction Sciences. 2021;9:158–159. [Google Scholar]
- 29.Ricke D. Etiology Model for Elevated Histamine Levels Driving High Reactogenicity Vaccines (including COVID-19) Associated Menstrual Adverse Events. Research Square. 2022 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the cohort is available for analysis after approval from a Norwegian ethics committee and application to the Norwegian Institute of Public Health.