Abstract
Immune checkpoint inhibitors (ICIs) have successfully treated a number of different types of cancer, which is of great significance for cancer treatment. With the widespread use of ICIs in clinical practice, the increasing checkpoint inhibitor pneumonia (CIP) will be a challenge to clinicians. To guide the diagnosis and treatment of CIP, we conducted in‐depth discussions based on the latest evidence, forming a consensus among Chinese experts on the multidisciplinary management of CIP.
Keywords: checkpoint inhibitor pneumonitis, Chinese experts consensus, immune checkpoint inhibitor‐related adverse effects
Chinese expert consensus on the multidisciplinary management of pneumonitis associated with immune checkpoint inhibitor.
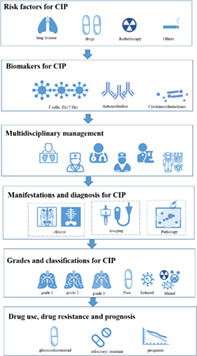
INTRODUCTION
One of the complications of immune checkpoint inhibitor (ICI) therapy is checkpoint inhibitor pneumonitis (CIP). CIP can be manifested by focal or diffuse inflammation of the lung parenchyma accompanied by cough, shortness of breath, and hypoxemia or asymptomatic. 1 , 2 Sever CIP may cause fatalities. 3 In clinical practice, the diagnosis and management of CIP is a great challenge.
In clinical trials, the incidence of CIP was reported to be 3% to 5%. 4 , 5 , 6 , 7 , 8 When real‐world data were included, the incidence is up to 13% to 19%. 3 , 9 , 10 The CPI incidence of non–small cell lung cancer (NSCLC) and renal cell cancer is higher than that of melanoma, which may be because of different tumor locations. A meta‐analysis showed that programmed cell death protein‐1(PD‐1) inhibitors had a higher risk of CIP than programmed death ligand‐1 (PD‐L1) inhibitors. 11 In small cell lung cancer (SCLC) patients, the incidence of immune‐related adverse events (irAEs) such as pneumonitis caused by PD‐L1 inhibitors is lower than that of PD‐1 (4.3% vs. 2.1%). 12
Previous study showed that the incidence of CIP in Japanese patients (8%–14%) is higher than that in non‐Asian population. 13 To date, the different incidence of CIP among Asian and non‐Asian patient remains unclear. In patients treated with immunosuppressants, the level of early irAE is related to the clinical prognosis of patients. 14 The overall mortality rate of adverse effects (AEs) caused by PD‐1/PD‐L1 inhibitors was 0.45%, and CIP was the most common cause (28.0%). 15 A phase 3 Chinese patients trials showed that the incidence of CIP in the camerlizumab plus carboplatin group was similar to other combination therapies of ICIs and chemotherapy. 16 Furthermore, all grade pneumonitis accounts for about 3%, and pneumonitis with grade more than 3 was ~2%. 16 In another Chinese patient study, the incidence of CIP was 3.4% in the sintilimab plus pemetrexed group, and pneumonitis with grade more than 3 was 0.8%. 17 In conclusion, although the overall incidence of CIP is not high, serious CIP needs to be taken seriously by clinicians.
CONSENSUS 1: THE RISK FACTORS FOR CIP
A case–control study revealed the main risk factors of CIP through prior lung disease, thoracic radiotherapy (RT), and combination treatment of ICIs or epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs), with odds ratios (OR): 2.86, 3.34, and 2.73, respectively. 18
Previous lung disease
CIP is involved in several lung diseases, including chronic obstructive pulmonary disease (COPD), poor lung function, asthma, interstitial lung disease (ILD), pulmonary fibrosis, pneumothorax, and pleural effusion. 18 It was reported that the frequency of CIP in patients with a history of asthma/COPD is higher than that in patients without a history of asthma/COPD (5.4% vs. 3.1%). 18 , 19 However, the number of CD4+ cells expressing PD‐1 is high in COPD patients. This may lead to a higher incidence of CIP and a longer progression‐free survival (PFS) in patients with mild COPD treated with ICIs. 20 In addition, asthma may be associated with a higher grade of CIP, and smoking may augment this association. 21 In addition to lower pretreatment percentage predicted forced expiratory volume in 1 s (FEV1pp), diffusion capacity of lung for carbon monoxide (DLCO) decline may be an early indicator of CIP. 22 Therefore, routine pulmonary function testing with DLCO measurement during treatment may help risk stratify for CIP. 23 , 24 Isono et al. 25 recently reported that idiopathic interstitial pneumonias (IIP) were a risk factor of CIP. Another study showed a significant difference in morbidity between patients with preexisting ILD and those without preexisting ILD (31% vs. 12%; p = 0.014). 26 According to a retrospective analysis, the risk of CIP was closely associated with preexisting pulmonary fibrosis. 27 Although prior lung diseases are not contraindications to PD‐1/PD‐L1, clinicians should be cautious when using ICIs for patients with previous lung disease.
Combination with ICIs or EGFR‐TKIs
Chemotherapeutic drugs, TKIs, and other ICIs are often used in combination with ICIs. The incidence of CIP in patients with combined ICIs is higher than that in patients with ICI alone. 8 For example, the combination of PD‐1 inhibitor and cytotoxic T lymphocyte antigen‐4 (CTLA‐4) inhibitor leaded to a significant increase in grade 1 to 5 pneumonitis compared with ICI alone 28 ; the pneumonitis incidence rate in patients with EGFR‐TKI monotherapy was 4.59%, whereas it increased to 25.7% in patients received EGFR‐TKI combined with ICI treatment. 29 Therefore, combination therapy may be a high‐risk factor that needs further safety assessment.
Thoracic radiotherapy
Previous RT appears to be a potential risk factor for CIP. Among lung cancer patients who had pneumonitis, Aiad et al. 30 reported that patients receiving immunotherapy combined with radiation were more serious than those receiving radiation therapy alone (55.7% vs. 36.2%). On the contrary, the PACIFIC study, which evaluated the effectiveness of durvalumab as maintenance therapy after definitive chemotherapy concurrent with RT for unresectable stage III NSCLC, demonstrated a moderate increase in pulmonary toxicity (durvalumab group vs. placebo group: 13.1% vs. 7.7%), and most of them were mild (grade 3–4 pneumonitis, durvalumab group vs. placebo group: 4.4% vs. 3.8%). 31
Other risk factors
The occurrence of CIP may be also related to age, smoking history, treatment history, Eastern Cooperative Oncology Group performance status (ECOG PS), virus infection, and histological type. The proportion of patients over 70 years old in CIP group was significantly higher than that in non‐CIP group (54.5% vs. 30.3%; p = 0.025). Compared with non‐smokers, former/current smokers had a higher incidence of pneumonitis. 32 Pneumonitis of any grade may be more common in treatment‐naive patients. 33 The incidence of CIP in patients with squamous histology is higher than that in patients with adenocarcinoma, and cytomegalovirus (CMV) infection may be an important trigger for CIP. It is important for patients with severe CIP to be vigilant against CMV infection. 34 In patients with NSCLC, especially within 3 months of PD‐1 treatment, tumor invasion in the central airway was consistently associated with early‐onset CIP 34 , 35 In addition, ECOG PS ≥2 was closely correlated to the occurrence of CIP. 36
CONSENSUS 2: BIOMARKER OF CIP
Research is ongoing to determine which markers can be used to predict or diagnose CIP. Based on the mechanism of the disease, potential biomarkers mainly focus on cellular biomarkers, autoantibodies, cytokines/chemokines, and imaging biomarkers.
Cellular biomarkers
Independent risk factors of grade 3/4 and lung irAEs includeed the elevated leukocyte count (p = 0.014, OR = 6.04) and decreased relative lymphocyte count (RLC) (p = 0.012, OR = 5.01). 37 The incidence of CIP was high in patients with high peripheral blood eosinophil count before ICIs treatment. 38
In bronchoalveolar lavage (BAL) fluid of patients with pulmonary symptoms after ICIs treatment, the number of T cells, especially interferon (IFN)ɤ + interleukin‐17 (IL‐17) − CD8+ T cells and CX chemokine receptor (CR) 3+ C‐C motif chemokine receptor (CCR) 6+ T helper (Th)17/Th1 cells increased. It is possible that CD8+ T cells and Th17/Th1 cells play an important role in CIP. 39
Autoantibodies
A panel of 5‐tumor associated autoantibodies (TAAbs) in plasma was selected to predict CIP. The 5‐TAAbs panel included p53, BRCA2, HUD, TRIM21, and NY‐ESO‐1. In contrast to negative patients, the incidence of CIP was significantly higher in patients with 5‐TAAbs positive than that in patients with 5‐TAAbs negative. 40 Moreover, anti‐CD74 autoantibody was reported to correlate with the development of CIP, and may be used for early detected of CIP. 41
Cytokines/chemokines
Cytokines and chemokines can regulate the immune system. In CIP patients, elevated levels of IL‐6 and C‐reactive protein (CRP) in peripheral blood were considered potential biomarkers. 42 , 43 , 44 The inflammation effect is influenced by these two factors. IFN‐ɤ may help to detect CIP after ICI‐based treatment. 45 Moreover, increased levels of IL‐17A, IL‐35, IL‐6, IL‐10, neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), and lactic dehydrogenase (LDH) levels or reduced absolute lymphocyte count (ALC) and albumin (ALB) levels were associated with the development of CIP. 46 , 47
Currently, although some progress has been made in the development of CIP markers, existing CIP studies are mainly retrospective and lacks accurate and effective biomarkers. It still needs sufficient prospective evidence to develop more meaningful biomarkers. In the future, it is necessary to carry out more research, especially prospective research, to improve our understanding of the underlying biology of CIP, to guide more precise and effective treatment strategies.
CONSENSUS 3: THE MULTIDISCIPLINARY MANAGEMENT OF CIP
To improve the diagnosis and therapy of CIP, a multidisciplinary team consisting of pulmonologists, medical oncologists, thoracic radiologists, pathologists, thoracic surgeons, intensive care unit doctors, infectionist, immunologists, and rheumatologists with expertise in lung cancer, drug‐related pneumonitis, infectious diseases, and immunology was established to identify and prioritize knowledge gaps, and guide basic, translational, and clinical research focused on the etiology, diagnosis, and management of CIP. 48 , 49 Multidisciplinary will participate in translational and clinical trials, especially to provide clinical research samples such as serum, BAL, and lung pathologic specimens from affected patients, which may aid in identifying the biological differences that lead to variable clinical presentations, and may in turn, guide the development of phenotype‐specific targeted therapeutics to prevent or treat CIP. To avoid diagnostic delays, it is important to interact between disciplines. The complexity of CIP makes multidisciplinary collaborations essential for improving research and diagnosis.
CONSENSUS 4: THE MANIFESTATIONS OF CIP
Clinical manifestations
CIP has no typical and specific clinical features. Patients with CIP may be asymptomatic or accompanied by dyspnea and cough, whereas fever and chest pain are less common. 4 The symptoms may be similar to respiratory tract infection, congestive heart failure, lymphangitis carcinomatosa, or another AE associated with systemic anti‐cancer therapy. Although fever and chest pain also sometimes occur, infectious pneumonia can also cause fever and chest pain. Therefore, the possibility of patients with fever should be excluded. In terms of disease course, CIP can manifest as acute, subacute, and chronic. 50 Chronic CIP is defined as persistent or worsening pneumonia after steroid reduction, and it is necessary to carry out immunosuppression for more than 12 weeks after ICI discontinuation. 51 Patients with early‐onset of CIP, within 6 weeks of ICI treatment, often have severe symptoms and poor prognosis, whereas patients with late‐onset of CIP, after 6 weeks of ICI treatment, often have few symptoms and better prognosis. 52
The duration of CIP is variable from the beginning of ICI administration to withdrawal of the drug. Therefore, it is important to monitor CIP throughout the whole clinical process of ICI treatment.
Imaging manifestations
Radiographic imaging is the most commonly used method for the diagnosis of pneumonitis. In 40% of patients, the pattern distribution was mixed and multifocal, and 75% had involvement of all lung lobes. 53 According radiographic characteristics, CIP can be divided into organizing pneumonitis (OP), ground glass opacification/opacity (GGO), interstitial pneumonitis (IP), hypersensitive pneumonitis (HP), and other types. 53 , 54 , 55 Nishino et al. 53 screened 20 advanced cancer patients developed CIP from 10 different nivolumab trials, and identified 13 (65%) OP, three (15%) nonspecific interstitial pneumonitis (NSIP), two (10%) HP, and two (10%) acute interstitial pneumonia (AIP)/acute respiratory distress syndrome (ARDS). Another retrospective study reported a single‐characterized radiographic pattern, with 45% of patients having bilateral involvement and 86% having lung involvement away from the peri‐tumoral zone. 56
In addition to radiographic imaging, bronchoscopy is also used for the diagnosis or auxiliary diagnosis of CIP, especially when X‐ray is difficult to diagnose. Bronchoscopy is helpful to diagnose CIP in patients with suspected infectious. Among the 12 patients diagnosed as CIP through bronchoscopy, 10 patients underwent BAL. Alveolitis was found in seven (87.5%) and OP was found in five (62.5%) patients. It also found acute lung injury and fibrosis. 57 The largest series of bronchoalveolar lavage fluid (BALF) cell count results and 80% of BALF results showed a lymphocytosis of >15%. The proportion of lymphocytes in all 10 patients was higher than 20%. 57 However, a subset of IIP, including idiopathic nonspecific interstitial pneumonitis (INP), cryptogenic organizing pneumonitis (COP) and connective tissue disorder‐related lung disease, is also predominant in lymphocytes and cannot be distinguished only by BALF findings.
Pathological examination may be helpful in understanding the specific mechanism of each classification if this clinical classification can be confirmed in a wider population.
Pathological manifestations
The pathological manifestations of CIP are greatly concerned all along, but little is known. Most CIP specimens come from transbronchial lung biopsy (TBLB), and therefore, the sample is often small. There is also an inevitable heterogeneity in the pathological manifestations. In pathological results of nine CIP patients, the symptoms of all patients were nonspecific, and even two patients were asymptomatic. All cases showed bilateral ground glass or nodular opacities, and often accompanied by pleural effusion. Among them, seven patients were OP with subclinical or mild disease, and three patients admixed with vague non‐necrotizing airspace granulomas. Six patients with follow‐up were stable. Two patients died during treatment and follow‐up: one died from acute fibrinous pneumonitis, and the other died from diffuse alveolar damage. There was foamy macrophage vacuolization in all nine cases, and eosinophils were found in six cases. CIP presents with bilateral ground glass opacities or nodules and usually manifests with organizing pneumonitis histopathologically, often accompanied by vague non‐necrotizing airspace granulomas. Foamy macrophages and pneumocyte vacuolization are characteristic and rare eosinophils are often seen. In rare cases, acute fibrinous pneumonitis or diffuse alveolar damage may occur, which is fatal. 58
CONSENSUS 5: THE DIFFERENTIAL DIAGNOSIS OF CIP
As mentioned above, relatively nonspecific symptoms are insufficiency in vast majority of CIP patients. These symptoms include dyspnoea, chest discomfort, cough, and less commonly fever, which are common symptoms of other lung diseases. Hypoxia may lead to rapid progress of CIP. About one‐third of patients may be asymptomatic at onset. Because of the lack of specific clinical or radiologic markers, CIP is difficult to diagnose. Generally, CIP needs to be confirmed by exclusion diagnosis, but does not include infection, tumor progression, and radiation‐related pneumonitis. After ICI treatment, CIP should be considered for any new emergence of respiratory symptoms, especially dry cough, dyspnea, or decreased oxygen saturation. 59
The diagnostic workup to identify an etiology should include detection of a source of infections such as nasal swab, sputum/urine culture, blood culture, detection of special pathogens such as fungus and uberculosis spot, chest radiography, bronchoscopy examination, and BAL examination. 4 , 60 Lung biopsy is not mandatory, and drugs and infection history occasionally help interpret the results. Use of diagnostic tests is related to the suspected pneumonitis grade. 61
Common differential diagnoses of CIP include pulmonary infections, pulmonary embolism, diffuse alveolar damage (DAD), lung cancer with underlying progression, cancerous lymphangitis, pulmonary interstitial edema caused by heart failure, fulminant myocarditis, and radiation‐induced pneumonitis. 59 During treatment with glucocorticosteroid (GCS) or other immunosuppressors, attention should be paid to secondary opportunistic infections arising from immune suppression. Opportunistic pulmonary infections, including tuberculosis (TB) pneumonia, aspergillosis, CMV pneumonia (CMVP), and pneumocystis jirovecii pneumonia (PJP), have been the foremost differential diagnoses of CIP in the NSCLC population. Notably, ICIs could cause special pathogen infections in some patients through inducing CIP. Aggressive lung biopsy was recommended to diagnose CIP in patients with NSCLC that mimicked the OP pattern or existed with the tumor invasion. The ICIs may cause inflammatory reaction in patients who previously irradiated fields with infiltrating lymphocytes and potential involvement related cytokines. Radiation recall pneumonitis (RRP) is characterized by inflammatory reaction within the previously treated radiation field after administration of specific treatment. 62 Radiation pneumonitis occurs in the radioactive field, whereas CIP tends to occur nonsegmentally in both lung fields, especially outside the regions of high‐dose chest radiation. In the majority of RRP cases, the area of pneumonitis matches the irradiated area. Xuguang Chen et al. 63 found that patients with bilateral computed tomography (CT) changes involving at least three lobes were more likely to develop CIP, whereas patients with unilateral CT changes with clear boundaries were more likely to develop radiation pneumonitis. After RT or ICI, severe pneumonitis is associated with bilateral and multifocal CT changes. 63 Quantitative CT radiomics and machine learning may help determine the cause of pneumonia for patients and improve personalized clinical treatment. 64
CONSENSUS 6: THE CIP GRADING AND CLINICAL CLASSIFICATION
Imaging manifestations and clinical symptoms are usually used for CIP grating. According to the National Comprehensive Cancer Network guidelines, 65 CIP is graded by the combination of clinical manifestations and radiological findings as follows (Table 1).
TABLE 1.
The grades of CIP based on clinical manifestations and radiological findings
| Grades | Clinical manifestations | Radiological findings |
|---|---|---|
| Grade 1 | Asymptomatic | One lobe of the lung or <25% of the lung parenchyma |
| Grade 2 | New respiratory symptoms, or aggravation of existing symptoms such as shortness of breath, cough, chest pain, fever, and increased oxygen requirements | Lesions affect 25%–50% of the lung parenchyma |
| Grade 3 | Severe symptoms, limited daily activities | Lesions affect all lung lobes or >50% of the lung parenchyma |
| Grade 4 | Life‐threatening respiratory damage |
Abbreviation: CIP, checkpoint inhibitor pneumonia.
Grade 1 is asymptomatic. The lesion is confined to one lobe of the lung or <25% of the lung parenchyma. Grade 2 includes new respiratory symptoms or aggravation of existing symptoms, including shortness of breath, cough, chest pain, fever, and increased oxygen requirements. On the chest CT, lesions affect 25% to 50% of the lung parenchyma. Grade 3 includes severe symptoms and limited daily activities. Lesions affect all lung lobes or >50% of the lung parenchyma. Grade 4 is life‐threatening respiratory damage.
However, the course and pathology type of CIP were not considered in the guidelines. Even if the patient is grade 2 to 3 at the time of diagnosis, and even for patients with rapid progression or severe imaging manifestations such as diffuse alveolar damage, close monitoring is recommended. In addition, CIP is not well classified according to clinical factors. The clinical classification of CIP may help to formulate treatment strategies and predict the tumor response to ICIs. 66 Based on clinical factors, CIP can be classified into three clinical subtypes, as shown below (Table 2):
Pure type (PT): defined as idiopathic, with or without autoimmune disease. In PT group, most patients were grade 1 to 2, with imaging COP, GGO. The treatment of CIP in the PT group basically followed the guidelines based on systemic glucocorticoids, supplemented by other immunosuppressive agents when necessary.
Induced type (IT): CMV or Epstein–Barr virus (EBV) is reactivated, RT is reactivated, and there is no evidence of organ damage caused by virus or RT. In IT group, most patients were grade 3 to 4, with imaging GGO (AIP). The IT participants received non‐thoracic RT and subsequently developed CIP. In addition, pneumonitis did not occur in these patients during multiple courses of immunotherapy, but developed pneumonia after RT. GGO has the highest proportion in the IT. The incidence of AIP‐ARDS in the IT group was higher than that in the other two groups. For IT patients, in addition to corticosteroids and supportive treatment, antiviral therapy for virus‐induced CIP and anti‐fibrotic therapy for RT‐ induced CIP can be considered.
Mixed type (MT): combined with infectious pneumonia (such as bacteria, fungus, or other organisms), tumor progression, or radiation‐related pneumonitis. In MT group, CIP of most patients were grade 2 to 4, with imaging NSIP, GGO. The MT was characterized by a combination of symptoms and imaging changes associated with infection, tumor progression, or radiation pneumonitis. Patients in MT group developed CIP with CMVP, which was diagnosed by positive CMV culture in BALF or tissue, CMV‐DNA in BALF, or CMV inclusion bodies in lung tissues. For MT patients, antibiotic treatment for co‐infection, antitumor treatment for co‐tumor progression, and anti‐fibrotic therapy for patients complicated with co‐radiation pneumonitis can be considered.
TABLE 2.
The clinical subtypes of CIP based on clinical factors
| Subtypes | Clinical factors | Grades | Imaging features | Treatments |
|---|---|---|---|---|
| Pure type | Idiopathic, with or without autoimmune disease | Grade 1–2 | COP, GGO | Basically followed the guidelines basing on systemic glucocorticoids, supplemented by other immunosuppressive agents when necessary |
| Induced type | CMV or EBV reactivation, radiotherapy reactivation, without evidence of organ damage caused by virus or radiotherapy | Grade 3–4 | GGO, AIP | Corticosteroids and supportive treatment, antiviral therapy (for virus‐induced CIP), and anti‐fibrotic therapy (for radiotherapy‐ induced CIP) can be considered |
| Mixed type | Combined with infectious pneumonia (bacteria, fungus, or other organisms), tumor progression, or radiation‐related pneumonitis | Grade 2–4 | NSIP, GGO | Antibiotic treatment (for co‐infection), antitumor treatment (for co‐tumor progression), and anti‐fibrotic therapy (for patients complicated with co‐radiation pneumonitis) can be considered |
Abbreviations: AIP, acute interstitial pneumonia; CIP, checkpoint inhibitor pneumonia; COP, cryptogenic organizing pneumonitis; CMV, cytomegalovirus; EBV, Epstein–Barr virus; GGO, ground glass opacification/opacity; NSIP, nonspecific interstitial pneumonitis.
CONSENSUS 7: THE USE OF GLUCOCORTICOSTEROID
GCS is the main treatment for CIP. Most studies have found that corticosteroid therapy can improve or resolve symptoms in patients with CIP, especially those with lower‐grade disease. 67 In 70% to 80% CIP patients, regular GCS treatment can control the disease. 68 For patients with grade 1 CIP, close monitoring is recommended, whereas GCS treatment should be considered if clinical progression is observed. Prednisolone is the most commonly used GCS in treatment of CIP. The equivalent dose of prednisolone (1–2 mg/kg/day) is recommended for grade 2 to 3 CIP, whereas intravenous GCS is recommended for more severe or acute CIP. It is recommended to gradually reduce GCS after clinical symptom remission are relieved. GCS treatment usually lasts for 6 to 8 weeks, but does not exceed 12 weeks. For CIP, clinical improvements should be assessed within 48 to 72 hours of GCS treatment. Patients receiving GCS treatment should be considered infectious diseases. In addition, blood pressure, glucose, and electrolytes should be monitored. The total duration of GCS treatment in most CIP patients is ~8 weeks, and the duration of the initial steroid dose is usually <3 weeks. Therefore, treatment of pneumocystis carinii is generally not required, except for patients who take 20 mg GCS per day for >6 weeks. It is possible to supplement calcium and vitamin D3 regularly.
CONSENSUS 8: STEROID‐REFRACTORY/RESISTANT CIP
Some patients are refractory or become resistant to steroids. According to the indications of additional immunomodulators, patients were divided into two subgroups: (i) steroid refractory, which are patients whose pneumonia did not improve or worsen after systemic steroid treatment; (ii) steroid resistant, which are patients who initially responded to steroids, but subsequently developed recurrent pneumonitis when steroid gradually decreased in the absence of immune checkpoint re‐challenge. Patients with steroid‐refractory pneumonitis had more severe disease than those with steroid‐resistant pneumonitis (grade 3–4, 100% vs. 29%, p = 0.0002). Compared with steroid‐resistant patients, steroids‐refractory patients exhibited earlier onset and fewer durable responses. 69 , 70 Steroid‐refractory CIP accounts for 18.5% of patients with multidisciplinary care. 71 The guidelines provide a list of additional immune modulators, however, evidences to guide expectations for patients and providers is limited. Infliximab, mycophenolate mofetil, immunoglobulin, and cyclophosphamide are some additional immune modulating options recommended for patients whose steroids are ineffective. Currently, these agents are mainly used based on experience of treating other irAEs and other inflammatory lung diseases. According to prospective comparative data, it is unclear what the exact role of below listed agents are. 70 , 72
Intravenous immunoglobulin (IVIG). IVIG can be used to neutralize antigens with good safety. It is especially preferred in patients with potential infection. The dosage of IVIG is 20 g per day for 3 consecutive days or 10 g per day for 5 consecutive days and can be used repeatedly if necessary. The mortality of patients treated with IVIG is low, and the oxygenation of patient can be improved. 71
Antitumor necrosis factor (TNF‐α). Infliximab, a TNF‐α inhibitor, has been used successfully to treat steroid‐ refractory ICI‐colitis, and the effect of infliximab‐ containing regimen treatment is poor. 71 Prospective data should be obtained from clinical trials in this area to identify the optimum immunosuppressive approach for steroid‐ refractory CIP. A multicenter‐randomized study of infliximab versus IVIG in steroid‐refractory CIP treatment is ongoing (NCT04438382).
IL‐6 receptor inhibitor. Tocilizumab is a humanized monoclonal antibody against IL‐6 receptors, which can block inflammatory cascade reaction and reduce the systemic inflammatory reaction and lung damage. Tocilizumab may be a therapeutic option, however, randomized trials are still needed to better elucidate the relative efficacy and safety. 73
Antifibrotic drugs. Antifibrotic drugs may be effective in patients with CIP. Nintedanib is a tyrosine kinase inhibitor, which can efficiently slow down the progression of idiopathic pulmonary fibrosis (IPF) and has an acceptable tolerability profile. 74 The pembrolizumab‐related pneumonitis was significantly improved after adding nintedanib in an advanced NSCLC patient. The patient did not obtain any clinical symptom relief with methylprednisolone alone. Pirfenidone is also an effective drug for the treatment of pulmonary fibrosis (PF). A clinical trial of pirfenidone combined with methylprednisolone versus methylprednisolone in the treatment of CIP is recruiting (NCT05280873).
Other immunosuppressors. Immunosuppressors such as mycophenolate mofetil and cyclophosphamide are also recommended in some practice guidelines. However, there is still a lack of study on patients treated with immunosuppressors, and this limits our ability to identify truly desirable immunosuppressive therapies.
We recommend corticosteroids as the first line treatment for most CIP. It is recommended to obtain additional details of the immunohistopathology whenever possible. Biopsy should at least be performed on the clinically affected end organ, which will provide information for subsequent targeted therapies. The immunopathogenic mechanisms should be studied in more detail as far as possible, including detailed immunohistochemistry of the affected end organ, peripheral blood flow cytometry, measurement of levels of autoantibodies and assessment of peripheral blood cytokines (such as IL‐1, IL‐6, TNF, and IL‐17). These data may enable us to make more accurate therapeutic decisions when selecting the optimal targeted therapies. 75 To develop biologically‐informed treatment for steroid‐resistant/resistant CIP, it is necessary to better understand the pathophysiology of CIP and recommend relevant clinical and basic trials.
CONSENSUS 9: THE PROGNOSIS OF CIP
In CIP patients, Suresh et al. 3 demonstrated that although ICIs did not significantly affect short‐term survival including disease control rate, overall response rate, and PFS, it would decrease the overall survival(OS) by 10 months. Another study reported that CIP increased PFS and OS, and 25% of patients continued to have no tumor growth after treatment discontinuation. 76 In real world studies, CIP is associated with poor prognosis. 3 , 9 On the other hand, the efficacy of ICI in patients with irAEs has improved. 77 The severe grade of CIP (≥grade 3) is associated with the reduction of PFS and OS. 10
AUTHOR CONTRIBUTIONS
Lu Si, Wenfeng Fang and Yong Song participated in the design of the expert consensus. Wenxian Wang, Qian Wang and Chunwei Xu conceived of the expert consensus, and participated in its design and other authors coordination and helped to draft the expert consensus. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest, financial interests, relationships and affiliations relevant to the subject of their manuscript.
ACKNOWLEDGMENT
The authors thank Dr. Karthik Suresh, Johns Hopkins University School of Medicine for his contribution to the previous research and participation in past symposiums.
Wang W, Wang Q, Xu C, Li Z, Song Z, Zhang Y, et al. Chinese expert consensus on the multidisciplinary management of pneumonitis associated with immune checkpoint inhibitor. Thorac Cancer. 2022;13(23):3420–3430. 10.1111/1759-7714.14693
Wenxian Wang, Qian Wang and Chunwei Xu, contributed equally to this study.
Contributor Information
Lu Si, Email: silu15_silu@126.com.
Wenfeng Fang, Email: fangwf@sysucc.org.cn.
Yong Song, Email: yong.song@nju.edu.cn.
REFERENCES
- 1. Reuss JE, Suresh K, Naidoo J. Checkpoint inhibitor pneumonitis: mechanisms, characteristics, management strategies, and beyond. Curr Oncol Rep. 2020;22(6):56. [DOI] [PubMed] [Google Scholar]
- 2. Zhai X, Zhang J, Tian Y, Li J, Jing W, Guo H, et al. The mechanism and risk factors for immune checkpoint inhibitor pneumonitis in non‐small cell lung cancer patients. Cancer Biol Med. 2020;17(3):599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Suresh K, Psoter KJ, Voong KR, Shankar B, Forde PM, Ettinger DS, et al. Impact of checkpoint inhibitor pneumonitis on survival in NSCLC patients receiving immune checkpoint immunotherapy. J Thorac Oncol. 2019;14(3):494–502. [DOI] [PubMed] [Google Scholar]
- 4. Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in patients treated with anti‐programmed death‐1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35(7):709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borghaei H, Paz‐Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med. 2015;373(17):1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khunger M, Jain P, Rakshit S, Pasupuleti V, Hernandez AV, Stevenson J, et al. Safety and efficacy of PD‐1/PD‐L1 inhibitors in treatment‐naive and chemotherapy‐refractory patients with non‐small‐cell lung cancer: a systematic review and meta‐analysis. Clin Lung Cancer. 2018;19(3):e335–48. [DOI] [PubMed] [Google Scholar]
- 8. Nishino M, Giobbie‐Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor‐related pneumonitis in patients with advanced cancer: a systematic review and meta‐analysis. JAMA Oncol. 2016;2(12):1607–16. [DOI] [PubMed] [Google Scholar]
- 9. Fukihara J, Sakamoto K, Koyama J, Ito T, Iwano S, Morise M, et al. Prognostic impact and risk factors of immune‐related pneumonitis in patients with non‐small‐cell lung cancer who received programmed death 1 inhibitors. Clin Lung Cancer. 2019;20(6):442–450.e444. [DOI] [PubMed] [Google Scholar]
- 10. Tone M, Izumo T, Awano N, Kuse N, Inomata M, Jo T, et al. High mortality and poor treatment efficacy of immune checkpoint inhibitors in patients with severe grade checkpoint inhibitor pneumonitis in non‐small cell lung cancer. Thorac Cancer. 2019;10(10):2006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen X, Zhang Z, Hou X, Zhang Y, Zhou T, Liu J, et al. Immune‐related pneumonitis associated with immune checkpoint inhibitors in lung cancer: a network meta‐analysis. J Immunother Cancer. 2020;8(2):e001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fu Y, Zheng Y, Wang PP, Ding ZY. Toxicities of immunotherapy for small cell lung cancer. Front Oncol. 2021;11:603658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nishio M, Hida T, Atagi S, Sakai H, Nakagawa K, Takahashi T, et al. Multicentre phase II study of nivolumab in Japanese patients with advanced or recurrent non‐squamous non‐small cell lung cancer. ESMO Open. 2017;2:e000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang W, Gu X, Wang L, Pu X, Feng H, Xu C, et al. The prognostic impact of mild and severe immune‐related adverse events in non‐small cell lung cancer treated with immune checkpoint inhibitors: a multicenter retrospective study. Cancer Immunol Immunother. 2022;71(7):1693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Velasco G, Je Y, Bossé D, Awad MM, Ott PA, Moreira RB, et al. Comprehensive meta‐analysis of key immune‐related adverse events from CTLA‐4 and PD‐1/PD‐L1 inhibitors in cancer patients. Cancer Immunol Res. 2017;5(4):312–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy‐naive patients with advanced non‐squamous non‐small‐cell lung cancer (CameL): a randomised, open‐label, multicentre, phase 3 trial. Lancet Respir Med. 2021;9(3):305–14. [DOI] [PubMed] [Google Scholar]
- 17. Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang S, et al. Efficacy and safety of sintilimab plus pemetrexed and platinum as first‐line treatment for locally advanced or metastatic nonsquamous NSCLC: a randomized, double‐blind, phase 3 study (Oncology pRogram by InnovENT anti‐PD‐1‐11). J Thorac Oncol. 2020;15(10):1636–46. [DOI] [PubMed] [Google Scholar]
- 18. Cui P, Liu Z, Wang G, Ma J, Qian Y, Zhang F, et al. Risk factors for pneumonitis in patients treated with anti‐programmed death‐1 therapy: a case‐control study. Cancer Med. 2018;7(8):4115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sul J, Blumenthal GM, Jiang X, He K, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of patients with metastatic non‐small cell lung cancer whose tumors express programmed death‐ligand 1. Oncologist. 2016;21(5):643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mark NM, Kargl J, Busch SE, Yang GHY, Metz HE, Zhang H, et al. Chronic obstructive pulmonary disease alters immune cell composition and immune checkpoint inhibitor efficacy in non‐small cell lung cancer. Am J Respir Crit Care Med. 2018;197(3):325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Galant‐Swafford J, Troesch A, Tran L, Weaver A, Doherty TA, Patel SP. Landscape of immune‐related pneumonitis in cancer patients with asthma being treated with immune checkpoint blockade. Oncology. 2020;98(2):123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reuss JE, Brigham E, Psoter KJ, Voong KR, Shankar B, Ettinger DS, et al. Pretreatment lung function and checkpoint inhibitor pneumonitis in NSCLC. JTO Clin Res Rep. 2021;2(10):100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Franzen D, Schad K, Kowalski B, Clarenbach CF, Stupp R, Dummer R, et al. Ipilimumab and early signs of pulmonary toxicity in patients with metastastic melanoma: a prospective observational study. Cancer Immunol Immunother. 2018;67(1):127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reuss J, Suresh K, Psoter K, Forde P, Naidoo J. P1.16‐06 early changes in pulmonary function are associated with development of pneumonitis in NSCLC patients receiving immune checkpoint blockade. J Thorac Oncol. 2019;14(10):S587–8. [Google Scholar]
- 25. Isono T, Kagiyama N, Takano K, Hosoda C, Nishida T, Kawate E, et al. Outcome and risk factor of immune‐related adverse events and pneumonitis in patients with advanced or postoperative recurrent non‐small cell lung cancer treated with immune checkpoint inhibitors. Thorac Cancer. 2021;12(2):153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kanai O, Kim YH, Demura Y, Kanai M, Ito T, Fujita K, et al. Efficacy and safety of nivolumab in non‐small cell lung cancer with preexisting interstitial lung disease. Thorac Cancer. 2018;9(7):847–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamaguchi T, Shimizu J, Hasegawa T, Horio Y, Inaba Y, Yatabe Y, et al. Pre‐existing pulmonary fibrosis is a risk factor for anti‐PD‐1‐related pneumonitis in patients with non‐small cell lung cancer: a retrospective analysis. Lung Cancer (Amsterdam, Netherlands). 2018;125:212–7. [DOI] [PubMed] [Google Scholar]
- 28. Su Q, Zhu EC, Wu JB, Li T, Hou YL, Wang DY, et al. Risk of pneumonitis and pneumonia associated with immune checkpoint inhibitors for solid tumors: a systematic review and meta‐analysis. Front Immunol. 2019;10:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oshima Y, Tanimoto T, Yuji K, Tojo A. EGFR‐TKI‐associated interstitial pneumonitis in nivolumab‐treated patients with non‐small cell lung cancer. JAMA Oncol. 2018;4(8):1112–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aiad M, Fresco K, Prenatt Z, Tahir A, Ramos‐Feliciano K, Stoltzfus J, et al. Comparison of pneumonitis rates and severity in patients with lung cancer treated by immunotherapy, radiotherapy, and immunoradiotherapy. Cureus. 2022;14(6):e25665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non‐small‐cell lung cancer. N Engl J Med. 2017;377(20):1919–29. [DOI] [PubMed] [Google Scholar]
- 32. Voong KR, Hazell S, Hu C, Hayman J, Hales R, Marrone K, et al. MA 09.08 receipt of chest radiation and immune‐related pneumonitis in patients with NSCLC treated with anti‐PD‐1/PD‐L1. J Thorac Oncol. 2017;12(11):S1837. [Google Scholar]
- 33. Sternschein R, Moll M, Ng J, D'Ambrosio C. Immune checkpoint inhibitor‐related pneumonitis. Incidence, risk factors, and clinical and radiographic features. Am J Respir Crit Care Med. 2018;198(7):951–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin X, Lu T, Li S, Xie X, Chen X, Jiang J, et al. Cytomegalovirus infection as an underestimated trigger for checkpoint inhibitor‐related pneumonitis in lung cancer: a retrospective study. Clin Transl Oncol. 2021;23(2):389–96. [DOI] [PubMed] [Google Scholar]
- 35. Moda M, Saito H, Kato T, Usui R, Kondo T, Nakahara Y, et al. Tumor invasion in the central airway is a risk factor for early‐onset checkpoint inhibitor pneumonitis in patients with non‐small cell lung cancer. Thorac Cancer. 2020;11(12):3576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang C, Gao F, Jin S, Gao W, Chen S, Guo R. Checkpoint inhibitor pneumonitis in Chinese lung cancer patients: clinical characteristics and risk factors. Ann Palliat Med. 2020;9(6):3957–65. [DOI] [PubMed] [Google Scholar]
- 37. Fujisawa Y, Yoshino K, Otsuka A, Funakoshi T, Fujimura T, Yamamoto Y, et al. Fluctuations in routine blood count might signal severe immune‐related adverse events in melanoma patients treated with nivolumab. J Dermatol Sci. 2017;88(2):225–31. [DOI] [PubMed] [Google Scholar]
- 38. Chu X, Zhao J, Zhou J, Zhou F, Jiang T, Jiang S, et al. Association of baseline peripheral‐blood eosinophil count with immune checkpoint inhibitor‐related pneumonitis and clinical outcomes in patients with non‐small cell lung cancer receiving immune checkpoint inhibitors. Lung Cancer (Amsterdam, Netherlands). 2020;150:76–82. [DOI] [PubMed] [Google Scholar]
- 39. Kim ST, Sheshadri A, Shannon V, Kontoyiannis DP, Kantarjian H, Garcia‐Manero G, et al. Distinct Immunophenotypes of T cells in bronchoalveolar lavage fluid from leukemia patients with immune checkpoint inhibitors‐related pulmonary complications. Front Immunol. 2020;11:590494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou J, Zhao J, Jia Q, Chu Q, Zhou F, Chu X, et al. Peripheral blood autoantibodies against to tumor‐associated antigen predict clinical outcome to immune checkpoint inhibitor‐based treatment in advanced non‐small cell lung cancer. Front Oncol. 2021;11:625578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tahir SA, Gao J, Miura Y, Blando J, Tidwell RSS, Zhao H, et al. Autoimmune antibodies correlate with immune checkpoint therapy‐induced toxicities. Proc Natl Acad Sci U S A. 2019;116(44):22246‐22251. [DOI] [PMC free article] [PubMed]
- 42. Naqash AR, Yang LV, Sanderlin EJ, Atwell DC, Walker PR. Interleukin‐6 as one of the potential mediators of immune‐related adverse events in non‐small cell lung cancer patients treated with immune checkpoint blockade: evidence from a case report. Acta Oncol (Stockholm, Sweden). 2018;57(5):705–8. [DOI] [PubMed] [Google Scholar]
- 43. Ke W, Zhang L, Dai Y. The role of IL‐6 in immunotherapy of non‐small cell lung cancer (NSCLC) with immune‐related adverse events (irAEs). Thorac Cancer. 2020;11(4):835–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kowalski B, Valaperti A, Bezel P, Steiner UC, Scholtze D, Wieser S, et al. Analysis of cytokines in serum and bronchoalveolar lavage fluid in patients with immune‐checkpoint inhibitor‐associated pneumonitis: a cross‐sectional case‐control study. J Cancer Res Clin Oncol. 2022;148(7):1711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hirashima T, Kanai T, Suzuki H, Yoshida H, Matsushita A, Kawasumi H, et al. The levels of interferon‐gamma release as a biomarker for non‐small‐cell lung cancer patients receiving immune checkpoint inhibitors. Anticancer Res. 2019;39(11):6231–40. [DOI] [PubMed] [Google Scholar]
- 46. Wang YN, Lou DF, Li DY, Jiang W, Dong JY, Gao W, et al. Elevated levels of IL‐17A and IL‐35 in plasma and bronchoalveolar lavage fluid are associated with checkpoint inhibitor pneumonitis in patients with non‐small cell lung cancer. Oncol Lett. 2020;20(1):611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lin X, Deng H, Yang Y, Wu J, Qiu G, Li S, et al. Peripheral blood biomarkers for early diagnosis, severity, and prognosis of checkpoint inhibitor‐related pneumonitis in patients with lung cancer. Front Oncol. 2021;11:698832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Naidoo J, Zhang J, Lipson EJ, Forde PM, Suresh K, Moseley KF, et al. A multidisciplinary toxicity team for cancer immunotherapy‐related adverse events. J Natl Compr Canc Netw. 2019;17(6):712–20. [DOI] [PubMed] [Google Scholar]
- 49. Londono MC, Reig M, Group RM. Multidisciplinary clinical approach to cancer patients with immune‐related adverse events induced by checkpoint inhibitors. Cancers (Basel). 2020;12(11):3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhou C, Yang Y, Lin X, Fang N, Chen L, Jiang J, et al. Proposed clinical phases for the improvement of personalized treatment of checkpoint inhibitor‐related pneumonitis. Front Immunol. 2022;13:935779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Naidoo J, Cottrell TR, Lipson EJ, Forde PM, Illei PB, Yarmus LB, et al. Chronic immune checkpoint inhibitor pneumonitis. J Immunother Cancer. 2020;8(1):e000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huang A, Xu Y, Zang X, Wu C, Gao J, Sun X, et al. Radiographic features and prognosis of early‐ and late‐onset non‐small cell lung cancer immune checkpoint inhibitor‐related pneumonitis. BMC Cancer. 2021;21(1):634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nishino M, Ramaiya NH, Awad MM, Sholl LM, Maattala JA, Taibi M, et al. PD‐1 inhibitor‐related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res. 2016;22(24):6051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nishino M, Hatabu H. Programmed death‐1/programmed death ligand‐1 inhibitor‐related pneumonitis and radiographic patterns. J Clin Oncol. 2017;35(14):1628–9. [DOI] [PubMed] [Google Scholar]
- 55. Tirumani SH, Ramaiya NH, Keraliya A, Bailey ND, Ott PA, Hodi FS, et al. Radiographic profiling of immune‐related adverse events in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res. 2015;3(10):1185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Suresh K, Voong KR, Shankar B, Forde PM, Ettinger DS, Marrone KA, et al. Pneumonitis in non‐small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol. 2018;13(12):1930–9. [DOI] [PubMed] [Google Scholar]
- 57. Nishiyama O, Shimizu S, Haratani K, Isomoto K, Tanizaki J, Hayashi H, et al. Clinical implications of bronchoscopy for immune checkpoint inhibitor‐related pneumonitis in patients with non‐small cell lung cancer. BMC Pulm Med. 2021;21(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Larsen BT, Chae JM, Dixit AS, Hartman TE, Peikert T, Roden AC. Clinical and histopathologic features of immune checkpoint inhibitor‐related pneumonitis. Am J Surg Pathol. 2019;43(10):1331–40. [DOI] [PubMed] [Google Scholar]
- 59. Sun Y, Shao C, Li S, Xu Y, Xu K, Zhang Y, et al. Programmed cell death 1 (PD‐1)/PD‐ligand 1(PD‐L1) inhibitors‐related pneumonitis in patients with advanced non‐small cell lung cancer. Asia Pac J Clin Oncol. 2020;16(6):299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, et al. Toxicities of the anti‐PD‐1 and anti‐PD‐L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Naidoo J, Nishino M, Patel SP, Shankar B, Rekhtman N, Illei P, et al. Immune‐related pneumonitis after chemoradiotherapy and subsequent immune checkpoint blockade in unresectable stage III non‐small‐cell lung cancer. Clin Lung Cancer. 2020;21(5):e435–44. [DOI] [PubMed] [Google Scholar]
- 62. Teng F, Li M, Yu J. Radiation recall pneumonitis induced by PD‐1/PD‐L1 blockades: mechanisms and therapeutic implications. BMC Med. 2020;18(1):275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chen X, Sheikh K, Nakajima E, Lin CT, Lee J, Hu C, et al. Radiation versus immune checkpoint inhibitor associated pneumonitis: distinct radiologic morphologies. Oncologist. 2021;26(10):e1822–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chen X, Sheikh K, Lin CT, Lee J, Hales RK, Naidoo J, et al. CT radiomics and machine learning for distinguishing radiotherapy vs. immune checkpoint inhibitor‐induced pneumonitis in non‐small cell lung cancer patients. Int J Rad Oncol Biol Phys. 2020;108(3):S163. [Google Scholar]
- 65. Thompson J, Schneider BJ, Brahmer JR, Achufusi A, Armand P, Berkenstock M, et al.Management of Immunotherapy‐Related Toxicities,Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Ne. 2022; 20(4):387‐405. [DOI] [PubMed]
- 66. Lin X, Deng H, Chen L, Wu D, Chen X, Yang Y, et al. Clinical types of checkpoint inhibitor‐related pneumonitis in lung cancer patients: a multicenter experience. Transl Lung Cancer Res. 2021;10(1):415–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pollack MH, Betof A, Dearden H, Rapazzo K, Valentine I, Brohl AS, et al. Safety of resuming anti‐PD‐1 in patients with immune‐related adverse events (irAEs) during combined anti‐CTLA‐4 and anti‐PD1 in metastatic melanoma. Ann Oncol. 2018;29(1):250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Suresh K, Naidoo J, Lin CT, Danoff S. Immune checkpoint immunotherapy for non‐small cell lung cancer: benefits and pulmonary toxicities. Chest. 2018;154(6):1416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Luo J, Beattie JA, Fuentes P, Rizvi H, Egger JV, Kern JA, et al. Beyond steroids: immunosuppressants in steroid‐refractory/resistant immune related adverse events. J Thorac Oncol. 2021;16:1759–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Beattie J, Rizvi H, Fuentes P, Luo J, Schoenfeld A, Lin IH, et al. Success and failure of additional immune modulators in steroid‐refractory/resistant pneumonitis related to immune checkpoint blockade. J Immunother Cancer. 2021;9(2):e001884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Balaji A, Hsu M, Lin CT, Feliciano J, Marrone K, Brahmer JR, et al. Steroid‐refractory PD‐(L)1 pneumonitis: incidence, clinical features, treatment, and outcomes. J Immunother Cancer. 2021;9(1):e001731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sears CR, Peikert T, Possick JD, Naidoo J, Nishino M, Patel SP, et al. Knowledge gaps and research priorities in immune checkpoint inhibitor–related pneumonitis. An official American thoracic society research statement. Am J Respir Crit Care Med. 2019;200(6):e31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stroud CR, Hegde A, Cherry C, Naqash AR, Sharma N, Addepalli S, et al. Tocilizumab for the management of immune mediated adverse events secondary to PD‐1 blockade. J Oncol Pharm Pract. 2019;25(3):551–7. [DOI] [PubMed] [Google Scholar]
- 74. Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–82. [DOI] [PubMed] [Google Scholar]
- 75. Esfahani K, Elkrief A, Calabrese C, Lapointe R, Hudson M, Routy B, et al. Moving towards personalized treatments of immune‐related adverse events. Nat Rev Clin Oncol. 2020;17(8):504–15. [DOI] [PubMed] [Google Scholar]
- 76. Ono K, Ono H, Toi Y, Sugisaka J, Aso M, Saito R, et al. Association of immune‐related pneumonitis with clinical benefit of anti‐programmed cell death‐1 monotherapy in advanced non‐small cell lung cancer. Cancer Med. 2021;10(14):4796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Grangeon M, Tomasini P, Chaleat S, Jeanson A, Souquet‐Bressand M, Khobta N, et al. Association between immune‐related adverse events and efficacy of immune checkpoint inhibitors in non‐small‐cell lung cancer. Clin Lung Cancer. 2019;20(3):201–7. [DOI] [PubMed] [Google Scholar]


