Abstract
Due to concerns regarding neurohormonal activation and fluid retention, adrenergic alpha‐1 receptor antagonists (A1Bs) are generally avoided in the setting of heart disease, namely, symptomatic heart failure (HF) with reduced ejection fraction (HFrEF). However, this contraindication is mainly supported by ancient studies, having recently been challenged by newer ones. We aim to perform a comprehensive meta‐analysis aimed at ascertaining the extent to which A1Bs might influence cardiovascular (CV) outcomes. We systematically searched PubMed, Cochrane Central Register of Controlled Trials and Web of Science for both prospective and retrospective studies, published until 1 December 2020, addressing the impact of A1Bs on both clinical outcomes—namely, acute heart failure (AHF), acute coronary syndrome (ACS), CV and all‐cause mortality—and on CV surrogate measures, specifically left ventricular ejection fraction (LVEF) and exercise tolerance, by means of exercise duration. Both randomized controlled trials (RCTs) and studies including only HF patients were further investigated separately. Study‐specific odds ratios (ORs) and mean differences (MDs) were pooled using traditional meta‐analytic techniques, under a random‐effects model. A record was registered in PROSPERO database, with the code number CRD42020181804. Fifteen RCTs, three non‐randomized prospective and two retrospective studies, encompassing 32 851, 19 287, and 71 600 patients, respectively, were deemed eligible; 62 256 patients were allocated to A1B, on the basis of multiple clinical indications: chronic HF itself [14 studies, with 72 558 patients, including seven studies with 850 HFrEF or HF with mildly reduced ejection fraction (HFmrEF) patients], arterial hypertension (four studies, with 44 184 patients) and low urinary tract symptoms (two studies, with 6996 patients). There were 25 998 AHF events, 1325 ACS episodes, 955 CV deaths and 33 567 all‐cause deaths. When considering only RCTs, A1Bs were, indeed, found to increase AHF risk (OR 1.78, [1.46, 2.16] 95% CI, P < 0.00001, i 2 2%), although displaying no significant effect on neither ACS nor CV or all‐cause mortality rates (OR 1.02, [0.91, 1.15] 95% CI, i 2 0%; OR 0.95, [0.47, 1.91] 95% CI, i 2 17%; OR 1.1, [0.84, 1.43] 95% CI, i 2 17%, respectively). Besides, when only HF patients were evaluated, A1Bs revealed themselves neutral towards not only ACS, CV, and all‐cause mortality events (OR 0.49, [0.1, 2.47] 95% CI, i 2 0%; OR 0.7, [0.21, 2.31] 95% CI, i 2 21%; OR 1.09, [0.53, 2.23] 95% CI, i 2 17%, respectively), but also AHF (OR 1.13, [0.66, 1.92] 95% CI, i 2 0%). As for HFrEF and HFmrEF, A1Bs were found to exert a similarly inconsequential effect on AHF rates (OR 1.01, [0.5–2.05] 95% CI, i 2 6%). Likewise, LVEF was not significantly influenced by A1Bs (MD 1.66, [−2.18, 5.50] 95% CI, i 2 58%). Most strikingly, exercise tolerance was higher in those under this drug class (MD 139.16, [65.52, 212.8] 95% CI, P < 0.001, i 2 26%). A1Bs do not seem to exert a negative influence on the prognosis of HF—and even of HFrEF—patients, thus contradicting currently held views. These drugs' impact on other major CV outcomes also appear trivial and they may even increment exercise tolerance.
Keywords: Adrenergic alpha‐antagonists, Heart failure, Acute coronary syndrome, Mortality, Left ventricular ejection fraction, Exercise tolerance
Introduction
Heart failure (HF) affects 64.34 million people worldwide. 1 Its clinical presentation may be classified as either acute or chronic. Acute HF (AHF) is the most common cause of hospital admission and its in‐hospital mortality varies between 4% and 7%. 2 It may represent a decompensation of chronic HF (CHF) or an abrupt development of the syndrome de novo. 3
Adrenergic alpha‐1 receptor antagonists (A1Bs) constitute a pharmacologic class that may be used, in the cardiovascular field, in patients with resistant arterial hypertension (AHT), because they induce arterial vasodilation and, thus, decrease systemic vascular resistance. 4 Resistant AHT may be defined as the inability to reach the arterial pressure goal despite the use of three antihypertensive drug classes, one being a diuretic, and is an important phenomenon, because its prevalence varies between 12 and 15%. 5 On the other hand, A1Bs were demonstrated to most strikingly inhibit the sympathetic tone at the bladder outlet, having established themselves as the first‐line treatment in patients with lower urinary tract symptoms (LUTS). 6 These are exceedingly common, affecting 50% of men aged 50 years or more and 80% aged 70 years or more, with benign prostatic hyperplasia serving as its most frequent underlying condition. 7
Given that, in HF, and, particularly, in heart failure with reduced ejection fraction (HFrEF), there is typically overactivation of the sympathetic nervous system, 8 there was once great interest in employing A1Bs in its treatment. 9 However, a number of trials 10 , 11 , 12 went on to reveal a higher—not lesser—risk of HF decompensation in patients treated with A1Bs. This was then attributed to tolerance in vascular alpha‐adrenergic‐blockade effects and renin‐angiotensin‐aldosterone system (RAAS) activation. 13 Of those studies, much attention was given to the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). 10 In fact, ever since the discontinuation of the doxazosin arm in this trial due to an increase in the risk of congestive HF—as compared with the one seen in the chlorthalidone group—questions have been raised about the safety of A1Bs. Indeed, this drug class was even explicitly contraindicated (class of recommendation III)—with a level of evidence judged to be as high as A—as an antihypertensive option in patients with HFrEF, as per the 2016 European Society of Cardiology (ESC) guidelines for the management of HF. 14 Nevertheless, newer studies have since disproved such claim. In particular, in 2018, a large cohort study 15 found that treatment of CHF patients with A1Bs was associated not only with a reduction in hospital readmission due to HF, but also with lower all‐cause mortality. Interestingly, the 2021 ESC guidelines for the management of HF 3 removed the aforementioned contraindication, only to assert that, in hypertensive HF patients, A1Bs ‘have no effects on survival and are, therefore, not indicated’. Besides, its authors acknowledged, for the first time, that, even in this perilous setting, ‘they can be used for the treatment of concomitant prostatic hyperplasia’.
On the whole, it seems that the impact of A1Bs in the natural history of HF is still insufficiently known. As such, our goal is to systematically review and meta‐analyse published literature on the effects of A1Bs on cardiovascular (CV) outcomes, attributing a particular emphasis to AHF events.
Methods
Protocol and registration
This systematic review, encompassing meta‐analytic efforts, was developed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) criteria. 16
A record was submitted to the PROSPERO database, having been successfully registered (CRD42020181804). It is available at https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020181804.
Literature search
We systematically searched PubMed, Cochrane Central Register of Controlled Trials and Web of Science Core Collection, from inception until 1 December 2020, for both interventional and observational studies appraising the effect of A1Bs on major clinical CV outcomes, namely, on AHF. The search was limited by: (i) article type, in PubMed (‘Clinical Study’, ‘Clinical Trial’, ‘Clinical Trial Protocol’, ‘Clinical Trial, Phase I’, ‘Clinical Trial, Phase II’, ‘Clinical Trial, Phase III’, ‘Clinical Trial, Phase IV’, ‘Comparative Study’, ‘Controlled Clinical Trial’, ‘Journal Article’, ‘Letter’, ‘Multicenter Study’, ‘Observational Study’, ‘Pragmatic Clinical Trial’ and ‘Randomized Controlled Trial’) and in Web of Science Core Collection (‘Article’, ‘Proceedings Paper’, ‘Letter’ and ‘Early Access’); (ii) species (‘Humans’), in PubMed; and (iii) language (English, Spanish, Portuguese). Medical Subject Headings (MeSH) terms were used in PubMed. Appendix I presents the search equation and strategy used in this study. Different publications with the same patient sample were considered as a single study.
Eligibility criteria
In order to be eligible for our review, full‐text articles were required to: (i) encompass patients with an indication for A1B treatment, specifically as far as CHF, AHT and LUTS are concerned; (ii) be randomized (RCTs), non‐randomized prospective (either interventional or observational) or retrospective, in nature; (iii) compare A1Bs with no treatment or control, either inactive or active; (iv) report AHF episodes, mortality (either CV or all‐cause), acute coronary syndrome (ACS) events, left ventricular ejection fraction (LVEF) dynamics or exercise tolerance—assessed via exercise duration (ED)—modification. Studies featuring beta‐blockers with concomitant alpha‐antagonist properties, like carvedilol or labetalol, either in the experimental or in the control arm, and studies with less than a four‐week follow‐up period were not considered.
Primary and secondary outcomes
The primary endpoint was AHF, whereas secondary outcomes included not only so‐called hard events, namely, all‐cause mortality, CV‐related mortality and ACS, but also LVEF variation and exercise tolerance—by means of ED—change.
Data collection and management
Two authors (JPS and DM) first systematically screened both title and abstract of the studies obtained from the literature search, with the aim of identifying publications fulfilling all aforementioned eligibility criteria. The full‐text of these were, then, independently examined, by the same authors, in order to further confirm the appropriateness of their inclusion in the present review. Any discrepancies between the two review team members were resolved through discussion and, whenever judged necessary, via the opinion of another author (RT). Data extraction focused on baseline demographic and clinical variables, the employed interventions, and the previously outlined primary and secondary outcomes. Studies with sequential publications were rigorously accessed to ensure no result duplication and the gathering of the most up‐to‐date and/or complete information.
A few observations should be made, in this setting: (i) Chapple and co‐workers 17 presented distinct controlled data for three types of tamsulosin formulations: oral controlled absorption system (OCAS), at a dose of either 0.4 mg or 0.8 mg, and modified release formulation (MR), at a dose of 0.4 mg. To increase statistical power, we combined the dichotomous data related to these three formulations; (ii) in the Combination of Avodart® and Tamsulosin (CombAT) trial, Roehrborn and co‐authors 18 reported on patients being treated for symptomatic benign prostatic hyperplasia with tamsulosin, dutasteride or both drugs combined. In this case, we merged the dichotomous data related to tamsulosin monotherapy and the therapeutic association, for the same reason; (iii) in the Veterans Administration Cooperative Vasodilator‐Heart Failure Trial (V‐HeFT I), Cohn and colleagues 9 also described three therapeutic arms: prazosin, the association of hydralazine and isosorbide dinitrate, and placebo. As for this scenario, we added the dichotomous data linked with hydralazine/isosorbide dinitrate and placebo, placing them under the control group; (iv) lastly, in a study with factorial design, authored by Faconti et al. (2019), 19 some of the baseline characteristics of the doxazosin and the spironolactone groups were presented as per active or placebo juice intake. As such, whenever possible, we added these dichotomous data to form our own pool of A1B and non‐A1B groups.
We encompassed, as an AHF event, both decompensation of previously known HF and de novo AHF, including any mortality resulting from them; all‐cause mortality covered all reported deaths, for whatever reason; CV mortality enclosed deaths caused by ACS, stroke, HF, arrhythmia and ‘sudden’ phenomena, as well as ‘other CV’ causes, as described in the different studies; lastly, an ACS event included fatal or non‐fatal myocardial infarction or unstable angina.
Risk of bias assessment
JPS and DM independently evaluated the quality of the studies included in this review. Observational ones were assessed using the Newcastle‐Ottawa Scale (NOS), 20 which comprises three domains: selection, comparability and outcome. As for non‐randomized clinical trials, their risk of bias was evaluated employing a simplified version of Cochrane's ‘Risk Of Bias In Non‐randomized Studies – of Intervention’ tool (ROBINS‐I), 21 focusing on another three main areas: confounding, selection bias and information bias. The latter was assessed on the grounds of recall and detection biases, aside from reporting bias. ‘Yes’, ‘probably yes’, ‘probably no’, ‘no’ and ‘no information’ were the possible classifiers. On the other hand, as far as RCTs are concerned, their quality was gauged using the Cochrane Collaboration's ‘Risk of Bias (RoB) 2’ tool, 22 which targets random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and ‘other biases’. In this case, a ‘low risk’, ‘high risk’ or ‘unclear risk’ verdict was to be attributed to each particular publication. This way, depending on its methodology, each study's risk of bias assessment may be presented via the NOS summary (Fig. 1 A ), a simplified ROBINS‐I summary (Fig. 1 B ) or the RoB summary (Fig. 1 C ). The latter was graphically represented through Cochrane's Risk‐Of‐Bias VISualization (robvis) tool. 23
Figure 1.
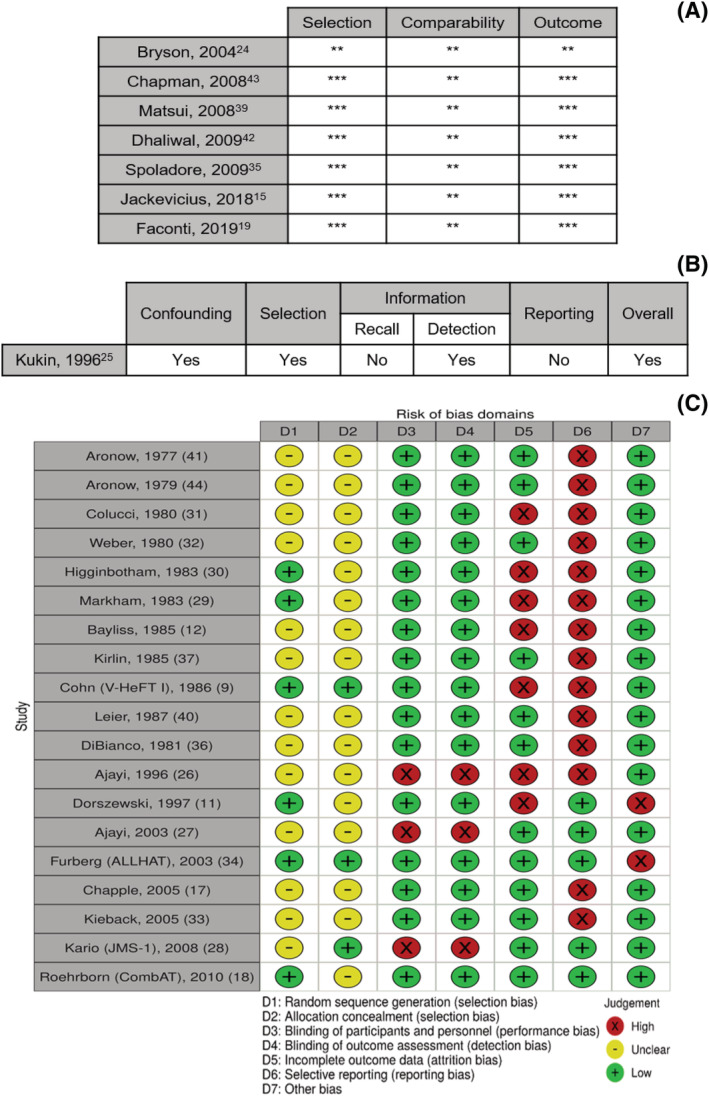
Risk of bias summary. (A) Newcastle‐Ottawa Scale; (B) Simplified version of the Cochrane Collaboration's ‘Risk Of Bias In Non‐randomized Studies – of Intervention’ tool; (C) Cochrane Collaboration's ‘Risk of Bias’ tool, displayed via Cochrane's Risk‐Of‐Bias VISualization (robvis).
In observational studies, and far as selection is concerned, there was plenty of bias possibilities, because all studies were judged as lacking population representativeness. Moreover, in one study, 24 exposure ascertainment was based on self‐reporting. On the contrary, comparability appears not to be a major issue, because the majority of the results were adjusted for a multitude of variables, including, for example, age, sex and systolic blood pressure. Regarding the outcome domain, bias is thought to be present in the study by Bryson and co‐workers, 24 because endpoint assessment was also performed via patient reporting, in a direct way.
In the only non‐randomized clinical trial, 25 confounding was thought to be significant, as the non‐A1B group (metoprolol) showed, namely, worse hemodynamic indexes at baseline. In addition, there also seemed to be selection bias, as, for instance, four patients in the active group (metoprolol + doxazosin) went on to receive only half the regimen dose, due to intolerance. The potential for detection bias was also recognized, because the study was unblinded.
Most RCTs showed an unclear risk of selection bias, because their authors frequently did not describe how random sequence generation and allocation concealment were processed. When evaluating performance bias, most studies revealed themselves to be at low risk, on the account of displaying a double‐blind design, carefully providing identical pills for both experimental and control groups. However, that was not the case for three studies: Two 26 , 27 disclosed a single‐blind design and one 28 lacked blinding altogether. It should also be noted that, in the study by Kario and co‐workers—the Japan Morning Surge‐1 (JMS‐1) trial 28 —blood pressure was self‐monitored by patients. As for detection bias, and with the exception of the three latter studies, 26 , 27 , 28 it was also generally avoided, and for the same reason: the employment of a double blinding strategy. Importantly, four studies 11 , 26 , 29 , 30 were analysed following per‐protocol principles and five 9 , 11 , 15 , 31 , 32 suffered from a significant percentage of withdrawals, some of which even possibly related with the outcomes of interest of this meta‐analysis. These facts likely disclose high risk of attrition bias. Of note, one other study 33 did manage to report dropouts but did not clarify their cause, this way conceding unclear risk in this domain. In parallel, reporting bias was also an issue, because some RCTs failed to report relevant quantitative data, such as statistics that would have permitted estimation of mean differences (MDs), standard deviations (SDs) or p‐values. As for ‘other biases’, it is important to state that two studies 11 , 34 were prematurely terminated. Furthermore, a cross‐over clinical trial 12 did not report results at the exact time of cross‐over.
Statistical analysis
We pooled dichotomous data under the form of odds ratios (ORs) and continuous data through MDs. These were meta‐analysed with the Mantel–Haenszel and the inverse‐variance methods, respectively, under a random‐effects model. As for the MDs, it should be stated that a fair number of studies 9 , 29 , 31 , 35 , 36 , 37 reported the continuous variables of interest only at baseline and at the end, therefore allowing the derivation of the MD but without associating it with a SD. Even though some SDs were, whenever possible, manually calculated, this scenario frequently precluded study incorporation into the quantitative synthesis. Study heterogeneity was accessed by the i 2 statistic—which was deemed excessive if overtaking 50%—whereas publication bias was graphically explored, via funnel plots. A minimum of three studies were deemed needed for meta‐analytic exploration. Both RCTs and studies including only HF patients were to be further investigated separately.
Significance was set at p‐value <0.05 and 95% confidence intervals (CIs) not including one for dichotomous variables and zero for continuous ones.
Statistical analysis was performed using Cochrane Review Manager, version 5.4.1. 38
Results
Search results
Literature review, using the search equation and strategy presented in Appendix I , yielded 1030 articles, with the JMS‐1 trial 28 —from which the study by Matsui et al. (2008) 39 derived its sample—added after manual research. After dismissing 259 duplicate results, 689 articles were then excluded through title and abstract screening, as well as study type. The eligibility of the remaining 83 studies was further evaluated via full‐text analysis, which led to rejection of 56 other records: 15 were trial sub‐analyses (12 from ALLHAT, 34 one from V‐HeFT I, 9 and two from the study by Bayliss and co‐workers 12 ); ALLHAT, 34 V‐HeFT I, 9 Leier et al., 40 and Aronow et al. 41 were picked between two articles encompassing the exact same population (for each study); 16 papers did not present available full‐texts; seven were conference meeting abstracts; in one study, both the active and the control groups were under A1B therapy; and 13 articles did not report relevant or comparable outcomes. This way, 27 records were finally assessed as fulfilling the criteria for inclusion in the qualitative synthesis. Their main characteristics are summarized in Table 1 . However, from these, one article 24 presented subgroups only in relative terms (percentage); two publications 33 , 42 failed at quantitatively stratifying by group allocation; one paper 12 corresponded to a cross‐over trial that did not report outcomes at the exact cross‐over period; one record 27 focused on New York Heart Association (NYHA) change; one study 19 described LVEF dynamics through least square means difference; and, in one article, 37 SDs of the MDs of LVEF and ED could not be obtained. These features rendered them unemployable in the quantitative synthesis. Therefore, only 20 records were finally engaged in this setting. From these, 15 were RCTs and encompassed 32 851 patients; three were non‐randomized prospective studies, featuring 19 287 participants; and two were retrospective in nature, and included a total of 71 600 patients; 62 256 patients were treated with A1Bs, which were introduced for three main clinical indications: CHF, AHT and LUTS. Fourteen studies (11 RCTs, one non‐randomized prospective and two retrospective) assembled 72 558 CHF patients. From these articles, seven (six RCTs and one non‐randomized prospective), accounting for 850 participants, focused only on HFrEF and HF with mildly reduced ejection fraction (HFmrEF). On the other hand, in four studies (two RCTs and two non‐randomized prospective), involving 44 184 patients, it was AHT that served as the main indication for treatment initiation. Lastly, the two remaining studies (both RCTs) encompassed 6996 patients with LUTS (Fig. 2 ). Of note, the Matsui et al. study 39 represented a sub‐analysis of the JMS‐1 trial, 28 further assessing the experiment effect on left ventricular structure and function, hence its inclusion in the quantitative synthesis only in the setting of LVEF change evaluation.
Table 1.
Main characteristics of the 27 studies included in the qualitative synthesis
| Study | Design | Indication (LVEF if HF, μ ± σ if available) | A1B group | Non‐A1B group | Number of patients | Age (years, μ ± σ) | Sex (males) | Follow‐up (months) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A1B | Non‐A1B | A1B | Non‐ A1B | A1B | Non‐A1B | ||||||
| Studies included in the quantitative synthesis | |||||||||||
| Jackevicius, C 15 2018 | Retrospective cohort | CHF (42.0 ± 15.5%) | A1B‐treated | A1B‐untreated | 35 713 | 35 713 | 74.6 ± 10.1 | 74.7 ± 10.3 | 35 651 | 35 660 |
24 30.1 |
| Roehrborn, C 18 2010 (CombAT) | RCT | LUTS | Tamsulosin + Tamsulosin and Dutasteride | Dutasteride | 3221 | 1623 | (a) | 66.0 ± 6.99 | 1611 | 1623 | 48 |
| Spoladore, R 35 2009 | Retrospective cohort | CHF (37 ± 9%) | Doxazosin | Non‐doxazosin | 52 | 122 | 69.3 ± 10.1 | 67.1 ± 9.1 | 33 | 91 |
50 (Dox) 41 (Non‐Dox) 16 |
| Chapman, N 43 2008 | RCT sub‐analysis | AHT | Doxazosin | Non‐doxazosin | 11 768 | 7489 | 62.7 ± 8.5 | 62.2 ± 8.5 | 9254 | 5488 |
66 66 |
| Kario, K 28 2008 (JMS‐1) | RCT | AHT | Doxazosin | Non‐doxazosin | 308 | 303 | 70.2 ± 9.2 | 70.1 ± 10.0 | 45.1% | 43.6% |
6 |
| Matsui, Y 39 2008 | RCT sub‐analysis | AHT | Doxazosin | Non‐doxazosin | 112 | 111 | 70.1 ± 10.4 | 70.4 ± 11.2 | 46.4% | 45.0% |
6 6 |
| Chapple, C 17 2005 | RCT | LUTS | Tamsulosin | Placebo | 1795 | 357 | (b) | 1795 | 357 |
2.76 2.8 |
|
| Furberg, C 34 2003 (ALLHAT) | RCT | AHT | Doxazosin | Chlortalidone | 9061 | 15 255 | 66.8 ± 7.7 | 66.9 ± 7.7 | 4858 | 8084 |
48 48 |
| Dorszewski, A 11 1997 | RCT | CHF (LVEF <35%) | Urapidil | Placebo | 18 (c) | 18 (c) | 55.7 ± 2.4 | 55.3 ± 3.3 | 17 | 16 |
2.76 2.8 |
| Ajayi, A 26 1996 | RCT | CHF (Not reported) | Prazosin and Enalapril | Placebo and Enalapril | 24 (d) | (d) | (d) |
0.92 0.9 |
|||
| Kukin, M 25 1996 | Non‐randomized controlled trial | CHF (μ = 12%, Range 7–23%) | Doxazosin and Metoprolol | Metoprolol | 16 (e) | 14 (e) | μ = 50, Range 29–76 | 25 |
3 3 |
||
| DiBianco, R 36 1991 | RCT | CHF (26.9 ± 11.8%) | Doxazosin | Placebo | 36 | 37 | 59.7 | 60.0 | 33 | 32 |
3 2.8 |
| Leier, C 40 1987 | RCT | CHF (Not reported) | Indoramin | Placebo | 11 | 10 | 55 ± 10 | 59 ± 6 | 6 | 6 |
2 2 |
| Cohn, J 9 1986 (V‐HeFT I) | RCT | CHF (μ = 29.9%) | Prazosin | Hydralazine and Isosorbide Dinitrate + Placebo | 183 | 459 | 58.3 | (f) | 183 | 459 |
Up to 68.4 68.4 |
| Higginbotham, M 30 1983 | RCT | CHF (25 ± 8%) | Prazosin | Placebo | 11 (g) | 11 (g) | 25 to 68 | 11 | 11 |
6 6 |
|
| Markham, R 29 1983 | RCT | CHF (23 ± 9%) | Prazosin | Placebo | 13 (h) | 12 (h) |
(h) (l) |
8 | 6 |
6 6 |
|
| Colucci, W 31 1980 | RCT | CHF (32.6 ± 3.9%) | Prazosin | Placebo | 10 | 12 | 59 ± 9.5 | 58 ± 10.4 | 7 | 10 |
2 2 |
| Weber, K 32 1980 | RCT | CHF (Not reported) | Trimazosin | Placebo | 10 (i) | 13 (i) | (i) | (i) | 1.38 | ||
| Aronow, W 44 1979 | RCT | CHF (44.5 ± 9.4%) | Prazosin | Placebo | 12 | 12 | Range 26–67 | 12 | 12 | 1.38 | |
| Aronow, W 41 1977 | RCT | CHF (Not reported) | Trimazosin | Placebo | 8 | 8 | Range 41–66 | 8 | 8 | 1.38 | |
| Additional studies included only in the qualitative synthesis | |||||||||||
| Faconti, L 19 2019 | RCT sub‐analysis | AHT | Doxazosin | Spironolactone | 43 | 44 | (j) | 32 | 28 |
6 6 |
|
| Dhaliwal, A 42 2009 | Prospective cohort | CHF (26 ± 13%) | A1B‐treated | A1B‐untreated | 98 | 290 | 73 ± 8 | 67 ± 11 | 100% | 99% |
Up to 37.42 10.0 |
| Kieback, A 33 2005 | RCT | CHF (Not reported) | Doxazosin (4 mg + 8 mg) | Placebo | 15 | 15 | (k) | 63.6 ± 7.7 | 11 | 11 |
2.76 2.8 |
| Bryson, C 24 2004 | Prospective cohort | The elderly | A1B‐treated | A1B‐untreated | (l) ***** | (l) | (l) | Up to 137.96 | |||
| Ajayi, A 27 2003 | RCT | CHF (38 ± 10%) | Prazosin and Enalapril | Atenolol and Enalapril + Enalapril | 8 | 20 | Range 50–56 | 3 | 11 | 0.92 | |
| Bayliss, J 12 1985 | Cross‐over RCT | CHF (Not reported) | Prazosin | Captopril | 19 (m) | μ = 62 Range 48–74 | 18 | 2 (cross‐over at 1 month) | |||
| Kirlin, P 37 1985 | RCT | CHF (22.5 ± 8.0%) | Trimazosin | Placebo | 9 | 8 | 49 ± 9 | 51 ± 11 | Not reported |
6 6 |
|
MR, modified release formulation.
The last seven were not featured in the quantitative one. A1B, adrenergic alpha‐1 receptor antagonist; AHT, arterial hypertension; CHF, chronic heart failure; DMII, diabetes mellitus type II; Dox, doxazosin; ED, exercise duration; LUTS, lower urinary tract symptoms; LVEF, left ventricular ejection fraction; MR, modified release; OCAS, oral controlled absorption system; RCT, randomized controlled trial.
(a) The tamsulosin monotherapy group age was 66.2 ± 7.00 years, whereas the tamsulosin and doxazosin association group age was 66.0 ± 7.05 years.
(b) The tamsulosin 0.4 mg OCAS group age was 64.7 ± 8.3 years (360 patients); the tamsulosin 0.8 mg OCAS group age was 64.6 ± 8.1 years (722 patients); The tamsulosin 0.4 mg MR group age was 64.7 ± 8.3 years (709 patients); the placebo group age was 64.9 ± 7.9 years (356 patients). These data are related to the ones who received at least one dose of medication and reported post‐baseline safety information.
(c) 36 patients were randomized, but LVEF data were available in only 29 (13 in the urapidil group and 16 in the placebo group).
(d) 24 patients were randomized, but age (49 ± 15 years and 53 ± 9 years, respectively) and ED data were available in only 17 [10 (seven men) in the prazosin plus enalapril group and seven (five men) in the placebo plus enalapril group].
(e) 30 patients entered the study, but LVEF data was available in only 26 (15 in the doxazosin + metoprolol group and 11 in the metoprolol group).
(f) The mean hydralazine and isosorbide dinitrate group age was 58.3 years (186 patients), whereas the mean placebo group age was 58.5 years (273 patients).
(g) 22 patients were randomized, but ED data was available in only 18 (nine in prazosin group and nine in placebo group).
(h) 25 patients were randomized, but only 23 comprised the subject of this report. Within the latter, the prazosin group (11 patients) age was 50 ± 14 years, whereas the placebo group (12 patients) age was 53 ± 13 years.
(i) The study population encompassed 27 patients [10 men; mean age 58 years (41 to 79 years)], but only 23 were randomized.
(j) Within the doxazosin group, 27 patients received a placebo juice (age 54.9 ± 13.8 years) and 16 an active juice (age 58.4 ± 14.7 years). Within the spironolactone group, 20 patients received a placebo juice (age 58.2 ± 9.9 years) and 24 an active juice (age 57.1 ± 13.2 years).
(k) The doxazosin 4 mg group (siex patients) age was 57.7 ± 11.2 years, whereas the doxazosin 8 mg group (nine patients) age was 67.0 ± 7.8 years.
(l) 1195 men (22% in the A1B group, age 71.5 ± 4.7 years; 78% in the non‐A1B group, age 72.9 ± 5.4 years) and 1910 women (8% in the A1B group, age 71.8 ± 4.9 years; 92% in the non‐A1B group, age 72.7 ± 5.6 years) were enrolled in the cohort of hypertensive patients, whereas 930 men (5% in the A1B group, age 72.8 ± 5.0 years; 95% in non‐A1B group, age 72.8 ± 5.7 years) were enrolled in the cohort of normotensive patients.
(m) 19 patients were randomized, but only 16 completed the study.
Figure 2.
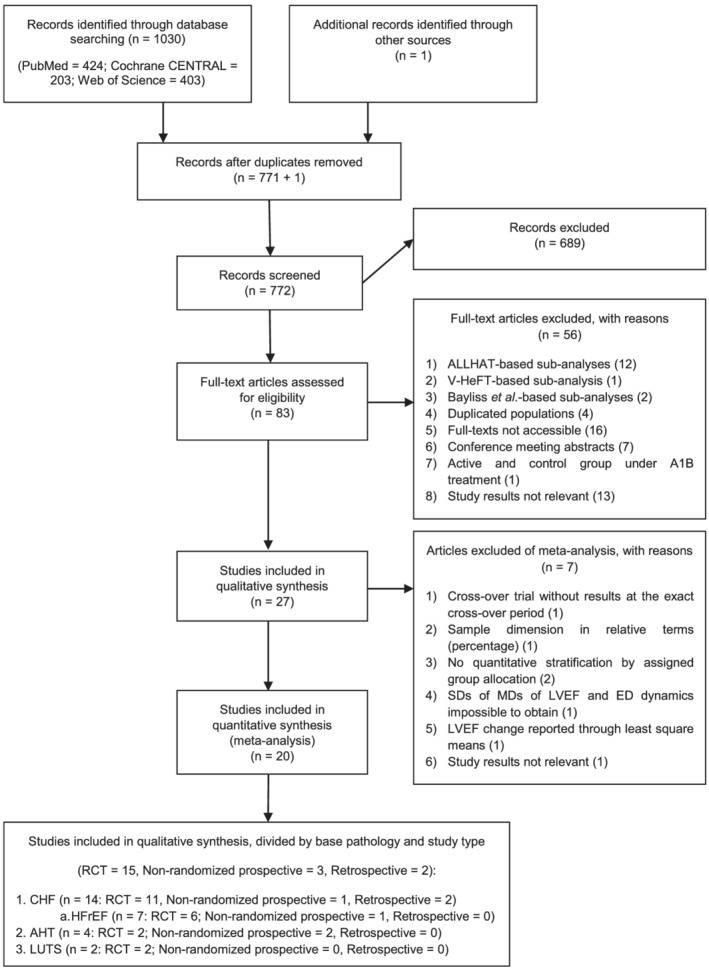
PRISMA flow diagram of literature search. A1B, adrenergic alpha‐1 receptor antagonist; AHT, arterial hypertension; CHF, chronic heart failure; ED, exercise duration; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; MD, mean difference; LUTS, lower urinary tract symptoms; RCT, randomized controlled trial; SD, standard deviation.
Acute heart failure events
We analysed 16 studies comparing A1Bs with other medications and/or placebo and reporting on AHF events. These articles comprised 123 660 patients and 25 998 AHF events, in total. The most represented records were, in due order: Jackevicus et al. (2018) 15 —a retrospective cohort study encompassing CHF patients, irrespective of LVEF; ALLHAT 34 —a randomized active‐controlled trial targeting AHT patients; Chapman et al. (2008) 43 —a RCT sub‐analysis also featuring an AHT population; and V‐HeFT I9—a randomized experiment, both active‐ and placebo‐controlled, directed at HFrEF patients. Doxazosin was the chosen A1B agent in both ALLHAT 34 and Chapman et al. 43
When all studies were included, a non‐significant association between A1B treatment and AHF was noticed, though at the cost of an elevated inter‐study heterogeneity (pooled OR 1.21, [0.84, 1.75] 95% CI, P = 0.31, i 2 88%, Fig. 3 A ). Therefore, we decided to focus on the 13 RCTs included. This analysis went on to reveal a significantly higher likelihood of AHF among the A1B‐treated patients, with very low inter‐study heterogeneity (pooled OR 1.78, [1.46, 2.16] 95% CI, P < 0.00001, i 2 2%, Fig. 3 B ) but at the expense of a large weight (82.8%) attributed to the ALLHAT trial. 34 On the other hand, a sub‐analysis featuring the nine RCTs that included only CHF patients failed to show a statistically significant difference between the study groups, as far as this outcome is concerned (pooled OR 1.13, [0.66, 1.92] 95% CI, P = 0.66, i 2 0%, Fig. 3 C ). Furthermore, when considering the six RCTs encompassing only participants with LVEF lower than 50% (that is, both HFrEF and HFmrEF patients), their joint analysis also did not disclose a statistically significant difference in AHF risk between the groups (pooled OR 1.01, [0.50, 2.05] 95% CI, P = 0.97, i 2 6%, Fig. 3 D ).
Figure 3.
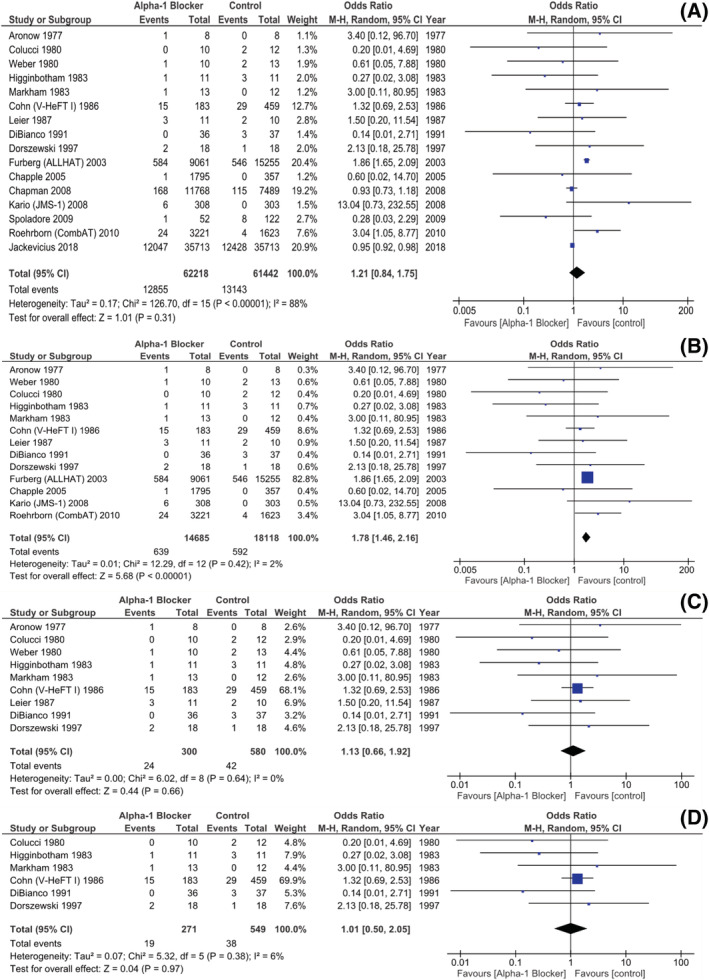
Studies reporting on AHF events: (A) All study designs, with unselected patients; (B) RCTs in unselected patients; (C) RCTs in CHF patients; (D) RCTs in HFrEF and HFmrEF patients. AHF, acute heart failure; CHF, chronic heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFrEF, heart failure with reduced ejection fraction; RCT, randomized controlled trial.
All‐cause and cardiovascular mortality
There were 13 studies reporting on the number of deaths witnessed, in each group, during follow‐up. Ten articles were RCTs and our joint analysis of them did not ultimately unveil a statistically significant difference regarding all‐cause mortality between the A1B and the non‐A1B groups (pooled OR 1.10, [0.84, 1.43] 95% CI, P = 0.48, i 2 17%, Fig. 4 A ). Moreover, when sub‐analysing the eight RCTs featuring only CHF patients, a similar trend was observed (pooled OR 1.09, [0.53, 2.23] 95% CI, P = 0.81, i 2 17%, Fig. 4 B ).
Figure 4.
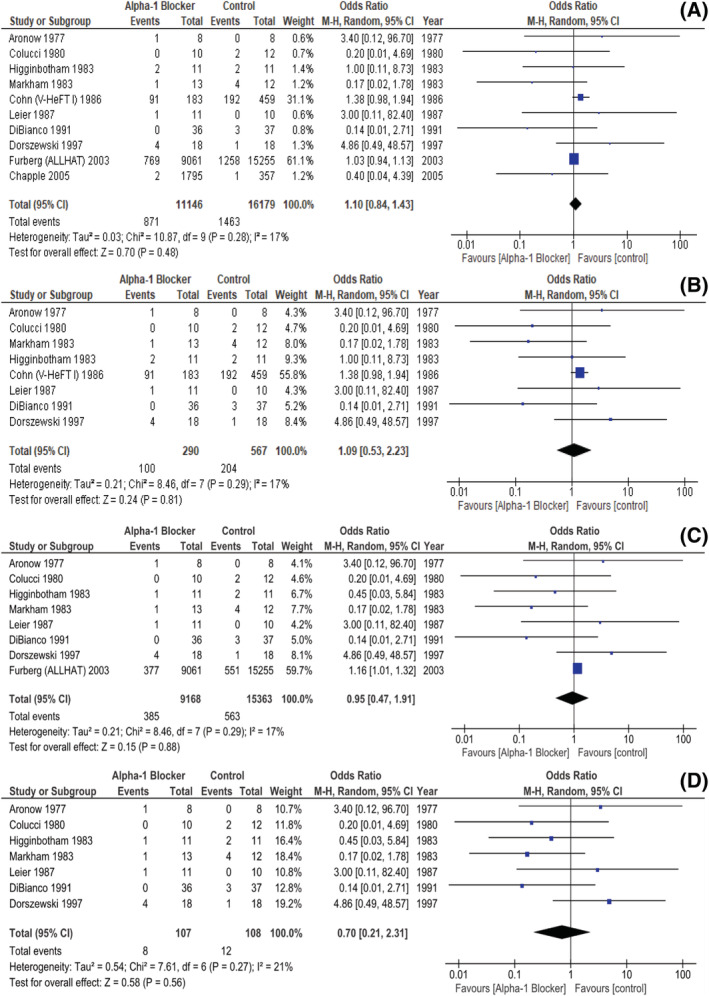
RCTs reporting on mortality: (A) All‐cause mortality in unselected patients; (B) All‐cause mortality in CHF patients; (C) CV mortality in unselected patients; (D) CV mortality in CHF patients. CHF, chronic heart failure; CV, cardiovascular; RCT, randomized controlled trial.
Moreover, we identified 10 studies discriminating the patients in whom the cause of death was judged to be CV in nature, in each group, eight of them being RCTs. In these, the A1B‐ and the non‐A1B‐treated patients also did not behave differently, as far as this outcome is concerned (pooled OR 0.95 [0.47, 1.91], 95% CI, P = 0.88, i 2 17%, Fig. 4 C ). In addition, a sub‐analysis encompassing the seven RCTs featuring only CHF patients was also not able to identify a statistically significant difference between both groups regarding CV mortality (pooled OR 0.70, [0.21, 2.31] 95% CI, P = 0.56, i 2 21%, Fig. 4 D ).
Of note, in Kukin, 25 Markham 29 and Higginbotham 30 trials, sudden death cases were reported without undoubtedly establishing if they were assigned to a CV or a non‐CV aetiology. In this position, we felt justified in including these events in our CV mortality outcome. Additionally, Ajayi et al. 26 conveyed one sudden death event, though without specifying in which study arm it occurred, thus resulting in its exclusion from this analysis. There was a total of 33 567 all‐cause deaths, of which 955 were CV in nature.
Acute coronary syndrome events
Seven studies, all of them RCTs, reported, as a whole, 1325 ACS events. Their analysis did not reveal a statistically significant difference between the A1B and the non‐A1B arms, as far as this outcome is concerned (pooled OR 1.02, [0.91, 1.15] 95%, P = 0.71, i 2 0%, Fig. 5 A ). It should be noted, however, again, that the ALLHAT trial 34 was attributed a large share, in this setting. Still, a sub‐analysis of the four articles including only CHF patients also did not report a meaningful discrepancy in ACS risk between both groups (pooled OR 0.49, [0.10, 2.47] 95% CI, P = 0.39, i 2 0%, Fig. 5 B ).
Figure 5.
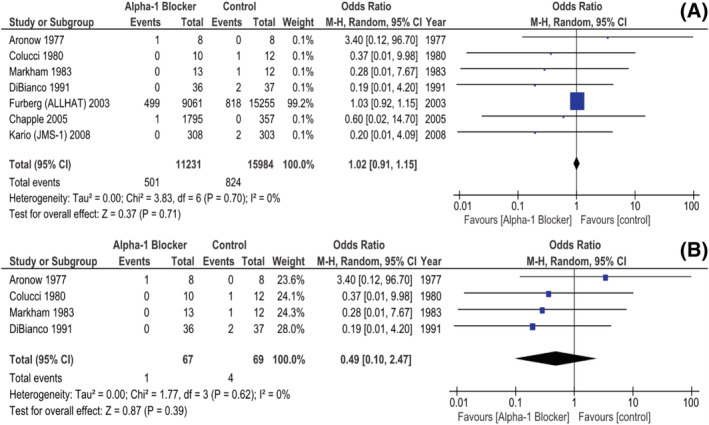
Studies (all RCTs) reporting on ACS events. (A) In unselected patients; (B) In CHF patients. ACS, acute coronary syndrome; CHF, chronic heart failure; RCT, randomized controlled trial.
Note that we chose to present, in the main paper, mainly, the analyses and the sub‐analyses related to the dichotomic outcomes assessed (AHF and all‐cause/CV mortality, not to mention ACS) considering only RCTs, except for the one linked with the occurrence of the primary endpoint in an unselected group of patients. The reason behind this decision was the repeated detection of an elevated inter‐study heterogeneity while jointly regarding RCTs, prospective non‐randomized and retrospective studies, particularly for AHF (i 2 88%) and all‐cause mortality (i 2 59%), when unselected patients were appraised. The remaining forest plots encompassing all study designs are presented in the supporting information (Appendices I and II).
Left ventricular ejection fraction change
We identified four studies (two RCTs, one RCT sub‐analysis and one prospective non‐randomized) allowing for the input of mean LVEF change (with the baseline value as reference), as well as its respective SD, in A1B and non‐A1B groups, either by information reported directly or through manual calculation. Their joint analysis—beset with expressive inter‐study heterogeneity—showed no between‐group statistically significant difference in this outcome (pooled MD 1.66, [−2.18, 5.50] 95%, P = 0.40, i 2 58%, Fig. 6 ).
Figure 6.
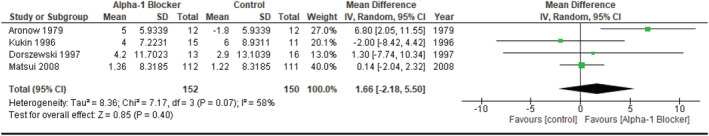
Studies reporting on the effect of adrenergic alpha‐1 receptor antagonists or control agents in left ventricular ejection fraction.
Exercise tolerance
Four studies (all RCTs) presented data from which it was possible to gather—either directly or indirectly—the difference between end of follow‐up and baseline mean ED, as well as its corresponding SD, in both A1B and non‐A1B groups. In this instance, there was a statistically significant increase in exercise tolerance, by means of ED, in seconds, in the A1B group when compared with other(s) drug(s) and/or placebo (pooled MD 139.16, [65.52, 212.80] 95% CI, P = 0.0002, i 2 26%, Fig. 7 ).
Figure 7.
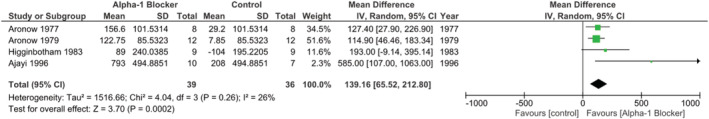
Studies reporting on the effect of adrenergic alpha‐1 receptor antagonists or control agents in exercise duration.
It should be noted that, in our analyses, studies using either placebo or active agents as comparators to A1Bs were considered among the control group. Therefore, whenever possible, we performed sub‐analyses encompassing only placebo‐controlled studies, in order to sort out if the therapeutic properties of the active drug(s) might be responsible for an eventual disproportionate result (supporting information – Appendices IV–VII).
The funnel plots of all analyses are, likewise, presented in the supporting information (Appendices VIII–XVI).
Discussion
Based on our findings, A1B‐treated patients seem to exhibit, when compared with non‐A1B‐treated ones: (i) greater AHF risk in unselected settings; (ii) similar AHF burden in a CHF—and, in particular, in a HFrEF/HFmrEF—background; (iii) comparable all‐cause and CV mortality, both in an unselected scenario and in a CHF context; (iv) equivalent ACS risk, not only in an unselected setup but also if only CHF patients are to be considered; (v) no significant difference in LVEF dynamics; (vi) an increase in ED.
To the best of our knowledge, this is the first systematic review with meta‐analysis that focused primarily on the AHF burden of patients treated with A1Bs, as compared with participants administered other drug(s) and/or placebo. Our introductory meta‐analytic result—the one addressing AHF in an unselected population and encompassing all study methodologies—was not given plentiful credit because of the high inter‐study heterogeneity involved. This problem was, then, fortunately, tackled by the decision to only include RCTs in the analysis. However, this time, a seeming paradox emerged: A1Bs were found to increase AHF in an unselected population but not in those with history of CHF or even HFrEF or HFmrEF. This finding may, though, be explained by the need to embrace the ALLHAT trial 34 —a decidedly negative study comparing doxazosin with chlortalidone, as first‐line antihypertensive pharmacologic options—in the former but not in the latter analyses. Interestingly, the authors of the original ALLHAT publication, 34 as well as many others 45 , 46 , 47 have conveyed potential explanations as to why the study might have been a ‘false negative’ result for A1Bs. For instance, doxazosin was titrated more slowly and discontinued more frequently than chlorthalidone, ultimately resulting in blood pressure being 3 mmHg higher in the doxazosin‐treated group. 34 This finding may have justified, at least partially, the higher AHF risk ascribed to the A1B drug, especially in light of the fact that such hazard mostly clustered in the first year of follow‐up. 34 , 45 , 46 From another point of view, the higher burden of AHF in the doxazosin arm of the ALLHAT trial may just reflect a cardioprotective effect of chlortalidone, rather than a harmful impact of the A1B. 34 , 47 This seems likely, because thiazide‐like diuretics promote sodium excretion, thus potentially contributing to congestion relief. 46 , 47 , 48 In fact, reporting on the cohort of the Cardiovascular Health Study, Bryson and co‐workers 24 also acknowledged an increase in AHF risk in hypertensive patients treated with an A1B agent, as compared with the participants given a thiazide, even if adjustment for systolic blood pressure was able to revert statistical significance. Of note, this study 24 also suggested that hypertensive patients treated with two or more drugs, one of which being an A1B, did not display an additional risk of AHF when compared with a multi‐antihypertensive regimen featuring no A1B. In the same vein, some authors have suggested that the biological mechanism traditionally thought of as through which A1Bs exert a deleterious effect on the natural history of HF—neurohormonal activation with subsequent fluid retention 10 , 11 , 12 —fail to hold in beta‐blocked patients. 49 Indeed, the mild increase in renin secretion—and, consequently, the slight RAAS activation—historically imputed to the A1B drug class has been demonstrated as being primarily triggered by the activation of the beta‐1‐adrenergic receptors. 11 , 12 , 29 , 31 , 50 , 51 , 52 , 53 It is, therefore, interesting to note that Dhaliwal et al. 42 reported that, in the LUTS arena, A1Bs were only associated with a significant increase in AHF events in the patient population not concomitantly medicated with beta‐adrenergic receptor antagonists. Even more strikingly, carvedilol, which is both an alpha‐ and a beta‐blocker, has been proved to provide massive prognostic benefit in HFrEF. 54 These observations may represent a rationale for the just reported asymmetrical harmless effect of A1Bs on CHF patients—particularly on those with HFrEF and HFmrEF—given that beta‐blockers are widely used in these settings. 3 , 55 On the whole, all such findings seem to concur to disprove the 2016 ESC guidelines for the management of HF 14 —which contraindicated A1Bs as antihypertensive options in HFrEF, with a level of evidence as high as A—and to validate the current ones 3 —which cautiously removed that statement—in that A1Bs appear safe in (well‐treated) HF patients. This endorsement may ultimately promote a more active management of LUTS, which frequently coexist in this patient population. 56
Our results regarding the neutral effect of A1B treatment on both all‐cause mortality and ACS risk appear easily acceptable, given that none of the RCTs showed a significant association—neither in a protective nor in a harmful way—between such variables. As for total deaths, point estimates diverged, though. In fact, while, for instance, the V‐HeFT I trial 9 showed a numerical tendency towards an increase in all‐cause mortality in the A1B‐treated group, the observational—hence not included in the main paper forest plot—and large Jackevicius et al. 2018 study 15 disclosed a protective effect—albeit minor—of A1Bs on this outcome, not only in a propensity score‐matched sample, but also in those without background beta‐blocker therapy. Such slight opposite‐directed effects may likely be attributed to the play of chance. A similar scenario applies to the relationship between A1B utilization and CV mortality, but with an important exception: in this case, a statistically significant, though small, increase was noted in (only) one RCT: the ALLHAT trial. 34 The reasons for this seem to extend beyond the play of chance and were covered above. Overall, the apparent incapacity of A1Bs to majorly influence death might concur to diminish our own initial findings of an increase in AHF risk in an A1B‐treated unselected population, because this syndrome is well‐known for its lethality. 3 On the other hand, the remarkable concordance among studies reporting on ACS events concurs to postulate that A1Bs are unable to influence the atherosclerotic process, either by plaque progression or erosion/rupture. 57
Our analyses revealed that A1Bs exert a neutral effect on LVEF and a positive one on exercise tolerance. However, only four of the 10 studies in which LVEF MDs were directly or indirectly reported were able to be incorporated in our meta‐analysis. Likewise, even though ED MDs were obtained in seven articles, only four of them reported data complete enough for inclusion in the quantitative synthesis. In some studies, 11 , 12 , 25 , 30 , 31 , 32 , 37 , 40 statistical analyses were primarily directed towards longitudinal intra‐group LVEF and ED changes, that is, not directly comparing between‐group MDs. Nevertheless, these unemployed studies provided data rich enough to merit discussion.
As for LVEF, and from the four included studies, the only significant association with A1B use was reported by Aronow and colleagues, 44 who unveiled its increment in the A1B‐treated arm. Interestingly, such an effect has already been replicated. For instance, Colucci and co‐workers 31 disclosed a significant increase in LVEF in the group assigned to prazosin but not in that allocated to placebo. In parallel, Kirlin et al., 37 employing trimazosin in randomized fashion, also divulged a meaningful rise in left ventricular systolic function in the active group, while no significant difference in this regard was detected in the placebo arm. Even more striking, perhaps, was that, in a recent sub‐analysis of the VaSera 58 trial, by Faconti et al. (2019), 19 doxazosin exerted an effect on LVEF comparable to that of spironolactone—a foundational therapy in HFrEF. 3 , 59 These findings may contribute to explain the null impact of A1Bs on the decompensation of CHF—as found in our study—in spite of the postulated RAAS activation. 11 , 12 , 29 , 31 , 50 , 51 , 52 , 53 On the other hand, one has to consider that, even if such a beneficial effect of the A1B class on LVEF exists, it may not be durable. On this matter, Higginbotham and colleagues reported an experiment 30 in which LVEF was evaluated at 1, 3, and 6 months, ultimately revealing a significant increase in the prazosin‐treated patients at the first two measurements, but not at the last one. In turn, the placebo arm showed an unchanged LVEF during the entire follow‐up period. This seemingly inconsistent influence of A1Bs on LVEF may even be the root cause of the substantial inter‐study heterogeneity (i 2 58%) detected in our specific analysis.
Rather surprisingly, currently available evidence seems concordant as far as an increase in ED with A1B treatment is concerned. In particular, all four studies included in our specific meta‐analysis pointed towards such an effect. Furthermore, Colucci et al., 31 with prazosin, and Weber and co‐workers, 32 with trimazosin, also unveiled a rise in exercise tolerance—a true surrogate for quality of life 60 —in the A1B‐treated individuals, as opposed to the ones assigned to placebo.
Our study suffers from shortcomings. Firstly, the inclusion of non‐randomized prospective and retrospective studies in our meta‐analysis resulted in elevated inter‐study heterogeneity. This fact provided the basis for our decision to relegate the majority of the forest plots featuring them to the supporting information, with the exception of the one related with our primary endpoint (AHF), when assessed in an unselected population. Concretely, even the evaluation of such a robust and unbiased outcome as all‐cause mortality reached an i 2 as high as 59%. Even though focusing only on RCTs largely solved this issue, such a strategy is not devoid of drawbacks, because it led us to undermine, for instance, the aforementioned large Jackevicius et al. (2018) article. 15
Secondly, and in close relationship with the former concern, A1B controls were found to be significantly heterogeneous. The importance of the differential effects likely exerted by each comparator—no intervention, placebo or other drug(s)—is suggested, for instance, by the fact that, when considering an unselected patient population, a sub‐analysis of only placebo‐controlled trials—thus excluding, namely, the ALLHAT 34 study—unveils a neutral effect of A1Bs on the risk of AHF (Appendix IVA ), standing in contrast to the statistically significant increment in this outcome, with A1B use, obtained when all RCTs are encompassed.
Additionally, the relative paucity of RCTs addressing CV outcomes or even surrogate measures specifically in patients with LUTS—only two were identified 17 , 18 —may hinder the external validity of our study, particularly given that this syndrome constitutes the most common reason for A1B prescription. 61
Moreover, and as previously stated, it was somewhat hard to pool (the SDs of) MDs of LVEF and ED, which might have led to missing data bias. Therefore, the ultimate findings of their analyses should be interpreted cautiously. On the other hand, other surrogate outcomes, such as the NYHA class and weight dynamics, might justify a closer look in further studies.
Lastly, the very definitions of not only HF 62 but also, for instance, ACS 63 have revealed themselves dynamic over time. In parallel, their diagnostic approach and therapeutic management have also changed dramatically in the last few decades. These issues likely further hinder the external validity of our findings, given that, particularly among the included RCTs, the most recent publication dates back to 2008.
Given the limitations of currently available evidence, the clinical utility of A1Bs not only in LUTS but also in resistant AHT, and the doubts still surrounding their impact on HF risk, we feel justified in asking for new studies in this setting.
Conclusions
Despite traditionally associated with fluid retention and HF worsening, A1Bs do not seem to exert a negative influence on the prognosis of HF—and even of HFrEF—patients. In fact, the previous stance seems to have majorly built on the negative results of the ALLHAT trial, which is not without internal validity shortcomings. In the current era, and particularly with commonplace concomitant blockade of the beta‐adrenergic axis, their effects on such populations are likely to be neutral. In the same vein, their impact on other CV outcomes apart from HF worsening, including not only LVEF but also hard ones like mortality and ACS, seem also to be nothing more than trivial, not to mention that they may even increase exercise tolerance.
Our findings seem to align with the new 2021 ESC guidelines for the management of HF, which revoked the contraindication placed upon A1Bs by its 2016 predecessor document, at least as far as AHT treatment in such context is concerned. However, new studies on this matter are still needed in order to finally settle the issue of the safety of this pharmacologic class.
Impact on daily practice
Our findings may legitimate A1B utilization for LUTS and/or AHT management in HF patients.
Conflict of interest
José Pedro Sousa, Diogo Mendonça, Rogério Teixeira and Lino Gonçalves have no conflicts of interest, of any nature, to declare. All authors have seen and agree with the contents of the manuscript. We certify that the submission is original work and is not under review at any other publication.
Supporting information
Data S1. Supporting Information.
Sousa, J. P. , Mendonça, D. , Teixeira, R. , and Gonçalves, L. (2022) Do adrenergic alpha‐antagonists increase the risk of poor cardiovascular outcomes? A systematic review and meta‐analysis. ESC Heart Failure, 9: 2823–2839. 10.1002/ehf2.14012.
References
- 1. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet. 2018; 392: 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Farmakis D, Parissis J, Lekakis J, Filippatos G. Acute heart failure: Epidemiology, risk factors, and prevention. Rev Esp Cardiol (Engl Ed). 2015; 68: 245. [DOI] [PubMed] [Google Scholar]
- 3. McDonagh T, Metra M, Adamo M, Gardner R, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JG. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 4. Williams B, Mancia G, Spiering W, Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I, ESC Scientific Document Group . 2018 ESC/ESH guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J. 2018; 39: 3021–3104.30165516 [Google Scholar]
- 5. Carey R, Calhoun D, Bakris G, Brook R, Daugherty S, Dennison‐Himmelfarb C, Egan BM, Flack JM, Gidding SS, Judd E, Lackland DT. Resistant hypertension: Detection, evaluation, and management: A scientific statement from the American Heart Association. Hypertension. 2018; 72: e53–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gravas S, Cornu J, Gacci M, Gratzke C, Herrmann T, Mamoulakis C, Oelke M, Tikkinen KAO, Gravas S. EAU Guidelines on Management of Non Neurogenic Male Lower Urinary Tract Symptoms (LUTS), incl. Benign Prostatic Obstruction (BPO) [Internet]. 2020. [cited 2021 Jan 11]. Available from: https://uroweb.org/guideline/treatment‐of‐non‐neurogenic‐male‐luts/ (15 November 2021).
- 7. Egan K. The epidemiology of benign prostatic hyperplasia associated with lower urinary tract symptoms: Prevalence and incident rates. Urol Clin North Am. 2016; 43: 289–297. [DOI] [PubMed] [Google Scholar]
- 8. Cohn J, Levine T, Francis G, Goldsmith S. Neurohumoral control mechanisms in congestive heart failure. Am Heart J. 1981; 102: 509–514. [DOI] [PubMed] [Google Scholar]
- 9. Cohn J, Archibald D, Ziesche S, Franciosa J, Harston W, Tristani F, Dunkman WB, Jacobs W, Francis GS, Flohr KH, Goldman S, Cobb FR, Shah PM, Saunders R, Fletcher RD, Loeb HS, Hughes VC, Baker B. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a veterans administration cooperative study. N Engl J Med. 1986; 314: 1547–1552. [DOI] [PubMed] [Google Scholar]
- 10. The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . Major cardiovascular events in hypertensive patients randomized to doxazosin vs Chlorthalidone the antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). JAMA. 2000; 283: 1967–1975. [PubMed] [Google Scholar]
- 11. Dorszewski A, Göhmann E, Dorszewski B, Werner G, Kreuzer H, Figulla H. Vasodilation by urapidil in the treatment of chronic congestive heart failure in addition to angiotensin‐converting enzyme inhibitors is not beneficial: Results of a placebo‐controlled, double‐blind study. J Card Fail. 1997; 3: 91–96. [DOI] [PubMed] [Google Scholar]
- 12. Bayliss J, Norell M, Canepa‐Anson R, Reid C, Poole‐Wilson P, Sutton G. Clinical importance of the renin‐angiotensin system in chronic heart failure: Double blind comparison of captopril and prazosin. Br Med J (Clin Res Ed). 1985; 290: 1861–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leier CV, Binkley PF, Cody RJ. Alpha‐adrenergic component of the sympathetic nervous system in congestive heart failure. Circulation. 1990; 82: I68–I76. [PubMed] [Google Scholar]
- 14. Ponikowski P, Voors A, Anker S, Bueno H, Cleland J, Coats A, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members; Document Reviewers . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur Heart J. 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 15. Jackevicius C, Ghaznavi Z, Lu L, Warner A. Safety of alpha‐adrenergic receptor antagonists in heart failure. JACC Heart Fail. 2018; 6: 917–925. [DOI] [PubMed] [Google Scholar]
- 16. Page M, McKenzie J, Bossuyt P, Boutron I, Hoffmann T, Mulrow C, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo‐Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chapple C, Al‐Shukri S, Gattegno B, Holmes S, Martínez‐Sagarra J, Scarpa R, van Vierssen Trip OB, Vik V, van der Putten‐Slob I. Tamsulosin Oral controlled absorption system (OCAS) in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia (LUTS/BPH): Efficacy and tolerability in a placebo and active comparator controlled phase 3a study. Eur Urol Suppl. 2005; 4: 33–44. [Google Scholar]
- 18. Roehrborn C, Siami P, Barkin J, Damião R, Major‐Walker K, Nandy I, Morrill BB, Gagnier RP, Montorsi F. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4 year results from the CombAT study. Eur Urol. 2010; 57: 123–131. [DOI] [PubMed] [Google Scholar]
- 19. Faconti L, Mills C, Govoni V, Gu H, Morant S, Jiang B, Cruickshank JK, Webb AJ. Cardiac effects of 6 months' dietary nitrate and spironolactone in patients with hypertension and with/at risk of type 2 diabetes, in the factorial design, double‐blind, randomised‐controlled, VASERA TRIAL. Br J Clin Pharmacol. 2019; 85: 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Losos M, Tugwell P, Sb Wells Ga, Zello G, Petersen J. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses [Internet]. The Ottawa Hospital Research Institute. 2000. [cited 2021 Jan 11]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (6 November 2021).
- 21. Sterne J, Hernán M, Reeves B, Savović J, Berkman N, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan A‐W, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JPT. ROBINS‐I: A tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016; 355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sterne J, Savović J, Page M, Elbers R, Blencowe N, Boutron I, Cates CJ, Cheng H‐Y, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019; 366: i4898. [DOI] [PubMed] [Google Scholar]
- 23. McGuinness LA, Higgins JPT. Risk‐of‐bias VISualization (robvis): An R package and Shiny web app for visualizing risk‐of‐bias assessments [Internet]. Research Synthesis Methods. John Wiley & Sons, Ltd; 2020. [cited 2021 Jan 11]. Available from: 10.1002/jrsm.1411 (6 November 2021). [DOI] [PubMed]
- 24. Bryson C, Smith N, Kuller L, Chaves P, Manolio T, Lewis W, Manolio TA, Lewis W, Boyko EJ, Furberg CD, Psaty BM. Risk of congestive heart failure in an elderly population treated with peripheral alpha‐1 antagonists. J Am Geriatr Soc. 2004; 52: 1648–1654. [DOI] [PubMed] [Google Scholar]
- 25. Kukin M, Kalman J, Mannino M, Freudenberger R, Buchholz C, Ocampo O. Combined alpha‐beta blockade (doxazosin plus metoprolol) compared with beta blockade alone in chronic congestive heart failure. Am J Cardiol. 1996; 77: 486–491. [DOI] [PubMed] [Google Scholar]
- 26. Ajayi A, Sofowora G, Balogun M. Concurrent alpha 1 adrenergic blockade and angiotensin converting enzyme inhibition in the treatment of congestive heart failure. Int J Cardiol. 1996; 57: 173–176. [DOI] [PubMed] [Google Scholar]
- 27. Ajayi A, Sofowora G, Adigun A, Asiyanbola B. Adjunctive sympathoplegic therapy to ACE inhibition in blacks with congestive heart failure: A comparison of alpha‐1 with beta‐1 blockade on exercise tolerance and cardiac sympathovagal reflex activity. Ethn Dis. 2003; 13: 71–79. [PubMed] [Google Scholar]
- 28. Kario K, Matsui Y, Shibasaki S, Eguchi K, Ishikawa J, Hoshide S, Ishikawa S, Kabutoya T, Schwartz JE, Pickering TG, Shimada K, Japan Morning Surge‐1 (JMS‐1) Study Group . An alpha‐adrenergic blocker titrated by self‐measured blood pressure recordings lowered blood pressure and microalbuminuria in patients with morning hypertension: The Japan morning Surge‐1 study. J Hypertens. 2008; 26: 1257–1265. [DOI] [PubMed] [Google Scholar]
- 29. Markham R, Corbett J, Gilmore A, Pettinger W, Firth B. Efficacy of prazosin in the management of chronic congestive heart failure: A 6‐month randomized, double‐blind, placebo‐controlled study. Am J Cardiol. 1983; 51: 1346–1352. [DOI] [PubMed] [Google Scholar]
- 30. Higginbotham M, Morris K, Bramlet D, Coleman R, Cobb F. Long‐term ambulatory therapy with prazosin versus placebo for chronic heart failure: Relation between clinical response and left ventricular function at rest and during exercise. Am J Cardiol. 1983; 52: 782–788. [DOI] [PubMed] [Google Scholar]
- 31. Colucci W, Wynne J, Holman B, Braunwald E. Long‐term therapy of heart failure with prazosin: A randomized double blind trial. Am J Cardiol. 1980; 45: 337–344. [DOI] [PubMed] [Google Scholar]
- 32. Weber K, Kinasewitz G, West J, Janicki J, Reichek N, Fishman A. Long‐term vasodilator therapy with trimazosin in chronic cardiac failure. N Engl J Med. 1980; 303: 242–250. [DOI] [PubMed] [Google Scholar]
- 33. Kieback A, Rödiger O, Jaenecke H, Grohmann A, Wernecke K‐D, Baumann G, Felix SB. Hemodynamic effects of alpha1‐adrenoceptor antagonist, doxazosin, in patients with chronic congestive heart failure. J Cardiovasc Pharmacol. 2005; 46: 399–404. [DOI] [PubMed] [Google Scholar]
- 34. The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . Diuretic versus alpha‐blocker as first‐step antihypertensive therapy: Final results from the antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). Hypertension. 2003; 42: 239–246. [DOI] [PubMed] [Google Scholar]
- 35. Spoladore R, Roccaforte R, Fragasso G, Gardini C, Palloshi A, Cuko A, Arioli F, Salerno A, Margonato A. Safety and efficacy of doxazosin as an “add‐on” antihypertensive therapy in mild to moderate heart failure patients. Acta Cardiol. 2009; 64: 485–491. [DOI] [PubMed] [Google Scholar]
- 36. DiBianco R, Parker J, Chakko S, Tanser P, Emmanuel G, Singh J, Marlon A. Doxazosin for the treatment of chronic congestive heart failure: Results of a randomized double‐blind and placebo‐controlled study. Am Heart J. 1991; 121: 372–380. [DOI] [PubMed] [Google Scholar]
- 37. Kirlin P, Das S, Pitt B. Chronic alpha‐adrenoceptor blockade with trimazosin in congestive heart failure. Int J Cardiol. 1985; 8: 89–92. [DOI] [PubMed] [Google Scholar]
- 38. Review Manager (RevMan) [Computer program]. Version 5.4.1. The Cochrane Collaboration; 2020.
- 39. Matsui Y, Eguchi K, Shibasaki S, Ishikawa J, Hoshide S, Pickering T, Shimada K, Kario K. Effect of doxazosin on the left ventricular structure and function in morning hypertensive patients: The Japan morning surge 1 study. J Hypertens. 2008; 26: 1463–1471. [DOI] [PubMed] [Google Scholar]
- 40. Leier C, Binkley P, Randolph P, Unverferth D. Long‐term indoramin therapy in congestive heart failure: A double‐blind, randomized, parallel placebo‐controlled trial. J Am Coll Cardiol. 1987; 9: 426–432. [DOI] [PubMed] [Google Scholar]
- 41. Aronow W, Greenfield R, Alimadadian H, Danahy D. Effect of the vasodilator trimazosin versus placebo on exercise performance in chronic left ventricular failure. Am J Cardiol. 1977; 40: 789–793. [DOI] [PubMed] [Google Scholar]
- 42. Dhaliwal A, Habib G, Deswal A, Verduzco M, Souchek J, Ramasubbu K, Aguilar D, Ma TS, Jneid H, Bolos M, Bozkurt B. Impact of alpha 1‐adrenergic antagonist use for benign prostatic hypertrophy on outcomes in patients with heart failure. Am J Cardiol. 2009; 104: 270–275. [DOI] [PubMed] [Google Scholar]
- 43. Chapman N, Chang C, Dahlöf B, Sever P, Wedel H, Poulter N, ASCOT Investigators . Effect of doxazosin gastrointestinal therapeutic system as third‐line antihypertensive therapy on blood pressure and lipids in the Anglo‐Scandinavian cardiac outcomes trial. Circulation. 2008; 118: 42–48. [DOI] [PubMed] [Google Scholar]
- 44. Aronow W, Lurie M, Turbow M, Whittaker K, van Camp S, Hughes D. Effect of prazosin vs placebo on chronic left ventricular heart failure. Circulation. 1979; 59: 344–350. [DOI] [PubMed] [Google Scholar]
- 45. Kjeldsen S. Warning for antihypertensive drug? Lancet. 2001; 358: 1181–1182. [DOI] [PubMed] [Google Scholar]
- 46. Poulter N, Williams B. Doxazosin for the management of hypertension: Implications of the findings of the ALLHAT trial. Am J Hypertens. 2001; 14: 1170–1172. [DOI] [PubMed] [Google Scholar]
- 47. Chen P, Chaugai S, Zhao F, Wang D. Cardioprotective effect of thiazide‐like diuretics: A meta‐analysis. Am J Hypertens. 2015; 28: 1453–1463. [DOI] [PubMed] [Google Scholar]
- 48. Massie BM. Prevention of heart failure with chlorthalidone in ALLHAT: Placing the results into perspective. J Clin Hypertens (Greenwich). 2009; 11: 462–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dupont A. Effects of carvedilol on renal function. Eur J Clin Pharmacol. 1990; 38: S96–S100. [DOI] [PubMed] [Google Scholar]
- 50. Osborn J, DiBona G, Thames M. Beta ‐1 receptor mediation of renin secretion elicited by low‐frequency renal nerve stimulation. J Pharmacol Exp Ther. 1981; 216: 265–269. [PubMed] [Google Scholar]
- 51. Colucci W. Alpha‐adrenergic receptor blockade with prazosin. Consideration of hypertension, heart failure, and potential new applications. Ann Intern Med. 1982; 97: 67–77. [DOI] [PubMed] [Google Scholar]
- 52. Shannon R, Chaudhry M. Effect of alpha1‐adrenergic receptors in cardiac pathophysiology. Am Heart J. 2006; 152: 842–850. [DOI] [PubMed] [Google Scholar]
- 53. Bryson C, Psaty B. A review of the adverse effects of peripheral Alpha‐1 antagonists in hypertension therapy. Curr Control Trials Cardiovasc Med. 2002; 3: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. carvedilol heart failure study group. N Engl J Med. 1996; 334: 1349–1355. [DOI] [PubMed] [Google Scholar]
- 55. González‐García A, Montero Pérez‐Barquero M, Formiga F, González‐Juanatey JR, Quesada MA, Epelde F, Oropesa R, Díez‐Manglano J, Cerqueiro JM, Manzano L, for the RICA registry investigators . Has beta‐blocker use increased in patients with heart failure in internal medicine settings? Prognostic implications: RICA registry. Rev Esp Cardiol (Engl Ed). 2014; 67: 196–202. [DOI] [PubMed] [Google Scholar]
- 56. Semczuk‐Kaczmarek K, Rys‐Czaporowska A, Platek AE, Szymanski FM. Prevalence of lower urinary tract symptoms in patients with cardiovascular disease. Cent European J Urol. 2021; 74: 190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gregorini L, Marco J, Kozàkovà M, Palombo C, Anguissola GB, Marco I, Bernies M, Cassagneau B, Distante A, Bossi IM, Fajadet J, Heusch G. Alpha‐adrenergic blockade improves recovery of myocardial perfusion and function after coronary stenting in patients with acute myocardial infarction. Circulation. 1999; 99: 482–490. [DOI] [PubMed] [Google Scholar]
- 58. Mills CE, Govoni V, Faconti L, Casagrande ML, Morant SV, Webb AJ, Cruickshank JK. Reducing arterial stiffness independently of blood pressure: The VaSera trial. J Am Coll Cardiol. 2017; 70: 1683–1684. [DOI] [PubMed] [Google Scholar]
- 59. Solomon SD, Claggett B, Lewis EF, Desai A, Anand I, Sweitzer NK, O'Meara E, Shah SJ, McKinlay S, Fleg JL, Sopko G, Pitt B, Pfeffer MA, for the TOPCAT Investigators . Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J. 2016; 37: 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ostman C, Jewiss D, Smart NA. The effect of exercise training intensity on quality of life in heart failure patients: A systematic review and meta‐analysis. Cardiology. 2017; 136: 79–89. [DOI] [PubMed] [Google Scholar]
- 61. Lepor H. Alpha‐blockers for the treatment of benign prostatic hyperplasia. Urol Clin North Am. 2016; 43: 311–323. [DOI] [PubMed] [Google Scholar]
- 62. Bozkurt B, Coats A, Tsutsui H. Universal definition and classification of heart failure. J Card Fail. 2021; S1071–9164: 00050–6. [Google Scholar]
- 63. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD, Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction . Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018; 72: 2231–2264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.


