Abstract
An 83‐year‐old Japanese man visited our hospital with dyspnea and general fatigue. Computed tomography (CT) revealed a tumor in the anterior mediastinum, bilateral pleural effusion, pericardial fluid, and multiple liver nodules. We performed a CT‐guided tumor biopsy, and the patient was diagnosed with thymic small‐cell carcinoma, Masaoka–Koga stage classification IVb. The patient received four cycles of carboplatin and etoposide, and all lesions disappeared on CT. However, after 6 months, CT revealed a recurrent tumor in the anterior mediastinum. After one cycle of rechallenge chemotherapy, we performed extended total thymectomy followed by another three cycles of chemotherapy. More than 2.5 years after the last chemotherapy session, the patient's carcinoma did not recur. Thus, this case suggests that salvage surgery may be a treatment option for local recurrence of thymic carcinoma after complete remission with chemotherapy, even in patients with stage IV cancer.
Keywords: chemotherapy, small cell carcinoma, surgery, thymic carcinoma
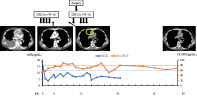
We present the case of an elderly man with thymic small‐cell carcinoma, a thymic neuroendocrine tumor, where multidisciplinary treatment, including chemotherapy comprising carboplatin and etoposide, and salvage surgery, was considered effective. Chest computed tomography scans show that 4 months after initiation of chemotherapy, the tumor and pleural effusions had disappeared (second from left). Six months after the confirmed complete response, a recurrent tumor, approximately 2 cm in size, was observed in the anterior mediastinum (third from left, circle). Two and a half years after the end of the treatment, the tumor had not recurred (right). The levels of neuron‐specific enolase (NSE) and pro‐gastrin‐releasing peptide (proGRP), tumor markers of small cell carcinoma, fluctuate with the disease status. Data on the NSE level after 25 months are not available. The orange and blue dotted lines on the graph represent the upper limit of normal for proGRP (80.0 pg/ml) and NSE (12.0 ng/ml), respectively.
INTRODUCTION
Thymic small‐cell carcinoma is classified as a thymic neuroendocrine tumor (TNET) according to the World Health Organization histologic classification. 1 TNETs are extremely rare malignant neoplasms, and their treatment remains unestablished. 2 , 3 Complete resection is reported as a prognostic factor of TNETs. However, in such cases debulking surgery does not increase overall survival, and chemotherapy or radiotherapy alone do not significantly affect overall survival, 2 , 4 therefore multidisciplinary treatment, including chemotherapy, radiotherapy, and surgery, should be considered for patients with advanced or recurrent thymic tumors.
We present the case of an elderly man with thymic small‐cell carcinoma, a TNET, where multidisciplinary treatment, including chemotherapy comprising carboplatin (CBDCA) and etoposide (VP‐16), and salvage surgery, was considered effective.
CASE REPORT
An 83‐year‐old Japanese man visited our hospital with dyspnea and general fatigue lasting several weeks. No significant findings were noted on physical examination. Laboratory test results on admission were unremarkable, except for an elevated neuron‐specific enolase level of 18.1 ng/ml. Chest radiography revealed right‐dominant bilateral pleural effusion (Figure 1a). Computed tomography (CT) revealed a partially enhanced tumor with a major axis of 8 cm in the anterior mediastinum (Figure 1b,c), along with bilateral pleural effusion, pericardial fluid (Figure 1d), and multiple liver nodules (Figure 1e). Right pleural effusion cytology revealed class V malignancy, suggestive of small‐cell carcinoma. The tumor was considered to be an inoperable primary thymic tumor, and we performed a CT‐guided tumor biopsy. Histopathological examination of the biopsy specimen revealed a sheet‐like arrangement of atypical small round cells with a high nuclear‐to‐cytoplasmic ratio and, focally, tumor cell nuclear crush artifacts (Figure 2a). Immunohistochemically, the tumor cells were diffusely positive for pancytokeratin AE1/AE3, synaptophysin (Figure 2b), and CD56 (Figure 2c), and focally positive for chromogranin A but negative for CD5, cytokeratin 5, p40, PAX8, thyroid transcription factor‐1, leukocyte common antigen, NUT, CD99, and S100 protein (data not shown). The Ki‐67 labeling index was 90%. The patient was diagnosed with a primary thymic small‐cell carcinoma, Masaoka–Koga stage IVb.
FIGURE 1.
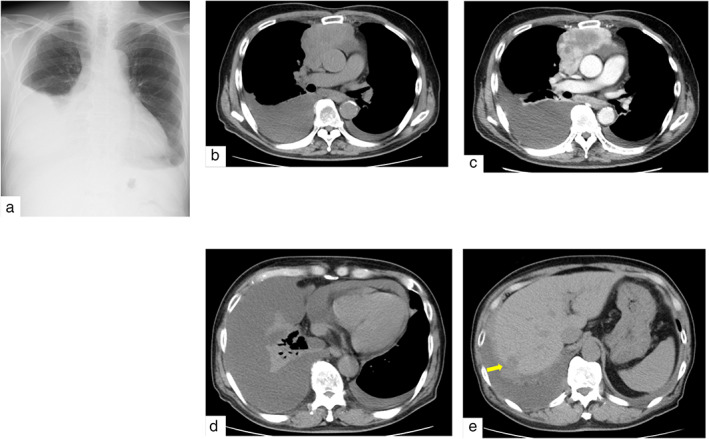
Imaging on admission. (a) Chest X‐ray shows right dominant pleural effusion. (b, c) Plain chest computed tomography (CT) shows an 8‐cm mass in anterior mediastinum, which is partially enhanced by contrast‐enhanced CT. (d, e) Plain CT shows bilateral pleural effusion, pericardial fluid, and a liver nodule (arrow)
FIGURE 2.
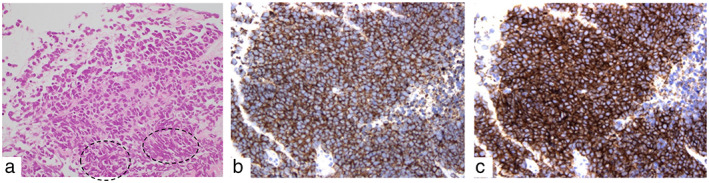
Histopathologic findings of the primary tumor after needle biopsy. (a) By hematoxylin and eosin (HE) staining, atypical small round cells with high nuclear‐to‐cytoplasmic ratio display a sheet‐like morphology, and nuclear crush artifacts are indicated by circles (magnification ×400). (b) By immunostaining, the tumor cells are diffusely positive for synaptophysin (magnification ×400). (c) By immunostaining, the tumor cells are diffusely positive for CD56 (magnification ×400)
The patient's clinical course is shown in Figure 3a. We administered chemotherapy with CBDCA and VP‐16. After four cycles of chemotherapy, all lesions disappeared on CT. No fluorine‐18 deoxyglucose accumulation was observed on positron emission tomography/CT (data not shown), and it was considered a complete response (CR). However, CT performed 6 months later revealed a tumor regrowth in the anterior mediastinum with an infiltrated pericardium (data not shown). We diagnosed the patient with local recurrence of the carcinoma and initiated chemotherapy with CBDCA and VP‐16. After one cycle of chemotherapy, we performed extended total thymectomy and partial resection of the pericardium. No pleural effusion or pleural dissemination was observed, but the thymic tumor was fixed to the pericardium. Pericardial fluid and pleural lavage cytology examinations revealed class II malignancy. The tumor was solid and approximately 23 × 16 × 15 mm in size (Figure 3b,c). Pathological examination confirmed small‐cell carcinoma (Figure 3d) with pericardium infiltration, indicating an R0 resection. The patient received three cycles of chemotherapy after surgery. More than 2.5 years after the last chemotherapy session, the carcinoma had not recurred (Figure 3a).
FIGURE 3.
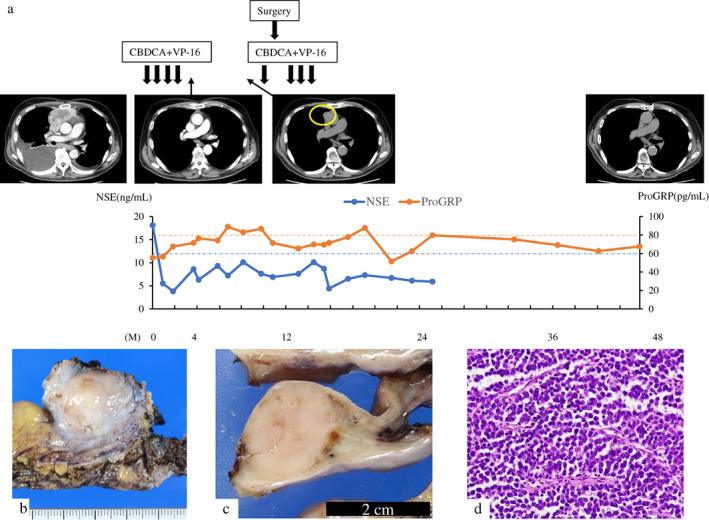
Clinical course (a) and histopathological findings of the tumor (b–d). (a) Chest computed tomography scans. Second from left: 4 months after initiation of chemotherapy, the tumor and pleural effusions disappeared. Third from left: 6 months after the confirmed complete response, a recurrent tumor, approximately 2 cm in size, was observed in the anterior mediastinum (circle). Right: 2.5 years after the end of the treatment, the tumor had not recurred. The levels of neuron‐specific enolase (NSE) and pro‐gastrin‐releasing peptide (proGRP), tumor markers of small cell carcinoma, fluctuate with the disease status. Data on NSE level after 25 months are not available. The orange and blue dotted lines on the graph represent the upper limit of normal for proGRP (80.0 pg/ml) and NSE (12.0 ng/ml), respectively. (b, c) The resected specimen (b) and the cut surface (c) show that the tumor is solid and demarcated. (d) By hematoxylin and eosin staining, atypical small round cells are seen proliferating in solid sheets with fine fibrovascular septa (magnification ×400)
DISCUSSION
No standard chemotherapy regimen has yet been established for thymic small‐cell carcinoma because of its rarity and the associated difficulties in conducting clinical trials. However, CBDCA plus VP‐16 chemotherapy has often been selected in such cases, based on the treatment for small‐cell lung cancer. 5 Based on a literature search, we found 14 reports of thymic small‐cell carcinoma cases (Table 1). To the best of our knowledge, ours is the first report of salvage surgery for small cell thymic carcinoma including the long‐term outcomes. In 12 previously reported cases, the patients were Japanese. Five of the 15 patients were treated with platinum (cisplatin or carboplatin) plus VP‐16 chemotherapy as initial therapy. In cases of recurrence, salvage surgery was not performed in any case.
TABLE 1.
Previously reported cases of thymic small cell carcinoma
| Initial therapy | Therapy on recurrence | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case | Age | Sex | Stage | Surgery | Radiation (Gy) | Chemotherapy (no. of cycles) | Chemotherapy (no. of cycles) | Other therapy | Prognosis after diagnosis | |
| 1 | Sumitomo et al. 8 | 72 | F | IVb | – | – | CPA + VCR + ADR (ND) | – | – | Died after 10 months |
| 2 | Yano et al. 9 | 66 | M | IVb | Exploratory resection | 61.2 | ADR (4) | – | – | Died after about 5.5 years |
| 3 | Yano et al. 9 | 55 | F | III | Complete resection | – | – | CDDP+CPA + ADR + VCR (2) | – | Died after about 9 years |
| 4 | Shimizu et al. 10 | 55 | M | ND | Incomplete resection | 50 | CDDP+VP‐16 | – | – | Alive 9 months postoperatively |
| 5 | Morita et al. 11 | 50 | F | IVb | – | 50 | CDDP+IFX + VP‐16 (2) | – | – | ND |
| 6 | Ojika et al. 12 | 46 | F | ND | Complete resection after chemotherapy | – | CBDCA+VP‐16 (2) | – | – | ND |
| 7 | Kaneko et al. 13 | 71 | F | II | Complete resection | 50 | – | CPA + ADM + VCR + CDDP+VP‐16 (ND) | – | Died after 16 months |
| 8 | Iwata et al. 14 | 63 | M | II | Complete resection | 50 | – | – | – | Alive 10 months postoperatively |
| 9 | Igawa et al. 15 | 57 | M | IVb | – | – | CBDCA+PTX (4) | 2) AMR (7) 3) GEM (ND) 4) CPT‐11 (ND) 5) CBDCA+PTX (ND) | – | Died after 2 years |
| 10 | Ejima et al. 16 | 71 | M | III | Complete resection | 45 | – | – | – | ND |
| 11 | Itoga et al. 17 | 75 | F | IVb | – | – | CBDCA+VP‐16 (4) | CBDCA+VP‐16 (2) | – | ND |
| 12 | Hashimoto et al. 18 | 71 | M | IIa | Complete resection | – | – | – | – | Alive 1 year postoperatively |
| 13 | Qin et al. 19 | 66 | M | IVa | – | 60 | CDDP+CPT‐11 (6) | 2) CDDP+CPT‐11 (5) 3) CDDP+PTX (4) after radiation therapy 4) anlotinib | Radiation 54 Gy | Alive 10 years post treatment |
| 14 | Hemmati et al. 20 | 41 | F | ND | Complete resection | – | – | CDDP+VP‐16 (ND) | – | Alive 1 year postoperatively |
| 15 | The present case | 83 | M | IVb | – | – | CBDCA+VP‐16 (4) | CBDCA+VP‐16 (4) | Complete resection after 1 cycle of chemotherapy | Alive 2.5 years post treatment |
Abbreviations: ADM, adriamycine; ADR, doxorubicin; AMR, amrubicin; CBDCA, carboplatin; CDDP, cisplatin; CPA, cyclophosphamide; CPT‐11, irinotecan; GEM, gemcitabine; IFX, ifosfamide; ND, no data; PTX, paclitaxel; VCR, vincristine; VP‐16, etoposide.
In this case, we decided against adding radiotherapy to the regimen owing to the patient's age and CR to chemotherapy. However, the tumor recurred locally within 6 months. In cases of thymic tumors, reoperation improves the prognosis in relapse cases. 6 Additionally, salvage operations have been performed for limited‐disease small‐cell lung cancer in cases of relapse at the primary site with resectable tumors. 7 We therefore performed surgery in combination with chemotherapy to prevent recurrence. Subsequently, the tumor has not recurred 2.5 years after the last chemotherapy session.
In conclusion, we successfully treated an elderly patient with unresectable thymic small‐cell carcinoma. After the first chemotherapy, his carcinoma recurred locally, but surgical resection combined with rechallenge chemotherapy was effective. This suggests that salvage surgery may be a treatment option for local recurrence of TNETs after chemotherapy, even in patients with stage IV cancer. It is important to select the appropriate treatment method for each individual case of a rare tumor. Further studies of treatments for thymic small‐cell carcinoma are warranted.
AUTHOR CONTRIBUTIONS
Investigation and validation: all authors. Conceptualization: E.T. Formal analysis: K.Y. Resources: S.I. and K.Y. Writing – original draft: J.T. and Y.T. Writing – review & editing: Y.T., E.T. and T.S. Visualization: Y.T. Supervision: T.S. All authors read and approved the final manuscript.
FUNDING INFORMATION
This report did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
ACKNOWLEDGMENTS
We thank Ms. Keiko Mizuno, Mr. Masahiko Ohara, Ms. Kaori Yasuoka, Ms. Yukari Wada, and Ms. Hiroyuki Tsutsui for preparing the histological and immunohistochemical specimens.
Terada J, Toyoda Y, Takeuchi E, Tanida N, Ito S, Yorita K, et al. Surgical resection combined with perioperative chemotherapy for a patient with locally recurrent, previously stage IV thymic small‐cell carcinoma: A case report. Thorac Cancer. 2022;13(23):3415–3419. 10.1111/1759-7714.14717
REFERENCES
- 1. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to the 2015 World Health Organization classification of tumours of the lung, pleura, thymus and heart. J Thorac Oncol. 2015;10:1240–2. [DOI] [PubMed] [Google Scholar]
- 2. Filosso PL, Yao X, Ahmad U, Zhan Y, Huang J, Ruffini E, et al. Outcome of primary neuroendocrine tumors of the thymus: a joint analysis of the international thymic malignancy interest group and the European Society of Thoracic Surgeons databases. J Thorac Cardiovasc Surg. 2015;149:103–9.e2. [DOI] [PubMed] [Google Scholar]
- 3. Dinter H, Bohnenberger H, Beck J, Bornemann‐Kolatzki K, Schütz E, Küffer S, et al. Molecular classification of neuroendocrine tumors of the thymus. J Thorac Oncol. 2019;14:1472–83. [DOI] [PubMed] [Google Scholar]
- 4. Wen J, Chen J, Chen D, Liu D, Xu X, Huang L, et al. Evaluation of the prognostic value of surgery and postoperative radiotherapy for patients with thymic neuroendocrine tumors: a propensity‐matched study based on the SEER database. Thorac Cancer. 2018;9:1603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Okamoto H, Watanabe K, Kunikane H, et al. Randomised phase III trial of carboplatin plus etoposide vs split doses of cisplatin plus etoposide in elderly or poor‐risk patients with extensive disease small‐cell lung cancer: JCOG 9702. Br J Cancer. 2007;97:162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bott MJ, Wang H, Travis W, Riely GJ, Bains M, Downey R, et al. Management and outcomes of relapse after treatment for thymoma and thymic carcinoma. Ann Thorac Surg. 2011;92:1984–91. discussion 1991. [DOI] [PubMed] [Google Scholar]
- 7. Shepherd FA, Ginsberg R, Patterson GA, Feld R, Goss PE, Pearson FG, et al. Is there ever a role for salvage operations in limited small‐cell lung cancer? J Thorac Cardiovasc Surg. 1991;101:196–200. [PubMed] [Google Scholar]
- 8. Sumitomo M, Uyama T, Fukumoto T, Takahashi K, Sakiyama S, Tsuyuguchi M, et al. Clinical study of thymic carcinoma. Jpn J Chest Surg. 1993;7:633–7. [Google Scholar]
- 9. Yano T, Hara N, Ichinose Y, Asoh H, Yokoyama H, Ohta M. Treatment and prognosis of primary thymic carcinoma. J Surg Oncol. 1993;52:255–8. [DOI] [PubMed] [Google Scholar]
- 10. Shimizu J, Hayashi Y, Morita K, Arano Y, Yoshida M, Oda M, et al. Primary thymic carcinoma: a clinicopathological and immunohistochemical study. J Surg Oncol. 1994;56:159–64. [DOI] [PubMed] [Google Scholar]
- 11. Morita Y, Yamagishi M, Harada H, et al. A case of small cell carcinoma of the thymus with extensive tracheal invasion. J Jpn Soc Respir Endoscopy. 1994;16:498–502. [Google Scholar]
- 12. Ojika T, Mukouyama N, Hattori N, Suzuki M, Tsuzuki T, Kouketsu H. Synchronous double cancer, thymic small cell carcinoma and breast cancer; a case report. Jpn J Lung Cancer. 1996;36:303–6. [Google Scholar]
- 13. Kaneko K, Yamada T, Miyazawa M, et al. A recurrent case of thymic small cell carcinoma with elevated NSE in pleural effusion. Jpn J Chest Surg. 2000;1:56–61. [Google Scholar]
- 14. Iwata T, Inoue K, Mizuguchi S, Morita R, Tsukioka T, Suehiro S. Thymic small cell carcinoma associated with pulmonary squamous cell carcinoma. Ann Thorac Surg. 2006;82:2266–8. [DOI] [PubMed] [Google Scholar]
- 15. Igawa S, Murakami H, Yamamoto N. Thymic small cell carcinoma shows marked response to amrubicin. J Thorac Oncol. 2009;4:778–9. [DOI] [PubMed] [Google Scholar]
- 16. Ejima M, Thujikawa Y, Tomoyasu H, Shimokawa R. A surgical case of thymic small cell carcinoma. Jpn J Chest Dis. 2010;69:976–9. [Google Scholar]
- 17. Itoga M, Asari Y, Morimoto T, Taima K, Nakamura K, Tanaka Y, et al. Sepsis caused by Listeria monocytogenes during chemotherapy for small cell carcinoma of the thymus. BMC Res Notes. 2015;8:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hashimoto S, Hayasaka K, Suzuki K, Endoh M, Yanagawa N, Shiono S. Thymic small cell carcinoma associated with Lambert‐Eaton myasthenic syndrome. Ann Thorac Surg. 2020;109:e347–8. [DOI] [PubMed] [Google Scholar]
- 19. Qin W, Zou B, Fan X, Fan B, Wang S, Wang L. Transformation from small cell to squamous cell carcinoma in a thymic carcinoma patient with a durable response to anlotinib: a case report. Cancer Manag Res. 2022;14:1595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hemmati P, Cassivi SD. Complete resolution of paraneoplastic syndrome of inappropriate antidiuretic hormone secretion following thymic small‐cell carcinoma thoracoscopic resection. Interact Cardiovasc Thorac Surg. 2022;35:ivac192. [DOI] [PMC free article] [PubMed] [Google Scholar]


