Abstract
Aims
In heart failure with preserved ejection fraction (HFpEF), the reduction of nitric oxide (NO)‐bioavailability and consequently endothelial dysfunction leads to LV stiffness and diastolic dysfunction of the heart. Besides shear stress, high‐density lipoprotein (HDL) stimulates endothelial cells to increased production of NO via phosphorylation of endothelial nitric oxide synthase (eNOS). For patients with heart failure with reduced ejection fraction, earlier studies demonstrated a positive impact of exercise training (ET) on HDL‐mediated eNOS activation. The study aims to investigate the influence of ET on HDL‐mediated phosphorylation of eNOS in HFpEF patients.
Methods and results
The present study is a substudy of the OptimEx‐Clin trial. The patients were randomized to three groups: (i) HIIT (high‐intensity interval training; (ii) MCT (moderate‐intensity continuous training); and (iii) CG (control group). Supervised training at study centres was offered for the first 3 months. From months 4–12, training sessions were continued at home with the same exercise protocol as performed during the in‐hospital phase. Blood was collected at baseline, after 3, and 12 months, and HDL was isolated by ultracentrifugation. Human aortic endothelial cells were incubated with isolated HDL, and HDL‐induced eNOS phosphorylation at Ser1177 and Thr495 was assessed. Subsequently, the antioxidative function of HDL was evaluated by measuring the activity of HDL‐associated paraoxonase‐1 (Pon1) and the concentration of thiobarbituric acid‐reactive substances (TBARS). After 3 months of supervised ET, HIIT resulted in increased HDL‐mediated eNOS‐Ser1177 phosphorylation. This effect diminished after 12 months of ET. No effect of HIIT was observed on HDL‐mediated eNOS‐Thr495 phosphorylation. MCT had no effect on HDL‐mediated eNOS phosphorylation at Ser1177 and Thr495. HIIT also increased Pon1 activity after 12 months of ET and reduced the concentration of TBARS in the serum after 3 and 12 months of ET. A negative correlation was observed between TBARS concentration and HDL‐associated Pon1 activity in the HIIT group (r = −0.61, P < 0.05), and a trend was evident for the correlation between the change in HDL‐mediated eNOS‐Ser1177 phosphorylation and the change in peak V̇O2 after 3 months in the HIIT group (r = 0.635, P = 0.07).
Conclusions
The present study documented that HIIT but not MCT exerts beneficial effects on HDL‐mediated eNOS phosphorylation and HDL‐associated Pon1 activity in HFpEF patients. These beneficial effects of HIIT were reduced as soon as the patients switched to home‐based ET.
Keywords: Heart failure with preserved ejection fraction, Exercise training, HDL, eNOS
Introduction
About 50% of patients hospitalized for heart failure (HF) have been diagnosed with heart failure with preserved ejection fraction (HFpEF). 1 , 2 , 3 The prevalence of HFpEF averages 4.9%, increasing by approximately 1% annually. Moreover, the mortality in patients with HFpEF may be as high as in HFrEF, 4 and it is predicted that the prevalence of HFpEF and treatment costs will increase due to an aging of the population. 5 Despite the high mortality rate, most therapeutic approaches using medications established for the treatment of heart failure with reduced ejection fraction (HFrEF) have failed. 6 , 7 , 8 , 9 Nevertheless, promising results with respect to the use of the SGLT2 inhibitor Empagliflozin 10 and exercise training 11 for the treatment of HFpEF have been reported. A pathophysiological model for the development of ventricular stiffness has been proposed by Paulus and Tschöpe. 12 In this model, a systemic proinflammatory state causes coronary microvascular endothelial inflammation resulting in reduced nitric oxide (NO) bioavailability, cyclic guanosine monophosphate content, and protein kinase G (PKG) activity. This finally leads to a hypophosphorylation of titin and consequently ventricular stiffening.
NO synthesis and release from the endothelium are induced by shear stress. 13 , 14 Shear stress has been shown to cause phosphorylation of endothelial nitric oxide synthase (eNOS) at the serine‐1177 (Ser1177) position and in parallel dephosphorylation at threonine‐495 (Thr495). Besides shear stress eNOS activity can also be induced by high‐density lipoprotein (HDL) via binding to scavenger receptor B1 (SR‐BI). 15 Studies have shown that a low concentration of HDL cholesterol is associated with an increased risk of cardiovascular events. 16 However, interventions increasing the concentration of HDL did not reduce cardiovascular risk. 17 Therefore, it was postulated that not the level of HDL is the important factor rather the ability of HDL to modulate inflammation and activate eNOS and NO production. This idea is supported by the observation that despite similar levels of plasma HDL cholesterol, the HDL lost almost 40% of its ability to stimulate eNOS activity and 20% of its ability to suppress an inflammatory response in patients with type 2 diabetes. 18 Furthermore, exercise training in HFrEF patients 19 or a lifestyle modification in obese adolescents 20 restored HDL‐mediated eNOS phosphorylation and NO production, without modulating HDL plasma concentration. At the molecular level, malondialdehyde (MDA), a product of lipid peroxidation, binds to HDL resulting in PKCßII activation leading to reduced eNOS activation (reduced phosphorylation of eNOS‐Ser1177 and increased phosphorylation of eNOS‐Thr495). 21 , 22 , 23
Exercise training (ET) is a powerful tool to reduce morbidity in heart failure patients and to increase endothelial function and subsequently exercise capacity. Particularly in HFpEF patients, ET has been for a long time the only treatment option showing positive results with respect to improvements in exercise capacity and quality of life. Nothing is known so far about the impact of different exercise training modalities on HDL function regarding modulated eNOS activity via phosphorylation. Therefore, in the present study, we isolated HDL from patients with diagnosed HFpEF before and after high‐intensity interval training (HIIT) and moderate‐intensity continuous training (MCT) programmes. Subsequently, we evaluated its stimulating effect on eNOS phosphorylation and its antioxidative properties by assessing Pon1 activity.
Methods
Patients and sample collection
The present study is a substudy of the OptimEx‐Clin trial (ClinicalTrials.gov Identifier: NCT02078947) investigating different training modalities of exercise to improve peak V̇O2 in HFpEF patients. 11 Sedentary, stable HFpEF patients ≥40 years of age with signs and symptoms of heart failure (LVEF >50%, NYHA II/III, E/e′ ≥ 15 or E/e′ ≥ 8 and NT‐proBNP ≥220 pg/mL) on stable medical therapy (>4 weeks) for risk factor control were randomized (1:1:1) to one of the following groups:
HIIT (three training sessions per week, 10 min warm‐up at intensity of 35–50% of heart rate reserve (HRR), then 4× 4 min intervals at high intensity of 80–90% of HRR, interspaced by 3 min of active recovery at 20–50% HRR, 38 min each session);
MCT (5 times per week, intensity of 35–50% of HRR, 40 min each session); and
CG (exercise recommendations according to guidelines 24 ).
Exercise intensity (% HRR) was determined by a maximal cardiopulmonary exercise test (CPET) at baseline and was adapted after 6 weeks, 3 months, and 6 months of exercise training based on repeated CPET. Supervised training at study centres was offered for the first 3 months. From months 4–12, training sessions were continued at home with the same exercise protocol as performed during the in‐hospital phase.
For the present study, 34 patients were randomly selected from the total of 176 patients included in the final analysis of the main study (10 subjects from the control group, 14 subjects from the MCT group, and 10 subjects from the HIIT group). A comparison between the 34 patients randomly selected for the present study and the overall OptimEx study cohort is depicted in the Supporting Information, Table S1 . Blood samples were collected from all participants when entering the study (baseline), after 3 months and after 12 months of training. Serum was prepared by ultracentrifugation (10 min at 3000× g at 4°C) and stored at −80°C until used.
Isolation of high‐density lipoprotein
High‐density lipoprotein was isolated by ultracentrifugation using a KBr density gradient as described in the literature. 19 In brief, the density of the serum sample was raised with solid KBr to a final density of 1.24 g/mL. For generating a density gradient the serum sample was put into the centrifugation tube, overlayed with a KBr solution (density 1.063 g/mL) and subjected to ultracentrifugation (6 h, RT, and 77 000 rpm, Optima Max MLA‐80 rotor). After centrifugation, two white bands were visible, and the upper band was collected using a syringe. To remove the KBr solution, the collected HDL was washed serval times with PBS and concentrated using a filter system (Amicon Ultra‐4, 30 kDa). The quality of isolated HDL was evaluated by polyacrylamide gel electrophoresis followed by Coomassie Brilliant Blue staining or by specific detection of Apo‐protein A1 (Western blot analysis).
Cell culture and incubation with isolated high‐density lipoprotein
Human aortic endothelial cells (HAEC; Cell Systems Biotechnology, Troisdorf, Germany) were cultured in EGM‐2 cell culture medium (Lonza, Walkersville, MD) and incubated for 0, 5, 10, 15, or 30 min with 50 μg/mL isolated HDL. Thereafter, cells were harvested with ice‐cold lysis buffer (50 mmol/L Tris–HCl; pH 7.4; 1% NP‐40; 0.25% Na‐deoxycholate; 150 mmol/L NaCl; 1 mmol/L EDTA; 0.1% Triton X‐100; 0.2% SDS) containing protease inhibitor mix M (Serva, Heidelberg, Germany) as well as phosphatase inhibitor mix II (Serva). Protein concentration was determined using BSA as standard (BCA method; Pierce, Rockford, IL).
Western blot analysis
Ten micrograms of total protein were separated on a denaturing polyacrylamide gel and transferred to a PVDF membrane. To detect specific proteins, the following antibodies were applied: anti‐eNOS (Santa Cruz), antiphospho‐eNOS‐Ser1177 and antiphospho‐eNOS‐Thr495 (both BD Biosciences, Heidelberg, Germany). For the evaluation of HDL‐induced phosphorylation of the respective protein, the maximal stimulation was used as recently described. 19 All samples were analysed in triplicate.
Measurement of the activity of paraoxonase‐1
The paraoxonase activity was measured by the conversion of paraoxon to p‐nitrophenol, which can be monitored spectrophotometrically by the change in absorbance over time at 405 nm. 25 Isolated HDL or an aliquot of serum was added to a solution containing 1 mM paraoxon, 2 mmol/L CaCl2, and 100 mmol/L Tris pH 8.0. The change of absorbance over time was monitored at 405 nm using a plate reader (Infinite M Plex reader, Tecan, Männedorf, Switzerland), and enzyme activity was calculated using an extinction coefficient of 17 000 M/cm.
Measurement of thiobarbituric acid‐reactive substances concentration
Free and protein‐bound malondialdehyde were determined using a commercially available TBARS assay kit following the manufacturers protocol (Thiobarbituric Acid Reactive Substances, Abcam ab118970).
Statistical analysis
Statistical analysis was performed using GraphPad Instat version 9.00. The data obtained are reported as mean values ± SEM (standard error of the mean). Differences between groups were evaluated by an independent repeated measures ANOVA test followed by a Bonferroni post hoc test. Statistical significance was present at a value of P < 0.05.
Results
Impact of exercise training on patients characteristics
At baseline, no significant differences between the three groups (control, MCT, or HIIT) were seen with respect to age, BMI, exercise capacity measured as peak V̇O2, NHYA class, hyperlipidaemia, hypertension, or atrial fibrillation. The patients included into the study exhibited normal left ventricular ejection fraction (LVEF), impaired diastolic function with an E/e′ above 15, and an elevated pro NT‐proBNP above >220 pg/mL (Table 1 ). Exercise training over a time period of 3 or 12 months resulted in a significant increase of peak V̇O2 when compared with baseline (Table 2 ). This increase was independent of the training modality, since no significant difference between HIIT and MCT was detected after 3 or 12 months. No impact of exercise training was observed over time on BMI, blood pressure, and lipid status (LDL, HDL, and total cholesterol) (Table 2 ).
Table 1.
Patient's baseline characteristics
| CG (N = 10) | MCT (N = 14) | HIIT (N = 10) | |
|---|---|---|---|
| Women, n | 8 (80%) | 9 (64%) | 8 (80%) |
| Men, n | 2 (20%) | 5 (36%) | 2 (20%) |
| Age (years) | 71 ± 3 | 71 ± 2 | 72 ± 2 |
| BMI (kg/m2) | 30.9 ± 1.6 | 32.8 ± 1.9 | 31.4 ± 1.9 |
| NYHA class (I/II/III/IV), n | 0/5/5/0 | 0/6/8/0 | 0/5/5/0 |
| LVEF (%) | 59.8 ± 1.9 | 61.2 ± 2.4 | 63.6 ± 2.6 |
| E/e′ | 17.23 ± 2.26 | 17.56 ± 0.73 | 16.51 ± 1.41 |
| NT proBNP (pg/mL) | 1008 ± 296 | 862 ± 237 | 535 ± 134 |
| Hypertension, n | 10 (100%) | 14 (100%) | 10 (100%) |
| Hyperlipidaemia, n | 8 (80%) | 11 (79%) | 9 (90%) |
| Diabetes mellitus type II, n | 1 (10%) | 5 (36%) | 4 (40%) |
| Non‐smoker, n | 5 (50%) | 7 (50%) | 7 (70%) |
| Ex‐smoker, n | 4 (40%) | 7 (50%) | 3 (30%) |
| Smoker, n | 1 (10%) | 0 | 0 |
| CAD, n | 2 (20%) | 4 (29%) | 2 (20%) |
| Atrial fibrillation | |||
| Paroxysmal, n | 1 (10%) | 1 (7%) | 2 (20%) |
| Persistent, n | 1 (10%) | 1 (7%) | 1 (10%) |
| Permanent, n | 1 (10%) | 2 (14%) | 1 (10%) |
| Medication | |||
| Beta‐blocker, n | 8 (80%) | 9 (64%) | 10 (100%) |
| Thiazide/loop diuretics, n | 9 (90%) | 13 (93%) | 7 (70%) |
| Angiotensin receptor blocker, n | 6 (60%) | 10 (72%) | 5 (50%) |
| Angiotensin‐converting enzyme inhibitor, n | 3 (30%) | 3 (22%) | 4 (40%) |
| Aldosterone antagonist, n | 1 (10%) | 1 (7%) | 2 (20%) |
| Statins, n | 5 (50%) | 8 (57%) | 6 (60%) |
BMI, body mass index; CAD, coronary artery disease.
Table 2.
Impact of exercise training on patient characteristics
| CG | MCT | HIIT | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline (N = 10) | 3 months (N = 10) | 12 months (N = 10) | Baseline (N = 14) | 3 months (N = 14) | 12 months (N = 14) | Baseline (N = 10) | 3 months (N = 10) | 12 months (N = 10) | |
| BMI (kg/m2) | 30.9 ± 1.6 | 31.0 ± 1.9 | 31.5 ± 1.9 | 32.8 ± 1.9 | 32.5 ± 2 | 32.2 ± 1.9 | 31.4 ± 1.9 | 31.3 ± 1.9 | 31.1 ± 1.9 |
| Peak V̇O2 (mL/kg/min) | 16.9 ± 1.4 | 17.9 ± 1.3 | 18.5 ± 1.4 | 17.7 ± 1.3 | 20.2 ± 1.6* | 20.3 ± 1.5* | 17.7 ± 1.2 | 20.9 ± 2.3* | 20.1 ± 2.1* |
| SBP (mmHg) | 140 ± 3 | 137 ± 3 | 141 ± 4 | 134 ± 2 | 131 ± 3 | 133 ± 3 | 135 ± 3 | 135 ± 5 | 136 ± 4 |
| DPB (mmHg) | 78 ± 3 | 72 ± 3 | 79 ± 3 | 76 ± 2 | 77 ± 2 | 78 ± 2 | 74 ± 4 | 75 ± 4 | 74 ± 3 |
| Cholesterol (mg/dL) | 187 ± 19 | 188 ± 16 | 189 ± 15 | 192 ± 13 | 173 ± 15 | 172 ± 13 | 176 ± 12 | 177 ± 11 | 185 ± 14 |
| LDL‐Cholesterol (mg/dL) | 115 ± 15 | 115 ± 15 | 115 ± 12 | 124 ± 13 | 105 ± 12 | 105 ± 11 | 108 ± 11 | 101 ± 9 | 100 ± 10 |
| HDL‐Cholesterol (mg/dL) | 58.4 ± 5.6 | 59.7 ± 3.7 | 58.9 ± 3.8 | 57.3 ± 4.8 | 55.1 ± 4.8 | 57.3 ± 3.9 | 57.3 ± 3.0 | 58.3 ± 3.5 | 63.0 ± 3.3 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure.
P < 0.05 versus baseline.
Impact of exercise training on high‐density lipoprotein mediated endothelial nitric oxide synthase phosphorylation
Analysing the impact of different training modalities on HDL‐mediated eNOS phosphorylation revealed that only HIIT after 3 months supervised training increased eNOS phosphorylation at the enzyme activating residue serin1177 (Figure 1 A–C ). This increase after 3 months was no longer detectable after continuing exercise training with the same exercise protocol for additional 9 months at home. No impact on eNOS‐Ser1177 phosphorylation was observed in the MCT or control group (Figure 1 A–C ). Correlating the change in eNOS‐Ser1177 phosphorylation and the change in peak V̇O2 after 3 months (Figure 2 ), a trend between these parameters was seen (r = 0.635, P = 0.07).
Figure 1.
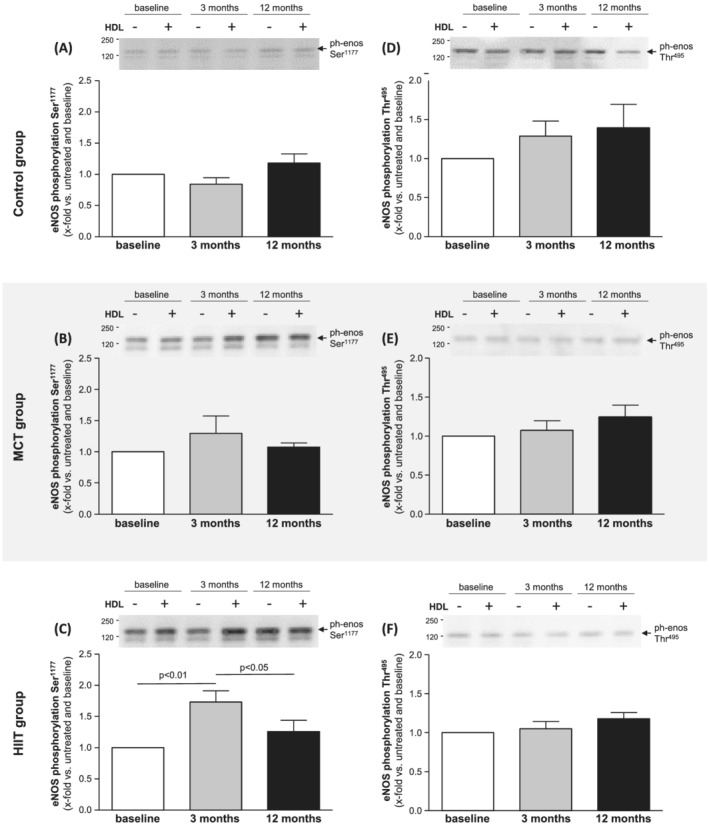
Human aortic endothelial cells (HAECs) were incubated with high‐density lipoprotein (HDL; 50 μg/mL) isolated from HFpEF patients at baseline (white bars), after 3 months (grey bars) and after 12 months (black bars). HDL‐induced phosphorylation of eNOS at Ser1177 (A–C) and Thr495 (D–F) was assessed in the control group (A,D), the MCT group (B,E), and the HIIT group (C,F). Values are shown as x‐fold increase in eNOS phosphorylation of HDL‐incubated cells versus untreated cells and expressed as means ± SEM. Representative western blots are shown above the bar graphs.
Figure 2.
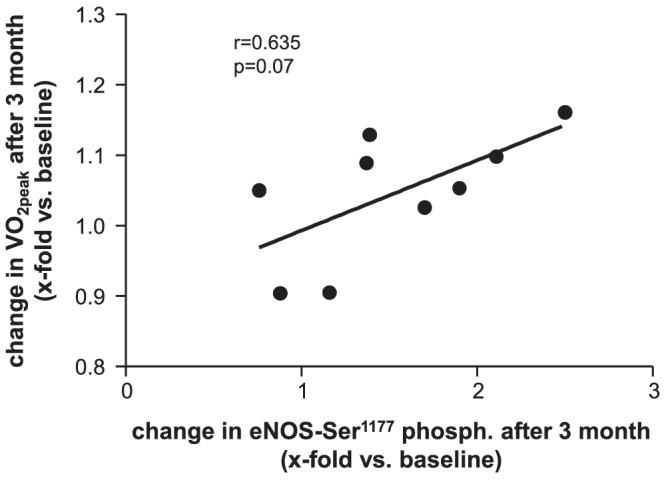
Correlation analysis between the change of eNOS‐Ser1177 phosphorylation and the change in VO2peak in the HIIT group after 3 months.
With respect to the phosphorylation of eNOS at the threonine495 site (phosphorylation leads to inhibition of enzyme activity), no significant change was observed over time in all three groups (Figure 1 D–F ).
Impact of exercise training on paraoxonase‐1 activity
Pon1 is the major enzyme responsible for anti‐oxidant activity of HDL. Measuring Pon1 activity in the isolated HDL (Figure 3 A–C ) and in serum samples (Figure 3 D–F ) showed that in the control group and MCT group, no significant increase in Pon1 activity was measured in either HDL (Figure 3 A,B ) or serum (Figure 3 D,E ). In contrast, the data collected for the HIIT group indicate a significant increase in enzyme activity after 12 months in both the isolated HDL (1.43‐fold increase vs. baseline) (Figure 3 C ) and serum (1.12‐fold increase vs. baseline) (Figure 3 F ).
Figure 3.
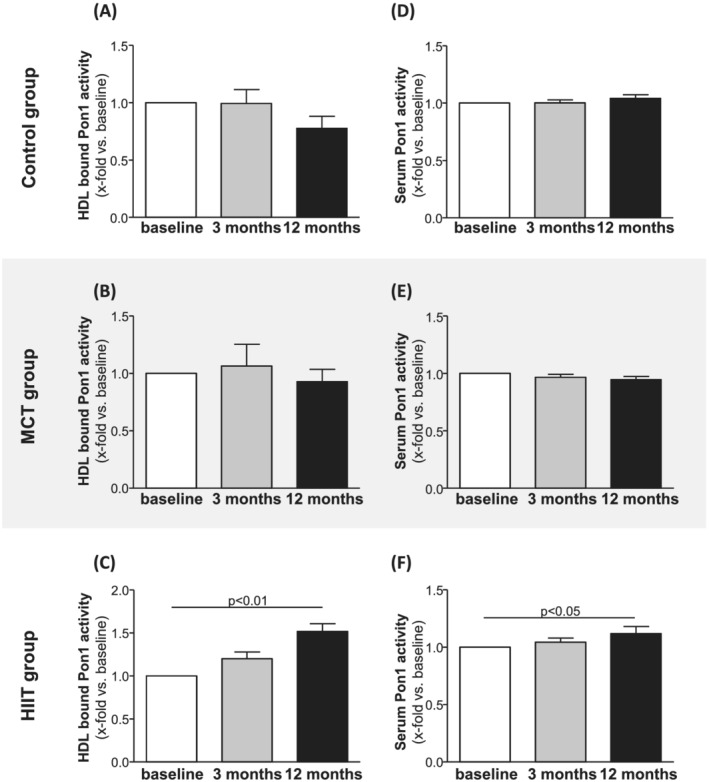
Pon1 activity bound to isolated HDL (A–C) and present in serum samples (D–F) was quantified at baseline (white bars), 3 months (grey bars), and 12 months (black bars) in samples from the control group (A,D), the MCT group (B,E), and the HIIT group (C,F). Values are shown as x‐fold increase versus baseline and expressed as means ± SEM.
Impact of exercise training on thiobarbituric acid reactive substances concentration
Measuring thiobarbituric acid reactive substances (TBARS) level in serum samples offers a convenient method of determining the relative lipid peroxide content as a marker for oxidative stress. As depicted in Figure 4 no difference in TBARS concentration was seen in the control (Figure 4 A ) and MCT group (Figure 4 B ) over time. Analysing the samples of the patients in the HIIT group a significant reduction of TBARS was evident after 3 (17% reduction) and 12 months (24% reduction) (Figure 4 C ). A negative correlation between changes in TBARS and Pon1 activity in the HIIT group was observed (Figure 5 ; r = −0.61, P < 0.05).
Figure 4.
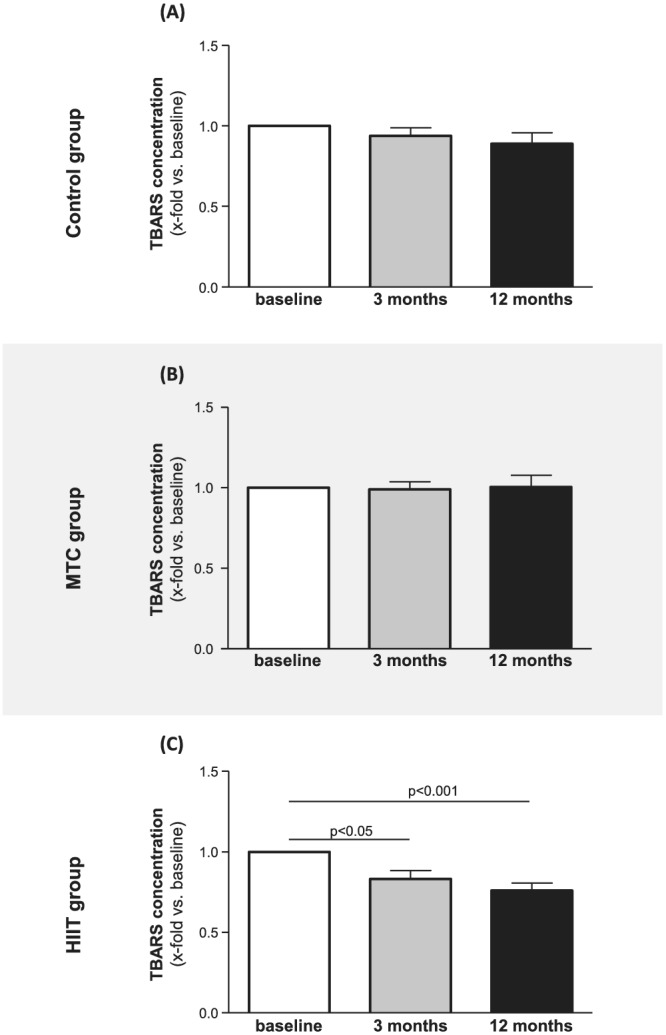
TBARS concentration was quantified at baseline (white bars), 3 months (grey bars), and 12 months (black bars) in serum samples from the control group (A), the MCT group (B), and the HIIT group (C). Values are shown as x‐fold increase vs. baseline and expressed as means ± SEM.
Figure 5.
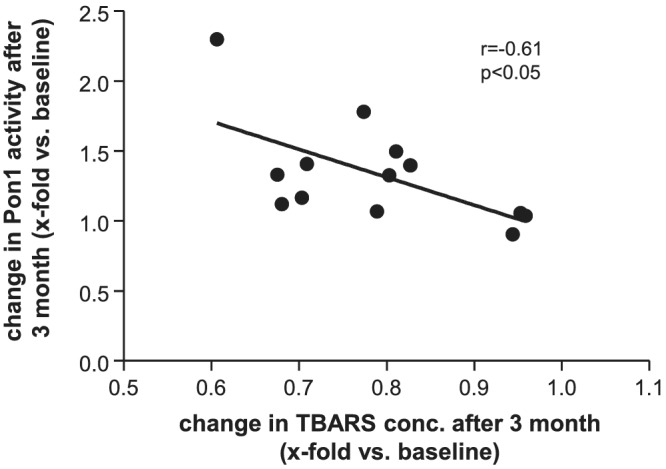
Correlation analysis between the change in TBARS serum concentration and the change in HDL‐bound Pon1 enzyme in the HIIT group after 3 months.
Discussion
Exercise training is widely accepted as treatment strategy for patients with heart failure independent of its aetiology—HFrEF or HFpEF. Beneficial effects of ET could be documented for vascular, myocardial and skeletal muscle function (reviewed in Gielen et al. 26 ). Several years ago, our group could even document that ET modulates HDL function in HFrEF, thereby influencing endothelial function via phosphorylation of eNOS. 19 If ET also impacts HDL function in HFpEF patients was unknown. The results of the present study can be summarized as follows:
HIIT but not MCT resulted in increased HDL‐mediated phosphorylation of eNOS at its activating site Ser1177 after 3 months of supervised ET.
This positive effect of HIIT diminished after switching from supervised to home‐based ET.
HDL isolated from patients after performing HIIT exhibited higher anti‐oxidative capacity as indicated by an increase in Pon1 activity.
Assessment of TBARS in the serum revealed a lower concentration after performing HIIT.
In conclusion, HIIT has clear effects on HDL function (anti‐oxidative properties and stimulating eNOS activity) in HFpEF patients, but unfortunately the eNOS activating effect is lost in case training compliance decreases. 11
Exercise training and its impact on high‐density lipoprotein
Analysing two large population‐based studies, the Copenhagen City Heart Study and the Copenhagen General Population Study, Madsen and colleagues documented that the association between HDL cholesterol concentration and all‐cause mortality was U‐shaped. Both, extreme high and low concentrations, were associated with high all‐cause mortality risk. 27 These observation and the finding that the pharmacologic increase in HDL levels did not improve mortality 28 supports the idea that not the quantity, but the functional capacity of HDL may be important for risk reduction in cardiovascular disease. 29 , 30 This idea is further supported by the finding that exercise training in heart failure patients, independent of reduced or preserved left ventricular ejection fraction, improves quality of life 11 , 31 and is even associated with lower mortality 32 irrespective of any significant modulation of HDL concentration. At least for HFrEF patients an earlier study from our group could show that ET improves HDL properties by increasing its ability to activate eNOS by phosphorylating the enzyme at its Ser1177 residue. The physiological relevance of eNOS phosphorylation at the Ser1177 has been supported by a close correlation between eNOS‐Ser1177 phosphorylation and noninvasively measured endothelial function in a cohort of healthy children. 33 With the results presented in the current study this observation can now be expanded to HFpEF patients. Also in HFpEF patients ET resulted in a higher HDL‐mediated phosphorylation of eNOS at Ser1177 without modulation of the eNOS‐Thr495 site, which inhibits eNOS activity. This is in contrast to HFrEF patients, where a HDL‐mediated reduction in Thr495 phosphorylation was seen after ET. 19
High‐density lipoprotein mediated protection against cardiovascular disease is partly attributable to its robust anti‐oxidant activities with Pon1 being the major enzyme. Measuring Pon1 activity in isolated HDL a significant increase after 12 months HIIT was observed. The association of ET and Pon1 activity is in line with earlier studies. An increase in Pon1 activity was detected immediately after a single bout of physical exercise, followed by a decrease after 2 h and a recovery of levels after 24 h. 34 In addition, a study of rugby players reported significantly higher levels of Pon1 activity after performing maximal exercise. 35 A potential regulator for this immediate change in Pon1 activity may be the accumulation of reactive oxygen species (ROS) induced by the acute exercise bout. Whereas in a chronic state a negative correlation between Pon1 activity and the load of ROS was observed. 36 Also, in the present study, a negative correlation between changes in TBARS and Pon1 activity was seen. What is the reason for observing different relations between ROS and Pon1 activity in acute and chronic conditions? The answer to this can only be speculative. In particular, for exercise training, it is well known that regular physical activity is considered a factor for preventing the development of cardiovascular disease. This goes along with the induction of antioxidative enzymes like catalase and SOD. On the other hand, a single bout of strenuous exercise results of oxygen consumption and excessive free radicals formation. Although low levels of reactive oxygen species (ROS) are beneficial for the organism ensuring normal cell and vascular function, the overproduction of ROS and increased oxidative stress levels play a significant role in the onset and progression of cardiovascular diseases (for review, see Tofas et al.). 37 Therefore, it is conceivable that different ROS concentrations exert different biological effects. Nevertheless, the precise molecular mechanism leading either to a positive of negative correlation between ROS and Pon1 activity is still not clear.
Training modalities—HIIT versus MCT
In a pioneering work, Wisloff and colleagues described the superior effect of HIIT when compared with MCT on endothelial function in patients with HFrEF. 38 In this small patient study, the improvement in flow‐dependent vasodilation was shown to be significantly greater after 3 months of HIIT compared with MCT. Nevertheless, the concept of HIIT being superior over MCT is still discussed controversial. Several large clinical multicentre trials could not confirm the superiority of HIIT when assessing the change in exercise capacity in different patient populations, 11 , 31 , 39 whereas a meta‐analysis of 10 studies in CAD patients reported a higher increase in peak V̇O2 after HIIT compared with MCT. 40 Also in HFpEF patients the discussion if HIIT is the better option for improvement in exercise capacity is still ongoing. The recently published OptimEx trial, 11 a large randomized multi‐centre trial, could not show a difference between MCT and HIIT whereas a meta‐analysis of 11 studies with 515 HFpEF patients reported more pronounced effects of HIIT when compared with MCT. 41 In the present study, the results clearly documented a difference between HIIT and MCT with respect to the modulation of HDL function. Significant changes in HDL‐mediated eNOS phosphorylation, Pon1 activity and TBARS concentration was only observed in HFpEF patients performing HIIT but not MCT. A possible explanation why only HIIT exhibited positive effects may be due to the fact that shear forces are probably higher in HIIT. Increased shear force is the driving factor for the modulation of ROS production and eNOS activation. In the report by Wisloff and colleagues, 38 HIIT showed a higher improvement of the anti‐oxidative capacity measured in serum samples. Therefore, it may be plausible that the larger reduction of ROS by HIIT results in a higher Pon1 activity associated with HDL and an increased HDL‐mediated eNOS phosphorylation. At least the significant reduction of TBARS by HIIT in the present study supports this hypothesis.
Nevertheless, it is also clear that the clinical impact of ET depends on the patient's compliance to ET advice. In the present study the effect of HIIT on HDL‐mediated eNOS phosphorylation was only detectable after 3 months of supervised training—high compliance. As soon as the patients trained at home without supervision and a lower compliance, 11 this beneficial effect was lost.
Limitations
The present study was performed at a small subset of patients from the whole study cohort of 176 HFpEF patients. The patients analysed in the current study were selected randomly from patients recruited only at the trial site Leipzig. Comparing the baseline characteristics of the recruited patients with that from the total cohort 11 only minor, but not study relevant differences were evident (see Supporting Information, Table S1 ). Therefore, we may assume that the results with respect to HDL function are representative for the whole HFpEF cohort of the OptimEx trial. Unfortunately, in the present subset of patients no measurement of endothelial function was performed. A correlation analysis between changes in HDL function (measured as change in eNOS phosphorylation at Ser1177) and changes in endothelial function would strengthen the physiological importance of exercise induced change in HDL function.
Conclusions
The results of the present study clearly documented that HIIT in HFpEF patients exerts beneficial effects on HDL mediated eNOS phosphorylation and HDL‐associated Pon1 activity. In addition, a significant effect of HIIT on a reduced ROS load was documented. These beneficial effects of HIIT were reduced as soon as the compliance was not 100% as it is the case with supervised training.
Funding
The OptimEx trial was funded by the European Commission, Framework Program 7, grant no. EU‐602405‐2; the Deutsche Forschungsgemeinschaft (DFG) through the TUM International Graduate School of Science and Engineering (IGSSE); and the Flemish Research Funds (FWO) through a Senior Clinical Investigator grant to Dr. van Craenenbroeck.
Conflict of interest
Dr. Winzer reported receiving personal fees from Amarin (lectures), Bayer (advisory board activities) Novartis (lectures and advisory board activities), Boehringer Ingelheim (advisory board activities), and CVRX (lectures) outside the submitted work. Dr. Linke reported receiving speaker fees from Abbott, Medtronic, Edwards Lifesciences, AstraZeneca, Boston Scientific, and Novartis; grants from Edwards Lifesciences and Novartis; advisory board fees from Transverse Medical, Picardia, Edwards Lifesciences, and Heart Leaflet Technology; and stock options from Claret Medical and Transverse Medical, and being a co‐owner of Dresden Cardiovascular Research Institute and Core Laboratories outside the submitted work. Dr. Pieske reported receiving personal fees from Bayer Healthcare (steering committee, lectures), Merck (steering committee, lectures), Novartis (steering committee, lectures), Servier, AstraZeneca (lectures), Bristol‐Myers Squibb (lectures), and Medscape (lectures) outside the submitted work. Dr. Van Craenenbroeck reported receiving grants from the Flemish Research Funds (FWO) during the conduct of the study. Dr. Halle reported receiving grants from the TUM International Graduate School of Science and Engineering during the conduct of the study and grants from Novartis (principal investigator of the Activity Study in HFrEF) and personal fees from Bristol‐Myers Squibb, Berlin Chemie‐Menarini, Novartis, Daiichi‐Sankyo, AstraZeneca, Roche, Abbott (advisory board on exercise and diabetes), Sanofi, Pfizer, Boehringer Ingelheim, and Bayer outside the submitted work. No other disclosures were reported.
Supporting information
Table S1. Baseline characteristics of patients with HDL function analyzed and the total Optimex study cohort.
Acknowledgements
Open Access funding enabled and organized by Projekt DEAL.
Sowa, P. W. , Winzer, E. B. , Hommel, J. , Männel, A. , van Craenenbroeck, E. M. , Wisløff, U. , Pieske, B. , Halle, M. , Linke, A. , and Adams, V. (2022) Impact of different training modalities on high‐density lipoprotein function in HFpEF patients: a substudy of the OptimEx trial. ESC Heart Failure, 9: 3019–3030. 10.1002/ehf2.14032.
[Correction added on 27 June 2022, after first online publication: Projekt DEAL funding statement has been added.]
References
- 1. Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL. Importance of heart failure with preserved systolic function in patients ≥65 years of age. CHS research group. Cardiovascular health study. Am J Cardiol. 2001; 87: 413–419. [DOI] [PubMed] [Google Scholar]
- 2. Lam CSP, Donal E, Kraigher‐Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011; 13: 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2013; 10: 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Riet EES, Hoes AW, Wagenaar KP, Limburg A, Landman MAJ, Rutten FH. Epidemiology of heart failure: The prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail. 2016; 18: 242–252. [DOI] [PubMed] [Google Scholar]
- 5. Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG, American Heart Association Advocacy Coordinating Committee, Council on Arteriosclerosis TaVB, Council on Cardiovascular Radiology and Intervention, Council on Clinical Cardiology, Council on Epidemiology and Prevention, Stroke Council . Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circ Heart Fail. 2013; 6: 606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cleland JGF, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP‐CHF) study. Eur Heart J. 2006; 27: 2338–2345. [DOI] [PubMed] [Google Scholar]
- 7. Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008; 359: 2456–2467. [DOI] [PubMed] [Google Scholar]
- 8. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014; 370: 1383–1392. [DOI] [PubMed] [Google Scholar]
- 9. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J. Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: The CHARM‐preserved trial. Lancet. 2003; 362: 777–781. [DOI] [PubMed] [Google Scholar]
- 10. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner‐La Rocca HP, Choi DJ, Chopra V, Chuquiure‐Valenzuela E, Giannetti N, Gomez‐Mesa JE, Janssens S, Januzzi JL, Gonzalez‐Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Pina IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021; 385: 1451–1461. [DOI] [PubMed] [Google Scholar]
- 11. Mueller S, Winzer EB, Duvinage A, Gevaert AB, Edelmann F, Haller B, Pieske‐Kraigher E, Beckers P, Bobenko A, Hommel J, Van de Heyning CM, Esefeld K, von Korn P, Christle JW, Haykowsky MJ, Linke A, Wisloff U, Adams V, Pieske B, Van Craenenbroeck EM, Halle M. OptimEx‐Clin study group. Effect of high‐intensity interval training, moderate continuous training, or guideline‐based physical activity advice on peak oxygen consumption in patients with heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2021; 325: 542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013; 62: 263–271. [DOI] [PubMed] [Google Scholar]
- 13. Buga GM, Gold ME, Fukuto JM, Ignarro LJ. Shear stress‐induced release of nitric oxide from endothelial cells grown on beads. Hypertension. 1991; 17: 187–193. [DOI] [PubMed] [Google Scholar]
- 14. Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, Lüscher TF. Nitric oxide is responsible for flow‐dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995; 91: 1314–1319. [DOI] [PubMed] [Google Scholar]
- 15. Yuhanna IS, Zhu Y, Cox BE, Hahner LD, Osborne‐Lawrence S, Lu P, Marcel YL, Anderson RG, Mendelsohn ME, Hobbs HH, Shaul PW. High‐density lipoprotein binding to scavenger receptor‐BI activates endothelial nitric oxide synthase. Nat Med. 2001; 7: 853–857. [DOI] [PubMed] [Google Scholar]
- 16. Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J. Major lipids,apolipoproteins, and risk of vascular disease. JAMA. 2009; 302: 1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Voight BF, Peloso GM, Orho‐Melander M, Frikke‐Schmidt R, Barbalic M, Jensen MK, Hindy G, Holm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton‐Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart AF, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett MS, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki ML, Perola M, Havulinna A, De Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, De Bakker PIW, Klungel OH, Maitland‐van der Zee A‐H, Peters BJM, De Boer A, Grobbee DE, Kamphuisen PW, Deneer VHM, Elbers CC, Onland‐Moret NC, Hofker MH, Wijmenga C, Verschuren WM, Boer JM, Van Der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, König IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Van de Werf F, Fox KA, El Mokhtari NE, Rubin D, Schrezenmeir J, Schreiber S, Schäfer A, Danesh J, Blankenberg S, Roberts R, Mc Pherson R, Watkins H, Hall AS, Overvad K, Rimm E, Boerwinkle E, Tybjaerg‐Hansen A, Cupples LA, Reilly MP, Melander O, Mannucci PM, Ardissino D, Siscovick D, Elosua R, Stefansson K, O'Donnell CJ, Salomaa V, Rader DJ, Peltonen L, Schwartz SM, Altshuler D, Kathiresan S. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet. 2011; 380: 572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vaisar T, Couzens E, Hwang A, Russell M, Barlow CE, DeFina LF, Hoofnagle AN, Kim F. Type 2 diabetes is associated with loss of HDL endothelium protective functions. PLoS ONE. 2018; 13: e0192616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adams V, Besler C, Fischer T, Riwanto M, Noack F, Höllriegel R, Oberbach A, Jehmlich N, Völker U, Winzer EB, Lenk K, Hambrecht R, Schuler GC, Linke A, Landmesser U, Erbs S. Exercise training in patients with chronic heart failure promotes restoration of HDL functional properties. Circ Res. 2013; 113: 1345–1355. [DOI] [PubMed] [Google Scholar]
- 20. Wesnigk J, Bruyndonckx L, Hoymans VY, De Guchtenaere A, Fischer T, Schuler G, Vrints CJ, Adams V. Impact of lifestyle intervention on HDL‐induced eNOS activation and cholesterol efflux capacity in obese adolescent. Cardiol Res Pract. 2016; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuboki K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T, Feener EP, Herbert TP, Rhodes CJ, King GL. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo: A specific vascular action of insulin. Circulation. 2000; 101: 676–681. [DOI] [PubMed] [Google Scholar]
- 22. Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi‐site eNOS phosphorylation. J Mol Cell Cardiol. 2007; 42: 271–279. [DOI] [PubMed] [Google Scholar]
- 23. Payne GA, Bohlen HG, Dincer UD, Borbouse L, Tune JD. Periadventitial adipose tissue impairs coronary endothelial function via PKC‐beta‐dependent phosphorylation of nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009; 297: H460–H465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Lochen ML, Löllgen H, Marques‐Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S, ESC Scientific Document Group . European guidelines on cardiovascular disease prevention in clinical practice: The sixth joint task force of the European Society of Cardiology and Other Societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts)developed with the special contribution of the European Association for Cardiovascular Prevention & rehabilitation (EACPR). Eur Heart J. 2016; 2016: 2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chang CT, Lim YP, Lee CW, Liao HY, Vheng FY, Chang CM, Tang FY, Yang CY, Chen CJ. Pon‐1 carbamylation is enhanced in HDL of uremia patients. J Food Drug Anal. 2019; 27: 542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training. Circulation. 2010; 122: 1221–1238. [DOI] [PubMed] [Google Scholar]
- 27. Madsen CM, Varbo A, Nordestgaard BG. Extreme high high‐density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: Two prospective cohort studies. Eur Heart J. 2017; 38: 2478–2486. [DOI] [PubMed] [Google Scholar]
- 28. Landrey MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J. Effects of extended‐release niacin with Laropiprant in high‐risk patients. New Engl J Med. 2014; 371: 203–212. [DOI] [PubMed] [Google Scholar]
- 29. Besler C, Heinrich K, Rohrer L, Doerries C, Riwanto M, Shih DM, Chroni A, Yonekawa K, Stein S, Schaefer N, Mueller M, Akhmedov A, Daniil G, Manes C, Templin C, Wyss C, Maier W, Tanner FC, Matter CM, Corti R, Furlong C, Lusis AJ, von Eckardstein A, Fogelman AM, Lüscher TF, Landmesser U. Mechanisms underlying adverse effects of HDL on eNOS‐activating pathways in patients with coronary artery disease. J Clin Invest. 2011; 121: 2693–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Riwanto M, Rohrer L, Roschitzki B, Besler C, Mocharla P, Mueller M, Perisa D, Heinrich K, Altwegg L, von Eckardstein A, Lüscher TF, Landmesser U. Altered activation of endothelial anti‐ and proapoptotic pathways by high‐density lipoprotein from patients with coronary artery disease: Role of high‐density lipoprotein‐proteome remodeling. Circulation. 2013; 127: 891–904. [DOI] [PubMed] [Google Scholar]
- 31. Ellingsen O, Halle M, Conraads V, Stoylen A, Dalen H, Delagardelle C, Larsen AI, Hole T, Mezzani A, Van Craenenbroeck EM, Videm V, Beckers P, Christle JW, Winzer E, Mangner N, Woitek F, Höllriegel R, Pressler A, Monk‐Hansen T, Snoer M, Feiereisen P, Valborgland T, Kjekshus J, Hambrecht R, Gielen S, Karlsen T, Prescott E, Linke A. High‐intensity interval training in patients with heart failure with reduced ejection fraction. Circulation. 2017; 135: 839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buckley BJR, Harrison SL, Fazio‐Eynullayeva E, Underhill P, Sankaranarayanan R, Wright DJ, Thijssen DHJ, Lip GYH. Cardiac rehabilitation and all‐cause mortality in patients with heart failure: A retrospective cohort study. Eur J Prev Cardiol. 2021; 28: 1704–1710. [DOI] [PubMed] [Google Scholar]
- 33. Müller U, Matsuo Y, Lauber M, Walther C, Oberbach A, Schuler G, Adams V. Correlation between endothelial function measured by finger plethysmography in children and HDL‐mediated eNOS activation ‐‐ a preliminary study. Metabolism. 2013; 62: 634–637. [DOI] [PubMed] [Google Scholar]
- 34. Tomas M, Elosua R, Senti M, Molina L, Vila J, Anglada R, Montserrat F, Covas MI, Marrugat J. Paraoxonase1‐192 polymorphism modulates the effects of regular and acute exercise on paraoxonase1 activity. J Lipid Res. 2002; 43: 713–720. [PubMed] [Google Scholar]
- 35. Otocka‐Kmiecik A, Bortnik K, Szkudlarek U, Nowak D, Orlowska‐Majdak M. Effect of exercise on plasma paraoxonase1 activity in rugby players: Dependance on training experience. Redox Rep. 2013; 18: 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Selek S, Aslan M, Horoz M, Gur M, Erel O. Oxidative status and serum PON1 activity in beta‐thalassemia minor. Clin Biochem. 2007; 40: 287–291. [DOI] [PubMed] [Google Scholar]
- 37. Tofas T, Draganidis D, Deli CK, Georgakouli K, Fatouros IG, Jamurtas AZ. Exercise‐induced regulation of redox status in cardiovascular diseases: The role of exercise training and detraining. Antioxidants (Basel). 2019; 9: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, Tjönna AE, Helgerud J, Slordahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen O, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients. Circulation. 2007; 115: 3086–3094. [DOI] [PubMed] [Google Scholar]
- 39. Conraads VM, Pattyn N, De Maeyer C, Beckers PJ, Coeckelberghs E, Cornelissen VA, Denollet J, Frederix G, Goetschalckx K, Hoymans VY, Possemiers N, Schepers D, Shivalkar B, Voigt JU, Van Craenenbroeck EM, Vanhees L. Aerobic interval training and continuous training equally improve aerobic exercise capacity in patients with coronary artery disease: The SAINTEX‐CAD study. Int J Cardiol. 2015; 179: 203–210. [DOI] [PubMed] [Google Scholar]
- 40. Liou K, Ho S, Fildes J, Ooi SY. High intensity interval versus moderate intensity continuous training in patients with coronary artery disease: A Meta‐analysis of physiological and clinical parameters. Heart Lung Circ. 2016; 25: 166–174. [DOI] [PubMed] [Google Scholar]
- 41. Boulmpou A, Theodorakopoulou MP, Boutou AK, Alexandrou ME, Papadopoulos CE, Bakaloudi DR, Pella E, Sarafidis P, Vassilikos V. Effects of different exercise programs on cardiorespiratory reserve in HFpEF: A systematic review and meta‐analysis. Hellenic J Cardiol. 2021. in press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics of patients with HDL function analyzed and the total Optimex study cohort.


