Abstract
Aims
The angiotensin receptor‐neprilysin inhibitor (ARNI) sacubitril/valsartan (Sac/Val) demonstrated to be superior to enalapril in reducing hospitalizations, cardiovascular and all‐cause mortality in patients with ambulatory heart failure and reduced ejection fraction (HFrEF), in particular when it is maximally up‐titrated. Unfortunately, the target dose is achieved in less than 50% of HFrEF patients, thus undermining the beneficial effects on the outcomes. In this study, we aimed to evaluate the role of Sac/Val and its titration dose on reverse cardiac remodelling and determine which echocardiographic index best predicts the up‐titration success.
Methods and results
From January 2020 to June 2021, we retrospectively identified 95 patients (65.6 [59.1–72.8] years; 15.8% females) with chronic HFrEF who were prescribed Sac/Val from the HF Clinics of 5 Italian University Hospitals and evaluated the tolerability of Sac/Val high dose (the ability of the patient to achieve and stably tolerate the maximum dose) as the primary endpoint in the cohort. We used a multivariable logistic regression analysis, with a stepwise backward selection method, to determine the independent predictors of Sac/Val maximum dose tolerability, using, as candidate predictors, only variables with a P‐value < 0.1 in the univariate analyses. Candidate predictors identified for the multivariable backward logistic regression analysis were age, sex, body mass index (BMI), chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), dyslipidaemia, atrial fibrillation, systolic blood pressure (SBP), baseline tolerability of ACEi/ARBs maximum dose, left ventricle global longitudinal strain (LVgLS), LV ejection fraction (EF), tricuspid annulus plane systolic excursion (TAPSE), right ventricle (RV) fractional area change (FAC), RV global and free wall longitudinal strain (RVgLS and RV‐FW‐LS). After the multivariable analysis, only one categorical (ACEi/ARBs maximum dose at baseline) and three continuous (younger age, higher SBP, and higher TAPSE), resulted significantly associated with the study outcome variable with a strong discriminatory capacity (area under the curve 0.874, 95% confidence interval (CI) (0.794–0.954) to predict maximum Sac/Val dose tolerability.
Conclusions
Our study is the first to analyse the potential role of echocardiography and, in particular, of RV dysfunction, measured by TAPSE, in predicting Sac/Val maximum dose tolerability. Therefore, patients with RV dysfunction (baseline TAPSE <16 mm, in our cohort) might benefit from a different strategy to titrate Sac/Val, such as starting from the lowest dose and/or waiting for a more extended period of observation before attempting with the higher doses.
Keywords: Heart failure, ARNI, Neprilysin inhibitors, Right ventricular function, Sacubitril valsartan
Introduction
In the PARADIGM‐HF trial, the angiotensin receptor‐neprilysin inhibitor (ARNI) sacubitril/valsartan (Sac/Val) was demonstrated to be superior to enalapril in reducing hospitalizations for worsening heart failure (HF), cardiovascular mortality, and all‐cause mortality in patients with ambulatory heart failure and reduced ejection fraction (HFrEF). 1 Because the PARADIGM‐HF trial required dose titration up to a target dose (97/103 mg) before randomization, little evidence is available regarding low (24/26 mg) and middle (49/51 mg) doses in a real‐world setting. It has been recently shown that the 97/103 or 49/51 mg dose is associated with lower mortality or hospitalization rate for HF in patients receiving Sac/Val compared with the 24/26 mg dose group. 2 In addition, in a post hoc analysis of the PARADIGM‐HF trial, patients who had a reduction to the low or middle dose of Sac/Val showed a significantly higher risk of cardiovascular death or re‐hospitalization for HF, when compared with those who maintained the target dose. 3 Unfortunately, the target dose is currently achieved in less than 50% of HFrEF patients. 4 , 5 Therefore, it be helpful to identify variables able to predict which patients are likely to reach maximal up‐titration. To date, some clinical variables, such as blood pressure, renal function, and patients' volume status, can predict the tolerability of high dose Sac/Val. 6 , 7 Other than clinical variables, some echocardiographic parameters may be associated with Sac/Val target dose achievement. Indeed, recent studies have shown that the beneficial effects of Sac/Val are related to its ability to promote reverse remodelling and improve exercise tolerance, cardiopulmonary functional capacity and autonomic function. 8 , 9 , 10
The present study aims to evaluate the role of Sac/Val and its titration dose on cardiac reverse remodelling and determine which echocardiographic index best predicts the up‐titration success.
Methods
From January 2020 to June 2021, we retrospectively selected patients with chronic HFrEF. They were prescribed with Sac/Val, according to the European Society of Cardiology guidelines 11 from the HF Clinics of 5 Italian University Hospitals (University Hospital of Salerno, Riuniti Hospital of Foggia, Monaldi Hospital and Federico II University of Naples, and University of Messina). Informed consent was obtained from all participants.
Exclusion criteria for the current study included (i) concomitant initiation of a therapy known to induce reverse remodelling (e.g. Cardiac Resynchronization Therapy [CRT]) during the study follow‐up or in the previous 6 months; (ii) echocardiographic images of low quality that do not allow for reliable offline assessment; (iii) any change of home medications in the 2 weeks before Sac/Val initiation and any change of other neurohormonal blockers (e.g. beta‐blockers and mineral‐corticoid receptor antagonists) doses during the follow‐up. From clinical records, we collected data on demographics (age and gender), medical history (presence of co‐morbidities and HF aetiology), ongoing treatments with other medications, clinical characteristics (blood pressure‐BP, heart rate‐HR, NYHA functional class), and echocardiographic results, immediately before starting Sac/Val treatment (baseline) and after 6 months from dose optimization, with doses being up‐titrated every 2 weeks after initiation. We selected only patients where Sac/Val up‐titration was attempted to avoid therapeutic inertia, even for a limited period.
The presence of chronic kidney disease (CKD) was defined by a glomerular filtration rate of less than 60 mL/min per 1.73 m2 (using the last serum creatinine value available at the time of enrolment). The ischaemic aetiology of the HF syndrome was defined as a previous history of myocardial infarction and/or prior revascularization through percutaneous and/or surgical procedures.
Patients had no history of malignancy and/or severe renal/hepatic impairment. According to the above criteria, we identified 95 patients with HFrEF.
All investigations were carried out according to the principles of the Helsinki Declaration. For this study, the primary endpoint was tolerability of Sac/Val high dose in the cohort, defined as the ability of the patient to achieve and stably tolerate the maximum dose during the follow‐up. Concerning our primary endpoint, the study cohort has been divided into two groups: patients who reached the maximum dose versus patients who did not. Our secondary outcome was a measure of patients' response to Sac/Val, defined by the occurrence of the left ventricle (LV) reverse remodelling within 6 months.
According to previous studies, 12 , 13 , 14 we considered LV reverse remodelling as an improvement of LV ejection fraction (EF) to 45% or a reduction in LV end‐systolic volume (ESV) of 15% from baseline. About the occurrence of LV reverse remodelling, we classified our study population into two main groups (responders vs. not responders).
Echocardiographic measurements
Echocardiographic examinations were performed with a 3.5 MHz monoplane ultrasound probe of Vivid E‐9 (GE‐Vingmed Ultrasound, Horten, Norway), according to international guidelines. 15 All parameters were analysed offline by two expert operators blinded to clinical data. Left ventricular ejection fraction (LVEF) was calculated by the Simpson biplane method according to the following formula: LVEF = [left ventricular end‐diastolic volume (LVEDV)‐LV end‐systolic volume (LVESV)]/LVEDV × 100 as mean of two measures in four and two apical chambers. Mitral E and A velocities, E/A ratio, tissue Doppler analysis of mitral annular E' velocity, and mitral E/e′ ratio were measured. Additionally, the diameter of the inferior vena cava and its respiratory variation was measured in the subcostal view and used to estimate right atrial pressure. Peak systolic pulmonary artery pressure (sPAP) was estimated by adding right atrial pressure to the systolic tricuspid regurgitation gradient.
Right ventricular (RV) systolic parameters were also estimated, assessed by calculating the tricuspid annulus plane systolic excursion (TAPSE) 16 and the RV fractional area change (FAC), calculated as (RV end‐diastolic area − end‐systolic area)/RV end‐diastolic area × 100. 15 Tricuspid annular S′ velocity (RVs′) was measured using pulsed‐wave tissue Doppler. 16
RV function was assessed using an off‐axis apical 4‐chamber view for better visualization of RV.
LV and RV strain analyses were also performed with 2D strain software EchoPAC (GE Healthcare) using high frame rate acquisitions (>40 frames per second) of the apical four‐chamber, two‐chamber and long‐axis view for the LV global longitudinal strain (LVgLS), and the off‐axis apical 4‐chamber view both for the RV free wall (RV‐FW‐LS) and for RV global longitudinal strain (RVgLS), as outlined in consensus documents. 17 , 18
Statistical analysis
Continuous variables were expressed as median (interquartile range [IQR]) and compared using the Student's t‐test (for normally distributed variables) or Wilcoxon–Mann–Whitney test (for non‐normally distributed variables). Normality was checked with the Kolmogorov–Smirnov and the Shapiro–Wilk statistic. When appropriate, categorical data were summarized as percentage and compared using χ 2 test or Fisher's exact test. Paired samples t‐test was performed to assess changes in continuous echocardiographic data between baseline and 6 months follow‐up. Using a stepwise backward selection method, a multivariable logistic regression analysis was employed to determine the independent predictors of Sac/Val maximum dose tolerability, using covariates with a P‐value <0.1 in the univariate analyses. Based on clinical judgement, we forced gender and the presence of CKD in the model. To assess the presence of multicollinearity, the variance inflation factor (VIF) was determined, and a value between 5 and 10 was considered indicative of multicollinearity. 19 Baseline TAPSE and RVs′ were highly correlated, and we chose to analyse only the TAPSE contribution in the final model, being the most routinely used parameter to describe RV function. We assessed linearity for continuous covariates concerning log odds of our primary outcome. We verified that the final model was a good fit by using the Hosmer–Lemeshow test and the C‐statistic. Rates of missing values among candidate variables were <4%: TAPSE, RVgLS, and RV‐FW‐LS: 3.2%, and LVgLS: 2.1%. No imputation has been realized in our study.
Due to the small sample size, to reduce the risk of overfitting, we performed an internal validation by bootstrapping 1000 samples of the original study group, as previously described. 20 As estimated in the bootstrap sample, the model was evaluated in both the bootstrap sample and the original one. The performance in the bootstrap sample represents an estimation of the apparent performance, and the performance in the original sample represents test performance. The difference between these performances is an estimation of the optimism in the apparent performance. This difference was averaged to obtain a stable estimate of the optimism.
To give a potential role in clinical practice to our final logistic regression model, each continuous predictor variable was converted to a categorical variable based on values above or below the optimal cutoff for each variable, defined as the highest value of Youden's J index (sensitivity + specificity − 1) on receiver‐operator characteristic (ROC) curve analysis for that variable in the cohort. 21 These categorical variables were then re‐entered into a multivariable logistic regression model to determine their relative contribution to the final model results. For all tests, a P value <0.05 was considered statistically significant. Statistical analysis was performed using SPSS software version 23.0 (SPSS Inc., Chicago, Illinois) and R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The baseline characteristics of the study population are reported in Table 1 . The median age was 65.6 years [59.1–72.8], and most patients were males (80, 84.2%). Hypertension was reported in 82.1% of cases, dyslipidaemia in 66.3%, and ischaemic aetiology of HF in 40%. At the time of recruitment, almost all of the patients were in NYHA class II (58%) or III (41%). Non‐cardiovascular co‐morbidities were significantly detected, with chronic obstructive pulmonary disease (COPD) in 25.3% of patients, and CKD in 44.2%.
Table 1.
Baseline characteristics of study population (n = 95)
| Clinical variables | |
|---|---|
| Age, years | 65.6 [59.1–72.8] |
| Female, N (%) | 15 (15.8) |
| BMI, kg/m2 | 27 [24.5–30.8] |
| BSA, m2 | 1.86 [1.76–2] |
| SBP, mmHg | 115 [110–130] |
| DBP, mmHg | 70 [60–80] |
| HR, b.p.m. | 68 [61–78] |
| Hypertension, N (%) | 78 (82.1) |
| Dyslipidaemia, N (%) | 63 (66.3) |
| Diabetes, N (%) | 35 (36.8) |
| Ischaemic aetiology, N (%) | 38 (40) |
| Smoking, N (%) | 17 (17.9) |
| ICD, N (%) | 38 (40) |
| CRT‐D, N (%) | 18 (18.9) |
| CKD, N (%) | 42 (44.2) |
| Atrial fibrillation, N (%) | 40 (42.1) |
| COPD, N (%) | 24 (25.3) |
| Obesity, N (%) | 30 (31.6) |
| NYHA II, N (%) | 55 (57.9) |
| NYHA III, N (%) | 39 (41) |
| Pharmacological treatment | |
|---|---|
| Beta‐blockers, N (%) | 93 (97.9) |
| MRA, N (%) | 71 (74.7) |
| Furosemide, N (%) | 74 (77.9) |
| Patients on maximum dose of ACEi/ARBs, N (%) | 36 (37.9) |
| Ivabradine, N (%) | 9 (9.5) |
BMI, body mass index; BSA, body surface area; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; ICD, implantable cardioverter defibrillator; CRT‐D, cardiac resynchronization therapy defibrillator; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; NYHA, New York Heart Association; MRA, mineralocorticoid receptor antagonist; ACEi/ARBs, angiotensin converting enzyme inhibitors/angiotensin receptor blockers.
Furosemide was prescribed in almost 78% of cases (at a mean dose of 53.2 ± 73.2 mg), mineralocorticoid receptor antagonist (MRA) in 75%, beta‐blockers in 98%, and ivabradine in 9.5%.
At 6 months from dose optimization of Sac/Val, 37.9% of the population was taking the highest dose of Sac/Val, 27.4% the intermediate dose and 34.7% the lowest dose.
The main reasons for failure to tolerate the highest Sac/Val dose were: symptomatic hypotension (39 patients, 41%); impairment of renal function (decline in estimated glomerular filtration rate (eGFR) of 30% or greater; 6 patients, 6.3%); gastrointestinal symptoms (12 patients, 12.6%); and others (2 patients, 2.1%).
Effects of Sac/Val on clinical and echocardiographic parameters at follow‐up
The main variation of clinical, haemodynamic, and echocardiographic parameters at follow‐up are reported, in Figures 1 , 2 , and Table 2 , respectively. A significant reduction of both systolic (SBP) and diastolic blood pressure (DBP) at follow‐up was observed (Figure 1 A,B ), together with a trend in HR reduction (Figure 1 C ). Conversely, a significant improvement in NYHA functional class was reported (NYHA II: from 58% to 74%; NYHA III: from 41% to 14%; Figure 2 ); notably, 11 patients (12%) showed NYHA class I, at follow‐up (Figure 2 ).
Figure 1.
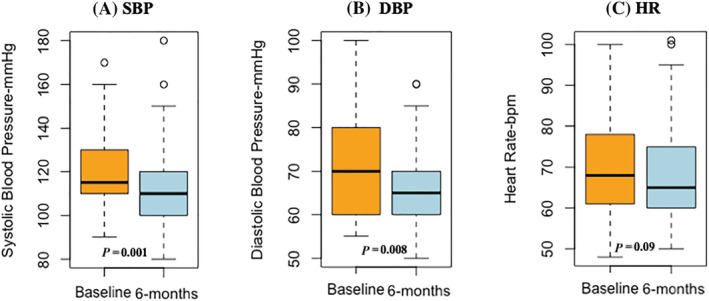
Reduction of SBP (A, P = 0.001), DBP (B, P = 0.008) and HR (C, P = 0.09) at follow‐up. SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate.
Figure 2.
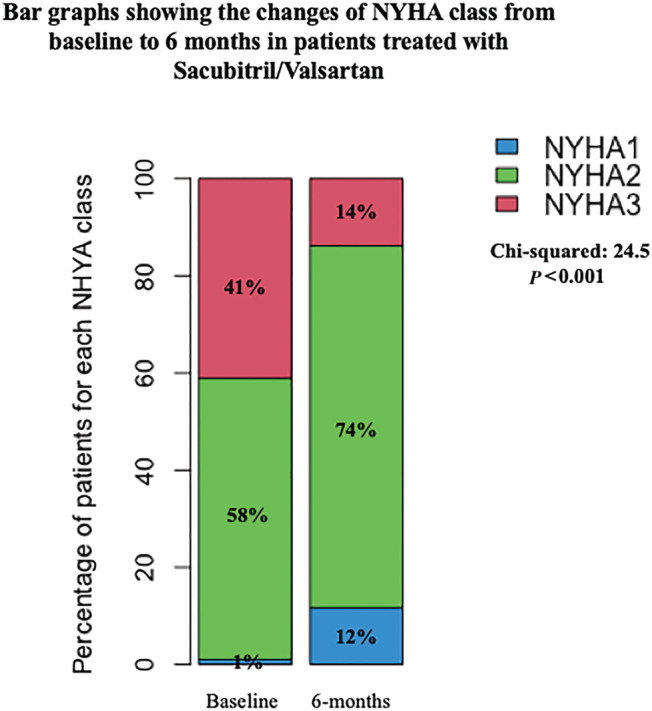
Changes of NYHA class from baseline to 6 months in patients treated with Sac/Val (P < 0.001). NYHA, New York Heart Association.
Table 2.
Changes in echocardiography 6 months after initiation of Sac/Val treatment (n = 95)
| Baseline | 6 months | P | |
|---|---|---|---|
| LVEDV, mL | 186 [158–233] | 172 [142–227] | <0.001 |
| LVESV, mL | 135 [108–180] | 114 [83–166] | <0.001 |
| LVEF, % | 28.8 [22.2–33] | 35 [29–40] | <0.001 |
| LVgLS, % | −7.4 [−9.8; −5.6] | −9.15 [−12.5; −7.1] | <0.001 |
| E/e′ ratio | 14 [11–19] | 12 [8–16] | <0.001 |
| Basal RVD, mm | 38 [34–44.7] | 37 [34–43] | 0.001 |
| RVgLS, % | −13.3 [−17.1; −8.9] | −15.6 [−19; −11.5] | <0.001 |
| RV‐FW‐LS, % | −17.7 [−21.3; −12.7] | −20 [−23.5; −15.2] | <0.001 |
| FAC, % | 35 [30–42] | 39 [34–46] | <0.001 |
| TAPSE, mm | 18 [15–20] | 18.5 [17–22] | <0.001 |
| RVs′, cm/s | 10.4 [8.8–12] | 11 [9–14] | <0.001 |
| sPAP, mmHg | 35 [28.7–48.5] | 31.5 [23–42.2] | <0.001 |
LVEDV, left ventricle end‐diastolic volume; LVESV, left ventricle end‐systolic volume; LVEF, left ventricular ejection fraction; LVgLS, left ventricular global longitudinal strain; E, early‐wave transmitral diastolic velocity; e′, early‐diastolic velocity at tissue Doppler imaging; RVD, right ventricle diameter; RVgLS, right ventricular global longitudinal strain; RV‐FW‐LS, right ventricular free wall longitudinal strain; FAC, fractional area change; TAPSE, tricuspid annular plane systolic excursion; RVs′, tricuspid annular S′ velocity; sPAP, pulmonary artery systolic pressure. We have reported in bold the statistically significant P‐value.
After 6 months, both LV and RV function significantly improved (Table 2 ): LVEF significantly increased from 28.8% [22.2–33] to 35% [29–40]; (P < 0.001). LVESV significantly decreased from 135 mL [108–180] to 114 mL [83–166] (P < 0.001), and sPAP decreased from 35 mmHg [28.7–48.5] to 31.5 mmHg [23–42.2] (P < 0.001). Moreover, both LV and RV strain analysis showed a significant improvement (LVgLS from −7.4% [−9.8; −5.6] to −9.15% [−12.5; −7.1], P < 0.001; RVgLS from −13.3% [−17.1; −8.9] to −15.6% [−19; −11.5], P < 0.001; RV‐FW‐LS from −17.7% [−21.3; −12.7] to −20% [−23.5; −15.2], P < 0.001).
In patients who achieved the maximum dose of Sac/Val, we observed some differences in baseline clinical and haemodynamic parameters (Table 3 ) and in the magnitude of improvement of some LV and RV echocardiographic parameters (Table 4 ). Patients who tolerated the maximum dose of Sac/Val also showed a positive trend in term of LV reverse remodelling (Figure 3 ), and of a NYHA class <3 at follow‐up (Supporting Information, Figure S1 ).
Table 3.
Baseline clinical and haemodynamic characteristics in relation to the Sac/Val dosage
| Maximum dose not reached, n = 59 | Maximum dose reached, n = 36 | P | |
|---|---|---|---|
| Age, years | 69.4 [62.5–74.5] | 59.7 [49.3–66.4] | 0.002 |
| Female, N (%) | 10 (16.9) | 5 (13.9) | 0.69 |
| BMI, kg/m2 | 27.5 [25.1–30.8] | 25.5 [23–28.8] | 0.07 |
| SBP, mmHg | 110 [110–120] | 122.5 [110–133.7] | 0.047 |
| DBP, mmHg | 65 [60–70] | 72.5 [62.5–80] | 0.016 |
| HR, b.p.m. | 70 [61–80] | 66.5 [61.2–75] | 0.89 |
| Hypertension, N (%) | 50 (84.7) | 28 (77.8) | 0.39 |
| Dyslipidaemia, N (%) | 43 (72.9) | 20 (55.6) | 0.08 |
| Diabetes, N (%) | 22 (37.3) | 13 (36.1) | 0.91 |
| Ischaemic aetiology, N (%) | 26 (44.1) | 12 (33.3) | 0.3 |
| Smoking, N (%) | 10 (16.9) | 7 (19.4) | 0.9 |
| ICD, N (%) | 21 (35.6) | 17 (47.2) | 0.75 |
| CRT‐D, N (%) | 13 (22) | 5 (13.9) | |
| CKD, N (%) | 28 (47.5) | 14 (38.9) | 0.41 |
| Atrial fibrillation, N (%) | 32 (54.2) | 8 (22.2) | 0.009 |
| COPD, N (%) | 19 (32.2) | 5 (13.9) | 0.046 |
| Obesity, N (%) | 19 (32.2) | 11 (30.6) | 0.87 |
| NYHA II, N (%) | 34 (57.6) | 21 (58.3) | 0.73 |
| NYHA III, N (%) | 24 (40.7) | 15 (41.7) |
BMI, body mass index; BSA, body surface area; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; ICD, implantable cardioverter defibrillator; CRT‐D, cardiac resynchronization therapy defibrillator; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; NYHA, New York Heart Association. We have reported in bold the statistically significant P‐value.
Table 4.
Change in echocardiographic parameters at 6 months, in relation to the Sac/Val dosage
| Δ‐Change in echocardiography a | Maximum dose not reached, n = 59 | Maximum dose reached, n = 36 | P |
|---|---|---|---|
| ΔLVEDV, mL | −9 [−32.6; 11] | −20 [−32; −5] | 0.054 |
| ΔLVESV, mL | −15.6 [−38; −6] | −25.5 [−33.7; −13.5] | 0.037 |
| ΔLVgLS, % | −1.5 [−2.9; −0.6] | −2 [−2.7; −1.1] | 0.2 |
| ΔLVEF, % | 5 [3–9.4] | 7.75 [5.25–11] | 0.032 |
| ΔE/e′ ratio | −2 [−4; 0] | −2.4 [−5; −1.1] | 0.13 |
| ΔBasal RVD, mm | −2 [−3; 0.5] | −1 [−3; 0] | 0.72 |
| ΔRVgLS, % | −1 [−3.6; −0.27] | −1.9 [−2.9; −0.95] | 0.52 |
| ΔRV‐FW‐LS, % | −1 [−4.6; −0.07] | −2 [−3.4; −0.5] | 0.22 |
| ΔFAC, % | 4 [2–5] | 4.75 [2–6] | 0.27 |
| ΔTAPSE, mm | 1 [0.5–2.5] | 2 [1–3] | 0.2 |
| ΔRVs', cm/s | 1 [0.35–2] | 1.5 [1–3] | 0.05 |
| ΔsPAP, mmHg | −5 [−13.5; 1.25] | −5 [−10; 0] | 0.57 |
LVEDV, left ventricle end‐diastolic volume; LVESV, left ventricle end‐systolic volume; LVgLS, left ventricular global longitudinal strain; LVEF, left ventricular ejection fraction; E, early‐wave transmitral diastolic velocity; e′, early‐diastolic velocity at tissue Doppler imaging; RVD, right ventricle diameter; RVgLS, right ventricular global longitudinal strain; RV‐FW‐LS, right ventricular free wall longitudinal strain; FAC, fractional area change; TAPSE, tricuspid annular plane systolic excursion; RVs′, tricuspid annular S′ velocity; sPAP, pulmonary artery systolic pressure. We have reported in bold the statistically significant P‐value.
Δ‐Change in echocardiography: follow‐up ‐ baseline value.
Figure 3.
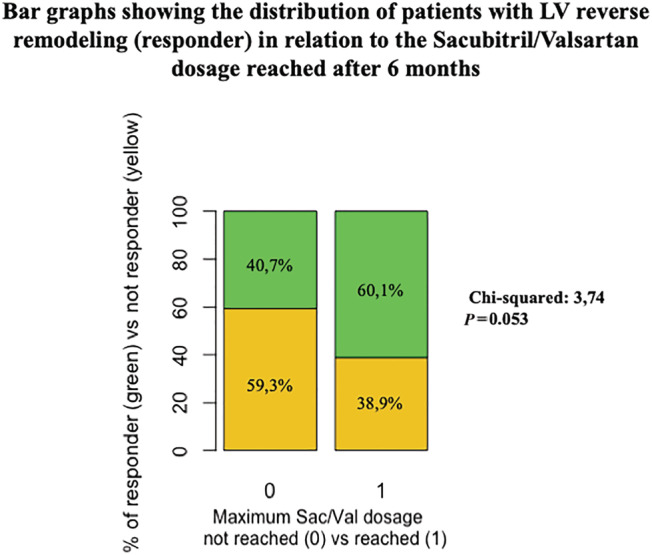
The distribution of patients with left ventricular reverse remodelling (responder) in relation to Sac/Val dosage reached after 6 months (P = 0.053).
Determinants of the maximum dose tolerability: univariate and multivariable analysis
Univariable regression analysis between baseline characteristics and the primary outcome variable is shown in the Supporting Information, Table S1 .
Candidate predictors for the multivariable backward logistic regression analysis were selected as outlined in the Methods section. When we entered the following variables: age, sex, body mass index‐BMI, CKD, COPD, dyslipidaemia, atrial fibrillation, SBP, baseline tolerability of ACEi/ARBs maximum dose, LVgLS, LVEF, TAPSE, FAC, RVgLS, and RV‐FW‐LS in the multivariable model, only one categorical (ACEi/ARBs maximum dose at baseline), and three continuous (younger age, higher SBP, and higher TAPSE) resulted significantly associated with the study outcome variable (Supporting Information, Table S2 ). The final regression model demonstrated a strong discriminatory capacity [area under the curve 0.874, 95% confidence interval (CI) 0.794–0.954]. Differences between observed and expected probabilities were small, indicating good calibration (P = 0.695, using the Hosmer–Lemeshow statistic). After adjustment for optimism, our final risk prediction model was able to discriminate patients achieving the highest dose of Sac/Val from those unable to tolerate the target dose, with a consistent C statistics of 0.853. The three continuous variables associated with the primary study endpoint in the final model were converted into categorical ones, as outlined in the Methods section, and re‐entered in a binary logistic regression model, to show the contribution of each term in the determination of the primary outcome (Table 5 ).
Table 5.
Multivariable predictive model of reaching maximum recommended dose of Sac/Val
| Variables | Odds ratio | 95% confidence interval | p |
|---|---|---|---|
| Age ≥60 years | 0.202 | 0.06–0.675 | 0.009 |
| SBP ≥ 125 mmHg | 2.942 | 1.021–8.480 | 0.046 |
| Baseline TAPSE ≥16 mm | 3.744 | 1.054–13.3 | 0.041 |
| ACE/ARBs maximum dose at baseline | 3.496 | 1.289–9.481 | 0.014 |
SBP, systolic blood pressure; TAPSE, tricuspid annular plane systolic excursion; ACEi/ARBs, angiotensin converting enzyme inhibitors/angiotensin receptor blockers. We have reported in bold the statistically significant P‐value.
Discussion
This study shows that the PARADIGM‐HF target dose of Sac/Val is achieved in about 38% of HFrEF patients during the 6 month follow‐up. Compared with patients enrolled in the PARADIGM‐HF trial, 1 our patients demonstrated a similar age and prevalence of co‐morbidities, but higher rankings on the NYHA classification and higher rates of implanted cardiac devices (albeit similar values of LVEF). Moreover, patients of the PARADIGM‐HF trial were selected a priori to tolerate the maximum tested dose. 1
Because little information is available about the titration and tolerability of maximal doses of Sac/Val in clinical practice for HFrEF patients, these findings still emphasize the issue of appropriate Sac/Val dose titration in a real‐world setting. Due to emerging data indicating that higher doses of Sac/Val are associated with improved outcomes, 22 investigations on mechanisms related to dose titration in the community are eagerly awaited.
To the best of our knowledge, our study is the first to analyse the potential role of echocardiography in predicting Sac/Val maximum dose tolerability. Indeed, previous studies designed to test the association with the achievement of the highest Sac/Val doses focused only on clinical variables, such as, demographic characteristics, BP and concomitant medication. 6 , 23
Accordingly, we showed that age, baseline SBP and baseline tolerability of ACEi/ARBs maximum dose, are critical determinants of Sac/Val target dose achievement. Baseline SBP is, of course, the primary determinant for achieving of higher Sac/Val dose. In our cohort, a significant reduction in BP was observed during follow‐up, and hypotension still represents, in several observational studies, the main determinant of ineffective up‐titration. 24 Elderly patients are frailer, with a higher co‐morbidity burden and, therefore, more prone to adverse effects during up‐titration, compared with the selected trial population. 7 Finally, as expected, the baseline tolerability of ACEi/ARBs maximum dose plays a key role in achieving of Sac/Val target dose, in line with previous literature findings. 6
About the echocardiography role, we confirmed the ability of Sac/Val to improve, during follow‐up, several parameters related to both LV and RV function. 25 , 26 , 27 This information is lacking in the PARADIGM‐HF trial because the study design did not include patients with echocardiographic follow‐up. We also showed an improvement in NYHA class during the follow‐up and a trend towards a better clinical improvement in patients with positive reverse remodelling during Sac/Val treatment. 28 , 29
The analysis of potential baseline echocardiographic contributors to Sac/Val maximum dose tolerability showed that RV baseline function, as measured by TAPSE, predicts the ability to reach the Sac/Val target dose. Although the role of RV function in the prognostic definition of patients with HFrEF has been previously elucidated, 30 , 31 its potential effect on appropriate drug management for patients with HFrEF remains undetermined. It has been recently reported that, in patients with HFrEF, Sac/Val is associated with the improvement of the RV function. 32 , 33 However, in both studies, Sac/Val target dose was under‐represented. In 163 patients enrolled by Masarone and colleagues, 33 Sac/Val was administered at a dose of 24/26 mg twice daily in 102 patients (62.5%) and at a dose of 49/51 mg twice daily in the remaining 61 patients (37.5%), while in a more recent work by Yang and collaborators, 32 22 patients (27%) were treated with 49/51 mg twice daily, 56 patients (68%) with 24/26 mg twice daily, and 4 patients (5%) were managed with a very low dose of 12/13 mg twice daily. From a clinical point of view, a low dose of Sac/Val can be still effective 34 ; therefore, it could be argued that patients with RV dysfunction (baseline TAPSE < 16 mm, in our cohort) still need to be considered for Sac/Val treatment and, even, for possible up‐titration, possibly with a different follow‐up timing and dose modification pattern, to maximize the Sac/Val up‐titration process.
However, in patients with significant bi‐ventricular dysfunction, uncertainties about the fine management of this drug need to be elucidated by assessing the exact mechanisms linked to an improvement of RV function. 35 , 36 , 37
Study limitations
Several limitations should be acknowledged, limiting the generalization of results. The study's retrospective nature and the relatively small sample size and the absence of a control group treated with ACEi/ARBs limit definitive conclusions. In addition, although several baseline information has been collected, some specific variables might be missed, with a potential influence on the final multivariable model results. Nonetheless, even of small size, our study is developed in a real‐world context and provided a multicentre collection of the data from several outpatient clinics for HF, supporting our findings' relevance.
Conclusions
The present study shows a significant improvement of both RV and LV function during Sac/Val treatment in patients with HFrEF, with evidence of a positive effect on LV remodelling. Baseline RV dysfunction may be associated with poor maximum Sac/Val up‐titration, suggesting different approach in this cohort. More extensive studies are encouraged to generate and validate, both internally and externally, specific models able to predict the tolerability to the Sac/Val maximum dose.
Conflict of interest
Carlo Gabriele Tocchetti has received funding from Amgen and personal fees from VivaLyfe and is listed as an inventor on two heart failure patents. Valeria Visco, Ilaria Radano, Alfonso Campanile, Amelia Ravera, Angelo Silverio, Daniele Masarone, Giuseppe Pacileo, Michele Correale, Pietro Mazzeo, Giuseppe Dattilo, Francesco Giallauria, Alessandra Cuomo, Valentina Mercurio, Paola Di Pietro, Albino Carrizzo, Rodolfo Citro, Gennaro Galasso, Carmine Vecchione, and Michele Ciccarelli declare no conflict of interest.
Supporting information
Figure S1. The distribution of patient with NYHA class of 3 and less than 3, in relation to the Sac/Val dosage reached after 6 months. NYHA: New York Heart Association.
Table S1. Univariable associations between baseline predictors and the ability in reaching maximum recommended dose of Sac/Val.
Table S2. Multivariable predictive model of reaching maximum recommended dose of Sac/Val before dichotomization of the main continuous predictors.
Visco, V. , Radano, I. , Campanile, A. , Ravera, A. , Silverio, A. , Masarone, D. , Pacileo, G. , Correale, M. , Mazzeo, P. , Dattilo, G. , Giallauria, F. , Cuomo, A. , Mercurio, V. , Tocchetti, C. G. , Di Pietro, P. , Carrizzo, A. , Citro, R. , Galasso, G. , Vecchione, C. , and Ciccarelli, M. (2022) Predictors of sacubitril/valsartan high dose tolerability in a real world population with HFrEF. ESC Heart Failure, 9: 2909–2917. 10.1002/ehf2.13982.
References
- 1. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, Investigators P‐H, Committees. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 2. Kido K, Bianco C, Caccamo M, Fang W, Sokos G. Evaluating Sacubitril/Valsartan Dose Dependence on Clinical Outcomes in Patients With Heart Failure With Reduced Ejection Fraction. Ann Pharmacother 2021; 55: 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vardeny O, Claggett B, Packer M, Zile MR, Rouleau J, Swedberg K, Teerlink JR, Desai AS, Lefkowitz M, Shi V, McMurray JJ, Solomon SD. Prospective Comparison of AwAtDIoGM, Morbidity in Heart Failure I. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction, the PARADIGM‐HF trial. Eur J Heart Fail 2016; 18: 1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wachter R, Fonseca AF, Balas B, Kap E, Engelhard J, Schlienger R, Klebs S, Wirta SB, Kostev K. Real‐world treatment patterns of sacubitril/valsartan: a longitudinal cohort study in Germany. Eur J Heart Fail 2019; 21: 588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Antol DD, Casebeer AW, DeClue RW, Stemkowski S, Russo PA. An Early View of Real‐World Patient Response to Sacubitril/Valsartan: A Retrospective Study of Patients with Heart Failure with Reduced Ejection Fraction. Adv Ther 2018; 35: 785–795. [DOI] [PubMed] [Google Scholar]
- 6. Pharithi RB, Ferre‐Vallverdu M, Maisel AS, O'Connell E, Walshe M, Sweeney C, Barton J, McDonald K, O'Hare D, Watson C, Gallagher J, Ledwidge M, McDonald K. Sacubitril‐Valsartan in a routine community population: attention to volume status critical to achieving target dose. ESC Heart Fail 2020; 7: 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mapelli M, Salvioni E, de Martino F, Mattavelli I, Bonomi A, Sassi V, Gugliandolo P, Vignati C, Magini A, Rovai S, Paolillo S, Agostoni P. Sacubitril/valsartan use in a real‐world population of patients with heart failure and reduced ejection fraction. J Cardiovasc Med (Hagerstown) 2020; 21: 882–888. [DOI] [PubMed] [Google Scholar]
- 8. Giallauria F, Vitale G, Pacileo M, Di Lorenzo A, Oliviero A, Passaro F, Calce R, Parlato A, Testa C, D'Ambrosio G, Romano G, Clemenza F, Sarullo S, Venturini E, Gentile M, Nugara C, Iannuzzo G, D'Andrea A, Vigorito C, Sarullo FM. Sacubitril/Valsartan Improves Autonomic Function and Cardiopulmonary Parameters in Patients with Heart Failure with Reduced Ejection Fraction. J Clin Med 2020; 9: E1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vitale G, Romano G, Di Franco A, Caccamo G, Nugara C, Ajello L, Storniolo S, Sarullo S, Agnese V, Giallauria F, Novo G, Clemenza F, Sarullo FM. Early Effects of Sacubitril/Valsartan on Exercise Tolerance in Patients with Heart Failure with Reduced Ejection Fraction. J Clin Med 2019; 8: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moon MG, Hwang IC, Choi W, Cho GY, Yoon YE, Park JB, Lee SP, Kim HK, Kim YJ. Reverse remodelling by sacubitril/valsartan predicts the prognosis in heart failure with reduced ejection fraction. ESC Heart Fail 2021; 8: 2058–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, Group ESCSD . 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 12. Mastenbroek MH, Van't Sant J, Versteeg H, Cramer MJ, Doevendans PA, Pedersen SS, Meine M. Relationship Between Reverse Remodeling and Cardiopulmonary Exercise Capacity in Heart Failure Patients Undergoing Cardiac Resynchronization Therapy. J Card Fail 2016; 22: 385–394. [DOI] [PubMed] [Google Scholar]
- 13. Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, Ghio S, Leclercq C, Bax JJ, Yu CM, Gorcsan J 3rd. St John Sutton M, De Sutter J, Murillo J. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation 2008; 117: 2608–2616. [DOI] [PubMed] [Google Scholar]
- 14. Stellbrink C, Breithardt OA, Franke A, Sack S, Bakker P, Auricchio A, Pochet T, Salo R, Kramer A, Spinelli J, Investigators P‐C, Group CPIGCHFR . Impact of cardiac resynchronization therapy using hemodynamically optimized pacing on left ventricular remodeling in patients with congestive heart failure and ventricular conduction disturbances. J Am Coll Cardiol 2001; 38: 1957–1965. [DOI] [PubMed] [Google Scholar]
- 15. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–270. [DOI] [PubMed] [Google Scholar]
- 16. Ling LF, Marwick TH. Echocardiographic assessment of right ventricular function: how to account for tricuspid regurgitation and pulmonary hypertension. JACC Cardiovasc Imaging 2012; 5: 747–753. [DOI] [PubMed] [Google Scholar]
- 17. Muraru D, Onciul S, Peluso D, Soriani N, Cucchini U, Aruta P, Romeo G, Cavalli G, Iliceto S, Badano LP. Sex‐ and Method‐Specific Reference Values for Right Ventricular Strain by 2‐Dimensional Speckle‐Tracking Echocardiography. Circ Cardiovasc Imaging 2016; 9: e003866. [DOI] [PubMed] [Google Scholar]
- 18. Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, Pedri S, Ito Y, Abe Y, Metz S, Song JH, Hamilton J, Sengupta PP, Kolias TJ, d'Hooge J, Aurigemma GP, Thomas JD, Badano LP. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 1–11. [DOI] [PubMed] [Google Scholar]
- 19. Yu H, Jiang S, Land KC. Multicollinearity in hierarchical linear models. Soc Sci Res 2015; 53: 118–136. [DOI] [PubMed] [Google Scholar]
- 20. Steyerberg EW, Harrell FE Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001; 54: 774–781. [DOI] [PubMed] [Google Scholar]
- 21. Youden WJ. Index for rating diagnostic tests. Cancer 1950; 3: 32–35. [DOI] [PubMed] [Google Scholar]
- 22. Martens P, Belien H, Dupont M, Vandervoort P, Mullens W. The reverse remodeling response to sacubitril/valsartan therapy in heart failure with reduced ejection fraction. Cardiovasc Ther 2018; 36: e12435. [DOI] [PubMed] [Google Scholar]
- 23. Martens P, Verluyten L, Van de Broek H, Somers F, Dauw J, Dupont M, Mullens W. Determinants of maximal dose titration of sacubitril/valsartan in clinical practice. Acta Cardiol 2021; 76: 20–29. [DOI] [PubMed] [Google Scholar]
- 24. Nandal S, Chow CL, Hannah V, Vaddadi G, Van Gaal W. Tolerability and efficacy of sacubitril/valsartan in clinical practice. Intern Med J 2021; 51: 87–92. [DOI] [PubMed] [Google Scholar]
- 25. Polito MV, Silverio A, Rispoli A, Vitulano G, Auria F, De Angelis E, Loria F, Gigantino A, Bonadies D, Citro R, Carrizzo A, Galasso G, Iaccarino G, Vecchione C, Ciccarelli M. Clinical and echocardiographic benefit of Sacubitril/Valsartan in a real‐world population with HF with reduced ejection fraction. Sci Rep 2020; 10: 6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Landolfo M, Piani F, Esposti DD, Cosentino E, Bacchelli S, Dormi A, Borghi C. Effects of sacubitril valsartan on clinical and echocardiographic parameters of outpatients with heart failure and reduced ejection fraction. Int J Cardiol Heart Vasc 2020; 31: 100656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Correale M, Mallardi A, Mazzeo P, Tricarico L, Diella C, Romano V, Ferraretti A, Leopizzi A, Merolla G, Di Biase M, Brunetti ND. Sacubitril/valsartan improves right ventricular function in a real‐life population of patients with chronic heart failure: The Daunia Heart Failure Registry. Int J Cardiol Heart Vasc 2020; 27: 100486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging 2011; 4: 98–108. [DOI] [PubMed] [Google Scholar]
- 29. Rodil Fraile R, Malafarina V, Tiberio LG. Sacubitril‐valsartan in heart failure and multimorbidity patients. ESC Heart Fail 2018; 5: 956–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dini FL, Carluccio E, Simioniuc A, Biagioli P, Reboldi G, Galeotti GG, Raineri C, Gargani L, Scelsi L, Mandoli GE, Cannito A, Rossi A, Temporelli PL, Ghio S. Network Labs Ultrasound in Heart Failure Study G. Right ventricular recovery during follow‐up is associated with improved survival in patients with chronic heart failure with reduced ejection fraction. Eur J Heart Fail 2016; 18: 1462–1471. [DOI] [PubMed] [Google Scholar]
- 31. Meyer P, Filippatos GS, Ahmed MI, Iskandrian AE, Bittner V, Perry GJ, White M, Aban IB, Mujib M, Dell'Italia LJ, Ahmed A. Effects of right ventricular ejection fraction on outcomes in chronic systolic heart failure. Circulation 2010; 121: 252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang Y, Shen C, Lu J, Fu G, Xiong C. Sacubitril/valsartan in the treatment of right ventricular dysfunction in patients with heart failure with reduced ejection fraction: a real‐world study. J Cardiovasc Pharmacol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Masarone D, Errigo V, Melillo E, Valente F, Gravino R, Verrengia M, Ammendola E, Vastarella R, Pacileo G. Effects of Sacubitril/Valsartan on the Right Ventricular Arterial Coupling in Patients with Heart Failure with Reduced Ejection Fraction. J Clin Med 2020; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim H, Oh J, Lee S, Ha J, Yoon M, Chun KH, Lee CJ, Park S, Lee SH, Kang SM. Clinical evidence of initiating a very low dose of sacubitril/valsartan: a prospective observational analysis. Sci Rep 2021; 11: 16335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Correale M, Mazzeo P, Magnesa M, Fortunato M, Tricarico L, Leopizzi A, Mallardi A, Mennella R, Tucci S, Brunetti ND. Predictors of right ventricular function improvement with sacubitril/valsartan in a real‐life population of patients with chronic heart failure. Clin Physiol Funct Imaging 2021; 41: 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Izzo C, Vitillo P, Di Pietro P, Visco V, Strianese A, Virtuoso N, Ciccarelli M, Galasso G, Carrizzo A, Vecchione C. The Role of Oxidative Stress in Cardiovascular Aging and Cardiovascular Diseases. Life (Basel) 2021; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Visco V, Esposito C, Vitillo P, Vecchione C, Ciccarelli M. It is easy to see, but it is better to foresee: a case report on the favourable alliance between CardioMEMS and levosimendan. Eur Heart J Case Rep 2020; 4: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The distribution of patient with NYHA class of 3 and less than 3, in relation to the Sac/Val dosage reached after 6 months. NYHA: New York Heart Association.
Table S1. Univariable associations between baseline predictors and the ability in reaching maximum recommended dose of Sac/Val.
Table S2. Multivariable predictive model of reaching maximum recommended dose of Sac/Val before dichotomization of the main continuous predictors.


