Abstract
ERBB2 exon 20 insertions may impact the clinical management of lung cancer patients. However, the frequency of ERBB2 exon 20 insertions in lung cancer patients in Brazil is scarce. Here, we analyzed 722 Brazilian non‐small cell lung cancer (NSCLC) patients from Barretos Cancer Hospital that were indicated to require routine lung cancer molecular testing. ERBB2 exon 20 insertions were evaluated by a targeted panel using next‐generation sequencing (NGS). Clinicopathological and molecular data were collected from patient medical records. Among the 722 NSCLC patients, 85.2% had lung adenocarcinomas, 53.9% were male, 66.8% were quitter or current smokers, and 63.2% were diagnosed at an advanced stage of the disease. We identified 0.8% (6/722) of patients who harbored the insertion p.(Tyr772_Ala775dup) at exon 20 of the ERBB2 gene. All ERBB2 mutated patients were diagnosed with lung adenocarcinoma, were never smokers, and wild‐type for EGFR, KRAS, and ALK hotspot alterations. Less than 1% of Brazilian NSCLC patients harbor ERBB2 exon 20 insertions, yet they could benefit in future from the new drugs in development.
Keywords: Brazil, ERBB2, exon 20 insertions, non‐small cell lung cancer
ERBB2 exon 20 insertions are rare in Brazilian patients and mutually exclusive with EGFR, KRAS, and ALK alterations.
![]()
INTRODUCTION
Lung cancer remains the deadliest cancer worldwide. 1 The International Agency for Research on Cancer estimated about 2.2 million new cases and 1.8 million deaths to the year 2020 around the world. 1 , 2 In Brazil, lung cancer is one of the most frequent diagnosed cancers, and the leading cause of cancer‐related deaths. 1 , 2
Targeted therapies towards specific molecular alterations in non‐small cell lung cancer (NSCLC) tumors, such as EGFR mutations and ALK translocations, have improved the clinical management and prognosis for patients. 3 The human epidermal growth factor receptor 2 gene (ERBB2) is a proto‐oncogene that has emerged as a candidate for targeted therapies in lung adenocarcinoma patients. 4 ERBB2 gene is amplified in about 2% of NSCLC tumors, and ERBB2 mutations have been reported in 2%–4% of NSCLC tumors. 4 Most ERBB2 mutations are duplications or insertions of 12 nucleotides at the exon 20. 4 This insertion adds four aminoacids (TyrValMetAla) in the kinase domain at codon 775, leading putatively to increased gene activation. 4 , 5 , 6 Additionally, ERBB2 exon 20 insertions are mutually exclusive with EGFR and KRAS mutations as either ALK translocations. 4 , 5 , 6 A recent comprehensive review highlighted the importance of ERBB2 mutations in the clinical management of NSCLC patients, and several drugs targeting ERBB2 insertion in exon 20, such as trastuzumab deruxtecan, poziotinib and pyrotinib, have been under clinical trials. 6
The frequency of ERBB2 exon 20 insertions in lung cancer patients in Brazil is scarce. Therefore, we aimed to evaluate the frequency of ERBB2 exon 20 insertions and their clinicopathological and molecular features in a series of Brazilian NSCLC patients.
METHODS
We evaluated a retrospective series of 722 Brazilian NSCLC patients, coming from almost all Brazilian regions (Supplementary Figure 1), diagnosed at Barretos Cancer Hospital (Barretos, São Paulo, Brazil). All patients were indicated to routine lung cancer molecular testing (EGFR, KRAS, and ERBB2) and ALK translocations at the Department of Molecular Diagnosis from the institution between the years 2018 and 2021. The clinicopathological and molecular data were collected from the medical records of patients. The present study was approved by the Barretos Cancer Hospital IRB (project no. 630/2012) and waived written informed consent due to the retrospective nature of the study. All procedures were performed following the Declaration of Helsinki.
DNA was isolated from formalin‐fixed paraffin‐embedded (FFPE) tumor using the commercial kit QIAamp DNA Micro Kit (Qiagen). DNA concentration and purity were evaluated by Nanodrop 2000 (Thermo Scientific) and by Qubit 2.0 Fluorometer (Thermo Fisher Scientific) with Qubit dsDNA HS assay kit (Thermo Fisher Scientific).
The mutational status of the exon 20 from ERBB2 gene (NM_004448) was assessed by next‐generation sequencing using the targeted panel TruSight Tumor 15 (Illumina) on the MiSeq instrument, according to the manufacturer's instructions. For the read alignment and variant calling, we used the BaseSpace BWA Enrichment version 2.1 (Illumina) and the Sophia DDM software version 4.2 (Sophia Genetics SA). Only ERBB2 insertions in exon 20 with depth higher than 500x and allele frequency higher than 5% were selected.
Detection of ALK rearrangements were routinely performed in FFPE sections using Ventana ALK (D5F3) CDx Assay (Roche) according to the manufacturer's instructions on automated equipment. Slides were evaluated by a specialist pathologist. ALK rearrangement was defined as positive by the presence of strong granular cytoplasmic staining in tumor cells (any percentage).
For statistical analysis, we used the frequency and percentage to describe categorical variables and median to describe continuous variables, using the software IBM SPSS Statistics Version 22 (IBM Corp).
RESULTS
The clinicopathological and molecular features of the 722 Brazilian NSCLC patients is summarized in Table 1. Among NSCLC patients, 85.2% (n = 615/722) had lung adenocarcinomas, 1.9% (n = 14/722) had squamous cell carcinoma, and 12.9% (n = 93/722) were from other NSCLC histology. The median age was 64.0 years old, and 53.9% were male (n = 389/722). Concerning tobacco use, 66.8% (n = 482/722) were quitters or current smokers, 53.6% (n = 387/722) were diagnosed with a performance status of 0/1, 46.6% (n = 336/722) presented with loss of weight 6 months prior the diagnosis, and 63.2% (n = 456/722) were diagnosed with advanced stage of the disease.
TABLE 1.
Clinicopathological and molecular features of NSCLC patients
| Variable | Category | NSCLC patients (n = 722) | |
|---|---|---|---|
| N | (%) | ||
| Age (years) a | Median (range) | 64.0 (26–94) | |
| ≤64 | 373 | 51.7% | |
| >64 | 349 | 48.3% | |
| Sex | Female | 333 | 46.1% |
| Male | 389 | 53.9% | |
| Tobacco use | Never smoker | 177 | 24.5% |
| Quitter smoker | 249 | 34.5% | |
| Current smoker | 233 | 32.3% | |
| Missing | 63 | 8.7% | |
| Loss of weight b | No | 216 | 29.9% |
| Yes ≤10% of weight | 194 | 26.9% | |
| Yes >10% of weight | 142 | 19.7% | |
| Missing | 170 | 23.5% | |
| ECOG PS | 0 | 111 | 15.4% |
| 1 | 276 | 38.2% | |
| 2 | 140 | 19.4% | |
| 3/4 | 74 | 10.2% | |
| Missing | 121 | 16.8% | |
| Histology | Adenocarcinoma | 615 | 85.2% |
| Squamous cell carcinoma | 14 | 1.9% | |
| Others c | 93 | 12.9% | |
| Stage at diagnosis d | I/II | 28 | 3.9% |
| III | 154 | 21.3% | |
| IV | 456 | 63.2% | |
| Missing | 84 | 11.6% | |
| Metastasis at diagnosis | No | 182 | 25.2% |
| Yes, central nervous system | 146 | 20.2% | |
| Yes, others | 273 | 37.8% | |
| Missing | 121 | 16.8% | |
| EGFR status | Wild‐type | 559 | 77.4% |
| Mutant | 163 | 22.6% | |
| KRAS status | Wild‐type | 533 | 73.8% |
| Mutant | 189 | 26.2% | |
| ERBB2 exon 20 insertions | Wild‐type | 716 | 99.2% |
| Mutated | 6 | 0.8% | |
| ALK status | Wild‐type | 621 | 86.0% |
| Mutant | 38 | 5.3% | |
| Missing/inconclusive | 63 | 8.7% | |
| Vital status | Alive | 336 | 46.5% |
| Death | 352 | 48.8% | |
| Missing | 34 | 4.7% | |
n, number of patients.
Age was categorized into two groups considering the median of the entire series as the cutoff.
Last 6 months before the diagnosis.
Adenosquamous, NOS (not otherwise specified), large cell, sarcomatoid carcinoma, and neuroendocrine carcinoma.
According to AJCC seventh edition.
Molecularly, 26.2% (n = 189/722) were KRAS‐mutated, 22.6% (n = 163/722) were EGFR‐mutated, and 5.3% (n = 38/722) were ALK‐translocated (Table 1). ERBB2 exon 20 insertions were identified in 0.8% (n = 6/722) of all patients.
We identified six lung adenocarcinoma patients harboring the ERBB2 inframe insertion p.(Tyr772_Ala775dup) in exon 20 (Figure 1a/b and Table A.1). The features of mutant ERBB2 patients are described in Table 2. Most patients were female, never smokers, and diagnosed at an advanced stage of disease with metastasis of lung/pleura, bone, and lymph node. All six patients were diagnosed with lung adenocarcinoma and were wild‐type for EGFR, KRAS and ALK‐translocations (Table 2; Figure 2).
FIGURE 1.
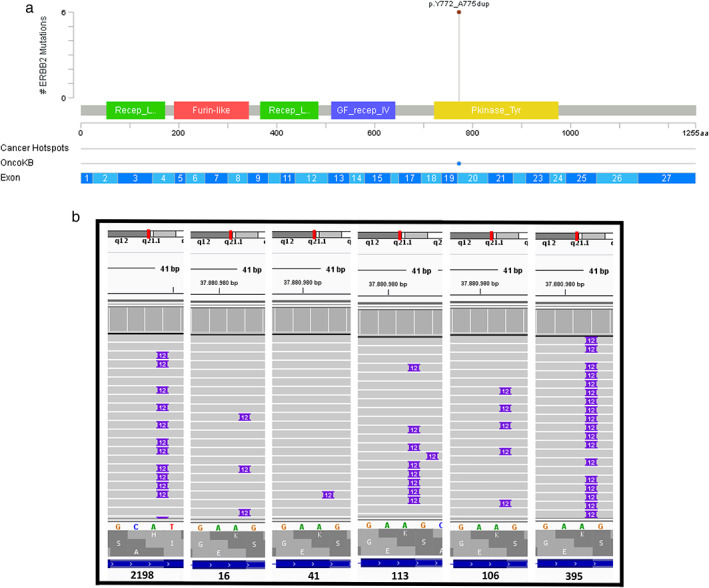
(a) ERBB2 exon 20 insertions indentified in non‐small cell lung cancer (NSCLC) patients (n = 6); Created in cbioportal.gov. (b) ERBB2 exon 20 insertions identified observed in IGV software
TABLE 2.
Clinicopathological and molecular features from ERBB2‐mutant patients (n = 6)
| Variable | ERBB2‐mutant patients p.(Tyr772_Ala775dup) | |||||
|---|---|---|---|---|---|---|
| ID‐113 | ID‐16 | ID‐395 | ID‐41 | ID‐2198 | ID‐106 | |
| Age (year) | 75.0 | 75.0 | 66.0 | 52.0 | 61.0 | 58.0 |
| Sex | Female | Male | Female | Female | Male | Female |
| Tobacco Use | Never smoker | Never smoker | Never smoker | Never smoker | Missing | Never smoker |
| Loss of weight a | Yes, <= 10% | No | Missing | Yes, <= 10% | No | No |
| ECOG PS | 2 | Missing | 3 | 1 | Missing | 1 |
| Histology | Adenocarcinoma | Adenocarcinoma | Adenocarcinoma | Adenocarcinoma | Adenocarcinoma | Adenocarcinoma |
| stage at diagnosis b | IV | IV | IV | IV | II | IV |
| Metastasis at diagnosis | Lung / pleura | Lung / pleura, Bone, lymph node | Bone | Lung / pleura | No | Liver, lymph node |
| EGFR status | Wild‐type | Wild‐type | Wild‐type | Wild‐type | Wild‐type | Wild‐type |
| KRAS status | Wild‐type | Wild‐type | Wild‐type | Wild‐type | Wild‐type | Wild‐type |
| ALK status | Wild‐type | Wild‐type | Wild‐type | Wild‐type | Missing | Wild‐type |
| Vital status | Alive‐active disease | Alive‐active disease | Death‐cancer | Death‐cancer | Alive‐active disease | Death‐cancer |
| Overall survival (months) | 27.8 | 27.2 | 3.5 | 28.6 | 2.8 | 2.0 |
Last 6 months before the diagnosis.
According to AJCC seventh edition.
FIGURE 2.
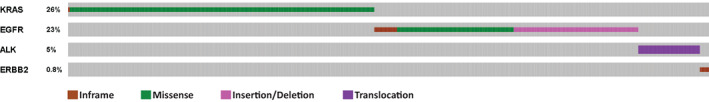
Representative molecular alterations in non‐small cell lung cancer (NSCLC) patients (n = 722). Only mutant patients are shown (n = 396)
DISCUSSION
Molecular ERBB2 therapies, namely targeting exon 20 insertion, are being explored in NSCLC patients. 4 Herein, we report the frequency of ERBB2 exon 20 insertions in a series of 722 Brazilian NSCLC patients.
The frequency of NSCLC patients harboring ERBB2 mutations ranges from 2%–5% in the literature. 6 , 7 , 8 The Cancer Genome Atlas (TCGA) reported a frequency of 2.6% (n = 6/230) for ERBB2‐mutated patients in lung adenocarcinomas. 7 Concerning only ERBB2 exon 20 insertions, TCGA reported a frequency of 1.3% (n = 3/230). 7 A recent comprehensive review on the topic reported that ERBB2 exon 20 insertions are present in about 1.5% of NSCLC patients and account for 90% of EBBB2 mutations. 6 The study by Li et al. reported an ERBB2 mutational frequency of 3% (n = 4/148) in lung adenocarcinomas patients – all identified mutations were insertions at exon 20. 8 Recently, Carrot‐Zhang et al. analyzed ERBB2 in Latin Americans (Mexico and Colombia patients), but no ERBB2 exon 20 insertion was reported. 9 Moreover, a Brazilian study using the Foundation One or Foundation ACT, described 5% of NSCLC patients harboring ERBB2 mutations (n = 26/513), with 1.4% (7/513) exhibiting the exon 20 insertions (p. A775_G776INSYVMA), but no additional information was reported. 10
Our study constituted a larger assessment of ERBB2 exon 20 insertions in Brazilian patients. Only six out of 722 (0.8%) were mutated, being mainly female and never smokers, in agreement with studies from other geographic regions. 4 , 6 , 8 Considering the frequency reported in our study and by Mascarenhas et al., 10 we may infer that about 1% of NSCLC patients harbor ERBB2 exon 20 insertions in Brazil. As expected, ERBB2 mutations were mutually exclusive with EGFR, KRAS and ALK alterations. In our study, all ERBB2 exon 20 insertions were the p.(Tyr772_Ala775dup) inframe insertion. According to the review by Friedlaender et al., the most common ERBB2 exon 20 insertions in NSCLC patients are p.(Tyr772dupTyrValMetAla) and the p.(Ala775_Gly776insTyrValMetAla). 6 Due to the low number of ERBB2 exon 20 insertion mutated patients in our study, no statistically significant association was performed.
Notably, we found six patients in our series harboring ERBB2 exon 20 insertion mutations that could benefit from treatment with tyrosine kinase inhibitors (TKIs), such as poziotinib, pyrotinib, and trastuzumab deruxtecan. A phase II basket trial (ZENITH20) evaluated 90 patients harboring ERBB2 exon 20 insertion mutations treated with poziotinib which showed an objective response rate (ORR) of 27.8% (95% CI: 18.9–38.2), with 25 of 90 patients achieving partial response, a disease control rate of 70% (95% CI: 59.4–79.2), and a median progression‐free survival (PFS) of 5.5 months (95% CI: 3.9–5.8). Patients pretreated with three or more prior treatment lines had greater responses (ORR, 37.1%). 11 Another phase II trial study evaluated 60 advanced (IIIB–IV) lung adenocarcinoma patients harboring ERBB2 mutations and previously treated with platinum‐based chemotherapy. 12 In this study, 49 patients were identified with ERBB2 exon 20 insertions (12‐bp insertion, n = 44; 9‐bp insertion, n = 5) and there were 11 patients with missense mutations. 12 From the 60 patients, 18 showed an ORR (30%, 95% CI: 18.8%–43.2%), all with partial response, and a median PFS and overall survival of 6.9 months (95% CI: 5.5–8.3). In patients with different mutation types, the ORR was higher in 44 patients harboring 12‐ and 9‐bp exon 20 insertions (27.3% and 60.0%, respectively). 12 Finally, the open‐label phase 2 DESTINY‐Lung01 study evaluated trastuzumab deruxtecan in 91 nonsquamous metastatic NSCLC patients harboring ERBB2 mutations that relapsed during standard treatment or had refractory to standard treatment (platinum‐based chemotherapy, anti‐PD‐1 or anti‐PD‐L1 treatment), and reported an ORR in 55% of the patients. 4
Importantly, ERBB2 can be deregulated by other mutations, namely missense in other regions, and harbor gene amplification mechanisms, 6 , 8 which were not explored in the present study, and could lead to a higher percentage of ERRB2 genomic alterations in Brazilian lung cancer patients.
In conclusion, we report that less than 1% of Brazilian NSCLC patients harbor the ERBB2 exon 20 insertions. Nevertheless, they could putatively benefit from ERBB2 targeted therapies.
CONFLICT OF INTEREST
The authors have nothing to disclose and confirm they have not received any grants or support related to this study.
Supporting information
Supplementary Figure 1 – Distribution of patients from Barretos Cancer Hospital (n = 722). Created using Microsoft Office Excel 2019.
Table A.1 ERBB2 exon 20 insertions molecular features.
ACKNOWLEDGMENTS
This study was partially supported by the Public Ministry of Labor Campinas (Research, Prevention, and Education of Occupational Cancer – 15ª zone, Campinas, Brazil), FINEP ‐ CT‐INFRA (02/2010), Barretos Cancer Hospital Research Fund (PAIP), and National Council for Scientific and Technological Development (CNPq, Brazil). RMR was supported by the National Council for Scientific and Technological Development (CNPq, Brazil) as Research Productivity Scholarship ‐ Level 1B. LFL was supported by the Public Ministry of Labor Campinas (Research, Prevention, and Education of Occupational Cancer – 15ª zone, Campinas, Brazil), and ROC was supported by Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil) with a PhD scholarship. Funding sources have no contribution to filling out authorship for the present study. We thank all members of the GTOP group (Translational Group of Pulmonary Oncology‐Barretos Cancer Hospital, Brazil) for scientific discussion and suggestions.
de Oliveira Cavagna R, Zaniolo BG, de Paula FE, Berardinelli GN, Santana I, da Silva ECA, et al. ERBB2 exon 20 insertions are rare in Brazilian non‐small cell lung cancer. Thorac Cancer. 2022;13(23):3402–3407. 10.1111/1759-7714.14605
Funding information Barretos Cancer Hospital Research Fund (PAIP); FINEP ‐ CT‐INFRA, Grant/Award Number: (02/2010); Higher Education Personnel (CAPES, Brazil); National Council for Scientific and Technological Development (CNPq, Brazil); Public Ministry of Labor Campinas (Research, Prevention, and Education of Occupational Cancer ‐ 15ª zone, Campinas, Brazil)
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. INCA IN de CJAG da S . Estimativa 2020: incidência de câncer no Brasil. Rio de Janeiro: INCA; 2019. p. 122. [Google Scholar]
- 3. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN guidelines insights: non‐small cell lung cancer, version 2.2021. J Natl Compr Canc Netw. 2021;19(3):254–66. [DOI] [PubMed] [Google Scholar]
- 4. Passaro A, Peters S. Targeting HER2 ‐mutant NSCLC — The light is on. N Engl J Med. 2022;386(3):286–9. [DOI] [PubMed] [Google Scholar]
- 5. Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97(5):339–46. [DOI] [PubMed] [Google Scholar]
- 6. Friedlaender A, Subbiah V, Russo A, Banna GL, Malapelle U, Rolfo C, et al. EGFR and HER2 exon 20 insertions in solid tumours: from biology to treatment. Nat Rev Clin Oncol. 2022;19(1):51–69. [DOI] [PubMed] [Google Scholar]
- 7. Cancer Genome Atlas Research Network . Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li BT, Ross DS, Aisner DL, Chaft JE, Hsu M, Kako SL, et al. HER2 amplification and her2 mutation are distinct molecular targets in lung cancers. J Thorac Oncol. 2016;11(3):414–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carrot‐Zhang J, Soca‐Chafre G, Patterson N, Thorner AR, Nag A, Watson J, et al. Genetic ancestry contributes to somatic mutations in lung cancers from admixed Latin American populations. Cancer Discov. 2021;11(3):591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mascarenhas E, Gelatti AC, Araújo LH, Baldotto C, Mathias C, Zukin M, et al. Comprehensive genomic profiling of Brazilian non‐small cell lung cancer patients (GBOT0118 / LACOG0418). Thorac Cancer. 2021;12(5):580–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Le X, Cornelissen R, Garassino M, Clarke JM, Tchekmedyian N, Goldman JW, et al. Poziotinib in non–small‐cell lung cancer harboring HER2 exon 20 insertion mutations after prior therapies: ZENITH20‐2 trial. J Clin Oncol. 2022;40(7):710–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou C, Li X, Wang Q, Gao G, Zhang Y, Chen J, et al. Pyrotinib in HER2 ‐mutant advanced lung adenocarcinoma after platinum‐based chemotherapy: a multicenter, open‐label, single‐arm, phase II study. J Clin Oncol. 2020;38(24):2753–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 – Distribution of patients from Barretos Cancer Hospital (n = 722). Created using Microsoft Office Excel 2019.
Table A.1 ERBB2 exon 20 insertions molecular features.


