Abstract
Aims
Resistin is a circulating inflammatory biomarker that is associated with cardiovascular disease. We investigated the associations of resistin and incident heart failure (HF) and its subtypes, as well as specific measures of subclinical HF (myocardial fibrosis and relevant biomarkers).
Methods
We analysed data from 1968 participants in the Multi‐Ethnic Study of Atherosclerosis with measurements of plasma resistin levels at clinic visits from 2002 to 2005. Participants were subsequently followed for a median of 10.5 years for HF events. The associations between resistin levels and incident HF, HF with reduced ejection fraction (HFrEF), and HF with preserved ejection fraction (HFpEF) were examined using multivariable Cox proportional hazards models. Linear regression models assessed the associations between resistin levels and myocardial fibrosis from cardiac magnetic resonance imaging, as well as hs‐cTnT and NT‐proBNP.
Results
The mean age of the cohort was 64.7 years, and 50.0% were female. Seventy‐four participants (4%) developed incident HF during follow‐up. In a Cox proportional hazards model adjusted for age, gender, education level, race/ethnicity, and traditional risk factors, higher resistin levels were significantly associated with incident HF (HR 1.44, CI 1.18–1.75, P = 0.001) and HFrEF (HR 1.47, CI 1.07–2.02, P = 0.016), but not with HFpEF (HR 1.25, CI 0.89–1.75, P = 0.195). Resistin levels showed no significant associations with myocardial fibrosis, NT‐proBNP, or hs‐cTnT levels.
Conclusions
In a multi‐ethnic cohort free of cardiovascular disease at baseline, elevated resistin levels were associated with incident HF, more prominently with incident HFrEF than HFpEF, but not with subclinical myocardial fibrosis or biomarkers of HF.
Keywords: Cardiac fibrosis, Heart failure, Resistin, Troponin
Introduction
In the United States from 2013 to 2016, the prevalence of heart failure (HF) has increased from 5.7 million (2009–2012) to 6.2 million. 1 Approximately half of incident hospitalized HF cases are HF with reduced ejection fraction (HFrEF), and the other half are HF with preserved ejection fraction (HFpEF) or HF with mildly reduced ejection fraction (HFmrEF). 2
Despite the wide acceptance of using traditional risk factors to identify patients at risk for HF, 3 early detection and the prevention of subclinical HF remains challenging. From epidemiologic studies, several biomarkers (e.g. high‐sensitivity troponin and natriuretic peptides) have been shown to correlate with future risk of incident HF. 4 More specifically, and using the Multi‐Ethnic Study of Atherosclerosis (MESA) population over a 10‐year follow‐up period, mildly elevated high‐sensitivity cardiac troponin T (hs‐cTnT) levels were found to be associated with a higher risk of increased left ventricular (LV) mass and LV dilation, as well as myocardial fibrosis. 5
Resistin is a circulating peptide hormone released primarily from macrophages. 6 , 7 Emerging evidence suggests that elevated circulating resistin levels are associated with various cardiovascular diseases (CVD) such as atherosclerosis, acute coronary syndrome, myocardial infarction, ischaemic stroke, hypertrophic cardiomyopathy, and HF. 8 , 9 , 10 , 11 , 12 , 13 In this regard, we have recently demonstrated direct cellular and in vivo cardiac effects of resistin, where resistin over‐expression in normal rodents induced myocyte injury and cardiac dysfunction, primarily due to increased apoptosis and myocardial fibrosis. 14 , 15 , 16 Furthermore, by using animal models of pressure overload and volume overload, we were able to demonstrate elevated LV resistin levels and increased fibrosis in the pressure overload model of HF, compared with the volume overload model where resistin is minimally elevated. 17 Chronic ischaemic injury in animal models of myocardial infarction also revealed local resistin expression in the infarct area that led to activation of pro‐fibrotic pathways and eventually cardiac replacement fibrosis. 17
Based on these lines of evidence, we hypothesized that resistin levels may be associated with specific HF subtypes and cardiac fibrosis. Therefore, the primary focus of this study was to determine the association of resistin levels with incident HF and its subtypes. In a subset of study participants, we assessed the association of resistin in cardiac fibrosis measured by cardiac magnetic resonance (CMR), as well as the relationships of resistin with hs‐cTnT and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP).
Methods
Study population
MESA is a longitudinal study initiated in July 2000 to determine the characteristics and risk factors of subclinical CVD and its procession to clinically overt CVD in a population comprising four racial/ethnic groups (non‐Hispanic White, Black, Hispanic American, and Chinese American) from six U.S. field centres. 18 , 19 Between 2000 and 2002 (Exam 1), the MESA cohort recruited 6814 men, and women aged 45–84 years who had no clinical history of CVD at baseline. Participants were then followed by further clinical evaluation and blood sample collection at Exam 2 (2002–2004), Exam 3 (2004–2005), Exam 4 (2005–2007), and Exam 5 (2010–2011).
Informed consents for participation were obtained from all participants and the MESA study. Associated protocols were approved by the Institutional Review Boards of each participating centre (details available at http://www.mesa‐nhlbi.org).
Demographic and clinical characteristics
Demographics, CVD risk factors, and clinical characteristics were obtained as previously reported. 19 Covariates were taken from Exam 2 or 3 to be concomitant to when resistin was measured and included age, gender, race/ethnicity, smoking, diabetes mellitus, height and weight, hypertension, total and LDL levels, and statin use. Diabetes mellitus was defined based on fast plasma glucose ≥126 mg/dL or taking anti‐diabetic drugs. 20 Hypertension was defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg or taking anti‐hypertensive medications.
Biomarker measurements
Blood samples were collected from a random subset of 1968 participants during MESA Exam 2 or 3 (2002–2005) (about one‐half at each exam) who were enrolled in an ancillary study utilizing abdominal computed tomography to investigate the associations between abdominal body composition, adiposity, inflammatory biomarkers, and subclinic and incident CVD. 13 Briefly, resistin levels were measured from stored EDTA plasma samples that were processed with Bio‐Rad Luminex flow cytometry (Millipore, Billerica, MA) at the Laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT). Plasma NT‐proBNP and hs‐cTnT levels had been previously measured in MESA participants at Exam 3. 5 , 21
CMR measurements and myocardial fibrosis study
CMR imaging and myocardial fibrosis study were performed at Exam 5. 22 , 23 , 24 Briefly, mid‐LV short‐axis T1 maps were acquired before gadolinium administration (native or pre‐contrast) and after gadolinium injection (post‐contrast) using the modified look‐locker imaging (MOLLI) sequence. Myocardial partition coefficient was computed by plotting the 1/T1 times of myocardium against the blood pool and calculating the slope of resultant linear regression line. Extracellular volume fraction (ECV) was derived by multiplying partition coefficient with (1‐haematocrit/100). 24 Myocardial scar was defined by sub‐endocardial or transmural late gadolinium enhancement areas that match any specific epicardial coronary artery perfusion territory. 25
Outcomes ascertainment and HF events
At intervals of 9–12 months during the follow‐up period, each participant in the MESA cohort or a family member of the participant was contacted by a telephone interviewer regarding all interim hospital admissions, cardiovascular outpatient diagnoses and procedures, and deaths. Additional information was obtained by cohort clinic visits, patient‐initiated contact, and medical record data abstraction. Outcomes were adjudicated by a central committee composed of physicians. Myocardial infarction was based on evaluation of symptoms, electrocardiograms, and cardiac biomarker levels. HF and its subtypes were defined with combinations of HF symptoms and one or more imaging criteria, such as pulmonary oedema/congestion on chest X‐ray, echocardiography, or radionuclide ventriculogram demonstrating evidence of reduced left ventricular systolic function, or evidence of left ventricular diastolic dysfunction. HFrEF was defined as HF diagnosis with documented left ventricular ejection fraction (LVEF) < 45% and HFpEF with LVEF ≥ 45%. 26 The time to incident HF events among participants depended on the time of resistin measurement (Exam 2 or 3). Participants who had HF events before their resistin measurements were excluded. HF incidents that occurred any time after resistin measurement (Exam 2 or 3) were used for further analysis.
Statistical analysis
Descriptive statistics by incident HF status were presented as mean +/− standard deviations (SD) or frequency (%) and compared using either t‐tests or chi‐squared tests, respectively. Multivariable adjusted Cox proportional hazards models were applied to estimate the associations of resistin level with incident HF, HFrEF, and HFpEF during the period after the resistin measurement (Exam 2 or 3). As we are primarily interested in aetiologic associations, not cumulative incidence, a cause‐specific approach was used to accommodate the competing risk of death, and for HF subtypes. Model 1 was adjusted for age, gender, education level, and race/ethnicity. Model 2 was further adjusted for smoking status, diabetes mellitus, body mass index, hypertension, systolic blood pressure, total and LDL cholesterol, and statin use. Model 3 was adjusted based on Model 2 plus an indicator for myocardial infarction prior to HF. Model 4 was adjusted for Model 1 plus ASCVD risk score.
Linear regression models were used to estimate the adjusted associations of resistin with biomarkers and CMR variables of fibrosis. Different subsets of participants were available for these measures depending on when the measurements were made. Cardiac biomarkers hs‐cTnT and NT‐proBNP were available at Exam 3 and were analysed in relation to the subset of resistin measures that were also taken at Exam 3. Finally, markers of fibrosis were taken from magnetic resonance images at Exam 5. Logistic regression was used to relate resistin to the presence of myocardial scar at Exam 5. For each endpoint, Model 1 included resistin, age, gender, race/ethnicity, and education. Model 2 additionally adjusted for ASCVD risk score. Robust standard errors were used in all models.
Results
Among the MESA study population at Exams 2 and 3, plasma resistin levels were measured in 1,968 participants (Figure 1 ). 13 After excluding participants with missing covariates, a total of 1841 participants were included in the analysis (732 from Exam 2 and 1109 from Exam 3). CMR was performed in 923 of these participants at Exam 5 with fibrosis measures including ECV available in 195 participants, pre‐contrast T1 time and post‐contrast 12 min and 25 min T1 time in 399 participants, and myocardial scar in 510 participants. Among 1109 participants with resistin at Exam 3, NT‐proBNP and hs‐cTnT levels were measured in 226 and 721 participants, respectively.
Figure 1.
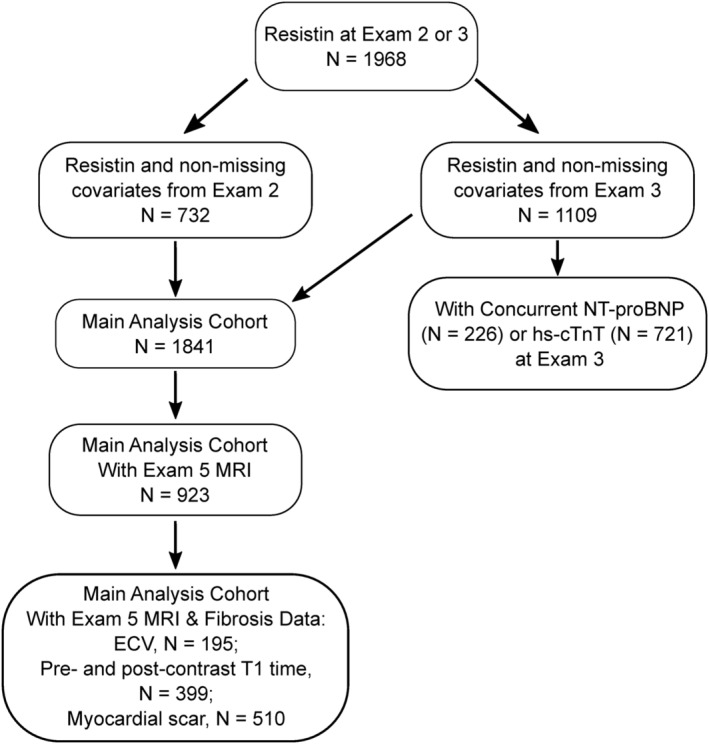
Study flow chart. The flow chart illustrates the number of MESA participants included in this study based on the availability of resistin levels, non‐missing covariates, and other markers.
The baseline demographics characteristics, CV risk factors, and clinical characteristics, stratified by the development of incident HF, are shown in Table 1 . Over a median follow‐up time of 10.5 years, 74 participants out of total 1841 participants developed incident HF, which was significantly associated with elevated resistin levels (19.8 ± 8.8 vs. 16.1 ± 6.7 ng/mL, P < 0.001). Participants with incident HF tended to be significantly older and have higher weight and systolic blood pressure, higher 10‐year ACVD rate, and a greater prevalence of hypertension and diabetes. There were no significant differences in total or LDL cholesterol, statin use, gender, race, education, smoking history and current smoking status between participants with and without incident HF. Kaplan–Meier failure curves demonstrated divergence of cumulative incidence of developing HF in resistin quartiles, 13 with significance increase in Quartile 4 (P < 0.001 based on log‐rank test) (Figure 2 ).
Table 1.
Demographics by incident CHF
| Variable | Incident CHF | P value | |
|---|---|---|---|
| No | Yes | ||
| (N = 1767) | (N = 74) | ||
| Mean ± SD | Mean ± SD | ||
| Age (year) | 64.4 ± 9.6 | 72.3 ± 7.9 | <0.001 |
| Height (cm) | 166.3 ± 10.0 | 166.5 ± 10.2 | 0.841 |
| Weight (lbs) | 171.5 ± 37.1 | 181.9 ± 40.6 | 0.020 |
| Smoking (pack‐years) | 11.7 ± 21.4 | 15.9 ± 23.1 | 0.100 |
| Systolic BP (mmHg) | 123.6 ± 20.8 | 133.5 ± 22.8 | <0.001 |
| Diastolic BP (mmHg) | 70 ± 9.8 | 70.4 ± 12.3 | 0.711 |
| Total cholesterol (mg/dL) | 189.7 ± 34.4 | 185.1 ± 44.7 | 0.259 |
| LDL cholesterol (mg/dL) | 112.2 ± 30.9 | 110.6 ± 38.5 | 0.672 |
| 10‐year ASCVD rate (%) | .1 ± 0.1 | .3 ± 0.2 | <0.001 |
| Resistin (ng/mL) | 16.1 ± 6.7 | 19.8 ± 8.7 | <0.001 |
| N (%) | N (%) | ||
| Gender | 0.098 | ||
| Female | 891 (50.4) | 30 (40.5) | |
| Male | 876 (49.6) | 44 (59.5) | |
| Race | 0.516 | ||
| Caucasian | 705 (39.9) | 33 (44.6) | |
| Chinese | 244 (13.8) | 6 (8.1) | |
| African American | 361 (20.4) | 14 (18.9) | |
| Hispanic | 457 (25.9) | 21 (28.4) | |
| Education | 0.140 | ||
| <High school | 320 (18.1) | 20 (27.0) | |
| High school/some college | 803 (45.4) | 31 (41.9) | |
| BS degree | 320 (18.1) | 15 (20.3) | |
| Graduate school | 324 (18.3) | 8 (10.8) | |
| Current smoker | 0.292 | ||
| No | 1574 (89.1) | 63 (85.1) | |
| Yes | 193 (10.9) | 11 (14.9) | |
| Diabetes | <0.001 | ||
| No | 1530 (86.6) | 52 (70.3) | |
| Yes | 237 (13.4) | 22 (29.7) | |
| Hypertension | <0.001 | ||
| No | 947 (53.6) | 19 (25.7) | |
| Yes | 820 (46.4) | 55 (74.3) | |
| On statin therapy | 0.213 | ||
| No | 1375 (77.8) | 53 (71.6) | |
| Yes | 392 (22.2) | 21 (28.4) | |
Figure 2.
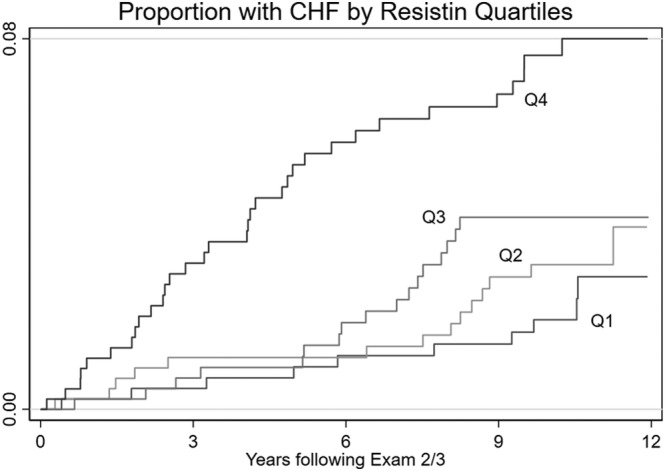
Kaplan–Meier failure curves for incident HF, stratified by resistin quartiles.
Adjusted associations between resistin levels and incident HF and HF subtypes (HFrEF and HFpEF) are shown in Table 2 . In all four models, elevated resistin levels were consistently associated with incident HF and remained a significant predictor for incident HF after multivariable adjustment (Model 1, HR 1.36, CI 1.14–1.62, P = 0.001; Model 2, HR 1.35, CI 1.12–1.63, P = 0.002; Model 3, HR 1.44, CI 1.18–1.75, P = 0.001; Model 4, HR 1.35, CI 1.13–1.62, P = 0.001). Elevated resistin levels were also associated with HFrEF across all models (Model 1, HR 1.34, CI 1.00–1.80, P = 0.048; Model 2, HR 1.39, CI 1.02–1.90, P = 0.039; Model 3, HR 1.47, CI 1.07–2.02, P = 0.016; Model 4, HR 1.34, CI 1.00–1.80, P = 0.049). No significant association between resistin level and HFpEF was found after multivariable adjustment in any of the four models.
Table 2.
Cox proportional hazards models for incident heart failure (HF), heart failure with reduced ejection fraction (HFrEF), and heart failure with preserved ejection fraction (HFpEF) events
| Incident HF events = 74 | Systolic HF (HFrEF) events = 29 | Diastolic HF (HFpEF) events = 33 | ||||
|---|---|---|---|---|---|---|
| Resistin per (SD = 6826) | HR a (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value |
| Model 1 b | 1.36 (1.14,1.62) | 0.001 | 1.34 (1.00,1.80) | 0.048 | 1.21 (0.91,1.62) | 0.190 |
| Model 2 c | 1.35 (1.12,1.63) | 0.002 | 1.39 (1.02, 1.90) | 0.039 | 1.18 (0.87,1.61) | 0.295 |
| Model 3 d | 1.44 (1.18,1.75) | 0.001 | 1.47 (1.07, 2.02) | 0.016 | 1.25 (0.89,1.75) | 0.195 |
| Model 4 e | 1.35 (1.13,1.62) | 0.001 | 1.34 (1.00,1.80) | 0.049 | 1.19 (0.89,1.59) | 0.242 |
HR is reported as per standard deviation increment in resistin levels.
Model 1: adjusted for age, gender, education level, and race/ethnicity.
Model 2: Model 1 plus smoking (current or not; pack‐years over lifetime), diabetes mellitus, body mass index, hypertension, systolic blood pressure, total and LDL level, and statin use.
Model 3: Model 2 plus interval myocardial infarction.
Model 4: Model 1 plus ASCVD risk score.
We examined the association between resistin levels and levels of NT‐proBNP and hs‐cTnT at Exam 3 (Table 3 ). Resistin was not found to have a significant correlation with either biomarker.
Table 3.
Association of resistin with cardiac biomarkers and fibrosis variables
| Model 1 | Model 2 | ||||
|---|---|---|---|---|---|
| N | Resistin coefficient per SD (95% CI) | P value | Resistin coefficient per SD (95% CI) | P value | |
| Limited to Exam 3 when BNP and hs‐cTnT were measured | |||||
| NT‐proBNP (pg/mL) | 226 | −11.0 (−32.9, 10.9) | 0.322 | −11.7 (−33.4, 10.0) | 0.289 |
| Hs‐cTnT (ng/L) | 721 | 0.49 (−0.13, 1.11) | 0.123 | 0.44 (−0.16, 1.04) | 0.154 |
| Limited to Exam 5 when MRI was conducted | |||||
| Extracellular volume fraction (%) | 195 | −0.06 (−0.54, 0.42) | 0.815 | −0.22 (−0.71, 0.28) | 0.390 |
| Pre‐contrast T1 time (ms) | 399 | 2.79 (−1.44, 7.02) | 0.196 | 2.04 (−2.30, 6.38) | 0.356 |
| 12 min post‐contrast T1 Time (ms) | 399 | −7.03 (−12.06, −1.99) | 0.006 | −5.89 (−10.94, −0.84) | 0.022 |
| 25 min post‐contrast T1 Time (ms) | 396 | −6.65 (−11.67, −1.62) | 0.010 | −5.70 (−10.71, −0.70) | 0.026 |
| Odds ratio per SD (95% CI) | P value | Odds ratio per SD (95% CI) | p‐value | ||
| Myocardial scar assessment | 510 | 0.92 (0.65, 1.31) | 0.649 | 0.91 (0.63, 1.31) | 0.614 |
hs‐cTnT, high‐sensitive cardiac troponin T; NT‐proBNP, amino‐terminal B‐type natriuretic peptide.
Model 1: adjusted for age, gender, education level, and race/ethnicity.
Model 2: Model 1 covariates plus ASCVD risk score.
We then examined the association of resistin levels measured at Exam 2 or 3 and CMR analysis at Exam 5 (Table 3 ). Resistin levels were negatively associated with post‐contrast T1 time at 12 min (Model 1, β coefficient per SD −7.03, CI −12.06 to −1.99, P = 0.006; Model 2, β coefficient per SD −5.89, CI −10.94 to −0.84, P = 0.022) and 25 min (Model 1, β coefficient per SD −6.65, CI −11.67 to −1.62, P = 0.010; Model 2, β coefficient per SD −5.70, CI −10.71 to −0.70, P = 0.026) after gadolinium administration. Resistin levels did not show significant correlation with native pre‐contrast T1 time, ECV, or myocardial scar.
Discussion
Over a median follow‐up period of 10.5 years, elevated baseline resistin levels were significant predictors of incident HF and HFrEF in a multi‐ethnic population. Conversely, the associations with HFpEF did not reach statistical significance. Elevated resistin levels were associated with shortened post‐contrast T1 times at 12 and 25 min on CMR, but did not demonstrate significant associations with other myocardial fibrosis variables including native T1 times, ECV, and myocardial scar.
Several clinical and epidemiological studies have suggested associations between higher resistin levels and high rates of adverse cardiac events in patients hospitalized for HF or subjects with known CVD. 9 , 27 In a MESA study 13 with 7 years of follow‐up, as well as the Framingham Offspring Study 10 with a mean follow‐up time of 6 years, elevated circulating resistin levels were shown to be strongly associated with incident HF. Baseline clinical characteristics stratified by resistin quartiles in the prior MESA study showed that increasing resistin quartiles were associated with significant increases in age, race, and socio‐economic status as well as risk factors such as BMI, systolic blood pressure, and the Framingham risk score. 13 We were able to extend these findings by showing associations of baseline resistin levels with incident HF with longer follow‐up period of 10.5 years and determine its association with HF subtypes. The associations of resistin levels with incident HF and HFrEF remained statistically significant even after comprehensive multivariable adjustment models (Table 2 ).
Baseline hs‐cTnT level has been proposed to be strongly associated with adverse ventricular remodelling in response to chronic subclinical cardiac injury in adults without clinical overt CVD. 4 , 5 We found that resistin levels at baseline were not associated with hs‐cTnT levels or NT‐proBNP. As a circulating peptide hormone, resistin levels at baseline perhaps reflect a systemic response to cardiovascular risk factors such as hypertension, diabetes, and vascular inflammation, 28 not limited to subclinical cardiomyocyte injury detected by mildly elevated hs‐cTnT levels.
ECV is calculated from pre‐ and post‐contrast myocardial T1 imaging, which gives relative quantification of the extracellular matrix component that is often affected by reactive fibrosis. 29 Despite post‐contrast myocardial T1 times appeared to be significantly shortened with higher resistin levels, resistin levels did not demonstrate a significant association with ECV and native T1 times, which are considered to be more reliable MRI variables for myocardial fibrosis. 24 It should be noted that the models in Table 3 were performed among all participants with both baseline resistin level and CMR data at Exam 5 available, not limited to participants who had developed incident HF. Therefore, the association between resistin and myocardial fibrosis in HF patients could be diminished due to the small number of HF incident cases in the current study. Further investigation is warranted to determine the association of resistin with cardiac function and myocardial fibrosis in a larger patient population of HF.
Despite diastolic dysfunction and myocardial fibrosis being detected in both HFrEF and HFpEF, there are significant differences at the molecular and cellular levels accounting for the pathophysiological progression between HFrEF and HFpEF. 30 , 31 Our epidemiologic study shows resistin may potentially serve as an early biochemical signature associated with HF and HFrEF. Clinical application of resistin measurement certainly requires further validation in larger patient cohorts. 32 Animal experiments have also provided insights into the relationships among resistin levels, HF and myocardial fibrosis, and modulation of resistin in alleviating HF at the molecular and cellular levels. 14 , 15 , 16 , 33 Therefore, resistin could also become a potential therapeutic target to alleviate cardiac remodelling and improve systolic function in patients with HF.
Limitations
Because the MESA study involves a large and ethnically diverse population from six communities with longitudinal follow‐ups in the USA, there might be residual confounding. CMR and cardiac fibrosis data were restricted to a limited number of participants due to data availability, and some of the fibrosis variables were only examined once. Limitations also include the asynchronous nature of cross‐sectional analyses in the present study with resistin measured at Exam 2 or 3, NT‐proBNP and hs‐cTnT at Exam 3, and CMR analysis at Exam 5 as well as the limited number of participants with measurements available for resistin, NT‐proBNP, and hs‐cTnT.
Conclusions
We have shown that in a multi‐ethnic adult population without evidence of clinically overt cardiovascular disease, elevated baseline resistin levels are significantly associated with incident HF and HFrEF over a median 10.5‐year follow‐up (Figure 3 ), whereas the association between resistin levels and incident HFpEF was not statistically significant. Moreover, elevated resistin levels were not associated with CMR‐derived myocardial fibrosis variables or biomarkers of HF. Future studies to investigate the relationship between resistin and cardiac remodelling CMR variables in a large population of HF patients are needed to determine the clinical significance and investigate the potential application of resistin as a novel biomarker and therapeutic target in HF patients.
Figure 3.
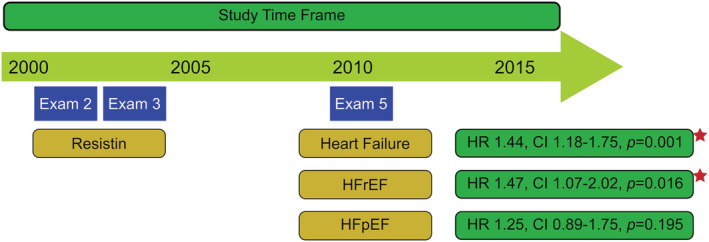
Resistin and risk of heart failure. In a multi‐ethnic cohort free of cardiovascular disease at baseline, elevated resistin levels measured at Exam 2 or 3 were significantly associated with incident HF and HFrEF that had occurred over a median 10.5‐year follow‐up after resistin measurement until the completion of Exam 5 in 2011. HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Conflict of interest
The authors report no relevant financial disclosures.
Funding
X.C. was supported in part by the UCLA Specialty Training and Advanced Research (STAR) programme, a Tibor Fabian Research Award, and a T32 training grant T32HL007895 from the National Institutes of Health. D.L. was supported by grants R01HL097357 and R01HL137220 from the National Institutes of Health. The MESA study was supported by the National Heart, Lung, and Blood Institute (grant R01HL088451, contracts 75N92020D00001, HHSN268201500003I, N01‐HC‐95159, 75N92020D00005, N01‐HC‐95160, 75N92020D00002, N01‐HC‐95161, 75N92020D00003, N01‐HC‐95162, 75N92020D00006, N01‐HC‐95163, 75N92020D00004, N01‐HC‐95164, 75N92020D00007, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168, N01‐HC‐95169) and the National Center for Advancing Translational Sciences (grants UL1‐TR‐000040, UL1‐TR‐001079, UL1‐TR‐001420).
Acknowledgements
The authors would like to thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa‐nhlbi.org. We would also like to thank prior MESA investigators 5 for making measurements of hs‐cTnT and NT‐proBNP available for our analyses.
Cai, X. , Allison, M. A. , Ambale‐Venkatesh, B. , Jorgensen, N. W. , Lima, J. A. C. , Muse, E. D. , McClelland, R. L. , Shea, S. , and Lebeche, D. (2022) Resistin and risks of incident heart failure subtypes and cardiac fibrosis: the Multi‐Ethnic Study of Atherosclerosis. ESC Heart Failure, 9: 3452–3460. 10.1002/ehf2.14064.
References
- 1. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L. Heart disease and stroke statistics‐2020 update: A report from the American Heart Association. Circulation 2020; 141: e139–e596. [DOI] [PubMed] [Google Scholar]
- 2. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 3. Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B, American College of Cardiology , American Heart Association Task Force on Practice Guidelines , American College of Chest Physicians , International Society for Heart and Lung Transplantation , Heart Rhythm Society . ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: A report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to update the 2001 guidelines for the evaluation and management of heart failure): Developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: Endorsed by the Heart Rhythm Society. Circulation 2005; 112: e154–e235. [DOI] [PubMed] [Google Scholar]
- 4. deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA 2010; 304: 2494–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seliger SL, Hong SN, Christenson RH, Kronmal R, Daniels LB, Lima JAC, de Lemos JA, Bertoni A, deFilippi CR. High sensitive cardiac troponin T as an early biochemical signature for clinical and subclinical heart failure: The multi‐ethnic study of atherosclerosis. Circulation 2017; 135: 1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shuldiner AR, Yang R, Gong DW. Resistin, obesity, and insulin resistance—The emerging role of the adipocyte as an endocrine organ. N Engl J Med 2001; 345: 1345–1346. [DOI] [PubMed] [Google Scholar]
- 7. Motojima K. The physiological role of resistin and its connection with metabolic diseases. J Endocrinol Invest 2003; 26: 1171–1173. [DOI] [PubMed] [Google Scholar]
- 8. Burnett MS, Lee CW, Kinnaird TD, Stabile E, Durrani S, Dullum MK, Devaney JM, Fishman C, Stamou S, Canos D, Zbinden S, Clavijo LC, Jang GJ, Andrews JA, Zhu J, Epstein SE. The potential role of resistin in atherogenesis. Atherosclerosis 2005; 182: 241–248. [DOI] [PubMed] [Google Scholar]
- 9. Takeishi Y, Niizeki T, Arimoto T, Nozaki N, Hirono O, Nitobe J, Watanabe T, Takabatake N, Kubota I. Serum resistin is associated with high risk in patients with congestive heart failure—A novel link between metabolic signals and heart failure. Circ J 2007; 71: 460–464. [DOI] [PubMed] [Google Scholar]
- 10. Frankel DS, Vasan RS, D'Agostino RB Sr, Benjamin EJ, Levy D, Wang TJ, Meigs JB. Resistin, adiponectin, and risk of heart failure the Framingham offspring study. J Am Coll Cardiol 2009; 53: 754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jamaluddin MS, Weakley SM, Yao Q, Chen C. Resistin: Functional roles and therapeutic considerations for cardiovascular disease. Br J Pharmacol 2012; 165: 622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lebeche D. Diabetic cardiomyopathy: Is resistin a culprit? Cardiovasc Diagn Ther 2015; 5: 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Muse ED, Feldman DI, Blaha MJ, Dardari ZA, Blumenthal RS, Budoff MJ, Nasir K, Criqui MH, Cushman M, McClelland RL, Allison MA. The association of resistin with cardiovascular disease in the multi‐ethnic study of atherosclerosis. Atherosclerosis 2015; 239: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chemaly ER, Hadri L, Zhang S, Kim M, Kohlbrenner E, Sheng J, Liang L, Chen J, K‐Raman P, Hajjar RJ, Lebeche D. Long‐term in vivo resistin overexpression induces myocardial dysfunction and remodeling in rats. J Mol Cell Cardiol 2011; 51: 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kang S, Chemaly ER, Hajjar RJ, Lebeche D. Resistin promotes cardiac hypertrophy via the AMP‐activated protein kinase/mammalian target of rapamycin (AMPK/mTOR) and c‐Jun N‐terminal kinase/insulin receptor substrate 1 (JNK/IRS1) pathways. J Biol Chem 2011; 286: 18465–18473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim M, Oh JK, Sakata S, Liang I, Park WJ, Hajjar RJ, Lebeche D. Role of resistin in cardiac contractility and hypertrophy. J Mol Cell Cardiol 2008; 45: 270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chemaly ER, Kang S, Zhang S, McCollum LT, Chen J, Bénard L, Purushothaman KR, Hajjar RJ, Lebeche D. Differential patterns of replacement and reactive fibrosis in pressure and volume overload are related to the propensity for ischaemia and involve resistin. J Physiol 2013; 591: 5337–5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Olson JL, Bild DE, Kronmal RA, Burke GL. Legacy of MESA. Glob Heart 2016; 11: 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi‐ethnic study of atherosclerosis: Objectives and design. Am J Epidemiol 2002; 156: 871–881. [DOI] [PubMed] [Google Scholar]
- 20. Bertoni AG, Kramer H, Watson K, Post WS. Diabetes: Insights from the multi‐ethnic study of atherosclerosis. Glob Heart 2016; 11: 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Choi EY, Bahrami H, Wu CO, Greenland P, Cushman M, Daniels LB, Almeida ALC, Yoneyama K, Opdahl A, Jain A, Criqui MH, Siscovick D, Darwin C, Maisel A, Bluemke DA, Lima JAC. N‐terminal pro‐B‐type natriuretic peptide, left ventricular mass, and incident heart failure: Multi‐ethnic study of atherosclerosis. Circ Heart Fail 2012; 5: 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch‐Herold M, Pearson G, Sinha S, Arai A, Lima JAC, Bluemke DA. Cardiovascular function in multi‐ethnic study of atherosclerosis: Normal values by age, sex, and ethnicity. AJR Am J Roentgenol 2006; 186: S357–S365. [DOI] [PubMed] [Google Scholar]
- 23. Liu CY, Liu YC, Wu C, Armstrong A, Volpe GJ, van der Geest RJ, Liu Y, Hundley WG, Gomes AS, Liu S, Nacif M, Bluemke DA, Lima JAC. Evaluation of age‐related interstitial myocardial fibrosis with cardiac magnetic resonance contrast‐enhanced T1 mapping: MESA (multi‐ethnic study of atherosclerosis). J Am Coll Cardiol 2013; 62: 1280–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ambale‐Venkatesh B, Liu CY, Liu YC, Donekal S, Ohyama Y, Sharma RK, Wu CO, Post WS, Hundley GW, Bluemke DA, Lima JAC. Association of myocardial fibrosis and cardiovascular events: The multi‐ethnic study of atherosclerosis. Eur Heart J Cardiovasc Imaging 2019; 20: 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Turkbey EB, Nacif MS, Guo M, McClelland RL, Teixeira PBRP, Bild DE, Barr RG, Shea S, Post W, Burke G, Budoff MJ, Folsom AR, Liu CY, Lima JA, Bluemke DA. Prevalence and correlates of myocardial scar in a US cohort. JAMA 2015; 314: 1945–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Duprez DA, Gross MD, Kizer JR, Ix JH, Hundley WG, Jacobs DR Jr. Predictive value of collagen biomarkers for heart failure with and without preserved ejection fraction: MESA (multi‐ethnic study of atherosclerosis). J Am Heart Assoc 2018; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang MH, Na B, Schiller NB, Whooley MA. Association of resistin with heart failure and mortality in patients with stable coronary heart disease: Data from the heart and soul study. J Card Fail 2011; 17: 24–30. [DOI] [PubMed] [Google Scholar]
- 28. Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation 2005; 111: 932–939. [DOI] [PubMed] [Google Scholar]
- 29. Taylor AJ, Salerno M, Dharmakumar R, Jerosch‐Herold M. T1 mapping: Basic techniques and clinical applications. JACC Cardiovasc Imaging 2016; 9: 67–81. [DOI] [PubMed] [Google Scholar]
- 30. Simmonds SJ, Cuijpers I, Heymans S, Jones EAV. Cellular and molecular differences between HFpEF and HFrEF: A step ahead in an improved pathological understanding. Cell 2020; 9: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. López B, Ravassa S, Moreno MU, José GS, Beaumont J, González A, Díez J. Diffuse myocardial fibrosis: Mechanisms, diagnosis and therapeutic approaches. Nat Rev Cardiol 2021; 18: 479–498. [DOI] [PubMed] [Google Scholar]
- 32. Ibrahim NE, Januzzi JL Jr. Established and emerging roles of biomarkers in heart failure. Circ Res 2018; 123: 614–629. [DOI] [PubMed] [Google Scholar]
- 33. Zhao B, Bouchareb R, Lebeche D. Resistin deletion protects against heart failure injury by targeting DNA damage response. Cardiovasc Res 2022; 118: 1947–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]


