Abstract
The case of a 35‐year‐old female with heart failure is presented, where the symptoms overlap with the heterogeneous manifestations of coronavirus disease 2019 (COVID‐19). Those similarities and a recent shift in priorities during the SARS‐CoV‐2 pandemic delayed the recognition of acute heart failure in this patient. During the differential diagnostic process, obliterative disease was discovered in the bilateral subclavian and right renal arteries, and the latter resulted in uncontrolled hypertension, which played a significant role in the development of heart failure. The aetiology of vascular alterations turned out to be Takayasu's arteritis. Diagnosing Takayasu's arteritis is typically not straightforward due to its nonspecific signs and symptoms. Therefore, it can be concluded from our case report that the rising incidence of COVID‐19 and focus on ruling out infection can potentially defer alternative, but appropriate diagnostic tests, particularly for certain conditions like rare diseases. Early identification and intervention is especially important for treating acute heart failure, whereas delay increases the risk of severe complications and mortality.
Keywords: Acute heart failure, Takayasu's arteritis, COVID‐19
Introduction
The coronavirus disease 2019 (COVID‐19) pandemic has placed a high burden on health care systems worldwide. 1 , 2 , 3 The clinical manifestation of an infection with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) can even overlap with the symptoms of heart failure (HF) 4 ; thus, it can be difficult to differentiate, especially in cases of patients below the age of 40 without any known cardiac conditions. Furthermore, complications of SARS‐CoV‐2 infection often involve the cardiovascular (CV) system. 4 Generally, the chance of infection and its complications occur with a much higher probability than de novo HF in the younger population. Even with clear statements from the current European Society of Cardiology (ESC) Guideline on HF 5 and guidance for the diagnosis and treatment of CV diseases during the COVID‐19 pandemic, 6 bias can arise due to a lack of experience with COVID‐19 patients. Late recognition of HF can result in delayed therapeutic intervention, leading to poorer outcome, especially if the aetiology of HF is a rare disease such as Takayasu's arteritis (TA).
Case Report
Initial medical examination
A 35‐year‐old female, with a medical history of total thyroidectomy due to obstructive goitre and ongoing thyroxine substitution (100 μg O.D.) experienced bilateral dorsal pain above the level of the kidneys, being severe in character and refractory to over‐the‐counter oral analgesics (paracetamol, ibuprofen, and diclofenac) in daily maximum doses. One week after the dorsal pain began, the symptoms worsened and broadened into sharp pain in the epigastric region with persistent nausea, and the patient was admitted to the regional emergency unit (ER). During physical examination, right subcostal and epigastric regions were highly sensitive to pressure, being the point of maximal pain at the gallbladder. No abdominal guarding was present. Abdominal ultrasound ruled out any pathological signs, except for an increased amount of fluid within the posterior cul‐de‐sac. This led to further gynaecological examination by a specialist, but no evident cause was found. Chest X‐ray (CXR) was also performed, without any significant abnormalities. The first laboratory tests showed that the glomerular filtration rate (GFR) was slightly reduced along with elevated C reactive protein (CRP) (Table 1 ). Urine analysis made with a dipstick was without any positive finding. The patient was discharged the same day and was advised to follow‐up with blood tests in a week, which later showed no significant changes, except for elevated lactate dehydrogenase enzyme (Table 1 ). Since the initial examination was performed in April 2020, there was no routine screening available at that time to exclude SARS‐CoV‐2 infection.
Table 1.
Dynamic changes in laboratory findings
| Lab item | Normal range | 01/April/2020 | 09/April/2020 | 22/April/2020 | 24/April/2020 | 27/April/2020 | 29/April/2020 | 03/Jun/2020 |
|---|---|---|---|---|---|---|---|---|
| White blood cell count (109/L) | 4.50–10.80 | 9.13 | 10.22 | 10.41 | ― | 15.96 | ― | ― |
| Lymphocyte count (109/L) | 0.90–3.10 | 1.23 | 1.81 | 1.51 | ― | 2.27 | ― | ― |
| Neutrophyl count (109/L) | 1.90–7.70 | 7 | 7.13 | 8 | ― | 11.51 | ― | ― |
| Hs‐CRP (mg/L) | <4.6 | 36 | 11.4 | 56 | 36.4 | 52.97 | ― | 4.7 |
| ALT (U/L) | <40 | 22 | 15 | ― | 845 | 1335 | 667 | 12 |
| AST (U/L) | <40 | 19 | 28 | ― | 760 | 567 | 143 | 13 |
| Creatinine (μmol/L) | 44–84 | 121 | 119 | 121 | 144 | 110 | 123 | 98 |
| Urea (mmol/L) | 2.8–8.0 | 3.6 | 9.2 | 6.5 | 13.7 | 10.5 | 10.6 | 6.7 |
| Glomerular filtration rate (mL/p/1.73 m2) | >90 | 50 | 51 | 50 | 41 | 56 | 49 | 64 |
| NT‐pro BNP (ng/L) | <191.1 | ― | ― | ― | ― | ― | 31 460 | ― |
| Lactate dehydrogenase (U/L) | 135.0–220.0 | 301 | 584 | 475 | 1627 | 627 | 352 | 164 |
| Serum amylase (U/L) | 5–220 | 31 | ― | ― | ― | ― | ― | ― |
Deterioration of the patient's general state led to hospitalization
After 10 days, the patient was admitted to the same ER with severe generalized weakness, headache, shortness of breath following minimal physical stress, and dry cough, but without any history of fever. Physical examination showed tachypnoea, bilateral crepitation in the lungs with congestion, along with normal heart sounds, diffuse abdominal sensitivity and bilateral peripheral oedema in her lower extremities. Normal values were obtained with blood pressure (BP) measurements on the upper limbs. ECG showed no specific alterations. CXR proved pneumonia and hydrothorax in the right lung. Furthermore, moderately elevated CRP was detected (Table 1 ). Taking the symptoms, CXR result, and heightened awareness about the pandemic into consideration, the patient was admitted with high probability of SARS‐CoV‐2 infection. However, real‐time reverse transcriptase‐polymerase chain reaction (RT‐PCR) analysis ruled out an active infection. Therefore, a chest computed tomography (CT) scan was performed to search for potential radiological signs of COVID‐19. A large area of pneumonia involving both lungs with extended pleural effusion was detected, but the specific COVID‐19 characteristics of bilateral, basal peripheral ground‐glass opacities were not found. The contour of the heart was enlarged, but the CT scan evaluation did not suggest any other cardiac abnormality. During examination, the CT scan was extended to the upper abdominal region, where an occluded right renal artery was detected. Because the supplied kidney was atrophic, an acute occlusive event was excluded. An aneurysm in the proximal part of the splenic artery was also noted. Because of the pleural effusion, thoracentesis was performed, where 2000 mL serosanguineous, but lightly clouded fluid was removed, which was confirmed to be a transudate according to Light criteria. 7 Further analysis of the transudate excluded any pathogenic infection or malignancy. The patient's treatment began here with intravenous (i.v.) levofloxacin and pantoprazole, which were administered for 9 days.
Transfer to the regional COVID‐19 ward
Regardless that SARS‐CoV‐2 infection was excluded, the patient was still moved to a regional COVID‐19 facility at day 5 of hospitalization. Consequently, this decision to transfer unnecessarily elevated her exposure risk to SARS‐CoV‐2. She also experienced severe nausea, and a gastroscopic examination was performed, where gastroesophageal reflux was proven. A new round of RT‐PCR tests again excluded SARS‐CoV‐2 infection. To explore alternative pathologies that could explain her status, transthoracic echocardiographic (TTE) examination was indicated, which showed severely reduced left ventricular ejection fraction (LVEF). On day 6 of hospitalization, HF was first posited as an explanation for the patient's severe status and symptoms. Hypotension was detected following a non‐invasive BP measurement; therefore, mannitol solution together with furosemide 8 and dopamine were administered i.v.
At this point, the patient was now in New York Heart Association (NYHA) III functional class, without adequate response to HF therapy, and the patient was transferred to our department's intensive care unit.
Admission to the cardiology ICU
At the time of admission, the patient's TTE showed a severely reduced LVEF of 18% with diffuse hypokinesis. The structure of the valves was normal aside from mild aortic regurgitation, moderate mitral regurgitation, and moderate tricuspid regurgitation with an elevated right ventricular systolic pressure. The thickness of the left ventricular wall was 10 mm. Around the heart, 10 mm concentric pericardial effusion was visualized. On ECG, sinus rhythm was detected, without any specific signs of ischemia, arrhythmia, peri‐ or myocarditis. NT‐proBNP was measured and found to be significantly elevated as high as 31 460 ng/L (Table 1 ). Treatment with i.v. positive inotropic agent (dobutamine) and diuretics (furosemide) was initiated, based on acute HF guidelines. 5
Hypertension was ascertained by invasive measurement during coronary angiography.
To establish the aetiology of the patient's acute HF, invasive coronary angiography was performed, but this excluded epicardial coronary artery disease. There was no coronary physiology test performed to assess coronary microcirculation. During examination, the right radial artery access failed, because the diagnostic catheter could not reach the brachiocephalic trunk due to an occluded right subclavian artery. It must be noted here that arterial BP was normal at the level of the occlusion. Switching to the right femoral artery approach, 220/100 mmHg BP was ascertained by invasive measurement. Systolic BPs of lower and upper limbs were compared, where the discrepancy was as high as 110 mmHg systolic.
Based on these findings, antihypertensive drug therapy was added to the HF therapy. Doses were titrated to achieve perindopril 5 mg twice a day (BID), indapamide 1.25 mg once daily, amlodipine 5 mg BID, bisoprolol 2.5 mg BID, and spironolactone 25 mg BID. The regimen above was prescribed at hospital discharge as well.
Final diagnosis
Abdominal‐chest CT angiography was performed, which confirmed bilateral subclavian artery occlusion (Figure 1 ) in addition to the previously described right renal artery occlusion (Figure 2 ), but with a polar artery still supplying the right kidney. Following from our findings, TA was proposed based on the widely used classification criteria by the American College of Rheumatology. 9 Three major criteria led to our conclusion: (i) age under 40 years; (ii) at least 10 mmHg difference systolic BP between the two arms/extremities; and (iii) occlusion of the aortic branches proven by angiography. 9 To summarize the likely progression of her condition, vascular inflammation from TA caused occlusion of the right renal artery and consequentially led to arterial hypertension, which could not be revealed by non‐invasive BP measurements because of the occluded subclavian arteries. Eventually, the patient's uncontrolled hypertension caused acute HF.
Figure 1.
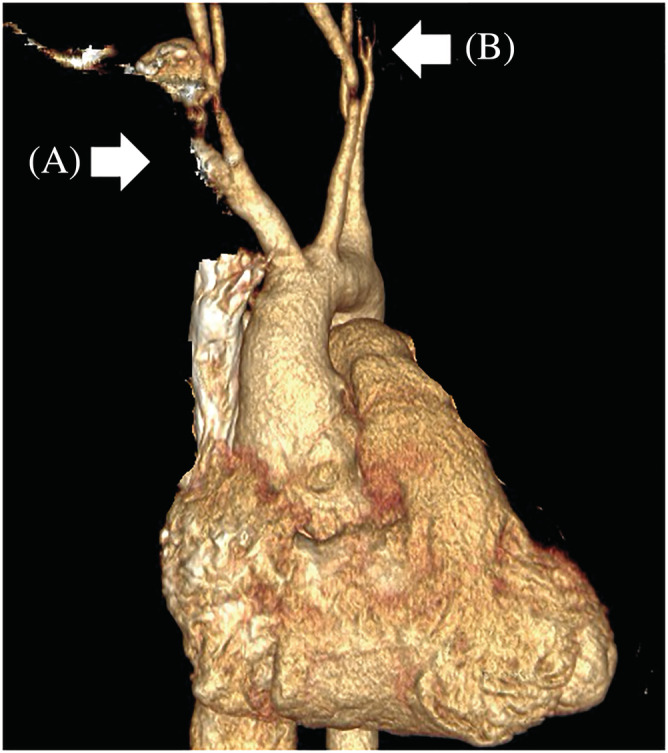
3D rendered CT angiography of the chest. CT angiography with volume rendered image of the chest. Occluded right (A) and left (B) subclavian arteries are showed by arrows.
Figure 2.
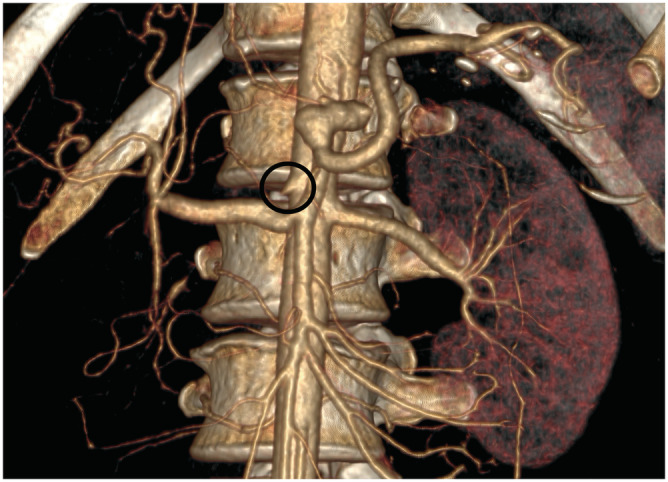
3D rendered CT angiography of the abdomen. CT angiography with volume rendered image shows the occluded right renal artery. The ostial part of the artery is visible (black circle). Due to the complete lack of perfusion, the right kidney is not visible under this window setting.
Our diagnosis of TA was supported after consultation with an immunologist. Another explanation could have been vasculitis of the small vessels causing myocarditis‐related HF; however, in this patient's case anti‐neutrophil cytoplasmic antibody (ANCA) levels were within the reference range and no small vessels were involved. Instead, either TA or immunoglobulin G4 (IgG4) related vasculitis was the most likely underlying pathology. IgG4‐related disease is a fibro‐inflammatory condition that can affect nearly any organ system with inflammatory aortitis and periaortitis, but it is usually restricted to the aorta. However, the patient's IgG4 values were normal, and the clinical character of the lesions suggested this mechanism was unlikely in her case. A positron emission tomography CT scan would have differentiated between TA‐ and IgG4‐related vasculitis but due to the current Hungarian health system financial environment, it could not have been performed in a timely manner. Considering that the treatment course for both diseases is equivalent, and the patient was in poor condition, the immunologist suggested prompt initiation of methylprednisolone and azathioprine therapy in combination with HF and antihypertensive medication.
The patient responded quickly to adequate antihypertensive and HF medical management. After 2 weeks, TTE showed a moderately reduced LVEF of 40%. 10 At 4 weeks, cardiac magnetic resonance imaging was performed and excluded significant structural abnormalities, the LVEF was normal. There was no late gadolinium enhancement with inversion recovery sequence.
Myocarditis could not be fully excluded as an explanation in this case. Unfortunately, a troponin measurement was not made during the examination process, a biopsy of the myocardium was not obtained, nor was a cardiac MR performed during hospitalization. It must be acknowledged that the patient also received immunosuppressive therapy indicated by the immunologist. However, the patient's rapid response to antihypertensive and HF pharmacotherapy supports our conclusions regarding the cause of the event.
Discussion
TA is a rare, idiopathic, granulomatous large‐vessel vasculitis affecting mainly young patients, and more frequently females. In most cases, diagnosis is delayed because of the diverse and nonspecific signs and symptoms. 11 The arteries involved typically include most of the aortic branches, and certain complications can develop such as cerebral thrombosis or embolism, malignant hypertension, acute coronary syndromes (ACS), or HF. 12
We present a case where delayed recognition of acute HF and TA led to end‐organ damage. However, there were several factors that influenced the course of events for this patient. First, it was almost inevitable the pandemic would take priority and guide decision making on the part of the medical staff. A major effort was initially placed on ruling out COVID‐19, for fear of misdiagnosing and spreading a SARS‐CoV‐2 infection. This factor alone significantly shifted attention away from other diseases and resulted in a substantial treatment delay. Additionally, the heterogeneous symptoms prolonged the diagnostic process. After initially examining for SARS‐CoV‐2 infection, other pathologies were considered, such as an acute abdominal event or pulmonary infection. The young age of the patient likely biased the attending physicians towards a more common aetiology and not to general artery disease or heart failure from any cause. Each investigation, even if necessary to rule out life‐threatening conditions, ultimately delayed the final diagnosis of acute HF and TA. Lastly, the time that elapsed before admitting the patient to a well‐equipped medical center also delayed diagnosis.
In our opinion, there are at least two highly important lessons to learn here. One is the necessity to arrive at a proper diagnosis and start evidence‐based therapy as quickly as possible, especially in life‐threatening conditions such as acute HF. The second lesson relates to the recent pandemic. The redistribution of resources in health care systems during the COVID‐19 pandemic resulted in fewer and delayed hospitalizations for cardiac conditions. 13 , 14 , 15 , 16 Clinicians justifiably prioritized the diagnosis of SARS‐CoV‐2 infection in their patients during the pandemic, but unfortunately, this type of bias could potentially albeit unintentionally hinder elucidation of other pathologies. It must be emphasized that heart‐related diseases are still the leading cause of death worldwide. 17 Our case report demonstrates that efforts must be made not to misdiagnose or delay the identification of CV conditions even when resources are unbalanced.
Conflict of interest
Informed consent was obtained from the patient of the case report before allowing the use of her medical information. All data were handled by the strict regulation of the local regulations. No conflict of interest declared.
Acknowledgements
We would like to express our special thanks to Jason Keller (BioNTech SE) for medical writing support and Tamas Papp (University of Debrecen, Department of Radiology) for his advice regarding the selection of CT images.
Rácz, Á. O. , Szabó, G. T. , Erdei, N. , Győry, F. , and Kolozsvári, R. V. (2022) Heart failure caused by Takayasu's arteritis in the time of COVID‐19: a case report. ESC Heart Failure, 9: 3602–3607. 10.1002/ehf2.14054.
References
- 1. Blumenthal D, Fowler EJ, Abrams M, Collins SR. Covid‐19 ‐ Implications for the Health Care System. N Engl J Med. 2020; 383: 1483–1488. [DOI] [PubMed] [Google Scholar]
- 2. Kaye AD, Okeagu CN, Pham AD, Silva RA, Hurley JJ, Arron BL, et al. Economic impact of COVID‐19 pandemic on healthcare facilities and systems: International perspectives. Best Pract Res Clin Anaesthesiol. 2021; 35: 293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xiao H, Dai X, Wagenaar BH, Liu F, Augusto O, Guo Y, Unger JM. The impact of the COVID‐19 pandemic on health services utilization in China: Time‐series analyses for 2016‐2020. Lancet Reg Health West Pac. 2021; 9: 100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Italia L, Tomasoni D, Bisegna S, Pancaldi E, Stretti L, Adamo M, Metra M. COVID‐19 and Heart Failure: From Epidemiology During the Pandemic to Myocardial Injury, Myocarditis, and Heart Failure Sequelae. Front Cardiovasc Med. 2021; 8: 713560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 6. ESC Guidance . ESC Guidance for the Diagnosis and Management of CV Disease during the COVID‐19 Pandemic. 2020. https://www.escardio.org/Education/COVID‐19‐and‐Cardiology/ESC‐COVID‐19‐Guidance (15 March 2022). [Google Scholar]
- 7. Light RW, Macgregor MI, Luchsinger PC, Ball WC. Pleural effusions: The diagnostic separation of transudates and exudates. Ann Intern Med. 1972; 77: 507–513. [DOI] [PubMed] [Google Scholar]
- 8. Turagam MK, Velagapudi P, Kalra AS, Ramalingam VS, Sinnakirouchenan R, Holley JL. Outcomes of furosemide‐mannitol infusion in hospitalized patients with heart failure: An observational single‐center cohort study of 122 patients. Int J Cardiol. 2011; 151: 232–234. [DOI] [PubMed] [Google Scholar]
- 9. Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990; 33: 1129–1134. [DOI] [PubMed] [Google Scholar]
- 10. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015; 28: 1–39.e14. [DOI] [PubMed] [Google Scholar]
- 11. An X, Han Y, Zhang B, Qiao L, Zhao Y, Guo X, et al. Takayasu arteritis presented with acute heart failure: Case report and review of literature. ESC Heart Fail. 2017; 4: 649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mason JC. Takayasu arteritis‐‐advances in diagnosis and management. Nat Rev Rheumatol. 2010; 6: 406–415. [DOI] [PubMed] [Google Scholar]
- 13. Kiss P, Carcel C, Hockham C, Peters SAE. The impact of the COVID‐19 pandemic on the care and management of patients with acute cardiovascular disease: A systematic review. Eur Heart J Qual Care Clin Outcomes. 2021; 7: 18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cannata A, Watson SA, Daniel A, Giacca M, Shah AM, McDonagh TA, et al. Impact of the COVID‐19 pandemic on in‐hospital mortality in cardiovascular disease: A meta‐analysis. Eur J Prev Cardiol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Katsoulis M, Gomes M, Lai AG, Henry A, Denaxas S, Lagiou P, et al. Estimating the Effect of Reduced Attendance at Emergency Departments for Suspected Cardiac Conditions on Cardiac Mortality During the COVID‐19 Pandemic. Circ Cardiovasc Qual Outcomes. 2021; 14: e007085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pessoa‐Amorim G, Camm CF, Gajendragadkar P, De MGL, Arsac C, Laroche C, et al. Admission of patients with STEMI since the outbreak of the COVID‐19 pandemic: A survey by the European Society of Cardiology. Eur Heart J Qual Care Clin Outcomes. 2020; 6: 210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics‐2019 Update: A Report From the American Heart Association. Circulation. 2019; 139: e56–e528. [DOI] [PubMed] [Google Scholar]


