Abstract
Aims
Implantable pulmonary artery pressure (PAP) sensors have been shown to reduce heart failure hospitalizations (HFH) in selected patients. The goal of this study was to evaluate the safety and efficacy of a novel wireless PAP monitoring system in patients with heart failure (HF).
Methods and results
This is a prospective, multi‐centre, open‐label, single‐arm trial evaluating the safety and efficacy of the Cordella™ PA Sensor System including the comprehensive Cordella™ Heart Failure System (CHFS) in patients with New York Heart Association (NYHA) Class III heart failure with a heart failure hospitalization and/or increase of N‐terminal pro‐Brain Natriuretic Peptide (NT‐proBNP) within 12 months of enrolment. The primary efficacy endpoint was the accuracy of PA sensor mean PAP measurements, compared with fluid‐filled catheter mean PAP measurements obtained by standard right heart catheterization (RHC) at 90 days post‐implant, assessed in all patients with a successful implant. The primary safety endpoint was freedom from adverse events associated with use of the Cordella PA Sensor System through 30 days post‐implant, assessed in all patients who entered the cath lab for PA sensor implant. The PA sensor was successfully implanted in 70 patients. Equivalence between the PA sensor and RHC for mean pulmonary artery pressures was excellent with measurements confined within the equivalence bounds of −4.0 to 4.0 mmHg (mean PAP: 0.0 to 2.9 mmHg, P = 0.003). The device safety profile was excellent with 98.6% freedom from Device System Related Complications, defined as invasive treatment, device explant or death. There were no pressure sensor failures. Patients' adherence to daily measurement transmissions of PAP and vital signs was 94%.
Conclusions
This trial supports the safety and efficacy of the Cordella PA Sensor System and in conjunction with the CHFS enables comprehensive HF management in NYHA class III heart failure patients.
Keywords: Remote patient monitoring, Heart failure, GDMT, Pulmonary artery pressure
Introduction
Despite significant advances in drug‐based and device‐based therapies, heart failure (HF) remains a major and growing public health problem associated with substantial disability, frequent hospitalizations, and high economic costs. 1 The focus of HF management has shifted away from reactive management of episodes of decompensation requiring hospitalization to proactive HF management to keep patients out of the hospital. Achieving this requires remote management of underlying heart disease, co‐morbidities, social and psychological aspects of the disease, and haemodynamic and fluid status.
Pulmonary artery pressure guided heart failure management using an implantable pulmonary artery pressure monitor reduces heart failure hospitalizations (HFH) in NYHA Class III patients, irrespective of ejection fraction, in randomized and post market studies. 2 , 3 , 4 , 5 The Cordella Heart Failure management system (the commercially available Cordella Heart Failure System (CHFS) and the investigational Cordella PA Sensor System (Endotronix Inc, Chicago, IL, USA)) provides comprehensive clinical information, including pulmonary artery pressures, body weight, blood pressure, heart rate, and oxygen saturation (SpO2), from NYHA Class III HF patients to their HF team, allowing for a proactive, comprehensive remote HF management platform. The Cordella Heart Failure management system has been shown to enable safe and accurate remote monitoring of HF status in first‐in‐human feasibility trial. 6
The hypothesis in the present study, a prospective, multi‐centre, open‐label, single‐arm clinical trial (SIRONA 2 trial), evaluating the safety and efficacy of the Cordella PA Sensor System in NYHA Class III Heart Failure Patients is that implantation of the Cordella PA Sensor (henceforward the PA sensor) is safe and that the sensor measures PAP with sustained accuracy.
Methods
Study design and inclusion criteria
SIRONA 2 (ClinicalTrials.gov identifier: NCT04012944) is a CE‐Mark trial to evaluate the safety and efficacy of the Cordella PA Sensor System in NYHA Class III HF patients implanted with the PA sensor and discharged to home with the CHFS. The study was undertaken in accordance with the Declaration of Helsinki (GCP‐ICH, ISO14155:2020) and approved by the relevant Competent Authorities and independent ethics committees. All patients provided written informed consent. Eligible patients for SIRONA 2 were men or woman over 18 years of age with a diagnosis of NYHA class III HF with reduced or preserved ejection fraction for at least 6 months treated for a minimum of 3 months and stable for at least 1 month prior to enrolment. Patients had to have at least one HF‐related hospitalization, HF treatment in a hospital day‐care setting, or unplanned outpatient clinic HF visit within 12 months prior to consent and/or increase of NT‐proBNP or Brain Natriuretic Peptide (BNP) at time of screening. Prespecified thresholds defined as NT‐proBNP ≥1000 pg/mL (or BNP ≥ 250 pg/mL) for subjects with an LVEF ≤40% and NT‐proBNP ≥700 pg/mL (or BNP ≥ 175 pg/mL) for subjects with an LVEF >40%, with threshold correction for body mass index (BMI). A full list of inclusion and exclusion criteria can be found on the supporting information.
Study device
The device has been described elsewhere, 6 but briefly, CHFS is a comprehensive digital HF management technology that measures, records, and transmits vital signs (blood pressure, heart rate, weight, and oxygen saturation) and PAP data from the patients' home to the clinical teams for proactive management for patients with HF. Data are transmitted for review by the clinical team on the web‐based patient management portal (PMP).
The Cordella™ PA Sensor System comprises the PA sensor, Cordella delivery system, Cordella calibration equipment (CalEQ), and myCordella patient reader, a small (~600 g) handheld reader and charging dock. The PA sensor is a permanent device, implanted via right heart catheterization (RHC), to the branch of the right pulmonary artery (RPA), where the interlobar artery typically turns downward and posterior with vessel diameter of 12–26 mm. The sensor body is approximately 20 × 4 × 2 mm in size and nitinol anchors extend from either end to hold the sensor in place against the wall of the RPA (Figure 1A and 1 B ).
Figure 1.
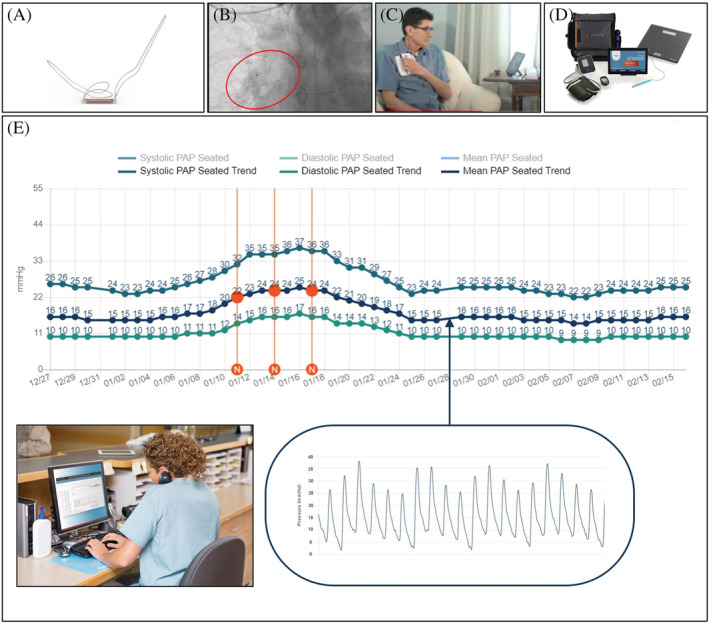
(A) Cordella pulmonary artery sensor. (B) Pulmonary angiogram depicting sensor deployment in the right pulmonary artery. (C) Depiction of patient using the handheld myCordella patient reader to measure seated PAP in the home environment. (D) Cordella patient kit with vital sign peripherals. (E) Cordella pulmonary artery pressure system trend waveforms as seen by the clinician through the patient management portal (PMP). The red vertical lines represent clinician notes. (Inset) Daily reading of mean pulmonary artery pressure. Respiratory fluctuations and secondary features such as the dicrotic notch are evident.
The PAP is measured by the handheld patient reader for 18 s to obtain the mean PAP and waveform (Figure 1 C ). This along with the vital signs are securely transmitted from the patient's myCordella™ tablet, via the Cordella data analysis platform (CDAP) to the PMP (Figure 1 D ). This web‐based application provides the patient's clinician with a comprehensive remote overview of the daily submissions. By default, PAP is displayed as daily measures and 7 day average over time for seated mean PAP. Systolic and diastolic PAP daily and trend values for seated and/or supine are also available along with optional metrics (Figure 1 E ). On the same page, accompanying daily vital signs are also displayed. The system also allows the clinician to set patient specific targets on the vital signs as a complement to PAP‐driven monitoring. In practice, the comprehensive overview provides the clinician with the overview and notification to assess patient haemodynamic status and drive guideline directed medical treatment (GDMT) when the parameters are outside of target PAP range. Furthermore, PMP provides easy functionality for patient and physician communication via chat and related notification options in order to facilitate engagement and optimize disease management.
Cordella™ pulmonary artery sensor implantation
The implantation procedure has been described elsewhere 6 but briefly, at the time of implant, patients underwent a RHC and implantation of the PA sensor. A 14 Fr introducer was inserted into the femoral vein and RHC undertaken using a 7.5 Fr Swan‐Ganz catheter (Edwards LifeSciences, Irvine, CA, USA), Through the distal lumen of the Swan‐Ganz catheter a support wire was positioned in the RPA and the Swan‐Ganz catheter exchanged for a 5 Fr angiographic catheter. Pulmonary angiography was performed in the antero‐posterior and left anterior oblique caudal views. The support wire was then positioned in a right lower lobe branch of the pulmonary artery (A8‐A10), and the angiographic catheter exchanged for the Cordella Delivery System. The PA sensor, pre‐mounted on the Delivery System, was advanced through the right heart and positioned at the inferior‐posterior inflection of the RPA. Position was confirmed by hand injection of contrast through the side arm of the Delivery System. Withdrawal of the release wires served to free the self‐aligning nitinol anchors and secure the PA sensor in place. Following implantation, the system was calibrated to a reference fluid‐filled pressure measurement using the CalEQ.
After implantation and prior to discharge, patients were trained on the set up and use of the CHFS and Patient Reader. Patients were trained on how to collect their weight, BP, HR, SpO2, and how to measure PAP in both seated and supine positions. Then patients were trained on how to transmit this data daily, at the same time, typically each morning, to their clinicians for review.
Follow‐up and endpoints
The primary safety endpoint was freedom from adverse events (SAEs and AEs) associated with use of the Cordella™ PA Sensor System through 30 days post‐sensor implant. Secondary endpoints reported include frequency of AEs and device/system‐related complications (DSRC) and PA pressure sensor failure rate throughout the study.
The primary efficacy endpoint was the accuracy of the PA sensor mean PAP measurements relative to standard‐of‐care fluid‐filled catheter mean PAP measurements obtained by standard RHC at 90 days post‐sensor implant. Key secondary efficacy endpoints reported include percentage of device success as documented by the ability of the system to successfully transmit collected PAP data to a secure database, change in PAP, frequency of HF hospitalizations, HF treatments in a hospital day‐care setting, or urgent outpatient clinic HF visits (HFH), quality of life and functional changes, and patient compliance with data transmission.
Subjects will participate in the study for a total of 48 months. All subjects were followed for 1 month for the primary safety endpoint, 3 months for the primary efficacy endpoint, and will continue until the 48 month assessment for the secondary efficacy endpoints. Subjects participated in the Screening visit, the Sensor Implant visit, and at least the 3 month follow‐up visit to conclude the primary endpoint analysis and results are presented here along with 6 month HFH rate and HFH and death rate. Total follow‐up visits are 1, 3, 6, 12, 18, 24, 36, and 48 months or until study termination. Assessments performed at follow‐up visits include physical examinations, concomitant medication assessment, clinical laboratory assessments, vital signs, review of Cordella PA Sensor readings, NYHA functional classification, KCCQ, 6MWT, and AE assessment.
Statistical analysis
Descriptive statistics were used to evaluate baseline clinical and demographic characteristics. Results are reported as mean ± standard deviation for continuous variables and as percent (count × 100/sample size) for binary variables.
Primary safety analysis
The primary safety endpoint was freedom from adverse events associated with use of the PA Sensor System through 30 days post‐implant in the intent‐to‐treat (ITT) population. Events are listed as Adverse Events, Serious Adverse Events, and Device/System‐Related Complications and results are reported as number and percentage of subjects reporting. Safety endpoints were adjudicated by a central endpoint committee.
Primary effectiveness endpoint and analysis
The PA sensor measurements were compared with the measurements of standard‐of‐care commercial products that use fluid‐filled invasive catheters to measure PAP, which have an accuracy of ±4 mmHg, in the modified intent‐to‐treat (mITT) population. Measurements were compared at 90 days post‐implant visit and equivalence of the readings within the region of ±4.0 mmHg was shown using a two‐sided paired t‐test with alpha = 5%. Schuirmann's two one‐sided test (TOST) approach was used to test equivalence 7 with the result of mean PAP being the confirmatory TOST test for equivalence. The TOST test was run using the ‘dataTOSTpaired’ function in the ‘TOSTER’ package in R (R Core Team 2021) with equivalence bounds of ±4 mmHg and alpha of 5%. The study was designed to provide a statistical power of >90%.
Secondary effectiveness endpoint and analysis
The correlation between the PA sensor measurement RHC was assessed at 90 days with Pearson's correlation coefficient computed for correlation. Results for KCCQ, 6MWT, and NYHA classification are reported as mean ± standard deviation. Comparisons between baseline and 3 month results are made with a two‐sample t‐test. Heart failure hospitalizations/events and heart failure hospitalizations or death through 6 months were evaluated using the Anderson–Gill proportional hazards model.
Patient survey
A survey was carried out to evaluate the subject experience with both the Cordella heart failure system and seated and supine PA pressure measurements. The survey was optional and administered to patients at 90 days. The survey consisted of 11 multiple choice questions and were asked via the Cordella patient tablet.
Results
Between 21 June 2019 and 16 July 2021, 81 patients were enrolled in the SIRONA 2 trial at seven sites. Six patients withdrew consent prior to implantation of the Cordella sensor, leaving 75 patients in the ITT population. The implant was aborted in five patients [patients withdrew consent prior to implant (n = 3), patients met exclusion criteria while awaiting implant (n = 2) and withdrawn by physician (n = 1)] leaving 70 patients in the implanted population (mITT). Baseline demographics are reported for the implanted population. Of the 70 patients, 50 (71.4%) were men, 66 (94.3%) were white, mean age was 71.0 years, and mean BMI was 28.7 kg/m2. As required all patients were NHYA Class III. Twenty‐two (31.4%) had a preexisting ICD device and 13 (18.6%) had a CRT or CRT‐D device. Twenty‐seven (38.6%) of patients had LVEF >40% and differences between LVEF >40% and LVEF ≤40% are also reported (Table 1 ).
Table 1.
Baseline demographics
| Characteristic | Implanted (n = 70) | HFPEF (LVEF >40) (n = 27) | HFREF (LVEF ≤40) (n = 43) |
|---|---|---|---|
| Demographics | |||
| Age (years), mean (SD) | 71.0 (10.0) | 74.0 (9.6) | 69.0 (9.9) |
| Male sex, n (%) | 50 (71.4) | 16 (59.3) | 34 (79.1) |
| White race, n (%) | 66 (94.3) | 25 (92.6) | 41 (95.3) |
| Body‐mass index (kg/m2), mean (SD) | 28.7 (5.8) | 29.3 (4.1) | 28.3 (6.6) |
| Medical history | |||
| Patients without HFH a in previous year but met condition of NT‐proBNP or BNP at screening, n (%) | 15 (21.4) | 7 (25.9) | 6 (14.0) |
| Patients who had at least 1 HFH a in past 12 months but did not meet the condition of NT‐proBNP or BNP at screening, n (%) | 10 (14.3) | 4 (14.8) | 8 (18.6) |
| Patients who had at least 1 HFH a in past 12 months and met the condition of NT‐proBNP or BNP at screening, n (%) | 45 (64.3) | 16 (59.3) | 29 (67.4) |
| CRT or CRT‐D device, n (%) | 13 (18.6) | 1 (3.7) | 12 (27.9) |
| ICD device, n (%) | 22 (31.4) | 1 (3.7) | 21 (48.8) |
| Diabetes mellitus, n (%) | 32 (45.7) | 14 (51.9) | 18 (41.8) |
| Hypertension, n (%) | 51 (72.9) | 21 (77.8) | 30 (69.8) |
| Stroke, n (%) | 9 (12.9) | 2 (7.4) | 7 (16.3) |
| Atrial fibrillation, n (%) | 46 (65.7) | 19 (70.3) | 27 (62.8) |
| Chronic kidney disease, n (%) | 22 (31.4) | 8 (29.6) | 14 (32.6) |
| Laboratory | |||
| Left ventricular ejection fraction (%), mean (SD) | 36.7 (14.1) | 52.1 (5.9) | 27.1 (7.4) |
| N‐terminal pro‐B‐type natriuretic peptide (pg/mL), mean (SD) | 2316.9 (3907.2) | 1239.5 (1096.7) | 3055.7 (4883.4) |
| Haemodynamics | |||
| Systolic blood pressure (mmHg), mean (SD) | 119.9 (19.7) | 133.7 (22.9) | 116.7 (13.9) |
| Diastolic blood pressure (mmHg), mean (SD) | 67.6 (11.8) | 73.9 (15.6) | 67.9 (10.1) |
| Heart rate (beats per min), mean (SD) | 71.4 (10.5) | 78.0 (19.7) | 72.0 (11.8) |
| Systolic pulmonary artery pressure (mmHg), mean (SD) | 41.1 (19.5) | 52.8 (22.7) | 38.1 (13.3) |
| Diastolic pulmonary artery pressure (mmHg), mean (SD) | 16.4 (10.7) | 18.6 (7.5) | 13.2 (7.5) |
| Mean pulmonary artery pressure (mmHg), mean (SD) | 24.7 (14.4) | 30.7 (11.9) | 22.6 (9.1) |
| Right atrial pressure (mmHg), mean (SD) | 7.6 (5.6) | 10.1 (5.4) | 6.0 (5.1) |
| Pulmonary capillary wedge pressure (mmHg), mean (SD) | 15.4 (7.2) | 17.0 (7.0) | 14.2 (7.5) |
| Cardiac output (L/min), mean (SD) | 4.9 (1.5) | 5.0 (1.7) | 4.7 (1.4) |
| Cardiac Index (L/min/m2), mean (SD) | 2.8 (3.2) | 2.5 (0.9) | 2.3 (0.6) |
| Heart failure medication | |||
| Agents acting on the renin‐angiotensin system, n (%) | 55 (77.1) | 17 (63.0) | 38 (88.4) |
| Angiotensin receptor‐neprilysin inhibitor | 27 (38.6) | 3 (11.1) | 24 (55.8) |
| Angiotensin II receptor blocker | 9 (12.9) | 5 (18.5) | 4 (9.3) |
| ACE inhibitor | 19 (27.1) | 10 (37.0) | 9 (20.9) |
| Beta‐blocker, n (%) | 54 (77.1) | 18 (66.7) | 36 (83.7) |
| Diuretic, n (%) | 61 (88.6) | 25 (92.6) | 36 (83.7) |
| Aldosterone antagonist, n (%) | 38 (54.3) | 8 (29.6) | 30 (69.8) |
| SGLT2 inhibitor, n (%) | 12 (17.1) | 2 (7.4) | 10 (23.3) |
| Functional class and quality of life | |||
| New York Heart Association functional class III, n (%) | 70 (100) | 27 (100) | 43 (100) |
| 6‐min walk test (m), mean (SD) | 287.3 (133.4) | 252.1 (155.1) | 311.7 (111.7) |
| Kansas City Cardiomyopathy Overall Summary Score (points), mean (SD) | 55.75 (24.4) | 52.0 (23.0) | 58.1 (25.2) |
BNP, B‐type natriuretic peptide; CRT(D), cardiac resynchronization therapy (defibrillator); ICD, implantable cardioverter defibrillator; NT‐pro BNP, N‐terminal prom hormone B‐type natriuretic peptide; SD, standard deviation; SGLT2, sodium‐glucose transport protein 2.
HFH, heart failure hospitalizations and urgent heart failure hospital visits defined as emergency department or hospital outpatient observation visits requiring IV diuretic.
Primary safety endpoint
The PA sensor was successfully implanted during the first procedure for all implanted patients. For the primary safety endpoint, freedom from AEs associated with use of the PA Sensor System through 30 days post‐implant in the ITT population, there were a total of six (8.0%) adverse events in four (5.3%) patients. There were two (2.7%) serious adverse events (SAE) related to the implant procedure with one SAE being adjudicated as DSRC (LV lead dislodgement), both in the same patient. There were no PA pressure sensor failures post‐implant. All observed complications recovered without sequelae (Table 2 ).
Table 2.
Primary safety endpoint
| Events through 30 days (n = 75) | Number of events, n (%) | Days after implant | Related to procedure | Related to study device | Therapy | Outcomes |
|---|---|---|---|---|---|---|
| Adverse events | ||||||
| Skin irritation | 1 (1.3) | 1 | Not related | Related | Clothing between reader and skin | Recovered without sequalae |
| Haemoptysis a | 1 (1.3) | 3 | Related | Related | None | Recovered without sequalae |
| Vessel trauma a | 1 (1.3) | 3 | Probably Related | Probably Related | Bronchoscopy | Recovered without sequalae |
| Haematoma | 1 (1.3) | 0 | Related | Not Related | None | Recovered without sequalae |
| Device‐related/system‐related complications | ||||||
| LV lead dislodgment a | 1 (1.3) | 0 | Related | Related | Lead revision | Recovered without sequalae |
| Serious adverse events | ||||||
| LV lead revision† | 1 (1.3) | 15 | Related | Related | Lead Replacement | Recovered without sequalae |
| Total Adverse Events | 6 (8.0) | |||||
LV, left ventricle.
Same patient.
Primary efficacy endpoint
For the 90 day efficacy endpoint, 58 patients (83%) underwent RHC. Reasons for patients not getting the 90 day RHC include cancelled due to COVID‐19 (n = 8), patient refusal to undergo RHC (n = 2), and RHC cancelled due to SAE (n = 2). Despite 12 patients (mITT population) not being included in the primary efficacy endpoint, 58 patients provide a sufficient number to provide >90% statistical power to satisfy the endpoint. PAP values measured by the PA sensor and RHC were well‐matched and the primary efficacy endpoint for mean PAP was met in all patients with a 90% CI equivalence margin of 0.0 mmHg (t‐value = 6.17, P‐value <0.001) to 2.9 mmHg (t‐value = −2.92, P‐value = 0.003) with an overall P = 0.003 for equivalence, well within the predefined equivalence margin of −4.0 to 4.0 mmHg (Figure 2 A ). Equivalence was also met for systolic PAP in all patients with a 90% CI equivalence margin of −2.0 mmHg (t‐value = 3.77, P‐value <0.001) to 1.32 mmHg (t‐value = −4.41, P‐value <0.001) with an overall P < 0.001 for equivalence and for diastolic PAP in all patients with a 90% CI equivalence margin of 0.63 mmHg (t‐value = 6.28, P‐value <0.001) to 3.99 mmHg (t‐value = −1.68, P‐value = 0.49) with an overall P = 0.49 for equivalence.
Figure 2.
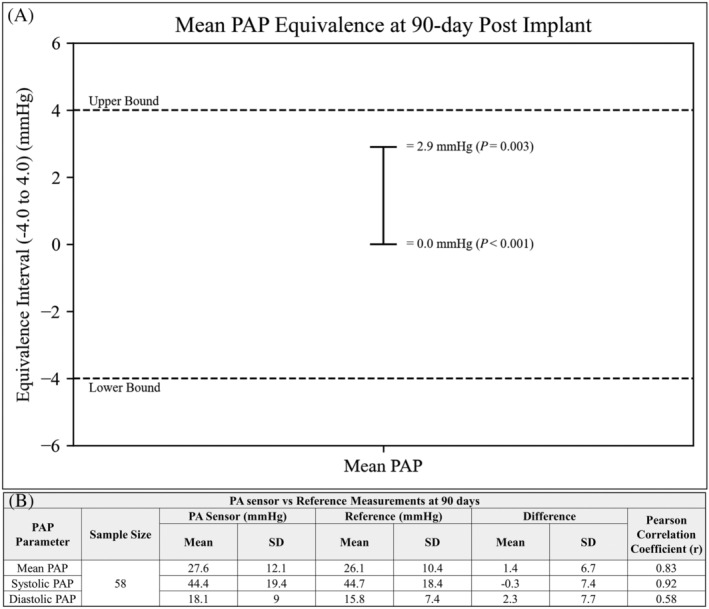
(A) TOST plot for mean PAP meeting primary efficacy endpoint. (B) Differences and correlation between PA sensor versus Reference for mean, systolic, and diastolic PAP at 90 days, all P values <0.001.
Secondary efficacy endpoint
Similarly, for the 90 day secondary efficacy endpoint, the PAP values measured by the PA sensor and reference at 90 days were well‐matched with the mean difference between PA sensor and reference for mean PAP 1.4 mmHg (r = 0.83), systolic PAP −0.3 mmHg (r = 0.92), and diastolic PAP 2.3 mmHg (r = 0.58) (all P values <0.001), Figure 2 B .
The overall performance of the device to transmit collected data was excellent with a 99.7% success rate of transmission (6000 days of successful transmission out of 6018 days).
Heart failure hospitalizations
Eight patients (11.4%) experienced a HFH (defined as in hospital, a hospital day‐care setting, or urgent outpatient clinic HF visits) at 90 days. In the 6 months following implant, 11 patients (15.7%) had an HFH. This translated into an event per patient per 6 months rate of 0.16. When examining the composite HFH plus death (N = 3), there were 14 events with a 0.20 event rate per patient per 6 months (Figure 3A and 3B ).
Figure 3.
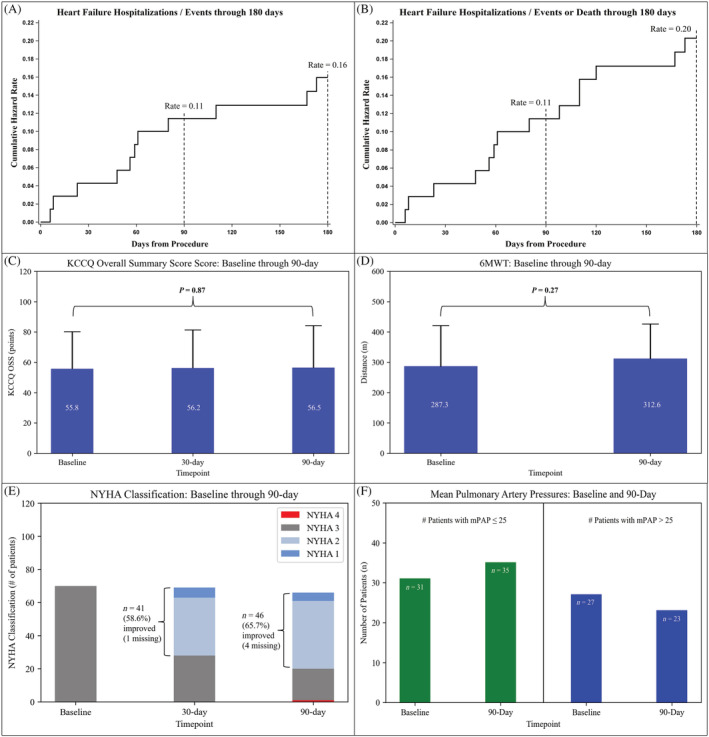
(A) Cumulative hazard rate curve through 180 days for HF hospitalizations, HF treatments in a hospital day‐care setting, or urgent outpatient clinic HF visits. (B) Cumulative hazard rate curve through 180 days for HF hospitalizations, HF treatments in a hospital day‐care setting, or urgent outpatient clinic HF visits and death. (C) Baseline, 1, 3 month KCCQ OSS. (D) Baseline, 3 month 6MWT. (E) Baseline, 1, 3 month NYHA Classification. (F) Number of patients with mean pulmonary artery pressure (mPAP) ≤ 25 and mPAP >25 mmHg at both baseline and 90 days.
Quality of life and functional capacity
Quality of life was measured by the Kansas Cardiomyopathy Questionnaire (KCCQ). Overall summary score was unchanged from baseline through 90 days (55.8 ± 24.4 vs. 56.5 ± 27.7 points, P = 0.87), Figure 3 C . Similarly, 6 min walk test (6MWT) distance was unchanged from baseline through 90 days (287.3 ± 133.4 vs. 312.6 ± 113.3, P = 0.27), Figure 3 D . Improvements in NYHA classification were demonstrated in 46 (65.7%) of patients and 1 patient (1.4%) went from NYHA class III to NYHA Class IV (Figure 3 E ).
Pulmonary artery pressure changes
Of the 58 patients included in the primary efficacy endpoint, 31 (53.4%) had mean PAP ≤ 25 mmHg at baseline and 35 (60.3%) had mean PAP ≤ 25 mmHg at 90 days. Conversely, 27 (46.6%) patients had mean PAP > 25 mmHg at baseline and 23 (39.7%) had mean PAP > 25 mmHg at 90 days (Figure 3 F ).
Patient compliance and satisfaction
Patient compliance was excellent with 95% of patients compliant (data transmission ≥5 out of 7 days) at 1 month, 94% at 3 months, and 93% at 6 months (Figure 4 A ). Results of the patient survey show 75% of respondents find the Cordella home system easy to use, 67% know their pressure ranges and would notice if their daily measurements were higher or lower than normal, and 84% prefer to take their PAP reading in the seated position (Figure 4 B ). Full results of the survey can be found in the supporting information.
Figure 4.
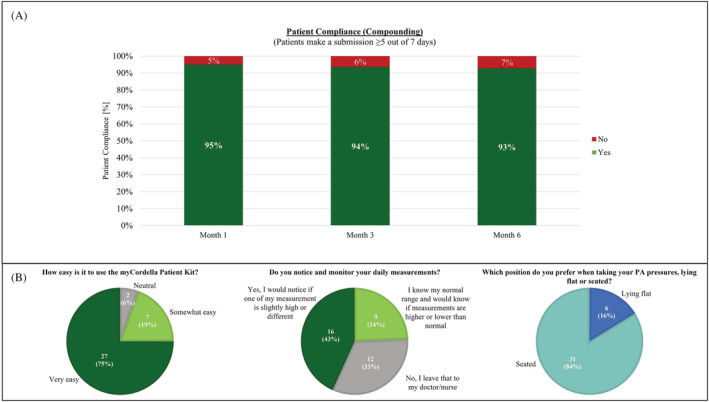
(A) Patient compliance at 1, 3, and 6 months. (B) Patient survey questions relating to ease of use, patient engagement, and seated versus supine PAP reading posture.
Discussion
SIRONA 2 is a prospective, multi‐centre study demonstrating that implantation of the Cordella PA Sensor was feasible and safe, PAP measurements were equivalent to RHC, and data transmission performance and patients' adherence to daily transmissions were excellent. Additionally, PAP measurements of the PA sensor, on average, showed a mean difference from the RHC of 1.4 mmHg for mean PAP, −0.3 mmHg for systolic PAP, and 2.3 mmHg for diastolic PAP and compares favourably with other implantable PAP monitoring systems. 8
The device safety profile through 30 days was excellent with only 1 patient experiencing a DSRC related to the procedure [left ventricle (LV) lead dislodgment]. All four patients who experienced an adverse event recovered without sequalae. There were no PA pressure sensor failures, and the overall safety profile compares very favourably to the most recent data published with other implantable PAP monitoring systems. 2 , 4 , 5 , 9
Heart failure hospitalizations remain unacceptably high, imparting significant costs to patients, caregivers, and the health system overall. Both non‐invasive home telemonitoring and monitoring of pulmonary artery pressures using a wireless haemodynamic monitoring system are recommended in the most recent European Society of Cardiology guidelines for the diagnosis and treatment of acute and chronic heart failure to reduce the risk of recurrent hospitalization and to improve clinical outcomes. 10 Home telemonitoring involves protocolized phone calls, vital sign measurement, and symptom monitoring to detect early HF decompensation. A systematic review conducted in 2017 identified 39 trials relevant to home telemonitoring and found a reduction of all‐cause mortality of 20% and HFH of 37%. 10 , 11 Similarly, remote monitoring of PAP has been shown to reduce HFH. 2 , 4 , 5 , 9 In the CHAMPION trial, the treatment group had 84 HFH in 6 months and a rate of 0.32 events per patient per 6 months. 2 The MEMS‐HF trial showed 27.8% of patients had HFH within 6 months of implant with an event per patient year rate of 0.60. 9 The most recent randomized control trial involving PAP, GUIDE‐HF, following patients for 12 months, found 42.9% of patient had HFH with an event per patient year rate of 0.47 and a composite HFH plus death in 50.9% of patient with a rate of 0.56. 5 In the present study, utilizing both home vitals and PA monitoring, there were 11 HFH (15.7%) through 6 months, with an events per patient 6 months rate of 0.16. For the composite HFH plus death, there were 14 events (20%) through 6 months with a rate of 0.20. These results may reflect the compounding effect of incorporating both home telemonitoring and PAP monitoring into one system with a high compliance rate that patients find easy to use. Some limitations may include the fact that, by certain markers, the patient cohort in SIRONA 2 is less sick, with smaller BMI (28.7 kg/m2 vs. 28.3–31.7 kg/m2) and lower filling pressures at the time of implant (mean PAP 24.7 mmHg vs. 28.0–31.3 mmHg), than has been seen in previous PA sensor trials. 2 , 4 , 5 , 9 Additionally, 9 months after SIRONA 2 began enrolment, the COVID‐19 pandemic began. HF events were reduced in the general heart failure population during the COVID‐19 pandemic 12 , 13 , 14 and, while the effects of this were not analysed here, the pandemic had an impact on heart failure hospitalizations in a recent PA pressure sensor trial. 5
The CHFS is the only remote heart monitoring platform to provide both ESC guideline recommended telemonitoring modalities, invasive (PAP) and noninvasive vitals (BP, weight, HR, and SPO2), from the patients' home. Additionally, the easy‐to‐use patient and clinician interface, patient to clinician chat feature, and small handheld patient reader facilitated engagement and drove high compliance throughout the study (93% patient compliance at 6 months). A patient survey distributed during the study found that 94% of those surveyed found the CHFS home kit easy or somewhat easy to use, 67% notice and monitor their daily measurements, and that 84% preferred to take their readings in the seated position compared with supine. These results continue to build on the excellent patient compliance found in the first‐in‐human study (99% at 90 days) and, in combination with the safety and accuracy reported herein, further strengthens the position for Cordella as the most comprehensive system in the proactive monitoring and management of NYHA class III HF patients. 6
Besides reducing HFH and mortality, a primary goal of heart failure treatment is to optimize patient health status, namely, their symptoms, function, and quality of life. 15 In this study, severity of symptoms as classified by NYHA, improved in 65.7% of cases (N = 41 went from NYHA Class III to II, N = 5 went from NYHA Class III to I). Examining quality‐of‐life and functional capacity outcomes there was no statistically significant improvement (nor was there a diminishment) in quality of life as measured by KCCQ nor in functional capacity as measured by 6MWT through 90 days. However, given the proportion of patients who had fair to excellent KCCQ overall summary scores (50–100) at baseline, the threshold for improvement may be difficult to ascertain. 15 Six‐minute walk test has been shown to be an independent predictor of mortality in patients with chronic HF and that 6MWT distance is stable for HF patients who survive 1 year between tests. 16 , 17 While there was a quantitative improvement in 6MWT from baseline to 3 months in this study, the results did not meet statistical significance. The most recent data from the other PAP monitoring system also showed no improvement in KCCQ and 6MWT through 12 months. 5
The SIRONA 2 clinical trial did not include a consistent PAP‐guided management guideline to enable remote adjustment of guideline‐directed medical therapy. 18 The ongoing PROACTIVE‐HF trial (ClinicalTrials.gov identifier: NCT04089059) incorporates prespecified PAP‐guided protocol, providing clinically actionable information to drive adherence to GDMT and adds to the comprehensive nature of this technology. 19
Conclusions
The SIRONA 2 study validates a new tool for comprehensive, patient‐centered PAP‐guided HF management enabling new haemodynamic insights in NYHA class III HF patients. The device is safe with a low rate of device and system‐related complications and no pressure sensor failures. PAP measurements were accurate and equivalent to the gold standard, Swan–Ganz catheter. A large clinical trial with prespecified treatment guidelines to validate clinical outcomes is underway and enrollment is ongoing (PROACTIVE‐HF).
Supporting information
Table S1. Inclusion and exclusion criteria.
Table S2. Patient Survey.
Sharif, F. , Rosenkranz, S. , Bartunek, J. , Kempf, T. , Assmus, B. , Mahon, N. G. , and Mullens, W. (2022) Safety and efficacy of a wireless pulmonary artery pressure sensor: primary endpoint results of the SIRONA 2 clinical trial. ESC Heart Failure, 9: 2862–2872. 10.1002/ehf2.14006.
References
- 1. Abraham WT, Bensimhon D, Pinney SP, Feitell SC, Peacock WF, Amir O, Burkhoff D. Patient monitoring across the spectrum of heart failure disease management 10 years after the CHAMPION trial. ESC Heart Failure. 2021; 8: 3472–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abraham WT, Adamson PB, Boureg RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS, CHAMPION Trial Study Group . Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomised controlled trial. Lancet. 2011; 377: 658–666. [DOI] [PubMed] [Google Scholar]
- 3. Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, Henderson J, Cowart P, Stevenson LW. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014; 7: 935–944. [DOI] [PubMed] [Google Scholar]
- 4. Shavelle DM, Desai AS, Abraham WT, Bourge RC, Raval N, Rathman LD, Heywood JT, Jermyn RA, Pelzel J, Jonsson OT, Costanzo MR, Henderson JD, Brett ME, Adamson PB, Stevenson LW, for the CardioMEMS Post‐Approval Study Investigators . Lower rates of heart failure and all‐cause hospitalizations during pulmonary artery pressure‐guided therapy for ambulatory heart failure: One‐year outcomes from the CardioMEMS post‐approval study. Circ Heart Fail. 2020; 13: e006863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lindenfeld J, Zile MR, Desai AS, Bhatt K, Ducharme A, Horstmanshof D, Krim SR, Maisel A, Mehra MR, Paul S, Sears SF, Sauer AJ, Smart F, Zughaib M, Castaneda P, Kelly J, Johnson N, Sood P, Ginn G, Henderson J, Adamson PB, Costanzo MR. Haemodynamic‐guided management of heart failure (GUIDE‐HF): A randomised controlled trial. Lancet. 2021; 398: 991–1001. [DOI] [PubMed] [Google Scholar]
- 6. Mullens W, Sharif F, Dupont M, Rothman AMK, Wijns W. Digital health care solution for proactive heart failure management with the Cordella heart failure system: Results of the SIRONA first‐in‐human study. Eur J Heart Fail. 2020; 22: 1912–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schuirmann DJ. A comparison of the two one‐sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm. 1987; 15: 657–680. [DOI] [PubMed] [Google Scholar]
- 8. Abraham WT, Adamson PB, Hasan A, Bourge RC, Pamboukian SV, Aaron MF, Raval NY. Safety and accuracy of a wireless pulmonary artery pressure monitoring system in patients with heart failure. Am Heart J. 2011; 161: 558–566. [DOI] [PubMed] [Google Scholar]
- 9. Angermann C, Assmus B, Anker SD, Asselbergs FW, Brachmann J, Brett ME, Brugts JJ, Ertl G, Ginn G, Hilker L, Koehler F, Rosenkranz S, Zhou Q, Adamson PB, Böhm M, MEMS‐HF Investigators . Pulmonary artery pressure‐guided therapy in ambulatory patients with symptomatic heart failure: The CardioMEMS European monitoring study for heart failure (MEMS‐HF). Eur J Heart Fail. 2020; 22: 1891–1901. [DOI] [PubMed] [Google Scholar]
- 10. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 11. Lin MH, Yuan WL, Huang TC, Zhang HF, Mai JT, Wang JF. Clinical effectiveness of telemedicine for chronic heart failure: A systematic review and meta‐analysis. J Invest Med. 2017; 65: 899–911. [DOI] [PubMed] [Google Scholar]
- 12. Hall ME, Vaduganathan M, Khan MS, Papadimitriou L, Long RC, Hernandez GA, Moore CK, Lennep BW, McMullan MR, Butler J. Reductions in heart failure hospitalizations during the COVID‐19 pandemic. J Card Fail. 2020; 26: 462–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cox ZL, Lai P, Lindenfeld J. Decreases in acute heart failure hospitalizations during COVID‐19. Eur J Heart Fail. 2020; 22: 1045–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sokolski M, Gajewski P, Zymlinski R, Biegus J, Berg JMT, Bor W, Braunschweig F, Caldeira D, Cuculi F, D'Elia E, Edes IF, Garus M, Greenwood JP, Halfwerk FR, Hindricks G, Knuuti J, Kristensen SD, Landmesser U, Lund LH, Lyon A, Mebazaa A, Merkely B, Nawrocka‐Millward S, Pinto FJ, Ruschitzka F, Semedo E, Senni M, Sepehri Shamloo A, Sorensen J, Stengaard C, Thiele H, Toggweiler S, Tukiendorf A, Verhorst PM, Wright DJ, Zamorano P, Zuber M, Narula J, Bax JJ, Ponikowski P. Impact of coronavirus disease 2019 (COVID‐19) outbreak on acute admissions at the emergency and cardiology departments across Europe. Am J Med. 2021; 134: 482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spertus JA, Jones PG, Sandhu AT, Arnold SV. Interpreting the Kanas City cardiomyopathy questionnaire in clinical trials and clinical care: JACC state‐of‐the‐art review. J MA Coll Cardiol. 2020; 76: 2379–2390. [DOI] [PubMed] [Google Scholar]
- 16. Ingle L, Cleland JG, Clark AL. The long‐term prognostic significance of 6‐minute walk test distance in patient with chronic heart failure. Biomed Res Int. 2014; 2014: 505969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ingle L, Cleland JG, Clark AL. The relationship between repeated 6‐minute walk test performance and outcome in patients with chronic heart failure. Ann Phys Rehabil Med. 2014; 57: 244–253. [DOI] [PubMed] [Google Scholar]
- 18. Yancy CW, Jessup MJ, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the Management of Heart Failure: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2017; 136: e137–e161. [DOI] [PubMed] [Google Scholar]
- 19. Yancy CW, Januzzi JL Jr, Allen LA, Butler J, Davis LL, Fonarow GC, Ibrahim NE, Jessup M, Lindenfeld J, Maddox TM, Masoudi FA, Motiwala SR, Patterson JH, Walsh MN, Wasserman A. 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: Answers to 10 pivotal issues about heart failure with reduced ejection fraction: A report of the American College of Cardiology Task Force on expert consensus decision pathways. J Am Coll Cardiol. 2018; 71: 201–230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Inclusion and exclusion criteria.
Table S2. Patient Survey.


