Abstract
Introduction
The PACIFIC study demonstrated that durvalumab consolidation therapy significantly improved progression‐free survival (PFS) and overall survival (OS) in patients with unresectable stage III non‐small cell lung cancer (NSCLC) after concurrent chemoradiotherapy (CCRT). However, there was no clinical benefit in both PFS and OS in epidermal growth factor receptor (EGFR) mutation‐positive patient groups in a post hoc exploratory analysis. Moreover, the clinical effects of immune checkpoint inhibitors (ICIs) in EGFR mutation‐positive stage IV NSCLC were demonstrated to be poor. Personalized treatment according to the mutation status is also required in stage III NSCLC. Lazertinib, a third‐generation EGFR tyrosine kinase inhibitor (TKI), is newly developed and approved for use in Korea.
Methods
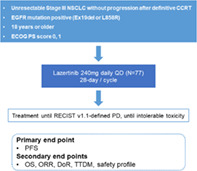
This prospective, open, single‐arm, multicenter, phase II clinical trial aims to evaluate the efficacy and safety of lazertinib as a consolidative therapy after CCRT treatment in unresectable, EGFR mutation‐positive NSCLC stage III patients. The primary endpoint of this study is PFS, and the secondary endpoints are OS, objective response rate (ORR), duration of response (DoR), time to death or distant metastasis (TTDM), and safety profiles.
Discussion
Our study may extend the indications for third‐generation EGFR‐TKIs to treat patients with stage III NSCLC. Moreover, using this drug to treat stage III NSCLC would emphasize the value of mutation analysis and personalized medicine.
Keywords: chemoradiotherapy, consolidation therapy, EGFR, lazertinib
In this prospective multicenter PLATINUM trial, we will evaluate the efficacy and safety of lazertinib as a consolidative therapy after CCRT treatment in unresectable, EGFR mutation‐positive NSCLC stage III patients. This trial may extend the indications for third‐generation EGFR‐TKIs to inoperable EGFR mutation‐positive stage III NSCLC patients, and thereby will draw attention to mutation analysis and personalized medicine in stage III NSCLC treatment.
INTRODUCTION
With the development of target therapy and immune checkpoint inhibitors (ICIs), the treatment landscape of non‐small cell lung cancer (NSCLC) is constantly evolving. 1 However, the 24‐month survival rate of patients with stage III NSCLC is still low, ranging from 27.0% to 45.3%. 2 Stage III NSCLC has various treatment options including concurrent chemoradiotherapy (CCRT). Consolidation therapy after CCRT is also being studied. 3 The PACIFIC study demonstrated that durvalumab consolidation therapy significantly improved progression‐free survival (PFS) and overall survival (OS) in patients with unresectable stage III NSCLC. 4 In the PACIFIC study, patients were allowed to participate regardless of their mutation status. Out of a total of 713 participants, only 35 (4.9%) patients were epidermal growth factor receptor (EGFR) mutation‐positive. Of these patients 24 (68.6%) received durvalumab and 11 (31.4%) received a placebo. There was no clinical benefit in terms of PFS and OS for these EGFR mutation‐positive patient groups (hazard ratio [H1R] = 0.91, HR = 1.02, respectively). 5 A recent post hoc subgroup analysis also showed that PFS and OS outcomes with durvalumab were similar to those with placebo in the EGFR mutant population. 5 The benefit of ICI consolidation therapy in this population remains unclear.
The prevalence of EGFR mutation has previously been reported to be approximately 30%–50% in an Asian population with lung adenocarcinoma, and in this study it was determined that there was no relationship with cancer stage. 6 EGFR mutation is common in women, nonsmokers, and Asians, and in a report analyzing 85 clinical studies, it was confirmed to be 33.8% (29.8% to 37.8%) in stage III NSCLC. 7 Although there may be differences depending on the EGFR type, the effect of ICIs in EGFR mutation‐positive stage IV NSCLC is poor. 8 Just as “personalized treatment” for each patient is performed according to the mutation status and programmed death‐ligand 1 (PD‐L1) expression in advanced NSCLC stage IV, the consolidation therapy of unresectable stage III NSCLC requires an individual optimized treatment. Recently, a prospective study using concurrent thoracic radiotherapy with a first‐generation EGFR TKI instead of cytotoxic chemotherapy demonstrated the tolerability and possible efficacy in EGFR mutation‐positive NSCLC. 9 In a multi‐institutional retrospective analysis of patients with unresectable stage III EGFR‐mutation‐positive NSCLC who underwent CCRT, it was reported that durvalumab consolidation therapy showed no benefit in PFS (p = 0.993). 10 Furthermore, it was reported that there was a statistically significant improvement in PFS when third‐generation EGFR tyrosine kinase inhibitor (TKI) was administered after CCRT (p = 0.023) in a retrospective study. 10
There have been studies analyzing the usefulness of first‐ or second‐generation EGFR TKIs after surgery in stage II–IIIA NSCLC patients. 11 , 12 , 13 However, first‐ or second‐generation EGFR TKIs did not demonstrate improvement in OS in patients with operable NSCLC. On the other hand, the ADAURA study showed that osimertinib as adjuvant therapy after surgery significantly prolonged disease‐free survival in EGFR mutation‐positive patients. 14 Based on multi‐institutional retrospective analysis and the ADAURA study performed in the adjuvant setting, third‐generation EGFR‐TKIs can be a feasible option as consolidation treatment after CCRT in EGFR‐mutant NSCLC.
Lazertinib, approved for use in Korea in 2021, is a third‐generation EGFR‐TKI similar to osimertinib and has shown excellent anticancer effects in preclinical studies and in early clinical settings. 15 , 16 It is a novel, highly potent, blood–brain barrier (BBB)‐penetrating, irreversible EGFR‐TKI, which selectively blocks EGFR sensitizing and T790M mutations. It showed an excellent antitumor effect and good BBB permeability in an EGFR‐mutant brain metastasis model. 14 Lazertinib also demonstrated clinically significant efficacy and safety in patients with advanced EGFR T790M‐positive NSCLC after previous EGFR TKIs through a phase I/II study (median PFS was 11.1 months and the ORR was 55.3%). 16 Regarding recurrence patterns, there were more distant metastases in EGFR mutation NSCLC than in wild‐type (76% vs. 40%, p = 0.001). 17 In particular, brain recurrence was 35% in EGFR‐mutant NSCLC and 15% in wild‐type. 17 Since lazertinib has good BBB permeability, it can effectively inhibit brain metastasis and be a good option for consolidation therapy after CCRT in EGFR mutation‐positive stage III NSCLC.
The aim of this study was to confirm the efficacy and safety of lazertinib consolidation therapy in patients with unresectable, EGFR mutation‐positive stage III NSCLC. The positive results of this study may extend the indications for third‐generation EGFR‐TKIs to inoperable EGFR mutation‐positive stage III NSCLC patients, thereby also drawing attention to mutation analysis and personalized medicine in stage III NSCLC treatment.
METHODS
Study design
The PLATINUM trial is a prospective, open, single‐arm, multicenter, phase II clinical trial (Figure 1). This trial aims to evaluate the efficacy and safety of lazertinib as a consolidative therapy after CCRT treatment in unresectable, EGFR mutation‐positive NSCLC stage III patients. The target number of enrolled patients is 77, and nine university hospitals will undertake competitive enrollment. Aiming for the statistically adequate number of cases to be 70, the number was set at 77, considering the 10% dropout rate. The patient registration period is from June 2022 to May 2023, and patient registration ends when all the target patient groups are registered. We plan to follow up for 3 years from the time of the last participant registration, when the final analysis would be carried out. Interim analysis is performed when more than 39 (50% of 77) patients have enrolled (at least 24 months after the start of the study). Regarding median progression‐free survival (mPFS), we speculate with reference to previous studies involving osimertinib in untreated, EGFR‐mutated advanced NSCLC and durvalumab in the PACIFIC trial. We estimate that the mPFS of this study should show the efficacy of at least 18.9 months of the FLAURA trial, and that it should have a threshold higher than PFS of 16.8 months (durvalumab group) of the PACIFIC trial.
FIGURE 1.
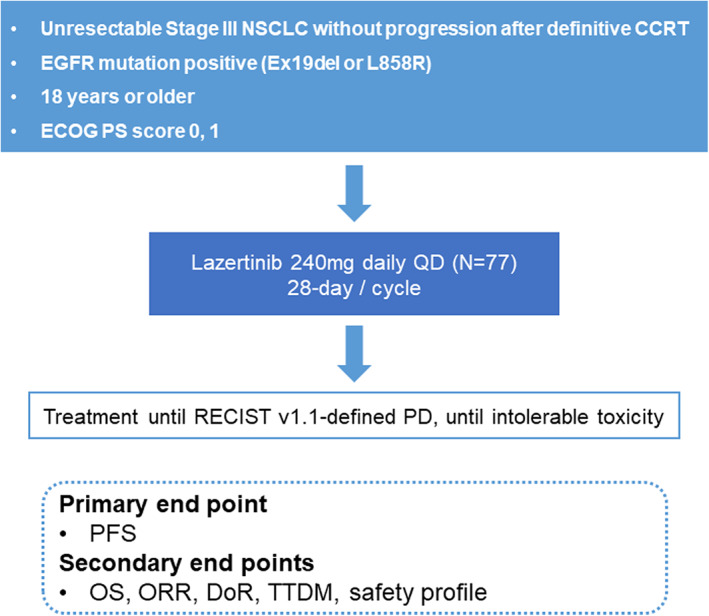
Study flowchart. PD, progressive disease; PFS, progression‐free survival; OS, overall survival; ORR, objective response rate; DoR, duration of response; TTDM, time to death or distant metastasis
The Data and Safety Monitoring Board comprises two medical experts and one statistician. This protocol was approved by the Institutional Review Board of all participating institutes. This study will follow the Declaration of Helsinki as a statement of ethical principles for medical research involving human subjects, including the study of identifiable human substances and data.
The primary endpoint of this study is PFS, defined as the time from the study registration to the disease progression or death in the case of death without disease progression. The secondary endpoints are OS, ORR, duration of response (DoR), and time to death or distant metastasis (TTDM). OS is defined as the time from the trial registration date to death due to any reason. If the subject's mortality is unknown by the time of analysis, statistical follow‐up analysis is performed by the last known surviving date. ORR is the ratio (%) of patients with at least one complete or partial response based on response evaluation criteria in solid tumor (RECIST) version 1.1. DoR is the time from the date of the first documented response until the first date of documented progression (or death in the absence of disease progression). TTDM is defined as the time from the enrollment date until the first date of distant metastasis or death without distant metastasis. Safety profiles evaluate adverse events, adverse drug reactions, serious adverse events, and permanent discontinuation due to adverse events, based on common terminology criteria for adverse events (CTCAE) version 5.0.
Eligibility criteria
The inclusion criteria are as follows: (a) Age > 18 years; (b) histologically confirmed as having locally advanced, unresectable, stage III NSCLC (staging according to the International Association for the Study of Lung Cancer [IASLC] staging manual version 8 in thoracic oncology); (c) tissue is positive for EGFR mutation (exon 19del or L858R); (d) Eastern Cooperative Oncology group (ECOG) performance status 0 or 1; (e) life‐expectancy of 6 months or more at the time of registration; (f) patients who received two or more cycles of platinum‐based CCRT for curative purposes and within 1–42 days after the end of the last radiation therapy; (g) patients without disease progression after CCRT; (h) patients with a total radiation dose of 60 Gy ± 10% (54–66 Gy); and (i) voluntary agreement in writing by the patient or his/her legal representative to participate in this clinical trial. Patients with a history of interstitial lung disease, symptomatic pneumonitis following CCRT, other primary malignancies, or previous EGFR‐TKI treatment will be excluded from the study.
Data collection
As baseline characteristics, information on sex, age, and history of smoking, related lung diseases, cardiovascular disease, previous cancer, and other comorbidities will be collected. Information related to CCRT, including the cytotoxic chemotherapy regimen, dose reduction, total radiation dose, and the best response, will be collected. For lung cancer, information on pathology type, stage, mutation analysis, and PD‐L1 status will be collected. Mutation analysis and PD‐L1 status are based on biopsy at the time of diagnosis prior to CCRT. In the case of the EGFR test, it will be performed on cancer tissue during enrollment, and on tissue or liquid sample when it progresses.
Each cycle is based on every 4 weeks ± 3 days (28 days ± 3 days) unless there is a delay in administration due to toxicity. Regardless of the delay in administering the test drug, one visit every 8 weeks ± 1 week is required to conduct follow‐up imaging tests and evaluations. Safety profiles and concomitant medications will be evaluated at every cycle.
Treatment plan
Following the approval of lazertinib for use in Korea (approval date January 18, 2021), the recommended dose of lazertinib in this clinical trial is 240 mg (80 mg, three tablets), taken orally at a fixed time once every day, regardless of the meals. Lazertinib is administered from the study enrollment date (cycle 1, first day of C1D1) to disease progression or unacceptable toxicity. Patients will receive lazertinib for at least 3 years. At the end of 3 years, additional use of lazertinib is possible at the judgment of the investigator. If a dose reduction is necessary according to safety and tolerability, the dosage can be reduced to 160 mg (80 mg, 2 tablets) once a day.
Statistical analysis
All statistical analyses are two‐sided, and the confidence interval (CI) is set to 95%. The analysis tool will use the statistical program SAS version 9.4 (SAS Institute Inc.). The mean, standard deviation, median, minimum, quartile, maximum, and 95% CI will be presented for continuous data, whereas frequency and percentage (%) will be presented for categorical data. The Kaplan–Meier method and log‐rank test will analyze PFS, OS, DoR, and TTDM. For subjects who will have not experienced an event by the time of analysis, the last date of evaluation or the last date the subject is known to be alive, will be considered. ORR and safety evaluation will analyze the frequency and proportion of subjects.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ACKNOWLEDGMENTS
This study is sponsored by the Yuhan Corporation. We would like to thank Editage (www.editage.co.kr) for English language editing.
Choi J, Lee JE, Choi C‐M, Oh I‐J, Lee KY, Jang TW, et al. A phase II, multicenter study of lazertinib as consolidation therapy in patients with locally advanced, unresectable, EGFR mutation‐positive non‐small cell lung cancer (stage III) who have not progressed following definitive, platinum‐based, chemoradiation therapy (PLATINUM trial). Thorac Cancer. 2022;13(23):3431–3435. 10.1111/1759-7714.14663
Clinical Trial Registration: This study is registered at ClinicalTrials.gov (https://clinicaltrials.gov, registration number NCT05338619).
REFERENCES
- 1. Lee JG, Kim HC, Choi C‐M. Recent trends of lung cancer in Korea. Tuberc Respir Dis. 2021;84:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim HC, Ji W, Lee JC, Kim HR, Song SY, Choi CM. Prognostic factor and clinical outcome in stage III non‐small cell lung cancer: a study based on real‐world clinical data in the Korean population. Cancer Res Treat. 2021;53:1033–41. 10.4143/crt.2020.1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Käsmann L, Eze C, Taugner J, Roengvoraphoj O, Dantes M, Schmidt‐Hegemann NS, et al. Chemoradioimmunotherapy of inoperable stage III non‐small cell lung cancer: immunological rationale and current clinical trials establishing a novel multimodal strategy. Radiat Oncol. 2020;15:167. 10.1186/s13014-020-01595-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non‐small‐cell lung cancer. N Engl J Med. 2017;377:1919–29. 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 5. Naidoo J, Antonia SJ, Wu YL, Cho BC, Thiyagarajah P, Mann H, et al. Durvalumab (durva) after chemoradiotherapy (CRT) in unresectable, stage III, EGFR mutation‐positive (EGFRm) NSCLC: a post hoc subgroup analysis from PACIFIC. J Clin Oncol. 2022;40:8541. 10.1200/JCO.2022.40.16_suppl.8541 [DOI] [PubMed] [Google Scholar]
- 6. Pi C, Xu CR, Zhang MF, Peng XX, Wei XW, Gao X, et al. EGFR mutations in early‐stage and advanced‐stage lung adenocarcinoma: analysis based on large‐scale data from China. Thorac Cancer. 2018;9:814–9. 10.1111/1759-7714.12651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang YL, Yuan JQ, Wang KF, Fu XH, Han XR, Threapleton D, et al. The prevalence of EGFR mutation in patients with non‐small cell lung cancer: a systematic review and meta‐analysis. Oncotarget. 2016;7:78985–93. 10.18632/oncotarget.12587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hastings K, Yu HA, Wei W, Sanchez‐Vega F, DeVeaux M, Choi J, et al. EGFR mutation subtypes and response to immune checkpoint blockade treatment in non‐small‐cell lung cancer. Ann Oncol. 2019;30:1311–20. 10.1093/annonc/mdz141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akamatsu H, Haruyasu Murakami MD, Hideyuki Harada MD, et al. Gefitinib with concurrent thoracic radiotherapy in Unresectable locally advanced NSCLC with EGFR mutation; West Japan Oncology Group 6911L. J Thorac Oncol. 2021;16:1745–52. 10.1016/j.jtho.2021.05.019 [DOI] [PubMed] [Google Scholar]
- 10. Aredo JV, Mambetsariev I, Hellyer JA, Amini A, Neal JW, Padda SK, et al. Durvalumab for stage III EGFR‐mutated NSCLC after definitive chemoradiotherapy. J Thorac Oncol. 2021;16:1030–41. 10.1016/j.jtho.2021.01.1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tada H, Mitsudomi T, Misumi T, Sugio K, Tsuboi M, Okamoto I, et al. Randomized phase III study of gefitinib versus cisplatin plus vinorelbine for patients with resected stage II‐IIIA non–small‐cell lung cancer with EGFR mutation (IMPACT). J Clin Oncol. 2022;10.1200/JCO.21.01729(40):231–41. [DOI] [PubMed] [Google Scholar]
- 12. Xu ST, Wu L, Shen Y, Liu YY, Chen C, Cheng Y, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II‐IIIA (N1‐N2) EGFR‐mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open‐label, phase 3 study. Lancet Oncol. 2018;19:139–48. 10.1016/S1470-2045(17)30729-5 [DOI] [PubMed] [Google Scholar]
- 13. Zhong WZ, Wang Q, Mao WM, Xu ST, Wu L, Wei YC, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II‐IIIA (N1‐N2) EGFR‐mutant NSCLC: final overall survival analysis of CTONG1104 phase III trial. J Clin Oncol. 2021;39(7):713–22. 10.1200/JCO.20.01820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu YL, Tsuboi M, He J, John T, Grohe C, Majem M, et al. Osimertinib in resected EGFR‐mutated non‐small‐cell lung cancer. N Engl J Med. 2020;383:1711–23. 10.1056/NEJMoa2027071 [DOI] [PubMed] [Google Scholar]
- 15. Yun J, Hong MH, Kim SY, Park CW, Kim S, Yun MR, et al. YH25448, an irreversible EGFR‐TKI with potent intracranial activity in EGFR mutant non‐small cell lung cancer. Clin Cancer Res. 2019;25:2575–87. 10.1158/1078-0432.Ccr-18-2906 [DOI] [PubMed] [Google Scholar]
- 16. Cho BC, Han JY, Kim SW, Lee KH, Cho EK, Lee YG, et al. A phase 1/2 study of lazertinib 240 mg in patients with advanced EGFR T790M‐positive NSCLC after previous EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2022;17:558–67. 10.1016/j.jtho.2021.11.025 [DOI] [PubMed] [Google Scholar]
- 17. Tanaka K, Hida T, Oya Y, Oguri T, Yoshida T, Shimizu J, et al. EGFR mutation impact on definitive concurrent chemoradiation therapy for inoperable stage III adenocarcinoma. J Thorac Oncol. 2015;10:1720–5. 10.1097/JTO.0000000000000675 [DOI] [PubMed] [Google Scholar]


