Abstract
Aims
There is limited evidence for the correlation between short‐term mortality and red cell distribution width (RDW) in critical patients with heart failure. Herein, a retrospective cohort study was conducted to investigate whether RDW was independently associated with short‐term mortality in critically ill patients with heart failure.
Methods and results
As a retrospective cohort study, it involved a total of 9465 patients with heart failure from the MIMIC‐IV database. The target‐dependent and independent variables were in‐hospital mortality, 90 day mortality and RDW measured at baseline, respectively. The relationship between all‐cause death and baseline RDW in hospital and after 90 days of admission to ICU was evaluated by using the Kaplan–Meier plot and Cox proportional hazard analysis. The average age of participants was 74.4 (64.2, 83.5) years old, among whom about 54.6% were male. Results of the adjusted Cox proportional hazard model revealed that RDW had a positive association with both in‐hospital and 90 day mortality risk after the adjustment of confounders (HR = 1.09, 95% CI: 1.04–1.15, P < 0.001; HR = 1.11, 95% CI: 1.08–1.14, P < 0.001, respectively). A non‐linear relationship was found between RDW and 90 day mortality, which had a threshold of 14.96%. The effect sizes and confidence intervals below and above the threshold were 1.36 (1.14 to 1.62) and 1.09 (1.04 to 1.15), respectively. It was also found by subgroup analysis that there were stronger correlations in male and patients with normal renal function.
Conclusions
Our data suggest that the short‐term mortality of critically ill patients with HF is independently predicted by RDW. At the same time, large prospective research and longer follow‐up time are required to further validate the findings of this study.
Keywords: Red cell distribution width, Critically ill patients with heart failure, Prognosis
Introduction
Red blood cell distribution width (RDW) is an index of variation in the size and shape of erythrocytes, corresponding to the degree of anisocytosis (increased variation of red blood cell size). 1 It used to be recognized as a useful diagnostic tool for haematological diseases, such as anaemia. Recent evidence suggests that anisocytosis is common in human diseases, such as liver and kidney failure, chronic obstructive pulmonary disease, community‐acquired pneumonia, diabetes, cancer, venous thromboembolism, cardiovascular disease, and other acute or chronic diseases. 2
Heart failure (HF) is one of the most frequent cardiovascular diseases with an association with high morbidity and mortality. The accurate prediction of prognosis in critical patients with HF is critically important. Previous studies have shown that an increase in RDW is associated with poor outcomes in HF patients. 3 , 4 , 5 , 6 , 7 However, there are limited results of previous studies on the correlation between RDW and short‐term outcomes in critical patients with HF. Previous studies on RDW were mostly designed for patients with acute or chronic heart failure, suggesting a cut‐off value of about 14%. But for critical patients with HF, the mean RDW is much higher than that of patients in general wards or outpatients. There is no established limit for critically ill patients with HF. It is necessary to carry out a retrospective cohort study to investigate whether RDW is independently associated with 90 day mortality in critically ill patients with heart failure.
Participants and methods
Study design
The independent and dependent variables are RDW at baseline and in‐hospital mortality, 90 day mortality after admission to ICU.
Data source
The open‐source Medical Information Mart for Intensive Care database (MIMIC‐ IV) was selected in this study, which is an updated version of MIMIC‐III with pre‐existing institutional review board approval. Currently, the MIMIC‐IV contains comprehensive and high‐quality data of patients admitted to intensive care units (ICUs) at the Beth Israel Deaconess Medical Center between 2008 and 2019 (inclusive). The database consists of comprehensive clinical data, like medical records, drug therapies, laboratory results, patient characteristics, and disease codes of International Classification of Diseases. 8 The author (XYZ) finished the online course at the National Institutes of Health and passed the Examination for Protecting Human Research Participants. The project was approved by the Institutional Review Board of the Massachusetts Institute of Technology and the Beth Israel Deaconess Medical Center. The privacy of patients was protected by hiding their identity details.
Patient and public involvement
Patients and/or the public were not directly involved in this study.
Inclusion and exclusion criteria
Adult patients with HF at first admission to ICU were included according to the ICD‐9 and ICD‐10 disease code. Since HF may not be listed as the principal diagnosis, we included records with HF in any of the first five diagnosis positions according to the diagnosis sequence. Patients with no ICU records and missing data of RDW were excluded from this study. Figure 1 shows the flow chart of how to select patients in the study. A total of 9465 adult patients were included in this study after screening 15 233 patients diagnosed with HF.
Figure 1.
Flow chart of the screening and enrolment of study participants.
Variables
RDW at baseline was considered to be a continuous variable. The outcome variable was all‐cause mortality in hospital and after 90 days of admission to ICU (dichotomous variable). Based on previous studies, the following data were extracted from general availability and clinical relevance: (i) demographic data; (ii) variables that affect RDW or 90 day mortality based on clinical experience or previous literature.
Statistical analysis
Categorical variables were represented by percentage or frequency. Differences between different RDW groups (quartile) were examined using the Kruskal–Wallis H test (skewed distribution) and χ 2 (normal distribution). Step 1: Four univariate and multivariate Cox proportional hazard models were constructed. Model 1: no adjustment for covariates; Model 2: adjustment for only age and gender; Model 3: Model 2 plus medical history and disease severity score; Model 4: Model 3 plus BMI, HR, SBP, DBP, leucocyte, haemoglobin, MCV, eGFR, potassium, calcium and length of stay. Step 2: To figure out whether there is the non‐linearity of RDW and mortality, a Cox proportional‐hazard regression model was constructed through smooth curve fitting and cubic spline functions. This curve fitting allows for a complete investigation of the relationship between RDW and mortality, underlining characteristics that may be missed using classification approaches. Step 3: Stratified Cox proportional hazard models were used for subgroup analysis. The continuous variable was converted into a categorical variable based on clinical cut points or median value, followed by an interaction test. The likelihood ratio test was conducted after the effect modification test for subgroup indexes. And the sensitivity analysis was also carried out for ensuring the robustness of the data. Afterwards, RDW was transferred into a categorical variable and the calculation of P value was to observe the likelihood of non‐linearity and validate the outcomes of RDW as a continuous variable. Free statistics software version 1.5 and statistical software package R 4.1.1 were used for all the analyses. A two‐tailed test indicated that P < 0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 9465 patients were chosen for the final data analysis (Figure 1 ). According to the quartile of RDW, the baseline characteristics of these patients are shown in Table 1 . The average age of them was 74.4 (64.2, 83.5) years old, among whom about 54.6% were male. Five hundred fourteen of the 9465 patients (5.4%) died in hospital and 1436 patients (15.2%) died after 90 days of admission to ICU. RDW of decedents was significantly higher than that of survivors, which is considered as a function of hazard (Figure 2 ). Patients with the highest RDW (Q4) had higher values in heart rate, NT‐proBNP, PT, creatinine, blood urea nitrogen, potassium, Charlson co‐morbidity index, SOFA and consisted of more female, atrial fibrillation, diabetes mellitus, deficiency anaemia, liver disease, chronic renal insufficiency, chronic pulmonary disease than those of other groups. The opposite patterns were observed in SBP, DBP, urine‐output, haematocrit (HCT), haemoglobin (HGB), erythrocyte, MHC, MCHC, MCV, leucocyte, eGFR, glucose, lactate, myocardial infarct, hypertension and hyperlipidaemia. There was no statistically significant difference among the four groups in terms of receiving heart assist system implantation and ECMO (P > 0.05).
Table 1.
Baseline characteristics of patients, divided according to red blood cell distribution width
| Variables | Total (n = 1177) | RDW (%) | P | |||
|---|---|---|---|---|---|---|
| Q1 (≤13.6%) (n = 2211) | Q2 (13.6%–14.6%) (n = 2378) | Q3 (14.6%–16.1%) (n = 2508) | Q4 (≥16.1%) (n = 2368) | |||
| Age, median (IQR), year | 74.4 (64.2, 83.5) | 71.2 (61.6, 81.1) | 74.7 (64.5, 84.0) | 76.1 (65.4, 84.6) | 75.3 (65.5, 83.8) | <0.001 |
| Gender | <0.001 | |||||
| Male, n (%) | 5164 (54.6) | 1360 (61.5) | 1267 (53.3) | 1315 (52.4) | 1222 (51.6) | |
| Female, n (%) | 4301 (45.4) | 851 (38.5) | 1111 (46.7) | 1193 (47.6) | 1146 (48.4) | |
| Body mass index, median (IQR), kg/m2 | 28.5 (24.6, 33.4) | 28.1 (24.7, 32.7) | 29.0 (25.1, 33.6) | 28.2 (24.4, 33.3) | 28.4 (24.4, 34.2) | 0.033 |
| Heart rate, median (IQR), bpm | 81.5 (72.1, 92.5) | 80.9 (72.5, 90.6) | 81.3 (71.8, 92.4) | 81.2 (72.1, 91.9) | 82.8 (72.0, 94.8) | 0.002 |
| SBP, median (IQR), mm Hg | 115.2 (106.3, 127.3) | 115.2 (107.3, 126.4) | 115.8 (107.0, 127.2) | 116.4 (106.9, 128.8) | 113.1 (103.9, 126.4) | <0.001 |
| DBP, median (IQR), mm Hg | 60.5 (54.0, 68.2) | 61.3 (55.0, 69.1) | 60.7 (53.8, 68.5) | 60.1 (53.1, 67.8) | 60.1 (53.7, 67.5) | <0.001 |
| SpO2, median (IQR), % | 96.8 (95.4, 98.1) | 96.8 (95.5, 98.1) | 96.8 (95.3, 98.1) | 96.8 (95.4, 98.1) | 96.8 (95.3, 98.1) | 0.822 |
| Urine‐output (first 24 hours),median (IQR), mL | 1570.0 (948.0, 2300.0) | 1685.0 (1100.0, 2415.0) | 1571.0 (990.0, 2324.0) | 1500.0 (903.8, 2250.0) | 1500.0 (806.0, 2200.0) | <0.001 |
| Medical history | ||||||
| Myocardial infarct | <0.001 | |||||
| No (n, %) | 6595 (69.7) | 1414 (64) | 1644 (69.1) | 1800 (71.8) | 1737 (73.4) | |
| Yes (n, %) | 2870 (30.3) | 797 (36) | 734 (30.9) | 708 (28.2) | 631 (26.6) | |
| Hypertension | <0.001 | |||||
| No (n, %) | 4053 (42.8) | 899 (40.7) | 966 (40.6) | 1043 (41.6) | 1145 (48.4) | |
| Yes (n, %) | 5412 (57.2) | 1312 (59.3) | 1412 (59.4) | 1465 (58.4) | 1223 (51.6) | |
| Atrial fibrillation | <0.001 | |||||
| No (n, %) | 3991 (42.2) | 1106 (50) | 1031 (43.4) | 1007 (40.2) | 847 (35.8) | |
| Yes (n, %) | 5474 (57.8) | 1105 (50) | 1347 (56.6) | 1501 (59.8) | 1521 (64.2) | |
| Diabetes mellitus | <0.001 | |||||
| No (n, %) | 5521 (58.3) | 1415 (64) | 1397 (58.7) | 1418 (56.5) | 1291 (54.5) | |
| Yes (n, %) | 3944 (41.7) | 796 (36) | 981 (41.3) | 1090 (43.5) | 1077 (45.5) | |
| Deficiency anaemia | <0.001 | |||||
| No (n, %) | 7368 (77.8) | 1922 (86.9) | 1978 (83.2) | 1876 (74.8) | 1592 (67.2) | |
| Yes (n, %) | 2097 (22.2) | 289 (13.1) | 400 (16.8) | 632 (25.2) | 776 (32.8) | |
| Depression | <0.001 | |||||
| No (n, %) | 6734 (71.1) | 1667 (75.4) | 1727 (72.6) | 1711 (68.2) | 1629 (68.8) | |
| Yes (n, %) | 2731 (28.9) | 544 (24.6) | 651 (27.4) | 797 (31.8) | 739 (31.2) | |
| Hyperlipidaemia | <0.001 | |||||
| No (n, %) | 2790 (29.5) | 605 (27.4) | 657 (27.6) | 759 (30.3) | 769 (32.5) | |
| Yes (n, %) | 6675 (70.5) | 1606 (72.6) | 1721 (72.4) | 1749 (69.7) | 1599 (67.5) | |
| Liver disease | <0.001 | |||||
| No (n, %) | 8849 (93.5) | 2143 (96.9) | 2269 (95.4) | 2330 (92.9) | 2107 (89) | |
| Yes (n, %) | 616 (6.5) | 68 (3.1) | 109 (4.6) | 178 (7.1) | 261 (11) | |
| Chronic renal insufficiency | <0.001 | |||||
| No (n, %) | 6020 (63.6) | 1732 (78.3) | 1597 (67.2) | 1494 (59.6) | 1197 (50.5) | |
| Yes (n, %) | 3445 (36.4) | 479 (21.7) | 781 (32.8) | 1014 (40.4) | 1171 (49.5) | |
| Chronic pulmonary disease | <0.001 | |||||
| No (n, %) | 6151 (65.0) | 1596 (72.2) | 1572 (66.1) | 1586 (63.2) | 1397 (59) | |
| Yes (n, %) | 3314 (35.0) | 615 (27.8) | 806 (33.9) | 922 (36.8) | 971 (41) | |
| Disease severity score | ||||||
| Charlson co‐morbidity index, mean ± SD | 7.0 ± 2.5 | 6.2 ± 2.3 | 6.8 ± 2.4 | 7.2 ± 2.4 | 7.9 ± 2.6 | <0.001 |
| SOFA, mean ± SD | 5.0 ± 3.3 | 4.5 ± 3.1 | 4.8 ± 3.3 | 5.1 ± 3.3 | 5.6 ± 3.6 | <0.001 |
| Laboratory tests | ||||||
| Haematocrit, median (IQR), % | 31.7 (27.2, 36.5) | 33.6 (28.9, 38.2) | 32.9 (28.0, 37.3) | 31.0 (26.7, 35.9) | 29.5 (25.7, 34.1) | <0.001 |
| Haemoglobin, median (IQR), g/dL | 10.3 (8.8, 11.9) | 11.2 (9.7, 12.7) | 10.8 (9.3, 12.3) | 10.1 (8.7, 11.7) | 9.3 (8.1, 10.8) | <0.001 |
| Erythrocyte, median (IQR), ×1012/L | 3.5 (3.0, 4.0) | 3.6 (3.1, 4.2) | 3.6 (3.0, 4.1) | 3.4 (2.9, 4.0) | 3.3 (2.8, 3.9) | <0.001 |
| MCH, median (IQR), pg | 29.9 (28.2, 31.4) | 30.7 (29.6, 31.9) | 30.1 (28.9, 31.4) | 29.6 (28.0, 31.0) | 28.5 (26.1, 30.6) | <0.001 |
| MCHC, median (IQR), % | 32.6 (31.5, 33.7) | 33.3 (32.5, 34.2) | 32.9 (32.0, 33.8) | 32.4 (31.5, 33.5) | 31.6 (30.5, 32.7) | <0.001 |
| MCV, median (IQR), fL | 91.0 (87.0, 95.0) | 92.0 (89.0, 96.0) | 91.2 (88.0, 95.0) | 91.0 (87.0, 95.0) | 90.0 (84.0, 96.0) | <0.001 |
| Leucocyte, median (IQR), ×109/L | 10.3 (7.5, 14.1) | 10.9 (8.1, 14.6) | 10.4 (7.8, 14.0) | 10.0 (7.4, 13.9) | 9.7 (7.0, 14.1) | <0.001 |
| Platelet, median (IQR), ×109/L | 187.0 (141.0, 244.0) | 182.0 (143.0, 231.0) | 190.0 (142.0, 243.0) | 188.0 (143.0, 247.0) | 190.0 (133.0, 256.2) | 0.104 |
| PT, median (IQR), s | 15.2 (13.1, 18.4) | 14.4 (12.6, 17.0) | 14.8 (13.0, 18.4) | 15.4 (13.2, 18.4) | 16.4 (13.6, 21.0) | <0.001 |
| NT‐proBNP, median (IQR), pg/mL | 2705.0 (936.0, 7308.0) | 2015.0 (675.5, 5168.0) | 2498.0 (882.0, 6419.0) | 2818.0 (991.2, 7892.0) | 3633.0 (1247.0, 9279.0) | <0.001 |
| Creatinine, median (IQR), mg/dL | 1.2 (0.9, 1.9) | 1.1 (0.8, 1.4) | 1.2 (0.9, 1.7) | 1.3 (1.0, 2.1) | 1.5 (1.0, 2.6) | <0.001 |
| Blood urea nitrogen, median (IQR), mg/dL | 26.0 (18.0, 42.0) | 20.0 (15.0, 29.0) | 24.0 (17.0, 36.0) | 28.0 (19.0, 46.0) | 34.0 (22.0, 56.0) | <0.001 |
| eGFR, median (IQR), mL/(min*1.73 m2) | 53.0 (31.4, 77.8) | 67.3 (46.9, 88.6) | 55.3 (35.8, 79.7) | 47.9 (28.0, 72.9) | 40.4 (22.2, 64.1) | <0.001 |
| Glucose, median (IQR), mEq/L | 132.2 (114.1, 162.8) | 132.7 (117.5, 160.4) | 133.0 (116.0, 162.5) | 132.0 (113.0, 162.5) | 130.8 (109.0, 166.4) | 0.003 |
| Potassium, median (IQR), mEq/L | 4.5 (4.1, 5.0) | 4.5 (4.1, 4.9) | 4.5 (4.1, 5.0) | 4.5 (4.1, 5.1) | 4.6 (4.2, 5.2) | <0.001 |
| Sodium, median (IQR), mEq/L | 140.0 (137.0, 142.0) | 139.0 (137.0, 142.0) | 140.0 (137.0, 142.0) | 140.0 (137.0, 142.0) | 140.0 (137.0, 142.0) | <0.001 |
| Calcium, total, median (IQR), mg/dL | 8.7 (8.3, 9.1) | 8.7 (8.4, 9.1) | 8.7 (8.3, 9.1) | 8.7 (8.3, 9.1) | 8.7 (8.3, 9.1) | 0.042 |
| pH, median (IQR) | 7.4 (7.4, 7.5) | 7.4 (7.4, 7.5) | 7.4 (7.4, 7.5) | 7.4 (7.4, 7.5) | 7.4 (7.4, 7.5) | <0.001 |
| Lactate, median (IQR), mmol/L | 2.1 (1.5, 3.2) | 2.2 (1.6, 3.2) | 2.2 (1.4, 3.2) | 2.1 (1.4, 3.2) | 2.0 (1.4, 3.1) | <0.001 |
| Procedures | ||||||
| Short‐term external heart assist system | 0.734 | |||||
| No (n, %) | 9354 (98.8) | 2181 (98.6) | 2349 (98.8) | 2480 (98.9) | 2344 (99) | |
| Yes (n, %) | 111 (1.2) | 30 (1.4) | 29 (1.2) | 28 (1.1) | 24 (1) | |
| Implantable heart assist system | 0.719 | |||||
| No (n, %) | 9427 (99.6) | 2203 (99.6) | 2367 (99.5) | 2496 (99.5) | 2361 (99.7) | |
| Yes (n, %) | 38 (0.4) | 8 (0.4) | 11 (0.5) | 12 (0.5) | 7 (0.3) | |
| Extracorporeal membrane oxygenation | 0.589 | |||||
| No (n, %) | 9437 (99.7) | 2204 (99.7) | 2369 (99.6) | 2500 (99.7) | 2364 (99.8) | |
| Yes (n, %) | 28 (0.3) | 7 (0.3) | 9 (0.4) | 8 (0.3) | 4 (0.2) | |
| Outcomes | ||||||
| Length of ICU stay, mean ± SD, days | 3.3 ± 4.4 | 3.0 ± 4.3 | 3.4 ± 4.3 | 3.2 ± 4.2 | 3.6 ± 4.8 | <0.001 |
| Length of hospital stay, mean ± SD, days | 10.0 ± 10.5 | 8.9 ± 13.0 | 9.5 ± 8.5 | 9.8 ± 9.2 | 11.6 ± 10.9 | <0.001 |
| In‐hospital mortality (n, %) | 514 ( 5.4) | 79 (3.6) | 114 (4.8) | 129 (5.1) | 192 (8.1) | <0.001 |
| 90 day mortality (n, %) | 1436 (15.2) | 178 (8.1) | 291 (12.2) | 421 (16.8) | 546 (23.1) | <0.001 |
BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; MCV, mean corpuscular volume; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; pH, potential of hydrogen; PT, prothrombin time; SBP, systolic blood pressure; SpO2, pulse oxygen saturation.
Figure 2.
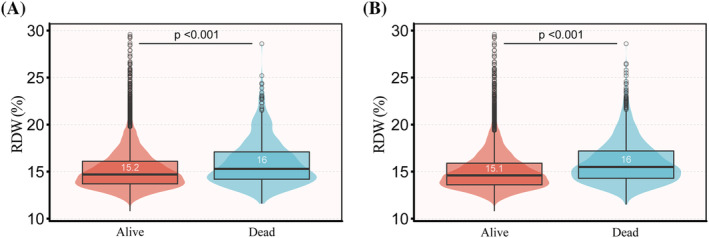
Red blood cell distribution width measures as a function of mortality. (A) In‐hospital mortality; (B) 90 day mortality.
Association between red cell distribution width and short‐term mortality
Results of the univariable analysis of covariates and mortality were shown in Supporting Information, Table S1 . Four models were constructed in this study for analysis of how RDW independently affects in‐hospital and 90 day mortality. 95% confidence intervals and effect sizes (HR) are presented in Table 2 . In the minimally adjusted model, with adjustment age, gender, RDW was positively associated with in‐hospital mortality and 90 day mortality (P < 0.001). After adjusted all potential covariates (Table 2 , model 4), these associations remained significant with RDW as a continuous variable.
Table 2.
Association between RDW and short‐term mortality
| RDW, % | RDW quintiles | P for trend | ||||||
|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||||
| Model 1 | In‐hospital mortality | HR (95% CI) | 1.14 (1.11–1.18) | Reference | 1.37 (1.03–1.82) | 1.51 (1.14–2) | 2.45 (1.88–3.18) | <0.001 |
| P | <0.001 | 0.032 | 0.004 | <0.001 | ||||
| 90 day mortality | HR (95% CI) | 1.15 (1.13–1.17) | Reference | 1.55 (1.29–1.87) | 2.19 (1.83–2.6) | 3.09 (2.61–3.66) | <0.001 | |
| p | < 0.001 | <0.001 | <0.001 | <0.001 | ||||
| Model 2 | In‐hospital mortality | HR (95% CI) | 1.14 (1.1–1.18) | Reference | 1.26 (0.94–1.68) | 1.35 (1.02–1.79) | 2.23 (1.72–2.91) | <0.001 |
| P | <0.001 | 0.116 | 0.035 | <0.001 | ||||
| 90 day mortality | HR (95% CI) | 1.15 (1.13–1.17) | Reference | 1.47 (1.22–1.77) | 2.04 (1.71–2.43) | 2.91 (2.46–3.45) | <0.001 | |
| P | <0.001 | <0.001 | <0.001 | <0.001 | ||||
| Model 3 | In‐hospital mortality | HR (95% CI) | 1.14 (1.1–1.17) | Reference | 1.24 (0.93–1.66) | 1.37 (1.03–1.82) | 2.17 (1.65–2.86) | <0.001 |
| P | <0.001 | 0.143 | 0.032 | <0.001 | ||||
| 90 day mortality | HR (95% CI) | 1.13 (1.11–1.15) | Reference | 1.39 (1.15–1.68) | 1.86 (1.55–2.22) | 2.53 (2.12–3.02) | <0.001 | |
| P | <0.001 | 0.001 | <0.001 | <0.001 | ||||
| Model 4 | In‐hospital mortality | HR (95% CI) | 1.09 (1.04–1.15) | Reference | 1.19 (0.81–1.74) | 1.13 (0.76–1.67) | 1.8 (1.23–2.65) | 0.002 |
| P | <0.001 | 0.369 | 0.545 | 0.003 | ||||
| 90 day mortality | HR (95% CI) | 1.11 (1.08–1.14) | Reference | 1.49 (1.15–1.94) | 1.9 (1.47–2.45) | 2.37 (1.83–3.08) | <0.001 | |
| P | <0.001 | 0.003 | <0.001 | <0.001 | ||||
BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HR, heart rate; MCV, mean corpuscular volume; SBP, systolic blood pressure.
In terms of sensitivity analysis, RDW was transferred to a categorical variable from a continuous variable. Compared with the bottom quartile of RDW, subjects in the top had a significantly higher risk for death in hospital and after 90 days, adjusting for all the potential covariates in model 4. Compared with patients with lower RDW, rates of mortality were rather higher in patients with higher RDW, given the function of RDW at presentation, and there were differences in both in‐hospital and 90 day survival curves (Figure 3 ; P < 0.0001).
Figure 3.
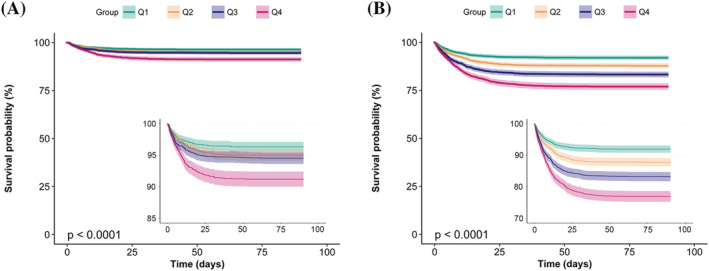
Kaplan–Meier survival curves for in‐hospital mortality and 90 day mortality. (A) In‐hospital mortality; (B) 90 day mortality.
Furthermore, we removed 2899 patients without NT‐proBNP testing records and took NT‐proBNP into consideration and adjusted in multivariate analyses. Similar results were found in association between RDW and mortality (Supporting Information, Table S2 ).
Dose–response relationship between red cell distribution width and mortality
Smooth curve and the result of Cox proportional hazards regression model with cubic spline functions showed that the relationship between RDW and in‐hospital mortality was linear after adjusting for all the variables in model 4 (P for non‐linearity = 0.651) (Supporting Information, Figure S1 ).
We observed a non‐linear dose–response relationship between RDW and 90 day mortality after adjusted all potential covariates in model 4 (P for non‐linearity <0.001) (Figure 4 ). With the continuous change of RDW, the association strength of 90 day mortality increased non‐linearly. By using a two‐piecewise linear regression model, a threshold for the association at RDW of 14.96% was identified (Table 3 ). Below the threshold, the 90 day mortality rose more rapidly with a multivariable adjusted HR of 1.36 (95% CI 1.14–1.62, P < 0.001) compared with above the threshold.
Figure 4.
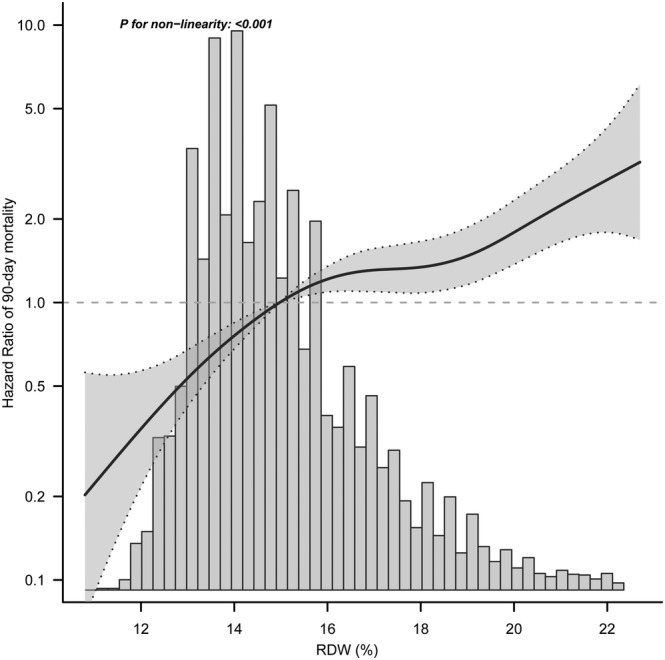
Nonlinear dose–response relationship between RDW and 90 day mortality. Adjusted for age, gender, medical history, disease severity score, BMI, HR, SBP, DBP, leucocyte, haemoglobin, MCV, eGFR, potassium, calcium, and length of stay. The solid line and dashed line represent the estimated values and their corresponding 95% confidence intervals.
Table 3.
Threshold effect analysis of RDW on 90 day mortality
| Threshold of RDW | HR | 95% CI | P‐value |
|---|---|---|---|
| <14.96% | 1.36 | 1.14–1.62 | <0.001 |
| ≥14.96% | 1.09 | 1.04–1.15 | <0.001 |
Adjustment factors included age, gender, medical history, disease severity score, BMI, HR, SBP, DBP, leucocyte, haemoglobin, MCV, eGFR, potassium, calcium and length of stay.
Subgroup analysis
Age, gender, BMI, eGFR, MCV, and HGB were used as stratification variables to observe the effect size trend, and a Forrest plot of data was generated (Figure 5 ). It should be noted that according to the previous specification, only a small number of interactions were observed including gender and eGFR (P = 0.033 and 0.042, respectively). The stronger association between RDW and 90 day mortality was detected in male and patients with normal renal function, while the weaker association was detected in female and patients with impaired renal function.
Figure 5.
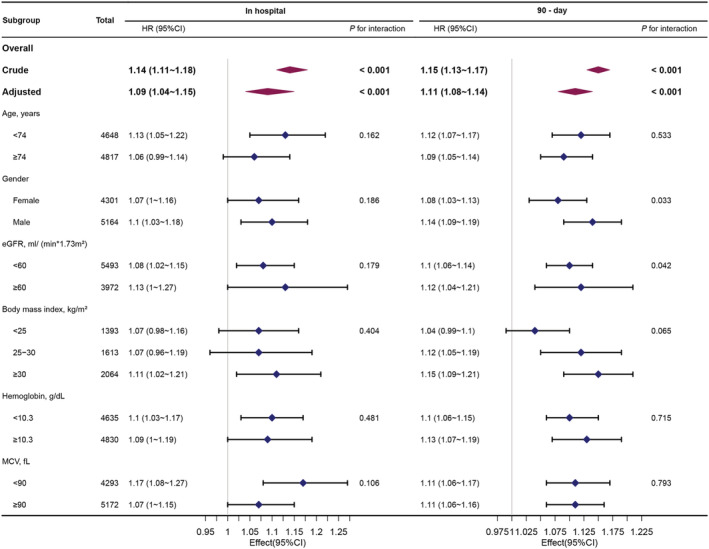
Association between RDW and in‐hospital mortality, 90 day mortality according to baseline characteristics. Each stratification adjusted for all covariants in model 4. BMI, body mass index; eGFR, estimated glomerular filtration rate; MCV, mean corpuscular volume; HGB, haemoglobin.
Discussion
The findings of this study indicate that RDW is positively associated with both in‐hospital mortality and 90 day mortality after adjustment of other covariates. Subgroup analysis makes it possible to have a better understanding of RDW and 90‐day mortality in different populations. It is also found that a stronger association is detected in male and patients with normal renal function. Felker et al. suggested that RDW is a very strong independent predictor of morbidity and mortality in a sample of 2679 patients with symptomatic chronic heart failure. 4 Similar results are also observed in the studies of van Kimmenade RR, Pascual‐Figal DA, and Zalawadiya SK, 6 , 7 , 9 which are consistent with our findings. However, there are also some other studies inconsistent with our findings. Al‐Najjar Y et al. found that all the risk is mediated by the levels of NT‐proBNP without any difference based on RDW in patients with NT‐proBNP >399 pmol/L. 3 After analysis of the studies that are inconsistent with our findings, it can be speculated that different findings may be due to the following factors: (i) the study population is different. For example, some studies targeted at HF patients with LVEF <45%; (ii) the majority of studies failed to consider the effects of hypertension, atrial fibrillation, hypoferric anaemia, depression, s, chronic renal insufficiency, COPD, SBP, DBP, MCV, eGFR, potassium and calcium on the relationship between RDW and mortality. However, previous studies have demonstrated that these variables are associated with RDW or all‐cause mortality.
Higher RDW, also known as anisocytosis, indicates defective maturation or degradation of RBCs. Diabetes, chronic kidney disease, anaemia, inflammatory‐related disorders such as infection and cancer, as well as heart failure, are associated with higher RDW. 10 , 11 , 12 , 13 , 14 , 15 However, the specific mechanistic links between RDW and poor prognosis in chronic disease including HF have not yet been fully understood. Multiple interlinked pathomechanisms, including oxidative stress, enhanced immune system activation, chronic inflammation, aberrant body iron distribution, malnutrition, and cachexia, are thought to be related to an increase in RDW and associated worse outcomes in patients with HF. 16 , 17 , 18 , 19 , 20 , 21 Because erythrocytes both convey oxygen to tissues and play a key role in cardiovascular regulation through release of extracellular nucleotides and other mediators, 22 a direct effect of changes in erythrocyte function on the heart appears conceivable. There is evidence that a chronic inflammatory condition causes inefficient erythropoiesis, resulting in the entry of immature RBCs into the circulation and an increase in RDW. 23 Inflammatory markers such as tumour necrosis factor‐a and interleukin‐6 can disrupt erythropoiesis by direct myelosuppression of erythroid precursors, lowering iron bioavailability for haemoglobin synthesis, promoting apoptosis, increasing erythropoietin resistance in precursor cell lines, and lowering erythropoietin production from the kidney. 24 , 25 Erythrolysis, on the other hand, is linked to an increase in the production of free radicals, 26 which are known to be detrimental to the heart. Higher oxidative stress is another potential mechanism linking RDW with mortality because it reduces RBC survival and leads to anisocytosis resulting from an increase in circulating premature erythrocytes. 27 , 28
Stratified analyses revealed generally consistent associations with the main results. However, we found that the HRs in some subgroups, such as female, or those with participants with impaired renal function, were lower than those in the corresponding subgroups. Patients with higher RDW consisted of more female and had higher creatinine levels on admission than patients with lower RDW, which is consistent with previous studies. The association between GFR and RDW was investigated and an independent inverse relationship was demonstrated between estimated GFR and RDW. 18 Chronic renal insufficiency is an inflammatory condition associated with higher oxidative stress and higher cardiovascular mortality. 29 , 30 The existence of these factors may modify and attenuate the impact of RDW on mortality.
The clinical value of this study is as follows: it is the first time to investigate the independent relationship between RDW and short‐term mortality in critically ill patients with heart failure; the results of this study should be helpful for evaluating RDW as an effective tool to stratify risk at admission in critically ill patients with HF; this study may provide support for future research on the establishment of short‐term mortality prediction models.
Here are a number of advantages of this study. (i) The sample size is relatively large compared with previous similar studies; (ii) this is an observational study and prone to the potential for confusion. And residual confounders can be minimized using stringent statistical adjustment; (iii) both continuous and categorical variables are treated as target‐independent variables. This approach can enhance the robustness of results and reduce the contingency in the data analysis; (iv) the effect modifier factor analysis makes better use of data to draw stable conclusions in different subgroups.
The present study also has certain limitations. Firstly, this is an observational study; it is not possible to identify the relationship between causes and outcomes. Secondly, the value of baseline RDW is only evaluated in this study; hence, it is not possible to assess the impact of dynamic changes in RDW on survival results. Thirdly, the subjects of this study are critical patients with heart failure, so there is a certain deficiency in the extrapolation and universality of the study.
Conclusions
The present study suggests that the short‐term mortality of critically ill patients with HF is independently predicted by RDW. We have shown for the first time the prognostic relevance of RDW in critically ill patients with HF suggesting that RDW may help to stratify risk already at admission. At the same time, large prospective research and longer follow‐up time are required to further validate the findings of this study.
Conflict of interest
None declared.
Funding
This work was supported by the National Natural Science Foundation of China (81900444, 81873516, 81873522, and 82170463), the Clinical Research Center of Shandong University (2020SDUCRCA009), the National Key Research and Development Program of China (2021YFF0501400 and 2017YFC1308303) and the Natural Science Foundation of Shandong Province (ZR2019PH030, ZR2020QH007, and ZR2019BH052), China International Medical Foundation (Z‐2016‐23‐2001‐01 and Z‐2019‐42‐1908‐2). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supporting information
Table S1. Univariate Cox Regression Analysis.
Table S2. Association between RDW and mortality after excluding without NT‐proBNP testing records.
Figure S1. Association between RDW and in‐hospital mortality. (adjusted for all covariants in model 4).
Acknowledgements
The authors thank all the staff members in our institution. We gratefully thank Dr. Jie Liu of Department of Vascular and Endovascular Surgery, Chinese PLA General Hospital, Dr. Qilin Yang of Department of Critical Care, the Second Affiliated Hospital of Guangzhou Medical University, and Dr. Yuxiong Chen of Department of Cardiology, Peking Union Medical College Hospital, Peking Union Medical College & Chinese Academy of Medical Sciences for their contribution to the statistical support, study design consultations and comments regarding the manuscript.
Zhang, X. , Wang, Y. , Chen, N. , Liu, Y. , Xiao, J. , Lin, Z. , Lu, H. , and Ji, X. (2022) Red cell distribution width is associated with short‐term mortality in critically ill patients with heart failure. ESC Heart Failure, 9: 3210–3220. 10.1002/ehf2.14023.
Contributor Information
Xinyu Zhang, Email: zhangxinyu@sdu.edu.cn.
Huixia Lu, Email: luhuixia@sdu.edu.cn.
Xiaoping Ji, Email: jixiaoping@sdu.edu.cn.
References
- 1. Poz D, de Falco E, Pisano C, Madonna R, Ferdinandy P, Balistreri CR. Diagnostic and prognostic relevance of red blood cell distribution width for vascular aging and cardiovascular diseases. Rejuvenation Res. 2019; 22: 146–162. [DOI] [PubMed] [Google Scholar]
- 2. Salvagno GL, Sanchis‐Gomar F, Picanza A, Lippi G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015; 52: 86–105. [DOI] [PubMed] [Google Scholar]
- 3. Al‐Najjar Y, Goode KM, Zhang J, Cleland JGF, Clark AL. Red cell distribution width: An inexpensive and powerful prognostic marker in heart failure. Eur J Heart Fail. 2009; 11: 1155–1162. [DOI] [PubMed] [Google Scholar]
- 4. Felker GM, Allen LA, Pocock SJ, Shaw LK, McMurray J, Pfeffer MA, Swedberg K, Wang D, Yusuf S, Michelson EL, Granger CB, CHARM Investigators . Red cell distribution width as a novel prognostic marker in heart failure: Data from the CHARM program and the Duke databank. J Am Coll Cardiol. 2007; 50: 40–47. [DOI] [PubMed] [Google Scholar]
- 5. Melchio R, Rinaldi G, Testa E, Giraudo A, Serraino C, Bracco C, Spadafora L, Falcetta A, Leccardi S, Silvestri A, Fenoglio L. Red cell distribution width predicts mid‐term prognosis in patients hospitalized with acute heart failure: The RDW in acute heart failure (RE‐AHF) study. Intern Emerg Med. 2019; 14: 239–247. [DOI] [PubMed] [Google Scholar]
- 6. Pascual‐Figal DA, Bonaque JC, Redondo B, Caro C, Manzano‐Fernandez S, Sánchez‐Mas J, Garrido IP, Valdes M. Red blood cell distribution width predicts long‐term outcome regardless of anaemia status in acute heart failure patients. Eur J Heart Fail. 2009; 11: 840–846. [DOI] [PubMed] [Google Scholar]
- 7. van Kimmenade RR, Mohammed AA, Uthamalingam S, van der Meer P, Felker GM, Januzzi JL Jr. Red blood cell distribution width and 1‐year mortality in acute heart failure. Eur J Heart Fail. 2010; 12: 129–136. [DOI] [PubMed] [Google Scholar]
- 8. Johnson AE, Pollard TJ, Shen L, Lehman LWH, Feng M, Ghassemi M, Moody B, Szolovits P, Anthony Celi L, Mark RG. MIMIC‐III, a freely accessible critical care database. Sci Data. 2016; 3: 160035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zalawadiya SK, Zmily H, Farah J, Daifallah S, Ali O, Ghali JK. Red cell distribution width and mortality in predominantly African‐American population with decompensated heart failure. J Card Fail. 2011; 17: 292–298. [DOI] [PubMed] [Google Scholar]
- 10. Nada AM. Red cell distribution width in type 2 diabetic patients. Diabetes Metab Syndr Obes. 2015; 8: 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Engström G, Smith JG, Persson M, Nilsson PM, Melander O, Hedblad B. Red cell distribution width, haemoglobin A1c and incidence of diabetes mellitus. J Intern Med. 2014; 276: 174–183. [DOI] [PubMed] [Google Scholar]
- 12. Hsieh YP, Chang CC, Kor CT, Yang Y, Wen YK, Chiu PF. The predictive role of red cell distribution width in mortality among chronic kidney disease patients. PLoS ONE. 2016; 11: e0162025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salvatori M, Formiga F, Moreno‐Gónzalez R, Chivite D, de Amicis MM, Cappellini MD, Corbella X. Red blood cell distribution width as a prognostic factor of mortality in elderly patients firstly hospitalized due to heart failure. Kardiol Pol. 2019; 77: 632–638. [DOI] [PubMed] [Google Scholar]
- 14. Kim CH, Park JT, Kim EJ, Han JH, Han JS, Choi JY, Han SH, Yoo TH, Kim YS, Kang SW, Oh HJ. An increase in red blood cell distribution width from baseline predicts mortality in patients with severe sepsis or septic shock. Crit Care. 2013; 17: R282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koma Y, Onishi A, Matsuoka H, Oda N, Yokota N, Matsumoto Y, Koyama M, Okada N, Nakashima N, Masuya D, Yoshimatsu H, Suzuki Y. Increased red blood cell distribution width associates with cancer stage and prognosis in patients with lung cancer. PLoS ONE. 2013; 8: e80240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Förhécz Z, Gombos T, Borgulya G, Pozsonyi Z, Prohászka Z, Jánoskuti L. Red cell distribution width in heart failure: Prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J. 2009; 158: 659–666. [DOI] [PubMed] [Google Scholar]
- 17. Allen LA, Felker GM, Mehra MR, Chiong JR, Dunlap SH, Ghali JK, Lenihan DJ, Oren RM, Wagoner LE, Schwartz TA, Adams KF Jr. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J Card Fail. 2010; 16: 230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relationship between red blood cell distribution width and kidney function tests in a large cohort of unselected outpatients. Scand J Clin Lab Invest. 2008; 68: 745–748. [DOI] [PubMed] [Google Scholar]
- 19. Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009; 133: 628–632. [DOI] [PubMed] [Google Scholar]
- 20. Montagnana M, Cervellin G, Meschi T, Lippi G. The role of red blood cell distribution width in cardiovascular and thrombotic disorders. Clin Chem Lab Med. 2011; 50: 635–641. [DOI] [PubMed] [Google Scholar]
- 21. Wołowiec Ł, Rogowicz D, Banach J, Buszko K, Surowiec A, Błażejewski J, Bujak R, Sinkiewicz W. Prognostic significance of red cell distribution width and other red cell parameters in patients with chronic heart failure during two years of follow‐up. Kardiol Pol. 2016; 74: 657–664. [DOI] [PubMed] [Google Scholar]
- 22. Wan J, Ristenpart WD, Stone HA. Dynamics of shear‐induced ATP release from red blood cells. Proc Natl Acad Sci U S A. 2008; 105: 16432–16437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iversen PO, Woldbaek PR, Tønnessen T, Christensen G. Decreased hematopoiesis in bone marrow of mice with congestive heart failure. Am J Physiol Regul Integr Comp Physiol. 2002; 282: R166–R172. [DOI] [PubMed] [Google Scholar]
- 24. Ghali JK. Anemia and heart failure. Curr Opin Cardiol. 2009; 24: 172–178. [DOI] [PubMed] [Google Scholar]
- 25. Laftah AH, Sharma N, Brookes MJ, McKie AT, Simpson RJ, Iqbal TH, Tselepis C. Tumour necrosis factor alpha causes hypoferraemia and reduced intestinal iron absorption in mice. Biochem J. 2006; 397: 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bogdan C. Oxidative burst without phagocytes: The role of respiratory proteins. Nat Immunol. 2007; 8: 1029–1031. [DOI] [PubMed] [Google Scholar]
- 27. Fibach E, Rachmilewitz E. The role of oxidative stress in hemolytic anemia. Curr Mol Med. 2008; 8: 609–619. [DOI] [PubMed] [Google Scholar]
- 28. Ghaffari S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid Redox Signal. 2008; 10: 1923–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cottone S, Lorito MC, Riccobene R, Nardi E, Mulè G, Buscemi S, Geraci C, Guarneri M, Arsena R, Cerasola G. Oxidative stress, inflammation and cardiovascular disease in chronic renal failure. J Nephrol. 2008; 21: 175–179. [PubMed] [Google Scholar]
- 30. Kaysen GA, Eiserich JP. The role of oxidative stress‐altered lipoprotein structure and function and microinflammation on cardiovascular risk in patients with minor renal dysfunction. J Am Soc Nephrol. 2004; 15: 538–548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariate Cox Regression Analysis.
Table S2. Association between RDW and mortality after excluding without NT‐proBNP testing records.
Figure S1. Association between RDW and in‐hospital mortality. (adjusted for all covariants in model 4).


