Abstract
Aims
Continuous‐flow left ventricular assist devices (CF‐LVADs) have become a standard of care in end‐stage heart failure. Limited data exist comparing outcomes of HeartMate3 (HM3) and HeartWare HVAD (HW). We aimed to compare midterm outcomes of these devices.
Methods and results
Investigator‐initiated retrospective‐observational comparative analysis of all patients who underwent primary LVAD implantation of either HM3 or HW at our centre between January 2010 and December 2020. Data were derived from a prospective registry. Primary endpoints were all‐cause mortality and heart transplantation. Secondary endpoints included device‐related major adverse cardiac and cerebrovascular events, which included major bleeding, major neurological dysfunction (defined as persisting neurological impairment for ≥24 h), device‐related major infection (excluding driveline infections), major device malfunctions leading to re‐intervention or partial device exchange (pump failure, outflow‐graft twist or failure, controller failure, battery failure, patient cable failure, but excluding pump thrombosis), and pump thrombosis. Further secondary endpoints included right heart failure, gastrointestinal bleeding, driveline infections, and surgical re‐interventions. The secondary outcomes were analysed not only for the first event but also for recurrent events. The analysis included competing risks analysis and recurrent event regression analysis, with adjustment for confounders age, gender, body mass index (BMI), and Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) level. Out of 106 primary CF‐LVAD implantations, 36 (34%) received HM3 and 70 (66%) received HW. Median follow‐up was 1.48 years [interquartile range 0.67, 2.41]. HM3 was more often implanted in men (91.7% vs. 72.9%, P = 0.024); patients were older (median 61 years [54, 66.5] vs. 52.5 years [43, 60], P < 0.001), had a higher BMI (median 26.7 kg/m2 [23.4, 29.0] vs. 24.3 kg/m2 [20.7, 27.4], P = 0.013), had more comorbidities, and were more likely targeted for destination therapy (36.1% vs. 14.3%, P = 0.010). Death occurred in 33.3% of HM3 patients, compared with 22.9% of HW patients, P = 0.247 (probability of survival at 4 years, 54.7% vs. 74.1%, P = 0.296). After adjustment for confounders, we observed a significant six‐fold risk increase in device malfunctions for HW [hazard ratio (HR) 6.49, 95% confidence interval (CI) [1.89, 22.32], P = 0.003], but no significant differences in pump thrombosis (P = 0.173) or overall survival (P = 0.801).
Conclusions
Comparing midterm outcomes between HM3 and HW for LVAD support from a prospective registry, HW patients had a significantly higher risk of device malfunctions. No significant differences were evident between devices in overall survival and in respect to most outcomes.
Keywords: Left ventricular assist device, LVAD, HeartMate3, HeartWare HVAD, Outcome comparison
Introduction
Continuous‐flow (CF) left ventricular assist devices (LVADs) have become a standard of care in end‐stage heart failure (HF). Through the technological advancements with the more durable centrifugal‐pump technology, today's devices have become more reliable and have a decreased rate of device‐related complications when compared with previous second‐generation or first‐generation devices. Since the emergence of magnetically levitated CF‐LVADs, overall survival at 4 years has approached 60% and thus offers a durable option for bridge to transplantation (BTT) as well as for destination therapy (DT). 1 , 2 , 3 The rate of device‐related adverse events remains high, with stroke, bleeding, infection, device malfunction, and pump thrombosis occurring in 19%, 26%, 47%, 5%, and <2% of patients within 2 years, respectively. 1 , 4 , 5 Two magnetically levitating CF‐LVADs are in use today and represent the current gold standard in LVAD therapy—HeartMate3 (HM3) and HeartWare HVAD (HW). The newer HM3 is a fully magnetically levitated centrifugal‐flow pathway pump with a frictionless rotor and a fixed intrinsic pulse, whereas HW uses a combination of hydrodynamic and magnetic levitation of the internal rotor. It is known from prospective trials of recent years that these magnetically levitating centrifugal CF‐LVADs are non‐inferior to axial‐flow CF‐LVADs and have a lower rate of device‐related complications. 2 , 6 , 7 However, very limited data exist comparing outcomes with both devices, and the gap of knowledge remains. 8 , 9 , 10 Therefore, we aimed to analyse all magnetically levitating CF‐LVADs implanted at our institution and compare outcomes between devices.
Methods
Study design
An observational retrospective analysis was conducted including all patients who underwent primary implantation of a centrifugal CF‐LVAD at our tertiary care academic centre between January 2010 and December 2020. Implanted CF‐LVADs included either the HM3 (Abbott, Chicago, IL, USA) or the HW (Medtronic, Minneapolis, MN, USA) device. HW has been implanted at our centre since 2010, and HM3 since 2015. We excluded all patients with biventricular VADs, isolated right VAD (RVAD), or after a device exchange. Patient data, including demographics, preoperative characteristics, and post‐operative outcomes, were collected from the prospective European Registry for Patients with Mechanical Circulatory Support (EUROMACS) database for our centre. All primary LVAD implantations in our patient cohort were performed over median sternotomy and under full cardiopulmonary bypass (CPB). The anticoagulation regime was consistent in all patients and included vitamin K antagonist (VKA) phenprocoumon with international normalized ratio (INR) range between 2.0 and 3.0, and acetylsalicylic acid (ASA).
The follow‐up duration was patient based and comprised the complete available follow‐up for each patient, until primary endpoints of overall death or heart transplantation, whichever occurred first, but no longer than 31 December 2020 (censor date). Secondary endpoints included all device‐related major adverse cardiac and cerebrovascular events (MACCE), defined as major bleeding [defined as any major bleeding event, including gastrointestinal (GI)], major neurological dysfunction (defined as persisting neurological impairment for ≥24 h), device‐related major infection (excluding driveline infections), major device malfunctions leading to re‐intervention or partial device exchange (pump failure, outflow‐graft twist or failure, controller failure, battery failure, patient cable failure, but excluding pump thrombosis), and pump thrombosis, as defined in the EUROMACS registry. Additional secondary endpoints included the occurrence of right heart failure (RHF) and RHF‐related rehospitalizations, incidence of surgical re‐interventions for driveline infection, overall incidence of driveline infections, and the overall rate of GI bleedings. Patients were grouped according to the implanted device type. Patient demographics and baseline data included the haemodynamic, echocardiographic, and laboratory status collected prior to LVAD implantation and are summarized in Table 1 . The study conforms with the principles outlined in the Declaration of Helsinki and was approved by the local ethics committee on human research (KEK Bern, 2017‐00785).
Table 1.
Baseline patient demographics and perioperative characteristics
| Characteristics | Total (N = 106) | HeartMate 3 (N = 36; 34%) | HeartWare (N = 70; 66%) | P‐value | Missing per group (HM3/HW) |
|---|---|---|---|---|---|
| Age (years) | 56.0 [47.0, 64.0] | 61.0 [54.0, 66.5] | 52.5 [43.0, 60.0] | <0.001 | 0/0 |
| Age < 18 years | 2 (1.9%) | 0 (0%) | 2 (2.9%) | 0.547 | |
| Gender | 0.024 | 0/0 | |||
| Female | 22 (20.8%) | 3 (8.3%) | 19 (27.1%) | ||
| Male | 84 (79.3%) | 33 (91.7%) | 51 (72.9%) | ||
| BMI (kg/m 2 ) | 25.3 [21.9, 27.9] | 26.7 [23.4, 29.0] | 24.3 [20.7, 27.4] | 0.013 | 1/3 |
| BSA (m 2 ) | 1.9 [1.8, 2.1] | 2.0 [1.9, 2.1] | 1.9 [1.7, 2.1] | 0.019 | 1/3 |
| Education | >0.99 | 20/9 | |||
| Primary education | 3 (3.9%) | 0 (0%) | 3 (4.9%) | ||
| Secondary education | 49 (63.6%) | 11 (68.8%) | 38 (62.3%) | ||
| Tertiary education | 25 (32.5%) | 5 (31.3%) | 20 (32.8%) | ||
| Blood type | 0.842 | 0/0 | |||
| A | 58 (54.7%) | 20 (55.6%) | 38 (54.3%) | ||
| AB | 2 (1.9%) | 0 (0%) | 2 (2.9%) | ||
| B | 14 (13.2%) | 4 (11.1%) | 10 (14.3%) | ||
| O | 32 (30.2%) | 12 (33.3%) | 20 (28.6%) | ||
| Comorbidities | |||||
| HTN | 1 (1%) | 0 (0%) | 1 (1.5%) | >0.99 | 1/5 |
| DM | 28 (26.7%) | 11 (31.4%) | 17 (24.3%) | 0.435 | 1/0 |
| Symptomatic peripheral vascular disease | 7 (6.9%) | 3 (9.1%) | 4 (5.8%) | 0.679 | 3/1 |
| Carotid artery disease | 4 (3.8%) | 2 (5.6%) | 2 (2.9%) | 0.605 | 0/1 |
| COPD | 15 (14.3%) | 11 (30.6%) | 4 (5.8%) | 0.001 | 0/1 |
| CKD | 29 (28.2%) | 17 (48.6%) | 12 (17.7%) | 0.001 | 1/2 |
| Dialysis | 3 (2.8%) | 1 (2.8%) | 2 (2.9%) | >0.99 | 0/0 |
| Smoking history | 50 (58.9%) | 17 (77.3%) | 33 (52.4%) | 0.041 | 14/7 |
| AF | 32 (30.8%) | 15 (41.7%) | 17 (25.0%) | 0.080 | 0/2 |
| Pulmonary HTN | 64 (91.4%) | 22 (88.0%) | 42 (93.3%) | 0.659 | 11/25 |
| Cancer excluding skin cancer | 5 (4.8%) | 4 (11.4%) | 1 (1.43) | 0.041 | 1/0 |
| Previous cardiac surgery | 13 (12.3%) | 3 (8.3%) | 10 (14.3%) | 0.376 | 0/0 |
| Cause of heart failure | 0.757 | 1/0 | |||
| Congenital heart disease | 2 (1.9%) | 0 (0%) | 2 (2.9%) | ||
| ICM | 37 (35.2%) | 14 (40.0%) | 23 (32.9%) | ||
| NIDCM | 65 (61.9%) | 21 (60.0%) | 44 (62.9%) | ||
| HCM | 1 (1.0%) | 0 (0%) | 1 (1.4%) | ||
| INTERMACS stage | 0.913 | 0/0 | |||
| 1—Critical cardiogenic shock | 4 (3.8%) | 1 (2.8%) | 3 (4.3%) | ||
| 2—Progressive decline | 17 (16.0%) | 4 (11.1%) | 13 (18.6%) | ||
| 3—Stable but inotrope dependent | 20 (18.9%) | 7 (19.4%) | 13 (18.6%) | ||
| 4—Resting symptoms | 39 (36.8%) | 16 (44.4%) | 23 (32.9%) | ||
| 5—Exertion intolerant | 14 (13.2%) | 4 (11.1%) | 10 (14.3%) | ||
| 6—Exertion limited | 11 (10.4%) | 4 (11.1%) | 7 (10.0%) | ||
| 7—Advanced NYHA class 3 | 1 (1.0%) | 0 (0%) | 1 (1.4%) | ||
| INTERMACS stage | 0.418 | 0/0 | |||
| INTERMACS ≤ 3 | 41 (38.7%) | 12 (33.3%) | 29 (41.4%) | ||
| INTERMACS ≥ 4 | 65 (61.3%) | 24 (66.7%) | 41 (58.6%) | ||
| Inotropic support prior to implant | 31 (29.5%) | 6 (17.1%) | 25 (35.7%) | 0.049 | 1/0 |
| MCS at time of LVAD | 13 (12.4%) | 3 (8.3%) | 10 (14.5%) | 0.363 | 0/1 |
| Ventilated at time of LVAD | 17 (16.2%) | 4 (11.1%) | 13 (18.8%) | 0.307 | 1 |
| Treatment strategy | 0.010 | 0/0 | |||
| BTT | 83 (78.3%) | 23 (63.9%) | 60 (85.7%) | ||
| DT | 23 (21.7%) | 13 (36.1%) | 10 (14.3%) | ||
| Laboratory values | |||||
| Creatinine (μmol/L) | 147.5 [101.0, 197.0] | 166.5 [127.0, 221.5] | 126.5 [92.0, 183.0] | 0.009 | 0/0 |
| BUN (mg/dL) | 62.5 [34.2, 116.0] | 85.7 [54.0, 128.8] | 48.8 [31.8, 102.0] | 0.027 | 8/16 |
| AST (U/L) | 31.5 [22.5, 44.0] | 35.0 [23.0, 44.0] | 31.0 [22.0, 46.0] | 0.944 | 22/28 |
| ALT (U/L) | 29.5 [20.0, 56.0] | 32.0 [19.0, 60.5] | 29.5 [21.0, 51.0] | 0.692 | 8/24 |
| Bilirubin total (mg/dL) | 1.2 [0.7, 1.8] | 0.9 [0.5, 1.5] | 1.3 [0.8, 1.8] | 0.254 | 29/40 |
| Cholesterol | 3.0 [2.3, 3.4] | 2.4 [2.0, 3.0] | 3.3 [2.8, 3.7] | 0.013 | 22/56 |
| Haemoglobin (g/dL) | 12.1 [10.5, 13.0] | 12.1 [10.4, 12.7] | 12.0 [10.6, 13.0] | 0.834 | 1/2 |
| WBC (×109/L) | 7.2 [6.1, 9.2] | 7.4 [6.3, 9.2] | 7.1 [6.1, 9.2] | 0.459 | 2/2 |
| Platelet count (×109/L) | 171.0 [133.0, 222.0] | 179.5 [134.0, 253.0] | 157.5 [133.0, 213.0] | 0.325 | 2/2 |
| INR | 1.4 [1.1, 2.0] | 1.2 [1.1, 1.8] | 1.6 [1.1, 2.2] | 0.115 | 1/2 |
| aPTT (s) | 47.8 [39.1, 58.2] | 48.2 [39.7, 58.2] | 44.5 [39.0, 57.9] | 0.519 | 7/32 |
| Haemodynamic and echocardiographic data | |||||
| Heart rate (1/min) | 75.5 [70.0, 89.0] | 80.0 [72.0, 89.0] | 74.0 [66.0, 90.0] | 0.179 | 1/3 |
| sAP (mmHg) | 88.0 [80.0, 98.0] | 86.0 [79.0, 100.0] | 90.0 [81.0, 98.0] | 0.611 | 1/5 |
| dAP (mmHg) | 57.0 [50.5, 63.0] | 56.0 [48.0, 63.0] | 58.0 [52.0, 63.0] | 0.396 | 1/5 |
| Mean AP (mmHg) | 67.0 [61.0, 73.8] | 65.0 [58.7, 76.3] | 67.3 [63.0, 73.7] | 0.466 | 1/5 |
| sPAP (mmHg) | 50.5 [42.0, 60.0] | 51.0 [38.0, 60.0] | 50.0 [42.0, 59.0] | 0.955 | 13/27 |
| dPAP (mmHg) | 23.5 [17.0, 30.0] | 23.0 [18.0, 29.0] | 24.0 [17.0, 30.0] | 0.807 | 13/27 |
| Mean PAP (mmHg) | 35.0 [25.3, 40.0] | 36.0 [27.0, 40.0] | 33.5 [24.7, 40.5] | 0.731 | 13/26 |
| PCWP (mmHg) | 23.0 [19.0, 29.0] | 25.0 [20.0, 31.0] | 20.5 [18.0, 28.0] | 0.229 | 17/32 |
| SVR (dyn s/cm5) | 1278 [1053, 1641] | 1292 [1159, 1641] | 1201 [1018, 1630] | 0.538 | 19/45 |
| PVR (dyn s/cm5) | 215.0 [166.0, 560.0] | 300.0 [173.0, 614.5] | 209 [130, 359] | 0.401 | 20/35 |
| CI (L/min/m2) | 1.8 [1.5, 2.1] | 1.6 [1.3, 2.0] | 1.9 [1.6, 2.2] | 0.205 | 17/32 |
| CO (L/min) | 3.3 [2.8, 3.9] | 3.3 [2.8, 4.1] | 3.4 [2.8, 3.9] | 0.847 | 17/31 |
| LVEF (%) | 15.0 [15.0, 20.0] | 15.0 [15.0, 20.0] | 15.0 [15.0, 20.0] | 0.368 | 2/2 |
| LVEDD (mm) | 69.5 [63.0, 73.0] | 70.0 [67.0, 76.0] | 67.0 [62.0, 73.0] | 0.094 | 8/8 |
| RV function severely impaired | 13 (13.3%) | 4 (12.1%) | 9 (13.9) | 0.812 | 3/5 |
| TAPSE (mm) | 15.0 [12.0, 17.0] | 15.0 [12.0, 16.5] | 15.0 [12.0, 18.0] | 0.692 | 8/11 |
| AR ≥ moderate | 2 (2.0%) | 0 (0%) | 2 (3.0%) | 0.549 | 2/3 |
| MR ≥ moderate | 61 (59.8%) | 21 (61.8%) | 40 (58.8%) | 0.775 | 2/2 |
| TR ≥ moderate | 53 (52.0%) | 17 (50.0%) | 36 (52.9%) | 0.779 | 2/2 |
| OR duration (min) | 192.0 [160.0, 233.0] | 209.5 [177.5, 240.0] | 181.5 [155.0, 228.0] | 0.042 | 0/0 |
| CPB duration (min) | 92.5 [72.0, 116.0] | 104.0 [84.0, 124.5] | 87.0 [68.0, 113.0] | 0.009 | 0/0 |
| Hospitalization duration (days) | 24.0 [17.0, 35.0] | 24.5 [18.0, 37.0] | 23.0 [17.0, 35.0] | 0.331 | 2/0 |
AF, atrial fibrillation; ALT, alanine transaminase; AP, arterial pressure; AR, aortic regurgitation; AST, aspartate transaminase; BMI, body mass index; BSA, body surface area; BUN, blood urea nitrogen; CAD, carotid artery disease; CI, cardiac index; CKD, chronic kidney disease; CO, cardiac output; COPD, chronic obstructive pulmonary disease; CPB, cardiopulmonary bypass; d, diastolic; DM, diabetes mellitus; HTN, arterial hypertension; INR, international normalized ratio; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support status; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; OR, operating room; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVD, peripheral vascular disease; PVR, pulmonary vascular resistance; RV, right ventricular function; s, systolic; SVR, systemic vascular resistance; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; WBC, white blood cells.
Numeric variables are presented as median [quartiles] and were compared with Mann–Whitney U tests. Categorical variables are presented as numbers and column percentages and were compared with Pearson's χ 2 test or Fisher's exact test, as appropriate. Percentages may not necessarily add up to 100% due to rounding error and may not necessarily use the total N of the respective column as the denominator due to missing data (see column ‘missing’).
Statistical analysis
The STROBE checklist was used for reporting observational studies. The distribution of continuous or discrete data was assessed with histograms, Q–Q plots, and Shapiro–Wilk tests, with most variables showing a skewed distribution. We therefore consistently report medians and quartiles for numeric data and used non‐parametric testing (Mann–Whitney U tests) for comparisons between groups. 11 Categorical data are summarized as counts and percentages and were compared between groups using Pearson's χ 2 tests or Fisher's exact tests, as appropriate. 12 Survival analysis techniques were used for time‐to‐event data. 13 Specifically, Kaplan–Meier curves and log‐rank tests were used to compare time‐to‐first event between the groups. Cox regression was used to derive the hazard ratios (HRs) and 95% confidence intervals (CIs). The proportional hazards assumption was assessed by testing for nonzero slope in a regression of the scaled Schoenfeld residuals on time. Additionally, analyses were performed in which heart transplantation was considered as competing risk for death, death as competing risk for heart transplantation, and both—death and heart transplantation—as competing risks for the secondary outcomes (Fine and Gray model). Moreover, events that can recur in patients (i.e. all secondary outcomes) were modelled using recurrent events analysis (Andersen–Gill model). We present unadjusted HRs as well as adjusted HR (aHR), adjusting for potential confounders, which we had selected a priori based on clinical relevance: patient age and body mass index (BMI) at the time of device implantation, gender, and Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) score 1–3 versus 4–7. 14 Likewise, unadjusted and adjusted subdistribution HR (SHR and aSHR) are reported for competing risks models. Two‐sided P‐values < 0.025 were considered statistically significant for primary outcomes (Bonferroni correction for two primary outcomes 15 ), and P < 0.05 was considered significant for secondary outcomes. All statistical analyses were performed in Stata/IC 16.0 (StataCorp., College Station, TX, USA).
Results
Demographic and operative characteristics
A total of 106 primary magnetically levitating CF‐LVADs were implanted during the study period. Thirty‐six patients (34%) received the HM3, and 70 patients (66%) received the HW. In brief, median age was higher in the HM3 population, with 61 years [54, 66.5], compared with a median age of 52.5 years [43, 60] in the HW group, P < 0.001. Among those with the HM3, there were more males compared with HW (91.7% vs. 72.9%, P = 0.024), and more patients with higher BMI and body surface area (BSA) received HM3 (median 26.7 kg/m2 [23.4, 29.0] vs. 24.3 kg/m2 [20.7, 27.4], P = 0.013, and 2.0 m2 [1.9, 2.1] vs. 1.9 m2 [1.7, 2.1], P = 0.019, respectively). Patients receiving the HM3 device also had a significantly higher incidence of chronic obstructive pulmonary disease (COPD) (30.6% vs. 5.8%, P = 0.001), chronic kidney disease (CKD) (48.6% vs. 17.7%, P = 0.001), history of smoking (77.3% vs. 52.4%, P = 0.041), and history of non‐skin cancer (11.4% vs. 1.43%, P = 0.041). More patients supported by the HM3 group were intended for DT (36.1% vs. 14.3%, P = 0.01), and fewer patients required inotropic support prior to implantation in the HM3 group (17.1% vs. 35.7%, P = 0.049), although no evidence was noted for a difference in the distribution of INTERMACS stages between the devices. The most common cause of HF was non‐ischaemic dilatative cardiomyopathy (NIDCM) (61.9%), and 12.3% of patients had had previous cardiac surgery. Remaining patient demographics were comparable between the groups (Table 1 ).
Endpoints
Median duration on LVAD support was 530 days [326, 894.5] for HM3 and 544.5 days [219, 873] for HW. Overall, 33.3% of HM3 patients died, compared with 22.9% in the HW group; however, no significant difference in overall mortality was observed between devices (Table 2 ). Median time to death in HM3 patients was 259 days [13, 530], compared with 391.5 days [214.5, 632] in HW. The Kaplan–Meier estimator of unadjusted survival probability (Figure 1 A ) at 1 and 4 years was 83.2% and 54.7% in HM3, and 86.0% and 74.1% in HW, respectively (P = 0.296). However, after adjustment for age, BMI, gender, and initial INTERMACS stage, no significant difference in survival between the treatment groups was observed using Cox regression models (aHR 0.97, 95% CI [0.43, 2.21], P = 0.949; Table 3 ) or competing risks regression (aSHR 0.90, 95% CI [0.41, 1.99], P = 0.801; Table 3 , Figure 1 B ).
Table 2.
Primary and secondary outcomes analysis
| Outcome events | Total (N = 106) | HeartMate 3 (N = 36; 34%) | HeartWare (N = 70; 66%) | P‐value |
|---|---|---|---|---|
| Follow‐up time (days) | 541.0 [246.0, 879.0] | 530.0 [326.0, 894.5] | 544.5 [219.0, 873.0] | 0.739 |
| Death | ||||
| Death overall | 28 (26.4%) | 12 (33.3%) | 16 (22.9%) | 0.247 |
| Death within 30 days | 6 (21.4%) | 4 (33.3%) | 2 (12.5%) | |
| Death after 30 days | 22 (78.6%) | 8 (66.7%) | 14 (87.5%) | |
| Days to death | 384.0 [58.0, 541.5] | 259.5 [13.0, 530.0] | 391.5 [214.5, 632.0] | 0.227 |
| Heart transplant | ||||
| Heart transplant overall | 47 (44.3%) | 4 (11.1%) | 43 (61.4%) | <0.001 |
| Heart transplant within 30 days | 0 (0%) | 0 (0%) | 0 (0%) | |
| Heart transplant after 30 days | 47 (100%) | 4 (100%) | 43 (100%) | |
| Days to heart transplant | 551.0 [219.0, 786.0] | 566.5 [372.5, 695.5] | 551.0 [209.0, 799.0] | 0.900 |
| MACCE | ||||
| MACCE overall | 67 (63.2%) | 21 (58.3%) | 46 (65.7%) | 0.456 |
| MACCE within 30 days | 32 (47.8%) | 12 (57.1%) | 20 (43.5%) | |
| MACCE after 30 days | 35 (52.2%) | 9 (42.9%) | 26 (56.5%) | |
| Days to MACCE | 115.0 [1.0, 260.0] | 19.0 [1.0, 301.0] | 123.5 [3.0, 254.0] | 0.750 |
| Major neurological dysfunction > 24 h | ||||
| Major neurological dysfunction overall | 22 (20.8%) | 5 (13.9%) | 17 (24.3%) | 0.211 |
| Major neurological dysfunction within 30 days | 10 (45.5%) | 4 (80.0%) | 6 (35.3%) | |
| Major neurological dysfunction after 30 days | 12 (54.6%) | 1 (20.0%) | 11 (64.7%) | |
| Days to major neurological dysfunction | 112.5 [1.0, 282.0] | 1.0 [1.0, 7.0] | 167.0 [1.0, 282.0] | 0.411 |
| Major bleeding | ||||
| Major bleeding overall | 44 (41.5%) | 16 (44.4%) | 28 (40.0%) | 0.660 |
| Major bleeding within 30 days | 22 (50.0%) | 10 (62.5%) | 12 (42.9%) | |
| Major bleeding after 30 days | 22 (50.0%) | 6 (37.5%) | 16 (57.1%) | |
| Days to major bleeding | 35.5 [3.5, 259.5] | 14.5 [1.0, 268.0] | 118.0 [5.5, 259.5] | 0.464 |
| Major device‐related infection excluding driveline | ||||
| Major device‐related infection excluding driveline overall | 5 (4.7%) | 2 (5.6%) | 3 (4.3%) | >0.99 |
| Major device‐related infection excluding driveline within 30 days | 1 (20.0%) | 0 (0%) | 1 (33.3%) | |
| Major device‐related infection excluding driveline after 30 days | 4 (80.0%) | 2 (100%) | 2 (66.7%) | |
| Days to major device‐related infection excluding driveline | 519.0 [354.0, 521.0] | 268.0 [354.0, 519.0] | 521.0 [10.0, 1977.0] | 0.800 |
| Major device malfunction, excluding pump thrombosis | ||||
| Major device malfunction overall | 23 (21.7%) | 3 (8.3%) | 20 (28.6%) | 0.017 |
| Major device malfunction within 30 days | 1 (4.4%) | 0 (0%) | 1 (5.0%) | |
| Major device malfunction after 30 days | 22 (95.7%) | 3 (100%) | 19 (95.0%) | |
| Days to major device malfunction | 391.0 [172.0, 784.0] | 612.0 [202.0, 784.0] | 328.0 [163.5, 863.5] | 0.635 |
| Pump thrombosis | ||||
| Pump thrombosis overall | 9 (8.5%) | 1 (2.8%) | 8 (11.4%) | 0.130 |
| Pump thrombosis within 30 days | 2 (22.2%) | 0 (0%) | 2 (25.0%) | |
| Pump thrombosis after 30 days | 7 (77.8%) | 1 (100%) | 6 (75.0%) | |
| Days to pump thrombosis | 233.0 [103.0, 526.0] | 1126.0 | 179.5 [53.0, 402.5] | 0.222 |
| Right heart failure | ||||
| RHF overall | 28 (26.4%) | 13 (36.1%) | 15 (21.4%) | 0.104 |
| RHF within 30 days | 5 (17.9%) | 2 (15.4%) | 3 (20.0%) | |
| RHF after 30 days | 23 (82.1%) | 11 (84.6%) | 12 (80.0%) | |
| Days to RHF | 199.0 [68.5, 331.0] | 223.0 [72.0, 330.0] | 189.0 [65.0, 332.0] | 0.892 |
| RHF‐related rehospitalization overall | 23 (21.7%) | 11 (30.6%) | 12 (17.1%) | 0.113 |
| RHF‐related rehospitalization within 30 days | 0 (0%) | 0 (0%) | 0 (0%) | |
| RHF‐related rehospitalization after 30 days | 23 (100%) | 11 (100%) | 12 (100%) | |
| Days to RHF‐related rehospitalizations | 223.0 [133.0, 350.0] | 252.0 [133.0, 350.0] | 212.0 [132.0, 473.5] | 0.976 |
| Driveline infection | ||||
| Driveline infection overall | 40 (37.7%) | 17 (47.2%) | 23 (32.9%) | 0.148 |
| Driveline infection within 30 days | 2 (5%) | 2 (11.8%) | 0 (0%) | |
| Driveline infection after 30 days | 38 (95%) | 15 (88.2%) | 23 (100%) | |
| Time to driveline infection | 198.0 [73.0, 325.5] | 131.0 [96.0, 250.0] | 199.0 [71.0, 339.0] | 0.665 |
| Driveline infection‐related surgical re‐interventions overall | 20 (18.9%) | 12 (33.3%) | 8 (11.4%) | 0.006 |
| Driveline infection‐related surgical re‐interventions within 30 days | 0 (0%) | 0 (0%) | 0 (0%) | |
| Driveline infection‐related surgical re‐intervention after 30 days | 20 (100%) | 12 (100%) | 8 (100%) | |
| Days to driveline infection‐related surgical re‐interventions | 226.5 [110.5, 458.5] | 233.5 [89.0, 512.0] | 187.0 [110.5, 423.0] | 0.851 |
| Gastrointestinal bleeding | ||||
| Major GI bleeding overall | 22 (20.8%) | 7 (19.4%) | 15 (21.4%) | 0.811 |
| GI bleeding within 30 days | 1 (4.6%) | 1 (14.3%) | 0 (0%) | |
| GI bleeding after 30 days | 21 (95.5%) | 6 (85.7%) | 15 (100%) | |
| Days to GI bleeding | 274.5 [159.0, 381.0] | 235.0 [36.0, 381.0] | 289.0 [161.0, 693.0] | 0.458 |
GI, gastrointestinal; MACCE, major device‐related adverse cardiac and cerebrovascular events; RHF, right heart failure.
Numeric variables are presented as median [quartiles] and were compared with Mann–Whitney U tests. Categorical variables are presented as numbers and percentages and were compared with Pearson's χ 2 test or Fisher's exact test as appropriate. Days to the respective endpoint, as well as the percentage within or after 30 days, are calculated only among those patients who actually had experienced the event. For outcomes in which multiple events are possible, numbers refer to the first episode. Percentages may not necessarily add up to 100% due to rounding error. MACCE: composite endpoint including any of the following events: (1) major neurological dysfunction > 24 h, (2) major bleeding, (3) major device‐related infection excluding driveline infections, (4) major device malfunction, and (5) pump thrombosis.
Figure 1.
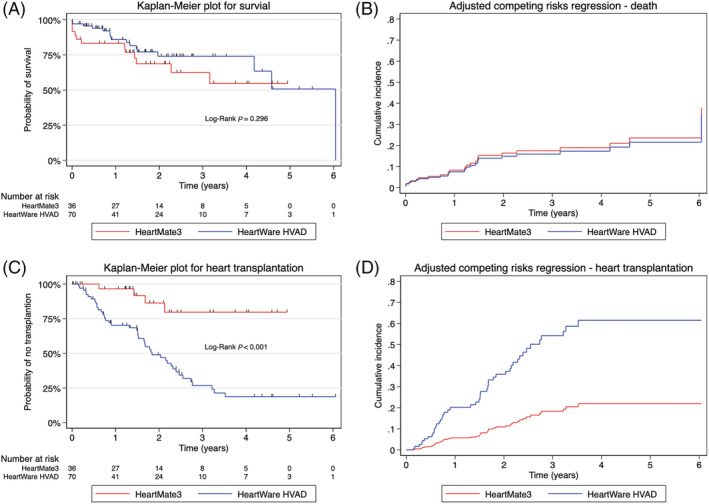
Kaplan–Meier plots and adjusted cumulative incidence functions (considering heart transplantation as competing risk for death and vice versa) for primary outcomes. Kaplan–Meier survival probability of primary outcomes of overall survival (A) and heart transplantation (C), and correlating competing risk analysis after adjusting for confounders, respectively (B, D). No significant difference was observed in overall survival between devices. Significant difference in transplant probability was observed in HeartWare HVAD in the adjusted and unadjusted model. The cumulative incidence functions for death (B) and heart transplantation (D) are typically displayed.
Table 3.
Time‐to‐event analysis results
| Outcome | Effect size [95% CI] | P‐value |
|---|---|---|
| Death | ||
| Unadjusted Cox regression | 0.67 [0.31, 1.43] | 0.300 |
| Adjusted Cox regression | 0.97 [0.43, 2.21] | 0.949 |
| Unadjusted competing risks regression | 0.51 [0.24, 1.08] | 0.077 |
| Adjusted competing risks regression | 0.90 [0.41, 1.99] | 0.801 |
| Heart transplantation | ||
| Unadjusted Cox regression | 5.94 [2.13, 16.54] | 0.001 |
| Adjusted Cox regression | 3.56 [1.21, 10.42] | 0.021 |
| Unadjusted competing risks regression | 6.73 [2.40, 18.85] | <0.001 |
| Adjusted competing risks regression | 3.85 [1.34, 11.07] | 0.012 |
| MACCE | ||
| Unadjusted Cox regression | 1.55 [0.90, 2.69] | 0.115 |
| Adjusted Cox regression | 1.78 [1.00, 3.18] | 0.051 |
| Unadjusted competing risks regression | 1.11 [0.67, 1.85] | 0.675 |
| Adjusted competing risks regression | 1.47 [0.88, 2.45] | 0.143 |
| Unadjusted recurrent events regression | 1.33 [0.75, 2.36] | 0.324 |
| Adjusted recurrent events regression | 1.61 [0.88, 2.96] | 0.122 |
| Major neurological dysfunction > 24 h | ||
| Unadjusted Cox regression | 1.91 [0.70, 5.19] | 0.203 |
| Adjusted Cox regression | 1.96 [0.69, 5.61] | 0.207 |
| Unadjusted competing risks regression | 1.74 [0.64, 4.76] | 0.278 |
| Adjusted competing risks regression | 1.91 [0.64, 5.73] | 0.247 |
| Unadjusted recurrent events regression | 1.73 [0.63, 4.73] | 0.285 |
| Adjusted recurrent events regression | 1.76 [0.60, 5.19] | 0.303 |
| Major bleeding | ||
| Unadjusted Cox regression | 1.01 [0.54, 1.90] | 0.970 |
| Adjusted Cox regression | 1.28 [0.65, 2.51] | 0.477 |
| Unadjusted competing risks regression | 0.80 [0.43, 1.46] | 0.463 |
| Adjusted competing risks regression | 1.13 [0.61, 2.09] | 0.706 |
| Unadjusted recurrent events regression | 0.89 [0.45, 1.76] | 0.742 |
| Adjusted recurrent events regression | 1.16 [0.61, 2.20] | 0.651 |
| Major device‐related infection excluding driveline | ||
| Unadjusted Cox regression | 0.54 [0.08, 3.83] | 0.534 |
| Adjusted Cox regression | 0.60 [0.07, 5.38] | 0.649 |
| Unadjusted competing risks regression | 0.59 [0.10, 3.37] | 0.553 |
| Adjusted competing risks regression | 1.27 [0.19, 8.66] | 0.808 |
| Unadjusted recurrent events regression | 0.37 [0.06, 2.41] | 0.295 |
| Adjusted recurrent events regression | 0.41 [0.07, 2.27] | 0.309 |
| Major device malfunction excluding pump thrombosis | ||
| Unadjusted Cox regression | 3.87 [1.14, 13.12] | 0.030 |
| Adjusted Cox regression | 4.54 [1.30, 15.80] | 0.017 |
| Unadjusted competing risks regression | 3.39 [1.02, 11.25] | 0.047 |
| Adjusted competing risks regression | 4.66 [1.40, 15.49] | 0.012 |
| Unadjusted recurrent events regression | 6.31 [1.92, 20.78] | 0.002 |
| Adjusted recurrent events regression | 6.49 [1.89, 22.32] | 0.003 |
| Pump thrombosis | ||
| Unadjusted Cox regression | 4.57 [0.57, 36.71] | 0.153 |
| Adjusted Cox regression | 4.65 [0.55, 38.98] | 0.157 |
| Unadjusted competing risks regression | 4.01 [0.49, 32.49] | 0.194 |
| Adjusted competing risks regression | 4.60 [0.51, 41.22] | 0.173 |
| Unadjusted recurrent events regression | 4.07 [0.54, 30.63] | 0.173 |
| Adjusted recurrent events regression | 4.24 [0.45, 39.85] | 0.207 |
| Right heart failure | ||
| Unadjusted Cox regression | 0.55 [0.26, 1.16] | 0.117 |
| Adjusted Cox regression | 0.70 [0.32, 1.55] | 0.380 |
| Unadjusted competing risks regression | 0.50 [0.24, 1.06] | 0.070 |
| Adjusted competing risks regression | 0.72 [0.33, 1.59] | 0.417 |
| Unadjusted recurrent events regression | 0.78 [0.36, 1.72] | 0.546 |
| Adjusted recurrent events regression | 1.04 [0.47, 2.30] | 0.924 |
| Right heart failure‐related rehospitalization | ||
| Unadjusted Cox regression | 0.53 [0.23, 1.19] | 0.123 |
| Adjusted Cox regression | 0.83 [0.36, 1.93] | 0.669 |
| Unadjusted competing risks regression | 0.48 [0.21, 1.08] | 0.076 |
| Adjusted competing risks regression | 0.86 [0.37, 2.01] | 0.734 |
| Unadjusted recurrent events regression | 0.81 [0.35, 1.85] | 0.612 |
| Adjusted recurrent events regression | 1.16 [0.49, 2.73] | 0.729 |
| Driveline infection | ||
| Unadjusted Cox regression | 0.60 [0.32, 1.13] | 0.115 |
| Adjusted Cox regression | 0.41 [0.19, 0.88] | 0.021 |
| Unadjusted competing risks regression | 0.59 [0.32, 1.1] | 0.096 |
| Adjusted competing risks regression | 0.42 [0.19, 0.93] | 0.032 |
| Unadjusted recurrent events regression | 0.64 [0.33, 1.25] | 0.192 |
| Adjusted recurrent events regression | 0.56 [0.26, 1.22] | 0.146 |
| Driveline infection‐related surgical re‐intervention | ||
| Unadjusted Cox regression | 0.31 [0.13, 0.77] | 0.011 |
| Adjusted Cox regression | 0.24 [0.08, 0.71] | 0.010 |
| Unadjusted competing risks regression | 0.29 [0.12, 0.70] | 0.006 |
| Adjusted competing risks regression | 0.21 [0.07, 0.65] | 0.007 |
| Unadjusted recurrent events regression | 0.58 [0.19, 1.76] | 0.337 |
| Adjusted recurrent events regression | 0.46 [0.11, 1.87] | 0.277 |
| Gastrointestinal bleeding | ||
| Unadjusted Cox regression | 1.28 [0.51, 3.17] | 0.598 |
| Adjusted Cox regression | 2.10 [0.79, 5.58] | 0.135 |
| Unadjusted competing risks regression | 0.99 [0.41, 2.40] | 0.979 |
| Adjusted competing risks regression | 1.79 [0.74, 4.34] | 0.200 |
| Unadjusted recurrent events regression | 0.78 [0.26, 2.40] | 0.671 |
| Adjusted recurrent events regression | 0.88 [0.26, 2.98] | 0.837 |
CI, confidence interval; MACCE, major device‐related adverse cardiac and cerebrovascular events.
Time‐to‐event analysis results for primary and secondary outcomes. Effect sizes are hazard ratios (Cox regression and recurrent events regression) or subdistribution hazard ratios (competing risks analysis), with HeartMate3 as the reference category. An effect size > 1 thus indicates a higher risk for the HeartWare device compared with HeartMate3 (i.e. favours HeartMate3), whereas an effect size < 1 indicates a lower risk (i.e. favours HeartWare). The proportional hazards assumption was satisfied for all outcomes except pump thrombosis. Adjusted analyses adjust for patient age and body mass index at the time of device implantation, gender, and Interagency Registry for Mechanically Assisted Circulatory Support score 1–3 versus 4–7.
With a higher distribution of DT candidates supported by HM3, only 1 in 10 patients supported by HM3 received heart transplantation, with a median waiting time of 566.5 days [372.5, 695.5]. In contrast, 61.4% of patients supported by HW received heart transplants, with a median waiting time of 551 days [209, 799]. The statistically significant higher transplantation rate in the HW group (P < 0.001; Figure 1 C ) continued to be observed after accounting for competing risks (Figure 1 D ), as well as when only BTT candidates were analysed (Supporting Information, Figure S1 , Table S2 ). One HW patient received device explantation due to myocardial recovery.
The composite of MACCE occurred in a majority of patients with both devices (58.3% in HM3 vs. 65.7% in HW, P = 0.456), with shorter time to the first MACCE in HM3 patients (19 days [1, 301] vs. 123.5 days [3, 254], P = 0.750), and higher incidence of early post‐operative MACCE at POD 30 in the HM3 group (57.1% vs. 43.5%). No evidence of difference between devices was observed in risk of composite MACCE either as first or as recurrent event (Table 2 , Figure 2 ), although freedom from MACCE was higher in HM3 (Figures 3A, 3B , Table 2 ).
Figure 2.
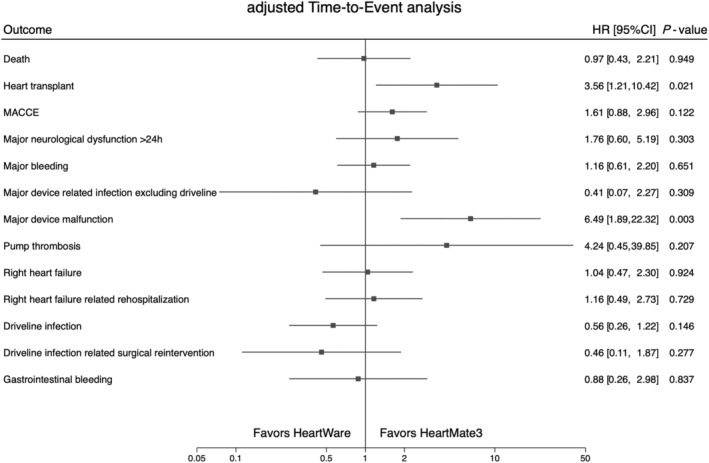
Forest plot analysis of primary and secondary outcomes by device type. Time‐to‐event analysis for primary and secondary outcomes. Primary outcomes were analysed by Cox proportional hazards regression. Secondary outcomes can recur and were analysed with a modification of Cox proportional hazards regression suggested by Andersen and Gill. All analyses were adjusted for patient age and body mass index at the time of device implantation, gender, and Interagency Registry for Mechanically Assisted Circulatory Support score 1–3 versus 4–7. CI, confidence interval; HR, hazard ratio; MACCE, major device‐related adverse cardiac and cerebrovascular events.
Figure 3.
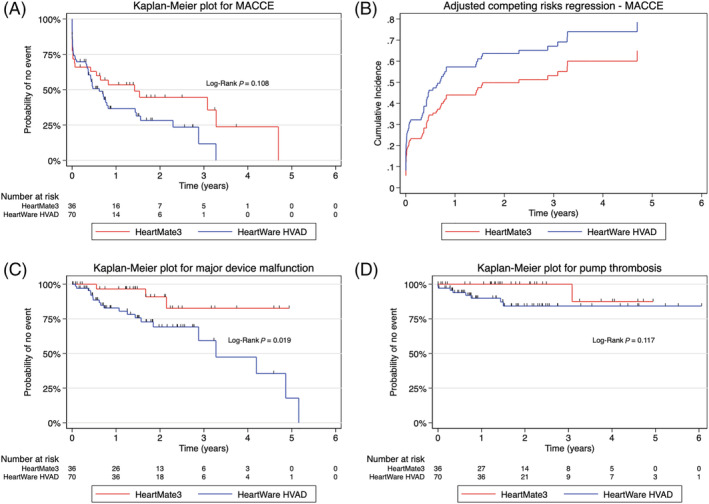
Kaplan–Meier plots for secondary outcomes and adjusted cumulative incidence functions for secondary outcomes. Kaplan–Meier freedom‐from‐event analysis for secondary outcomes: composite of MACCE (A), major device malfunction excluding pump thrombosis (C), and pump thrombosis (D). Competing risk analysis for composite of MACCE after adjusting for confounders is displayed in Panel (B). The cumulative incidence functions for MACCE are typically displayed. MACCE, major device‐related adverse cardiac and cerebrovascular events.
Severe neurological dysfunction occurred in 13.9% of HM3 patients and in 24.3% of HW patients (P = 0.211; Table 2 ). Major bleeding of any cause was observed in over 40% of patients in both devices (44.4% vs. 40.0%, P = 0.660; Table 2 ), and the first major bleeding occurred later in the HW group (14.5 days [1.0, 268] vs. 118 days [5.5, 295.5], P = 0.464). No significant difference was observed for recurrent major bleedings (aHR 1.16, 95% CI [0.61, 2.20], P = 0.651) or for recurrent GI bleedings (aHR 0.88, 95% CI [0.26, 2.98], P = 0.837), with same anticoagulation therapy in both devices. Rate of GI bleeding was similar for the two devices (19.4% vs. 21.4%, P = 0.811). Device‐related infections excluding driveline were rare for both devices (5.6% vs. 4.3%, P > 0.99), but driveline infections were observed significantly more often for HM3 (47.2% vs. 32.9%, P = 0.148; aSHR 0.42, 95% CI [0.19, 0.93], P = 0.032). However, when analysing for recurrent driveline infections, the significance between devices vanishes (aHR 0.56, 95% CI [0.26, 1.22], P = 0.146). There was no significant difference in median time to first driveline infection (131 days [96, 250] vs. 199 days [71, 339], P = 0.665). Almost three‐fold more surgical driveline revisions due to infection were performed in HM3 (33.3% vs. 11.4%, P = 0.006; aHR 0.21, 95% CI [0.07, 0.65], P = 0.007), but the significance again disappeared in the recurrent event analysis (aHR 0.46, 95% CI [0.11, 1.87], P = 0.277).
Major device malfunctions excluding pump thrombosis were significantly more frequent with the HW (28.6% vs. 8.3%, P = 0.017; aHR 4.66, 95% CI [1.40, 15.49], P = 0.012) and occurred later (612 days [202, 784] vs. 328 days [163.5, 863.5], P = 0.635). Moreover, when analysing recurrent device malfunctions, HW was associated with a significant six‐fold increase in risk (aHR 6.49, 95% CI [1.89, 22.32], P = 0.003). Pump thrombosis occurred in 11.4% of HW patients, and one HM3 patient (2.8%) had an outflow‐graft twist with resulting thrombosis (P = 0.130); however, no significant difference between the devices was observed for pump thrombosis. Median time to pump thrombosis in HW was 179.5 days [53, 402.5]. No significant difference for pump thrombosis was shown between devices in competing risk analysis (aHR 4.60, 95% CI [0.51, 41.22], P = 0.173).
No significant difference between devices was observed in occurrence of initial (aSHR 0.72, 95% CI [0.33, 1.59], P = 0.417) or recurrent RHF (aHR 1.04, 95% CI [0.47, 2.30], P = 0.924), although RHF occurred in 36.1% of HM3 patients, compared with 21.4% of HW patients (P = 0.104). More rehospitalizations with RHF were observed for HM3 (30.6% vs. 17.1%, P = 0.113). Further results are summarized in Tables 2 and 3 , as well as in Supporting Information, Figures S1 – S3 .
Discussion
The authors present the institution's uncensored experience from a prospective registry of all implanted HM3 and HW devices for primary LVAD support over the past 10 years. No significant differences in overall survival rates between the devices were found after adjusting for the confounders age, gender, BMI, and INTERMACS level. A 20% higher but non‐significant probability of mortality was observed in patients supported by the HM3 at 4 years in the unadjusted model, but the HM3 patients were older and had more comorbidities. An important observation is the significant difference in major device malfunctions (excluding pump thrombosis) between devices, with HW associated with a six‐fold risk increase in recurrent events (P = 0.003), and HM3 having over 20% less device malfunction events compared with HW (P = 0.017). Even though pump thrombosis occurred in ~1 out of 10 HW patients, we observed no evidence of a statistically significant risk increase for HW. With respect to composite of MACCE, as well as major bleeding, GI bleeding, stroke, major infection, driveline infection, and RHF, no significant difference was found between devices, confirming existing data. 4 , 5 , 8 , 9 , 10 , 16 , 17
While there was no evidence of difference in most clinical outcomes, the difference in major device‐related malfunctions stands out. A significantly elevated risk of device malfunctions in HW was consistently observed across the unadjusted and confounder‐adjusted analyses, with and without considering death and heart transplantation as competing risks, and when analysing the time to the first malfunction as well as recurrent events. Major device malfunctions included any severe malfunction of a device component leading to readmission, reoperation, or an exchange of the device component and may thus be considered clinically relevant. While the MOMENTUM and ENDURANCE trials demonstrated that device malfunctions have become less common with the magnetically levitating LVADs compared with axial‐flow pumps, 6 , 7 our results demonstrate that the elevated risk of device malfunctions excluding pump thrombosis in HW remains high.
Despite this risk in HW, overall survival did not statistically differ between devices and was comparable with survival from other studies at 1 and 2 years. 4 , 6 , 9 , 16 , 17 However, at 4 years, a drop of survival was found in HM3 patients to 54.7%. While the difference is not significant, it should be further investigated. Importantly, when adjusting for confounders, the mortality risk in both groups was not significantly different. This merely indicates that there is insufficient evidence to claim there is a difference but does not imply that devices are ‘equivalent’ or ‘similar’ with respect to these outcomes. These results support conclusions of the study published by Potapov et al. involving >1500 LVAD patients, where an increased risk for haemorrhagic stroke and pump thrombosis was found in HW, but no evident difference was observed in overall survival between the devices. 17 A recent study by Pagani et al. however observed a significantly elevated mortality in patients supported by HW at both 12 and 24 months after implant. 18 While our study fails to differentiate between the haemorrhagic and ischaemic stroke, and other studies did not differentiate between pump thrombosis and other major device malfunctions that led to device exchange or death, all suggest an elevated risk profile in HW. However, to our best knowledge, no studies comparing both devices have analysed for recurrent events, and no studies have differentiated between device malfunctions that excluded pump thrombosis and other major device malfunctions. 8 , 9 , 17 , 18
It is worth mentioning that our LVAD population differs from those in other studies by primarily having INTERMACS stages >3 in both devices. Despite these non‐significant differences, HM3 was associated with higher freedom from composite of MACCE, neurological dysfunction, and pump thrombosis but had lower freedom from RHF, RHF‐related rehospitalizations, driveline infections, and driveline infection‐related surgical re‐interventions. Our study observed an elevated incidence of stroke and of pump thrombosis in patients supported by HW; however, these risks were not significant in the adjusted models and in competing risks analyses. While our data thus do not provide strong evidence for an association, there may be a possible association between HW and stroke or pump thrombosis. This is in line with the results published by others, where HW has been associated with significantly elevated risks for stroke and pump thrombosis. It is known from the prospective MOMENTUM and ELEVATE trials that HM3 and HW have lower rates of pump thrombosis compared with HeartMate II 6 , 7 and that the low 2.3% incidence of pump thrombosis with HM3 is consistent with results reported elsewhere. 4 , 9 , 16 , 17 There appears to be fewer driveline infections after adjustment for potential confounders in the HW group; however, this only holds true when the first event is considered. Numan et al. observed a similar trend towards higher driveline infection rates with HM3 but also found no statistically significant difference. 9 It is worth mentioning that our study differs from the study by Numan et al. 9 as our data were taken from a prospective registry that was analysed not only for the primary event but also for recurrent ones. In the recurrent events analysis, the significance vanishes. Therefore, while there is some evidence that HW is associated with a lower risk of initial driveline infections, there is no evidence that it is associated with a lower risk of driveline infections overall. This should be investigated further in a larger population.
While transplantation was significantly more frequent following HW implantation, this difference should be interpreted carefully. The median waiting time was just under 2 years for both devices, but more transplantations were performed after HW support, also due to longer institutional experience and patient follow‐up with HW. As our HW patients were significantly younger, were more often female, had lower BMI, and more frequently (albeit not significantly) had a lower INTERMACS score, they were more often intended for transplantation. Concurrently, as the durability of HM3 became evident through initial non‐inferiority studies, our patients intended for DT more often received HM3. Another major consideration should be given to the difference in pump size, which reflects on the patient selection. The HW pump is smaller than the HM3 and is often preferred in smaller patients such as young adolescents and patients with smaller BSA and BMI. This was observed in our HW cohort, in which the patients were significantly younger and had a lower BMI. We aimed to account for such differences by adjusting the statistical analysis for BMI as well as other potential confounders. These differences in demographics are important, as our institutional or surgeon's bias is not reflected in the transplant probability analysis, which only include BTT candidates (Supporting Information, Figure S1 , Table S2 ).
Our study is subject to the inherent limitations of observational research, in particular, potential confounding due to systemic baseline differences between the HW and HM3 groups, inherently leading to possible differences in respective outcomes. An important limitation is the possible time bias and sample size. As the HW was implanted since 2010, and the HM3 since 2015, there is a potential time bias in patient selection and overall device experience. By 2016, the devices were implanted in 1:1 ratio and have since then shifted towards implanting more HM3 devices over the years. As the smaller size of the HW pump might be preferred by the surgeon in patients with smaller chest, such as young adolescents and patients with smaller BSA and BMI, we never fully transitioned to HM3 only. This is reflected by our data where HW patients were significantly younger and had a lower BMI. While we adjusted the analysis for potential confounders (age, gender, BMI, and INTERMACS score) and thus correct for these differences between the device groups in the analysis, we were limited in our ability to control for other confounding such as era analysis more rigorously due to the limited sample size and event rates. Hence, as generally true in observational research, our results do not support causal inferences or give conclusions and recommendations but should instead be interpreted in terms of associations. However, information bias, selection bias, and type‐I error were minimized by the prospective design of the registry, which included all VAD patients treated at our institution, over a long follow‐up period. It is important to notice that the specialized care team for VAD patients remained largely consistent throughout the years. Healthcare providers entering data into the registry had been trained in the proper use of the database, and manual (MM) as well as plausibility checks (PS) were performed to identify and correct potentially erroneous entries. Because this study was based on all available patients, a formal a priori sample size and power calculation was not performed, and results should be confirmed in a larger patient cohort.
In conclusion, our comparison of prospectively collected data on midterm outcomes of two major CF‐LVAD devices—the HM3 and the HW—reveals a significantly higher risk of device malfunction in HW. No difference in overall survival between patients under HM3 or HW support was noted, and no evidence of differences was observed between devices with respect to most clinical outcomes. Multicentre studies with longer observational periods or prospective design are needed to investigate this further.
Amendment
This manuscript was submitted after 3 June 2021, when Medtronic stopped the distribution and sale of the HVAD system after a Class I recall by the FDA on 3 June 2021 to stop new implants of the Medtronic HVAD system, due to reports of patient injuries and deaths associated with device malfunctions. 19 In fact, Medtronic has ‘received over 100 complaints involving delay or failure to restart of the HW pump, including reports of 14 patient deaths and 13 cases where an explant was necessary’. 20 We would like to clarify that such kind of device malfunctions were not observed in our cohort. As the smaller size of the HW pump was preferred by the surgeon in patients with smaller chest, such as young adolescents and patients with smaller BSA and BMI, our centre did not fully transition to HM3 only. This is reflected by our data, where HW patients were significantly younger and had a lower BMI. The withdrawal of the HW pump will probably have a significant impact on the paediatric and adolescent field. 21
Conflict of interest
J.C.S. reports (full departmental disclosure) departmental grants outside of the submitted work from Orion Pharma, Abbott Nutrition International, Braun Medical AG, CSEM AG, Edwards Lifesciences Services GmbH, Kenta Biotech Ltd, Maquet Critical Care AB, Omnicare Clinical Research AG, Nestle, Pierre Fabre Pharma AG, Pfizer, Bard Medica S.A., Abbott AG, Anandic Medical Systems, Pan Gas AG Healthcare, Bracco, Hamilton Medical AG, Fresenius Kabi, Getinge Group Maquet AG, Dräger AG, Teleflex Medical GmbH, Glaxo Smith Kline, Merck Sharp and Dohme AG, Eli Lilly and Company, Baxter, Astellas, Astra Zeneca, CSL Behring, Novartis, Covidien, and Nycomed. The money was paid into departmental funds, and there was no personal financial gain. P.M. reports consulting for Abbott outside of the submitted work. D.R. reports proctoring for Abbott HM3, has previously received paid travel expenses by Edwards, Medtronic, and Abbott, and is the board member of Swiss Society of Cardiac Surgery. All authors report no conflicts of interest, and the presented study did not receive any funding support.
Funding
None.
Supporting information
Table S1. Baseline variables investigated. Legend: body surface area (BSA), body mass index (BMI), arterial hypertension (HTN), pulmonary hypertension (pulmonary HTN), diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD), renal disease (CKD), atrial fibrillation (AF), peripheral vascular disease (PVD), carotid artery disease (CAD), Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) status, white blood cells (WBC), international normalized ratio (INR), blood urea nitrogen (BUN), aspartate transaminase (AST), alanine transaminase (ALT), arterial pressure (AP), systolic (s), diastolic (d), pulmonary artery pressure (PAP), pulmonary capillary wedge pressure (PCWP), systemic vascular resistance (SVR), pulmonary vascular resistance (PVR), cardiac index (CI), cardiac output (CO), left ventricular ejection fraction (LVEF), left ventricular end‐diastolic diameter (LVEDD), right ventricular (RV) function, tricuspid annular plane systolic excursion (TAPSE), aortic regurgitation (AR), mitral regurgitation (MR), tricuspid regurgitation (TR), cardiopulmonary bypass (CPB), operating room (OR).
Table S2. Analysis of outcome for heart transplantation, restricted to BTT patients (n = 83).
Figure S1. Kaplan–Meier curve analysis showing probability of no transplantation to BTT patients (n = 83).
Figure S2. Freedom‐from‐event analysis of secondary outcomes. Kaplan–Meier freedom‐from‐event analysis for secondary outcomes: major neurologic dysfunction >24 h (A), major bleeding (B), major device‐related infection excluding driveline infections (C) and gastrointestinal (GI) bleeding (D). No statistical significance was observed throughout between devices in respect to these outcomes.
Figure S3. Freedom‐from‐event analysis of secondary outcomes. Kaplan–Meier freedom‐from‐event analysis for secondary outcomes: right‐heart failure (RHF) (A), RHF‐related rehospitalization (B), driveline infections (C), and surgical reinterventions due to driveline infections (D). Driveline‐infection‐related surgical reinterventions were significantly more likely to occur in HeartMate 3 patients (P = 0.006), but no statistical significance was observed between devices in respect to other outcomes.
Acknowledgements
The authors would like to thank the past and present team members of the Center for Advanced Heart Failure at University Hospital Bern for their comments and intellectual contributions to this manuscript.
Mihalj, M. , Heinisch, P. P. , Schober, P. , Wieser, M. , Martinelli, M. , de By, T. M. M. H. , Schefold, J. C. , Luedi, M. M. , Kadner, A. , Carrel, T. , Mohacsi, P. , Hunziker, L. , and Reineke, D. (2022) Third‐generation continuous‐flow left ventricular assist devices: a comparative outcome analysis by device type. ESC Heart Failure, 9: 3469–3482. 10.1002/ehf2.13794.
References
- 1. Kormos RL, Cowger J, Pagani FD, Teuteberg JJ, Goldstein DJ, Jacobs JP, Higgins RS, Stevenson LW, Stehlik J, Atluri P, Grady KL, Kirklin JK. The Society of Thoracic Surgeons Intermacs database annual report: evolving indications, outcomes, and scientific partnerships. J Heart Lung Transplant 2019; 38: 114–126. [DOI] [PubMed] [Google Scholar]
- 2. Teuteberg JJ, Cleveland JC Jr, Cowger J, Higgins RS, Goldstein DJ, Keebler M, Kirklin JK, Myers SL, Salerno CT, Stehlik J, Fernandez F, Badhwar V, Pagani FD, Atluri P. The Society of Thoracic Surgeons Intermacs 2019 annual report: the changing landscape of devices and indications. Ann Thorac Surg 2020; 109: 649–660. [DOI] [PubMed] [Google Scholar]
- 3. Molina EJ, Shah P, Kiernan MS, Cornwell WK 3rd, Copeland H, Takeda K, Fernandez FG, Badhwar V, Habib RH, Jacobs JP, Koehl D, Kirklin JK, Pagani FD, Cowger JA. The Society of Thoracic Surgeons Intermacs 2020 annual report. Ann Thorac Surg 2021; 111: 778–792. [DOI] [PubMed] [Google Scholar]
- 4. Zimpfer D, Gustafsson F, Potapov E, Pya Y, Schmitto J, Berchtold‐Herz M, Morshuis M, Shaw SM, Saeed D, Lavee J, Heatley G, Gazzola C, Garbade J. Two‐year outcome after implantation of a full magnetically levitated left ventricular assist device: results from the ELEVATE registry. Eur Heart J 2020; 41: 3801–3809. [DOI] [PubMed] [Google Scholar]
- 5. Goldstein DJ, Naka Y, Horstmanshof D, Ravichandran AK, Schroder J, Ransom J, Itoh A, Uriel N, Cleveland JC Jr, Raval NY, Cogswell R, Suarez EE, Lowes BD, Kim G, Bonde P, Sheikh FH, Sood P, Farrar DJ, Mehra MR. Association of clinical outcomes with left ventricular assist device use by bridge to transplant or destination therapy intent: the multicenter study of MagLev Technology in patients undergoing mechanical circulatory support therapy with HeartMate 3 (MOMENTUM 3) randomized clinical trial. JAMA Cardiol 2020; 5: 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mehra MR, Uriel N, Naka Y, Cleveland JC Jr, Yuzefpolskaya M, Salerno CT, Walsh MN, Milano CA, Patel CB, Hutchins SW, Ransom J, Ewald GA, Itoh A, Raval NY, Silvestry SC, Cogswell R, John R, Bhimaraj A, Bruckner BA, Lowes BD, Um JY, Jeevanandam V, Sayer G, Mangi AA, Molina EJ, Sheikh F, Aaronson K, Pagani FD, Cotts WG, Tatooles AJ, Babu A, Chomsky D, Katz JN, Tessmann PB, Dean D, Krishnamoorthy A, Chuang J, Topuria I, Sood P, Goldstein DJ. A fully magnetically levitated left ventricular assist device—final report. N Engl J Med 2019; 380: 1618–1627. [DOI] [PubMed] [Google Scholar]
- 7. Rogers JG, Pagani FD, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, Boyce SW, Najjar SS, Jeevanandam V, Anderson AS, Gregoric ID, Mallidi H, Leadley K, Aaronson KD, Frazier OH, Milano CA. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med 2017; 376: 451–460. [DOI] [PubMed] [Google Scholar]
- 8. Mueller M, Hoermandinger C, Richter G, Mulzer J, Tsyganenko D, Krabatsch T, Starck C, Stein J, Schoenrath F, Falk V, Potapov E. Retrospective 1‐year outcome follow‐up in 200 patients supported with HeartMate 3 and HeartWare left ventricular assist devices in a single centre. Eur J Cardio‐thorac Surg 2020; 57: 1160–1165. [DOI] [PubMed] [Google Scholar]
- 9. Numan L, Ramjankhan FZ, Oberski DL, Oerlemans M, Aarts E, Gianoli M, van der Heijden JJ, de Jonge N, van der Kaaij NP, Meuwese CL, Mokhles MM, Oppelaar AM, de Waal EEC, Asselbergs FW, van Laake LW. Propensity score‐based analysis of long‐term outcome of patients on HeartWare and HeartMate 3 left ventricular assist device support. ESC Heart Fail 2021; 8: 1596–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Itzhaki Ben Zadok O, Ben‐Avraham B, Shaul A, Hammer Y, Rubachevs.ki V, Aravot D, Kornowski R, Ben‐Gal T. An 18‐month comparison of clinical outcomes between continuous‐flow left ventricular assist devices. Eur J Cardio‐thorac Surg 2019; 56: 1054–1061. [DOI] [PubMed] [Google Scholar]
- 11. Schober P, Vetter TR. Nonparametric statistical methods in medical research. Anesth Analg 2020; 131: 1862–1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schober P, Vetter TR. Chi‐square tests in medical research. Anesth Analg 2019; 129: 1193. [DOI] [PubMed] [Google Scholar]
- 13. Schober P, Vetter TR. Survival analysis and interpretation of time‐to‐event data: the tortoise and the hare. Anesth Analg 2018; 127: 792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schober P, Vetter TR. Confounding in observational research. Anesth Analg 2020; 130: 635. [DOI] [PubMed] [Google Scholar]
- 15. Schober P, Vetter TR. Adjustments for multiple testing in medical research. Anesth Analg 2020; 130: 99. [DOI] [PubMed] [Google Scholar]
- 16. Colombo PC, Mehra MR, Goldstein DJ, Estep JD, Salerno C, Jorde UP, Cowger JA, Cleveland JC Jr, Uriel N, Sayer G, Skipper ER, Downey FX, Ono M, Hooker R Jr, Anyanwu AC, Givertz MM, Mahr C, Topuria I, Somo SI, Crandall DL, Horstmanshof DA. Comprehensive analysis of stroke in the long‐term cohort of the MOMENTUM 3 study. Circulation 2019; 139: 155–168. [DOI] [PubMed] [Google Scholar]
- 17. Potapov EV, Nersesian G, Lewin D, Özbaran M, de By TMMH, Stein J, Pya Y, Gummert J, Ramjankhan F, Zembala MO, Damman K, Carrel T, Meyns B, Zimpfer D, Netuka I. Propensity score‐based analysis of long‐term follow‐up in patients supported with durable centrifugal left ventricular assist devices: the EUROMACS analysis. Eur J Cardio‐thorac Surg 2021; 60: 579–587. [DOI] [PubMed] [Google Scholar]
- 18. Pagani FD, Cantor R, Cowger J, Goldstein D, Teuteberg J, Mahr C, Atluri P, Kilic A, Maozami N, Habib R, Naftel D, Kirklin JK. Concordance of treatment effect: an analysis of the Society of Thoracic Surgeons Intermacs database. Ann Thorac Surg. 2021:S0003–4975(21)00929–2. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19. FDA Class I recall for Medtronic HVAD system. Stop New Implants of the Medtronic HVAD System—Letter to Health Care Providers. June 3, 2021. https://www.fda.gov/medical‐devices/letters‐health‐care‐providers/stop‐new‐implants‐medtronic‐hvad‐system‐letter‐health‐care‐providers. (Accessed Oct 16 2021).
- 20. Medtronic Press Release. Urgent Medical Device Communication Notification Letter Medtronic HVAD™ System. June 3, 2021. https://www.medtronic.com/content/dam/medtronic‐com/global/HCP/Documents/hvad‐urgent‐medical‐device‐notice‐june‐2021.pdf (Accessed Oct 16 2021).
- 21. Deshpande SR, Slepian MJ, Alsoufi B. HeartWare HVAD market withdrawal and impact on the pediatric field. ASAIO J 2021; 67: 825–826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline variables investigated. Legend: body surface area (BSA), body mass index (BMI), arterial hypertension (HTN), pulmonary hypertension (pulmonary HTN), diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD), renal disease (CKD), atrial fibrillation (AF), peripheral vascular disease (PVD), carotid artery disease (CAD), Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) status, white blood cells (WBC), international normalized ratio (INR), blood urea nitrogen (BUN), aspartate transaminase (AST), alanine transaminase (ALT), arterial pressure (AP), systolic (s), diastolic (d), pulmonary artery pressure (PAP), pulmonary capillary wedge pressure (PCWP), systemic vascular resistance (SVR), pulmonary vascular resistance (PVR), cardiac index (CI), cardiac output (CO), left ventricular ejection fraction (LVEF), left ventricular end‐diastolic diameter (LVEDD), right ventricular (RV) function, tricuspid annular plane systolic excursion (TAPSE), aortic regurgitation (AR), mitral regurgitation (MR), tricuspid regurgitation (TR), cardiopulmonary bypass (CPB), operating room (OR).
Table S2. Analysis of outcome for heart transplantation, restricted to BTT patients (n = 83).
Figure S1. Kaplan–Meier curve analysis showing probability of no transplantation to BTT patients (n = 83).
Figure S2. Freedom‐from‐event analysis of secondary outcomes. Kaplan–Meier freedom‐from‐event analysis for secondary outcomes: major neurologic dysfunction >24 h (A), major bleeding (B), major device‐related infection excluding driveline infections (C) and gastrointestinal (GI) bleeding (D). No statistical significance was observed throughout between devices in respect to these outcomes.
Figure S3. Freedom‐from‐event analysis of secondary outcomes. Kaplan–Meier freedom‐from‐event analysis for secondary outcomes: right‐heart failure (RHF) (A), RHF‐related rehospitalization (B), driveline infections (C), and surgical reinterventions due to driveline infections (D). Driveline‐infection‐related surgical reinterventions were significantly more likely to occur in HeartMate 3 patients (P = 0.006), but no statistical significance was observed between devices in respect to other outcomes.


