Abstract
Aim
Randomized controlled trials comparing the use of the MitraClip device in addition to guideline directed medical therapy (GDMT) to GDMT alone in patients with secondary mitral regurgitation (MR) have shown conflicting results. However, if these differences could be due to the underlying MR aetiology is still unknown. Therefore, we aimed to evaluate if the effects of percutaneous edge‐to‐edge repair with MitraClip implantation could differ in patients with ischaemic (I‐MR) and non‐ischaemic mitral regurgitation (NI‐MR).
Methods and results
PubMed, Embase, BioMed Central, and the Cochrane Central Register of Controlled Trials were searched for all studies including patients with secondary MR treated with the MitraClip device. Data were pooled using a random‐effects model. Primary endpoint was the composite of all‐cause death and heart failure‐related hospitalization. Secondary endpoints were the single components of the primary endpoint, New York Heart Association functional Classes III and IV, and mitral valve re‐intervention. Seven studies enrolling 2501 patients were included. Patients with I‐MR compared with patients with NI‐MR had a similar risk of the primary endpoint (odds ratio: 1.17; 95% confidence interval: 0.93 to 1.46; I 2: 0%). The risk of all‐cause death was increased in patients with I‐MR (odds ratio: 1.31; 95% confidence interval: 1.07 to 1.62; I 2: 0%), while no differences were observed between the two groups in terms of the other secondary endpoints.
Conclusions
The risk of mortality after MitraClip implantation is lower in patients with NI‐MR than in those with I‐MR. No absolute differences in the risk of heart failure related hospitalization were observed between groups.
Keywords: Secondary mitral regurgitation, Heart failure, Percutaneous edge‐to‐edge repair, MitraClip
Introduction
Mitral regurgitation (MR) due to left‐sided heart diseases has become the most common valve disorder in the developed countries. 1 , 2 It may arise as a consequence of left atrium enlargement and mitral annular dilatation in the presence of normal left ventricular size and function (atrial functional MR), or, most commonly, due to left ventricular remodelling and dilatation. 3
This latter form of secondary MR is present in up to 50% of patients affected by heart failure with reduced ejection fraction, 1 , 2 often with different underlying anatomical features among patients with ischaemic and non‐ischaemic MR (NI‐MR). 4 , 5 Indeed, while in patients with ischaemic MR (I‐MR), the main cause of MR is usually the tethering of the posterior leaflet associated with dyskinesia of the left ventricular posterolateral wall, resulting in predominant asymmetrical tethering, in NI‐MR a symmetrical tethering of both mitral valve leaflets is usually found, due to a global left ventricular remodelling and systolic dysfunction. Despite these differences, in both cases it is still debated if MR correction could effectively improve the prognosis of patients with heart failure. Mitral surgical repair and replacement have not shown to improve clinical outcomes or left ventricular function and dimension in the setting of secondary MR. 6 , 7
Accordingly, current European Society of Cardiology/European Association for Cardio‐Thoracic Surgery (ESC/EACTS) and American College of Cardiology/American Heart Association (AHA/ACC) guidelines on treatment of valvular heart disease recommend as first line of treatment for secondary MR the optimal heart failure medical therapy, including diuretics, beta‐blockers, angiotensin‐converting enzyme inhibitor/angiotensin receptor blocking agents, aldosterone antagonists, angiotensin receptor‐neprilysin inhibitor, and—if indicated—cardiac resynchronization therapy. 8 , 9
It is therefore uncertain if the severity of MR should be considered only as a marker of LV dysfunction or as an important target to improve the prognosis of these patients. 10 , 11 , 12 , 13 In this context, the MitraClip device (Abbott Vascular, Menlo Park, California, USA) has shown to be a safe and feasible option for patients with MR, 14 , 15 with significant improvement observed in patients with both primary and secondary MR. 16 , 17 Hence, indication for transcatheter mitral valve repair with the MitraClip device has been subsequently expanded for the treatment of secondary MR in high‐risk and inoperable patients, achieving the Food and Drug Administration approval in 2019.
Recently published randomized controlled trials (RCTs) comparing the use of the MitraClip device in addition to guideline directed medical therapy (GDMT) to GDMT alone in patients with secondary MR have shown conflicting results. 18 , 19 Only few studies have assessed if these differences could be due to the underlying secondary MR aetiology. 20 , 21 The aim of this study is to provide a comprehensive synthesis and quantitative assessment of evidence about efficacy and safety of percutaneous edge‐to‐edge repair with MitraClip device in patients with I‐MR or NI‐MR in terms of mortality and heart failure related hospitalization.
Methods
Search strategy and selection criteria
Randomized trials and observational studies including patients with secondary MR treated with the MitraClip device were evaluated for inclusion in this meta‐analysis. Eligible studies had to satisfy the following pre‐specified inclusion criteria: (i) studies reporting clinical data after percutaneous edge‐to‐edge repair with the MitraClip device; (ii) studies reporting outcomes stratified for secondary MR aetiology (i.e. ischaemic and non‐ischaemic). Exclusion criteria were as follows: (i) studies including less than 25 patients; (ii) studies with overlapping populations. No restrictions were applied for publication status.
Search strategy, study selection, data extraction, and data analysis were performed in accordance with the Cochrane Collaboration and the Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRISMA) guidelines. 22
On 15 April 2020, we searched PubMed, Embase, BioMed Central, and the Cochrane Central Register of Controlled Trials. In addition, we employed backward snowballing (i.e. review of references from identified articles and pertinent reviews). The search strategy is available in the Supporting information. This study is registered with PROSPERO, number CRD42018115060.
Data extraction
Three investigators (MC, FC, and DC) independently assessed studies for possible inclusion, with a senior investigator (GGS) resolving discrepancies. Non‐relevant articles were excluded based on title and abstract. The same investigators independently extracted data on study design, measurements, patient characteristics, and outcomes, using a standardized data‐extraction form. Data extraction conflicts were discussed and resolved with a senior investigator (GGS). In the case of studies with overlapping populations, only the manuscript reporting the largest number of patients was selected. We had access to individual patient level data from two out of seven of the included studies, and further missing data were requested by email to the corresponding author of each study. 20 , 21 , 23
Data about authors, year of publication, inclusion and exclusion criteria, sample size, baseline patients' features, endpoint definitions, effect estimates, and follow‐up time were collected.
Outcomes of interest
The prespecified primary efficacy endpoint was the composite of all‐cause death and heart failure‐related hospitalization. Secondary clinical endpoints were the single components of the primary endpoint, New York Heart Association (NYHA) functional Classes III and IV, and mitral valve re‐intervention (surgical or percutaneous). Secondary echocardiographic endpoints were mitral regurgitation grade ≥2+ at discharge and during follow‐up. Each endpoint was assessed at 2 years or the longest available follow‐up (in case of lack of 2 year follow‐up) and according to the definitions reported in the original study protocols, as summarized in the Table S1.
Risk of bias
The risk of bias in each study has been assessed using the revised Cochrane risk of bias tool (RoB 2.0) for RCTs and the Risk of Bias In Non‐randomized Studies of Interventions assessment Tool from Cochrane handbook (ROBINS‐I) for observational studies. 24 , 25 (Supporting information methods, Tables S2 and S3). The presence of publication bias was investigated with the Harbord test, and by visual estimation of funnel plots (Figures S1–S7).
Statistical analysis
Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using the DerSimonian and Laird random‐effects model, with the estimate of heterogeneity being taken from the Mantel–Haenszel method. The presence of heterogeneity among studies was evaluated with the Cochran Q χ 2 test, with P ≤ 0.10 considered of statistical significance, and using the I 2 test to evaluate inconsistency. Subgroup analyses for the primary endpoint were performed according to the study design (i.e. RCTs vs. observational studies). To assess the interaction between this potential effect modifier and treatment, a random‐effects meta‐regression analysis with the ‘empirical Bayes’ (Paule–Mandel) method was conducted (Supporting information methods).
We performed a leave one out sensitivity analysis for the primary endpoint by iteratively removing one study at a time to confirm that our findings were not driven by any single study. Further sensitivity analyses to evaluate the effects of underlying MR aetiology on clinical outcomes was conducted by calculating ORs with 95% CI using a fixed‐effects model with the Mantel and Haenszel method and calculating risk ratios with 95% CI with both fixed‐effects and random‐effects models. Finally, an additional sensitivity analysis was performed to evaluate the impact of follow‐up duration among trials on the primary endpoint and its single components with a Poisson regression model with random intervention effects to calculate inverse‐variance weighted averages of study‐specific log stratified incidence rate ratios (IRRs). The statistical level of significance was two‐tailed P < 0.05. Statistical analyses were performed with the Stata software version 13.1 (StataCorp LP, College Station, Texas, USA).
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Results
Search results
Figure 1 displays the PRISMA study search and selection process. A total of two RCTs 18 , 19 and five observational studies 20 , 21 , 23 , 26 , 27 were identified and included in this analysis. The main features of included studies are presented in Table 1 .
Figure 1.
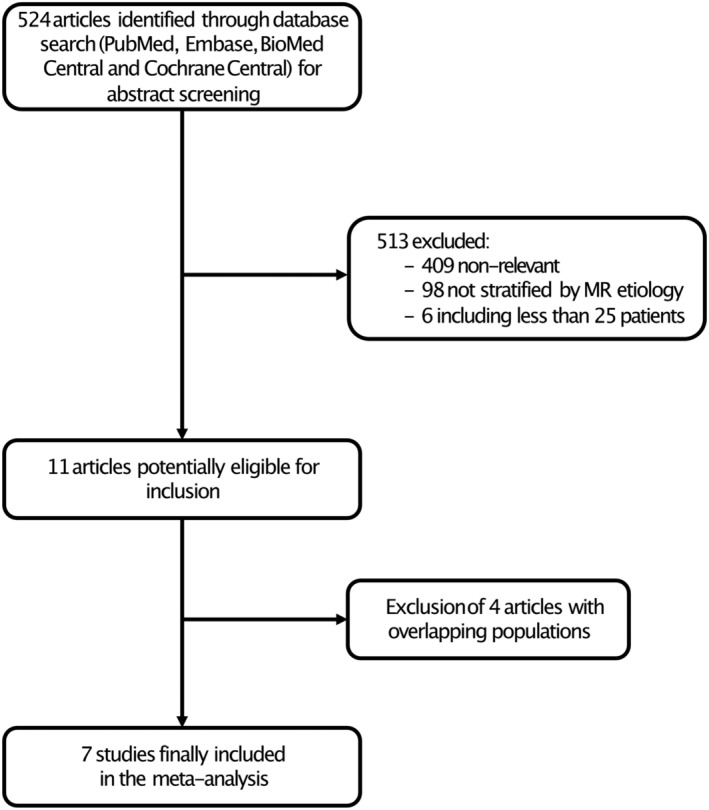
Flow chart of the study selection process. MR indicates mitral regurgitation.
Table 1.
Key study features
| Study | Year of publication | Study design | N of patients | Multicentre | Follow‐up | ||
|---|---|---|---|---|---|---|---|
| Overall | I‐MR | NI‐MR | |||||
| MITRA‐FR 23 | 2019 | RCT | 152 | 95 | 57 | Yes | 24 months |
| COAPT 15 | 2018 | RCT | 302 | 184 | 118 | Yes | 24 months |
| Godino et al. 20 | 2018 | Observational | 349 | 226 | 123 | Yes | 24 months |
| Kitamura et al. 24 | 2018 | Observational | 532 | 310 | 222 | Yes | 24 months |
| TRAMI 25 | 2016 | Observational | 474 | 409 | 65 | Yes | 12 months |
| Pilot European Sentinel 21 | 2016 | Observational | 452 | 235 | 217 | Yes | 12 months |
| GRASP‐IT 17 | 2015 | Observational | 240 | 136 | 104 | Yes | 24 months |
I‐MR, ischaemic mitral regurgitation; NI‐MR, non‐ischaemic mitral regurgitation; RCT, randomized clinical trial.
A total of 2501 patients with secondary MR treated with percutaneous edge‐to‐edge repair with the MitraClip device were included.
Baseline characteristics
Main baseline characteristics of included patients are summarized in Table 2 . A total of 454 patients were enrolled from RCTs while 2047 were included from observational studies. Mean baseline LVEF was 32.8%, most patients suffered from moderate–severe or severe MR and presented with NYHA Class ≥III.
Table 2.
Baseline clinical characteristics of included patients
| Study | Age (years) | Male (%) | Diabetes (%) | Atrial fibrillation (%) | NYHA ≥III (%) | LVEF (%) | Left ventricular end‐diastolic volume (mL) | MR grade ≥3 (%) | N of clips implanted per patient |
|---|---|---|---|---|---|---|---|---|---|
| MITRA‐FR 23 | 70.1 | 78.9 | 32.9 | 34.5 | 63.1 | 33.3 | 136.2 ± 37.4 † | 48 | 1.2 |
| COAPT 15 | 71.7 | 66.6 | 35.1 | 57.3 | 57 | 31.3 | 194.4 ± 69.2 | 100 | 1.7 |
| Godino et al. 20 | 69 | 77 | 33 | 47 | 78 | 30.8 | 217.1 ± 77.1 | 94.8 | 1.7 |
| Kitamura et al. 24 | 73.6 | 68.9 | 47.9 | 64.5 | 85.3 | 32.1 | — | — | — |
| TRAMI 25 | 74.5 | 70.7 | — | — | 91.3 | — | — | 100 | 1.4 |
| Pilot European Sentinel 21 | 72.8 | 68 | 33 | 27 | 88 | 37.1 | 171.1 ± 90.2 | 40.8 | 1.4 |
| GRASP‐IT 17 | 71.1 | 68.3 | 37.9 | 41.6 | 18 | 32.5 | 174 ± 83 | 67.9* | 1.3 |
| Overall | 71.8 | 71.2 | 36.6 | 45.3 | 68.7 | 32.8 | — | 75.2 | 1.4 |
LVEF, left ventricular ejection fraction; MR, mitral regurgitation; NYHA, New York Heart Association.
Including only MR Grade 4.
mL/m2.
Follow‐up duration
The Pilot European Sentinel 21 and the TRAMI 27 registries reported 1 year follow‐up outcomes, while the MITRA‐FR RCT, 28 the COAPT RCT, 18 Godino et al., 23 the GRASP‐IT registry, 20 and Kitamura et al. 26 reported 2 year follow‐up.
Publication bias and asymmetry
Funnel‐plot distributions of the pre‐specified outcomes as well as Harbord's tests indicated absence of publication bias and small study effect for all the outcomes (Figures S1–S7).
Risk of bias assessment
Tables S2 and S3 summarize the results of the risk of bias assessment with the RoB 2.0 tool for RCTs and with the ROBINS‐I tool for observational studies. Two studies were considered at low overall risk of bias, one at moderate overall risk of bias, three at serious overall risk of bias, and one at critical overall risk of bias.
Outcomes
Clinical outcomes
Patients with I‐MR undergoing MitraClip implantation, compared with patients with NI‐MR, were associated with similar risk of the composite primary endpoint (OR: 1.17; 95% CI: 0.93 to 1.46; I 2: 0%), heart failure‐related re‐hospitalization (OR: 1.02; 95% CI: 0.71 to 1.45; I 2: 32.8%), NYHA functional class (OR: 0.91; 95% CI: 0.56 to 1.49; I 2: 64.4%), and mitral valve re‐intervention (OR: 1.37; 95% CI: 0.60 to 3.13; I 2: 0%). Conversely, the risk of all‐cause death was significantly higher in patients with I‐MR compared with patients with NI‐MR (OR: 1.31; 95% CI: 1.07 to 1.62; I 2: 0%) (Figure 2 ).
Figure 2.
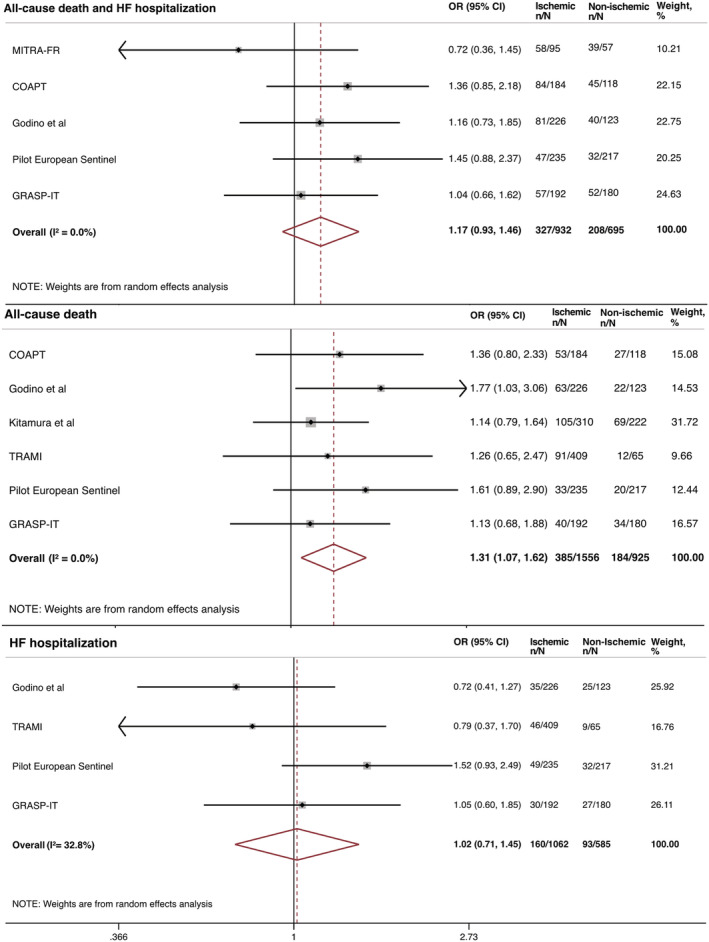
Clinical outcomes in patients with I‐MR compared with patients with NI‐MR undergoing MitraClip implantation. CI, confidence interval; HF, heart failure; I‐MR, ischaemic mitral regurgitation; NI‐MR, non‐ischaemic mitral regurgitation; OR, odds ratio.
Echocardiographic outcomes
No differences were observed between patients with I‐MR and patients with NI‐MR with respect to MR severity ≥2+ at discharge (OR: 1.19; 95% CI: 0.93 to 1.51; I 2: 18.9%) and during follow‐up (OR: 0.89; 95% CI:0.65 to 1.23; I 2: 0%).
Subgroup analysis and meta‐regression
A stratified analysis of the primary endpoint according to the study design (i.e. RCT vs. observational) showed findings consistent with the primary analysis (Figure S8). Meta‐regression analysis showed that the study design had no impact on treatment effect (Table S4). Additional meta‐regression analyses did not show any significant interaction between baseline medical therapy (beta‐blockers, angiotensin‐converting enzyme inhibitor/angiotensin receptor blocking agents, and mineralocorticoid receptor antagonist), baseline clinical and echocardiographic characteristics and treatment effect (Table S4). Furthermore, a stratified analysis of the composite primary endpoint according to estimated risk of bias was consistent with the primary analysis (Table S4).
Sensitivity analyses
Findings remained consistent with the main analysis after calculation of ORs using a fixed effects model as well as risk ratios with both fixed and random‐effects models (Table S5).
At leave‐one sensitivity analysis results remained consistent with the primary analysis (Table S6).
When calculating IRR to account for the impact of follow‐up duration on treatment effect, the rate of the composite primary endpoint was significantly higher in patients with I‐MR compared with patients with NI‐MR (IRR: 1.57; 95% CI: 1.27 to 1.93; P < 0.001), as well as of heart failure related re‐hospitalization (IRR: 1.77; 95% CI: 1.08 to 2.88; P = 0.022), while the increased risk of all‐cause death in patients with I‐MR was magnified compared with the main analysis (IRR: 2.15; 95% CI: 1.39 to 3.33; P = 0.022) (Figure 3 ).
Figure 3.
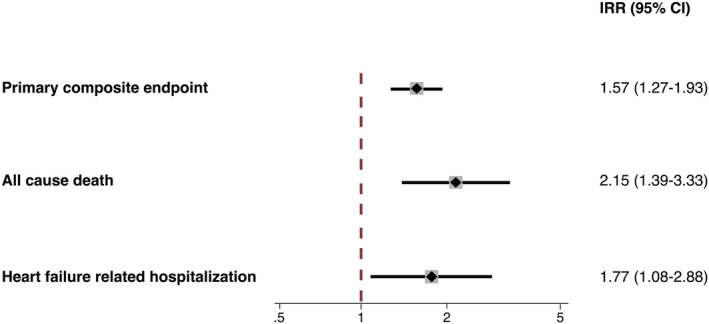
Incidence rate ratio of the composite endpoint, all‐cause death, and heart failure related hospitalization in patients with I‐MR and NI‐MR undergoing MitraClip implantation. CI, confidence interval; I‐MR, ischaemic mitral regurgitation; NI‐MR, non‐ischaemic mitral regurgitation.
Discussion
In this study, we evaluated the impact of the underlying aetiology in patients with secondary MR undergoing percutaneous edge‐to‐edge repair with the MitraClip device. The main findings of this study can be summarized as follows:
The risk of the primary endpoint (composite of all‐cause death and heart failure related hospitalization) and of mitral valve re‐intervention, NYHA Class III or IV at follow‐up and heart failure related hospitalization (outcomes included in the secondary endpoint) does not differ between patients with I‐MR and NI‐MR.
Patients with I‐MR undergoing MitraClip implantation have higher risk of all‐cause death compared with patients with NI‐MR.
When accounting for the different follow‐up across the included studies, the rate of the primary composite endpoint and its individual components (i.e. all‐cause death and heart failure related hospitalization) are significantly higher in patients with I‐MR compared with patients with NI‐MR.
Secondary MR is a common finding in patients affected by heart failure with reduced ejection fraction and is associated with an increased risk of morbidity and mortality. 11 , 29 As a consequence, many pharmacological approaches and interventional strategies have been sought in order to reduce the grade of MR, improve heart failure symptoms, and potentially reduce its impact on mortality. 30 Current international guidelines on treatment of valvular heart disease recommend as a first and essential step in the management of patients with secondary MR the use of GDMT for heart failure. 8 , 9 If symptoms persist after heart failure treatment optimization, possible options for mitral valve intervention should immediately be evaluated. 8 , 9 Data on mitral valve surgical repair/replacement in this contest are scarce. While survival improvement was observed adding mitral valve repair to coronary artery bypass grafting in patients with coronary artery disease and concomitant significant secondary MR, 31 no prognostic benefit was reported after isolated mitral valve surgery in secondary MR due to significant procedural risk and high rates of recurrent MR and mortality. 6 , 7 Accordingly, the 2021 ESC/EACTS guidelines on treatment of secondary MR recommends (Class I, level of Evidence B) mitral valve surgery only in patients undergoing coronary artery bypass grafting or other cardiac surgery, while for isolated mitral valve intervention, surgical procedure might be considered (Class IIb, level of Evidence C). 8
Therefore, percutaneous non‐invasive strategies have become an appealing alternative for the treatment of patients with secondary MR.
The recently published COAPT and MITRA‐FR trials compared the use of the MitraClip device in addition to GDMT and GDMT alone in patients with secondary MR and showed conflicting results. 18 , 19 In the COAPT trial, the primary endpoint (all heart failure hospitalizations within 24 months) and all secondary endpoints (MR severity, quality of life, 2‐year mortality, and left ventricle size changes) were markedly improved in the MitraClip arm, 18 whereas in the MITRA‐FR trial no benefits in the primary endpoint (all‐cause death or heart failure hospitalization) nor in any of the secondary endpoints were noted. 19 These divergent results are largely explained by discrepancies in study design, patients' selection, successful implantation rate, and proportion of patients treated with adequately titrated GDMT before and after MitraClip implantation. 18 , 19 For instance, while in COAPT randomization was stratified according to cardiomyopathy aetiology (ischaemic vs. non ischaemic), in MITRA‐FR a significantly higher percentage of patients with prior myocardial infarction was enrolled in the experimental arm compared to the GDMT alone arm (49.3 vs. 34.2%, P = 0.007). Proper patients' selection is of paramount importance to identify those patients most likely to benefit from MitraClip implantation. Indeed, in view of the increasing burden of secondary MR worldwide, 32 a thorough cost‐effectiveness assessment must be taken into account when considering which subset of patients might really benefit from a high‐cost procedure such as percutaneous mitral repair. 33 Based on its positive results, both American and European guidelines 8 , 9 currently suggest considering the anatomical and clinical inclusion criteria of the COAPT trial to identify the ideal candidate for MitraClip treatment. 18
Nevertheless, whether the underlying aetiology of secondary MR has an impact on patients' outcomes undergoing MitraClip implantation has been still poorly investigated. Four observational studies have found no differences in terms of mortality or re‐hospitalization rates between patients with I‐MR and NI‐MR undergoing percutaneous edge‐to‐edge mitral valve repair. 21 , 23 , 26 , 27 Conversely, in the GRASP‐IT registry, patients with I‐MR showed significantly increased rates of all‐cause death or heart failure related re‐hospitalization compared with patients with NI‐MR. 20 The results of the present meta‐analysis add further knowledge on optimal patient selection when formulating the indication for MitraClip implantation in patients with secondary MR. Despite the risk of all‐cause death was significantly increased in patients with I‐MR, no differences for the primary composite endpoint nor for heart failure related re‐hospitalization were found in our main analysis. However, when accounting for the different follow‐up, patients with ischaemic MR showed a higher rate of the primary composite endpoint as well as heart failure‐related re‐hospitalization and all‐cause death. Multiple studies and epidemiologic surveys have shown that patients with ischaemic cardiomyopathy compared with patients with non‐ischaemic cardiomyopathy have decreased survival, 34 , 35 which might in part explain the lack of differences for the composite primary endpoint and heart failure related re‐hospitalization when pooling the data considering only event rate without taking into account time to event. Of note, similar data have been reported in studies evaluating the single components of GDMT in patients with heart failure: a number of trials evaluating the role of beta‐blockers, renin–angiotensin–aldosterone system inhibitor, and cardiac resynchronization therapy have suggested an increased benefit of these treatment strategies among patients with non‐ischaemic compared with those with ischaemic cardiomyopathy. 36 , 37 , 38 There are several anatomic and clinical differences among I‐MR and NI‐MR patients that could potentially explain the higher mortality and risk for heart failure observed in case of I‐MR: first, patients with I‐MR are at increased risk for scar‐related arrhythmic events; second, compared with patients with NI‐MR, those with I‐MR suffer more commonly of recurrent myocardial infarction and often need for percutaneous or surgical revascularization, with the intrinsic risk of further adverse events; third, I‐MR patients are usually affected by higher burden of comorbidities compared to NI‐MR patients (e.g. chronic kidney disease, diabetes mellitus, and peripheral artery disease); lastly, asymmetric tethering, typically observed in patients with I‐MR, represents a more challenging anatomy to be approached percutaneously, often leading to suboptimal results and subsequent increased risk for severe MR recurrence. 39 The occurrence of this latter complication is indeed associated to a higher incidence of heart failure, repeat hospitalization and mortality. 40 In this circumstance, mitral valve re‐intervention may be needed; surgical valve replacement is commonly performed due to leaflet injury, and it is burdened by high in‐hospital (10–15%) and 1 year mortality, with also increased risk of periprocedural cerebrovascular accidents and acute kidney injury requiring dialysis. 41 , 42 Previous surgical studies have reported how the rate of recurrence of moderate or severe MR for patients with I‐MR is higher for those who undergo mitral valve repair instead of replacement. 43 , 44 Accordingly, chordal‐sparing mitral valve replacement is nowadays preferred over mitral valve repair in patients with I‐MR. 9 Whether this indication also applies to transcatheter intervention is still unknown, and only future prospective studies comparing percutaneous mitral valve repair to transcatheter mitral valve replacement in patients with secondary I‐MR would clarify this point.
Secondary I‐MR and NI‐MR are therefore two different pathologies that must be distinguished when mitral valve intervention is weighted. Based on our findings, considering the underlying aetiology of mitral valve disease in addition to current anatomical criteria for MitraClip procedure 8 , 9 might help the Heart Team in the proper patient's selection for percutaneous mitral valve repair.
The results of our investigation should be interpreted in light of some limitations. First, this is a study‐level meta‐analysis providing average treatment effects, although for two of the included studies we had access to the updated patient‐level database. 20 , 23 The lack of patient‐level data from the rest of the included studies data prevents us from assessing the impact of some features, such as baseline mean effective regurgitant orifice area, left ventricle volumes, drugs and therapeutic strategies on treatment effects. Similarly, despite the high prevalence of elderly patients referred for percutaneous mitral valve repair, we could not adequately evaluate the impact of age on the safety and effectiveness of percutaneous mitral valve repair. Second, the inclusion of RCTs and observational studies might represent a source of bias and limit the inference of results. However, stratified analysis combined with meta‐regression analysis did not detect any potential impact neither of study design nor any of the other tested variables on effect estimates. Lastly, results from meta‐regression analyses, considering the low number of studies included, should only be considered as hypothesis‐generating. Additional evidence will be provided by two ongoing randomized trials (ClinicalTrials.gov identifiers NCT02371512 and NCT02444338).
Conclusions
Our study suggests that MitraClip implantation could be more effective in reducing the risk of mortality in patients with non‐ischaemic than in patients with ischaemic MR. No absolute differences in the risk of heart failure‐related hospitalization or other adverse clinical outcomes were observed in the two groups. However, when accounting for time to event, patients with non‐ischaemic MR showed a reduced rate of both heart failure related hospitalization and all‐cause death.
Conflict of interest
PAP is a proctor for Cardia and Boston Scientific. CT has received speaking fees from Boston Scientific and Abbott. BR has received speaking honoraria from Boston Scientific. CG is a proctor physician for Abbott Vascular. GS has received a research grant from Boston Scientific (not related to this study), and speaker/consulting fees from Abbott Vascular, Boston Scientific, and Pfizer/BMS. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Funding statement
This work was not supported by any external funding.
Supporting information
Table S1. Definition of key endpoints.
Table S2. Risk of bias assessment for randomized clinical trials.
Table S3. Risk of bias assessment for observational studies.
Table S4. Meta‐regression results.
Table S5. Pooled analysis according to fixed‐effects and random‐effects model for clinical end‐points.
Table S6. Leave‐one sensitivity analysis for primary composite end‐point.
Figure S1. Funnel plot for composite primary end‐point (Harbord test, P = 0.22). OR = odds ratio; SE = standard error.
Figure S2. Funnel plot for all‐cause death (Harbord test, P = 0.30). OR = odds ratio; SE = standard error.
Figure S3. Funnel plot for heart failure hospitalization (Harbord test, P = 0.30). OR = odds ratio; SE = standard error.
Figure S4. Funnel plot for NYHA functional class at follow up (Harbord test, P = 0.59). OR = odds ratio; SE = standard error.
Figure S5. Funnel plot for mitral valve re‐intervention (Harbord test, P = 0.13). OR = odds ratio; SE = standard error.
Figure S6. Funnel plot for mitral regurgitation at discharge (Harbord test, P = 0.69). OR = odds ratio; SE = standard error.
Figure S7. Funnel plot for mitral regurgitation at follow‐up (Harbord test, P = 0.51). OR = odds ratio; SE = standard error.
Figure S8. Subgroup analysis for primary endpoint according to study design.
Acknowledgements
JSS is personally supported by a grant from the Fundación Alfonso Martin Escudero (Madrid, Spain).
Chiarito, M. , Sanz‐Sanchez, J. , Pighi, M. , Cannata, F. , Rubbio, A. P. , Munafò, A. , Cao, D. , Roccasalva, F. , Pini, D. , Pagnotta, P. A. , Ettori, F. , Petronio, A. S. , Tamburino, C. , Reimers, B. , Colombo, A. , Di Mario, C. , Grasso, C. , Mehran, R. , Godino, C. , and Stefanini, G. G. (2022) Edge‐to‐edge percutaneous mitral repair for functional ischaemic and non‐ischaemic mitral regurgitation: a systematic review and meta‐analysis. ESC Heart Failure, 9: 3177–3187. 10.1002/ehf2.13772.
Mauro Chiarito and Jorge Sanz‐Sanchez contributed equally to the study.
Contributor Information
Cosmo Godino, Email: godino.cosmo@hsr.it.
Giulio G. Stefanini, Email: giulio.stefanini@gmail.com.
References
- 1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez‐Sarano M. Burden of valvular heart diseases: a population‐based study. Lancet 2006; 368: 1005–1011. [DOI] [PubMed] [Google Scholar]
- 2. Dziadzko V, Clavel M‐A, Dziadzko M, Medina‐Inojosa JR, Michelena H, Maalouf J, Nkomo V, Thapa P, Enriquez‐Sarano M. Outcome and undertreatment of mitral regurgitation: a community cohort study. Lancet 2018; 391: 960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deferm S, Bertrand PB, Verbrugge FH, Verhaert D, Rega F, Thomas JD, Vandervoort PM. Atrial functional mitral regurgitation: JACC review topic of the week. J Am Coll Cardiol 2019; 73: 2465–2476. [DOI] [PubMed] [Google Scholar]
- 4. Nishimura RA, Vahanian A, Eleid MF, Mack MJ. Mitral valve disease—current management and future challenges. Lancet 2016; 387: 1324–1334. [DOI] [PubMed] [Google Scholar]
- 5. Utsunomiya H, Itabashi Y, Kobayashi S, Yoshida J, Ikenaga H, Rader F, Hussaini A, Makar M, Trento A, Siegel RJ, Kar S, Shiota T. Comparison of mitral valve geometrical effect of percutaneous edge‐to‐edge repair between central and eccentric functional mitral regurgitation: clinical implications. Eur Heart J Cardiovasc Imaging 2019; 20: 455–466. [DOI] [PubMed] [Google Scholar]
- 6. Goldstein D, Moskowitz AJ, Gelijns AC, Ailawadi G, Parides MK, Perrault LP, Hung JW, Voisine P, Dagenais F, Gillinov AM, Thourani V, Argenziano M, Gammie JS, Mack M, Demers P, Atluri P, Rose EA, O'Sullivan K, Williams DL, Bagiella E, Michler RE, Weisel RD, Miller MA, Geller NL, Taddei‐Peters WC, Smith PK, Moquete E, Overbey JR, Kron IL, O'Gara PT, Acker MA, CTSN . Two‐year outcomes of surgical treatment of severe ischemic mitral regurgitation. N Engl J Med 2016; 374: 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Michler RE, Smith PK, Parides MK, Ailawadi G, Thourani V, Moskowitz AJ, Acker MA, Hung JW, Chang HL, Perrault LP, Gillinov AM, Argenziano M, Bagiella E, Overbey JR, Moquete EG, Gupta LN, Miller MA, Taddei‐Peters WC, Jeffries N, Weisel RD, Rose EA, Gammie JS, DeRose JJ Jr, Puskas JD, Dagenais F, Burks SG, el‐Hamamsy I, Milano CA, Atluri P, Voisine P, O'Gara PT, Gelijns AC, CTSN . Two‐year outcomes of surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med 2016; 374: 1932–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2021: ehab395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O'Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, Toly C. 2020 ACC/AHA guideline for the Management of Patients with Valvular Heart Disease: Executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021; 143: e35–e71. [DOI] [PubMed] [Google Scholar]
- 10. Goel SS, Bajaj N, Aggarwal B, Gupta S, Poddar KL, Ige M, Bdair H, Anabtawi A, Rahim S, Whitlow PL, Tuzcu EM, Griffin BP, Stewart WJ, Gillinov M, Blackstone EH, Smedira NG, Oliveira GH, Barzilai B, Menon V, Kapadia SR. Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure: comprehensive analysis to determine the potential role of MitraClip for this unmet need. J Am Coll Cardiol 2014; 63: 185–186. [DOI] [PubMed] [Google Scholar]
- 11. Goliasch G, Bartko PE, Pavo N, Neuhold S, Wurm R, Mascherbauer J, Lang IM, Strunk G, Hülsmann M. Refining the prognostic impact of functional mitral regurgitation in chronic heart failure. Eur Heart J 2018; 39: 39–46. [DOI] [PubMed] [Google Scholar]
- 12. Wu AH, Aaronson KD, Bolling SF, Pagani FD, Welch K, Koelling TM. Impact of mitral valve annuloplasty on mortality risk in patients with mitral regurgitation and left ventricular systolic dysfunction. J Am Coll Cardiol 2005; 45: 381–387. [DOI] [PubMed] [Google Scholar]
- 13. Beeri R, Yosefy C, Guerrero JL, Nesta F, Abedat S, Chaput M, del Monte F, Handschumacher MD, Stroud R, Sullivan S, Pugatsch T, Gilon D, Vlahakes GJ, Spinale FG, Hajjar RJ, Levine RA. Mitral regurgitation augments post‐myocardial infarction remodeling: failure of hypertrophic compensation. J Am Coll Cardiol 2008; 51: 476–486. [DOI] [PubMed] [Google Scholar]
- 14. Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Siegel R, Rose GA, Engeron E, Loghin C, Trento A, Skipper ER, Fudge T, Letsou GV, Massaro JM, Mauri L. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 2011; 364: 1395–1406. [DOI] [PubMed] [Google Scholar]
- 15. Estévez‐Loureiro R, Settergren M, Winter R, Jacobsen P, Dall'Ara G, Sondergaard L, Cheung G, Pighi M, Ghione M, Ihlemann N, Moat NE, Price S, Streit Rosenberg T, di Mario C, Franzen O. Effect of gender on results of percutaneous edge‐to‐edge mitral valve repair with MitraClip system. Am J Cardiol 2015; 116: 275–279. [DOI] [PubMed] [Google Scholar]
- 16. Chiarito M, Pagnesi M, Martino EA, Pighi M, Scotti A, Biondi‐Zoccai G, Latib A, Landoni G, Mario CD, Margonato A, Maisano F, Feldman T, Alfieri O, Colombo A, Godino C. Outcome after percutaneous edge‐to‐edge mitral repair for functional and degenerative mitral regurgitation: a systematic review and meta‐analysis. Heart 2018; 104: 306–312. [DOI] [PubMed] [Google Scholar]
- 17. Kalbacher D, Schäfer U, Bardeleben RS, Eggebrecht H, Sievert H, Nickenig G, Butter C, May AE, Bekeredjian R, Ouarrak T, Kuck KH. Long‐term outcome, survival and predictors of mortality after MitraClip therapy: results from the German Transcatheter Mitral Valve Interventions (TRAMI) registry. Int J Cardiol 2019; 277: 35–41. [DOI] [PubMed] [Google Scholar]
- 18. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Marx SO, Cohen DJ, Weissman NJ, Mack MJ. Transcatheter mitral‐valve repair in patients with heart failure. N Engl J Med 2018; 379: 2307–2318. [DOI] [PubMed] [Google Scholar]
- 19. Obadia J‐F, Messika‐Zeitoun D, Leurent G, Iung B, Bonnet G, Piriou N, Lefèvre T, Piot C, Rouleau F, Carrié D, Nejjari M, Ohlmann P, Leclercq F, Saint Etienne C, Teiger E, Leroux L, Karam N, Michel N, Gilard M, Donal E, Trochu JN, Cormier B, Armoiry X, Boutitie F, Maucort‐Boulch D, Barnel C, Samson G, Guerin P, Vahanian A, Mewton N. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med 2018; 379: 2297–2306. [DOI] [PubMed] [Google Scholar]
- 20. Capodanno D, Adamo M, Barbanti M, Giannini C, Laudisa ML, Cannata S, Curello S, Immè S, Maffeo D, Bedogni F, Petronio AS, Ettori F, Tamburino C, Grasso C, GRASP‐IT Investigators . Predictors of clinical outcomes after edge‐to‐edge percutaneous mitral valve repair. Am Heart J 2015; 170: 187–195. [DOI] [PubMed] [Google Scholar]
- 21. Pighi M, Estevez‐Loureiro R, Maisano F, Ussia GP, Dall'Ara G, Franzen O, Laroche C, Settergren M, Winter R, Nickenig G, Gilard M, di Mario C, Transcatheter Valve Treatment Sentinel Registry (TCVT) Investigators of the EURObservational Research Programme (EORP) of the European Society of Cardiology . Immediate and 12‐month outcomes of ischemic versus nonischemic functional mitral regurgitation in patients treated with MitraClip (from the 2011 to 2012 Pilot Sentinel Registry of Percutaneous Edge‐To‐Edge Mitral Valve Repair of the European Society of Cardiology). Am J Cardiol 2017; 119: 630–637. [DOI] [PubMed] [Google Scholar]
- 22. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Godino C, Scotti A, Taramasso M, Adamo M, Russo M, Chiarito M, Melillo F, Beneduce A, Pivato CA, Arrigoni L, Toscano E, Salerno A, Cappelletti A, Magni V, Stella S, Fragasso G, Montorfano M, Agricola E, Ettori F, Margonato A, Maisano F, Colombo A. Two‐year cardiac mortality after MitraClip treatment of functional mitral regurgitation in ischemic and non‐ischemic dilated cardiomyopathy. Int J Cardiol 2018; 269: 33–39. [DOI] [PubMed] [Google Scholar]
- 24. Anon . Risk of bias tools—current version of RoB 2. Available at: https://www.riskofbias.info/welcome/rob‐2‐0‐tool/current‐version‐of‐rob‐2. Accessed May 3, 2019.
- 25. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ 2016; 355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kitamura M, Kaneko H, Schlüter M, Schewel D, Schmidt T, Alessandrini H, Kreidel F, Neuss M, Butter C, Kuck KH, Frerker C. Predictors of mortality in ischaemic versus non‐ischaemic functional mitral regurgitation after successful transcatheter mitral valve repair using MitraClip: results from two high‐volume centres. Clin Res Cardiol 2019; 108: 264–272. [DOI] [PubMed] [Google Scholar]
- 27. Schwencke C, Bijuklic K, Ouarrak T, Lubos E, Schillinger W, Plicht B, Eggebrecht H, Baldus S, Schymik G, Boekstegers P, Hoffmann R, Senges J, Schofer J. Impact of cardiac comorbidities on early and 1‐year outcome after percutaneous mitral valve interventions: data from the German transcatheter mitral valve interventions (TRAMI) registry. Clin Res Cardiol 2017; 106: 249–258. [DOI] [PubMed] [Google Scholar]
- 28. Iung Bernard, Armoiry Xavier, Vahanian Alec, Boutitie Florent, Mewton Nathan, Trochu Jean‐Noël, Lefèvre Thierry, Messika‐Zeitoun David, Guerin Patrice, Cormier Bertrand, Brochet Eric, Thibault Hélène, Himbert Dominique, Thivolet Sophie, Leurent Guillaume, Bonnet Guillaume, Donal Erwan, Piriou Nicolas, Piot Christophe, Habib Gilbert, Rouleau Frédéric, Carrié Didier, Nejjari Mohammed, Ohlmann Patrick, Saint Etienne Christophe, Leroux Lionel, Gilard Martine, Samson Géraldine, Rioufol Gilles, Maucort‐Boulch Delphine, Obadia Jean François, Obadia Jean‐François, Barthelet Martine, Grinberg Daniel, Rioufol Gilles, Thibault Hélène, Thivolet Sophie, Furber Alain, Benard Thomas, Debrux Jean‐Louis, Pinaud Frédéric, Rouleau Frédéric, Chocron Sidney, Chopard Romain, Meneveau Nicolas, Schiele François, Gilard Martine, Boschat Jacques, Castellant Philippe, Jobic Yannick, Le Ven Florent, Romain Didier, Beygui Farzin, Sabatier Rémi, Saloux Eric, Motreff Pascal, Clerfond Guillaume, Azarnoush Kasra, Lusson Jean René, Vorilhon Charles, Teiger Emmanuel, Antoine Clémence, Champagne Stéphane, Couetil Jean‐Paul, Damy Thibault, Dubois‐Rande Jean‐Luc, Ernande Laura, Guendouz Soulef, Lim Pascal, Monin Jean‐Luc, Nguyen Annabelle, Riant Elisabeth, Ternacle Julien, Zerbib Céline, Bertrand Bernard, Bouvaist Hélène, Saunier Carole, Vaislic Claude, Khelifa Riadh Cheikh, Favereau Xavier, Hilpert Loïc, Maribas Philippe, Azmoun Alexandre, Angel Jean‐Yves, Baruteau Alban, Brenot Philippe, Deleuze Philippe, Garcon Philippe, Raoux François, Slama Michel, Van Belle Eric, Prat Alain, Coisne Augustin, Duvapentiah Anju, Juthier Francis, Modine Thomas, Polge Anne‐Sophie, Richardson Marjorie, Rousse Natacha, Spillemaeker Hugues, Sudre Arnaud, Vincent Flavien, Vincentelli André, Jeu Antoine, Ketelers Régis, Bonnet Jean‐Louis, Bonnet Guillaume, Habib Gilbert, Michel Nicolas, Pankert Mathieu, Salaun Erwan, Collart Frédéric, Houël Rémi, Bille Jacques, Commeau Philippe, Joly Patrick, Michel Nicolas, Philip Emmanuel, Collet Frédéric, Arméro Sébastien, Bayet Gilles, Giacomoni Marie‐Paule, Maximovitch Alexandre, Lefèvre Thierry, Cormier Bertrand, Romano Mauro, Leclercq Florence, Albat Bernard, Cade Stéphane, Cransac Frédéric, Macia Jean‐Christophe, Piot Christophe, Francois Fabrice, Pons Maxime, Raczka Franck, Sportouch Catherine, Juillière Yves, Folliguet Thierry, Huttin Olivier, Popovic Batric, Selton‐Suty Christine, Venner Clément, Guérin Patrice, Baron Olivier, Letocart Vincent, Letourneau Thierry, Piriou Nicolas, Trochu Jean‐Noël, Belliard Olivier, Allouch Philippe, Bensouda Christophe, Janower Sandra, Makowski Serge, Pasquier Damien, Pillière Rémy, Rosencher Julien, Moceri Pamela, Vahanian Alex, Brochet Eric, Himbert Dominique, Iung Bernard, Nataf Patrick, Spaulding Christian, Achouh Paul, Berrebi Alain, Jouan Jérôme, Karam Nicole, Mirabel Mariana, Puscas Tania, Riant Elisabeth, Suen Po Wen, Caussin Christophe, Berrebi Alain, Diakov Christelle, Philippe François, Veugeois Aurélie, Collet Jean Philippe, Choussat Rémi, Isnard Richard, Montalescot Gilles, Leroux Lionel, Coste Pierre, Dijos Marina, Labrousse Louis, Lafitte Stéphane, Picard François, Raud‐Raynier Pascale, Degrand Bruno, Bedossa Marc, Corbineau Hervé, Donal Erwan, Leurent Guillaume, Eltchaninoff Hélène, Bauer Fabrice, Tron Christophe, Nejjari Mohammed, Attias Davis, Gerbay Antoine, Pierrard Romain, Fuzellier Jean‐François, Drogoul Laurent, Elebeze Jean‐Pierre, Lopez Stéphane, Mariottini Claude, Meyer Pierre, Mihoubi Alain, Tapia Michel, Teboul Jacques, Ohlmann Patrick, Goette‐Dimarco Paola, Kretz Jean‐Georges, Mommerot Arnaud, Morel Olivier, Petit‐Eisenmann Hélène, Samet Annie, Trinh Annie, Carrié Didier, Gautier Mathieu, Badie Yoan Lavie, Lhermusier Thibault, Marcheix Bernard, Tchétché Didier, Abouliatim Issam, Bonfils Laurent, Dumonteil Nicolas, Farah Bruno, Fondard Olivier, Pathak Atul, Etienne Christophe Saint, Bernard Anne, Dion Fany, Loardi Claudia, Quilliet Laurent, Seemann Aurélien, Antoine Clémence, Arnoult Marc Antoine, Meurisse Yvon, Wautot Fabrice, Champagnac Didier, Dementhon Julie, Doisy Vincent, Frieh Jean‐Philippe, Garrier Olivier, Jamal Fadi, Lamartine Stéphanie, Leroux Pierre‐Yves, Lienhart Yves, Staat Patrick, Zouaghi Oualid, Percutaneous repair or medical treatment for secondary mitral regurgitation: outcomes at 2 years. European Journal of Heart Failure. 2019;21: (12):1619. –1627. 10.1002/ejhf.1616 [DOI] [PubMed] [Google Scholar]
- 29. Sannino A, Smith RL, Schiattarella GG, Trimarco B, Esposito G, Grayburn PA. Survival and cardiovascular outcomes of patients with secondary mitral regurgitation: a systematic review and meta‐analysis. JAMA Cardiol 2017; 2: 1130–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chehab O, Roberts‐Thomson R, Ng Yin Ling C, Marber M, Prendergast BD, Rajani R, Redwood SR. Secondary mitral regurgitation: pathophysiology, proportionality and prognosis. Heart 2020; 106: 716–723. [DOI] [PubMed] [Google Scholar]
- 31. Deja MA, Grayburn PA, Sun B, Rao V, She L, Krejca M, Jain AR, Leng Chua Y, Daly R, Senni M, Mokrzycki K, Menicanti L, Oh JK, Michler R, Wróbel K, Lamy A, Velazquez EJ, Lee KL, Jones RH. Influence of mitral regurgitation repair on survival in the surgical treatment for ischemic heart failure trial. Circulation 2012; 125: 263–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. D'Arcy JL, Coffey S, Loudon MA, Kennedy A, Pearson‐Stuttard J, Birks J, Frangou E, Farmer AJ, Mant D, Wilson J, Myerson SG. Large‐scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: the OxVALVE population cohort study. Eur Heart J 2016; 37: 3515–3522a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baron SJ, Wang K, Arnold SV, Magnuson EA, Whisenant B, Brieke A, Rinaldi M, Asgar AW, Lindenfeld J, Abraham WT, Mack MJ, Stone GW, Cohen DJ, the COAPT Investigators . Cost‐effectiveness of transcatheter mitral valve repair versus medical therapy in patients with heart failure and secondary mitral regurgitation: results from the COAPT trial. Circulation 2019; 140: 1881–1891. [DOI] [PubMed] [Google Scholar]
- 34. Elgendy IY, Mahtta D, Pepine CJ. Medical therapy for heart failure caused by ischemic heart disease. Circ Res 2019; 124: 1520–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lloyd‐Jones DM, Larson MG, Leip EP, Beiser A, D'Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D. Lifetime risk for developing congestive heart failure: the Framingham heart study. Circulation 2002; 106: 3068–3072. [DOI] [PubMed] [Google Scholar]
- 36. Auricchio A, Schillinger W, Meyer S, Maisano F, Hoffmann R, Ussia GP, Pedrazzini GB, van der Heyden J, Fratini S, Klersy C, Komtebedde J, Franzen O, PERMIT‐CARE Investigators . Correction of mitral regurgitation in nonresponders to cardiac resynchronization therapy by MitraClip improves symptoms and promotes reverse remodeling. J Am Coll Cardiol 2011; 58: 2183–2189. [DOI] [PubMed] [Google Scholar]
- 37. Metra M, Nodari S, Parrinello G, Giubbini R, Manca C, Dei Cas L. Marked improvement in left ventricular ejection fraction during long‐term β‐blockade in patients with chronic heart failure: clinical correlates and prognostic significance. Am Heart J 2003; 145: 292–299. [DOI] [PubMed] [Google Scholar]
- 38. Schleman KA, Lindenfeld JA, Lowes BD, Bristow MR, Ferguson D, Wolfel EE, Abraham WT, Zisman LS. Predicting response to carvedilol for the treatment of heart failure: a multivariate retrospective analysis. J Card Fail 2001; 7: 4–12. [DOI] [PubMed] [Google Scholar]
- 39. Levine RA, Hagége AA, Judge DP, Padala M, Dal‐Bianco JP, Aikawa E, Beaudoin J, Bischoff J, Bouatia‐Naji N, Bruneval P, Butcher JT. Mitral valve disease‐morphology and mechanisms. Nat Rev Cardiol 2015; 12: 689–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kar S, Mack MJ, Lindenfeld JA, Abraham WT, Asch FM, Weissman NJ, Enriquez‐Sarano M, Lim DS, Mishell JM, Whisenant BK, Rogers JH. Relationship between residual mitral regurgitation and clinical and quality‐of‐life outcomes after transcatheter and medical treatments in heart failure: COAPT trial. Circulation 2021; 144: 426–437. [DOI] [PubMed] [Google Scholar]
- 41. Melillo F, Baldetti L, Beneduce A, Agricola E, Margonato A, Godino C. Mitral valve surgery after a failed MitraClip procedure. Interact Cardiovasc Thorac Surg 2021; 32: 380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chikwe J, O'Gara P, Fremes S, Sundt T, Habib RH, Gammie J, Gaudino M, Badhwar V, Gillinov M, Acker M, Rowe G. Mitral surgery after transcatheter edge‐to‐edge repair: Society of Thoracic Surgeons database analysis. J Am Coll Cardiol 2021; 78: 1–9. [DOI] [PubMed] [Google Scholar]
- 43. Magne J, Girerd N, Sénéchal M, Mathieu P, Dagenais F, Dumesnil JG, Charbonneau E, Voisine P, Pibarot P. Mitral repair versus replacement for ischemic mitral regurgitation: comparison of short‐term and long‐term survival. Circulation 2009; 120: S104–S111. [DOI] [PubMed] [Google Scholar]
- 44. Lorusso R, Gelsomino S, Vizzardi E, D'Aloia A, de Cicco G, Lucà F, Parise O, Gensini GF, Stefàno P, Livi U, Vendramin I, Pacini D, di Bartolomeo R, Miceli A, Varone E, Glauber M, Parolari A, Giuseppe Arlati F, Alamanni F, Serraino F, Renzulli A, Messina A, Troise G, Mariscalco G, Cottini M, Beghi C, Nicolini F, Gherli T, Borghetti V, Pardini A, Caimmi PP, Micalizzi E, Fino C, Ferrazzi P, di Mauro M, Calafiore AM, ISTIMIR Investigators . Mitral valve repair or replacement for ischemic mitral regurgitation? the Italian study on the treatment of ischemic mitral regurgitation (ISTIMIR). J Thorac Cardiovasc Surg 2013; 145: 128–139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Definition of key endpoints.
Table S2. Risk of bias assessment for randomized clinical trials.
Table S3. Risk of bias assessment for observational studies.
Table S4. Meta‐regression results.
Table S5. Pooled analysis according to fixed‐effects and random‐effects model for clinical end‐points.
Table S6. Leave‐one sensitivity analysis for primary composite end‐point.
Figure S1. Funnel plot for composite primary end‐point (Harbord test, P = 0.22). OR = odds ratio; SE = standard error.
Figure S2. Funnel plot for all‐cause death (Harbord test, P = 0.30). OR = odds ratio; SE = standard error.
Figure S3. Funnel plot for heart failure hospitalization (Harbord test, P = 0.30). OR = odds ratio; SE = standard error.
Figure S4. Funnel plot for NYHA functional class at follow up (Harbord test, P = 0.59). OR = odds ratio; SE = standard error.
Figure S5. Funnel plot for mitral valve re‐intervention (Harbord test, P = 0.13). OR = odds ratio; SE = standard error.
Figure S6. Funnel plot for mitral regurgitation at discharge (Harbord test, P = 0.69). OR = odds ratio; SE = standard error.
Figure S7. Funnel plot for mitral regurgitation at follow‐up (Harbord test, P = 0.51). OR = odds ratio; SE = standard error.
Figure S8. Subgroup analysis for primary endpoint according to study design.


