Abstract
Background
The high false‐positive rate for pulmonary nodules (PNs) from using low‐dose computed tomography (LDCT) screening can lead to overuse of invasive procedures, overtreatment, and patient anxiety. Therefore, it is very important to develop new diagnostic methods.
Methods
A negative enrichment‐fluorescence in situ hybridization (NE‐FISH) approach was used to detect circulating tumor cells (CTCs) in patients with PNs. We evaluated whether or not the combination of CTC counts with serum tumor marker levels (CEA, CA 125, CYFRA 21‐1, SCC) could improve the diagnostic ability for distinguishing patients with malignant pulmonary nodules (MPNs) from those with benign pulmonary nodules (BPNs). Moreover, the potential clinical application of this combination for the diagnosis of solitary pulmonary nodules (SPNs) with a diameter ≤2 cm was also investigated.
Results
The combination of CTC counts and tumor marker levels had a sensitivity of 80.12% and the area under the receiver operating characteristics curve (AUCROC) of 0.853 (95% confidence interval [CI]: 0.800–0.897, p < 0.001) for the differential diagnosis of PNs. For early cancer stages, the sensitivity was 75.38% (AUCROC = 0.780, 95% CI: 0.713–0.838, p < 0.001). In addition, for SPNs within 2 cm the combination of CTC counts and tumor marker levels was still the most valuable diagnostic tool with a sensitivity of 78.95% and AUCROC of 0.888.
Conclusion
The combination of CTC counts and serum tumor marker levels is helpful for improving the diagnosis of PNs, especially in the early stages of cancer and for SPNs within 2 cm.
Keywords: circulating tumor cells, diagnosis, early stages, pulmonary nodules, tumor markers
We aimed to evaluate whether or not the combination measurement of circulating tumor cell counts and serum tumor marker levels can enhance the screening efficiency for malignant versus benign pulmonary nodules. The results show this combination was effective, especially for the early stages of cancer and solitary pulmonary nodules within 2 cm.
INTRODUCTION
Lung cancer is the most common cause of cancer‐related deaths in the world. In 2018, there were 2.1 million new lung cancer cases and 1.8 million deaths predicted. 1 In China in 2018, there were 774 000 new cases of lung cancer and 690 000 deaths. It is worth noting that the incidence and mortality of lung cancer in China are higher than the average levels of other countries. Therefore, it is very important to reduce the mortality rate of lung cancer in China.
Early diagnosis and early treatment can significantly reduce the mortality of lung cancer. 2 , 3 Early lung cancer exists in the form of pulmonary nodules (PNs). The formation of PNs is a gradual process. PNs are considered to be focal, round‐shaped, dense, solid, or subsolid lung shadows in radiological images with a diameter of ≤3 cm. They can be isolated or multiple without atelectasis, lung portal lymph node enlargement, or pleural effusion.
With the continual improvement of health awareness by individuals and the development of highly sensitive imaging detection technologies such as low‐dose computed tomography (LDCT) for lung cancer screening, more and more patients with PNs are being discovered before the nodules become malignant tumors or early stage cancer. 4 , 5 , 6 Although LDCT plays an important role in the early screening of lung cancer, it is limited by a high false‐positive rate and does not fully meet the clinical needs for the differential diagnosis of PNs. 7 , 8 Another examination method is percutaneous lung puncture biopsy of PNs but it is invasive and will cause certain damage to the patient's body and increase the economic burden. 9 As an auxiliary diagnostic method, lung cancer tumor markers mainly have a certain clinical value for advanced lung cancer. However, its clinical application is limited due to low specificity and sensitivity for lung cancer patients, especially those at early stages. 10 , 11 Therefore, non‐invasive auxiliary detection technology has gradually become the focus of clinical research for the diagnosis of PNs. 12 , 13 , 14
Circulating tumor cells (CTCs) are tumor cells that have escaped from the primary lesion or metastasis. CTCs spread through the bloodstream or lymphatic system and give rise to new metastatic tumors. 15 , 16 , 17 Studies have found that CTCs exist in the blood in the early stage of tumor formation, suggesting that CTC levels may be an important indicator and potential breakthrough for the early diagnosis of lung cancer. 18 , 19
Many novel technologies have been developed in recent years to detect CTCs in various cancers. The most common detection strategy relies on epithelial cell biomarkers. CellSearch, as the most representative and only FDA‐approved CTC detection technology, uses epithelial cell adhesion molecule (EpCAM) and cytokeratin (CK) antibodies and has been utilized to detect CTCs and monitor chemotherapeutic efficacy in prostate, colorectal, and breast cancer patients. 20 , 21 , 22 , 23 However, when cells undergo epithelial‐mesenchymal transition (EMT), the expression of these epithelial cell biomarkers usually changes dynamically, which leads to a lower CTC detection rate and restricts the clinical application of this technology. Our previous study showed that a new platform integrating EpCAM‐independent subtraction, immunostaining‐fluorescence in situ hybridization (FISH), and the IMSTAR high content screening (HCS) device can be used as a diagnostic tool to distinguish cancer patients from patients with benign tumors or from healthy individuals. 24 , 25
In this study, the same platform was used to evaluate the clinical application value for differentiating patients with malignant pulmonary nodules (MPNs) from patients with benign pulmonary nodules (BPNs). We explored whether the combination of measuring CTC numbers and serum levels of tumor markers (carcinoembryonic antigen [CEA], cancer antigen 125 [CA 125], cytokeratin 19 fragment [CYFRA 21‐1], and squamous cell carcinoma antigen [SCC]) could increase the screening efficiency for differentiating MPN patients from BPN patients. In addition, the potential clinical utility of this combination for the diagnosis of solitary pulmonary nodules (SPNs) within 2 cm was also analyzed.
METHODS
Patients and specimens
In this retrospective study, we included 224 patients with PNs confirmed by CT or LDCT as newly diagnosed and untreated cases at Liaocheng People's Hospital (Liaocheng, Shandong, China) between August 2016 and May 2018. The study inclusion criteria were as follows: (1) All selected patients had been screened by CT or LDCT to detect single or multiple lung nodules ≤3 cm within the past one year; (2) were age ≥ 18 years; (3) patients were able to tolerate lung resection and had planned to undergo surgery for resection of lung nodules and accept histopathological examination; and (4) patients had agreed to participate in this study and had signed an informed consent form. The study exclusion criteria were as follows: (1) Patients with severe heart, liver, lung, or kidney dysfunction or mental illness who could not tolerate surgery; (2) history of malignant tumors in the past 3 years; and (3) patients who had received lung cancer‐related clinical therapeutic interventions in the past such as surgery, radiotherapy, chemotherapy, targeted therapy, or immunotherapy.
All patients provided written informed consent to participate in this study. This research was approved by Liaocheng People's Hospital and followed the principles of the Declaration of Helsinki.
Of the 224 patients with PNs, 171 cases were MPNs and 53 cases were BPNs as confirmed by surgical treatment and pathological diagnosis. Clinical characteristics were recorded for each MPN patient. Tumor staging (i.e., I, II, III) was accomplished according to the eighth edition of the American Joint Committee on Cancer (AJCC) tumor‐node‐metastasis (TNM) classification for lung cancer. The group of 53 patients with BPNs was age‐ and gender‐matched to the MPN group, but the differences between the groups were not statistically significant (Table S1).
Peripheral blood samples were used for analysis of CTCs and serum tumor markers. The samples were collected before resection, kept at room temperature, and analyzed within 24 h. To avoid possible bias, all blood samples were collected, coded, and detected by different personnel in a blinded manner.
Detection of CTCs
Negative enrichment (NE)‐FISH using an automated image analysis system, the IMSTAR HCS device (IMSTAR S.A., Paris, France), was used for the enrichment and identification of CTCs, as reported in our previous study. 24 , 25 Cells were identified as CTCs if they were positive for 4′, 6‐diamidino‐2‐phenylindole (DAPI), negative for CD45, and positive for centromere of chromosome 8 (CEP8) (Figure 1). CTC counts were expressed as the number of CTCs per 3.2 ml of whole blood.
FIGURE 1.
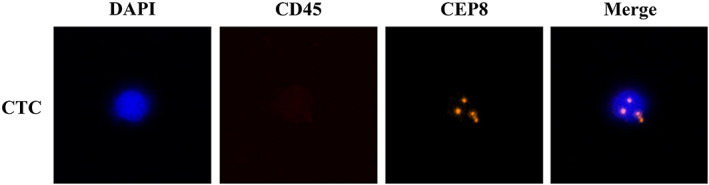
Detection of CTCs by NE‐FISH in patients with PNs. CTCs are defined as cells that are DAPI positive (blue), CD45 negative (lack of red stain), and CEP8 positive (orange). Abbreviations: CEP8, centromere of chromosome 8; CTC, circulating tumor cell; DAPI, 4′, 6‐diamidino‐2‐phenylindole; NE‐FISH, negative enrichment‐fluorescence in situ hybridization; PN, pulmonary nodules
Measurement of tumor marker levels
Serum levels of tumor markers including CEA, CA 125, CYFRA 21‐1, and SCC were measured using an immunology analyzer (Cobas e602; Roche Diagnostics). The upper limits of normal values were 5 ng/ml for CEA, 35 U/ml for CA 125, 3.3 ng/ml for CYFRA 21‐1, and 1.5 ng/ml for SCC.
Statistical analysis
SPSS 20.0 software (IBM Corp.) was used to perform statistical analysis. A receiver operating characteristic (ROC) curve was created to determine the cutoff value for the number of CTCs that would best differentiate patients with MPNs from those with BPNs. Differences in patient demographics and clinical characteristics were evaluated according to the CTC count or tumor marker levels using the Mann–Whitney U test and the Kruskal‐Wallis H test. p < 0.05 was considered statistically significant.
RESULTS
Detection of CTCs in patients with PNs
A total of 171 patients with MPNs (age: 60.33 ± 8.90 years; range 31 to 77) and 53 patients with BPNs (age: 59.47 ± 10.90 years; range 29 to 86) were recruited for this study. CTCs were detected in 132 patients with MPNs (median = 2 cells/3.2 ml blood, range 0 to 32) and 19 patients with BPNs (median = 0 cells/3.2 ml blood, range 0 to 2). CTC enumeration was able to differentiate patients with MPNs from patients with BPNs (p < 0.001, Figure 2a).
FIGURE 2.
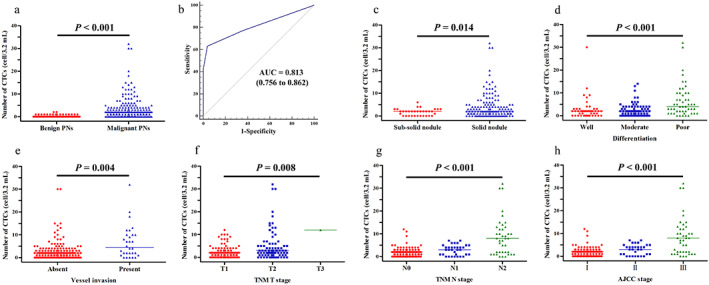
CTC enumerations can differentiate patients with benign PNs from patients with malignant PNs (p < 0.001). (a) Distribution of CTC counts (number of cells per 3.2 ml of whole blood) in patients with malignant PNs and patients with benign PNs. (b) The ROC curve was used to determine the cutoff value for CTC counts. (c–h) CTC enumerations were correlated with nodule type (p = 0.014), tumor differentiation (p < 0.001), vessel invasion (p = 0.004), TNM T stage (p = 0.008), TNM N stage (p < 0.001), and AJCC stage (p < 0.001). Abbreviations: AJCC, American Joint Committee on Cancer; AUC, area under the curve; CTC, circulating tumor cell; PNs, pulmonary nodules; ROC, receiver operating characteristic; TNM, tumor‐node‐metastasis
CTCs could be used as a diagnostic tool to distinguish MPN patients from BPN patients when the cutoff value was 1.5 CTCs/3.2 ml of blood with an AUCROC of 0.813 according to the ROC curve (95% confidence interval [CI]: 0.756–0.862, p < 0.001, Figure 2b). However, when the CTC cutoff value was set to 1.0 CTCs/3.2 ml of blood, the specificity was too low (64.15%). Therefore, we decided to use a cutoff value of 2.0 CTCs/3.2 ml of blood for the differential diagnosis of MPN patients from BPN patients. This cutoff value resulted in a sensitivity of 63.16% and a specificity of 96.23%.
With this cutoff value, the sensitivities of detection were 56.57, 64.52, and 78.05% for different AJCC TNM stages (I, II, III, respectively). Using this method, CTCs with different levels can be also detected in the early stage, which is concordant with previous studies. 26 , 27 , 28
Correlations between CTC counts and patient characteristics
Table 1 shows correlations between CTC counts and demographics and clinical characteristics of patients with MPNs. Patients with solid nodules presented with significantly higher CTC counts than those with subsolid nodules (p = 0.014, Figure 2c). There were also statistically significant differences between CTC counts and tumor differentiation (p < 0.001, Figure 2d), T stage (p = 0.008, Figure 2f), N stage (p < 0.001, Figure 2g), and AJCC stage (p < 0.001, Figure 2h). In addition, we found that CTC counts differed significantly depending on whether patients had vessel invasion or not (p = 0.004, Figure 2e).
TABLE 1.
Correlations between CTC counts and demographics and clinical characteristics of patients with MPNs
| Characteristics | n | Proportion (%) | CTC <2 | CTC ≥2 | p‐value | ||
|---|---|---|---|---|---|---|---|
| n | Proportion (%) | n | Proportion (%) | ||||
| Gender | |||||||
| Male | 94 | 54.97 | 39 | 41.49 | 55 | 58.51 | 0.379 |
| Female | 77 | 45.03 | 24 | 31.17 | 53 | 68.83 | |
| Age | |||||||
| ≥60 | 99 | 57.89 | 31 | 31.31 | 68 | 68.69 | 0.096 |
| <60 | 72 | 42.11 | 32 | 44.44 | 40 | 55.56 | |
| Smoking history | |||||||
| Yes | 49 | 28.65 | 20 | 40.82 | 29 | 59.18 | 0.126 |
| No | 122 | 71.35 | 43 | 35.25 | 79 | 64.75 | |
| Type | |||||||
| Subsolid nodule | 33 | 19.30 | 13 | 39.39 | 20 | 60.61 | 0.014 |
| Solid nodule | 138 | 80.70 | 50 | 36.23 | 88 | 63.77 | |
| Nodule size | |||||||
| ≤2 cm | 127 | 74.27 | 45 | 35.43 | 82 | 64.57 | 0.783 |
| >2 cm | 44 | 25.73 | 18 | 40.91 | 26 | 59.09 | |
| Differentiation | |||||||
| Well | 46 | 26.90 | 18 | 39.13 | 28 | 60.87 | <0.001 |
| Moderate | 76 | 44.44 | 33 | 43.42 | 43 | 56.58 | |
| Poor | 49 | 28.65 | 12 | 24.49 | 37 | 75.51 | |
| Vessel invasion | |||||||
| Absent | 141 | 82.46 | 56 | 39.72 | 85 | 60.28 | 0.004 |
| Present | 30 | 17.54 | 7 | 23.33 | 23 | 76.67 | |
| Number of nodules | |||||||
| Single | 163 | 95.32 | 60 | 36.81 | 103 | 63.19 | 0.220 |
| Multiple | 8 | 4.68 | 3 | 37.50 | 5 | 62.50 | |
| Histology | |||||||
| Adenocarcinoma | 127 | 74.27 | 46 | 36.22 | 81 | 63.78 | 0.742 |
| Squamous | 41 | 23.98 | 16 | 39.02 | 25 | 60.98 | |
| SCLC | 3 | 1.75 | 1 | 33.33 | 2 | 66.67 | |
| Tumor depth | |||||||
| T1 | 91 | 53.22 | 40 | 43.96 | 51 | 56.04 | 0.008 |
| T2 | 79 | 46.20 | 23 | 29.11 | 56 | 70.89 | |
| T3 | 1 | 0.58 | 0 | 0 | 1 | 100 | |
| Lymph node metastasis | |||||||
| N0 | 98 | 57.31 | 42 | 42.86 | 56 | 57.14 | <0.001 |
| N1 | 33 | 19.30 | 12 | 36.36 | 21 | 63.64 | |
| N2 | 40 | 23.39 | 9 | 22.50 | 31 | 77.50 | |
| TNM stage (AJCC) | |||||||
| I | 99 | 57.89 | 43 | 43.43 | 56 | 56.57 | <0.001 |
| II | 31 | 18.13 | 11 | 35.48 | 20 | 64.52 | |
| III | 41 | 23.98 | 9 | 21.95 | 32 | 78.05 | |
Abbreviations: AJCC, American Joint Committee on Cancer; CTC, circulating tumor cell; MPNs, malignant pulmonary nodules; SCLC, small cell lung cancer; TNM, tumor‐node‐metastasis.
Correlation of tumor marker levels with patient characteristics
The serum levels of the tumor markers CEA, CA 125, CYFRA 21‐1, and SCC in MPN patients and BPN patients are shown in Figure 3a–d. A significant difference in CYFRA 21‐1 levels was found between MPN patients and BPN patients (p = 0.034, Figure 3c). Referring to the upper limits of normal values, the sensitivities and specificities for these serum tumor markers were 19.30% and 92.45% for CEA, 18.71% and 94.34% for CA 125, 17.54% and 90.57% for CYFRA 21‐1, and 12.28% and 88.68% for SCC, respectively. The lower sensitivities that we found for these markers compared to those reported in previous studies 29 , 30 may be because most MPN patients in our study were at early cancer stages (I–II, 76.02%).
FIGURE 3.
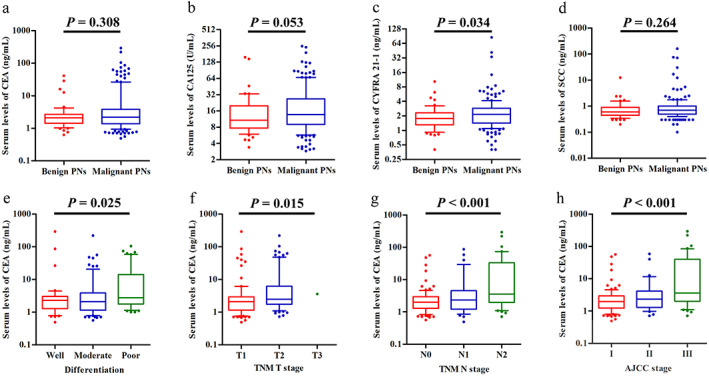
Tumor marker levels in patients with benign PNs or malignant PNs. (a–d) Serum levels of CEA, CA 125, CYFRA 21‐1, and SCC in patients with benign or malignant PNs (box plot with median, 10, 25, 75, and 90 centiles). (e–h) Serum levels of CEA are correlated with tumor differentiation (p = 0.025), T stage (p = 0.015), N stage (p < 0.001), and AJCC stage (p < 0.001). Abbreviations: AJCC, American Joint Committee on Cancer; CA 125, cancer antigen 125; CEA, carcinoembryonic antigen; CYFRA 21‐1, cytokeratin 19 fragment; PNs, pulmonary nodules; SCC, squamous cell carcinoma; TNM, tumor‐node‐metastasis
The relationships between serum levels of tumor markers and the demographics and clinical characteristics of MPN patients are shown in Table S2. There were statistically significant differences between CEA levels and tumor differentiation (p = 0.025, Figure 3e), T stage (p = 0.015, Figure 3f), N stage (p < 0.001, Figure 3g), and AJCC stage (p < 0.001, Figure 3h). Male patients had significantly higher levels of CYFRA 21‐1 (p = 0.011, Figure S1) and SCC (p = 0.010, Figure S1) than female patients. The levels of CA 125 and CYFRA 21‐1 differed significantly based on N stage (p = 0.042, Figure S1; p = 0.001, Figure S1; respectively) and AJCC stage (p = 0.028, Figure S1; p = 0.001, Figure S1; respectively). Patients with vessel invasion had significantly higher levels of CYFRA 21‐1 than those without invasion (p = 0.045, Figure S1). In addition, CYFRA 21‐1 levels differed significantly as a function of tumor differentiation (p = 0.019, Figure S1) and nodule size (p = 0.003, Figure S1).
Combination of CTC counts and tumor marker levels improves diagnostic performance
In this study, we investigated the diagnostic performance of CTC counts and serum tumor markers levels in MPNs and explored whether their combined use would increase screening efficiency. As shown in Figure 4a, CTC counts had a significantly higher diagnostic performance than serum levels of CEA, CA 125, CYFRA 21‐1, or SCC (AUCROC = 0.813 for CTC counts, and 0.546, 0.588, 0.596, 0.551, respectively, for tumor markers; all p < 0.0001). When we used all four tumor marker levels together as a diagnostic method for MPNs, the sensitivity was still lower than that of CTC counts alone (49.71% vs. 63.16%, Table S3; AUCROC = 0.670 vs. 0.813, p < 0.01, Figure 4b). However, the combination of tumor marker levels and CTC counts had a more accurate diagnostic ability than CTC counts alone, resulting in a sensitivity of 80.12% and AUCROC of 0.853. Therefore, this combination increased the screening efficiency for distinguishing MPN patients from BPN patients.
FIGURE 4.
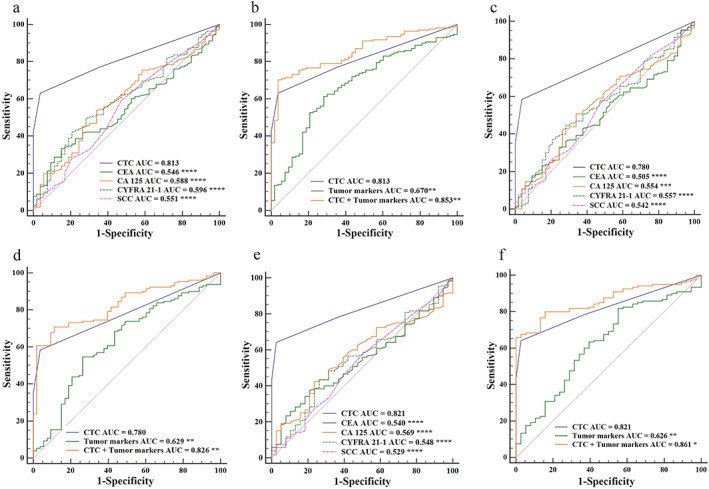
Comparison of diagnostic performance of CTC counts and tumor marker levels in patients with PNs. (a) Performance of CTC counts and levels of CEA, CA 125, CYFRA 21‐1, and SCC, and (b) their combinations in the differential diagnosis of 171 patients with MPNs and 53 patients with BPNs. (c) Performance of CTC counts and levels of CEA, CA 125, CYFRA 21‐1, and SCC, and (d) their different combinations in the differential diagnosis of 130 patients with MPNs at early cancer stages (I‐II) and 53 patients with BPNs. (e) Performance of CTC counts and levels of CEA, CA 125, CYFRA 21‐1, and SCC, and (f) their different combinations in the differential diagnosis of 120 patients with MPNs and 38 patients with BPNs, all of whom had SPNs within 2 cm. **** p < 0.0001, *** p < 0.001, ** p < 0.01, * p < 0.05. Abbreviations: AUC, area under the curve; BPNs, benign pulmonary nodules; CA 125, cancer antigen 125; CEA, carcinoembryonic antigen; CTC, circulating tumor cell; CYFRA 21‐1, cytokeratin 19 fragment; MPNs, malignant pulmonary nodules; PNs, pulmonary nodules; SCC, squamous cell carcinoma; SPNs, solitary pulmonary nodules
For the early‐stage cancer (I–II) patients with MPNs, the CTC count was also the most valuable diagnostic tool compared to tumor marker levels (AUCROC = 0.780 for CTC counts, and 0.505, 0.554, 0.557, 0.542, respectively, for tumor markers; all p < 0.001; Figure 4c). As shown in Figure 4d, using the combination of CTC counts and tumor marker levels further improved the diagnostic value for early‐stage cancer (I–II) patients with MPNs, resulting in a sensitivity of 75.38% and AUCROC of 0.826.
In this study, there were 120 MPN patients and 38 BPN patients who had SPNs within 2 cm. It is worth noting that the CTC count was still the most valuable diagnostic tool with a sensitivity of 64.17% and AUCROC of 0.821, compared to tumor marker levels (Figure 4e). Furthermore, the sensitivity was further improved by using the combination of CTC counts and tumor marker levels (77.50% for sensitivity and 0.861 for AUCROC, p < 0.05; Figure 4f), suggesting that this combination may have potential clinical applications for the differential diagnosis of SPNs within 2 cm.
DISCUSSION
Although medical standards have improved, the incidence of PNs has continually increased in recent years. While LDCT screening has been widely employed in lung cancer diagnosis, its use for screening to differentiate malignant PNs from benign PNs is still challenging because of the high false‐positive rate which may result in unnecessary treatment and psychological burden. 31 Using LDCT screening, 24.2% of heavy smokers had indeterminate PNs in the National Lung Screening Trial (NLST). Ultimately, 96.4% of these indeterminate PNs were finally confirmed as benign growths over the three rounds of screening. 32 Moreover, LDCT screening may lead to radiation‐induced lung cancer because of the repeated radiation exposure. 33 , 34 Therefore, there is an urgent need to develop minimally invasive and nonradiological techniques with high sensitivity and specificity to help distinguish patients with MPNs from those with BPNs, especially in the early stage of cancer.
As a real‐time “liquid biopsy,” CTC analyses have significant benefits of non‐invasiveness, easy and objective access, and ability to repeatedly capture tumor‐related information from lung cancer patients. In this study, to detect CTCs in MPN and BPN patients, we used NE‐FISH technology as an EpCAM‐independent method to avoid a lower CTC detection rate that may result from the EMT transition process. Using 2.0 CTCs/3.2 ml of blood as the cutoff value, the sensitivity and specificity of using CTC counts to distinguish MPN patients from BPN patients were 63.16% and 96.23%, respectively. In comparison, the first liquid biopsy approach approved by the China Food and Drug Administration (CFDA) for helping to distinguish lung nodules was a panel of seven autoantibodies (7‐AABs, including p53, PGP9.5, CAGE, GAGE7, SOX2, MAGEA1, and GBU4‐5) with a sensitivity range of 46.15 to 56.25% and a specificity range of 70.00 to 91.18%. 35 , 36 , 37 Notably, the diagnostic value for CTC counts was higher than the 7‐AABs panel, suggesting that CTC detection offers potential application in the clinic for distinguishing PNs.
Our study showed that CTC counts in MPN patients differed significantly as a function of nodule type (sub‐solid vs. solid, p = 0.014), tumor differentiation (p < 0.001), vessel invasion (p = 0.004), T stage (p = 0.008), N stage (p < 0.001), and AJCC stage (p < 0.001). It has been reported that the presence of vessel invasion indicates that tumor cells have already penetrated blood vessels or peripheral lymphatic vessels, and thus the cancer is in the early stage of metastasis. Vessel invasion has also been reported as an independent poor prognostic factor in patients with non‐small cell lung cancer (NSCLC). 38 , 39 , 40 , 41 Of the 171 MPN patients in our study, 168 patients (98%) had NSCLC. For this subtype, patients with vessel invasion presented with a significantly higher count of CTCs than those without invasion (p = 0.007, data not shown) suggesting that CTC counts may also be a predictive marker for early metastasis and a prognostic factor for NSCLC.
Because of the wide use of serum tumor markers for diagnosing lung cancer in the clinic, the performance of levels of CEA, CA 125, CYFRA 21‐1, and SCC in the diagnosis of MPNs was also investigated. These tumor markers exhibited a significantly lower diagnostic ability than CTC counts. Furthermore, the diagnostic performance of these four tumor markers together was still not as good as that of CTC counts alone. However, by using the combination of CTC counts and tumor marker levels, the diagnostic sensitivity for MPN detection was bolstered to 80.12% (AUCROC = 0.853), indicating that this combination increased the screening efficiency for MPNs versus BPNs. This improvement in diagnostic performance was also observed in the early‐stage cancer (I–II) patients (sensitivity = 75.38%, AUCROC = 0.826).
With the improvement of health awareness by individuals coupled with the availability of imaging examinations, SPNs are being increasingly detected, but the accurate diagnosis of SPNs within 2 cm relies on CT‐guided invasive percutaneous transthoracic needle biopsy or 18F‐fluorodeoxyglucose positron emission tomography (18F‐FDG PET)/CT. 42 , 43 As a noninvasive and nonradioactive diagnostic tool, the combination of CTC counts and tumor marker levels may have potential clinical applications for the diagnosis of SPNs within 2 cm (sensitivity = 77.50%, AUCROC = 0.845).
Our study shows that CTC counts detected by NE‐FISH can be used as a diagnostic aid for distinguishing MPN patients from BPN patients. Moreover, the combination of CTC counts and tumor marker levels improves the diagnostic value for patients with PNs, as well as for those at early cancer stages (I–II). In addition, the combination of these markers has a potential application for identifying patients with SPNs within 2 cm. More studies in a larger patient population should be conducted to confirm our findings.
This study also has several limitations and shortcomings. First, this was a retrospective and single‐center study. Further research on multicenter cohorts and different populations is warranted. Second, a small number of CTCs were also detected in patients without cancer. According to previous studies, CTCs found in control individuals without cancer (classified as “nonauthentic” CTCs) may be cells with chromosomal variations caused by inflammation or aging. 26 While these cells may be cleared by the immune system at any time, a long‐time dynamic study of these BPN patients with nonauthentic CTCs should be conducted in the future to determine whether they would ultimately develop into lung cancer or other lung diseases.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest.
Supporting information
Appendix S1. Supporting Information
ACKNOWLEDGMENTS
This study was funded by Major Science and technology innovation project of Shandong Province (No. 2019JZZY021002).
Ma G, Yang D, Li Y, Li M, Li J, Fu J, et al. Combined measurement of circulating tumor cell counts and serum tumor marker levels enhances the screening efficiency for malignant versus benign pulmonary nodules. Thorac Cancer. 2022;13(23):3393–3401. 10.1111/1759-7714.14702
Funding information Major Science and technology innovation project of Shandong Province, Grant/Award Number: 2019JZZY021002
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA‐Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Pastorino U, Silva M, Sestini S, Sabia F, Boeri M, Cantarutti A, et al. Prolonged lung cancer screening reduced 10‐year mortality in the MILD trial: new confirmation of lung cancer screening efficacy. Ann Oncol. 2019;30:1162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oudkerk M, Liu S, Heuvelmans MA, Walter JE, Field JK. Lung cancer LDCT screening and mortality reduction‐evidence, pitfalls and future perspectives. Nat Rev Clin Oncol. 2021;18:135–51. [DOI] [PubMed] [Google Scholar]
- 4. Pinsky PF. Lung cancer screening with low‐dose CT: a world‐wide view. Transl Lung Cancer Res. 2018;7:234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang KL, Wang SY, Lu WC, Chang YH, Su J, Lu YT. Effects of low‐dose computed tomography on lung cancer screening: a systematic review, meta‐analysis, and trial sequential analysis. BMC Pulm Med. 2019;19:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang X, Liu H, Shen Y, Shen Y, Li W, Chen Y, et al. Low‐dose computed tomography (LDCT) versus other cancer screenings in early diagnosis of lung cancer: a meta‐analysis. Medicine. 2018;97:e11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coughlin JM, Zang Y, Terranella S, Alex G, Karush J, Geissen N, et al. Understanding barriers to lung cancer screening in primary care. J Thorac Dis. 2020;12:2536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jacobs CD, Jafari ME. Early results of lung cancer screening and radiation dose assessment by low‐dose CT at a community hospital. Clin Lung Cancer. 2017;18:e327–31. [DOI] [PubMed] [Google Scholar]
- 9. Zhou Q, Dong J, He J, Liu D, Tian DH, Gao S, et al. The Society for Translational Medicine: indications and methods of percutaneous transthoracic needle biopsy for diagnosis of lung cancer. J Thorac Dis. 2018;10:5538–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liang W, Zhao Y, Huang W, Liang H, Zeng H, He J. Liquid biopsy for early stage lung cancer. J Thorac Dis. 2018;10:S876–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qi W, Li X, Kang J. Advances in the study of serum tumor markers of lung cancer. J Cancer Res Ther. 2014;10:95–101. [DOI] [PubMed] [Google Scholar]
- 12. Liang W, Zhao Y, Huang W, Gao Y, Xu W, Tao J, et al. Non‐invasive diagnosis of early‐stage lung cancer using high‐throughput targeted DNA methylation sequencing of circulating tumor DNA (ctDNA). Theranostics. 2019;9:2056–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thorlacius‐Ussing J, Kehlet SN, Rønnow SR, Karsdal MA, Willumsen N. Non‐invasive profiling of protease‐specific elastin turnover in lung cancer: biomarker potential. J Cancer Res Clin. 2019;145:383–92. [DOI] [PubMed] [Google Scholar]
- 14. Naeli P, Yousefi F, Ghasemi Y, Savardashtaki A, Mirzaei H. The role of microRNAs in lung cancer: implications for diagnosis and therapy. Curr Mol Med. 2020;20:90–101. [DOI] [PubMed] [Google Scholar]
- 15. Shen Z, Wu A, Chen X. Current detection technologies for circulating tumor cells. Chem Soc Rev. 2017;46:2038–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Castro‐Giner F, Aceto N. Tracking cancer progression: from circulating tumor cells to metastasis. Genome Med. 2020;12:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Diamantopoulou Z, Castro‐Giner F, Aceto N. Circulating tumor cells: ready for translation? J Exp Med. 2020;217:e20200356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanaka F, Yoneda K, Kondo N, Hashimoto M, Takuwa T, Matsumoto S, et al. Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin Cancer Res. 2009;15:6980–6. [DOI] [PubMed] [Google Scholar]
- 19. Fiorelli A, Accardo M, Carelli E, Angioletti D, Santini M, Domenico MD. Circulating tumor cells in diagnosing lung cancer: clinical and morphologic analysis. Ann Thorac Surg. 2015;99:1899–905. [DOI] [PubMed] [Google Scholar]
- 20. Andree KC, van Dalum G, Terstappen LW. Challenges in circulating tumor cell detection by the CellSearch system. Mol Oncol. 2016;10:395–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Riethdorf S, O'Flaherty L, Hille C, Pantel K. Clinical applications of the CellSearch platform in cancer patients. Adv Drug Delivery Rev. 2018;125:102–21. [DOI] [PubMed] [Google Scholar]
- 22. Gorges TM, Stein A, Quidde J, Hauch S, Röck K, Riethdorf S, et al. Improved detection of circulating tumor cells in metastatic colorectal cancer by the combination of the CellSearch® system and the AdnaTest®. PLoS One. 2016;11:e0155126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miller MC, Doyle GV, Terstappen LW. Significance of circulating tumor cells detected by the CellSearch system in patients with metastatic breast colorectal and prostate cancer. J Oncol. 2010;2010:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Y, Tian X, Gao L, Jiang X, Fu R, Zhang T, et al. Clinical significance of circulating tumor cells and tumor markers in the diagnosis of lung cancer. Cancer Med. 2019;8:3782–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li Y, Ma G, Zhao P, Fu R, Gao L, Jiang X, et al. Improvement of sensitive and specific detection of circulating tumor cells using negative enrichment and immunostaining‐FISH. Clin Chim Acta. 2018;485:95–102. [DOI] [PubMed] [Google Scholar]
- 26. Lei Y, Sun N, Zhang G, Liu C, Lu Z, Huang J, et al. Combined detection of aneuploid circulating tumor‐derived endothelial cells and circulating tumor cells may improve diagnosis of early stage non‐small‐cell lung cancer. Clin Transl Med. 2020;10:e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen YY, Xu GB. Effect of circulating tumor cells combined with negative enrichment and CD45‐FISH identification in diagnosis, therapy monitoring and prognosis of primary lung cancer. Med Oncol. 2014;31:240. [DOI] [PubMed] [Google Scholar]
- 28. Chen Q, Ge F, Cui W, Wang F, Yang Z, Guo Y, et al. Lung cancer circulating tumor cells isolated by the EpCAM‐independent enrichment strategy correlate with cytokeratin 19‐derived CYFRA21‐1 and pathological staging. Clin Chim Acta. 2013;419:57–61. [DOI] [PubMed] [Google Scholar]
- 29. Zang R, Li Y, Jin R, Wang X, Lei Y, Che Y, et al. Enhancement of diagnostic performance in lung cancers by combining CEA and CA125 with autoantibodies detection. Oncoimmunology. 2019;8:e1625689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen ZQ, Huang LS, Zhu B. Assessment of seven clinical tumor markers in diagnosis of non‐small‐cell lung cancer. Dis Markers. 2018;2018:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kramer BS, Berg CD, Aberle DR, Prorok P. Lung cancer screening with low‐dose helical CT: results from the National Lung Screening Trial (NLST). J Med Screen. 2011;18:109–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patz EF, Pinsky P, Gatsonis C, Sicks JD, Kramer BS, Tammemägiet MC, et al. Overdiagnosis in low‐dose computed tomography screening for lung cancer. JAMA. Intern Med. 2014;174:269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perisinakis K, Seimenis I, Tzedakis A, Karantanas A, Damilakis J. Radiation burden and associated cancer risk for a typical population to be screened for lung cancer with low‐dose CT: a phantom study. Eur Radiol. 2018;28:4370–8. [DOI] [PubMed] [Google Scholar]
- 34. Rampinelli C, De Marco P, Origgi D, Maisonneuve P, Casiraghi M, Veronesi G, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk‐benefit analysis. BMJ. 2017;356:j347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li F, Hu A. The clinical value of seven autoantibodies in the differential diagnosis of benign and malignant pulmonary nodules. Label Immuno Clin Med. 2020;27:967–73. [Google Scholar]
- 36. Jia J, Huang J, Zhu X. Diagnostic value of tumor‐associated autoantibodies for malignant pulmonary nodules. Chin J Clin Oncol. 2020;47:271–6. [Google Scholar]
- 37. Jin X, Qian K, Lu G, Zhang Y. Value of tumor associated autoantibodies in the differential diagnosis of benign and malignant sub‐centimeter pulmonary nodules. Pract J Card Cereb Pneumal Vasc Dis. 2020;28:83–7. [Google Scholar]
- 38. Bréchot JM, Chevret S, Charpentier MC, Vecchi CA, Carpom F, Prudent J, et al. Blood vessel and lymphatic vessel invasion in resected nonsmall cell lung carcinoma: correlation with TNM stage and disease free and overall survival. Cancer. 1996;78:2111–8. [PubMed] [Google Scholar]
- 39. Miyoshi K, Moriyama S, Kunitomo T, Nawa S. Prognostic impact of intratumoral vessel invasion in completely resected pathologic stage I non–small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137:429–34. [DOI] [PubMed] [Google Scholar]
- 40. Shoji F, Haro A, Yoshida T, Ito K, Morodomi Y, Yano T, et al. Prognostic significance of intratumoral blood vessel invasion in pathologic stage IA non‐small cell lung cancer. An. Thorac Surg. 2010;89:864–9. [DOI] [PubMed] [Google Scholar]
- 41. Wang J, Chen J, Chen X, Wang B, Li K, Bi J. Blood vessel invasion as a strong independent prognostic indicator in non‐small cell lung cancer: a systematic review and meta‐analysis. PLoS One. 2011;6:e28844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xu C, Yuan Q, Chi C, Zhang Q, Wang Y, Wang W, et al. Computed tomography‐guided percutaneous transthoracic needle biopsy for solitary pulmonary nodules in diameter less than 20 mm. Medicine. 2018;97:e0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tang K, Wang L, Lin J, Zheng X, Wu Y. The value of 18F‐FDG PET/CT in the diagnosis of different size of solitary pulmonary nodules. Medicine. 2019;98:e14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information


