Abstract
Aims
Recent studies suggested that both left ventricular ejection fraction (LVEF) lower than 60% or higher than 65% were associated with an increased mortality in the general population. Uncertainty remains regarding adverse outcomes across LVEF in coronary artery disease (CAD). The common understanding was that LVEF <40% was associated with an increased risk of mortality. But the threshold at LVEF of 40% was arbitrary because quite a lot of adverse outcomes existed in patients with ejection fraction >40%. We aimed to evaluate the relationship between LVEF and mortality or adverse events in CAD patients undergoing percutaneous coronary intervention (PCI).
Methods and results
A total of 10 252 CAD patients undergoing PCI from an observational cohort were studied. All‐cause mortality and major adverse cardiovascular and cerebrovascular events (MACCE) were set as outcomes. Kaplan–Meier curves, adjusted Cox regression models, and restricted cubic spline analyses were used for evaluation. A total of 137 (1.3%) patients had all‐cause mortality, and 816 (8.0%) patients had MACCE during a median of 2.4 years of follow‐up. The median LVEF was 64%. All‐cause mortality and MACCE rates changed substantially across LVEF categories, and a linear inverse relationship of LVEF with all‐cause mortality and MACCE risk was observed. All‐cause mortality or MACCE risk increased significantly below an LVEF of 55 or 65%, respectively. Patients with LVEF <55% had a more than 3.5‐fold higher mortality than those with LVEF ≥55%. Patients with LVEF <65% had a more than 1.3‐fold higher MACCE than those with LVEF ≥65%. Below 55 or 65%, there was a rise in mortality or MACCE. A gradient–response relationship was observed, with an all‐cause mortality risk range between 8.6‐fold and 3.0‐fold increase from LVEF <40 to 50–54.9% and MACCE risk range between 2.4‐fold and 1.4‐fold from LVEF <40 to 60–64.9%.
Conclusions
In CAD patients undergoing PCI, LVEF lower than 55% or LVEF lower than 65% was correlated with increased all‐cause mortality and MACCE respectively, whereas higher LVEF was not.
Keywords: Ejection fraction, Mortality, Cardiovascular events, Coronary artery disease, Percutaneous coronary intervention
Introduction
Left ventricular ejection fraction (LVEF) is a well‐recognized measurement for cardiac function and has been widely utilized as a risk stratification tool in heart failure (HF), valvular disease, and coronary artery disease (CAD). 1 , 2 , 3 , 4 Reduced LVEF (<40%) is a marker of contractile dysfunction and predicts mortality and adverse events in a variety of clinical settings. 1 , 2 Many randomized controlled trials (RCTs) for heart diseases designated reduced ejection fraction as the inclusion criterion, 5 , 6 , 7 , 8 but the chosen cut‐off at an LVEF of 40% is arbitrary and mainly concluded from evidence in HF. 9
In contrast with the prior agreement that only severely low levels of LVEF are related to increased mortality, the recent cohort studies reported conflicting findings, with both LVEF lower than 60% or higher than 70% associated with an elevated risk of mortality. 10 , 11 These studies were conducted in the general population undergoing echocardiography, but the heterogeneity inherent to these studies required further investigation in the specific population. The association between LVEF and adverse events in patients with CAD undergoing percutaneous coronary intervention (PCI) is not completely clear. Few studies have a large enough population to accurately define the relationship. The largest cohort in previous studies had 5127 patients, with all women aged >65 years diagnosed with acute coronary syndrome. 12 The largest pool analysis of RCTs had 6198 patients, but the patients were crudely divided into three groups (<40, 40–50, and ≥50%). 3 Besides, the RCTs do not reflect the real‐world practice, because enormous patients in the general population were excluded by the strict entry and exclusion criteria of RCTs. 13
In the present study, we endeavoured to identify the relationship between LVEF and the risk of mortality and cardiovascular events in CAD patients undergoing PCI in a large observational cohort from the National Center for Cardiovascular Diseases in China. We assessed whether or not there is a threshold above or below which there is no relationship between LVEF and mortality and cardiovascular events.
Methods
Study cohort
The present cohort study included 10 724 consecutive CAD patients undergoing PCI at the National Center for Cardiovascular Diseases in China (Fuwai Hospital) from January 2013 to December 2013. Clinical data were prospectively collected. Among them, 472 patients were excluded due to missing LVEF data and having a perioperative myocardial infarction (PMI). We excluded patients with PMI because it could affect subsequent ejection fraction or MACCE. PMI was defined by SCAI definition. 14 Finally, a total of 10 252 patients were enrolled in the study. All participants had written informed consent before the enrolment. The study complied with the Declaration of Helsinki and was approved by the ethical review board of Fuwai Hospital. The LVEF was measured by transthoracic echocardiography at admission before PCI. The PCI procedures were performed in the current standard manner, and the use of stents and equipment was at the discretion of the treating cardiologist.
Endpoint and follow‐up
All patients received follow‐up at regular intervals through telephone or outpatient clinic visits. The outcomes were all‐cause mortality and major adverse cardiovascular and cerebrovascular events (MACCE). MACCE was composed of cardiac death, myocardial infarction (MI), stent thrombosis (ST), target vessel revascularization (TVR), and stroke.
Statistical analysis
Description of continuous variables was presented as mean ± SD and compared using analysis of variance (ANOVA); categorical variables were presented as numbers with percentages and compared using the chi‐square test. Adjusted Cox proportional hazards regression and Kaplan–Meier survival curves analyses were performed to assess the association between LVEF and endpoints. The adjusted variables used in multivariate models were selected and chosen based on the variables with P values < 0.05 in the univariable analysis for mortality (Supplemental Table 1 ), including age, BMI, chronic kidney diseases (CKD, i.e. eGFR below 60), total occlusion, previous cerebrovascular disease, moderate to severe calcification, and baseline TIMI 3. Considering the clinical relevance, sex, lesion length, successful revascularization, and clinical presentation for PCI [stable coronary artery disease (stable CAD) and acute coronary syndrome (ACS)] were also used in multivariate models. Restricted cubic spline (RCS) analysis based on Cox proportional hazards models was used to assess the levels of LVEF on a continuous scale. In addition, LVEF were stratified by 5% width interval into eight groups: <40, 40–44.9, 45–49.9, 50–54.9, 55–59.9, 60–64.9, 65–69.9, and ≥70%. The reference group was the one associated with the lowest risk of all‐cause mortality or MACCE. SPSS Version 24.0 (Chicago, USA) and R statistical software version 4.0.3. (https://www.r‐project.org/) were used to conduct statistical analyses. All P values were two‐sided, and a P value < 0.05 was considered statistically significant.
Results
A total of 10 252 patients with a median follow‐up period of 2.4 years were included in the current study. Seven thousand nine hundred (77.1%) of them were men, and the mean age was 59 years. Table 1 displays that patients with low LVEF were more frequently men and current smokers, had longer lesion lengths, were older ages, and were more likely to have total occlusion and multivessel diseases. Alternatively, hypertension and hyperlipidaemia were more frequent in high LVEF. During the median 2.4 years of follow‐up, 137 (1.3%) patients had all‐cause mortality, and 816 (8.0%) patients had MACCE.
Table 1.
Baseline characteristics by LVEF categories
| LVEF <40 (N = 125) | LVEF 40–44.9 (N = 163) | LVEF 45–49.9 (N = 233) | LVEF 50–54.9 (N = 495) | LVEF 55–59.9 (N = 1269) | LVEF 60–64.9 (N = 3417) | LVEF 65–69.9 (N = 2959) | LVEF ≥70 (N = 1591) | P value | |
|---|---|---|---|---|---|---|---|---|---|
| Age, years | 60.3 (11.6) | 59.5 (11.8) | 58.3 (11.2) | 57.2 (11.3) | 58.9 (10.8) | 58.5 (10.3) | 58.0 (9.9) | 58.5 (9.8) | 0.006 |
| Male sex | 101 (80.8) | 133 (81.6) | 196 (84.1) | 432 (87.3) | 1,050 (82.7) | 2,627 (76.9) | 2,224 (75.2) | 1,137 (71.5) | <0.001 |
| BMI, kg/m2 | 25.5 (3.1) | 25.9 (3.3) | 25.7 (3.3) | 26.1 (3.3) | 26.1 (3.2) | 25.9 (3.2) | 25.9 (3.1) | 25.8 (3.3) | 0.046 |
| LVEF, % | 35.8 (3.9) | 41.5 (1.5) | 46.6 (1.4) | 52.0 (1.5) | 57.3 (1.5) | 61.8 (1.6) | 66.6 (1.5) | 72.5 (2.5) | <0.001 |
| Current smoking | 78 (62.4) | 99 (60.7) | 153 (65.7) | 323 (65.3) | 808 (63.7) | 1931 (56.5) | 1,631 (55.1) | 821 (51.6) | <0.001 |
| Diabetes mellitus | 43 (34.4) | 50 (30.7) | 85 (36.5) | 162 (32.7) | 395 (31.1) | 1,050 (30.7) | 868 (29.3) | 464 (29.2) | 0.218 |
| Hypertension | 78 (62.4) | 92 (56.4) | 129 (55.4) | 280 (56.6) | 779 (61.4) | 2,199 (64.4) | 1950 (65.9) | 1,087 (68.3) | <0.001 |
| Hyperlipidaemia | 71 (56.8) | 103 (63.2) | 155 (66.5) | 314 (63.4) | 840 (66.2) | 2,298 (67.3) | 2034 (68.7) | 1,096 (68.9) | 0.023 |
| Peripheral vascular disease | 3 (2.4) | 2 (1.2) | 5 (2.1) | 11 (2.2) | 43 (3.4) | 99 (2.9) | 75 (2.5) | 34 (2.1) | 0.413 |
| Previous cerebrovascular disease | 17 (13.6) | 19 (11.7) | 21 (9.0) | 46 (9.3) | 150 (11.8) | 367 (10.7) | 309 (10.4) | 166 (10.4) | 0.663 |
| Multivessel disease | 96 (76.8) | 130 (79.8) | 181 (77.7) | 388 (78.4) | 994 (78.3) | 2,579 (75.5) | 2,174 (73.5) | 1,166 (73.3) | 0.005 |
| Left main disease | 7 (5.6) | 13 (8.0) | 11 (4.7) | 25 (5.1) | 94 (7.4) | 207 (6.1) | 174 (5.9) | 105 (6.6) | 0.407 |
| Total occlusion | 43 (34.4) | 54 (33.1) | 79 (33.9) | 171 (34.5) | 313 (24.7) | 574 (16.8) | 392 (13.2) | 174 (10.9) | <0.001 |
| Lesion length, mm | 30.1 (19.8) | 29.9 (17.9) | 31.1 (19.0) | 32.2 (19.5) | 29.7 (20.0) | 29.0 (21.0) | 28.5 (18.3) | 27.5 (16.6) | <0.001 |
| Moderate to severe calcification | 23 (18.4) | 31 (19.0) | 43 (18.5) | 85 (17.2) | 212 (16.7) | 532 (15.6) | 454 (15.3) | 236 (14.8) | 0.5 |
| Baseline TIMI 3 | 67 (53.6) | 82 (50.3) | 112 (48.1) | 252 (50.9) | 777 (61.2) | 2,354 (68.9) | 2,157 (72.9) | 1,202 (75.5) | <0.001 |
| ACS presentation | 80 (64.0) | 101 (62.0) | 158 (67.8) | 345 (69.7) | 844 (66.5) | 2,144 (62.7) | 1771 (59.9) | 925 (58.1) | <0.001 |
| PCI duration, min | 38.2 (28.4) | 36.6 (26.6) | 41.0 (34.6) | 40.9 (36.1) | 37.7 (33.0) | 35.0 (30.3) | 34.4 (32.8) | 32.2 (25.7) | <0.001 |
| Successful revascularization | 118 (94.4) | 159 (97.5) | 225 (96.6) | 473 (95.6) | 1,231 (97.0) | 3,337 (97.7) | 2,872 (97.1) | 1,555 (97.7) | 0.055 |
BMI, body mass index; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction.
Continuous variables were presented as the mean with standard deviation. Categorical characteristics were described as counts with percentages.
As displayed in Figure 1 , with a stepwise 5% increase of LVEF from <40 to ≥70%, an inverse association between LVEF and all‐cause mortality rates and MACCE rates was observed. In Figure 2 , Kaplan–Meier survival curves show that LVEF under 55% was correlated with increased all‐cause mortality (P < 0.001) and LVEF under 65% was correlated with increased MACCE (P < 0.001).
Figure 1.
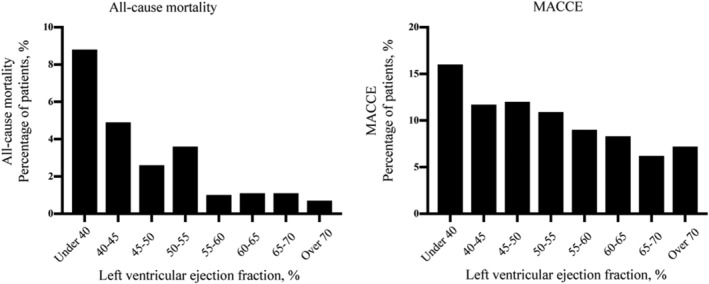
All‐cause mortality and MACCE rates according to LVEF categories. LVEF, left ventricular ejection function; MACCE, major adverse cardiovascular and cerebrovascular events.
Figure 2.
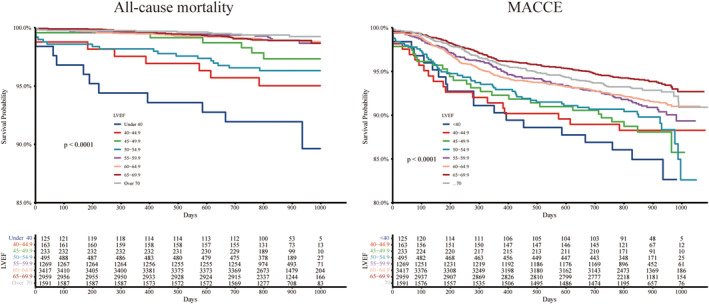
Kaplan–Meier curves by LVEF categories. LVEF, left ventricular ejection function; MACCE, major adverse cardiovascular and cerebrovascular events.
The associations of LVEF with all‐cause mortality and MACCE are illustrated in Figure 3 . A significantly higher adjusted risk was observed at LVEF <55% for all‐cause mortality and at LVEF <65% for MACCE. Patients with LVEF <55% had a more than 3.5‐fold higher mortality [adjusted hazard ratio (HR) 3.51, 95% confidence interval 2.41–5.13, P < 0.001] than those with LVEF ≥55%. Patients with LVEF <65% had a more than 1.3‐fold higher MACCE [adjusted HR 1.33, 95% confidence interval 1.15–1.53, P < 0.001] than those with LVEF ≥65%. A gradient–response relationship was observed, with an all‐cause mortality risk range between 8.6‐fold and 3.0‐fold increase from LVEF <40 to 50–54.9% (P for trend < 0.001) and MACCE risk range between 2.4‐fold and 1.4‐fold increase from LVEF <40 to 60–64.9% (P for trend < 0.001). Adjusted RCS for LVEF and events are shown in Figure 4 . A linear inverse pattern was observed at LVEF <55% for all‐cause mortality and at LVEF <65% for MACCE although the risk was comparable at higher LVEF. Similar findings were also seen in stable CAD and acute coronary syndrome (ACS) (Figure 5 ).
Figure 3.
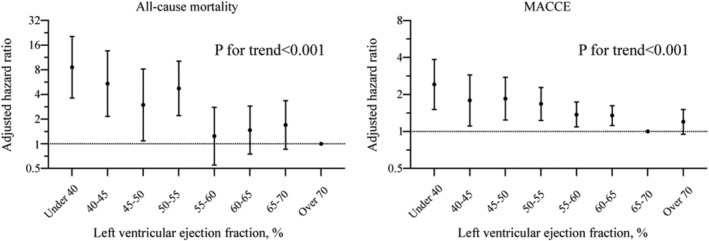
Adjusted hazard ratio and 95% confidence interval for all‐cause mortality and MACCE by LVEF categories. LVEF, left ventricular ejection function; MACCE, major adverse cardiovascular and cerebrovascular events.
Figure 4.
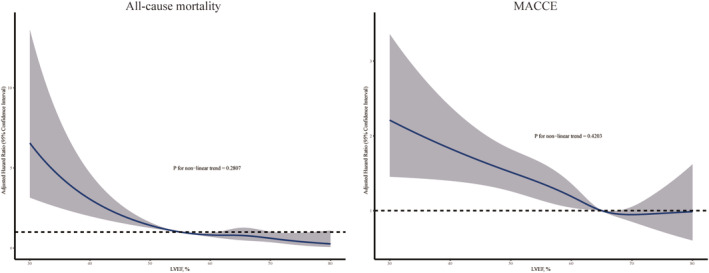
Adjusted restricted cubic spline curves for all‐cause mortality and MACCE by LVEF categories. LVEF, left ventricular ejection function; MACCE, major adverse cardiovascular and cerebrovascular events.
Figure 5.
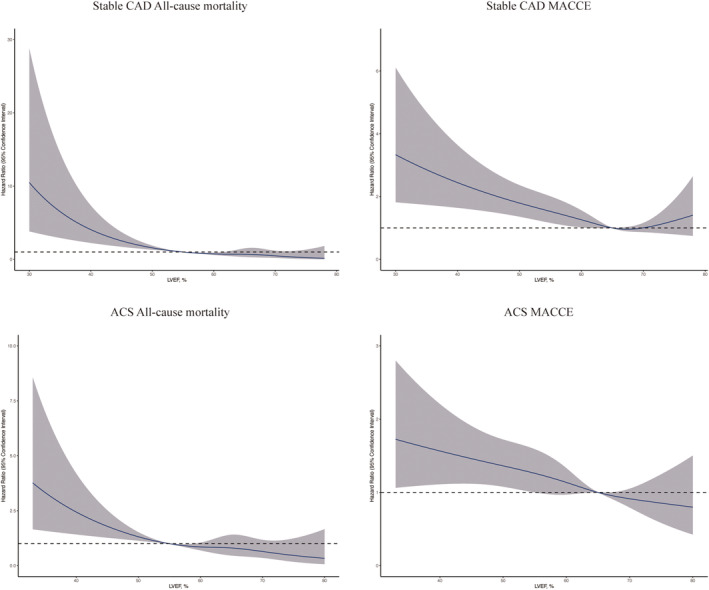
Adjusted restricted cubic spline curves for all‐cause mortality and MACCE by LVEF categories for stable CAD and ACS. ACS, acute coronary syndrome; CAD, coronary artery disease; LVEF, left ventricular ejection function; MACCE, major adverse cardiovascular and cerebrovascular events. [Correction added on 29 July 2022, after first online publication: the text in the lower right corner of every panel in Figure 5 has been deleted in this version.]
Discussion
In the present study, we reported an analysis of 10 252 CAD participants in a PCI cohort. We identified the linear inverse relationship of LVEF with all‐cause mortality and MACCE. The findings demonstrate that all‐cause mortality or MACCE was increased in patients with LVEF <55 or <65%, respectively. In contrast, LVEF >55 or >65% were not correlated with significantly higher risk. The cut‐off values for increased risk of all‐cause mortality and MACCE were at LVEF of 55% and LVEF of 65%, respectively. Patients with LVEF <55% had a more than 3.5‐fold higher mortality than those with LVEF ≥55%. Patients with LVEF <65% had a more than 1.3‐fold higher MACCE than those with LVEF ≥65%. Below 55 or 65%, there was a rise in mortality and MACCE.
To the best of our knowledge, this is the largest cohort study exploring the association between LVEF and mortality or cardiovascular events in CAD patients. The research is important given the recent yet unexplained finding of excess mortality in subjects with LVEF deviated from 60 to 65% in the general population. 10 Our results are relevant. The findings provide critical evidence for clinical risk evaluation and management. Furthermore, our study has crucial implications for the LVEF threshold used for the entry criterion in CAD‐related studies and trials.
Prior studies mainly focused on the association between LVEF and adverse events in HF patients, coming with various results. An inverse relation between LVEF and mortality or cardiovascular outcomes was observed in chronic or stable HF at LVEF <45%. 15 , 16 A U‐shaped relation for unadjusted mortality was observed in acute HF with a nadir at LVEF of 35%, but the increased risk in high LVEF was attenuated after adjustment. 17 Recent evidence of heterogeneous population studies found conflicting results. In a cohort of individuals without cardiovascular diseases at baseline from the Multi‐Ethnic Study of Atherosclerosis, they found a U‐shaped association for the risk of incident chronic HF with a nadir at 62.5%, but not for the risk of mortality with increased risk of mortality only at LVEF <50%. 18 On the contrary, echocardiographic data from the cohort in Geisinger health records in the USA and the registry from the National Echocardiography Database Australia (NEDA) show that both low and high levels of LVEF were associated with increased mortality. 10 , 11 Few studies have examined the relationship between LVEF and mortality in CAD patients undergoing PCI, with the majority of studies studying LVEF as dichotomization (LVEF <40 and ≥40%) 19 , 20 or as three categories (LVEF <40%, 40–50, and ≥50). 3 , 21 , 22 An interesting study focusing on women aged over 65 years showed that LVEF >65% is associated with an increased risk of death. 12 Moreover, most studies were conducted with a modest size of subjects; large enough PCI participants are required for the reliable examination. Based on 10252 participants, our study found that low, but not high, levels of LVEF were associated with increased mortality and MACCE in the large‐scale PCI cohort in contemporary practice. Consistent with pool analysis of CAD RCTs and direct analysis of the Multi‐Ethnic Study of Atherosclerosis, 3 , 18 we also found that low normal LVEF, more specifically 55 or 65%, was capable of risk‐stratified increased mortality or cardiovascular events.
Our results provide crucial clinical implications for understanding the optimal levels of LVEF in CAD patients. The finding shows that LVEF ≥55% or LVEF ≥65% is not associated with all‐cause mortality or MACCE respectively, which implies that LVEF over this threshold might be relatively optimal. Furthermore, the present study indicates that the LVEF cut‐off in CAD might need to be redefined based on findings in the CAD setting.
Limitations
First, our study was a retrospective analysis of prospectively collected data, and the inherent bias associated with this type of study should be carefully considered. However, the present study provided real‐world evidence in a non‐selected CAD population. Second, we did not have the data of LVEF at discharge or at follow‐up or physiological testing data.
Conclusions
Low and low normal, but not high, LVEF was associated with increased risk of all‐cause and MACCE in CAD patients undergoing PCI. Patients with LVEF ≥55% or LVEF ≥65% could be considered as the threshold above which there is no relationship between LVEF and mortality or cardiovascular events. Our findings might help to guide the clinical management and inclusion criterion in clinical trial design.
Conflict of interest
Nothing to report.
Funding
This work was supported by National Key R&D Program of China (2020YFC20047050), National Natural Science Foundation of China (81825003, 91957123, 81800327, 81900272), the Beijing Nova Program (Z201100006820002) from Beijing Municipal Science & Technology Commission, Research Unit of Medical Science Research Management/Basic and Clinical Research of Metabolic Cardiovascular Diseases from Chinese Academy of Medical Sciences (2021RU003), and Science and Technology Project of Xicheng District Finance (XCSTS‐SD2021‐01).
Supporting information
Supplementary Table 1. Univariable Cox analysis of all‐cause mortality.
Acknowledgements
We thank Drs Jingye Lin and Haodi Wu for the helpful discussions.
Liu, Y. , Song, J. , Wang, W. , Zhang, K. , Qi, Y. , Yang, J. , Wen, J. , Meng, X. , Gao, J. , Shao, C. , and Tang, Y.‐D. (2022) Association of ejection fraction with mortality and cardiovascular events in patients with coronary artery disease. ESC Heart Failure, 9: 3461–3468. 10.1002/ehf2.14063.
References
- 1. Marwick TH. Ejection fraction pros and cons. J Am Coll Cardiol 2018; 72: 2360–2379. [DOI] [PubMed] [Google Scholar]
- 2. Murphy SP, Ibrahim NE, Januzzi JL. Heart failure with reduced ejection fraction: A review. JAMA 2020; 324: 488. [DOI] [PubMed] [Google Scholar]
- 3. Siontis GC, Branca M, Serruys P, Silber S, Räber L, Pilgrim T, Valgimigli M, Heg D, Windecker S, Hunziker L. Impact of left ventricular function on clinical outcomes among patients with coronary artery disease. Eur J Prev Cardiol 2019; 26: 1273–1284. [DOI] [PubMed] [Google Scholar]
- 4. Falk V, Holm PJ, Iung B, Lancellotti P, Lansac E, Munoz DR, Rosenhek R. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2017; 38: 2739–2791. [DOI] [PubMed] [Google Scholar]
- 5. Solomon SD, Claggett B, Lewis EF, Desai A, Anand I, Sweitzer NK, O'meara E, Shah SJ, McKinlay S, Fleg JL, Sopko G. Influence of ejection fraction on outcomes and efficacy of Sacubitril/Valsartan (LCZ696) in heart failure with reduced ejection fraction: the prospective comparison of ARNI with ACEI to determine impact on Global Mortality and Morbidity in Heart Failure (PARADIGM‐HF) Trial. Circ Heart Fail 2016; 9(3):e002744. 10.1161/CIRCHEARTFAILURE.115.002744 [DOI] [PubMed] [Google Scholar]
- 6. Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W, Packer M. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta‐analysis of the EMPEROR‐Reduced and DAPA‐HF trials. The Lancet 2020; 396: 819–829. [DOI] [PubMed] [Google Scholar]
- 7. Pleger ST, Schulz‐Schönhagen M, Geis N, Mereles D, Chorianopoulos E, Antaredja M, Lewening M, Katus HA, Bekeredjian R. One year clinical efficacy and reverse cardiac remodelling in patients with severe mitral regurgitation and reduced ejection fraction after MitraClip © implantation. Eur J Heart Fail 2013; 15: 919–927. [DOI] [PubMed] [Google Scholar]
- 8. Bloch Thomsen PE, Jons C, Raatikainen MJP, Moerch Joergensen R, Hartikainen J, Virtanen V, Boland J, Anttonen O, Gang UJ, Hoest N, Boersma LVA, Platou ES, Becker D, Messier MD, Huikuri HV, for the Cardiac Arrhythmias and Risk Stratification After Acute Myocardial Infarction (CARISMA) Study Group . Long‐term recording of cardiac arrhythmias with an implantable cardiac monitor in patients with reduced ejection fraction after acute myocardial infarction: The cardiac arrhythmias and risk stratification after acute myocardial infarction (CARISMA) study. Circulation 2010; 122: 1258–1264. [DOI] [PubMed] [Google Scholar]
- 9. Packer M. Heart failure with a mid‐range ejection fraction. JACC: Heart Failure 2017; 5: 805–807. [DOI] [PubMed] [Google Scholar]
- 10. Wehner GJ, Jing L, Haggerty CM, Suever JD, Leader JB, Hartzel DN, Kirchner HL, Manus JNA, James N, Ayar Z, Gladding P, Good CW, Cleland JGF, Fornwalt BK. Routinely reported ejection fraction and mortality in clinical practice: where does the nadir of risk lie? Eur Heart J 2019: ehz550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stewart S, Playford D, Scalia GM, Currie P, Celermajer DS, Prior D, Codde J, Strange G, on behalf of the NEDA Investigators . Ejection fraction and mortality: a nationwide register‐based cohort study of 499 153 women and men. Eur J Heart Fail 2020: ejhf.2047. [DOI] [PubMed] [Google Scholar]
- 12. Saab FA, Steg PG, Avezum Á, López‐Sendón J, Anderson FA, Huang W, Eagle KA. Can an elderly woman's heart be too strong?: Increased mortality with high versus normal ejection fraction after an acute coronary syndrome. The Global Registry of Acute Coronary Events. Am Heart J 2010; 160: 849–854. [DOI] [PubMed] [Google Scholar]
- 13. Grapow MTR, von Wattenwyl R, Guller U, Beyersdorf F, Zerkowski H‐R. Randomized controlled trials do not reflect reality: Real‐world analyses are critical for treatment guidelines! J Thorac Cardiovasc Surg 2006; 132: 5–7. [DOI] [PubMed] [Google Scholar]
- 14. Moussa ID, Klein LW, Shah B, Mehran R, Mack MJ, Brilakis ES, Reilly JP, Zoghbi G, Holper E, Stone GW. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization. J Am Coll Cardiol 2013; 62: 1563–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Solomon SD, Anavekar N, Skali H, McMurray JJV, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, Pfeffer MA. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005; 112: 3738–3744. [DOI] [PubMed] [Google Scholar]
- 16. Curtis JP, Sokol SI, Wang Y, Rathore SS, Ko DT, Jadbabaie F, Portnay EL, Marshalko SJ, Radford MJ, Krumholz HM. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol 2003; 42: 736–742. [DOI] [PubMed] [Google Scholar]
- 17. Toma M, Ezekowitz JA, Bakal JA, O'Connor CM, Hernandez AF, Sardar MR, Zolty R, Massie BM, Swedberg K, Armstrong PW, Starling RC. The relationship between left ventricular ejection fraction and mortality in patients with acute heart failure: Insights from the ASCEND‐HF trial: Ejection fraction in acute heart failure. Eur J Heart Fail 2014; 16: 334–341. [DOI] [PubMed] [Google Scholar]
- 18. Yeboah J, Rodriguez CJ, Qureshi W, Liu S, Carr JJ, Lima JA, Hundley WG, Herrington DM. Prognosis of low normal left ventricular ejection fraction in an asymptomatic population‐based adult cohort: The multiethnic study of atherosclerosis. J Card Fail 2016; 22: 763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Daneault B, Généreux P, Kirtane AJ, Witzenbichler B, Guagliumi G, Paradis J‐M, Fahy MP, Mehran R, Stone GW. Comparison of three‐year outcomes after primary percutaneous coronary intervention in patients with left ventricular ejection fraction <40% versus ≥40% (from the HORIZONS‐AMI trial). Am J Cardiol 2013; 111: 12–20. [DOI] [PubMed] [Google Scholar]
- 20. Toma A, Stähli BE, Gick M, Gebhard C, Kaufmann BA, Mashayekhi K, Ferenc M, Buettner HJ, Neumann F‐J. Comparison of benefit of successful percutaneous coronary intervention for chronic total occlusion in patients with versus without reduced (≤40%) left ventricular ejection fraction. Am J Cardiol 2017; 120: 1780–1786. [DOI] [PubMed] [Google Scholar]
- 21. Thuijs DJFM, Milojevic M, Stone GW, Puskas JD, Serruys PW, Sabik JF, Dressler O, Crowley A, Head SJ, Kappetein AP. Impact of left ventricular ejection fraction on clinical outcomes after left main coronary artery revascularization: Results from the randomized EXCEL trial. Eur J Heart Fail 2020; 22: 871–879. [DOI] [PubMed] [Google Scholar]
- 22. Alkhalil M, Kearney A, MacElhatton D, Fergie R, Dixon L. The prognostic role of mid‐range ejection fraction in ST‐segment elevation myocardial infarction. Int J Cardiol 2020; 321: 12–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Univariable Cox analysis of all‐cause mortality.


