Abstract
Aims
Atrial fibrillation (AF)/atrial flutter is common during cardiac amyloidosis (CA). Electrical cardioversion (EC) is a strategy to restore sinus rhythm (SR). However, left atrial thrombus (LAT) represents a contraindication for EC. CA patients with AF/atrial flutter have a high prevalence of LAT. We aimed to evaluate EC characteristics, LAT prevalence and risk factors, and AF/atrial flutter outcome in CA patients undergoing EC, predominantly treated with direct oral anticoagulants (DOACs).
Methods and results
All patients with CA and AF/atrial flutter referred for the first time to our national referral centre of amyloidosis for EC from June 2017 to February 2021 were included in this study. In total, 66 patients (median age 74.5 [70;80.75] years, 67% male) were included with anticoagulation consisted of DOAC in 74% of cases. All patients underwent cardiac imaging before EC to rule out LAT. EC was cancelled due to LAT in 14% of cases. Complete thrombus resolution was observed in only 17% of cases. The two independent parameters associated with LAT were creatinine [hazard ratio (HR) = 1.01; confidence interval (CI) = 1.00–1.03, P = 0.036] and the use of antiplatelet agents (HR = 13.47; CI = 1.85–98.02). EC acute success rate was 88%, and we observed no complication after EC. With 64% of patients under amiodarone, AF/atrial flutter recurrence rate following EC was 51% after a mean follow‐up of 30 ± 27 months.
Conclusions
Left atrial thrombus was observed in 14% of CA patients listed for EC and mainly treated with DOAC. The acute EC success rate was high with no complication. The long‐term EC success rate was acceptable (49%).
Keywords: Cardiac amyloidosis, Atrial arrhythmia, Direct current cardioversion, Left atrial thrombus, Direct oral anticoagulants
Introduction
Cardiac amyloidosis (CA) corresponds to amyloid infiltration of the different layers of the heart and is a severe disease probably underdiagnosed with poor prognosis. 1 Atrial fibrillation (AF)/atrial flutter prevalence in CA patients varies from 15% 2 to 44% 3 with a higher rate up to 70% in transthyretin (ATTR) CA. 4 AF/atrial flutter are often highly symptomatic and poorly tolerated. AF during CA has specific characteristics due to atrial substrate infiltration and its therapeutic management is often challenging. Indeed, AF/atrial flutter is usually more advanced, with commonly a persistent evolution and a resistance to standard therapies such as electrical cardioversion (EC) or ablation to restore sinus rhythm (SR). 5 In addition, usual antiarrhythmic drugs (AADs) used to treat AF, such as AAD class Ic (flecainide), beta‐blockers, 6 calcium‐channel blockers, 7 or digoxin, should be avoided in this disease. 8 , 9
Parallel to this, intracardiac left atrial thrombi (LAT) are highly prevalent in CA between 20% and 30% of cases and anticoagulation guided by CHA2DS2‐VASc score is not applicable to predict thromboembolic risk. 10 However, data regarding LAT in CA patients are rare with small sample size studies. 11 , 12 In addition, cardiac imaging was not systematically performed before EC in most studies, and anticoagulation strategy applied was vitamin K antagonist (VKA) agents and/or heparin therapy. The aim of this study was to assess EC characteristics in CA patients with AF/atrial flutter, mainly treated with direct oral anticoagulants (DOACs).
Methods
In this retrospective observational study, we collected all first EC performed for AF/atrial flutter in CA patients from June 2017 to February 2021 at Henri Mondor Hospital, the French referral national centre for CA. The standard assessment for CA was exhaustive and based on electrocardiogram (ECG), echocardiography, screening for the presence of a monoclonal light chain in serum and urine, cardiac magnetic resonance (CMR) imaging, 99mTc‐bone compound scintigraphy, and cardiac computerized‐tomography scan (CT‐scan). If necessary, especially to confirm light chain (AL) amyloidosis, histological evidence was provided by accessory salivary gland biopsy or endomyocardial biopsy. CA diagnosis was suspected before EC and was confirmed according to international guidelines. 13 If CA was ruled out, the patient was not included in our study. If the patient was referred more than once for EC, we considered only the first hospitalization. For each patient, we recorded baseline characteristics: age, sex, weight, height, type of CA [light chain (AL), serum amyloid A (AA), wild‐type transthyretin (ATTRwt), or mutated transthyretin (ATTRmut)] and the date of diagnosis, type of arrhythmia (AF, atrial flutter, or atrial tachycardia) and its history duration before EC, stage of HF according to New York Heart Association (NYHA) classification, cardiovascular history [cardiovascular risk factors, heart disease, pacemaker (PM), or implantable cardioverter‐defibrillator (ICD)], biological values (haemoglobin, troponin, N‐terminal pro‐brain natriuretic peptide, creatinine, and creatinine clearance using the Cockroft–Gault formula), echocardiographic parameters [left ventricular ejection fraction (LVEF), LV strain, ventricular wall thickness, and left atrial (LA) size], and medication (antiplatelet agents, anticoagulants, and dosage and type of AAD). Each patient underwent systematic cardiac imaging [transesophageal echocardiography (TEE), cardiac CT‐scan, or CMR] before EC to rule out LAT, with focus on the left atrial appendage (LAA). EC acute success was defined as maintenance of SR, attested on a standard 12‐lead ECG, prior to patient leaving the recovery room. Potential early procedural complications including death, haemodynamic instability, sinus dysfunction, or systemic embolism (stroke) were recorded. All patients with proven ATTR were treated with Tafamidis. During follow‐up, recurrence of AF/atrial flutter was attested on ECG, Holter‐ECG, or cardiac device interrogation. In patients with intracardiac LAT, we analysed the modification of anticoagulant strategy and its efficacy on thrombus resolution during follow‐up. This strategy was left at the discretion of the care physician.
The study protocol was approved by a national ethics committee, and the study was performed in accordance with the ethical principles stated in the Declaration of Helsinki. All patients gave written informed consent to participate to the study.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation or median ± interquartile range (IQR) if needed. Statistical significance was assessed using the unpaired Student's t‐test or Mann–Whitney test if necessary. Categorical variables, expressed as numbers or percentages, were analysed using the χ 2 test or Fisher's exact test. Univariate analysis of variables was performed. Relevant parameters that were associated with LAT presence with a P value of <0.1 in the univariate analysis were entered into multiple logistic regression analysis.
Cumulative event rates were calculated according to the Kaplan–Meier method to assess freedom from any AF/atrial flutter recurrence during the follow‐up period. A P value ≤ 0.05 defined statistical significance. Statistical analysis was performed using MedCalc and Statview 5.0 statistical software.
Results
During the study period, 956 patients with CA were seen in our centre. Among these patients, 530 presented with AF/flutter and we performed 66 EC.
Patient baseline characteristics
Patient baseline characteristics are summarized in Table 1 . The median age was 74.5 [70;80.75] years; 67% were men. Forty‐five patients (68%) were implanted with a cardiac device (PM or ICD), ischaemic cardiomyopathy was present in nine patients (14%), and none had significant valvular disease. The median NYHA class was 2.6 ± 0.7. Amyloidosis was of ATTRwt type in 35 patients (53%), ATTRmut type in 13 patients (20%), and AL type in 18 patients (27%). ATTR mutation was Val122Ile in 12 patients and Thr49Ile in 1 patient. CA was diagnosed 55 [10;96] months before EC. Echocardiographic data indicated a median LVEF of 47% [40;55] and a median LV strain of −10% [−11.4;−7.7]. LA diameter, area, and volume were, respectively, 48 [44;53] mm, 29 [25;32] cm2, and 100 [82;119.75] mL. The atrial arrhythmia requiring EC was AF in 61 patients (76%) and flutter in 19 (24%) patients. Mean arrhythmia history duration before EC was 12 [3.5;23] months.
Table 1.
Patient baseline characteristics (n = 66)
| All patients | |
|---|---|
| n = 66 | |
| Age, years | 74.5 [70;80.75] |
| Male gender, n (%) | 44 (67) |
| Risk factors, n (%) | |
| Hypertension | 49 (74) |
| Diabetes mellitus | 15 (23) |
| Current smoker | 1 (1.5) |
| Cardiovascular history, n (%) | |
| Ischaemic cardiomyopathy | 9 (14) |
| Valvular disease | 0 (0) |
| Cardiac device PM or ICD, n (%) | 45 (68) |
| Mean NYHA class | 2.6 ± 0.7 |
| Weight (kg) | 71.5 [61.25;82.75] |
| Creatinine (μmol/L) | 126 [106.75;151] |
| Clearance of creatinine with the Cockroft formula (mL/min) | 45 [30.5;56.8] |
| Type of amyloidosis, n (%) | |
| ATTRwt | 35 (53) |
| ATTRmut | 13 (20) |
| Val122Ile | 12 (18) |
| Thr49Ile | 1 (2) |
| AL | 18 (27) |
| History of amyloidosis (months) | 55 [10;96] |
| Echocardiographic data | |
| LVEF (%) | 47 [40;55] |
| LV strain (%) | −10 [−11.4;−7.7] |
| LA diameter (mm) | 48 [44;53] |
| LA area (cm2) | 29 [25;32] |
| LA volume (mL) | 100 [82;119.75] |
| Septal LV thickness (mm) | 17 [15;19] |
| Posterior LV thickness (mm) | 16 [14;19] |
| Type of atrial arrhythmia (n, %) | |
| AF | 61 (76) |
| Atrial flutter | 19 (24) |
| Arrhythmia history duration (months) | 12 [3.5;23] |
AF, atrial fibrillation; AL, light chain amyloidosis; ATTRmut, mutated transthyretin amyloidosis; ATTRwt, wild‐type transthyretin amyloidosis; ICD, implantable cardioverter‐defibrillator; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PM, pacemaker.
Characteristics of electrical cardioversion
Table 2 presents baseline characteristics of EC. Antithrombotic therapy consisted of DOAC in 74% of the cases, VKA in 14%, heparin in 11%, antiplatelet agents in 9%, and 2% had none. The patient with no antithrombotic therapy had severe pancytopenia post‐chemotherapy requiring anticoagulation suspension and treatment with platelet transfusion before EC. Forty‐two (64%) EC were performed with amiodarone therapy. Cardiac imaging to rule out LAT was systematically carried out before EC as follows: 54 patients (82%) had TEE, 13 (20%) CT‐scan, 2 (3%) both, and 1 (1%) CMR. Nine (14%) EC were cancelled due to LAT and 57 were performed with an acute success rate of 88% (Figure 1 ). We observed no complication following EC.
Table 2.
Baseline characteristics of electrical cardioversion (n = 66)
| Antithrombotic therapy, n (%), n = 66 | |
| Direct oral anticoagulants | 49 (74) |
| VKA | 9 (14) |
| Heparin | 7 (11) |
| No antithrombotic therapy | 1 (2) |
| Antiplatelet agents | 6 (9) |
| Type of direct oral anticoagulants, n = 49 | |
| Rivaroxaban | 18 (37) |
| Apixaban | 30 (61) |
| Dabigatran | 1 (2) |
| Antiarrhythmic drugs (n, %) | |
| Amiodarone | 42 (64) |
| Beta‐blockers | 6 (9) |
| DCCV success rate (n = 57) | 50 (88) |
| Immediate complications following DCCV (n, %) | |
| Death | 0 (0) |
| Sinus node dysfunction | 0 (0) |
| Acute stroke | 0 (0) |
DCCV, direct current cardioversion; VKA, vitamin K antagonist.
Figure 1.
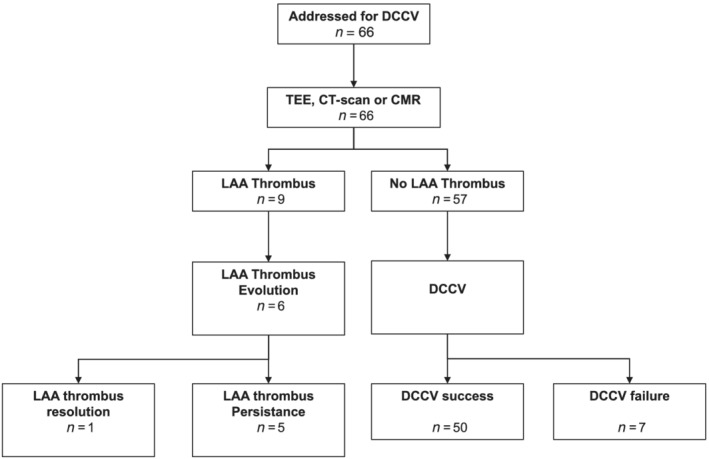
Flow chart of the study. CMR, cardiovascular magnetic resonance; CT‐scan, computerized‐tomography scan; DCCV, direct current cardioversion; LAA, left atrial appendage; TEE, transesophageal echocardiography.
As shown in Table 3 , the comparison between the population with and without LAT did not show any significant difference regarding age, sex, cardiovascular risk factors, cardiovascular history, type of amyloidosis, echocardiographic data, type of atrial arrhythmia, and drug therapy. However, renal function was significantly worse in the LAT group (creatinine: 172 [126;187] μmol/L vs. 124 [102;144] μmol/L, P = 0.01; clearance of creatinine: 31.6 [27.1;40.4] mL/min vs. 45.8 [30.7;60.8] mL/min, P = 0.04), and patients with LAT had a higher rate of antiplatelet agents use (33% vs. 5%, P = 0.006). No other significant differences were observed in anticoagulant therapy or the choice of DOAC (Table 4 ). On multivariate analysis, the two independent parameters significantly associated with LAT were creatinine [hazard ratio (HR) = 1.01; confidence interval (CI) = 1.00–1.03, P = 0.026] and the use of antiplatelet agents (HR = 13.47; CI = 1.85–98.02, P = 0.01). DOACs, prescribed in 74% of our studied population (n = 49), were correctly dosed in 75% of cases, underdosed in 10% of cases, and overdosed in 15% of cases. A non‐significant higher prevalence of LAT was found for patients in the underdosed DOAC group (26% vs. 8%, P = 0.18).
Table 3.
Direct current cardioversion characteristics according to the presence of left atrial thrombus
|
Left atrial thrombus n = 9 |
No left atrial thrombus n = 57 |
P | |
|---|---|---|---|
| Age, years | 76 [72;79] | 74 [70;81] | 0.46 |
| Male gender, n (%) | 8 (89) | 36 (63) | 0.13 |
| Risk factors, n (%) | |||
| Hypertension | 7 (78) | 42 (74) | 0.79 |
| Diabetes mellitus | 4 (44) | 11 (19) | 0.09 |
| Current smoker | 0 (0) | 1 (2) | 0.99 |
| Cardiovascular history, n (%) | |||
| Ischaemic cardiomyopathy | 2 (22) | 7 (12) | 0.42 |
| Valvular disease | 0 (0) | 0 (0) | 1.0 |
| Cardiac device PM or zICD, n (%) | 7 (78) | 38 (67) | 0.51 |
| Mean NYHA class | 2.6 ± 0.7 | 2.6 ± 0.7 | 0.78 |
| Weight (kg) | 67 [64;73] | 73 [60;84] | 0.53 |
| Creatinine (μmol/L) | 172 [126;187] | 124 [102;144] | 0.01 |
| Clearance of creatinine with the Cockroft formula (mL/min) | 31.6 [27.1;40.4] | 45.8 [30.7;60.8] | 0.04 |
| Type of amyloidosis, n (%) | |||
| ATTRwt | 5 (56) | 30 (53) | 0.87 |
| ATTRmut | 3 (33) | 10 (18) | 0.27 |
| Val122Ile | 3 (33) | 9 (16) | 0.20 |
| Thr49Ile | 0 (0) | 1 (2) | 0.99 |
| AL | 1 (11) | 17 (30) | 0.24 |
| History of amyloidosis (months) | 61.3 [15.2;70.9] | 54 [9;96.5] | 0.47 |
| Echocardiographic data | |||
| LVEF (%) | 47 [42;52] | 47 [40;55] | 0.76 |
| LV strain (%) | −9 [−10;−7.5] | −10 [−11;−8] | 0.38 |
| LA diameter (mm) | 51.5 [49;56] | 48 [44;53] | 0.34 |
| LA area (cm2) | 28.5 [26;32] | 29 [25;32] | 0.69 |
| LA volume (mL) | 107 [84;119] | 100 [81;120] | 0.86 |
| Septal LV thickness (mm) | 15 [15;18] | 17 [15;19] | 0.62 |
| Posterior LV thickness (mm) | 15 [15;18] | 16 [14;19] | 0.99 |
| Type of atrial arrhythmia (n, %) | |||
| AF | 8 (89) | 43 (75) | 0.37 |
| Atrial flutter | 1 (11) | 14 (25) | 0.37 |
| Arrhythmia history duration (months) | 14 [6;23] | 11.4 [3.4;23.2] | 0.88 |
AF, atrial fibrillation; AL, light chain amyloidosis; ATTRmut, mutated transthyretin amyloidosis; ATTRwt, wild‐type transthyretin amyloidosis; ICD, implantable cardioverter‐defibrillator; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PM, pacemaker.
Table 4.
Direct current cardioversion drug therapy characteristics according to the presence of left atrial thrombus
| Left atrial thrombus | No left atrial thrombus | P | |
|---|---|---|---|
| n = 9 | n = 57 | ||
| Antithrombotic therapy, n (%) | |||
| Direct oral anticoagulants | 5 (56) | 44 (77) | 0.17 |
| VKA | 2 (22) | 7 (12) | 0.42 |
| Heparin | 2 (22) | 5 (9) | 0.22 |
| No antithrombotic therapy | 0 (0) | 1 (2) | 0.99 |
| Antiplatelet agents | 3 (33) | 3 (5) | 0.006 |
| Type of direct oral anticoagulants, n = 49 | |||
| Rivaroxaban | 1 (11) | 17 (30) | 0.24 |
| Apixaban | 4 (44) | 26 (46) | 0.95 |
| Dabigatran | 0 (0) | 1 (2) | 0.99 |
| Antiarrhythmic drugs (n, %) | |||
| Amiodarone | 4 (44) | 38 (67) | 0.20 |
| Beta‐blockers | 1 (11) | 5 (9) | 0.82 |
VKA, vitamin K antagonist.
Follow‐up
After LAT diagnosis, antithrombotic therapy was adapted, as shown in Figure 2 . The physicians mainly proposed an increase in antithrombotic therapy consisting in the addition of antiplatelet agents. As shown in Figure 1 , among the nine diagnosed LAT, six patients had a cardiac imaging after a mean follow‐up of 4 months to assess the thrombus persistence, and we observed only one thrombus complete resolution (16%). For these six patients, the initial diagnosis of LAT was made on TEE for five patients and on CT‐scan for one patient. The cardiac imaging performed during follow‐up was CT‐scan in two patients and TEE in four patients. Finally, as shown in Figure 3 , AF/atrial flutter recurrence rate following EC was 51% after a mean follow‐up of 30 ± 27 months.
Figure 2.
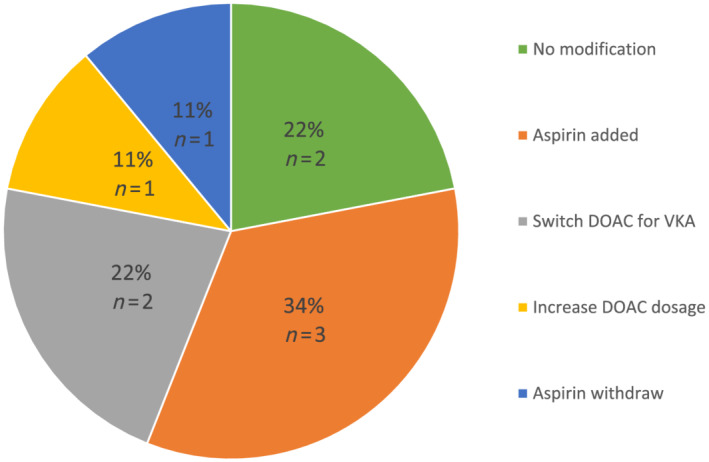
Antithrombotic strategy following left atrial thrombus diagnosis (n = 13). DOAC, direct oral anticoagulant; VKA, vitamin K antagonist.
Figure 3.
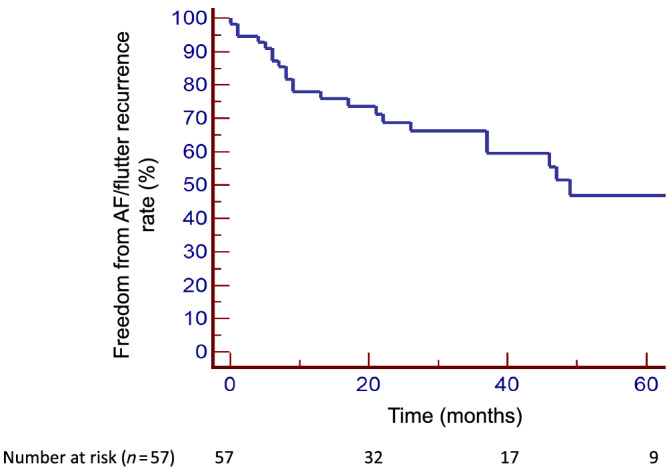
Kaplan–Meier curve of freedom from AF/atrial flutter recurrence following electrical cardioversion. AF, atrial fibrillation.
Discussion
The main findings of our study were as follows: (1) LAT in patients with CA and AF/atrial flutter were present in 14% of cases in our population treated in 74% of cases with DOAC; (2) the independent predictors of LAT were creatinine level and treatment with antiplatelet agents; (3) the EC immediate success rate was 88%; and (4) after a mean follow‐up of 30 months, the recurrence rate of AF/atrial flutter was 51%.
Left atrial thrombus in cardiac amyloidosis patients with atrial fibrillation/atrial flutter
In a prospective study, Schaeffer et al. assessed the overall incidence of thrombus formation in AF patients undergoing EC to 4.7%, with a lower rate in the DOAC group compared with the VKA group (2.5% vs. 5.3%, P = 0.02). When only focusing on patients with effective anticoagulation [uninterrupted DOAC and VKA therapy with international normalized ratio (INR) ≥ 2.0 for 3 weeks], the incidence of thrombus decreased to 2.7% with no difference between DOAC and VKA, and type of DOAC. 14 A recent meta‐analysis including 35 studies reported an LAT prevalence among patients with AF/flutter undergoing TEE following at least 3 weeks of continuous therapeutic oral anticoagulation with VKAs or DOACs of 2.73% without significant difference between VKA‐treated and DOAC‐treated patients. In addition, patients with non‐paroxysmal AF/atrial flutter had a four‐fold higher LAT prevalence compared with paroxysmal patients (4.81% vs. 1.03%, P < 0.001). 15
In CA patients, the rate of LAT is considered to be much higher. Indeed, El‐Am et al. reported 10 times more LAT on TEE in CA patients vs. standard patients with AF/atrial flutter (28% vs. 2.5%, P < 0.001). 16 In this former study, the anticoagulation agents consisted of VKA and/or heparin; no patient was on DOAC therapy and only 46% of patients with LAT had an efficient therapeutic level of anticoagulation for at least 3 weeks prior to EC.
To our knowledge, our cohort is the first to prioritize the use of DOAC (74% of the anticoagulation therapy) in CA patients with AF/atrial flutter undergoing EC. We observed an LAT rate of 14% in a CA population that received systematic LA cardiac imaging before EC. This high rate suggests that all CA patients should routinely undergo LA cardiac imaging prior to EC despite 3 weeks of pre‐procedural appropriate anticoagulation. In addition, there is no difference in the use of DOAC between the group with no LAT and the LAT group (56% vs. 77%, P = 0.17), but a more frequent use of antiplatelet agents in the LAT group (33% vs. 5%, P = 0.006). This former finding could indicate that patients with LAT could represent, at baseline, a population with a high thromboembolism risk. The LAT prevalence in our study is relatively lower than described in previous studies. Recently, Martinez‐Naharro et al. performed systematic CMR in CA patients with or without AF/atrial flutter and found 13.1% of thrombi mainly located in the LAA. They reported that 54% of patients with AF/flutter and LAT were treated DOAC; however, this population is different from our study and no specific analysis of LAT was assessed. 12 Data regarding DOAC in CA and AF/atrial flutter are sparse, but El‐Am et al. reported a 28% rate of LAT before planned EC in patients treated with VKA or heparin. 16 The potential benefit of DOAC therapy may be in part explained by the absence of INR adjustment that can be challenging in CA patients. However, to be effective, DOACs must be prescribed with the correct dosage according to therapeutic guidelines. In our population, we found that DOACs were more underdosed (25%) in patients with LAT than in patients without LAT (7.7%).
However, this association was not statistically significant as other LAT risk factors such as HF, or valvular disease, probably due to our small sample size.
In our study, the diagnosis of LAT was predominately based on TEE (82%), but additional imaging modalities including CT or CMR were also used. TEE is the gold standard for the evaluation of LAA stasis and thrombosis, 17 whereas CT is considered as an alternative. Indeed, a two‐phase scan protocol has been proposed for LAT diagnosis with a sensitivity and specificity of 100% and 98%, respectively. 18 In addition, CMR has been shown to be a comparable alternative to TEE or CT for LAT diagnosis. 19 The diagnostic accuracy of these imaging modalities suggests that our LAT prevalence of 14% is reliable.
When LAT is identified, according to the European Society of Cardiology guidelines, 20 effective anticoagulation is recommended for at least 3 weeks and a repeat TEE should be considered to ensure thrombus resolution before EC. However, in the case of thrombus persistence despite effective anticoagulation, the antithrombotic strategy is unclear. In our study, nine EC were cancelled due to LAT. The management of the antithrombotic treatment consisted mainly of an increase of anticoagulation therapy or the addition of antiplatelet agents.
Concerningly, after a mean follow‐up of 4 months, we observed a low rate of LAT resolution of 16% in CA, whereas in the general population, Nelles et al. estimated the rate of LAT resolution at 51% within 1 year in a population of patients mainly treated with oral anticoagulation at baseline. 21 In addition, Niku et al. observed a 60% rate of thrombus resolution in a standard AF population, but no difference in efficacy was found between DOAC and VKA. 22 Finally, the X‐TRA study 23 showed a rate of resolved or reduced thrombus of 60.4% after 6 weeks of Rivaroxaban treatment in patients with initial inappropriate anticoagulation therapy. In practice, according to the survey conducted in 2019 by the European Heart Rhythm Association in 54 centres, the most prevalent strategies were to switch from VKA to DOAC or vice versa with either higher (2.5–3.5) or standard target INR (2.0–3.0). Switching to low molecular weight heparin was less common. The use of unfractionated heparin or the addition of antiplatelet drugs was rare. 24 Indeed, there is no consensus to favour one strategy over another, and further studies are needed to define the best antithrombotic strategies to treat LAT. Recently, in the case of thrombus persistence, some authors have postulated the safety and feasibility of LAA closure. 25 , 26 This could facilitate EC, but at present, the place of this strategy in LAA thrombus management remains unclear. The reasons explaining the variable effects of antithrombotic therapy on LAA thrombus resolution are not fully understood; however, possible important factors may be chronic inflammation, age, structure, and architecture of the clot or associated comorbidities.We identified a significant independent association between creatinine level and antiplatelet agents use with LAT. First, regarding the renal function, we collected the creatinine level at the time of hospitalization, and because of creatinine fluctuations, we cannot guarantee that the creatinine value retained correctly reflects the renal function. It seems indeed possible that creatinine fluctuation had led to inappropriate dose reduction favouring thrombus formation. However, in chronic renal failure patients, the activation of procoagulant and proinflammatory pathways can be a potentially mechanistic explanation. 27 In addition, Feng et al. 11 found a borderline association between AL type and LAT in a multivariate analysis based on clinical variable (odds ratio 2.3; CI 0.96–5.6, P = 0.06). 27 Finally, Russo et al. suggested that the high LAT prevalence and low LAT resolution in CA patients could be due to endomyocardial injury secondary to amyloid infiltration, blood hypercoagulability involving hyperviscosity and renal insufficiency, and altered haemodynamics with atrial blood stasis. 28 Secondly, the association between antiplatelet agents use and LAT seems paradoxical, but we can suppose that these patients may have a very high thromboembolic risk.
Electrical cardioversion acute success
We observed an EC acute success rate of 88% in CA patients. In the Euro Heart Survey, success rates were similar among the general population. 29 Procedural complications were uncommon. In our study, we report no adverse event. In addition, El‐Am et al. showed that procedural complication rates were significantly higher in CA vs. control patients (14% vs. 2%, P = 0.007) 16 and included acute hypoxemia and stroke as post EC complications. The Euro Heart Survey reported an incidence of 4.2% of major complications in the general population including, in addition to rhythm and thromboembolic complications, the occurrence of acute HF. 29 The absence of complications observed in our study can be explained by the uncommon use of beta‐blockers (8%) and non‐dihydropyridine calcium‐channel blockers (0%) compared with previous studies that could account for haemodynamic instability and electrical conduction disorders. Also, in our population, we observed a higher prevalence of PM or defibrillator (68%) implantation that could explain the absence of severe bradycardia following EC.
Atrial fibrillation/atrial flutter recurrence management following electrical cardioversion: characteristics of cardiac amyloidosis patients
Atrial fibrillation is strongly associated with an increased risk of HF due to the loss of atrial contribution leading to reduction of ventricular filling and consequently stroke volume in patients with CA. However, the impact of AF on mortality is debated in this specific population. 3 , 4 Indeed, Mints et al. failed to demonstrate a significant survival difference comparing strategies of AF rhythm control and rate control, 30 but Donnellan et al. proved on multivariable models that maintenance of SR in ATTR CA was associated with a significant reduction of mortality. 4 Also, in this former study, EC was more effective when performed earlier in the disease course: 90% of patients with stage 1 ATTR‐CA remaining free of recurrent AF/atrial flutter at 30 days following EC compared with only 33% of those with stage 3 disease (P < 0.0001). 4 No similar study has been conducted so far in AL CA. In our study, CA stages were not recorded but duration of CA between diagnosis and EC was 61 months, corresponding to relatively advanced CA disease states.
Surprisingly, we found that AF/atrial flutter recurrence rate of 51% was relatively low after a mean follow‐up of 30 ± 27 months. Several studies assessed the risk of AF recurrence after cardioversion in the general population. In a meta‐analysis including 44 randomized controlled trials and 11 322 patients, Lafuente‐Lafuente et al. found that pooled recurrence rates after EC at 1 year were high: 71–84% in controls and reduced to 44–67% in patients treated with AAD. 31 One may have expected a higher rate of AF/atrial flutter recurrence following EC in CA patients as a result of the atrial myopathy associated with the disease. Our improved acute success rate can be explained by the wider use of amiodarone that was given in 64% of cases before EC to maintain SR. Indeed, it is recognized that amiodarone is more effective than other AADs to prevent AF recurrence 31 and the use of other AADs is usually contraindicated in CA patients.
Limitations
This is a retrospective single‐centre study and, thus, our results should be interpreted with caution. However, our centre is the national referral centre for CA and our cohort of patients represents, to date, the largest cohort of CA patients undergoing EC with LAT analysis. Clinical biases in patient recruitment and management are inherent to the methodology of this study. Moreover, the patient population remains small and a lack of statistical power can be questioned. As there was no specific protocol for AF/atrial flutter recurrence detection or LAT follow‐up, observations could have been underestimated. Finally, LAT presence is a surrogate endpoint that does not predict directly the clinical risk of peripheral thromboembolism.
Conclusions
In CA patients, after excluding LAT with systematic cardiac imaging, EC is effective in restoring SR in the majority of patients (88%) with no complications. The incidence of LAT was 14% in our population that were treated with a DOAC in 74% of cases. Finally, the long‐term SR maintenance success rate following EC was acceptable (49%) in a population predominately treated with amiodarone.
Conflict of interest
None declared.
Funding
None.
Touboul, O. , Algalarrondo, V. , Oghina, S. , Elbaz, N. , Rouffiac, S. , Hamon, D. , Extramiana, F. , Gandjbakhch, E. , D'Humieres, T. , Marijon, E. , Dhanjal, T. S. , Teiger, E. , Damy, T. , and Lellouche, N. (2022) Electrical cardioversion of atrial arrhythmias with cardiac amyloidosis in the era of direct oral anticogulants. ESC Heart Failure, 9: 3556–3564. 10.1002/ehf2.14082.
Affiliations 1–4 are centres part of the GPUR (Groupe Parisien Universitaire de Rythmologie).
References
- 1. Rubin J, Maurer MS. Cardiac amyloidosis: overlooked, underappreciated, and treatable. Annu Rev Med. 2020; 71: 203–219. [DOI] [PubMed] [Google Scholar]
- 2. Longhi S, Quarta CC, Milandri A, Lorenzini M, Gagliardi C, Manuzzi L, Bacchi‐Reggiani ML, Leone O, Ferlini A, Russo A, Gallelli I, Rapezzi C. Atrial fibrillation in amyloidotic cardiomyopathy: prevalence, incidence, risk factors and prognostic role. Amyloid. 2015; 22: 147–155. [DOI] [PubMed] [Google Scholar]
- 3. Sanchis K, Cariou E, Colombat M, Ribes D, Huart A, Cintas P, Fournier P, Rollin A, Carrié D, Galinier M, Maury P, Duparc A, Lairez O, On behalf of the Toulouse Amyloidosis Research Network collaborators . Atrial fibrillation and subtype of atrial fibrillation in cardiac amyloidosis: clinical and echocardiographic features, impact on mortality. Amyloid. 2019; 26: 128–138. [DOI] [PubMed] [Google Scholar]
- 4. Donnellan E, Wazni OM, Hanna M, Elshazly MB, Puri R, Saliba W, Kanj M, Vakamudi S, Patel DR, Baranowski B, Cantillon D, Dresing T, Jaber WA. Atrial fibrillation in transthyretin cardiac amyloidosis: predictors, prevalence, and efficacy of rhythm control strategies. JACC Clin Electrophysiol. 2020; 6: 1118–1127. [DOI] [PubMed] [Google Scholar]
- 5. Black‐Maier E, Rehorn M, Loungani R, Friedman DJ, Alenezi F, Geurink K, Pokorney SD, Daubert JP, Sun AY, Atwater BD, Jackson KP, Hegland DD, Thomas KL, Bahnson TD, Khouri MG, Piccini JP. Catheter ablation of atrial fibrillation in cardiac amyloidosis. Pacing Clin Electrophysiol. 2020; 43: 913–921. [DOI] [PubMed] [Google Scholar]
- 6. Aimo A, Vergaro G, Castiglione V, Rapezzi C, Emdin M. Safety and tolerability of neurohormonal antagonism in cardiac amyloidosis. Eur J Intern Med. 2020; 80: 66–72. [DOI] [PubMed] [Google Scholar]
- 7. Pollak A, Falk RH. Left ventricular systolic dysfunction precipitated by verapamil in cardiac amyloidosis. Chest. 1993; 104: 618–620. [DOI] [PubMed] [Google Scholar]
- 8. Donnelly JP, Sperry BW, Gabrovsek A, Ikram A, Tang WHW, Estep J, Hanna M. Digoxin use in cardiac amyloidosis. Am J Cardiol. 2020; 133: 134–138. [DOI] [PubMed] [Google Scholar]
- 9. Muchtar E, Gertz MA, Kumar SK, Lin G, Boilson B, Clavell A, Lacy MQ, Buadi FK, Hayman SR, Kapoor P, Dingli D, Rajkumar SV, Dispenzieri A, Grogan M. Digoxin use in systemic light‐chain (AL) amyloidosis: contra‐indicated or cautious use? Amyloid. 2018; 25: 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feng D, Edwards WD, Oh JK, Chandrasekaran K, Grogan M, Martinez MW, Syed II, Hughes DA, Lust JA, Jaffe AS, Gertz MA, Klarich KW. Intracardiac thrombosis and embolism in patients with cardiac amyloidosis. Circulation. 2007; 116: 2420–2426. [DOI] [PubMed] [Google Scholar]
- 11. Feng D, Syed IS, Martinez M, Oh JK, Jaffe AS, Grogan M, Edwards WD, Gertz MA, Klarich KW. Intracardiac thrombosis and anticoagulation therapy in cardiac amyloidosis. Circulation. 2009; 119: 2490–2497. [DOI] [PubMed] [Google Scholar]
- 12. Martinez‐Naharro A, Gonzalez‐Lopez E, Corovic A, Mirelis JG, Baksi AJ, Moon JC, Garcia‐Pavia P, Gillmore JD, Hawkins PN, Fontana M. High prevalence of intracardiac thrombi in cardiac amyloidosis. J Am Coll Cardiol. 2019; 73: 1733–1734. [DOI] [PubMed] [Google Scholar]
- 13. Yamamoto H, Yokochi T. Transthyretin cardiac amyloidosis: an update on diagnosis and treatment. ESC Heart Fail. 2019; 6: 1128–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schaeffer B, Rüden L, Salzbrunn T, Pinnschmidt HO, Akbulak R, Moser JM, Jularic M, Meyer C, Eickholt C, Sultan A, Lüker J, Steven D, Willems S, Hoffmann BA. Incidence of intracardiac thrombus formation prior to electrical cardioversion in respect to the mode of oral anticoagulation. J Cardiovasc Electrophysiol. 2018; 29: 537–547. [DOI] [PubMed] [Google Scholar]
- 15. Lurie A, Wang J, Hinnegan KJ, McIntyre WF, Belley‐Côté EP, Amit G, Healey JS, Connolly SJ, Wong JA. Prevalence of left atrial thrombus in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2021; 77: 2875–2886. [DOI] [PubMed] [Google Scholar]
- 16. El‐Am EA, Dispenzieri A, Melduni RM, Ammash NM, White RD, Hodge DO, Noseworthy PA, Lin G, Pislaru SV, Egbe AC, Grogan M, Nkomo VT. Direct current cardioversion of atrial arrhythmias in adults with cardiac amyloidosis. J Am Coll Cardiol. 2019; 73: 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aschenberg W, Schlüter M, Kremer P, Schröder E, Siglow V, Bleifeld W. Transesophageal two‐dimensional echocardiography for the detection of left atrial appendage thrombus. J Am Coll Cardiol. 1986; 7: 163–166. [DOI] [PubMed] [Google Scholar]
- 18. Hur J, Kim YJ, Lee HJ, Ha JW, Heo JH, Choi EY, Shim CY, Kim TH, Nam JE, Choe KO, Choi BW. Left atrial appendage thrombi in stroke patients: detection with two‐phase cardiac CT angiography versus transesophageal echocardiography. Radiology. 2009; 251: 683–690. [DOI] [PubMed] [Google Scholar]
- 19. Rathi VK, Reddy ST, Anreddy S, Belden W, Yamrozik JA, Williams RB, Doyle M, Thompson DV, Biederman RWW. Contrast‐enhanced CMR is equally effective as TEE in the evaluation of left atrial appendage thrombus in patients with atrial fibrillation undergoing pulmonary vein isolation procedure. Heart Rhythm. 2013; 10: 1021–1027. [DOI] [PubMed] [Google Scholar]
- 20. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, de Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GYH, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016; 18: 1609–1678. [DOI] [PubMed] [Google Scholar]
- 21. Nelles D, Lambers M, Schafigh M, Morais P, Schueler R, Vij V, Tiyerili V, Weber M, Schrickel JW, Nickenig G, Hammerstingl C, Sedaghat A. Clinical outcomes and thrombus resolution in patients with solid left atrial appendage thrombi: results of a single‐center real‐world registry. Clin Res Cardiol. 2021; 110: 72–83. [DOI] [PubMed] [Google Scholar]
- 22. Niku AD, Shiota T, Siegel RJ, Rader F. Prevalence and resolution of left atrial thrombus in patients with nonvalvular atrial fibrillation and flutter with oral anticoagulation. Am J Cardiol. 2019; 123: 63–68. [DOI] [PubMed] [Google Scholar]
- 23. Lip GY, Hammerstingl C, Marin F, Cappato R, Meng IL, Kirsch B, van Eickels M, Cohen A, X‐TRA study and CLOT‐AF registry investigators . Left atrial thrombus resolution in atrial fibrillation or flutter: results of a prospective study with rivaroxaban (X‐TRA) and a retrospective observational registry providing baseline data (CLOT‐AF). Am Heart J. 2016; 178: 126–134. [DOI] [PubMed] [Google Scholar]
- 24. Farkowski MM, Jubele K, Marín F, Gandjbakhch E, Ptaszynski P, Merino JL, Lenarczyk R, Potpara TS. Diagnosis and management of left atrial appendage thrombus in patients with atrial fibrillation undergoing cardioversion or percutaneous left atrial procedures: results of the European Heart Rhythm Association survey. Europace. 2020; 22: 162–169. [DOI] [PubMed] [Google Scholar]
- 25. Lee OH, Kim JS, Pak HN, Hong GR, Shim CY, Uhm JS, Cho IJ, Joung B, Yu CW, Lee HJ, Kang WC, Shin ES, Choi RK, Lim DS, Jang Y. Feasibility of left atrial appendage occlusion for left atrial appendage thrombus in patients with persistent atrial fibrillation. Am J Cardiol. 2018; 121: 1534–1539. [DOI] [PubMed] [Google Scholar]
- 26. Sahiner L, Coteli C, Kaya EB, Ates A, Kilic GS, Yorgun H, Aytemir K. Left atrial appendage occlusion in patients with thrombus in left atrial appendage. J Invasive Cardiol. 2020; 32: 222–227. [DOI] [PubMed] [Google Scholar]
- 27. Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, Furberg CD, Psaty BM. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003; 107: 87–92. [DOI] [PubMed] [Google Scholar]
- 28. Russo D, Limite LR, Arcari L, Autore C, Musumeci MB. Predicting the unpredictable: how to score the risk of stroke in cardiac amyloidosis? J Am Coll Cardiol. 2019; 73: 2910–2911. [DOI] [PubMed] [Google Scholar]
- 29. Pisters R, Nieuwlaat R, Prins MH, Le Heuzey JY, Maggioni AP, Camm AJ, Crijns HJ, Euro Heart Survey Investigators . Clinical correlates of immediate success and outcome at 1‐year follow‐up of real‐world cardioversion of atrial fibrillation: the Euro Heart Survey. Europace. 2012; 14: 666–674. [DOI] [PubMed] [Google Scholar]
- 30. Mints YY, Doros G, Berk JL, Connors LH, Ruberg FL. Features of atrial fibrillation in wild‐type transthyretin cardiac amyloidosis: a systematic review and clinical experience. ESC Heart Fail. 2018; 5: 772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lafuente‐Lafuente C, Mouly S, Longás‐Tejero MA, Mahé I, Bergmann JF. Antiarrhythmic drugs for maintaining sinus rhythm after cardioversion of atrial fibrillation: a systematic review of randomized controlled trials. Arch Intern Med. 2006; 166: 719–728. [DOI] [PubMed] [Google Scholar]


