Abstract
Background
The prognosis of invasive mucinous adenocarcinoma (IMA) remains controversial and should be clarified by comparison with the International Association for the Study of Lung Cancer (IASLC) histologic grading system for invasive nonmucinous adenocarcinoma (INMA).
Methods
This study included patients with IMA who underwent curative resection. Their clinicopathological outcomes were compared with those of patients with INMA. Propensity score matching was performed to compare the prognosis of IMA with IASLC grade 2 or 3. Kaplan–Meier survival curves and log‐rank tests were used to analyze recurrence‐free survival (RFS) and overall survival (OS).
Results
The prognoses of IMA and IASLC grade 2 were similar in terms of RFS and OS. Although patients with IMA had better RFS than patients with IASLC grade 3, the OS was not significantly different. After propensity score matching, IMA demonstrated similar RFS to IASLC grade 2 but superior to IASLC grade 3; there was no difference in the OS compared with grades 2/3. Multivariate analysis revealed that tumor size (hazard ratio [HR] = 1.20, p = 0.028), lymphovascular invasion (HR = 127.5, p = 0.003), and maximum standardized uptake value (HR = 1.24, p = 0.005) were poor prognostic predictors for RFS. Patients with IMA demonstrated RFS similar to and significantly better than that of patients with IASLC grades 2 and 3, respectively. For OS, IMA prognosis was between that of IASLC grades 2 and 3.
Conclusions
Since the prognosis of IMA among lung adenocarcinomas appears to be relatively worse, further clinical studies investigating IMA‐specific treatment and follow‐up plans are necessary to draw more inferences.
Keywords: classification, lung adenocarcinoma, mucinous adenocarcinoma, pathologic subtype, prognosis
Patients with Inavsive mucinous adenocarcinoma (IMA) have better Recurrence‐free surviva (RFS) than those with International Association for the Study of Lung Cancer (IASLC) grade 3 lung adenocarcinoma.
Patients with IMA have similar RFS to those with IASLC grade 2 lung adenocarcinoma.
The Overall survival (OS) of IMA was between IASLC grades 2 and 3 lung adenocarcinomas.
Tumor size, lymphovascular invasion, and SUVmax are prognostic factors for RFS in IMA.

INTRODUCTION
Invasive mucinous adenocarcinoma (IMA) is histopathologically characterized by tumors with goblet or columnar cells containing abundant intracytoplasmic mucin. 1 IMA is a rare variant of adenocarcinoma, accounting for approximately 5% of all pulmonary adenocarcinomas (ADCs). 2 In the 2011 lung adenocarcinoma classification system by the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS), IMAs were classified as invasive ADC variants. 3 Moreover, according to the 2015 World Health Organization (WHO) classification, IMA is an invasive ADC variant. 4
In June 2020, the IASLC pathology committee proposed a new histologic grading system for invasive pulmonary ADC 5 taking into account predominant histologic and high‐grade patterns based on the 2015 WHO classification. 4 This grading system classifies any tumor with ≥20% high‐grade patterns, including solid, micropapillary, and complex glandular patterns, as IASLC grade 3. Moreover, three further validation studies confirmed that this grading system provides significant prognostication. 5 , 6 , 7 , 8 However, IMA differs from invasive nonmucinous adenocarcinoma (INMA) because of major clinical, radiologic, pathologic, and genetic differences. Therefore, IMA was excluded from this grading system.
The prognosis of IMA remains controversial. Russell et al. 9 and Amin et al. 10 suggested that IMAs are usually associated with poor survival outcomes. In contrast, Warth et al. 11 reported that IMAs had better prognosis than conventional INMA. It is necessary to confirm the prognosis of IMA by comparing it with INMA based on the new grading system. This study aimed to compare the prognosis between patients with IMA and those with INMA classified according to the IASLC histologic grading system and review the clinicopathologic and radiologic features of surgically resected IMA tumors.
METHODS
Study population
The institutional review boards of two institutions (Hospital A: approval No. 4‐2021‐1633; Hospital B: approval No. 3–2021‐0509) approved this study. The requirement for informed consent was waived owing to the study's retrospective design.
Of the 3132 patients who underwent curative resection for lung cancer between 2012 and 2017 in both hospitals, 2323 lung ADCs were identified. Of these, 853 were excluded for the following reasons 1 : previous cancer history (n = 434), 2 concomitant presence of other malignancies in the lung (n = 112), 3 neoadjuvant treatment (n = 45), 4 incomplete resection (n = 4), and 5 30‐day mortality (n = 5). Patients with the following types of ADC were also excluded: mixed invasive mucinous and nonmucinous ADC (n = 16), ADC in situ or minimally invasive ADC (n = 202), and semiquantitative assessment not reported (n = 34). Ultimately, 112 patients with completely resected solitary IMAs and 1358 patients with INMA were included in this study (Supplementary Figure S1).
FIGURE 1.
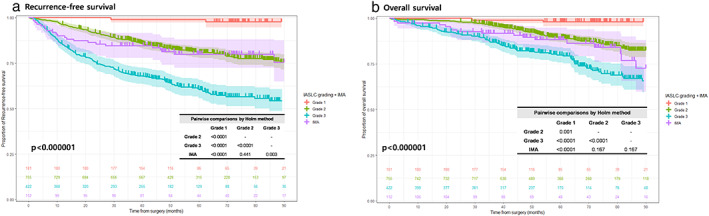
Recurrence‐free (a) and overall (b) survival curves of patients with IMA and INMA (IASLC grades 1–3). IALSC, International Association for the Study of Lung Cancer; INMA, invasive non‐mucinous adenocarcinoma; IMA, invasive mucinous adenocarcinoma
Data on clinical presentation, tumor stage, surgical treatment methods, and survival outcomes were obtained from electronic medical records. The tumor, node, and metastatic stages for each lung cancer were determined according to the eighth edition of the Cancer Staging Manual of the American Joint Committee on Cancer. 10 The survival and disease progression were also assessed according to medical records and data from the Korea National Statistical Office.
Pathologic evaluation
Two experienced lung pathologists (Y.C. and H.S.S.) interpreted all the tissue sections. The histopathologic criteria for IMA included tumor cells with a goblet or columnar cell morphological pattern with abundant intracytoplasmic mucin. In case of INMAs, histologic subtyping was carried out according to the 2011 IASLC/ATS/ERS and 2015 WHO classifications. The percentage of each histologic component was recorded in 5% increments (lepidic, acinar, papillary, micropapillary, and solid). In addition, we analyzed and scored the complex glandular patterns. Discrepancies in classification were resolved through consensus discussion.
Tumor spread through air spaces (STAS) was defined as the presence of tumor cells within the lung parenchyma's air spaces beyond the primary tumor's edge. Nonmucinous tumors were categorized into three subgroups based on the new histologic grading system of the IASLC: grade 1, lepidic predominant tumor with no or <20% of high‐grade patterns; grade 2, acinar or papillary predominant tumor, both with no or <20% of high‐grade patterns; and grade 3, any tumor with ≥20% high‐grade patterns (solid, micropapillary, and complex glandular patterns).
Statistical analysis
For continuous variables (age, pathologic size, and pulmonary function test), we present data as median and interquartile range after their normality was checked. They were later compared using the Mann–Whitney U test. Fisher's exact test was used to compare categorical variables of IMA and INMA. Overall survival (OS) was defined as the time from surgery to death from any cause or censored at the last follow‐up. Recurrence‐free survival (RFS) was defined as the time from surgery to recurrence or death from any cause or censored at the last follow‐up. RFS and OS were estimated using the Kaplan–Meier method. The log‐rank test and pairwise comparison using the Holm method were used to evaluate the differences among subgroups.
To adjust unbalanced compounding variables, a propensity score matching method was used to compare IMA and IASLC grades 2 and 3. The propensity score for each participant was measured using a logistic model that included the following variables: age, sex, comorbidities (diabetes mellitus and hypertension), smoking history, pathologic tumor size, and pathologic nodal stages. Then, nearest‐neighbor matching within 0.2 caliper width without replacement was used to perform 1:1 matching of patients in the two groups.
Multivariate Cox proportional hazards regression was performed to identify RFS and OS risk factors among patients with IMA. Variables with a p value <0.10 on univariate analysis were used as the input variables for the multivariable Cox regression analysis. Statistical analyses were performed using R version 4.0.4 (R Core Team, Vienna, Austria). Statistical significance was set at ≤0.05.
RESULTS
Patient baseline characteristics
Compared to patients with INMA (n = 1358, IASLC grades 1–3), patients with IMA (n = 112) had similar characteristics in all clinical variables other than the primary lesion location (p < 0.001). IMA was more frequently observed in the lower lobes (Table 1). According to histopathologic results, IMA demonstrated lower nodal stages (p < 0.001), lower frequency of lymphovascular invasion (p < 0.001), and visceral pleural invasion (p < 0.001) than INMA; however, positivity in STAS was more frequently observed in IMA than in INMA (Table 2). Generally, the stage I portion was lower in patients that underwent curative resection (p = 0.049). There was no difference between the two groups in the number of recurrences, deaths, or patients who underwent adjuvant treatments. The specific recurrence sites and causes of death are described in Supplementary Table S1 and S2.
TABLE 1.
The clinical characteristics of mucinous and nonmucinous (IASLC grade 1–3) invasive pulmonary adenocarcinoma
| Factor | IMA | INMA | p value a | IASLC grading subgroups in INMA | ||
|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | ||||
| N = 112 | N = 1358 | n = 181 | n = 755 | n = 422 | ||
| Age | 64.5 [56.8, 71.3] | 63.0 [55.0, 71.0] | 0.319 | 64.0 [58.0, 71.0] | 63.0 [55.0, 70.0] | 64.0 [55.0, 71.0] |
| Gender | 0.844 | |||||
| Female | 57 (50.9) | 707 (52.1) | 97 (53.6) | 444 (58.8) | 166 (39.3) | |
| Male | 55 (49.1) | 651 (47.9) | 84 (46.4) | 311 (41.2) | 256 (60.7) | |
| Diabetes mellitus | 21 (18.8) | 224 (16.5) | 0.512 | 34 (18.8) | 121 (16.0) | 69 (16.4) |
| Hypertension | 43 (38.4) | 566 (41.7) | 0.550 | 73 (40.3) | 315 (41.7) | 178 (42.2) |
| Smoking history | 0.224 | |||||
| Ex and current | 36 (32.1) | 520 (38.3) | 66 (36.5) | 239 (31.7) | 215 (50.9) | |
| Never | 76 (67.9) | 838 (61.7) | 115 (63.5) | 516 (68.3) | 207 (49.1) | |
| ECOG | 0.913 | |||||
| ECOG 0 | 98 (96.1) | 1288 (95.9) | 174 (96.1) | 720 (96.4) | 394 (94.9) | |
| ECOG 1 | 3 (2.9) | 36 (2.7) | 2 (1.1) | 20 (2.7) | 14 (3.4) | |
| ECOG 2 | 1 (1.0) | 19 (1.4) | 5 (2.8) | 7 (0.9) | 7 (1.7) | |
| FEV1 (%) | 106.0 [93.0, 118.5] | 104.0 [93.0, 114.8] | 0.442 | 103.0 [94.0, 118.8] | 105.0 [95.0, 115.0] | 101.0 [90.8, 113.0] |
| FEV1/FVC ratio (%) | 77.0 [73.2, 80.9] | 76.0 [70.9, 80.0] | 0.053 | 75.7 [71.0, 79.0] | 76.0 [71.0, 81.0] | 75.0 [69.0, 79.2] |
| Extent of surgery | 0.330 | |||||
| Wedge resection | 6 (5.4) | 62 (4.6) | 15 (8.3) | 36 (4.8) | 11 (2.6) | |
| Segmentectomy | 3 (2.7) | 74 (5.4) | 25 (13.8) | 39 (5.2) | 10 (2.4) | |
| Lobectomy | 99 (88.4) | 1195 (88.0) | 140 (77.3) | 671 (88.9) | 384 (91.0) | |
| Bilobectomy | 4 (3.6) | 21 (1.5) | 0 (0.0) | 9 (1.2) | 12 (2.8) | |
| Pneumonectomy | 0 (0.0) | 6 (0.4) | 1 (0.6) | 0 (0.0) | 5 (1.2) | |
| Primary site of lesion | <0.001 | |||||
| LUL | 12 (10.7) | 316 (23.3) | 47 (26.0) | 168 (22.3) | 101 (23.9) | |
| LLL | 45 (40.2) | 214 (15.8) | 24 (13.3) | 113 (15.0) | 77 (18.2) | |
| RUL | 6 (5.4) | 437 (32.2) | 69 (38.1) | 247 (32.7) | 121 (28.7) | |
| RML | 6 (5.4) | 122 (9.0) | 18 (9.9) | 69 (9.1) | 35 (8.3) | |
| RLL | 43 (38.4) | 269 (19.8) | 23 (12.7) | 158 (20.9) | 88 (20.9) | |
Note: All data are presented as n (%), n/N (%), or median [interquartile range (IQR)].
Abbreviations: ECOG, European Cooperative Oncology Group; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; IASLC, International Association for the Study of Lung Cancer; IMA, invasive mucinous adenocarcinoma; INMA, invasive non‐mucinous adenocarcinoma; LLL, Left lower lobe; LUL, Left upper lobe; RLL, Right lower lobe; RML, Right middle lobe; RUL, right upper lobe.
p value was measured between IMA and total INMA.
TABLE 2.
Clinical outcome and histopathologic findings of mucinous and nonmucinous (IASLC grades 1–3) invasive pulmonary adenocarcinoma
| Factor | IMA | INMA | p value a | IASLC grading subgroups in INMA | ||
|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | ||||
| N = 112 | N = 1358 | n = 181 | n = 755 | n = 422 | ||
| Adjuvant treatment | 0.913 | |||||
| No | 81 (72.3) | 968 (71.3) | 176 (97.2) | 599 (79.3) | 193 (45.7) | |
| Yes | 31 (27.7) | 390 (28.7) | 5 (2.8) | 156 (20.7) | 229 (54.3) | |
| Death | 16 (14.3) | 189 (13.9) | 0.672 | 4 (2.2) | 87 (11.5) | 98 (23.2) |
| Recurrence | 15 (13.4) | 260 (19.1) | 0.165 | 0 (0.0) | 121 (16.0) | 139 (32.9) |
| Type of recurrence | 0.213 | |||||
| Loco‐regional | 5 (33.3) | 65 (25.0) | 0 (0.0) | 30 (24.8) | 33 (23.7) | |
| Distant | 4 (26.7) | 112 (43.1) | 0 (0.0) | 58 (47.9) | 68 (48.9) | |
| Combined | 6 (40.0) | 83 (31.9) | 0 (0.0) | 33 (27.3) | 38 (27.4) | |
| Tumor size, cm | 2.40 [1.50, 4.45] | 2.20 [1.50, 3.00] | 0.057 | 1.70 [1.30, 2.20] | 2.10 [1.50, 2.80] | 2.50 [1.80, 3.50] |
| TNM stage | 0.125 | |||||
| IA1 | 11 (9.8) | 89 (6.6) | 20 (11.0) | 61 (8.1) | 8 (1.9) | |
| IA2 | 38 (33.9) | 433 (31.9) | 99 (54.7) | 258 (34.2) | 76 (18.0) | |
| IA3 | 22 (19.6) | 290 (21.4) | 45 (24.9) | 194 (25.7) | 51 (12.1) | |
| IB | 8 (7.1) | 242 (17.8) | 13 (7.2) | 141 (18.7) | 88 (20.9) | |
| IIA | 8 (7.1) | 35 (2.6) | 0 (0.0) | 19 (2.5) | 16 (3.8) | |
| IIB | 13 (11.6) | 111 (8.2) | 3 (1.7) | 44 (5.8) | 64 (15.2) | |
| IIIA | 10 (8.9) | 135 (9.9) | 0 (0.0) | 36 (4.8) | 99 (23.5) | |
| IIIB | 2 (1.8) | 23 (1.7) | 1 (0.6) | 2 (0.3) | 20 (4.7) | |
| Nodal stages | <0.001 | |||||
| N0 | 108 (96.4) | 1127 (83.0) | 178 (98.3) | 691 (91.5) | 258 (61.1) | |
| N1 | 0 (0.0) | 103 (7.6) | 2 (1.1) | 38 (5.0) | 63 (14.9) | |
| N2 | 4 (3.6) | 128 (9.4) | 1 (0.6) | 26 (3.4) | 101 (23.9) | |
| Lymphovascular invasion | <0.001 | |||||
| No | 107 (95.5) | 1121 (82.5) | 181 (100.0) | 692 (91.7) | 248 (58.8) | |
| Yes | 5 (4.5) | 237 (17.5) | 0 (0.0) | 63 (8.3) | 174 (41.2) | |
| Perineural invasion | 0.616 | |||||
| No | 112 (100.0) | 1345 (99.0) | 181 (100.0) | 748 (99.1) | 416 (98.6) | |
| Yes | 0 (0.0) | 13 (1.0) | 0 (0.0) | 7 (0.9) | 6 (1.4) | |
| Visceral pleural invasion | <0.001 | |||||
| No | 106 (94.6) | 1073 (79.0) | 178 (98.3) | 629 (83.3) | 266 (63.0) | |
| Yes | 6 (5.4) | 285 (21.0) | 3 (1.7) | 126 (16.7) | 156 (37.0) | |
| Spread through air spaces b | <0.001 | |||||
| Negative | 17 (33.3) | 524 (69.5) | 117 (100.0) | 342 (81.6) | 65 (29.8) | |
| Positive | 34 (66.7) | 230 (30.5) | 0 (0.0) | 77 (18.4) | 153 (70.2) | |
| Follow‐up periods, months | 58.7 [43.6, 75.7] | 59.1 [43.3, 74.1] | 0.803 | 59.3 [43.5, 75.4] | 59.7 [45.3, 78.1] | 54.0 [40.1, 71.2] |
Note: All data are presented as n (%), n/N (%), or median [interquartile range (IQR)].
Abbreviations: IASLC, International Association for the Study of Lung Cancer; IMA, invasive mucinous adenocarcinoma; INMA, invasive nonmucinous adenocarcinoma; TNM, tumor nodes and metastases .
p value was measured between IMA and total INMA.
Data were available: 45.5% (51/112) in IMA and 55.5% (754/1358) in INMA.
Survival analyses between IMAs and INMA
Figure 1 shows the survival curves for patients with IMA or INMA (IASLC grade 1–3). Patients with IMA demonstrated similar RFS to patients with IASLC grade 3 in the early postoperative period (within 10 months), but the difference became evident in the long term (Figure 1a). Finally, patients with IMA had a superior prognosis to patients with IASLC grade 3 (p = 0.003), but similar to patients with IASLC grade 2 (p = 0.441). With respect to OS, patients with IMA had a worse outcome than patients with IASLC grade 1 (p < 0.0001), but it was not significantly different from patients with IASLC grade 2 (p = 0.167) or 3 (p = 0.167) INMA (Figure 1b). Patients with IMA had a prognosis similar to that of patients with IASLC grades 2 and 3 in terms of OS.
Clinical outcomes between IMA and IASLC grade 2–3 after propensity score matching
Table 3 describes the clinicopathological characteristics of patients with IMA and IASLC grade 2 after propensity score matching. Compared to patients with IASLC grade 2, those with IMA had more lesions in the lower lobes (p < 0.001) and STAS positivity (p < 0.001). The Kaplan–Meier curves for RFS (p = 0.628) and OS (p = 0.585) did not differ between the two groups (Figure 2a,b).
TABLE 3.
Clinicopathologic characteristics of IMA and IASLC grade 2 after propensity score matching
| Factor | IMA | IASLC grade 2 | p value |
|---|---|---|---|
| N = 100 | N = 100 | ||
| Age | 64.00 [55.75, 71.00] | 64.00 [56.00, 70.00] | 0.909 |
| Gender | 0.572 | ||
| Female | 52 (52.0) | 47 (47.0) | |
| Male | 48 (48.0) | 53 (53.0) | |
| Diabetes mellitus | 19 (19.0) | 17 (17.0) | 0.854 |
| Hypertension | 38 (38.0) | 34 (34.0) | 0.659 |
| Smoking history | 0.644 | ||
| Ex and current | 32 (32.0) | 28 (28.0) | |
| Never | 68 (68.0) | 72 (72.0) | |
| ECOG | 0.835 | ||
| ECOG 0 | 87 (95.6) | 97 (97.0) | |
| ECOG 1 | 3 (3.3) | 3 (3.0) | |
| ECOG 2 | 1 (1.1) | 0 (0.0) | |
| FEV1, % | 106.0 [94.0, 119.5] | 106.0 [96.0, 115.0] | 0.944 |
| FEV1/FVC ratio, % | 78.0 [73.7, 80.9] | 75.0 [70.8, 81.0] | 0.102 |
| Extent of surgery | 0.444 | ||
| Wedge resection | 6 (6.0) | 3 (3.0) | |
| Segmentectomy | 3 (3.0) | 7 (7.0) | |
| Lobectomy | 88 (88.0) | 86 (86.0) | |
| Bilobectomy | 3 (3.0) | 4 (4.0) | |
| Location of primary lesion | <0.001 | ||
| LLL | 40 (40.0) | 19 (19.0) | |
| LUL | 10 (10.0) | 21 (21.0) | |
| RLL | 39 (39.0) | 24 (24.0) | |
| RML | 6 (6.0) | 7 (7.0) | |
| RUL | 5 (5.0) | 29 (29.0) | |
| Hospital (%) | 0.459 | ||
| Hospital A | 68 (68.0) | 62 (62.0) | |
| Hospital B | 32 (32.0) | 38 (38.0) | |
| Tumor size | 2.20 [1.40, 3.23] | 2.00 [1.50, 3.50] | 0.836 |
| Lymphovascular invasion | 1 | ||
| No | 96 (96.0) | 97 (97.0) | |
| Yes | 4 (4.0) | 3 (3.0) | |
| Visceral pleural invasion | 0.051 | ||
| No | 95 (95.0) | 86 (86.0) | |
| Yes | 5 (5.0) | 14 (14.0) | |
| Spread through air spaces | <0.001 | ||
| No | 17 (38.6) | 55 (84.6) | |
| Yes | 27 (61.4) | 10 (15.4) | |
| TNM stages | 0.133 | ||
| IA1 | 11 (11.0) | 9 (9.0) | |
| IA2 | 37 (37.0) | 38 (38.0) | |
| IA3 | 22 (22.0) | 15 (15.0) | |
| IB | 8 (8.0) | 21 (21.0) | |
| IIA | 8 (8.0) | 7 (7.0) | |
| IIB | 9 (9.0) | 5 (5.0) | |
| IIIA | 3 (3.0) | 5 (5.0) | |
| IIIB | 2 (2.0) | 0 (0.0) | |
| Adjuvant treatment | 0.476 | ||
| No | 78 (78.0) | 83 (83.0) | |
| Yes | 22 (22.0) | 17 (17.0) | |
| Death | 13 (13.0) | 10 (10.0) | 0.658 |
| Recurrence | 10 (10.0) | 17 (17.0) | 0.214 |
| Type of recurrence | 0.576 | ||
| Loco‐regional | 5 (50.0) | 5 (29.4) | |
| Distant | 2 (20.0) | 6 (35.3) | |
| Combined | 3 (30.0) | 6 (35.3) | |
| Follow‐up duration, months | 59.3 [45.3, 78.9] | 56.2 [41.5, 71.8] | 0.438 |
Note: All data are presented as n (%), n/N (%), or median [interquartile range (IQR)].
Abbreviations: ECOG, European Cooperative Oncology Group; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; IASLC, International Association for the Study of Lung Cancer; IMA, invasive mucinous adenocarcinoma; LLL, Left lower lobe; LUL, Left upper lobe; RLL, Right lower lobe; RML, Right middle lobe; RUL, right upper lobe, TNM, tumor nodes and metastases.
FIGURE 2.
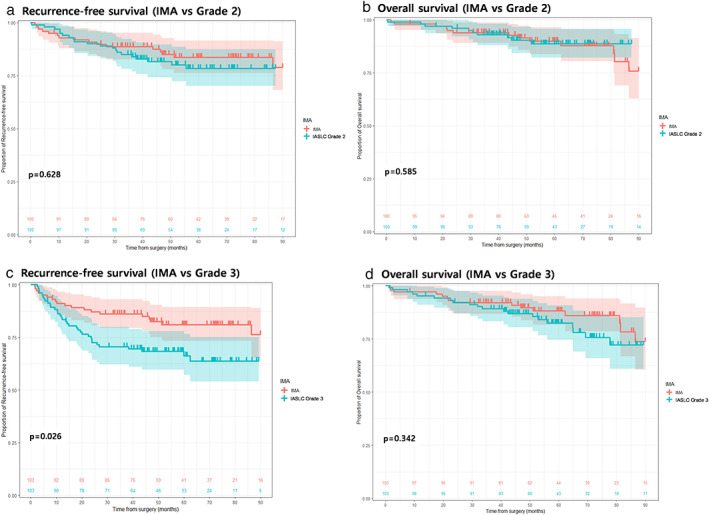
Recurrence‐free and overall survival curves between patients with IMA and IASLC grades 2 (a–b) and 3 (c–d) after propensity score matching. IALSC, International Association for the Study of Lung Cancer; IMA, invasive mucinous adenocarcinoma
Compared to patients with IASLC grade 3 after adjusting for covariates (Table 4), the FEV1/FVC ratio was slightly higher with fewer lobar lesions in the patients with IMA. However, lymphovascular (p < 0.001) and visceral pleural invasion (p < 0.001) were less frequently detected in patients with IMA. STAS did not differ between the two groups. With respect to RFS, patients with IMA had a superior outcome compared to patients with IASLC grade 3 (Figure 2c, p = 0.026), but the difference was not observed in OS (Figure 2d, p = 0.342).
TABLE 4.
Clinicopathologic characteristics of IMA and IASLC grade 3 after propensity score matching
| Factor | IMA | IASLC grade 3 | p value |
|---|---|---|---|
| N = 103 | N = 103 | ||
| Age | 64.00 [57.00, 71.00] | 64.00 [56.00, 72.00] | 0.968 |
| Gender | 0.485 | ||
| Female | 51 (49.5) | 45 (43.7) | |
| Male | 52 (50.5) | 58 (56.3) | |
| Diabetes mellitus | 19 (18.4) | 17 (16.5) | 0.855 |
| Hypertension | 41 (39.8) | 37 (35.9) | 0.667 |
| Smoking history | 0.884 | ||
| Ex and current | 35 (34.0) | 37 (35.9) | |
| Never | 68 (66.0) | 66 (64.1) | |
| ECOG | 0.872 | ||
| ECOG 0 | 89 (95.7) | 97 (96.0) | |
| ECOG 1 | 3 (3.2) | 2 (2.0) | |
| ECOG 2 | 1 (1.1) | 2 (2.0) | |
| FEV1, % | 106.00 [93.00, 119.50] | 101.00 [89.50, 113.00] | 0.076 |
| FEV1/FVC ratio, % | 77.00 [73.21, 80.75] | 74.00 [68.46, 77.00] | <0.001 |
| Extent of surgery | 0.881 | ||
| Wedge resection | 6 (5.8) | 7 (6.8) | |
| Segmentectomy | 3 (2.9) | 2 (1.9) | |
| Lobectomy | 90 (87.4) | 92 (89.3) | |
| Bilobectomy | 4 (3.9) | 2 (1.9) | |
| Location of primary lesion | <0.001 | ||
| LLL | 42 (40.8) | 25 (24.3) | |
| LUL | 11 (10.7) | 25 (24.3) | |
| RLL | 39 (37.9) | 20 (19.4) | |
| RML | 6 (5.8) | 5 (4.9) | |
| RUL | 5 (4.9) | 28 (27.2) | |
| Hospital (%) | 0.649 | ||
| Hospital A | 70 (68.0) | 74 (71.8) | |
| Hospital B | 33 (32.0) | 29 (28.2) | |
| Tumor size | 2.20 [1.45, 3.50] | 2.50 [1.80, 3.50] | 0.105 |
| Lymphovascular invasion | <0.001 | ||
| No | 98 (95.1) | 77 (74.8) | |
| Yes | 5 (4.9) | 26 (25.2) | |
| Visceral pleural invasion | <0.001 | ||
| No | 97 (94.2) | 76 (73.8) | |
| Yes | 6 (5.8) | 27 (26.2) | |
| Spread through air spaces | 0.834 | ||
| No | 16 (34.0) | 17 (31.5) | |
| Yes | 31 (66.0) | 37 (68.5) | |
| TNM stages | 0.136 | ||
| IA1 | 11 (10.7) | 3 (2.9) | |
| IA2 | 37 (35.9) | 29 (28.2) | |
| IA3 | 21 (20.4) | 16 (15.5) | |
| IB | 8 (7.8) | 32 (31.1) | |
| IIA | 8 (7.8) | 10 (9.7) | |
| IIB | 10 (9.7) | 4 (3.9) | |
| IIIA | 6 (5.8) | 8 (7.8) | |
| IIIB | 2 (1.9) | 1 (1.0) | |
| Adjuvant treatment | 0.035 | ||
| No | 78 (75.7) | 63 (61.2) | |
| Yes | 25 (24.3) | 40 (38.8) | |
| Death | 15 (14.6) | 20 (19.4) | 0.458 |
| Recurrence | 12 (11.7) | 28 (27.2) | 0.008 |
| Type of recurrence | 0.489 | ||
| Loco‐regional | 4 (33.3) | 7 (24.1) | |
| Distant | 3 (25.0) | 13 (44.8) | |
| Combined | 5 (41.7) | 9 (31.0) | |
| Follow‐up duration, months | 59.20 [43.50, 77.85] | 56.00 [42.15, 71.90] | 0.571 |
Note: All data are presented as n (%), n/N (%), or median [interquartile range (IQR)].
Abbreviations: ECOG, European Cooperative Oncology Group; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; IASLC, International Association for the Study of Lung Cancer; IMA, invasive mucinous adenocarcinoma; LLL, Left lower lobe; LUL, Left upper lobe; RLL, Right lower lobe; RML, Right middle lobe; RUL, right upper lobe,. TNM, tumor nodes and metastases.
Identification of prognostic factors for RFS among patients with IMA
In univariate analysis, sublobar resection, maximum standardized uptake value (SUVmax), lymphovascular invasion, tumor size, and nodal stage (N2 vs. N0) were considered as input variables for RFS. The multivariate Cox proportional hazards analysis revealed that tumor size (hazard ratio [HR] = 1.20, 95% confidence interval [CI] 1.02–1.40, p = 0.028), lymphovascular invasion (HR = 127.5, 95% CI 5.22–3116, p = 0.003), and SUVmax (HR = 1.24, 95% CI 1.07–1.43, p = 0.005) were significant independent poor prognostic predictors for RFS (Table 5).
TABLE 5.
Cox proportional hazard regression for recurrence‐free survival among invasive mucinous adenocarcinoma
| Factor | Univariate | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age | 1.03 (0.99–1.08) | 0.132 | ||
| Diabetes mellitus | 0.80 (0.27–2.33) | 0.681 | ||
| Gender (male) | 1.37 (0.62–3.02) | 0.432 | ||
| Never smoker | 0.73 (0.33–1.63) | 0.441 | ||
| Sublobar resection | 2.68 (0.91–7.95) | 0.075 | ||
| SUVmax | 1.28 (1.11–1.47) | <0.001 | 1.24 (1.07–1.43) | 0.005 |
| LVI | 17.2 (5.86–50.2) | <0.001 | 128 (5.22–3116) | 0.003 |
| STAS (+) | 1.65 (0.45–6.1) | 0.452 | ||
| Tumor size | 1.27 (1.13–1.42) | <0.001 | 1.20 (1.02–1.40) | 0.028 |
| VPI | 2.13 (0.63–7.23) | 0.230 | ||
| N stage (N2 vs N0) | 12.9 (4.12–40.6) | <0.001 | 0.06 (0.00–1.19) | 0.064 |
Abbreviations: CI, confidence interval; HR, hazard ratio; LVI, lymphovascular invasion; STAS, spread through air spaces; SUVmax, the maximum standardized uptake value; VPI, visceral pleural invasion.
DISCUSSION
Clinical investigations for IMAs are scarce, despite there being several studies on INMAs assessing the prognostic value of histologic patterns, 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 including a recently proposed IASLC grading system. 5 With the introduction of the new IASLC classification, the heterogeneity in INMAs was further clarified by dividing them into three categories. To the best of our knowledge, this study is the first to attempt to compare the prognosis of IMA to that of the new IASLC classification of INMA based on data from two institutions. Moreover, our study compared IMA with IASLC grades 2 and 3 after adjusting for compounding variables to better reflect the unique characteristics of IMA. This will deliver more precise information for determining the prognosis of IMA.
IMA has different clinical characteristics from INMA in imaging studies. Previous studies have suggested distinctive computed tomography findings of IMA, such as mixed airspace consolidation, ground‐glass opacity, and air bronchogram. 17 , 18 , 19 Several imaging characteristics, such as spontaneous regression of airspace opacities 20 and pneumonic‐type IMA, 21 were considered poor prognostic radiological factors. Moreover, the prognostic value of the SUVmax on 10 (18)F‐fluorodeoxyglucose positron emission tomography has been investigated in previous IMA studies. 22 SUVmax highly correlated with tumor or pathological invasive size, although (18)F‐fluorodeoxyglucose uptake was not notably high in the IMA. 17 , 22 We also found that SUVmax significantly predicted RFS, in line with a previous study. 23 However, this study could not comprehensively review all radiologic findings because of the diversity and ever‐changing protocols in diagnosis. Further systematic reviews of this radiological perspective are warranted.
Compared to INMA, IMA is mostly found in the lower lobes of the lungs. This study also confirmed this unique characteristic. Ichinokawa et al. suggested a correlation between this type of occurrence and KRAS mutation. 24 While IMAs were regarded to present at an advanced stage during diagnosis and might not be treated by surgery, 10 , 25 , 26 a study that analyzed the Surveillance, Epidemiology, and End Results database reported a high proportion of up to 70% IMA in the early stages. 27 Most studies concerning the surgical outcome of IMA also included stage I or II rather than advanced stages. 18 , 23 , 28 , 29 In this study, the patient population comprised patients with stages I–II disease, and approximately 10% of patients had stage III disease. Due to this difference, the RFS and OS results were different from those of previous studies.
The clinical outcome of IMA has been controversial 10 , 23 , 30 , 31 due to its low incidence, which is approximately 1.5% of total lung cancers. 27 Lee et al. reported a relatively favorable prognosis of IMA and that it was better than acinar or papillary predominant type INMA in disease‐free survival. 23 This contradicts the results of our study, and it could be attributed to differences in age, sex, and the number of advanced staged cases in the study population. However, when the prognosis of patients with IMA was compared to that of patients with lepidic‐predominant INMA, it had inferior clinical outcomes. Notably, Chang et al. classified IMA into various predominant patterns, as observed in INMA, and the presence of >10% micropapillary and cribriform patterns was associated with more aggressive behavior. 32 Interobserver agreement was not assessed in this study. However, if this predominant pattern could be applied to predict the prognosis of IMA, it would specifically differentiate between IMAs similar to IALSC grading for INMAs. 5
In the pathologic findings of this study, IMA was associated with lower rates of nodal metastasis and lymphovascular and visceral pleural invasion than INMA. Similar patterns were observed in previous studies. 9 , 13 , 23 , 33 This implies that IMA and INMA have different disease characteristics. Although lymphovascular invasion was low, its prognostic impact was evident in our study. More attention should be paid to IMAs with lymphovascular invasion. An aerogenous spread pattern, equivalent to STAS in INMA, was also significant in IMA despite these pathological differences. Previous studies reported a higher incidence of STAS in IMA (50–72.3%) 34 , 35 , 36 than in INMA (14.8–47.6%). 37 , 38 , 39 Similar results were observed in this study despite the limited data. This could be attributable to different mucin protein expression, which could affect cell polarity and cancer cell migration. 40 , 41 STAS was also a significant poor prognostic factor and was suggested to be related to older age and lobulated and spiculated computed tomography margins. 36 Matsui et al. reported a higher intrapulmonary recurrence in IMA, which could be supported by its higher STAS positivity, 42 therefore further studies incorporating these pathological findings are necessary.
In the case of advanced cancer, adjuvant therapy is performed according to genetic alterations, such as EGFR, ALK, ROS, and KRAS mutations. In the case of IMA, there is almost no EGFR mutation, 31 , 43 , 44 , 45 therefore tyrosine kinase inhibitors (TKIs) are not used, affecting survival. Due to these differences in adjuvant treatments, we believe that RFS is a better assessment tool for comparing clinical prognosis in various histologic findings. Specifically, aggressive INMA (such as IASLC grade 3) could benefit from TKI treatment, as OS in this study did not represent the difference with IMA. To minimize the effect of post‐surgery treatment, we suggest RFS as a reliable parameter to overcome the impact of confounding factors.
Our study had several limitations. Although we had the largest number of patients with IMA from the two institutions, categorization into a large‐scale population was lacking. However, we aimed to overcome this problem using propensity score matching to increase the statistical power. Second, this study did not present molecular mutation data, although we obtained those results from approximately half of the patients. As specific mutational studies of IMA have not yet been clearly defined, we leave this as a future study topic. We expect to obtain these results in the near future. Third, the presence of STAS was not fully confirmed in the total population. As STAS was recently proposed, we could not find satisfactory results in the population that underwent surgery in the early period.
In conclusion, patients with IMA demonstrated better RFS than those with IASLC grade 3, but similar to the patients with IASLC grade 2. Additionally, the OS of patients with IMA was between that of patients with IASLC grades 2 and 3. Since the prognosis of IMA among lung adenocarcinomas appears to be relatively advanced, further clinical studies investigating IMA‐specific treatment and follow‐up plans are necessary to draw more implications from these findings.
AUTHOR CONTRIBUTIONS
Wongi Woo: Conceptualization, methodology, data curation, formal analysis, resources, investigation, software, writing—original draft, writing—review and editing. Young Ho Yang: Conceptualization, methodology, data curation, formal analysis, investigation, writing—original draft, writing—review and editing. Yoon‐Jin Cha: Investigation, writing—review and editing. Duk Hwan Moon: Data curation, investigation, writing—review and editing. Hyo Sup Shim: Investigation, writing—original draft, writing—review and editing. Arthur Cho: Investigation, writing—original draft, writing—Review and editing. Ha Eun Kim: Writing—review and editing. Byung Jo Park: Writing—review and editing. Jin Gu Lee: Writing—review and editing. Dae Joon Kim: Writing—review and editing. Sungsoo Lee: Conceptualization, methodology, validation, supervision, project administration writing—review and editing. Chang Young Lee: Conceptualization, methodology, validation, supervision, project administration writing—review and editing.
CONFLICTS OF INTEREST
The authors disclose no financial or nonfinancial conflicts of interest, including funding, provision of study materials, medical writing, or article processing charges. This research did not receive grants from funding agencies in the public, commercial, or not‐for‐profit sectors.
Supporting information
Appendix S1: Supporting Information.
ACKNOWLEDGMENTS
None.
Woo W, Yang YH, Cha YJ, Moon DH, Shim HS, Cho A, et al. Prognosis of resected invasive mucinous adenocarcinoma compared with the IASLC histologic grading system for invasive nonmucinous adenocarcinoma: Surgical database study in the TKIs era in Korea. Thorac Cancer. 2022;13(23):3310–3321. 10.1111/1759-7714.14687
Wongi Woo and Young Ho Yang are considered as Co‐first authors.
Contributor Information
Sungsoo Lee, Email: chestlee@yuhs.ac.
Chang Young Lee, Email: cyleecs@yuhs.ac.
DATA AVAILABILITY STATEMENT
The corresponding authors will share this article's data upon reasonable request.
REFERENCES
- 1. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to the 2015 World Health Organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol. 2015;10(9):1240–2. [DOI] [PubMed] [Google Scholar]
- 2. Saito T, Tsuta K, Honda O, Ishida M, Yamaka R, Tanaka N, et al. Prognostic impact of mucin spread, tumor cell spread, and invasive size in invasive mucinous adenocarcinoma of the lung. Lung Cancer. 2020;146:50–7. [DOI] [PubMed] [Google Scholar]
- 3. Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6(2):244–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–60. [DOI] [PubMed] [Google Scholar]
- 5. Moreira AL, Ocampo PSS, Xia Y, Zhong H, Russell PA, Minami Y, et al. A grading system for invasive pulmonary adenocarcinoma: a proposal from the International Association for the Study of Lung Cancer pathology committee. J Thorac Oncol. 2020;15(10):1599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rokutan‐Kurata M, Yoshizawa A, Ueno K, Nakajima N, Terada K, Hamaji M, et al. Validation study of the International Association for the Study of Lung Cancer histologic grading system of invasive lung adenocarcinoma. J Thorac Oncol. 2021;16(10):1753–8. [DOI] [PubMed] [Google Scholar]
- 7. Deng C, Zheng Q, Zhang Y, Jin Y, Shen X, Nie X, et al. Validation of the novel International Association for the Study of Lung Cancer grading system for invasive pulmonary adenocarcinoma and association with common driver mutations. J Thorac Oncol. 2021;16(10):1684–93. [DOI] [PubMed] [Google Scholar]
- 8. Hou L, Wang T, Chen D, She Y, Deng J, Yang M, et al. Prognostic and predictive value of the newly proposed grading system of invasive pulmonary adenocarcinoma in Chinese patients: a retrospective multicohort study. Mod Pathol. 2022;10:1–8. [DOI] [PubMed] [Google Scholar]
- 9. Russell PA, Wainer Z, Wright GM, Daniels M, Conron M, Williams RA. Does lung adenocarcinoma subtype predict patient survival?: a clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol. 2011;6(9):1496–504. [DOI] [PubMed] [Google Scholar]
- 10.Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population‐based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–9. doi: 10.3322/caac.21388 [DOI] [PubMed] [Google Scholar]
- 11. Warth A, Muley T, Meister M, Stenzinger A, Thomas M, Schirmacher P, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage‐independent predictor of survival. J Clin Oncol. 2012;30(13):1438–46. [DOI] [PubMed] [Google Scholar]
- 12. Hung JJ, Yeh YC, Jeng WJ, Wu KJ, Huang BS, Wu YC, et al. Predictive value of the international association for the study of lung cancer/American Thoracic Society/European Respiratory Society classification of lung adenocarcinoma in tumor recurrence and patient survival. J Clin Oncol. 2014;32(22):2357–64. [DOI] [PubMed] [Google Scholar]
- 13. Kadota K, Yeh YC, D'Angelo SP, Moreira AL, Kuk D, Sima CS, et al. Associations between mutations and histologic patterns of mucin in lung adenocarcinoma: invasive mucinous pattern and extracellular mucin are associated with KRAS mutation. Am J Surg Pathol. 2014;38(8):1118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kadota K, Suzuki K, Kachala SS, Zabor EC, Sima CS, Moreira AL, et al. A grading system combining architectural features and mitotic count predicts recurrence in stage I lung adenocarcinoma. Mod Pathol. 2012;25(8):1117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mäkinen JM, Laitakari K, Johnson S, Mäkitaro R, Bloigu R, Pääkkö P, et al. Histological features of malignancy correlate with growth patterns and patient outcome in lung adenocarcinoma. Histopathology. 2017;71(3):425–36. [DOI] [PubMed] [Google Scholar]
- 16. Sica G, Yoshizawa A, Sima CS, Azzoli CG, Downey RJ, Rusch VW, et al. A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am J Surg Pathol. 2010;34(8):1155–62. [DOI] [PubMed] [Google Scholar]
- 17. Lee HY, Lee KS, Han J, Kim BT, Cho YS, Shim YM, et al. Mucinous versus nonmucinous solitary pulmonary nodular bronchioloalveolar carcinoma: CT and FDG PET findings and pathologic comparisons. Lung Cancer. 2009;65(2):170–5. [DOI] [PubMed] [Google Scholar]
- 18. Miyamoto A, Kurosaki A, Fujii T, Kishi K, Homma S. HRCT features of surgically resected invasive mucinous adenocarcinoma associated with interstitial pneumonia. Respirology. 2017;22(4):735–43. [DOI] [PubMed] [Google Scholar]
- 19. Watanabe H, Saito H, Yokose T, Sakuma Y, Murakami S, Kondo T, et al. Relation between thin‐section computed tomography and clinical findings of mucinous adenocarcinoma. Ann Thorac Surg. 2015;99(3):975–81. [DOI] [PubMed] [Google Scholar]
- 20. Beck KS, Sung YE, Lee KY, Han DH. Invasive mucinous adenocarcinoma of the lung: serial CT findings, clinical features, and treatment and survival outcomes. Thorac Cancer. 2020;11(12):3463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang T, Yang Y, Liu X, Deng J, Wu J, Hou L, et al. Primary invasive mucinous adenocarcinoma of the lung: prognostic value of CT imaging features combined with clinical factors. Korean J Radiol. 2021;22(4):652–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murakami S, Saito H, Karino F, Kondo T, Oshita F, Ito H, et al. 18F‐fluorodeoxyglucose uptake on positron emission tomography in mucinous adenocarcinoma. Eur J Radiol. 2013;82(11):e721–5. [DOI] [PubMed] [Google Scholar]
- 23. Lee HY, Cha MJ, Lee KS, Lee HY, Kwon OJ, Choi JY, et al. Prognosis in resected invasive mucinous adenocarcinomas of the lung: related factors and comparison with resected nonmucinous adenocarcinomas. J Thorac Oncol. 2016;11(7):1064–73. [DOI] [PubMed] [Google Scholar]
- 24. Ichinokawa H, Ishii G, Nagai K, Kawase A, Yoshida J, Nishimura M, et al. Distinct clinicopathologic characteristics of lung mucinous adenocarcinoma with KRAS mutation. Hum Pathol. 2013;44(12):2636–42. [DOI] [PubMed] [Google Scholar]
- 25. Dacic S. Pros: the present classification of mucinous adenocarcinomas of the lung. Transl Lung Cancer Res. 2017;6(2):230–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wislez M, Antoine M, Baudrin L, Poulot V, Neuville A, Pradere M, et al. Non‐mucinous and mucinous subtypes of adenocarcinoma with bronchioloalveolar carcinoma features differ by biomarker expression and in the response to gefitinib. Lung Cancer. 2010;68(2):185–91. [DOI] [PubMed] [Google Scholar]
- 27. Moon SW, Choi SY, Moon MH. Effect of invasive mucinous adenocarcinoma on lung cancer‐specific survival after surgical resection: a population‐based study. J Thorac Dis. 2018. Jun;10(6):3595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luo J, Wang R, Han B, Zhang J, Zhao H, Fang W, et al. Analysis of the clinicopathologic characteristics and prognostic of stage I invasive mucinous adenocarcinoma. J Cancer Res Clin Oncol. 2016;142(8):1837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang G, Wang X, Jia J, Zuo Z, Wang L, Gao S, et al. Development and validation of a nomogram for predicting survival in patients with surgically resected lung invasive mucinous adenocarcinoma. Transl Lung Cancer Res. 2021;10(12):4445–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin G, Li H, Kuang J, Tang K, Guo Y, Han A, et al. Acinar‐predominant pattern correlates with poorer prognosis in invasive mucinous adenocarcinoma of the lung. Am J Clin Pathol. 2018;149(5):373–8. [DOI] [PubMed] [Google Scholar]
- 31. Cai L, Wang J, Yan J, Zeng J, Zhu L, Liang J, et al. Genomic profiling and prognostic value analysis of genetic alterations in Chinese resected lung cancer with invasive mucinous adenocarcinoma. Front Oncol. 2020;10:603671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chang WC, Zhang YZ, Lim E, Nicholson AG. Prognostic impact of histopathologic features in pulmonary invasive mucinous adenocarcinomas. Am J Clin Pathol. 2020;154(1):88–102. [DOI] [PubMed] [Google Scholar]
- 33. Kakegawa S, Shimizu K, Sugano M, Miyamae Y, Kaira K, Araki T, et al. Clinicopathological features of lung adenocarcinoma with KRAS mutations. Cancer. 2011;117(18):4257–66. [DOI] [PubMed] [Google Scholar]
- 34. Isaka T, Yokose T, Miyagi Y, Washimi K, Nishii T, Ito H, et al. Detection of tumor spread through airspaces by airway secretion cytology from resected lung cancer specimens. Pathol Int. 2017;67(10):487–94. [DOI] [PubMed] [Google Scholar]
- 35. Kim SK, Kim TJ, Chung MJ, Kim TS, Lee KS, Zo JI, et al. Lung adenocarcinoma: CT features associated with spread through air spaces. Radiology. 2018;289(3):831–40. [DOI] [PubMed] [Google Scholar]
- 36. Lee MA, Kang J, Lee HY, Kim W, Shon I, Hwang NY, et al. Spread through air spaces (STAS) in invasive mucinous adenocarcinoma of the lung: incidence, prognostic impact, and prediction based on clinicoradiologic factors. Thorac Cancer. 2020;11(11):3145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kadota K, Nitadori JI, Sima CS, Ujiie H, Rizk NP, Jones DR, et al. Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage I lung adenocarcinomas. J Thorac Oncol. 2015;10(5):806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Warth A, Muley T, Kossakowski CA, Goeppert B, Schirmacher P, Dienemann H, et al. Prognostic impact of intra‐alveolar tumor spread in pulmonary adenocarcinoma. Am J Surg Pathol. 2015;39(6):793–801. [DOI] [PubMed] [Google Scholar]
- 39. Morales‐Oyarvide V, Mino‐Kenudson M. Tumor islands and spread through air spaces: distinct patterns of invasion in lung adenocarcinoma. Pathol Int. 2016;66(1):1–7. [DOI] [PubMed] [Google Scholar]
- 40. Lakshmanan I, Ponnusamy MP, Macha MA, Haridas D, Majhi PD, Kaur S, et al. Mucins in lung cancer: diagnostic, prognostic, and therapeutic implications. J Thorac Oncol. 2015;10(1):19–27. [DOI] [PubMed] [Google Scholar]
- 41. Geles A, Gruber‐Moesenbacher U, Quehenberger F, Manzl C, Al Effah M, Grygar E, et al. Pulmonary mucinous adenocarcinomas: architectural patterns in correlation with genetic changes, prognosis and survival. Virchows Arch. 2015;467(6):675–86. [DOI] [PubMed] [Google Scholar]
- 42. Matsui T, Sakakura N, Koyama S, Nakanishi K, Sasaki E, Kato S, et al. Comparison of surgical outcomes between invasive mucinous and non‐mucinous lung adenocarcinoma. Ann Thorac Surg. 2021;112(4):1118–26. [DOI] [PubMed] [Google Scholar]
- 43. Cha YJ, Shim HS. Biology of invasive mucinous adenocarcinoma of the lung. Transl Lung Cancer Res. 2017. [cited 2022 Feb 15];6(5) Available from::508–12. https://tlcr.amegroups.com/article/view/14625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Boland JM, Maleszewski JJ, Wampfler JA, Voss JS, Kipp BR, Yang P, et al. Pulmonary invasive mucinous adenocarcinoma and mixed invasive mucinous/nonmucinous adenocarcinoma‐a clinicopathological and molecular genetic study with survival analysis. Hum Pathol. 2018;71:8–19. [DOI] [PubMed] [Google Scholar]
- 45. Hwang DH, Sholl LM, Rojas‐Rudilla V, Hall DL, Shivdasani P, Garcia EP, et al. KRAS and NKX2‐1 mutations in invasive mucinous adenocarcinoma of the lung. J Thorac Oncol. 2016;11(4):496–503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information.
Data Availability Statement
The corresponding authors will share this article's data upon reasonable request.


