Abstract
Abstract
Aims
Exercise training (ET) has been consistently shown to increase peak oxygen consumption (V̇O2) in patients with heart failure with preserved ejection fraction (HFpEF); however, inter‐individual responses vary significantly. Because it is unlikely that ET‐induced improvements in peak V̇O2 are significantly mediated by an increase in peak heart rate (HR), we aimed to investigate whether baseline peak O2‐pulse (V̇O2 × HR−1, reflecting the product of stroke volume and arteriovenous oxygen difference), not baseline peak V̇O2, is inversely associated with the change in peak V̇O2 (adjusted by body weight) following ET versus guideline control (CON) in patients with HFpEF.
Methods and results
This was a secondary analysis of the OptimEx‐Clin (Optimizing Exercise Training in Prevention and Treatment of Diastolic Heart Failure, NCT02078947) trial, including all 158 patients with complete baseline and 3 month cardiopulmonary exercise testing measurements (106 ET, 52 CON). Change in peak V̇O2 (%) was analysed as a function of baseline peak V̇O2 and its determinants (absolute peak V̇O2, peak O2‐pulse, peak HR, weight, haemoglobin) using robust linear regression analyses. Mediating effects on change in peak V̇O2 through changes in peak O2‐pulse, peak HR and weight were analysed by a causal mediation analysis with multiple correlated mediators. Change in submaximal exercise tolerance (V̇O2 at the ventilatory threshold, VT1) was analysed as a secondary endpoint. Among 158 patients with HFpEF (66% female; mean age, 70 ± 8 years), changes in peak O2‐pulse explained approximately 72% of the difference in changes in peak V̇O2 between ET and CON [10.0% (95% CI, 4.1 to 15.9), P = 0.001]. There was a significant interaction between the groups for the influence of baseline peak O2‐pulse on change in peak V̇O2 (interaction P = 0.04). In the ET group, every 1 mL/beat higher baseline peak O2‐pulse was associated with a decreased mean change in peak V̇O2 of −1.45% (95% CI, −2.30 to −0.60, P = 0.001) compared with a mean change of −0.08% (95% CI, −1.11 to 0.96, P = 0.88) following CON. None of the other factors showed significant interactions with study groups for the change in peak V̇O2 (P > 0.05). Change in V̇O2 at VT1 was not associated with any of the investigated factors (P > 0.05).
Conclusions
In patients with HFpEF, the easily measurable peak O2‐pulse seems to be a good indicator of the potential for improving peak V̇O2 through exercise training. While changes in submaximal exercise tolerance were independent of baseline peak O2‐pulse, patients with high O2‐pulse may need to use additional therapies to significantly increase peak V̇O2.
Keywords: Endurance training, Exercise test, Precision medicine, Regression analysis, Oxygen pulse, Responder
Introduction
Exercise training (ET) has a Class I recommendation for patients with heart failure with preserved ejection fraction (HFpEF). 1 While pharmacological trials, except for the recently published EMPEROR‐HF trial, 2 have been overall unsuccessful, 3 ET has been shown to reduce exercise intolerance—the hallmark symptom in HFpEF—as measured by increased peak oxygen consumption (V̇O2). 4 , 5 However, as for any given treatment, ET is associated with a certain response heterogeneity with some patients showing better responses than others despite exercising to a similar extent. Heterogeneous responses to therapies led to the concept of personalized medicine which requires the identification of factors associated with treatment effects. This is especially important in HFpEF, as it is known to be a multifactorial and highly heterogeneous disease with several co‐existing co‐morbidities 6 , 7 and patients are almost exclusively suffering from multiple defects affecting the convective and diffusive oxygen delivery and utilization. 8 In addition to abnormal active relaxation and increased passive stiffness (i.e. diastolic dysfunction) resulting in a blunted stroke volume (SV) response during exercise, 9 a high prevalence of chronotropic incompetence, which is considered as the most relevant haemodynamic limitation in HFpEF, 10 further limits the cardiac output response to incremental exercise. On the other hand, emerging data suggest that abnormalities in extracardiac factors such as haemoglobin concentration, alveolar ventilation, lung or muscle diffusion capacity, or mitochondrial respiration, which lead to a reduced arteriovenous oxygen content difference [C(a‐v)O2], play a significantly greater role in HFpEF compared with heart failure with reduced ejection fraction (HFrEF). 8 , 11 , 12
It is generally assumed that individuals with a lower baseline peak V̇O2 have a higher potential to benefit from ET; however, this could not be confirmed in a recent meta‐analysis in patients with heart failure. 13 According to the Fick Principle, V̇O2 is the product of heart rate (HR), SV, and C(a‐v)O2, and therefore, the increase in peak V̇O2 following ET is mediated through an increase in any or a combination of these variables. Peak HR is known to be highly dependent upon age but not significantly different between trained and sedentary individuals. 14 Accordingly, a meta‐analysis from trials in healthy middle aged and older adults 15 revealed that endurance ET did not significantly improve peak HR and that the improved peak V̇O2 was related to significant changes in both peak SV and peak C(a‐v)O2. During cardiopulmonary exercise testing (CPET), the product of SV and C(a‐v)O2 can be indirectly obtained as O2‐pulse [V̇O2 × HR−1 = SV × C(a‐v)O2].
Even though peak HR, SV and C(a‐v)O2 can all be significantly reduced in patients with HFpEF, limited evidence suggests that the ET‐induced improvements in peak V̇O2 are mainly due to increases in C(a‐v)O2, 16 , 17 whereas most studies did not show increases in either peak HR (7/9 studies) 5 , 16 , 18 , 19 , 20 , 21 , 22 , 23 , 24 or peak SV (2/2 studies). 16 , 17 Based on these findings and the concept of a higher potential for improvement when starting with a lower baseline, the hypothesis of this study was that baseline peak O2‐pulse—not baseline peak V̇O2—is inversely associated with the ET‐induced change in peak V̇O2 following 3 months of supervised ET compared with guideline control (CON).
Methods
Study setting
This study is a secondary analysis of the initial 3 month supervised period of the OptimEx‐Clin (Optimizing Exercise Training in Prevention and Treatment of Diastolic Heart Failure) trial—a prospective, randomized, controlled, multicentre‐trial investigating the effects of high‐intensity interval training (HIIT), moderate continuous training (MCT) and CON in 180 sedentary patients with stable HFpEF (New York Heart Association Class II‐III; left ventricular ejection fraction ≥50%; elevated estimated LV filling pressure [E/e′ medial ≥15] or E/e′ medial ≥8 with elevated natriuretic peptides [NT‐proBNP ≥ 220 pg/mL or BNP ≥ 80 pg/mL]). 25 The study design 26 and the main results of the trial 5 have been published before.
In brief, participants were randomly assigned to HIIT (3 × 38 min/week with 4 × 4‐min intervals at 80–90% of heart rate reserve), MCT (5 × 40 min/week at 35–50% heart rate reserve) and CON (one‐time advice on physical activity). All patients were assessed at baseline and 3 months after randomization. CPET was performed on bicycle ergometers (starting at 20 watts, increasing by 10 watts per minute) and analysed at the study core lab in Munich, blinded to treatment arm assignment. Peak V̇O2 was defined as the highest 30 s average within the last minute of exercise. 27 Peak HR and peak O2‐pulse were defined as the 30 s average derived from the same time span as peak V̇O2. V̇O2 at the first ventilatory threshold (VT1), a measure of submaximal exercise tolerance, was determined by the V‐slope method. 28 The study was approved by the local ethic committees at all participating sites and conforms with the principles outlined in the Declaration of Helsinki. All participants provided written informed consent.
As the current research question did not aim at differences between HIIT and MCT, the results of the main analysis were not significantly different between both modes, 5 and the required sample size to identify covariate–treatment interactions is substantially higher than for comparing group means, 29 the main analyses were performed with one ET group (combination of HIIT and MCT) versus CON. Only patients with complete paired baseline and 3 month follow‐up CPET measurements were included.
Statistical analyses
The primary endpoint in the present analysis was the change in relative peak V̇O2 (mL/kg/min). The change in relative V̇O2 at VT1 (mL/kg/min) was analysed as a secondary endpoint. To ensure comparability in the evaluation of individual responses between single subjects with varying baseline values, all changes are expressed as %‐change from baseline to 3 month follow‐up. Next to baseline peak O2‐pulse, relative peak V̇O2 and its other determinants (absolute peak V̇O2, peak HR, weight, and haemoglobin as one of the determinants of C(a‐v)O2) were examined. Group means were compared with t‐tests for independent means. For comparisons of ordinal data, the Mann–Whitney U‐test was used. To determine the proportions of change in relative peak V̇O2 that can be explained by the changes in peak O2‐pulse, peak HR and weight, a causal mediation analysis with multiple correlated mediators 30 was performed (R library ‘multimediate’ 31 ). Furthermore, the relationships between changes in relative peak V̇O2, changes in peak HR, changes in peak O2‐pulse and changes in weight were analysed. The impact of baseline peak V̇O2 and its determinants on the change in relative peak V̇O2 was assessed using linear regression models with main effects of the independent variable and group as well as their interaction term (group × independent variable). These analyses were performed using robust linear regressions with MM‐type estimators (function ‘rlm’ in R library ‘MASS’ 32 and function ‘f.robftest’ in R library ‘sfsmisc’ 33 ). This method uses an iteratively reweighted least‐squares procedure fitting bisquare estimators that is insensitive to influential data points and remains highly efficient (in comparison to ordinary least square estimates) in case of no outliers. 34 Analyses of the primary endpoint were performed in a complete data set (including all patients with valid assessments of the variables of interest) and a per‐protocol set (excluding patients randomized to ET with adherence of <70% to the scheduled exercise sessions). Furthermore, we performed a sensitivity analysis within the original groups (HIIT vs. MCT vs. CON). Global interaction p‐values were calculated using the function ‘lmrob’ with the recommended setting ‘KS2014’ in R library ‘robustbase’. 35 All statistical analyses were performed using R Statistical Software (Version 3.6.1, Foundation for Statistical Computing, Vienna, Austria) 36 with local significant levels of α = 0.05. As a secondary analysis, the results presented in this manuscript should be interpreted as exploratory.
Results
All 158 patients [106 (ET) vs. 52 (CON); 66% women; mean age of 70 ± 8 years; Table 1 ] with complete CPET data at follow‐up were included in this sub‐study (Figure 1 ). Due to indeterminable VT1, analyses including this endpoint were performed on all 154 patients with determinable VT1 at baseline and follow‐up [104 (ET) vs. 50 (CON)]. The per‐protocol set of ET patients included 87 individuals who performed at least 70% of the prescribed exercise sessions.
Table 1.
Demographic and clinical characteristics at baseline
| Exercise (N = 106) | Guideline control (N = 52) | |
|---|---|---|
| Sex | ||
| Female | 70 (66%) | 34 (65%) |
| Male | 36 (34%) | 18 (35%) |
| Age, years | 70 (7) | 69 (10) |
| Weight, kg | 84.6 ± 18.0 | 78.7 ± 15.2 |
| Body mass index, kg/m2 | 30.6 ± 6.1 | 29.0 ± 4.9 |
| Resting heart rate, min | 65 ± 11 | 65 ± 11 |
| Blood pressure, mmHg | ||
| Systolic | 128 ± 13 | 127 ± 15 |
| Diastolic | 74 ± 10 | 74 ± 10 |
| Cardiovascular risk factors | ||
| Hypertension | 90 (85%) | 46 (88%) |
| Diabetes | 30 (28%) | 12 (23%) |
| Dyslipidaemia | 74 (70%) | 40 (77%) |
| Smoking | ||
| Never smoked | 55 (52%) | 30 (58%) |
| Ex‐smoker | 45 (42%) | 21 (40%) |
| Current Smoker | 6 (6%) | 1 (2%) |
| Sleep apnoea | 19 (18%) | 9 (17%) |
| Severity of HFpEF | ||
| New York Heart Association class | ||
| II | 80 (75%) | 35 (67%) |
| III | 26 (25%) | 17 (33%) |
| E/e′ average, [no.] | 13.4 ± 3.4 [103] | 13.3 ± 4.7 [49] |
| E/A, [no.] | 1.23 ± 0.65 [88] | 1.20 ± 0.62 [46] |
| NT‐proBNP, pg/mL, [no.] | 321 (161–689) [102] | 341 (175–622) [52] |
| Haemoglobin, mg/dL, [no.] | 13.6 ± 1.6 [103] | 13.2 ± 1.4 [52] |
| Other cardiac diagnoses | ||
| Coronary artery disease | 29 (27%) | 16 (31%) |
| Atrial fibrillation | ||
| Paroxysmal | 13 (12%) | 7 (13%) |
| Persistent | 8 (8%) | 3 (6%) |
| Permanent | 10 (9%) | 2 (4%) |
| Heart failure medication | ||
| Beta‐blocker | 68 (64%) | 37 (71%) |
| Thiazide/loop diuretics | 60 (57%) | 31 (60%) |
| Angiotensin receptor blocker | 44 (42%) | 21 (40%) |
| Angiotensin‐converting enzyme inhibitor | 36 (34%) | 16 (31%) |
| Aldosterone antagonists | 12 (11%) | 5 (10%) |
| Cardiopulmonary exercise testing parameters | ||
| Peak oxygen consumption, mL/kg/min | 18.5 ± 5.1 | 19.5 ± 5.8 |
| Peak oxygen pulse, mL/beat | 12.7 ± 3.5 | 12.9 ± 4.0 |
| Peak heart rate, b.p.m. | 123 ± 26 | 121 ± 28 |
| Peak respiratory exchange ratio | 1.11 ± 0.09 | 1.10 ± 0.13 |
Values are expressed as mean ± SD, median (inter‐quartile range) or absolute values (percentage). E, peak velocity blood flow from ventricular relaxation in early diastole; e′, mitral annular early diastolic velocity; A, peak velocity flow in late diastole caused by atrial contraction; NT‐proBNP, N‐terminal prohormone of brain natriuretic peptide; pg = picogram.
Figure 1.
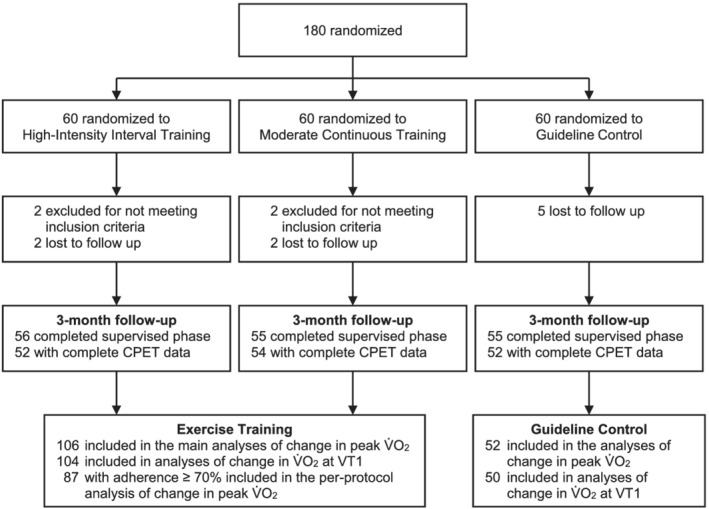
Study flow chart. Among 180 randomized patients, this study included all 158 patients with complete paired baseline and 3 month follow‐up cardiopulmonary exercise testing measurements. For the main analyses, high‐intensity interval training and moderate continuous training were combined to one exercise training group. A complete CONSORT flow chart of the study has been published previously. 5 CPET, cardiopulmonary exercise testing; V̇O2, oxygen consumption; VT1, ventilatory threshold.
Comparison of mean changes
Relative peak V̇O2 increased by 8.0 ± 15.7% in the ET group compared with a reduction of −2.0 ± 18.3% in the CON group. Mean changes were significantly different between ET and CON for relative peak V̇O2, absolute peak V̇O2, peak O2‐pulse and weight (P < 0.05), while no significant differences have been observed for the change in peak HR, haemoglobin (Figure 2 , Table 2 ) or the levels of exhaustion as measured by peak respiratory exchange ratio (RER) (Table 2 ). Mean change in relative V̇O2 at VT1 was significantly different between groups (P = 0.03; Table 2 ). The difference in change in relative peak V̇O2 between groups was primary mediated by changes in peak O2‐pulse (~72%), while changes in peak HR and weight accounted for approximately 18% and 10%, respectively. From baseline to follow‐up, the beta‐blocker dosage was changed in 14 patients with an increase in two patients randomized to ET (none in CON) and a decrease in 10 ET and 2 CON patients (P = 0.13). When these patients were excluded, the difference in mean change in peak HR between groups diminished to 0.3% (95% CI, −3.5 to 4.1, P = 0.89), whereas the mean changes in peak V̇O2, peak O2‐pulse, and weight remained significantly different between the groups (data not shown). In this subset, change in peak O2‐pulse accounted for approximately 88% of the difference in change in relative peak V̇O2 between groups.
Figure 2.
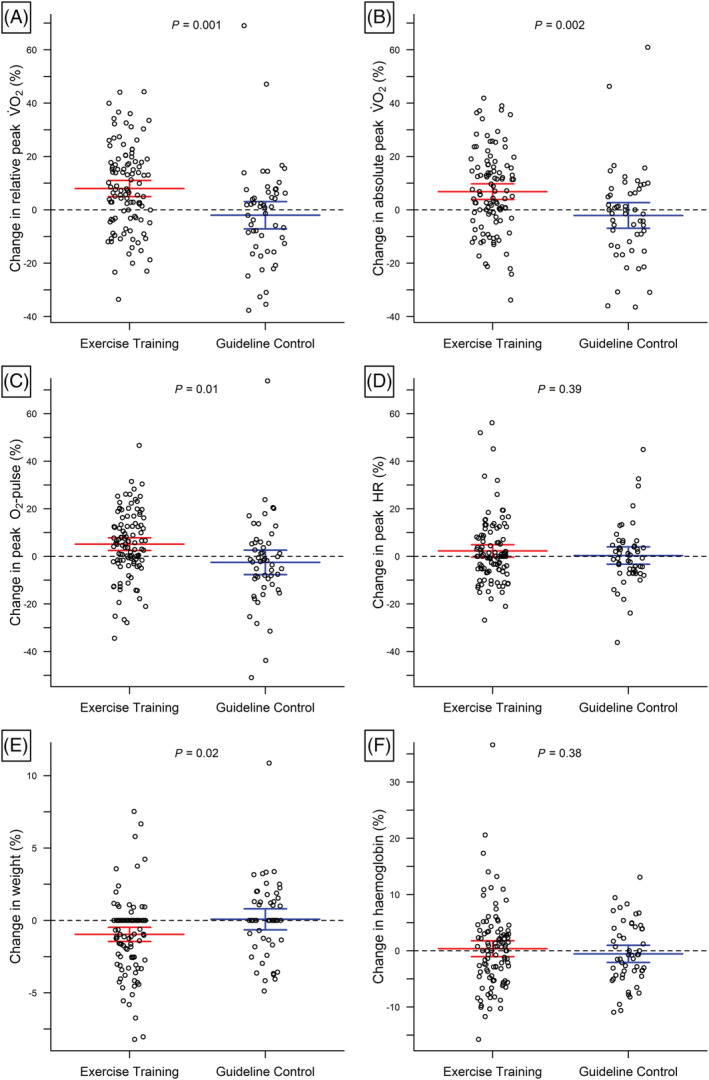
Change in relative peak V̇O2 and its determinants. Differences in individual changes (circles) plus mean and 95% confidence intervals (lines) of relative peak V̇O2 (A), absolute peak V̇O2 (B), O2‐pulse (C), peak heart rate (D), weight (E) and haemoglobin (F) between exercise training and guideline control.
Table 2.
Mean changes following exercise training and guideline control
| Mean ± SD | |||||||
|---|---|---|---|---|---|---|---|
| Exercise training [N = 106]* | Guideline control [N = 52]* | ||||||
| Baseline | 3 months | Change | Baseline | 3 months | Change | Difference (95% CI), P‐value | |
| Relative peak V̇O2, mL/kg/min | 18.5 ± 5.1 | 19.8 ± 5.7 | 8.0 ± 15.7% | 19.5 ± 5.8 | 18.9 ± 5.7 | −2.0 ± 18.3% | 10.0% (4.1 to 15.9), P = 0.001 |
| Absolute peak V̇O2, mL/min | 1,528 ± 447 | 1,617 ± 465 | 6.8 ± 15.2% | 1,519 ± 488 | 1,475 ± 485 | −2.1 ± 17.3% | 8.9% (3.3 to 14.5), P = 0.002 |
| Peak O2‐pulse, mL/beat | 12.7 ± 3.5 | 13.2 ± 3.1 | 5.1 ± 13.9% | 12.9 ± 4.0 | 12.5 ± 4.3 | −2.5 ± 18.5% | 7.7% (1.9 to 13.4), P = 0.01 |
| Peak heart rate, b.p.m. | 123 ± 26 | 124 ± 25 | 2.3 ± 13.5% | 121 ± 28 | 121 ± 30 | 0.3 ± 13.1% | 2.6% (−2.5 to 6.4), P = 0.39 |
| Weight, kg | 84.6 ± 18.0 | 83.8 ± 18.1 | −1.0 ± 2.6% | 78.7 ± 15.2 | 78.8 ± 15.6 | 0.1 ± 2.6% | −1.0% (−1.9 to −0.2), P = 0.02 |
| Haemoglobin, g/dL* | 13.6 ± 1.6 | 13.6 ± 1.4 | 0.4 ± 7.2% | 13.2 ± 1.4 | 13.1 ± 1.4 | −0.6 ± 5.5% | 0.9% (−1.1 to 3.0), P = 0.38 |
| Relative V̇O2 at VT1, mL/kg/min* | 11.1 ± 3.1 | 11.9 ± 3.1 | 9.4 ± 16.5% | 11.5 ± 2.9 | 11.4 ± 2.6 | 2.0 ± 21.3% | 7.4% (2.0 to 14.2), P = 0.03 |
| Peak Respiratory Exchange Ratio | 1.11 ± 0.09 | 1.10 ± 0.13 | −1.0 ± 5.9% | 1.10 ± 0.09 | 1.11 ± 0.12 | 0.9 ± 11.0% | −0.1% (−5.2 to 1.3), P = 0.24 |
Different N (due to missing values) for the analyses including haemoglobin (exercise training: 103; guideline control: 52) and VT1 (exercise training: 104; guideline control: 50). V̇O2˙O2, oxygen consumption; VT1, ventilatory threshold.
Covariate‐treatment interactions
The influence of baseline peak O2‐pulse (in mL/beat) on change in relative peak V̇O2 was significantly higher following ET compared with CON (interaction P = 0.04; Figure 3 A ). In the ET group, every 1 mL/beat higher baseline peak O2‐pulse was associated with a decreased mean change in relative peak V̇O2 of −1.45% (95% CI, −2.30 to −0.60, P = 0.001). In contrast, the change in relative peak V̇O2 following CON was not dependent on baseline peak O2‐pulse [β‐coefficient: −0.08% (95% CI, −1.11 to 0.96), P = 0.88]. After adjustment for sex, age, and baseline weight, this difference remained significant [ET: −1.89% (95% CI, −2.84 to −0.94); CON: −0.42% (95% CI, −1.77 to 0.62); interaction P = 0.049]. The interaction between baseline peak O2‐pulse and group on change in peak V̇O2 may also depend on baseline peak RER with a higher difference between groups in patients with peak RER > 1.10 (Supporting Information, Figure S1 ). No significant interactions on change in relative peak V̇O2 were found between groups and relative peak V̇O2, absolute peak V̇O2, peak HR, weight, and haemoglobin (interaction P > 0.05; Figure 3 , Table 3 ). None of these factors was significantly associated with the change in relative V̇O2 at VT1 in either group (Supporting Information, Table S1 ; Figure S2 ). The influence of baseline peak O2‐pulse on change in relative peak V̇O2 was similar in HIIT and MCT; however, neither peak O2‐pulse (interaction P = 0.15) nor any of the other factors showed a significant interaction with the original study groups (HIIT, MCT, and CON) on the change in relative peak V̇O2 (Supporting Information, Table S2 ; Figure S3 ). Results of the per‐protocol analysis were similar to the main results (Table 3 ; Supporting Information, Figure S4 ). Following ET, every 1 mL/beat higher baseline peak O2‐pulse was associated with a decreased mean change in relative peak V̇O2 of −1.88% (95% CI, −2.79 to −0.97, P < 0.001; interaction P = 0.01). Accordingly, the mean difference in change in relative peak V̇O2 between a patient who attended at least 70% of ET sessions and a patient randomized to CON was 20.5% for a baseline peak O2‐pulse of 8.6 mL/beat (10th percentile), and 3.7% for a baseline peak O2‐pulse of 17.9 mL/beat (90th percentile).
Figure 3.
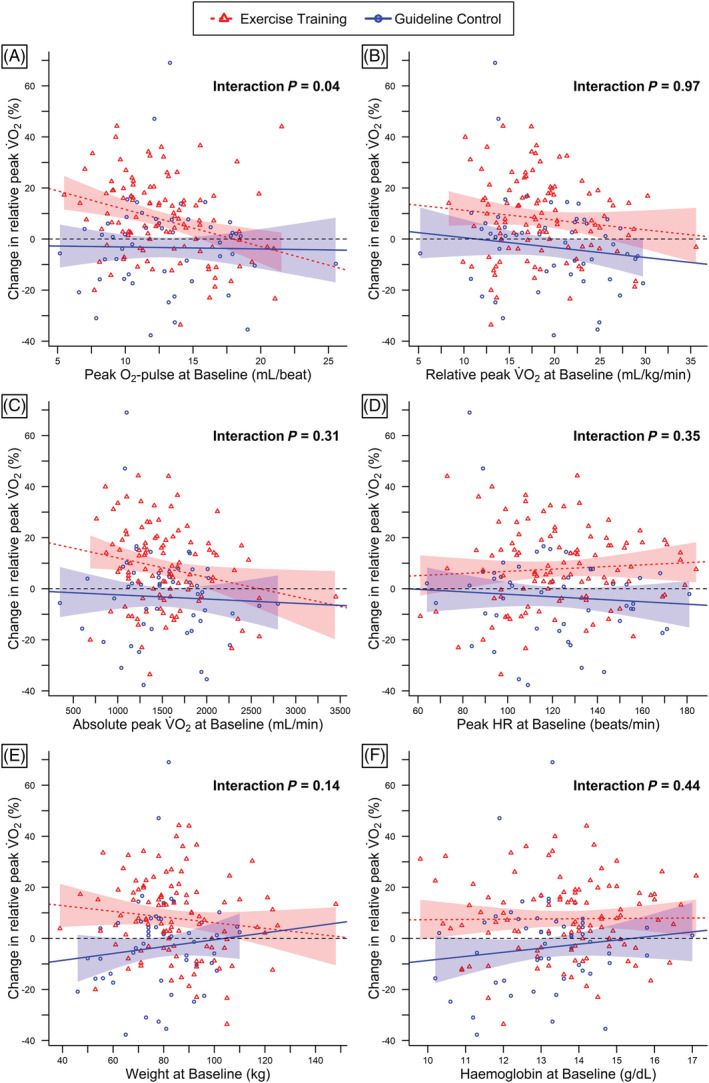
Predictors of change in relative V̇O2. Relationships between changes in relative peak V̇O2 and baseline peak O2‐pulse (A), relative peak V̇O2 at baseline (B), absolute peak V̇O2 at baseline (C), baseline peak heart rate (D), baseline weight (E), and baseline haemoglobin (F). Individual relationships and robust linear regression lines and 95% confidence bands are shown separately for exercise training ( ) and guideline control (
) and guideline control ( ). Black dashed lines (
). Black dashed lines ( ) represent null lines.
) represent null lines.
Table 3.
Results of the predictor analyses for the inter‐individual response variability in peak V̇O2 for the main and per protocol analysis
| Mean change in relative peak V̇O2 [β‐coefficient (95% CI), P‐value] | Interaction P for exercise training vs. guideline control | ||||
|---|---|---|---|---|---|
| Exercise training | Guideline control [N = 52]* | Main analysis | Per protocol analysis | ||
| Complete set [N = 106]* | Per protocol set [N = 87]* | ||||
| Peak O2‐pulse (per 1 mL/beat) | −1.45% (−2.30 to −0.60), P = 0.001 | −1.88% (−2.79 to −0.97), P < 0.001 | −0.08% (−1.11 to 0.96), P = 0.88 | 0.04 | 0.01 |
| Peak V̇O2 (per 1 mL/kg/min) | −0.38% (−0.99 to 0.22), P = 0.17 | −0.26% (−0.98 to 0.45), P = 0.47 | −0.39% (−1.10 to 0.32), P = 0.28 | 0.97 | 0.78 |
| Peak V̇O2 (per 100 mL/min) | −0.76% (−1.45 to 0.07), P = 0.03 | −0.74% (−1.51 to 0.02), P = 0.06 | −0.17% (−1.03 to 0.69), P = 0.70 | 0.31 | 0.34 |
| Peak heart rate (per 10 b.p.m.) | 0.42% (−0.79 to 1.63), P = 0.50 | 0.74% (−0.57 to 2.06), P = 0.27 | −0.48% (−1.96 to 0.99), P = 0.52 | 0.35 | 0.22 |
| Weight (per 10 kg) | −1.11% (−2.84 to 0.63), P = 0.21 | −2.04% (−4.15 to 0.08), P = 0.06 | 1.35% (−1.35 to 4.05), P = 0.32 | 0.14 | 0.054 |
| Haemoglobin (per 1 mg/dL)* | 0.10% (−1.81 to 2.00), P = 0.92 | 0.21% (−1.91 to 2.33), P = 0.85 | 1.60% (−1.46 to 4.65), P = 0.30 | 0.44 | 0.49 |
Different N for the analyses including haemoglobin (exercise training complete set: 103; exercise training per‐protocol set: 84; guideline control: 52). V̇O2, oxygen consumption.
Associations between changes in peak V̇O2 and its determinants
In the overall sample, changes in relative peak V̇O2 were positively correlated with changes in peak O2‐pulse and peak HR and negatively correlated with weight (P < 0.001). Furthermore, the changes in peak O2‐pulse were negatively correlated with the changes in peak HR (P < 0.001). Changes in weight were not significantly correlated with either changes in peak HR nor changes in peak O2‐pulse (P > 0.45). A correlation matrix is shown in Supporting Information, Figure S5 . None of these associations were significantly different between ET and CON (interaction P > 0.05; Supporting Information, Table S3 ).
Discussion
This is the first study to examine potential predictors of inter‐individual response variability in relative peak V̇O2 following ET versus CON in HFpEF and, to the best of our knowledge, the first study to examine the effects of baseline peak O2‐pulse on the change in peak V̇O2 following ET in any population. The main finding of this study (confirming the primary hypothesis) is that in patients randomized to ET, lower baseline peak O2‐pulse was associated with a larger improvement in relative peak V̇O2, whereas no such association was found in CON patients. This difference between ET and CON remained significant after adjusting for sex, age and baseline weight, and increased after excluding ET‐patients with an adherence lower than 70%. Furthermore, the predictive effect of baseline O2‐pulse seemed to be independent of the exercise mode (HIIT and MCT).
O2‐pulse is the fraction of V̇O2 and HR; therefore, low peak O2‐pulse can be caused by either low peak V̇O2, high peak HR or a combination of both. Nevertheless, the association of baseline peak V̇O2 (absolute or adjusted to body weight) with the change in relative peak V̇O2 was not significantly different between groups, confirming the results of a recent meta‐analysis in heart failure. 13 When adjusted to body weight, we observed an almost parallel decline in change in peak V̇O2 with increasing baseline peak V̇O2 in both groups. Accordingly, the baseline level of relative peak V̇O2 may only be a prognostic marker for its change in patients with HFpEF, with differences between ET and CON being similar for different base levels. In a cohort study including 120 patients with heart failure with reduced ejection fraction, 37 it has already been shown that the severity of chronotropic incompetence may be associated with an impaired response to ET. However, in the present trial, peak HR alone was not a significant predictor of the ET‐induced changes in relative peak V̇O2. Alternatively, a high O2‐pulse can also be defined as a high SV and/or C(a‐v)O2. Accordingly, a higher peak O2‐pulse at baseline is accompanied with a lower reserve to increase peak SV and/or C(a‐v)O2—the components of peak V̇O2 that are most likely to improve following ET. 15 This interpretation is supported by the results after splitting the sample based on baseline peak RER. While a peak RER ≥1.10 is considered excellent effort and maximal exhaustion, 38 patients with a lower peak RER could have stopped for other reasons (e.g. low motivation or musculoskeletal complaints), which seems to reduce the predictive power of baseline peak O2‐pulse for ET‐induced changes in peak V̇O2 in this subgroup. On the other hand, patients with a low peak O2‐pulse despite high volitional effort are very likely to be truly limited by their ability to increase SV and/or C(a‐v)O2. Therefore, in addition to the results of the per‐protocol analysis, the higher difference between groups in patients with peak RER ≥ 1.10 strengthens the assumption of a true association between baseline peak O2‐pulse and change in peak V̇O2 through ET. Consequently, the change in peak O2‐pulse was also the primary mediator for the change in relative peak V̇O2 following ET in this study. Importantly, the predictive power of baseline peak O2‐pulse did not apply to the change of V̇O2 at VT1. Instead, patients were able to equally improve their functional capacity through exercise training irrespective of baseline peak O2‐pulse. This may be explained by the fact that peak O2‐pulse is not a determinant of V̇O2 at VT1. Furthermore, the extent to which ET‐induced changes in V̇O2 at VT1 are mediated by changes in HR, SV and C(a‐v)O2 is less well investigated.
To date, only two studies have examined the effects of ET versus CON on SV and C(a‐v)O2 in HFpEF. 16 , 17 In a non‐randomized study, Fu et al. 16 found a significant improvement in peak V̇O2 after 12 weeks of HIIT compared with CON (n = 60) along with significant improvements in peak C(a‐v)O2 and without significant changes in either peak SV or peak HR. In a secondary analysis of the randomized controlled PARIS study (n = 40), Haykowsky et al. 17 showed that the improvement in peak V̇O2 following ET was due to significant increases in peak HR and peak C(a‐v)O2. However, despite the significant increase in peak HR, only 16% of the training related improvement in peak V̇O2 was attributed to an improved cardiac output. While changes in peak HR were not significantly different between the groups in most ET trials in HFpEF, 5 , 16 , 19 , 20 , 21 , 22 significant improvements as observed in two trials 23 , 24 could also be influenced by factors not directly related to ET, that is, changes in levels of exhaustion or changes in HR‐affecting medications (e.g. beta‐blockers). For instance, patients randomized to the ET group of the PARIS study 23 had a significantly higher change in peak HR compared with CON (+4 vs. −7 b.p.m.); however, the results for peak systolic blood pressure (+1 mmHg vs. −10 mmHg, P = 0.04), and peak RER (+0.03 vs. −0.02, P = 0.07) indicate that different levels of exhaustion between groups may have contributed to the significant difference in peak HR. In the present trial, changes in beta‐blocker dosage were more common in the ET group (11.3%) compared with CON (3.8%) and when these patients were excluded, the difference in change in peak HR between groups diminished from 1.9% to 0.3% (both P > 0.05).
The evidence that ET‐related improvements in peak V̇O2 are most likely mediated through increases in peak C(a‐v)O2 may also explain the overall positive effects of ET in patients with HFpEF as C(a‐v)O2 has been shown to be reduced in 75% and being the leading cause of exercise intolerance in 40% of patients with HFpEF. 12 Furthermore, it has been shown that a normalization of impaired muscle oxygen diffusion would result in a significantly larger improvement in peak V̇O2 than a normalization of convective oxygen delivery. 8 , 12 Due to interactions between the components of the Fick equation, Houstis et al. 8 demonstrated that doubling a patient's cardiac output would lead to a decrease in C(a‐v)O2 of 45%, and thus, peak V̇O2 would increase by only 10%. On the other hand, normalization of the 36% deficit in skeletal muscle oxygen diffusion that was observed in their study led to a predicted improvement in peak V̇O2 of 27%. Similarly, the results of the present trial show that a change in peak HR (independent of groups assignment) was not associated with an equivalent change in relative peak V̇O2 (a 10% increase in peak HR was associated with ~6.4% increase in peak V̇O2), which is likely to be explained by a reduced SV (shortening the time of the diastole) and a reduced C(a‐v)O2 (reducing the contact time in the muscle).
Future implications
The results of this exploratory analysis implicate that patients with HFpEF and high O2‐pulse may not be able to significantly improve their peak V̇O2 by performing regular ET. Although we still highly recommend regular ET for patients with HFpEF and high O2‐pulse to reduce the decline in peak V̇O2 with ageing and disease progression and to improve parameters beyond peak V̇O2 (e.g. V̇O2 at VT1), it should probably be supplemented by additional therapies if the intention is to increase maximal exercise tolerance.
Despite lacking evidence for its benefits in HFpEF, most patients were treated with beta‐blockers (66% in the present trial). This proportion is likely to be decreasing, as the effects of a long‐term administration of beta‐blockers in hypertension, stable coronary artery disease or atrial fibrillation (common co‐morbidities in HFpEF) are questioned and some studies led to the concern that beta‐blockers may be even deleterious in HFpEF. 39 By increasing the duration of diastole, a reduced HR may allow a better left ventricular filling; however, it may also impair the cardiac output response to exercise 40 and according to the results of the present analysis may contribute to a blunted ET response by its effect on peak O2‐pulse. Indeed, a recently published trial investigating the effects of beta‐blocker withdrawal in HFpEF 41 has shown a significant short‐term increase in peak HR (~31%) and peak V̇O2 (~17%). However, further research is necessary to show whether interventions to increase peak HR in patients with HFpEF (e.g. by reducing beta‐blockers or rate‐adaptive pacing 42 ) have a positive long‐term effect on clinical outcomes and exercise capacity.
Interestingly, we also found a trend towards lower ET‐induced changes in relative peak V̇O2 for patients with higher body weight at baseline (P = 0.14 in the full analysis, P = 0.054 in the per‐protocol analysis). Whether patients with HFpEF and higher baseline weight benefit less from exercise training or especially HIIT (see Supporting Information, Figure S3 E) should be investigated in future studies. Nevertheless, the results of the present study underscore the need for additional trials that specifically target weight loss in HFpEF. As most patients with HFpEF are overweight or obese (85% in the present trial), losing weight, which has a disproportionate impact on relative peak V̇O2 (a weight loss of 20% leads to an increase in relative peak V̇O2 of 25%), is another important option to increase exercise tolerance in HFpEF that has been largely ignored so far. To date, in the only lifestyle intervention trial targeting weight loss in patients with HFpEF (N = 100; mean BMI, 39.3 kg/m2), 21 ET and caloric restriction resulted in similar and additive changes in relative peak V̇O2 (main effect of ET: 1.2 mL/kg/min vs. diet: 1.3 mL/kg/min; joint effect: 2.5 mL/kg/min) by significantly improving absolute peak V̇O2 (ET) and reducing weight (ET and caloric restriction).
A combination of treatments targeting several deficits may overcome the interaction effects between the determinants of peak V̇O2 and will likely have a disproportionate impact compared with the correction of a single deficit. 8 For example, based on the interaction with SV and C(a‐v)O2, that is, O2‐pulse, increasing peak HR will possibly not only have a direct effect on peak V̇O2, 41 but also enhance the potential for improving peak V̇O2 following ET in patients with high O2‐pulse.
Methodological aspects, strengths, and limitations
This study has several strengths and limitations. The individual pre‐post change in peak V̇O2 can be divided into a ‘true’ change depending on the intervention, a ‘true’ change which is independent of the intervention (e.g. ageing), and a change due to random errors which is also independent of group assignment (e.g. measurement errors, day‐to‐day variability, different levels of exhaustion during the CPETs at baseline and follow‐up). 29 Therefore, to examine predictors of the ET‐induced response variability (instead of prognostic factors that are independent of the intervention and possibly influenced by regression to the mean) it is mandatory to include a comparator arm, which, however, has not been performed in many previous studies. Furthermore, the dependent and all independent parameters were analysed as continuous variables, which has several advantages over arbitrary categorization (e.g. retaining higher power and avoiding misclassification due to random errors).
Despite these strengths, the original trial was designed to detect differences between group means and therefore, methods to further reduce the bias of random errors (e.g. repeated pre‐measurements and post‐measurements) 29 have not been applied. The wide scatter of individual changes underlines the importance of conducting predictor analyses; however, this heterogeneity might have been amplified by the multimorbid condition of the patients with a high number of adverse events in both groups 5 and the fact that, strictly speaking, this study was not a predictor analysis for ‘ET versus control’ as it compared the offer for supervised ET (HIIT and MCT) with a recommendation to perform regular physical activity (CON). To account for different levels in adherence, we performed a per‐protocol analysis excluding ET patients with an adherence of <70%; however, it is unclear if and how many patients assigned to CON started exercising between baseline and follow‐up. Nevertheless, as mean peak V̇O2 slightly decreased following CON, it is unlikely that many patients performed regular exercise training in this group. On average, patients included in the present trial had a relatively preserved exercise capacity at baseline. However, the wide range of baseline values and their linear relationships with the change in peak V̇O2 suggest that the results of the regression analyses are likely to be generalizable. Lastly, the measurement of O2‐pulse does not allow to distinguish between SV and C(a‐v)O2; however, it can be more easily obtained in routine care. While both peak SV and peak C(a‐v)O2 are significantly reduced in HFpEF, 10 further research is necessary to show whether both factors play a relevant role for the improvements in peak V̇O2 following ET.
Conclusions
In patients with HFpEF, lower baseline peak O2‐pulse is associated with higher ET‐induced changes in relative peak V̇O2. This is an important finding towards a deficit‐oriented personalized medicine in HFpEF, provides an easily measurable indicator of the potential for improving maximal exercise tolerance through exercise training and underlines the value of CPET to guide therapy. While changes in submaximal exercise tolerance were independent of baseline peak O2‐pulse, patients with HFpEF and high O2‐pulse may need to use additional therapies (e.g. reduction of negative chronotropic drugs, rate‐adaptive pacing, and/or weight loss) to significantly increase maximal exercise tolerance.
Conflict of interest
Stephan Mueller reported receiving grants from Deutsche Forschungsgemeinschaft (DFG) through the TUM International Graduate School of Science and Engineering and the German Centre for Cardiovascular Research (DZHK) during the conduct of the study. Ephraim B. Winzer reported receiving personal fees from Novartis (honoraria for lectures and advisory board activities), Boehringer Ingelheim (honoraria for advisory board activities), and CVRX (honoraria for lectures) outside the submitted work. Luciene F. Azevedo reported receiving grants from Brazilian National Council for Scientific and Technological Development and Technical University of Munich—Laura Bassi Award. André Duvinage reported receiving grants from Novartis outside the submitted work. Frank Edelmann reported receiving grants from DFG, BMBF, Servier, and personal fees from Bayer Healthcare, Merck, Novartis, Servier, Berlin Chemie, Boehringer Ingelheim, Vifor Pharma, AstraZeneca, PharmaCosmos outside the submitted work. Axel Linke reported receiving speaker fees from Abbott, Medtronic, Edwards Lifesciences, AstraZeneca, Boston Scientific, and Novartis; grants from Edwards Lifesciences and Novartis; advisory board fees from Transverse Medical, Picardia, Edwards Lifesciences, and Heart Leaflet Technology; and stock options from Claret Medical and Transverse Medical, and being a co‐owner of Dresden Cardiovascular Research Institute and Core Laboratories outside the submitted work. Emeline M. Van Craenenbroeck reported receiving grants from the Flemish Research Funds (FWO) as a senior clinical investigator during the conduct of the study. Burkert Pieske reported receiving personal fees from Bayer Healthcare (steering committee, lectures), Merck (steering committee, lectures), Novartis (steering committee, lectures), Servier, AstraZeneca (lectures), Bristol‐Myers Squibb (lectures), and Medscape (lectures) outside the submitted work. Martin Halle reported receiving grants from the TUM International Graduate School of Science and Engineering and the German Centre for Cardiovascular Research (DZHK) during the conduct of the study and grants from Novartis (principal investigator of the Activity Study in HFrEF) and personal fees from Bristol‐Myers Squibb, Berlin Chemie‐Menarini, Novartis, Daiichi‐Sankyo, AstraZeneca, Roche, Abbott (advisory board on exercise and diabetes), Sanofi, Pfizer, Boehringer Ingelheim, and Bayer, and serves as an advisor for Medical Park SE, Germany outside the submitted work. No other disclosures were reported. Bernhard Haller, Anna Feuerstein, Paul Beckers, Mark J. Haykowsky, Andreas B. Gevaert, Jennifer Hommel, Katrin Esefeld, Isabel Fegers‐Wustrow, Jeffrey W. Christle, Elisabeth Pieske‐Kraigher, Evgeny Belyavskiy, Daniel A. Morris, Martin Kropf, and Radhakrishnan Aravind‐Kumar declared that they have no conflict of interest.
Funding
This work was supported by the European Commission, Framework Program 7 [grant number: EU 602405‐2]; the German Centre for Cardiovascular Research (DZHK) [grant number: 81Z0600603] [S.M. and M.H.]; and the Deutsche Forschungsgemeinschaft (DFG) through the TUM International Graduate School of Science and Engineering (IGSSE; Garching, Germany) [S.M., A.D., and M.H.]. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Supporting information
Table S1: Results of the predictor analyses for the inter‐individual response variability in V̇O2 at VT1.
*Different N for the analyses including haemoglobin (Exercise Training: 101, Guideline Control: 50); V̇O2 = oxygen consumption, VT1 = ventilatory threshold.
Table S2: Results of the predictor analyses for the inter‐individual response variability in peak V̇O2 in the three‐group design.
*Different N for the analyses including haemoglobin (High‐Intensity Interval Training: 50, Moderate Continuous Training: 53; Guideline Control: 52); V̇O2 = oxygen consumption.
Table S3: Correlations between changes in peak V̇O2, peak heart rate, peak O2‐pulse, and weight V̇O2 = oxygen consumption.
Figure S1: Relationships between baseline peak O 2 ‐pulse and change in peak V̇O 2 , separated by median baseline peak respiratory exchange ratio (RER). In patients with baseline peak RER < 1.10 (A), there was no significant interaction between baseline peak O2‐pulse × group on change in peak V̇O2 (Exercise Training: −0.87% [95% CI, −2.16 to 0.41], P = 0.20; Guideline Control: −0.44% [95% CI, −1.83 to 0.94), P = 0.53]). In patients with RER ≥ 1.10 (B), the association between baseline peak O2‐pulse and change in peak VO2 was significantly different between groups (Exercise Training: −1.89% [95% CI, −3.07 to −0.70), P = 0.003]; Guideline Control: 0.58% [95% CI, −1.07 to 2.23], P = 0.49). Individual relationships and robust linear regression lines and 95% confidence bands are shown separately for Exercise Training (Δ) and Guideline Control (○). Black dashed lines (− − −) represent null lines.
Figure S2: Predictors of change in V̇O 2 at VT1. Relationships between changes in relative V̇O2 at VT1 and peak O2‐pulse at baseline (A), relative peak V̇O2 at baseline (B), absolute peak V̇O2 at baseline (C), peak heart rate at baseline (D), baseline weight (E), and baseline haemoglobin (F). Individual relationships and robust linear regression lines and 95% confidence bands are shown separately for Exercise Training (Δ) and Guideline Control (○). Black dashed lines (− − −) represent null lines.
Figure S3: Predictors of change in peak V̇O 2 following High‐Intensity Interval Training (HIIT), Moderate Continuous Training (MCT) and Guideline Control (Con). Relationships between changes in relative peak V̇O2 and peak O2‐pulse at baseline (A), relative peak V̇O2 at baseline (B), absolute peak V̇O2 at baseline (C), peak heart rate at baseline (D), baseline weight (E), and baseline haemoglobin (F). Individual relationships and robust linear regression lines and 95% confidence bands are shown separately for HIIT (Δ), MCT (· □ ·) and Con (○). Black dashed lines (− − −) represent null lines.
Figure S4: Predictors of change in peak V̇O 2 (excluding patients with adherence < 70%). Relationships between changes in peak V̇O2 and peak O2‐pulse at baseline (A), relative peak V̇O2 at baseline (B), absolute peak VO2 at baseline (C), peak heart rate at baseline (D), baseline weight (E) and baseline haemoglobin (F). Individual relationships and linear regression lines and 95% confidence bands are shown separately for the Exercise Training Per‐Protocol Set (Δ) and Usual Care (○). Black dashed lines (− − −) represent null lines.
Figure S5: Associations between the changes in peak V̇O2 and its determinants including regression lines and 95% confidence bands. Red lines (—) in A‐C represent the predicted associations if all other determinants remain constant. Black dashed lines (− − −) represent null lines.
Acknowledgements
The authors thank the staff of the University Hospital ‘Klinikum rechts der Isar’ of the Technical University of Munich, Antwerp University Hospital, Heart Centre Leipzig, Charité Universitätsmedizin Berlin and Norwegian University of Science and Technology Trondheim who were involved in recruitment, evaluations, administration, and support during the conduct of the OptimEx‐Clin study. Open Access funding enabled and organized by Projekt DEAL.
Mueller, S. , Haller, B. , Feuerstein, A. , Winzer, E. B. , Beckers, P. , Haykowsky, M. J. , Gevaert, A. B. , Hommel, J. , Azevedo, L. F. , Duvinage, A. , Esefeld, K. , Fegers‐Wustrow, I. , Christle, J. W. , Pieske‐Kraigher, E. , Belyavskiy, E. , Morris, D. A. , Kropf, M. , Aravind‐Kumar, R. , Edelmann, F. , Linke, A. , Adams, V. , Van Craenenbroeck, E. M. , Pieske, B. , Halle, M. , and the OptimEx‐Clin Study Group (2022) Peak O2‐pulse predicts exercise training‐induced changes in peak V̇O2 in heart failure with preserved ejection fraction. ESC Heart Failure, 9: 3393–3406. 10.1002/ehf2.14070.
References
- 1. Pelliccia A, Sharma S, Gati S, Bäck M, Börjesson M, Caselli S, Collet JP, Corrado D, Drezner JA, Halle M, Hansen D, Heidbuchel H, Myers J, Niebauer J, Papadakis M, Piepoli MF, Prescott E, Roos‐Hesselink JW, Graham Stuart A, Taylor RS, Thompson PD, Tiberi M, Vanhees L, Wilhelm M. ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2020; 2020: 17–96. [DOI] [PubMed] [Google Scholar]
- 2. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner‐La Rocca H‐P, Choi D‐J, Chopra V, Chuquiure‐Valenzuela E, Giannetti N, Gomez‐Mesa JE, Janssens S, Januzzi JL, Gonzalez‐Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021; 385: 1451–1461. [DOI] [PubMed] [Google Scholar]
- 3. Bonsu KO, Arunmanakul P, Chaiyakunapruk N. Pharmacological treatments for heart failure with preserved ejection fraction‐a systematic review and indirect comparison. Heart Fail Rev. 2018; 23: 147–156. [DOI] [PubMed] [Google Scholar]
- 4. Fukuta H, Goto T, Wakami K, Kamiya T, Ohte N. Effects of exercise training on cardiac function, exercise capacity, and quality of life in heart failure with preserved ejection fraction: A meta‐analysis of randomized controlled trials. Heart Fail Rev. 2019; 24: 535–547. [DOI] [PubMed] [Google Scholar]
- 5. Mueller S, Winzer EB, Duvinage A, Gevaert AB, Edelmann F, Haller B, Pieske‐Kraigher E, Beckers P, Bobenko A, Hommel J, Van de Heyning CM, Esefeld K, von Korn P, Christle JW, Haykowsky MJ, Linke A, Wisløff U, Adams V, Pieske B, van Craenenbroeck EM, Halle M. Effect of high‐intensity interval training, moderate continuous training, or guideline‐based physical activity advice on peak oxygen consumption in patients with heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2021; 325: 542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype‐specific treatment of heart failure with preserved ejection fraction: A multiorgan roadmap. Circulation. 2016; 134: 73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pandey A, Vaduganathan M, Arora S, Qamar A, Mentz RJ, Shah SJ, Chang PP, Russell SD, Rosamond WD, Caughey MC. Temporal trends in prevalence and prognostic implications of comorbidities among patients with acute decompensated heart failure: The ARIC study community surveillance. Circulation. 2020; 142: 230–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Houstis NE, Eisman AS, Pappagianopoulos PP, Wooster L, Bailey CS, Wagner PD, Lewis GD. Exercise intolerance in heart failure with preserved ejection fraction: Diagnosing and ranking its causes using personalized O(2) pathway analysis. Circulation. 2018; 137: 148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure‐‐abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004; 350: 1953–1959. [DOI] [PubMed] [Google Scholar]
- 10. Pandey A, Khera R, Park B, Haykowsky M, Borlaug BA, Lewis GD, Kitzman DW, Butler J, Berry JD. Relative impairments in hemodynamic exercise reserve parameters in heart failure with preserved ejection fraction: A study‐level pooled analysis. JACC Heart failure. 2018; 6: 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011; 58: 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dhakal BP, Malhotra R, Murphy RM, Pappagianopoulos PP, Baggish AL, Weiner RB, Houstis NE, Eisman AS, Hough SS, Lewis GD. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: The role of abnormal peripheral oxygen extraction. Circ Heart Fail. 2015; 8: 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taylor RS, Walker S, Smart NA, Piepoli MF, Warren FC, Ciani O, Whellan D, O'Connor C, Keteyian SJ, Coats A, Davos CH, Dalal HM, Dracup K, Evangelista LS, Jolly K, Myers J, Nilsson BB, Passino C, Witham MD, Yeh GY. Impact of exercise rehabilitation on exercise capacity and quality‐of‐life in heart failure: Individual participant meta‐analysis. J Am Coll Cardiol. 2019; 73: 1430–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nes BM, Janszky I, Wisløff U, Støylen A, Karlsen T. Age‐predicted maximal heart rate in healthy subjects: The HUNT fitness study. Scand J Med Sci Sports. 2013; 23: 697–704. [DOI] [PubMed] [Google Scholar]
- 15. Montero D, Díaz‐Cañestro C. Endurance training and maximal oxygen consumption with ageing: Role of maximal cardiac output and oxygen extraction. Eur J Prev Cardiol. 2016; 23: 733–743. [DOI] [PubMed] [Google Scholar]
- 16. Fu TC, Yang NI, Wang CH, Cherng WJ, Chou SL, Pan TL, Wang JS. Aerobic interval training elicits different hemodynamic adaptations between heart failure patients with preserved and reduced ejection fraction. Am J Phys Med Rehabil. 2016; 95: 15–27. [DOI] [PubMed] [Google Scholar]
- 17. Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012; 60: 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Angadi SS, Mookadam F, Lee CD, Tucker WJ, Haykowsky MJ, Gaesser GA. High‐intensity interval training vs. moderate‐intensity continuous exercise training in heart failure with preserved ejection fraction: A pilot study. J Appl Physiol. 2015; 119: 753–758. [DOI] [PubMed] [Google Scholar]
- 19. Donelli da Silveira A, Beust de Lima J, da Silva PD, dos Santos MD, Zanini M, Nery R, Laukkanen JA, Stein R. High‐intensity interval training is effective and superior to moderate continuous training in patients with heart failure with preserved ejection fraction: A randomized clinical trial. European journal of. Prev Cardiol. 2020; 27: 1733–1743. [DOI] [PubMed] [Google Scholar]
- 20. Edelmann F, Gelbrich G, Düngen HD, Fröhling S, Wachter R, Stahrenberg R, Binder L, Töpper A, Lashki DJ, Schwarz S, Herrmann‐Lingen C, Löffler M, Hasenfuss G, Halle M, Pieske B. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: Results of the ex‐DHF (exercise training in diastolic heart failure) pilot study. J Am Coll Cardiol. 2011; 58: 1780–1791. [DOI] [PubMed] [Google Scholar]
- 21. Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2016; 315: 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smart NA, Haluska B, Jeffriess L, Leung D. Exercise training in heart failure with preserved systolic function: A randomized controlled trial of the effects on cardiac function and functional capacity. Congest Heart Fail. 2012; 18: 295–301. [DOI] [PubMed] [Google Scholar]
- 23. Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: A randomized, controlled, single‐blind trial. Circ Heart Fail. 2010; 3: 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kitzman DW, Brubaker PH, Herrington DM, Morgan TM, Stewart KP, Hundley WG, Abdelhamed A, Haykowsky MJ. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: A randomized, controlled, single‐blind trial. J Am Coll Cardiol. 2013; 62: 584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite‐Moreira AF, Borbély A, Édes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: A consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the heart failure and echocardiography associations of the European Society of Cardiology. Eur Heart J. 2007; 28: 2539–2550. [DOI] [PubMed] [Google Scholar]
- 26. Suchy C, Massen L, Rognmo O, Van Craenenbroeck EM, Beckers P, Kraigher‐Krainer E, Linke A, Adams V, Wisloff U, Pieske B, Halle M. Optimising exercise training in prevention and treatment of diastolic heart failure (OptimEx‐CLIN): Rationale and design of a prospective, randomised, controlled trial. Eur J Prev Cardiol. 2014; 21: 18–25. [DOI] [PubMed] [Google Scholar]
- 27. Mezzani A, Agostoni P, Cohen‐Solal A, Corrà U, Jegier A, Kouidi E, Mazic S, Meurin P, Piepoli M, Simon A, Laethem CV, Vanhees L. Standards for the use of cardiopulmonary exercise testing for the functional evaluation of cardiac patients: A report from the exercise physiology section of the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil. 2009; 16: 249–267. [DOI] [PubMed] [Google Scholar]
- 28. Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (Bethesda, Md: 1985). 1986; 60: 2020–2027. [DOI] [PubMed] [Google Scholar]
- 29. Ross R, Goodpaster BH, Koch LG, Sarzynski MA, Kohrt WM, Johannsen NM, Skinner JS, Castro A, Irving BA, Noland RC, Sparks LM, Spielmann G, Day AG, Pitsch W, Hopkins WG, Bouchard C. Precision exercise medicine: Understanding exercise response variability. Br J Sports Med. 2019; 53: 1141–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jérolon A, Baglietto L, Birmelé E, Alarcon F, Perduca V. Causal mediation analysis in presence of multiple mediators uncausally related. Int J Biostat. 2020; 17: 191–221. [DOI] [PubMed] [Google Scholar]
- 31. Jérolon A. multimediate: Causal mediation analysis in presence of multiple mediators uncausally related. 2021. R package version 0.0.0.9000. Available from: https://github.com/AllanJe/multimediate [DOI] [PubMed]
- 32. Venables WN, Ripley BD. Modern Applied Statistics with S, Fourth ed. New York: Springer; 2002. [Google Scholar]
- 33. Maechler M. sfsmisc: Utilities from 'Seminar fuer Statistik' ETH Zurich. 2020. R package version 1.1–7. Available from: https://CRAN.R‐project.org/package=sfsmisc
- 34. Yu C, Yao W. Robust linear regression: A review and comparison. Commun Stat Simul Comput. 2017; 46: 6261–6282. [Google Scholar]
- 35. Maechler M, Rousseeuw P, Croux C, Todorov V, Ruckstuhl A, Salibian‐Barrera M, Verbeke T, Koller M, Conceicao E, Anna di Palma M. robustbase: Basic Robust Statistics. 2021. R package version 0.93–9. Available from: http://robustbase.r‐forge.r‐project.org/
- 36. R Core Team . R: A Language and Environment for Statistical Computing. 2021. Available from: https://www.R‐project.org/
- 37. Schmid JP, Zurek M, Saner H. Chronotropic incompetence predicts impaired response to exercise training in heart failure patients with sinus rhythm. Eur J Prev Cardiol. 2013; 20: 585–592. [DOI] [PubMed] [Google Scholar]
- 38. Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, Macko R, Mancini D, Milani RV. Clinician's guide to cardiopulmonary exercise testing in adults: A scientific statement from the American Heart Association. Circulation. 2010; 122: 191–225. [DOI] [PubMed] [Google Scholar]
- 39. Meyer M, LeWinter MM. Heart rate and heart failure with preserved ejection fraction: Time to slow β‐blocker use? Circ Heart Fail. 2019; 12: e006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kalogeropoulos AP, Butler J. Heart rate in heart failure with preserved ejection fraction: Target or marker? Eur J Heart Fail. 2017; 19: 1504–1506. [DOI] [PubMed] [Google Scholar]
- 41. Palau P, Seller J, Domínguez E, Sastre C, Ramón JM, de La Espriella R, Santas E, Miñana G, Bodí V, Sanchis J, Valle A, Chorro FJ, Llácer P, Bayés‐Genís A, Núñez J. Effect of β‐blocker withdrawal on functional capacity in heart failure and preserved ejection fraction. J Am Coll Cardiol. 2021; 78: 2042–2056. [DOI] [PubMed] [Google Scholar]
- 42. Zweerink A, van der Lingen ACJ, Handoko ML, van Rossum AC, Allaart CP. Chronotropic incompetence in chronic heart failure. Circ Heart Fail. 2018; 11: e004969. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Results of the predictor analyses for the inter‐individual response variability in V̇O2 at VT1.
*Different N for the analyses including haemoglobin (Exercise Training: 101, Guideline Control: 50); V̇O2 = oxygen consumption, VT1 = ventilatory threshold.
Table S2: Results of the predictor analyses for the inter‐individual response variability in peak V̇O2 in the three‐group design.
*Different N for the analyses including haemoglobin (High‐Intensity Interval Training: 50, Moderate Continuous Training: 53; Guideline Control: 52); V̇O2 = oxygen consumption.
Table S3: Correlations between changes in peak V̇O2, peak heart rate, peak O2‐pulse, and weight V̇O2 = oxygen consumption.
Figure S1: Relationships between baseline peak O 2 ‐pulse and change in peak V̇O 2 , separated by median baseline peak respiratory exchange ratio (RER). In patients with baseline peak RER < 1.10 (A), there was no significant interaction between baseline peak O2‐pulse × group on change in peak V̇O2 (Exercise Training: −0.87% [95% CI, −2.16 to 0.41], P = 0.20; Guideline Control: −0.44% [95% CI, −1.83 to 0.94), P = 0.53]). In patients with RER ≥ 1.10 (B), the association between baseline peak O2‐pulse and change in peak VO2 was significantly different between groups (Exercise Training: −1.89% [95% CI, −3.07 to −0.70), P = 0.003]; Guideline Control: 0.58% [95% CI, −1.07 to 2.23], P = 0.49). Individual relationships and robust linear regression lines and 95% confidence bands are shown separately for Exercise Training (Δ) and Guideline Control (○). Black dashed lines (− − −) represent null lines.
Figure S2: Predictors of change in V̇O 2 at VT1. Relationships between changes in relative V̇O2 at VT1 and peak O2‐pulse at baseline (A), relative peak V̇O2 at baseline (B), absolute peak V̇O2 at baseline (C), peak heart rate at baseline (D), baseline weight (E), and baseline haemoglobin (F). Individual relationships and robust linear regression lines and 95% confidence bands are shown separately for Exercise Training (Δ) and Guideline Control (○). Black dashed lines (− − −) represent null lines.
Figure S3: Predictors of change in peak V̇O 2 following High‐Intensity Interval Training (HIIT), Moderate Continuous Training (MCT) and Guideline Control (Con). Relationships between changes in relative peak V̇O2 and peak O2‐pulse at baseline (A), relative peak V̇O2 at baseline (B), absolute peak V̇O2 at baseline (C), peak heart rate at baseline (D), baseline weight (E), and baseline haemoglobin (F). Individual relationships and robust linear regression lines and 95% confidence bands are shown separately for HIIT (Δ), MCT (· □ ·) and Con (○). Black dashed lines (− − −) represent null lines.
Figure S4: Predictors of change in peak V̇O 2 (excluding patients with adherence < 70%). Relationships between changes in peak V̇O2 and peak O2‐pulse at baseline (A), relative peak V̇O2 at baseline (B), absolute peak VO2 at baseline (C), peak heart rate at baseline (D), baseline weight (E) and baseline haemoglobin (F). Individual relationships and linear regression lines and 95% confidence bands are shown separately for the Exercise Training Per‐Protocol Set (Δ) and Usual Care (○). Black dashed lines (− − −) represent null lines.
Figure S5: Associations between the changes in peak V̇O2 and its determinants including regression lines and 95% confidence bands. Red lines (—) in A‐C represent the predicted associations if all other determinants remain constant. Black dashed lines (− − −) represent null lines.


