Abstract
Background
Prognostic markers of survival have been identified in wild‐type transthyretin amyloidosis (ATTRwt), but limited data exist with respect to hospitalizations with worsening heart failure (WHF). Predictive markers of WHF have yet to be identified.
Methods
From April 2017 to February 2021, 104 patients with ATTRwt were diagnosed and prospectively followed from the time of diagnosis to the time of death or the censoring date of 1 February 2021. Baseline patient characteristics, biomarkers, and advanced echocardiography were used to predict hospitalization with WHF.
Results
During the median follow‐up period of 23 months, 51% of patients were hospitalized due to WHF. Seventy‐three per cent of patients with WHF were admitted at least twice. Patients with WHF during the first year had significantly poorer survival (P < 0.001). Independent predictors of WHF during follow‐up were pacemaker implantation prior to diagnosis (PMI, P = 0.037) and right atrial volume index (RAVi, P = 0.008). Patients with PMI had a higher left ventricular mass index and poorer left ventricular and right ventricular systolic function indicating a more advanced stage of amyloid disease.
Conclusions
A high incidence and recurrence of hospital admissions with WHF were demonstrated in contemporary patients with ATTRwt, which was associated with reduced survival. Patients with pacemaker devices prior to ATTRwt diagnosis experienced more frequent hospitalizations with WHF. PMI and right atrial enlargement were identified as independent predictors of WHF during follow‐up.
Keywords: Wild‐type transthyretin cardiac amyloidosis, Heart failure, Pacemaker
Introduction
Wild‐type transthyretin cardiac amyloidosis (ATTRwt) is increasingly recognized among elderly patients with heart failure (HF). 1 , 2 , 3 ATTRwt is a progressive restrictive cardiomyopathy associated with increased morbidity and poor survival. 1 Several markers of survival have been identified in cardiac amyloidosis such as age, left ventricular ejection fraction (LVEF), global longitudinal strain (GLS), myocardial extracellular volume, estimated glomerular filtration rate (eGFR), and biomarkers N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) and cardiac troponins. 1 , 4 , 5 , 6 , 7 , 8 Survival assessment and disease staging based on eGFR and NT‐proBNP measurements have been suggested and validated by Gillmore et al. 7
Patients with ATTRwt often experience several hospital contacts due to ATTR‐related symptoms before the diagnosis is established. 1 , 3 A median of three inpatient hospital admissions and 17 incidences of hospital usage has been reported prior to diagnosis in both hereditary and wild‐type ATTR. 1 After the diagnosis of ATTR is established, the frequent need for hospital services continues as the median number of inpatient hospital admissions ranges from two to three per year. 1 Hospitalization due to worsening heart failure (WHF) is likely one of the main reasons for hospitalization after the diagnosis is established. It often requires several days of in‐hospital stay and is associated with an adverse prognosis in the general HF population. However, limited data exist on the prevalence and clinical characteristics of patients admitted with worsening ATTRwt‐related HF.
In the present study, we aimed to investigate the incidence of WHF admissions in contemporary ATTRwt patients and the association between WHF and mortality. We also aimed to characterize the patient population and to identify predictors of hospital admission with WHF.
Methods
The present study enrolled 104 consecutive patients with diagnosed ATTRwt at Aarhus University Hospital, Denmark, from 1 April 2017 to 1 February 2021. All patients underwent comprehensive echocardiographic examinations and had electrocardiograms (ECGs) taken and blood drawn for biomarker analysis. Patients were then prospectively followed until the date of death or the censoring date of 1 February 2021.
ATTRwt diagnosis was confirmed by amyloid‐positive endomyocardial biopsy using Congo red staining and immunohistochemistry/mass spectrometry (n = 30), a positive 99mTc‐DPD scintigraphy with a Perugini Grade 2 or 3 (n = 57) with negative immunofixation analysis and a normal p‐kappa/lambda light chain ratio or both scintigraphy and biopsy in patients with positive immunofixation analysis and/or abnormal kappa/light chain ratio (n = 17). Normal p‐kappa/lambda light chains were considered in the interval 0.26–1.65, and spike bands were reported as present/not present. All patients were genotyped to exclude hereditary ATTR.
The primary endpoint was hospitalization due to WHF. Admission to a hospital with WHF was defined as development or worsening of existing symptoms and/or clinical signs of HF requiring non‐scheduled, inpatient hospitalization with treatment of intravenous diuretics, pleural or ascites drainage, inotropic therapy, or initiation of dialysis. In addition, the WHF condition should be primarily related to the ATTRwt cardiomyopathy. Secondary endpoint was all‐cause mortality.
Electronic medical charts and imaging servers of all patients were reviewed at initial referral, the time of diagnosis and when hospitalizations or deaths occurred. The collected data at baseline included information about patient symptoms, medical history, co‐morbidities, medication, biochemistry, echocardiography, and ECG. Data were collected from either the regional database The Electronic Patient Journal or the national database The Health Journal providing the investigators with admission data from across all of Denmark. All‐cause mortality data were collected from the Danish Central Personal Registry.
From the time of diagnosis and during follow‐up, there was complete access for the investigators to all the aforementioned data.
The ATTRwt disease stage was evaluated in accordance with staging system of the National Amyloidosis Centre (NAC), London, UK, 7 and the staging system of the Mayo Clinic. 6
The study was approved by the Danish Data Protection Agency, and as such, no ethical approval was required according to Danish law.
Echocardiography
A commercially available ultrasound system was used at initial referral, the time of diagnosis, and during follow‐up (GE Vivid 9 or E95, Horten, Norway) with a 3.5‐MHz phased‐array transducer. All echocardiographic examinations in the Central Denmark Region are stored in a digital image vault with the possibility of review of any given examination.
All patients underwent a comprehensive two‐dimensional echocardiographic assessment in accordance with current guidelines. 9 LVEF was calculated using Simpson's biplane method. The peak systolic left ventricular (LV) GLS 10 magnitude was obtained using automated function imaging in standard two‐dimensional cine loops with a frame rate >55 frames/s. The regional speckle area of interest was manually adjusted to obtain optimal tracking results. GLS was calculated using a 17‐segment model at the time in systole when the value peaked. We used triplane images for strain calculation in patients with atrial fibrillation. If the triplane image quality was suboptimal, we selected loops from the apical four‐chamber, two‐chamber, and long‐axis views with comparable RR intervals for strain calculation. Left atrial volume index (LAVi) was assessed by biplane method indexed to the body surface area and right atrial volume index (RAVi) was assessed from the right‐sided focused four‐chamber view.
The principles for estimation of LV pressure and work have previously been described. 11 In this method, we used a previously generated empiric reference curve for LV pressure assessment. This reference curve is individualized by scaling the amplitude using measured systolic cuff pressure. Subsequently, a pressure–strain curve is obtained by fitting the individualized reference curve in time according to aorta and mitral valve opening and closing. Based on the pressure–strain curve, the global LV myocardial work index is automatically calculated as the average of all segmental values. We calculated the average of the six apical, mid‐ventricular, and basal segments of both GLS and LV myocardial work index. Data were analysed offline using dedicated software (EchoPAC PC SW‐Only, Version 202, GE Healthcare, Milwaukee, WI, USA).
ECG
The presence of atrial fibrillation was determined based on the ECG or Holter findings at the time of diagnosis. The ECG changes related to ATTRwt were defined in accordance with standard ECG criteria described previously. 3
Statistics
Normally distributed data were presented as mean ± standard deviation (SD), and non‐normally distributed data were presented as median and interquartile range (IQR).
Group differences were assessed using Student's t‐test for normally distributed data, the Mann–Whitney U test for non‐normally distributed data, and the χ 2 test for categorical data.
Survival analysis was calculated using Kaplan–Meier estimates, and differences between groups were analysed using the log‐rank test. Nelson–Aalen estimates were used to assess competing risk.
Hazard ratios, 95% CIs, and two‐sided P‐values were determined using Cox proportional‐hazard regression models for univariable and multivariable analysis. Parameters with significant prognostic value (P < 0.05) in univariable analysis were used to create a multivariable model to explore if these parameters were independently related to WHF. Baseline was set to the time of diagnosis. Two‐sided tests were used for all analyses, and P < 0.05 was considered significant. The data were analysed using STATA (STATA/IC 16, StataCorp LP, College Station, TX, USA.)
Results
Clinical characteristics
The clinical characteristics at the time of the ATTRwt diagnosis are presented in Table 1 .
Table 1.
Patient characteristics at the time of ATTRwt diagnosis stratified by worsening heart failure within the following 12 months
| Variable | All (n = 104) | No. of HF admission (n = 68) | HF admission (n = 36) | P‐value |
|---|---|---|---|---|
| Age (years), mean ± SD | 81.2 ± 6 | 80.7 ± 6 | 82.0 ± 6 | 0.33 |
| Male gender, % | 90 (n = 94) | 91 (n = 62) | 92 (n = 33) | 0.93 |
| BSA (m2), mean ± SD | 1.95 ± 0.17 | 1.97 ± 0.18 | 1.91 ± 0.14 | 0.06 |
| NYHA, I/II/III/IV, % | 26/47/26/1 | 25/49/25/1 | 28/44/28/0 | 0.86 |
| Hypertension, % | 64 (n = 67) | 65 (n = 44) | 64 (n = 23) | 0.93 |
| IHD, % | 23 (n = 24) | 18 (n = 12) | 33 (n = 12) | 0.07 |
| Diabetes, % | 15 (n = 16) | 15 (n = 10) | 17 (n = 6) | 0.79 |
| Aortic stenosis, % | 24 (n = 25) | 24 (n = 16) | 25 (n = 9) | 0.87 |
| Pacemaker device, % | 35 (n = 36) | 26 (n = 18) | 50 (n = 18) | 0.02 |
| Carpal tunnel syndrome, % | 38 (n = 40) | 40 (n = 27) | 36 (n = 13) | 0.72 |
| Spinal stenosis, % | 7 (n = 7) | 4 (n = 3) | 11 (n = 4) | 0.20 |
| Diuretics, % | 77 (n = 79) | 71 (n = 49) | 83 (n = 30) | 0.15 |
| ACE/ARB, % | 45 (n = 47) | 46 (n = 31) | 44 (n = 16) | 0.91 |
| Beta blockers, % | 44 (n = 45) | 40 (n = 27) | 47 (n = 17) | 0.46 |
| Anti‐coagulation, % | 62 (n = 64) | 57 (n = 39) | 69 (n = 25) | 0.16 |
| Heart rate (beats/min), mean ± SD | 73 ± 15 | 72 ± 13 | 74 ± 19 | 0.69 |
| SBP (mmHG), mean ± SD | 134 ± 18 | 137 ± 19 | 130 ± 14 | 0.07 |
| DBP (mmHG), mean ± SD | 80 ± 12 | 81 ± 12 | 79 ± 11 | 0.69 |
| Troponin T (ng/L), median (IQR) | 63 (56) | 58 (60) | 76 (69) | 0.10 |
| NT‐proBNP (ng/L), median (IQR) | 2544 (3794) | 2116 (3407) | 3665 (7237) | 0.01 |
| eGFR (mL/min), mean ± SD | 62 ± 19 | 64 ± 19 | 57 ± 19 | 0.08 |
| Sinus rhythm, % | 55 (n = 57) | 59 (n = 40) | 47 (n = 17) | 0.26 |
| Atrial fibrillation, % | 41 (n = 43) | 37 (n = 25) | 50 (n = 18) | 0.19 |
| Low voltage, % | 27 (n = 28) | 25 (n = 17) | 31 (n = 11) | 0.54 |
| 1st deg. AV block, % | 15 (n = 15) | 15 (n = 10) | 14 (n = 5) | 0.91 |
| Left bundle branch block, % | 21 (n = 22) | 19 (n = 13) | 25 (n = 9) | 0.49 |
| Pseudo‐infarction, % | 8 (n = 8) | 7 (n = 5) | 8 (n = 3) | 0.86 |
AV, atrioventricular; BSA, body surface area; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HF, heart failure; IHD, ischaemic heart disease; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; SBP, systolic blood pressure.
Thirty‐six (34.6%) patients were hospitalized at least once with WHF at 12 months of follow‐up. The main symptoms leading to WHF hospitalization were worsening dyspnoea, which occurred in 92% of cases (n = 33), and peripheral oedema, which occurred in 44% of cases (n = 16). Ascites was noted in 20% of cases (n = 7), and drainage was performed in all except one. Intravenous and increased dosages of loop diuretics were used in all 36 patients. Atrial fibrillation or flutter was seen in 14% of cases (n = 5), of which three were treated with electric conversion and two were treated medically. One patient underwent a coronary angiography due to atypical chest pain with increased troponin levels but with normal findings. One patient received a dual‐lead pacemaker due to advanced atrioventricular block. No patients were admitted due to aortic valve stenosis, and the severity was comparable between patients with WHF compared with patients without WHF at 12 months of follow‐up [WHF: mild–moderate: n = 8 (80%); severe: n = 2 (20%) vs. non‐WHF: mild–moderate: 12 (80%); severe n = 3 (20%), P = 0.746].
Patients with WHF at 12 months of follow‐up differed significantly from those without WHF with respect to higher NT‐proBNP (P = 0.01) and proportion of pacemaker implantations (PMI) at baseline (P = 0.02). The indication of PMI was advanced atrioventricular block in 67% (n = 24) of the patients, sick sinus syndrome in 14% (n = 5), ventricular tachycardia in 11% (n = 4), and HF with left bundle branch block and reduced LVEF in 8% (n = 3). No difference was noted with respect to PMI indication at baseline between patients with or without WHF. The types of implanted pacemaker devices were as follows: dual chamber (69%, n = 25), single chamber with ventricular pacing and sensing (17%, n = 6), biventricular pacing (11%, n = 4), and single chamber with atrial pacing and sensing (4%, n = 1). According to the pacemaker reports at baseline, a total of 10 patients (28%) were paced where six patients with PMI (excluding biventricular devices) were V‐paced of 59% of the time on average, however with varying intensities (99%, 99%, 87%, 41%,26%, and 1% of the time, respectively).
Patients were treated with diuretics in 77% (n = 79) of cases. Angiotensin receptor inhibitors (ACE‐I)/aldosterone–renin blockers (ARB), beta blockers, and anti‐coagulation therapy were used in 45% (n = 47), 44% (n = 45), and 62% (n = 64) of cases, respectively. No patient received transthyretin stabilizing therapy (tafamidis), transthyretin synthesis inhibitors (patisiran and inotersen), doxycycline, or diflunisal at baseline or during follow‐up.
Myocardial systolic and diastolic function at baseline
The echocardiographic variables at the time of diagnosis are shown in Table 2 . LVEF was mildly and GLS moderately reduced, but without any significant difference between patients with or without WHF at 12 months of follow‐up. Left atrial and ventricular volumes were comparable between groups. The apical to basal segment strain ratio (ABr) was significantly increased in patients with WHF compared with non‐WHF patients. LV myocardial work indices were comparable between the WHF and non‐WHF group. LV myocardial work index was also comparable between groups.
Table 2.
Echocardiographic parameters at the time of ATTRwt diagnosis
| Variable | All (n = 104) | No. of HF admission (n = 68) | HF admission (n = 36) | P‐value |
|---|---|---|---|---|
| LVEF (%), mean ± SD | 46 ± 11 | 46 ± 11 | 46 ± 11 | 0.99 |
| LV EDV (mL), median (IQR) | 93 (40) | 93 (38) | 93 (4) | 0.62 |
| LV ESV (mL), median (IQR) | 48 (24) | 50 (24) | 46 (24) | 0.47 |
| SVI (mL/m2), mean ± SD | 23 ± 8 | 23 ± 8 | 23 ± 9 | 0.99 |
| GLS (%), mean ± SD | −11.3 ± 3 | −11.3 ± 4 | −11.1 ± 3 | 0.73 |
| GLS apical (%), mean ± SD | −17.9 ± 6 | −17.4 ± 6 | −19.0 ± 5 | 0.17 |
| GLS mid (%), mean ± SD | −9.7 ± 4 | −9.7 ± 4 | −9.8 ± 3 | 0.86 |
| GLS base (%), mean ± SD | −4.8 ± 4 | −5.2 ± 4 | −4.2 ± 3 | 0.21 |
| Relative apical strain ratio, median (IQR) | 2.7 (1.5) | 2.6 (1.7) | 2.8 (1.4) | 0.35 |
| Apical basal strain ratio, median (IQR) | 3.6 (3.6) | 3.4 (3.3) | 4.4 (3.8) | 0.04 |
| Global work index (mmHg%), mean ± SD | 1089 ± 380 | 1113 ± 387 | 1055 ± 371 | 0.46 |
| Global work index base (mmHg%), mean ± SD | 598 ± 428 | 665 ± 451 | 501 ± 330 | 0.07 |
| IVS (mm), mean ± SD | 17 ± 3 | 17 ± 4 | 17 ± 3 | 0.99 |
| PW (mm), mean ± SD | 14 ± 3 | 14 ± 3 | 14 ± 3 | 0.49 |
| LVMi (g/m2), mean ± SD | 159 ± 41 | 160 ± 45 | 155 ± 35 | 0.62 |
| LAVi (mL/m2), median (IQR) | 33 (21) | 33 (19) | 33 (20) | 0.97 |
| E/e′, mean, median (IQR) | 12 (8) | 11 (7) | 12.5 (11) | 0.14 |
| RV free wall (mm), mean ± SD | 8 ± 2 | 7 ± 3 | 8 ± 2 | 0.30 |
| RAVi (mL/m2), median (IQR) | 37 (22) | 33 (16) | 44 (30) | 0.03 |
| TAPSE (mm), mean ± SD | 17 ± 5 | 18 ± 6 | 15 ± 5 | 0.04 |
| TRG (mmHg), mean ± SD | 30 ± 10 | 28 ± 8 | 33 ± 13 | 0.02 |
GLS, global longitudinal strain; GWI, global work index; IVC, inferior vena cava; IVS, interventricular septum; LAVi, left atrial volume index; LV EDV, left ventricular end‐diastolic volume; LV ESV, left ventricular end‐systolic volume; LVMi, left ventricular mass index; PW, posterior wall; RV, right ventricle; RAVi, right atrial volume index; SVI, stroke volume index; TAPSE, tricuspid annular plane systolic excursion; TRG, tricuspid regurgitant gradient.
Right atrial enlargement was significantly more pronounced in patients with WHF. Right ventricular (RV) wall thickness was comparable between the two groups. RV systolic function seemed impaired as the tricuspid annular plane systolic excursion (TAPSE) was reduced among the WHF group relative to the non‐WHF group (P = 0.04). The systolic pulmonary pressure evaluated by tricuspid regurgitant gradient was elevated significantly in the WHF patients compared with non‐WHF (P = 0.02).
Hospital admissions due to WHF and mortality
Median follow‐up was 23 (IQR 16) months. Ninety‐one patients had a minimum of 12 months of follow‐up, and 49 patients had a minimum of 24 months of follow‐up. During follow‐up, 51% of the ATTRwt patients experienced a hospitalization due to WHF, and 70% of the WHF group experienced at least two HF admissions. The number of HF admissions prior to and after baseline is shown in Figure 1 .
Figure 1.
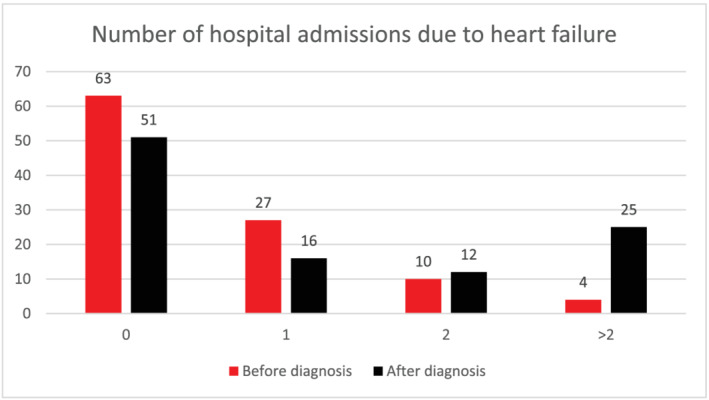
Hospital admissions due to heart failure before and after baseline. Hospital admissions due to heart failure before and after diagnosed wild‐type ATTR. Post‐diagnostic hospitalization is often re‐occurring as evidenced by the fact that 70% of patients hospitalized post‐diagnosis are hospitalized at least twice.
At 12 and 24 months of follow‐up, 35 and 50% of the ATTRwt patients had been admitted to a hospital with WHF. Hospitalization rate adjusted by Nelson–Aalen estimates showed no significant effect on the results. The cumulative incidence of hospital admissions with WHF is presented in Figure 2 .
Figure 2.
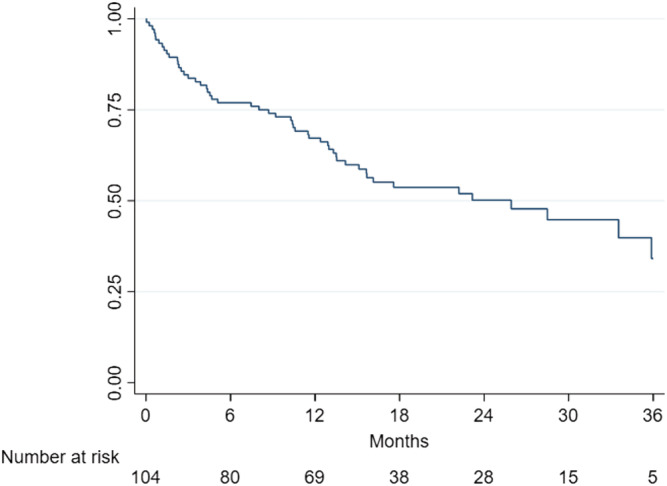
Incidence of hospital admissions with worsening heart failure. Incidence of hospital admissions due to worsening heart failure.
During follow‐up, 25 (24%) deaths occurred with a mortality rate of 12, 20, and 34% at 1, 2, and 3 years, respectively. Figure 3 A demonstrates the survival rate according to presence or absence of hospital admission due to WHF at 12 months of follow‐up. Survival at 12 months of follow‐up was significantly reduced in the WHF group (log‐rank, P < 0.001). Figure 3 B demonstrates the survival rate landmarked at 12 months showing a significantly reduced survival among patients with WHF at 12 months of follow‐up (log rank, P = 0.03). Causes of death were HF in 12 patients, one sudden death, and 10 unknown causes. Admission to a hospital with WHF had occurred in 19 patients before death was registered.
Figure 3.
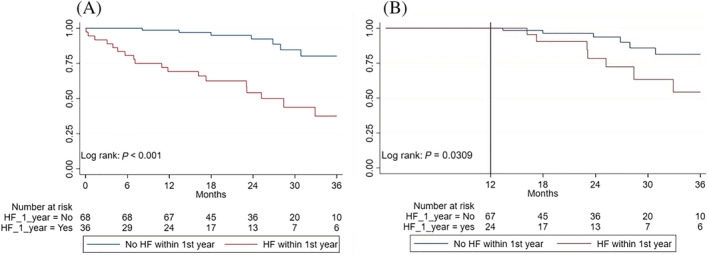
Survival stratified by WHF hospitalization within 12 months of diagnosis. Survival stratified by WHF hospitalization within 12 months of wild‐type ATTR diagnosis. (A) The overall study population. (B) Landmarked at 12 months. HF, heart failure.
According to the NAC staging system, 50% of the patients were in Stage I, 33% in Stage II, and 17% in Stage III at baseline. The survival status between the NAC stages (I vs. II–III) differed significantly (log‐rank, P = 0.012). The survival rates at 12 and 24 months were 95 and 90% for Stage I compared with 78 and 71% for NAC Stages II–III. No survival analysis of NAC Stage II vs. III was performed due to a low number of deaths 8 in each group. The freedom of hospitalization with WHF between the NAC stages (I vs. II–III) did not differ significantly (log‐rank, P = 0.141). The freedom of hospitalization with WHF at 12 and 24 months were 76 and 55% for NAC Stage I compared with 58 and 45% for NAC Stages II–III.
According to the Mayo Clinic staging system, 19% of the patients were in Stage I, 42% in Stage II, and 39% in Stage III at baseline. The survival status between the Mayo stages (I vs. II–III) differed significantly (log‐rank, P = 0.009). The survival rates at 12 and 24 months were 100 and 88% for Stage I compared with 85% and 74% for NAC Stages II–III. The freedom of hospitalization with WHF between the Mayo stages (I vs. II–III) did not differ significantly (log‐rank, P = 0.79). The freedom of hospitalization with WHF at 12 and 24 months were 75 and 47% for Mayo Stage I compared with 62 and 52% for Mayo Stages II–III.
Prognostic value of clinical characteristics, biomarkers, and myocardial function in relation to hospital admission with WHF
Univariable and multivariable analysis identified PMI and RAVi as independent predictors of hospital admission with WHF during follow‐up, which is presented in Table 3 . Univariable analysis of the clinical and echocardiographic parameters identified RAVi, tricuspid regurgitant gradient, GLS, ABr ≥ 3.6, and baseline PMI as predictors of hospitalization due to WHF during follow‐up. In the multivariable analysis, PMI at baseline and RAVi were identified as independent predictors of WHF.
Table 3.
Univariable and multivariable analysis of predictors of hospitalization with worsening heart failure
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| Clinical | ||||||
| Age, years | 0.98 | [0.94–1.03] | 0.449 | |||
| NYHA | 1.21 | [0.85–1.73] | 0.295 | |||
| Pacemaker implantation | 2.28 | [1.32–3.92] | 0.003 | 1.88 | 1.04–3.40 | 0.037 |
| Atrial fibrillation | 1.23 | [0.71–2.13] | 0.460 | |||
| Aortic stenosis | 1.20 | [0.64–2.26] | 0.572 | |||
| NAC stage (I vs. II + III) | 1.51 | 0.87–2.62 | 0.143 | |||
| eGFR | 0.99 | 0.98–1.01 | 0.214 | |||
| NT‐proBNP | 1.00 | 1.00–1.00 | 0.089 | |||
| Echocardiographic | ||||||
| LVEF | 0.98 | 0.96–1.01 | 0.175 | |||
| GLS < 11.3 | 1.24 | 0.72–2.14 | 0.444 | |||
| RAVi | 1.01 | 1.00–1.02 | 0.027 | 1.01 | 1.00–1.02 | 0.008 |
| TAPSE > 18 mm | 1.56 | 0.88–2.76 | 0.127 | |||
| TRG > 35 mmHg | 1.97 | 1.10–3.52 | 0.022 | 1.69 | 0.90–3.18 | 0.103 |
| GWI base | 1.00 | 1.00–1.00 | 0.058 | |||
| Apical basal ratio ≥ 3.6 | 1.89 | 1.07–3.16 | 0.027 | 1.33 | 0.71–2.50 | 0.379 |
| Concomitant medication | ||||||
| ACE inhibitors | 1.16 | 0.61–2.21 | 0.660 | |||
| Angiotensin II antagonists | 0.4 | 0.12–1.32 | 0.133 | |||
| Beta blockers | 1.53 | 0.82–2.84 | 0.183 | |||
ABr, apical to basal ratio; ACE, angiotensin‐converting enzyme; IVC, inferior vena cava; GLS, global longitudinal strain; GWI, global work index; LAVi, left atrial volume index; LVMi, left ventricular mass index; RAVi, right atrial volume index; RVWT, right ventricular wall thickness; TRG, tricuspid return gradient.
The prognostic value of medical treatment with ACE‐I [HR 1.16 (0.61;2.21), P = 0.66], ARB [HR 0.40 (0.12;1.32), P = 0.133], and beta blockers [HR 1.53 (0.82;2.84), P = 0.183] in relation to WHF was non‐significant. No significant difference in follow‐up time was noted among patients with hospitalization due to WHF compared with non‐WHF [25 (IQR 22) vs. 20 (IQR 11) months, P = 0.09].
Clinical, biochemical, and echocardiographic characteristics of patients with or without pacemaker device implantation at baseline
As PMI at baseline was identified as an independent predictor of WHF, patients were stratified by this variable to investigate whether PMI patients had more advanced disease compared with patients without. Table 4 demonstrates the baseline clinical, biochemical, and selected echocardiographic variables in patients with or without PMI. Patients with PMI at baseline had significantly higher NT‐proBNP, lower eGFR, higher LV mass index, reduced LV and RV systolic function, increased RAVi, and higher tricuspid regurgitant gradients compared with patients without PMI. Patients with PMI at baseline were at significantly higher risk of hospital admission due to WHF compared with non‐PMI patients (log‐rank, P < 0.0023) as shown in Figure 4 . A trend towards higher mortality among PMI patients was noted compared with patients without (log‐rank, P = 0.071).
Table 4.
Clinical and echocardiographic characteristics of patients with or without PMI at the time of ATTRwt diagnosis
| Variable | No. of PM (n = 68) | PM (n = 36) | P‐value |
|---|---|---|---|
| Age (years), mean ± SD | 81.6 ± 7 | 80.4 ± 6 | 0.39 |
| NYHA I/II/III/IV (%) | 28/41/29/1 | 22/58/19/0 | 0.36 |
| Atrial fibrillation, n (%) | 31 (46) | 12 (33) | 0.23 |
| eGFR (mL/min), mean ± SD | 64.4 ± 18 | 56.3 ± 20 | 0.04 |
| NT‐proBNP (ng/L), median (IQR) | 2225 (3494) | 2839 (3506) | 0.04 |
| LVEF (%), mean ± SD | 48 ± 11 | 38 ± 12 | <0.01 |
| LV‐GLS (%), mean ± SD | −11.4 ± 4 | −8.9 ± 3 | <0.01 |
| LV‐GWI (mmHg%), mean ± SD | 1,165 ± 437 | 825 ± 343 | <0.01 |
| LVMI (g/m2), mean ± SD | 151 ± 34 | 183 ± 44 | <0.01 |
| LAVi (mL/m2), median (IQR) | 30 (16) | 29 (16) | 0.92 |
| RAVi (mL/m2), median (IQR) | 35 (22) | 43 (17) | 0.04 |
| RV free wall thickness (mm), mean ± SD | 5.8 (3.5) | 7.0 (3.5) | 0.11 |
| TAPSE (mm), mean ± SD | 19 ± 5 | 15 ± 6 | 0.01 |
| TRG (mmHg), mean ± SD | 28 ± 8 | 30 ± 12 | 0.38 |
LAVi, left atrial volume index; LVEF, left ventricular ejection fraction; LV‐GLS, left ventricular global longitudinal strain; LV‐GWI, left ventricular global work index; LVMI, left ventricular mass index; PM, pacemaker; RAVi, right atrial volume index; TAPSE, tricuspid annular plane systolic excursion; TRG, tricuspid return gradient.
Figure 4.
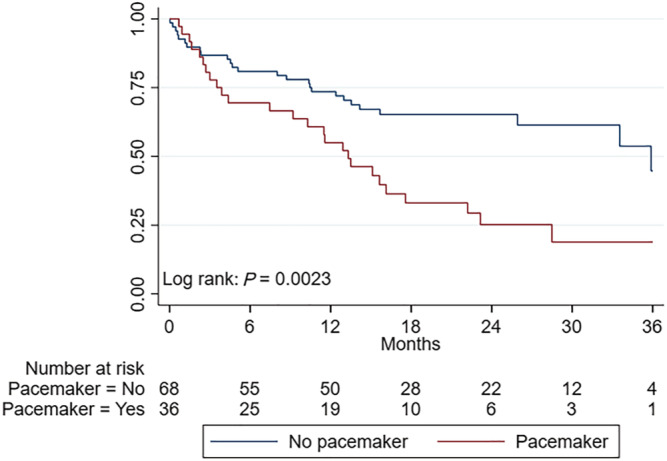
Hospitalization due to heart failure stratified by pacemaker status. Hospitalization due to heart failure stratified by pacemaker status.
Discussion
The main findings were as follows: Firstly, 35 and 50% of ATTRwt patients were admitted to a hospital with WHF at 12 and 24 months after diagnosis. Of these, 70% were admitted at least twice. Secondly, patients with WHF were more likely to have a PMI at baseline and had higher NT‐proBNP, higher RAVi, higher GLS, higher ABr, and higher tricuspid regurgitant gradients. Thirdly, PMI at baseline and RAVi were shown to be independent predictors of hospitalization due to WHF during follow‐up. Finally, patients with PMI at baseline had more advanced biventricular systolic dysfunction compared with patients without PMI.
The morbidity of ATTRwt seems considerable as reported by Lane et al. who demonstrated a high frequency of in‐hospital episodes and surgical procedures before and after ATTR diagnosis. 1 The median number of hospital admissions within 12 months of diagnosis was two, and 30% were admitted at least three times. Data from the ATTR‐ACT trial show that 34.6% of the patients with ATTR were hospitalized within the first year of randomization and with an overall cardiovascular‐related hospitalization incidence of 0.70 per year. 12 In the present study of contemporary ATTRwt patients, we report a 35% incidence of hospitalization due to WHF within 12 months of diagnosis. Patients admitted to a hospital with WHF during the first year were also demonstrated to have significantly reduced survival even after the survival rate was landmarked at 12 months. Despite that 50% of the patients were classified as NAC Stage I at the time of the diagnosis, a WHF‐related hospitalization incidence of 24 and 45% was registered at 12 and 24 months, and a high number of WHF readmissions was noted. Overall, the HF hospitalization burden seems significant in most ATTRwt patients and carries both a poor prognosis and a significant economic burden to the healthcare system. It therefore seems important to identify patients with the highest risk of WHF as early as possible to initiate transthyretin‐specific pharmacological treatment.
In the present study, the clinical and biochemical characteristics of patients with and without WHF differed on a few variables as patients with WHF had higher NT‐proBNP, higher RAVi, higher GLS, higher ABr, higher tricuspid regurgitant gradients, and previous PMI. Among clinical and echocardiographic parameters, RAVi and PMI at baseline were identified as independent predictors of hospital admission due to WHF.
Reduced TAPSE along with higher RAVi was noted among patients with WHF, indicating impairment of both RV systolic and diastolic function. The enlargement of the right atrium could be a consequence of LV backward failure as indicated by the higher tricuspid regurgitant gradient in the WHF group. The independent prognostic value of RAVi over LAVi could also be explained by the fact that RAVi reflects a more advanced disease stage with biventricular dysfunction. A normal atrial booster function is important to ensure prober ventricular filling, in particular in conditions with restrictive pathophysiology as in ATTRwt, but the observed severe right atrial dilatation might indicate the first signs of atrial dysfunction and decongestion. 13 Atrial fibrillation was also common in the present population with WHF, and the presence of right ventricular pacemaker leads might in some cases induce functional tricuspid valve regurgitation leading to atrial dilatation.
The reported prevalence of PMI at the time of ATTRwt diagnosis varies from 10 to 40% depending on patient characteristics and institution. 2 , 14 In our population, 35% had PMI prior to the diagnosis of ATTRwt. Advanced atrioventricular block was noted as the main indication in accordance with previous studies. 2 , 3 , 15 The prevalence of PMI was higher among patients with WHF, and PMI independently predicted admission due to WHF. Chronic right ventricular pacing can lead to impairment of LV systolic function and subsequent development of HF. 16 Based on the present pacemaker reports, only a limited number of patients experienced ventricular pacing, suggesting a limited effect on the study population. It is more likely that the ATTRwt patients with PMI at the time of diagnosis had more advanced stages of amyloid cardiomyopathy, which is supported by the higher LV mass index, higher NT‐proBNP, and poorer LV and RV systolic function demonstrated in this group. In contrast to isolated LV pathologies such as HF with preserved LVEF where left atrial volume has been related to outcomes, our data demonstrated that right atrial volume in excess to the LV failure is particular enlarged in patients with WHF and shown to be a significant prognostic WHF factor. Even among patients with PMI, right atrial enlargement was noted, which might suggest that the presence of PMI with right‐sided atrial dilatation is likely to reflect the natural course of amyloidosis. Patients with advanced HF symptoms and ATTRwt often have increased right ventricular wall thickening, indicating a higher degree of biventricular involvement. Whether the PMI with an inherent risk of inducing a tricuspid valve insufficiency can contribute to worsening of the right‐sided failure is impossible to conclude from our data, and with a prober pacemaker indication, the implantation is unavoidable.
Prospective screening for cardiac amyloidosis in elderly patients with conduction disorders or systolic HF has revealed a low (2%) prevalence of ATTRwt. 17 The low prevalence reported might be related to the high proportion of females that was included as males usually account for about 90% of patients with diagnosed ATTRwt. 1 , 3 , 7 Future screening studies of elderly males with advanced atrioventricular conduction disorders and signs of LV hypertrophy are warranted to identify ATTRwt patients with less advanced disease. 18 , 19
Limitations
Our study is limited by its single‐centre nature because only patients diagnosed or referred for a diagnostic evaluation at our department were included. All patients were treatment naïve with respect to transthyretin stabilizers or synthesis inhibitors as these agents are not approved by the Danish authorities. This should be considered when comparing our observations with other populations treated routinely with these agents. The follow‐up time can be considered only intermediate with a median of 23 months, but it is comparable to other prospective ATTR studies. In addition, a substantial number of HF events occurred during follow‐up. Amyloid cardiomyopathy was considered the primary cause of WHF, but other ATTRwt‐associated diseases may contribute to the development of HF such as severe aortic stenosis and ischaemic heart disease. In the present study, only two patients with WHF had severe aortic stenosis, and it is difficult to determine the primary cause of HF in such cases. No patients were documented to have ongoing myocardial ischemia and all patients were previously revascularized.
Conclusions
A high incidence and recurrence of hospital admissions with WHF was demonstrated in contemporary patients with ATTRwt and was associated with reduced survival. Patients with PMI at the time of diagnosis experienced more frequent hospitalizations with WHF, and PMI and right atrial enlargement were identified as independent predictors of WHF during follow‐up.
Conflict of interest
The authors declare no competing financial interests.
Funding
There was no funding to report for this study.
Acknowledgements
The authors would like to acknowledge the patients and the staff at the Cardiac Amyloidosis Clinic, Aarhus University Hospital, involved in patient diagnosis and care.
Ladefoged, B. T. , Dybro, A. , Dahl Pedersen, A. L. , Rasmussen, T. B. , Vase, H. Ø. , Clemmensen, T. S. , Gillmore, J. , and Poulsen, S. H. (2022) Incidence and predictors of worsening heart failure in patients with wild‐type transthyretin cardiac amyloidosis. ESC Heart Failure, 9: 2978–2987. 10.1002/ehf2.14000.
References
- 1. Lane T, Fontana M, Martinez‐Naharro A, Quarta CC, Whelan CJ, Petrie A, Rowczenio DM, Gilbertson JA, Hutt DF, Rezk T, Strehina SG, Caringal‐Galima J, Manwani R, Sharpley FA, Wechalekar AD, Lachmann HJ, Mahmood S, Sachchithanantham S, Drage EPS, Jenner HD, McDonald R, Bertolli O, Calleja A, Hawkins PN, Gillmore JD. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation 2019; 140: 16–26. [DOI] [PubMed] [Google Scholar]
- 2. González‐López E, Gagliardi C, Dominguez F, Quarta CC, de Haro‐del Moral FJ, Milandri A, Salas C, Cinelli M, Cobo‐Marcos M, Lorenzini M, Lara‐Pezzi E, Foffi S, Alonso‐Pulpon L, Rapezzi C, Garcia‐Pavia P. Clinical characteristics of wild‐type transthyretin cardiac amyloidosis: Disproving myths. Eur Heart J 2017; 38: 1895–1904. [DOI] [PubMed] [Google Scholar]
- 3. Ladefoged B, Dybro A, Povlsen JA, Vase H, Clemmensen TS, Poulsen SH. Diagnostic delay in wild type transthyretin cardiac amyloidosis ‐ a clinical challenge. Int J Cardiol 2020; 304: 138–143. [DOI] [PubMed] [Google Scholar]
- 4. Chacko L, Martone R, Bandera F, Lane T, Martinez‐Naharro A, Boldrini M, Rezk T, Whelan C, Quarta C, Rowczenio D, Gilbertson JA, Wongwarawipat T, Lachmann H, Wechalekar A, Sachchithanantham S, Mahmood S, Marcucci R, Knight D, Hutt D, Moon J, Petrie A, Cappelli F, Guazzi M, Hawkins PN, Gillmore JD, Fontana M. Echocardiographic phenotype and prognosis in transthyretin cardiac amyloidosis. Eur Heart J 2020; 41: 1439–1447. [DOI] [PubMed] [Google Scholar]
- 5. Pan JA, Kerwin MJ, Salerno M. Native T1 mapping, extracellular volume mapping, and late gadolinium enhancement in cardiac amyloidosis: A meta‐analysis. JACC Cardiovasc Imaging 2020; 13: 1299–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, Klarich KW, Miller WL, Maleszewski JJ, Dispenzieri A. Natural history of wild‐type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol 2016; 68: 1014–1020. [DOI] [PubMed] [Google Scholar]
- 7. Gillmore JD, Damy T, Fontana M, Hutchinson M, Lachmann HJ, Martinez‐Naharro A, Quarta CC, Rezk T, Whelan CJ, Gonzalez‐Lopez E, Lane T, Gilbertson JA, Rowczenio D, Petrie A, Hawkins PN. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J 2018; 39: 2799–2806. [DOI] [PubMed] [Google Scholar]
- 8. Clemmensen TS, Eiskjær H, Ladefoged B, Mikkelsen F, Sørensen J, Granstam SO, Rosengren S, Flachskampf FA, Poulsen SH. Prognostic implications of left ventricular myocardial work indices in cardiac amyloidosis. Eur Heart J Cardiovasc Imaging 2020; 22: 695–704. [DOI] [PubMed] [Google Scholar]
- 9. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39.e14. [DOI] [PubMed] [Google Scholar]
- 10. Negishi K, Negishi T, Kurosawa K, Hristova K, Popescu BA, Vinereanu D, Yuda S, Marwick TH. Practical guidance in echocardiographic assessment of global longitudinal strain. JACC Cardiovasc Imaging 2015; 8: 489–492. [DOI] [PubMed] [Google Scholar]
- 11. Russell K, Eriksen M, Aaberge L, Wilhelmsen N, Skulstad H, Remme EW, Haugaa KH, Opdahl A, Fjeld JG, Gjesdal O, Edvardsen T, Smiseth OA. A novel clinical method for quantification of regional left ventricular pressure‐strain loop area: A non‐invasive index of myocardial work. Eur Heart J 2012; 33: 724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington‐Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB, Rapezzi C. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018; 379: 1007–1016. [DOI] [PubMed] [Google Scholar]
- 13. Gillebert TC. Left atrial reservoir and booster function in HFrEF: Implications for diastolic function. JACC Cardiovasc Imaging 2020; 13: 1116–1118. [DOI] [PubMed] [Google Scholar]
- 14. Donnellan E, Wazni OM, Saliba WI, Hanna M, Kanj M, Patel DR, Wilner B, Kochar A, Jaber WA. Prevalence, incidence, and impact on mortality of conduction system disease in transthyretin cardiac amyloidosis. Am J Cardiol 2020; 128: 140–146. [DOI] [PubMed] [Google Scholar]
- 15. Rapezzi C, Lorenzini M, Longhi S, Milandri A, Gagliardi C, Bartolomei I, Salvi F, Maurer MS. Cardiac amyloidosis: The great pretender. Heart Fail Rev 2015; 20: 117–124. [DOI] [PubMed] [Google Scholar]
- 16. Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, Lamas GA, MOde Selection Trial Investigators . Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003; 107: 2932–2937. [DOI] [PubMed] [Google Scholar]
- 17. López‐Sainz Á, de Haro‐del Moral FJ, Dominguez F, Restrepo‐Cordoba A, Amor‐Salamanca A, Hernandez‐Hernandez A, Ruiz‐Guerrero L, Krsnik I, Cobo‐Marcos M, Castro V, Toquero‐Ramos J, Lara‐Pezzi E, Fernandez‐Lozano I, Alonso‐Pulpon L, González‐López E, Garcia‐Pavia P. Prevalence of cardiac amyloidosis among elderly patients with systolic heart failure or conduction disorders. Amyloid 2019; 26: 156–163. [DOI] [PubMed] [Google Scholar]
- 18. Garcia‐Pavia P, Rapezzi C, Adler Y, Arad M, Basso C, Brucato A, Burazor I, Caforio ALP, Damy T, Eriksson U, Fontana M, Gillmore JD, Gonzalez‐Lopez E, Grogan M, Heymans S, Imazio M, Kindermann I, Kristen AV, Maurer MS, Merlini G, Pantazis A, Pankuweit S, Rigopoulos AG, Linhart A. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J 2021; 42: 1554–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, Wechalekar AD, Berk JL, Quarta CC, Grogan M, Lachmann HJ, Bokhari S, Castano A, Dorbala S, Johnson GB, Glaudemans AWJM, Rezk T, Fontana M, Palladini G, Milani P, Guidalotti PL, Flatman K, Lane T, Vonberg FW, Whelan CJ, Moon JC, Ruberg FL, Miller EJ, Hutt DF, Hazenberg BP, Rapezzi C, Hawkins PN. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016; 133: 2404–2412. [DOI] [PubMed] [Google Scholar]


