Abstract
Aims
Data regarding prognostic events following catheter ablation (CA) for atrial fibrillation (AF) in patients with heart failure with preserved ejection fraction (HFpEF) are scarce. We conducted this study to compare the incidence of major adverse clinical events (MACE) following CA for AF between patients with HFpEF and those with systolic heart failure (HF).
Methods and results
This single‐centre observational study included 142 patients with HF who underwent CA for AF (median follow‐up: 4.0 [2.6, 6.3] years). The patients were grouped based on the presence of HFpEF (n = 84) and systolic HF (left ventricular ejection fraction <50%, n = 58). We compared the cumulative incidence and incidence rate of MACE, comprising all‐cause death, unplanned cardiovascular hospitalization (CVH), and HF hospitalization (HFH) between both groups and the number of HFH before and after CA in each group. Multivariate analysis was performed to identify the predictors of MACE in patients with HFpEF. The incidence of MACE was comparable between the groups (following the first procedure: HFpEF: 23%, 4.7/100 person‐years, vs. systolic HF: 28%, 6.6/100 person‐years, P = 0.18; last procedure: 20%, 4.8/100 person‐years, vs. 24%, 6.9/100 person‐years, P = 0.21). Although the incidence of HFH was lower in patients with HFpEF than in those with systolic HF (first procedure: 14%, 2.9/100 person‐years, vs. 24%, 5.7/100 person‐years, P = 0.07; last procedure: 11%, 2.5/100 person‐years, vs. 24%, 6.9/100 person‐years, P = 0.01), the incidence of CVH was higher (first procedure: 8%, 1.7/100 person‐years, vs. 5%, 1.2/100 person‐years, P = 0.74; last procedure: 6%, 1.4/100 person‐years, vs. 2%, 0.5/100 person‐years, P = 0.4). The number of HFH significantly decreased in both groups after CA (HFpEF: 1 hospitalization [the first and third quartiles: 0, 1] in pre‐CA, vs. 0 hospitalizations [0, 0] in post‐CA, P < 0.0001; systolic HF: 1 hospitalization [0, 1], vs. 0 hospitalizations [0, 0], P < 0.005). The proportion of HFH among total clinical events was significantly smaller in patients with HFpEF than in those with systolic HF (following the first procedure: 56% vs. 88%, P < 0.005; last procedure: 52% vs. 92%, P < 0.005).
Conclusions
CA for AF could be beneficial for patients with HFpEF, similar to those with systolic HF. However, clinical events other than HFH should be considered cautiously in such patients.
Keywords: Heart failure with preserved ejection fraction, Catheter ablation, Atrial fibrillation, Major adverse clinical events
Introduction
With the aging population, heart failure (HF) is becoming a common cause of death in developed countries. 1 , 2 Recently, HF with preserved ejection fraction (HFpEF) emerged as the major subtype of HF, rather than HF with reduced ejection fraction (HFrEF) or mildly reduced ejection fraction (HFmrEF). 3 , 4 , 5 Atrial fibrillation (AF), the most common atrial arrhythmia worldwide, is closely related to both HFpEF and HFrEF. 6 Hence, HFpEF and AF share common risk factors such as obesity, aging, sedentary lifestyle, and hypertension, the prevalence of AF is higher in patients with HFpEF than in those with HFrEF. 7 It is crucial for patients with HFpEF to manage AF because the negative prognostic impact of the development of AF is higher in patients with HFpEF than in those with HFrEF. 8 , 9 Recently, catheter ablation (CA) for AF has been recognized as a beneficial option for patients with HFrEF owing to the positive results of a randomized controlled trial. 6 , 10 Regarding HFpEF, limited information is available on whether CA for AF could provide clinical benefit for HF treatment in patients with HFpEF, as it does for patients with HFrEF. 11 , 12 , 13 Apart from HFrEF, patients with HFpEF tended to develop various prognostic events other than HF such as cardiovascular events and non‐cardiovascular death, which reflect various co‐morbidities of such patients. 14 , 15 One might infer that the aetiologic difference between HFpEF and HFrEF would impact the long‐term prognosis following CA. To clarify this issue, we first compared the cumulative major adverse clinical events (MACE) following CA for AF between patients with HFpEF and those with systolic HF (HFrEF and HFmrEF). Second, we aimed to evaluate the clinical impact of CA for AF on HF treatment in both groups by comparing the number of HF hospitalizations (HFH) before and after CA. Third, we aimed to identify predictors of MACE development in patients with HFpEF.
Methods
Study design
The datasets analysed in this study are available from the corresponding author upon reasonable request. The present study was categorized as a single‐centre, retrospective observational study. The review board of Yamaguchi University Hospital approved this study. The requirement for informed consent was waived via the opt‐out system. The tenets of the Declaration of Helsinki and the ethical standards of the responsible committee on human experimentation were followed.
Patients with HF were selected from consecutive patients who underwent catheter ablation for AF between January 2009 and December 2020. Patients who could not be achieved for the planned procedure were excluded. The included patients were dichotomized into two groups: (i) the HFpEF group, including patients with a history of symptomatic HF with left ventricular ejection fraction (LVEF) ≥ 50%, and (ii) the systolic HF group (HFrEF/mrEF; patients with HFrEF [LVEF<40%] or HFmrEF [history of symptomatic HF with LVEF of 40–49%]). We compared the cumulative incidence of MACE after CA between groups. In each group, we compared the number of HFH before and after the first CA. In the HFpEF group, we evaluated predictors of MACE following the first procedure using univariate and multivariate analyses.
Study endpoints
The primary endpoint of this study was a comparison of the incidence of MACE following the CA procedure between the HFpEF and systolic HF groups. Secondary endpoints were (i) to compare the incidence of each clinical event that comprised MACE between the groups, (ii) to compare the total number of HFH pre‐initial and post‐initial CA in each group, and (iii) to identify the predictors for developing MACE following the initial CA in the HFpEF group.
Definition of clinical events
MACE was defined as a composite of all‐cause death (ACD), cardiovascular hospitalization (CVH), and HFH. CVH was defined as unplanned hospitalization for spontaneous cardiovascular disease without the findings of decompensated HF. Cardiovascular disease induced by an iatrogenic cause such as procedure‐related stroke was not counted as an event. HFH was defined as hospitalization that required unplanned administration of intravenous agents such as diuretics and inotropes or the use of mechanical devices such as a ventilator for decompensated HF. Events that occurred during the blanking period (within 3 months following the latest procedure) were excluded.
Diagnosis of heart failure
HF was diagnosed according to the current guidelines of the Japanese Circulation Society/Japanese Heart Failure Society. 16 Patients who had a history of HF‐plausible symptoms based on the Framingham criteria (e.g. orthopnoea and pulmonary congestion on chest X‐ray) with elevation of brain natriuretic peptide (BNP) or who had a history of HFH were diagnosed with HFpEF. The ejection fraction was classified according to the latest universal definition. 17 Briefly, LVEF <40% was defined as HFrEF. LVEF ≥40% to <50% was defined as HFmrEF. LVEF ≥50% was defined as HFpEF.
Assessment of heart failure hospitalization
We aimed to evaluate the effect of CA on the development of HFH; the number of HFHs between pre‐initial and post‐initial CA were compared in each group. The total number of pre‐CA HFHs was counted by reviewing previous medical records or inquiring of the primary physician of each patient. The total number of post‐CA HFHs was counted during the entire follow‐up period. In addition, to evaluate the difference in the weight of HFH between groups, the proportion of HFH among the total clinical events following the procedure (first/last) was also compared.
Catheter ablation procedure
Our strategy for CA has been previously described. 18 In brief, all patients underwent pulmonary vein isolation (PVI) using radiofrequency energy (Navistar Thermocool™; Biosense Webster, Diamond Bar, CA, USA) or second‐generation cryoballoon energy (Arctic Front Advance, Medtronic, Inc., Minneapolis, MN, USA) using a three‐dimensional electroanatomical mapping system (CARTO, Biosense Webster). Patients with paroxysmal AF underwent radiofrequency or cryoballoon PVI. Patients with persistent AF since October 2015 underwent empiric superior vena cava isolation in addition to PVI. Substrate modification was not performed. Patients who were clinically confirmed to have common/uncommon atrial flutter or atrial tachycardia also underwent ablation targeting the arrhythmia.
Patient follow‐up
Our post‐procedural protocols have been previously described. 18 Ambulatory ECG and/or 24 h Holter ECG recordings were obtained at 1, 3, 6, and 12 months after the procedure. Atrial tachyarrhythmia (ATA) recurrence was defined as the detection of >30 s of ATA after the blanking period. A redo procedure was recommended for all patients who developed ATA recurrence. Data regarding clinical events were collected by contacting the primary care physician of each patient in October 2021.
Statistical analysis
Normally distributed variables are expressed as mean ± standard deviation, whereas non‐normally distributed variables are expressed as medians and interquartile ranges (first and third quartiles). Differences in continuous variables between groups were evaluated using the Mann–Whitney U‐test. Categorical variables are presented as frequencies and proportions (%) and were compared using the χ 2 test. The success rate following the procedure (the rate of freedom from ATA recurrence) was also compared using the χ 2 test. The number of HFHs between pre‐CA and post‐CA was compared using the one‐paired Wilcoxon test. The proportion of HFH among the total clinical events between patients with HFpEF and systolic HF was compared using the χ 2 test. For the Kaplan–Meier analysis of the cumulative incidence following the first procedure, time 0 was set as the day of the first procedure. For the analysis of the incidence following the last procedure, time 0 was set as the day of the latest procedure. The incidence of each event was expressed in three ways: the crude incidence rate (event number/total number of each group*100), the incidence rate (event number/the total number of person‐years in each group*100), and the cumulative incidence with 95% confidence intervals (CIs) at the period of last follow‐up in each group. The log‐rank test was used to compare the differences in the cumulative incidence of MACE and each clinical event. Regarding the clinical events which had the tendency of different incidence between the groups (P < 0.1 for log‐rank test), we performed the multivariate adjustment of baseline characteristics using Cox proportional hazards model as the sensitivity analysis to precisely evaluate the differences.
Univariate and multivariate analyses were performed for patients with HFpEF using the Cox proportional hazards model. Variables previously reported as prognostic factors for HFpEF were selected. 14 , 15 Variables with P‐values <0.2 in the univariate analysis were selected for subsequent multivariate analysis. Continuous variables were binarized using conventional cut‐off values. The results are expressed as hazard ratios and 95% CIs. All analyses were performed using SPSS version 19 (IBM Corp., Armonk, NY), and results with a P‐value <0.05 were considered statistically significant.
Results
Study population
A flow diagram of the present study is shown in Figure 1 . Among the 869 patients who underwent CA for AF during the entire study period, 143 patients were diagnosed with HF. Of which, a patient with HFpEF was excluded because the CA procedure had to be terminated prior to the creation of the ablation lesions owing to cardiac tamponade. Hence, 142 patients were included in the present study. Of these, 84 and 58 patients were categorized as having HFpEF and systolic HF, respectively. At diagnosis, the median BNP was comparable between the groups (HFpEF vs. systolic HF: 276 [197, 453] pg/mL vs. 314 [157, 645], P = 0.62). The patient characteristics are shown in Table 1 . Patients with HFpEF were significantly older than those with systolic HF (68 ± 9 vs. 64 ± 11 years, P = 0.02). The proportion of patients with coronary artery disease and diabetes mellitus (DM) was significantly lower in the HFpEF group than in the systolic HF group. In addition, the proportion of patients who took anti‐arrhythmic drugs, including amiodarone, was also significantly lower in the HFpEF group than in the systolic HF group.
Figure 1.
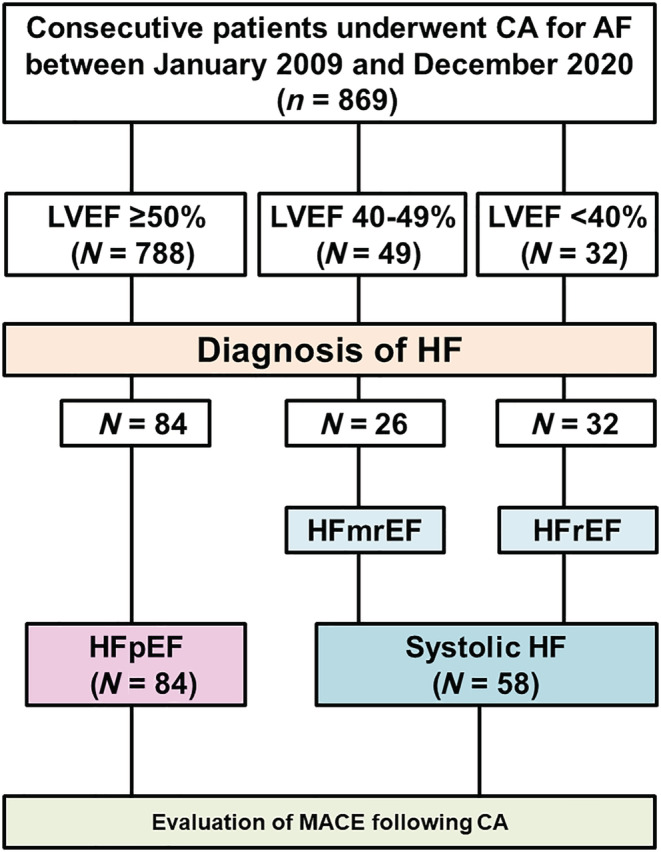
Flow diagram of the study. AF, atrial fibrillation; CA, catheter ablation; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; MACE, major adverse clinical events.
Table 1.
Patient characteristics
| Total (n = 142) | HFpEF (n = 84) | Systolic HF (n = 58) | P‐value | |
|---|---|---|---|---|
| Age (years), mean ± SD* | 67 ± 10 | 68 ± 9 | 64 ± 11 | 0.02 |
| Female sex, n (%) | 50 (35) | 34 (40) | 16 (28) | 0.1 |
| Persistent AF, n (%) | 89 (63) | 52 (62) | 37 (64) | 0.82 |
| History of AF, (months), median (IQR) | 9 (4, 35) | 9 (4, 38) | 7 (4, 23) | 0.22 |
| NYHA class, mean ± SD | 1.8 ± 0.7 | 1.8 ± 0.6 | 1.9 ± 0.7 | 0.15 |
| BMI (kg/m2), mean ± SD | 24 ± 4 | 25 ± 4 | 23 ± 4 | 0.1 |
| Pacemaker, n (%) | 10 (7) | 6 (7) | 4 (7) | 0.95 |
| ICD/CRT, n (%)* | 14 (10) | 0 | 14 (24) | <0.0001 |
| SBP (mmHg), mean ± SD* | 124 ± 21 | 129 ± 22 | 117 ± 18 | <0.001 |
| HR (/min), mean ± SD | 75 ± 19 | 73 ± 20 | 77 ± 18 | 0.11 |
| History of HFH, n (%) | 96 (68) | 58 (69) | 38 (66) | 0.66 |
| CTR, mean ± SD | 51 ± 5 | 52 ± 5 | 51 ± 5 | 0.4 |
| CHADS2, mean ± SD | 2 ± 1.2 | 2 ± 1.2 | 2 ± 1.2 | 0.56 |
| CHA2DS2‐VASc, mean ± SD | 3 ± 1.7 | 3 ± 1.5 | 2.9 ± 1.9 | 0.31 |
| Hypertension, n (%) | 55 (39) | 37 (44) | 18 (31) | 0.11 |
| DM, n (%)* | 27 (19) | 11 (13) | 16 (28) | 0.03 |
| Aetiology of SHD | ||||
| CAD, n (%)* | 14 (10) | 4 (5) | 10 (17) | 0.01 |
| HCM, n (%) | 7 (5) | 5 (6) | 2 (3) | 0.49 |
| VHD, n (%) | 6 (4) | 4 (5) | 2 (3) | 0.7 |
| Ablation‐related parameters | ||||
| Radiofrequency‐PVI, n (%) | 124 (87) | 74 (88) | 50 (86) | 0.74 |
| Cryo‐PVI, n (%) | 18 (13) | 10 (12) | 8 (14) | 0.74 |
| CTI‐ablation, n (%) | 37 (26) | 22 (26) | 15 (26) | 0.9 |
| Posterior wall isolation, n (%) | 0 | 0 | 0 | >0.99 |
| SVC isolation, n (%) | 65 (46) | 39 (46) | 26 (45) | 0.89 |
| Echocardiographic parameters | ||||
| LVDd (mm), mean ± SD* | 51 ± 7 | 48 ± 5 | 56 ± 7 | <0.0001 |
| LVEF (%), mean ± SD* | 51 ± 15 | 62 ± 9 | 37 ± 8 | <0.0001 |
| LAD (mm), mean ± SD | 44 ± 7 | 44 ± 6 | 44 ± 8 | 0.67 |
| LAVI (mL/m2), mean ± SD | 57 ± 18 | 55 ± 17 | 60 ± 19 | 0.11 |
| Mitral E/e′ ratio, mean ± SD | 11 ± 5 | 11 ± 4 | 12 ± 5 | 0.32 |
| Therapeutic agents | ||||
| ACEI/ARB, n (%) | 103 (73) | 56 (67) | 47 (81) | 0.06 |
| Beta‐blocker, n (%)* | 122 (86) | 67 (80) | 55 (95) | 0.01 |
| MRA, n (%) | 46 (32) | 23 (27) | 23 (40) | 0.12 |
| Diuretics, n (%) | 86 (61) | 51 (61) | 35 (60) | 0.96 |
| AADs, n (%) | 30 (21) | 11 (13) | 19 (33) | 0.004 |
| Amiodarone, n (%)* | 18 (13) | 1 (1) | 17 (29) | <0.0001 |
| Laboratory data | ||||
| eGFR (mL/min/1.73 m2), mean ± SD | 55 ± 20 | 57 ± 22 | 52 ± 18 | 0.11 |
| BNP level (pg/mL), median (IQR) | 168 (96, 335) | 158 (87, 238) | 184 (104, 399) | 0.11 |
AADs, anti‐arrhythmic drugs; ACEI, angiotensin converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; CAD, coronary artery disease; CRT, cardiac resynchronization therapy; CTI, cavotricuspid isthmus; CTR, cardiothoracic ratio; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HCM, hypertrophic cardiomyopathy; HF, heart failure; HFH, heart failure hospitalization; HFpEF, heart failure with preserved ejection fraction; HR, heart rate; ICD, implantable cardioverter‐defibrillator; IQR, interquartile range; LAD, left atrial diameter; LAVI, left atrial volume index; LVDd, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart association; PVI, pulmonary vein isolation; SBP, systolic blood pressure; SD, standard deviation; SHD, structural heart disease; SVC, superior vena cava; VHD, valvular heart disease.
Numerical data are expressed as mean ± SD or median (interquartile range [IQR]; first quartile, third quartile). Categorical data were expressed as percentages and numbers.
Statistical significance (P < 0.05).
Efficacy of ablation
All patients, included in the study, successfully underwent the ablation procedure. In the HFpEF group, one patient developed a complication. The patient developed a pericardial effusion, which resolved without pericardiocentesis. In the systolic HF group, there were no complications. During the median follow‐up period of 4.0 (2.6, 6.3) years (652 person‐years), 54% (45/84 patients) of the HFpEF group and 50% (29/58 patients) of systolic HF group developed ATA recurrence. In patients with ATA recurrence, patients in both groups (60% [27/45 patients] of the HFpEF group and 72% [21/29 patients] of the systolic HF group) underwent redo procedures (the median of 1 [1, 2] procedure in HFpEF group, and 1 [1, 2] procedure in systolic HF group). In the redo procedures, no patient developed complications in the HFpEF group. Two patients developed complications in the systolic HF group. Of those, one patient had cerebral infarction, which was treated conservatively. The other patient had groin arteriovenous fistula, which resolved naturally during the follow‐up period. Following the last procedure, 38% (32/84 patients) of the HFpEF group and 34% (20/58 patients) of the systolic HF group developed ATA recurrence. There was no difference in success rates at 12 months after the first procedure between the groups (68% in the HFpEF group vs. 61% in the systolic HF group, P = 0.46). The success rates of off‐antiarrhythmic drugs (AADs) also showed no difference (70% vs. 63%, P = 0.33). Following the last procedure, the success rate at 12 months was comparable (79% in the HFpEF group vs. 78% in the systolic HF group, P = 0.84). The rate of off‐AADs also followed a similar trend (80% vs. 81%, P = 0.92).
Oral anticoagulants following ablation
In the HFpEF group, six patients (7%) discontinued their oral anticoagulants following the ablation procedure. In the systolic HF group, one patient (1.7%) discontinued their oral anticoagulants following the procedure. The proportion of patients who stopped their anticoagulation therapy was comparable between the groups (P = 0.14).
Cumulative incidence of major adverse clinical events
During the entire follow‐up period, 32 clinical events in the HFpEF group and 33 events in the systolic HF group were documented. Regarding MACE, 19 patients in the HFpEF group and 16 patients in the systolic HF group experienced at least one clinical event (Supporting Information, Table S1 A : patients with HFpEF; Supporting Information, Table S1 B : systolic HF). The most common diagnosis of CVH was stroke (6 patients), followed by atrioventricular block (2 patients), sick sinus syndrome (2 patients), and aortic dissection (1 patient). Figure 2 and Table 2A show a comparison of the crude incidence rate, incidence rate, and cumulative incidence of MACE between the HFpEF and systolic HF groups. The incidence of MACE following the first procedure was comparable between groups (Figure 2 A , crude incidence rate: 23%, incidence rate: 4.7/100 person‐years vs. 28%, 6.6/100 person‐years, P = 0.18, Table 2A ). The incidence of MACE following the last procedure was also comparable between groups (Figure 2 B , crude incidence rate: 20%, incidence rate: 4.8/100 person‐years vs. 24%, 6.9/100 person‐years, P = 0.21, Table 2B ).
Figure 2.
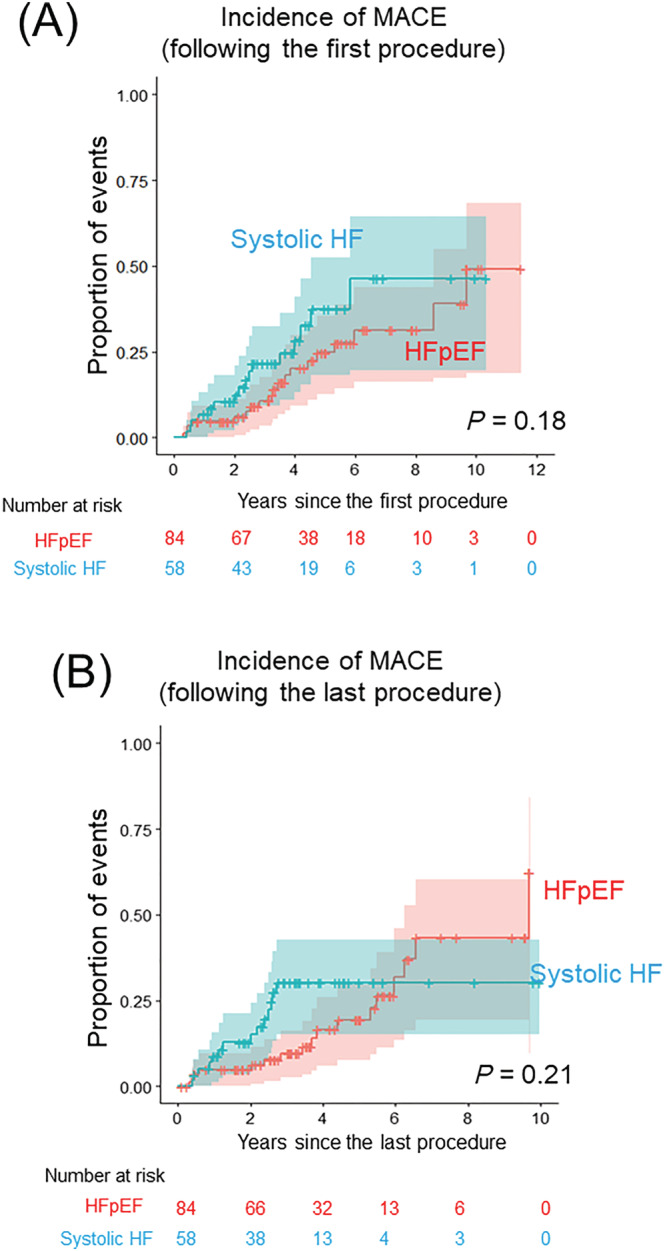
Cumulative incidence of MACE. (A) Comparison of the cumulative incidence and 95% CI of MACE following the first procedure between patients with HFpEF and systolic HF (red: patients with HFpEF, blue: systolic HF). (B) Comparison of the cumulative incidence and 95% CI of MACE following the last procedure between patients with HFpEF and systolic HF (red: patients with HFpEF, blue: systolic HF). AF, atrial fibrillation; CA, catheter ablation; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; MACE, major adverse clinical events.
Table 2A.
The incidence of each clinical event following the first procedure
| HFpEF | Systolic HF | P‐value | |||||
|---|---|---|---|---|---|---|---|
| Crude incidence rate, % | Incidence rate, events/100 person‐years | Cumulative incidence, % (95% CI) | Crude incidence rate, % | Incidence rate, number/100 person‐years | Cumulative incidence, % (95% CI) | ||
| MACE | 23 | 4.7 | 49 (19, 68) | 28 | 6.6 | 46 (20, 64) | 0.18 |
| ACD | 7 | 1.5 | 29 (0, 50) | 2 | 0.4 | 3 (0, 8) | 0.33 |
| CVH | 8 | 1.7 | 57 (0, 89) | 5 | 1.2 | 6 (0, 12) | 0.74 |
| HFH | 14 | 2.9 | 29 (7, 46) | 24 | 5.7 | 39 (11, 57) | 0.07 |
CI, confidence interval; ACD, all‐cause death; CVH, cardiovascular hospitalization; HFH, heart failure hospitalization; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; MACE, major adverse clinical events.
P‐values express results of log‐rank analysis for cumulative incidence.
Table 2B.
The incidence of each clinical event following the last procedure
| HFpEF | Systolic HF | P‐value | |||||
|---|---|---|---|---|---|---|---|
| Crude incidence rate, % | Incidence rate, events/100 person‐years | Cumulative incidence, % (95% CI) | Crude incidence rate, % | Incidence rate, events/100 person‐years | Cumulative incidence, % (95% CI) | ||
| MACE | 20 | 4.8 | 62 (9, 84) | 24 | 6.9 | 30 (15, 43) | 0.21 |
| ACD | 7 | 1.7 | 49 (0, 78) | 2 | 0.5 | 2 (0, 7) | 0.33 |
| CVH | 6 | 1.4 | 16 (0, 30) | 2 | 0.5 | 2 (0, 7) | 0.4 |
| HFH* | 11 | 2.5 | 21 (5, 34) | 24 | 6.9 | 29 (14, 41) | 0.01 |
CI, confidence interval; ACD, all‐cause death; CVH, cardiovascular hospitalization; HFH, heart failure hospitalization; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; MACE, major adverse clinical events.
Statistical significance (P < 0.05).
Cumulative incidence of each clinical event
Figure 3 presents the cumulative incidence of each clinical event following the procedures. The incidence of ACD was higher in the HFpEF group than in the systolic HF group, but not significantly (Figure 3 A : following the first procedure: HFpEF, crude incidence rate: 7%, incidence rate: 1.5/100 person‐years vs. systolic HF, 2%, 0.4/100 person‐years, P = 0.33; following the last procedure: 7%, 1.7/100 person‐years vs. 2%, 0.5/100 person‐years, P = 0.33). The incidence of CVH followed the similar trend following the first procedure: 8%, 1.7/100 person‐years vs. 5%, 1.2/100 person‐years, P = 0.74; following the last procedure: 6%, 1.4/100 person‐years, vs. 2%, 0.5/100 person‐years, P = 0.4 (Figure 3 B ). In contrast, the incidence of HFH following the first procedure was lower in the HFpEF group than in the systolic HF group: 14%, 2.9/100 person‐years, vs. 24%, 5.7/100 person‐years, P = 0.07. The rate of HFH following the last procedure was significantly lower in the HFpEF group than in the systolic HF group (11%, 2.5/100 person‐years, vs. 24%, 6.9/100 person‐years, P = 0.01) (Figure 3 C ). A sensitivity analysis was performed for the incidence of HFH. After adjustment for 11 baseline characteristics (age, sex, persistent AF, New York Heart Association class, systolic blood pressure, hypertension, DM, coronary artery disease, estimated glomerular filtration rate [eGFR], and use of beta‐blocker and amiodarone), the tendency of low incidence of HFH in the HFpEF group, compared with systolic HF persisted (adjusted HR [95% CI]: 0.4 [0.12–1.26], P = 0.11 for following the first procedure, 0.34 [0.09–1.22], P = 0.09 for following the last procedure).
Figure 3.
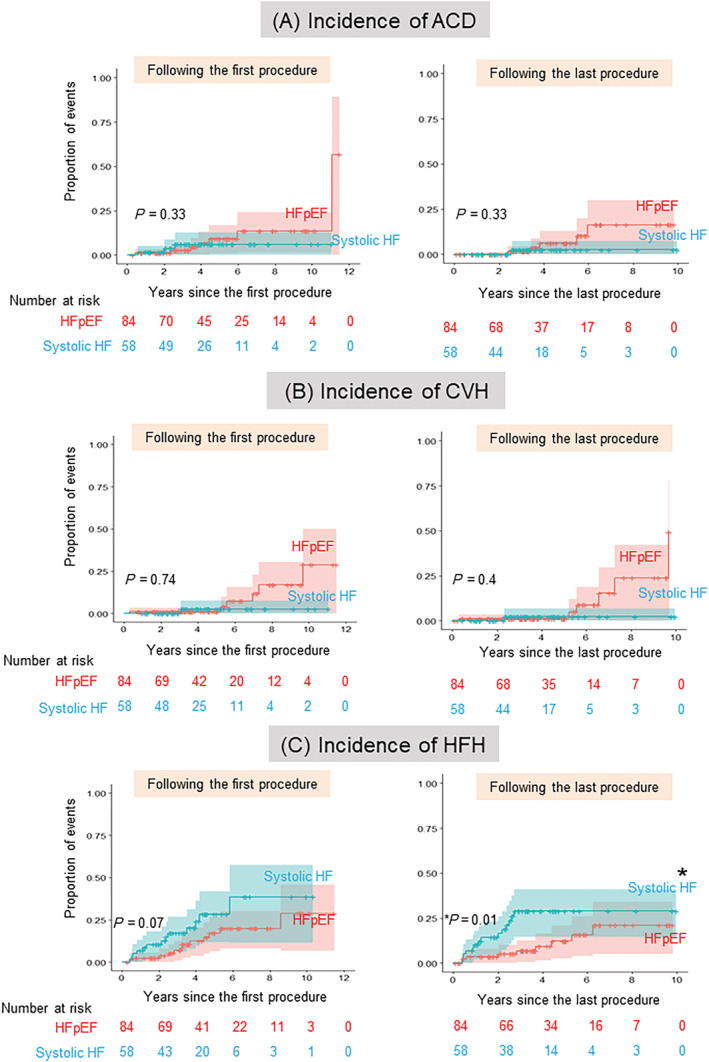
Cumulative incidence of each clinical event following the procedure. (A) Comparison of the cumulative incidence and 95% CI of ACD following the first (left)/last (right) procedure between patients with HFpEF and systolic HF (red: patients with HFpEF, blue: systolic HF). (B) Comparison of the cumulative incidence and 95% CI of CVH following the first (left)/last (right) procedure between patients with HFpEF and systolic HF (red: patients with HFpEF, blue: systolic HF). (C) Comparison of the cumulative incidence and 95% CI of HFH following the first (left)/last (right) procedure between patients with HFpEF and systolic HF (red: patients with HFpEF, blue: systolic HF). Asterisk indicates statistical significance (*P = 0.01). ACD, all‐cause death; CVH, cardiovascular hospitalization; HFH, heart failure hospitalization; AF, atrial fibrillation; CA, catheter ablation; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; MACE, major adverse clinical events.
The total number of heart failure hospitalization following the first procedure
Figure 4 compares the number of HFHs between pre‐CA and post‐CA procedure using a Sankey diagram. In total, the median follow‐up period for pre‐CA and post‐CA was 2.3 (0.8, 4.4) and 4.0 (2.6, 6.3) years, respectively. In the HFpEF group, the number was significantly smaller for post‐CA than for pre‐CA (Figure 4 A , one hospitalization [the first and third quartile: 0, 1] pre‐CA, vs. 0 hospitalizations [0, 0] post CA, P < 0.0001). The number of patients with systolic HF also showed a similar tendency (Figure 4 B , 1 hospitalization [0, 1], vs. 0 hospitalizations [0, 0], P < 0.005).
Figure 4.
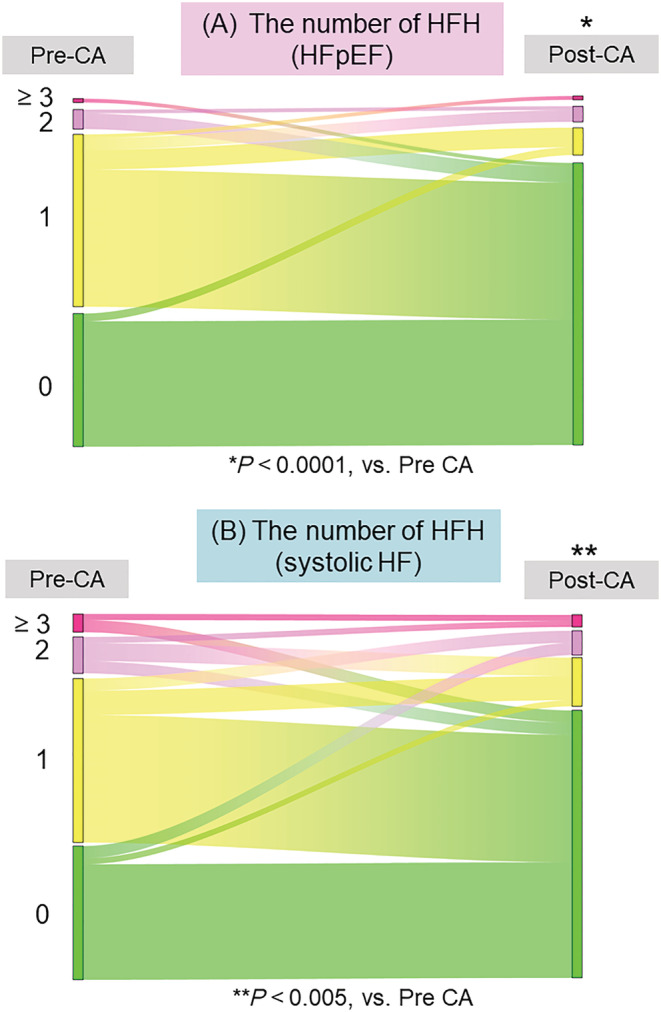
Comparison of the number of HFH between pre‐CA and post‐CA. (A) Sankey diagram shows the comparison of the number of HFH between pre‐CA and post‐CA in patients with HFpEF. (B) Sankey diagram shows the comparison of the number of HFH between pre‐CA and post‐CA in patients with systolic HF. Asterisks indicate statistical significance (*P < 0.0001, **P < 0.005). Each colour indicates the number of HFH: red, ≥3; pink, 2; yellow, 1; green, 0. ACD, all‐cause death; CVH, cardiovascular hospitalization; HFH, heart failure hospitalization; AF, atrial fibrillation; CA, catheter ablation; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; MACE, major adverse clinical events.
Proportion of heart failure hospitalization among total clinical events
Figure 5 shows the proportion of each clinical event following the first and last procedures. Following the first and last procedures, the proportion of HFH was significantly smaller in the HFpEF group than in the systolic HF group (first procedure: 56% vs. 88%, P < 0.005, last procedure: 52% vs. 92%, P < 0.005).
Figure 5.
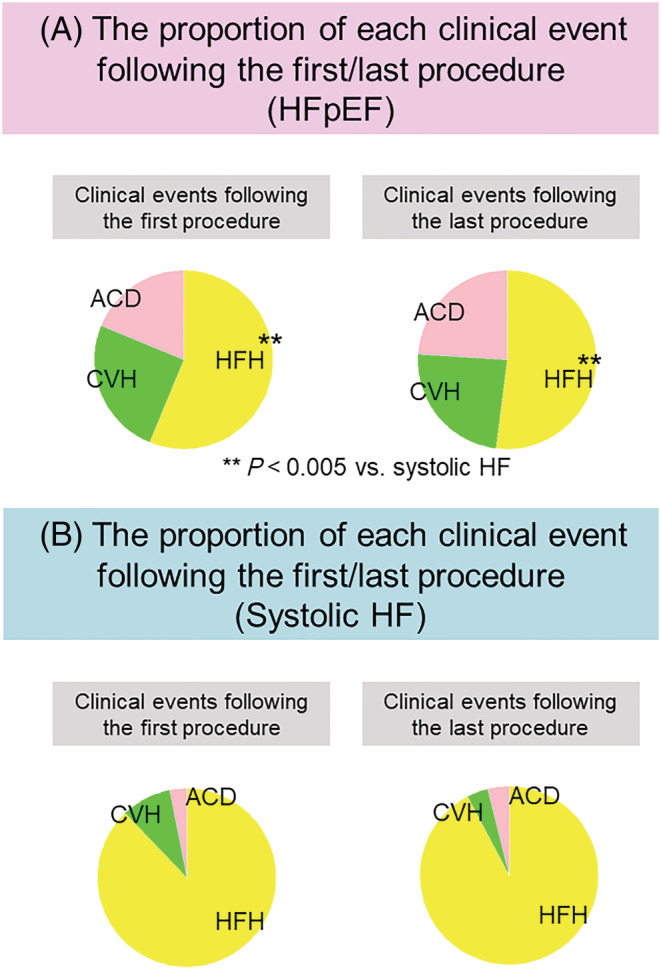
Proportion of each clinical event following the first and last procedure. (A) The proportion of each clinical event following the first (left) and last (right) procedure in patients with HFpEF. (B) The proportion of each clinical event following the first (left) and last (right) procedure in patients with systolic HF. The asterisks indicate statistical significance (**P < 0.005). Each colour indicates each clinical event: red: ACD; yellow, HFH; green, CVH. ACD, all‐cause death; CVH, cardiovascular hospitalization; HFH, heart failure hospitalization; AF, atrial fibrillation; CA, catheter ablation; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; MACE, major adverse clinical events.
Predictors for developing major adverse clinical events
Table 3 shows the univariate and multivariate analyses to explore predictors of MACE following the first procedure in the HFpEF group. Univariate analysis revealed that age ≥75 years, female sex, persistent AF, ATA recurrence, left atrial diameter (LAD) ≥ 45 mm, DM, and eGFR <45 mL/min/1.73 m2 were potential predictors. Age ≥75 years, ATA recurrence, and eGFR <45 mL/min/1.73 m2 emerged as independent predictors of MACE after multivariate adjustment.
Table 3.
Results of univariate/multivariate analysis to identify predictors for MACE following the first procedure
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| Age ≥75 years* | 4.1 | 1.5–10.9 | 0.004 | 4.8 | 1.4–15.7 | 0.009 |
| Female sex | 1.1 | 0.45–2.8 | 0.79 | 0.81 | 0.23–2.7 | 0.73 |
| Persistent AF | 0.5 | 0.2–1.2 | 0.13 | 0.45 | 0.17–1.1 | 0.11 |
| ATA recurrence* | 3.2 | 0.92–11.1 | 0.07 | 4.2 | 1.04–16.8 | 0.04 |
| Discontinuation of OACs following the procedure | 2.2 | 0.5–9.8 | 0.29 | |||
| LAD ≥45 mm | 3.3 | 1.3–8.5 | 0.01 | 1.9 | 0.57–6.4 | 0.29 |
| LVEF ≥65% | 1.3 | 0.5–3.3 | 0.59 | |||
| DM | 3.0 | 0.96–9.7 | 0.06 | 2.2 | 0.50–10.1 | 0.28 |
| CAD | 0.69 | 0.08–4.7 | 0.65 | |||
| eGFR <45 mL/min/1.73 m2 * | 4.8 | 1.9–12.4 | 0.001 | 3.5 | 1.1–10.6 | 0.02 |
| BNP level ≥400 pg/mL | 2.9 | 1.1–7.3 | 0.03 | 1.4 | 0.45–4.7 | 0.52 |
AF, atrial fibrillation; ATA, atrial tachyarrhythmia; BNP, brain natriuretic peptide; CAD, coronary artery disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HR, hazard ratio; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; MACE, major adverse clinical events; OACs, oral anticoagulants.
Statistical significance after adjustment in multivariate analysis (P < 0.05).
Discussion
Main findings
The important findings of this study are as follows: First, the incidence of MACE was comparable between the HFpEF and systolic HF groups (following the first procedure: 23%, 4.7/100 person‐years, vs. 28%, 6.6/100 person‐years, P = 0.18; following the last procedure: 20%, 4.8/100 person‐years, vs. 24%, 6.9/100 person‐years, P = 0.21). However, the incidence of HFH had a lower tendency in the HFpEF group than in the systolic HF group (following the first procedure: 14%, 2.9/100 person‐years, vs. 24%, 5.7/100 person‐years, P = 0.07; following the last procedure: 11%, 2.5/100 person‐years, vs. 24%, 6.9/100 person‐years, P = 0.01). Second, the total number of HFHs after the first CA was significantly smaller than the number of HFH before the CA in both the HFpEF and systolic HF groups. Third, the proportion of HFH among total MACE following both the first and last procedures was significantly smaller in the HFpEF group than in the systolic HF group. Fourth, multivariate analysis showed that age ≥75 years, ATA recurrence, and eGFR <45 mL/min/1.73 m2 emerged as independent predictors of MACE following the first procedure in the HFpEF group.
Difference in major adverse clinical events following the catheter ablation procedure between the heart failure with preserved ejection fraction and systolic heart failure groups
To our knowledge, this is the first study to compare both the long‐term prognostic events (all‐cause death, unplanned cardiovascular events, and heart failure hospitalization) and the efficacy of CA for reducing the number of HFH between HFpEF and systolic HF (HFrEF with HFmrEF) in patients who underwent CA for AF. There is a huge aetiologic difference between HFpEF and HFrEF. 7 , 15 Regarding HFpEF, multiple factors, such as vascular‐ventricular stiffness and endothelial microvascular inflammation, which are derived from various co‐morbidities predispose patients to left ventricular diastolic dysfunction. 8 Hence, patients' prognosis with HFpEF was as poor as that with HFrEF because patients with HFpEF tended to develop various cardiovascular events and non‐cardiac death which reflected various co‐morbidities. 14 , 15 , 19 In agreement with this phenomenon, our results also showed that the long‐term prognostic events in patients with HFpEF were comparable with those with systolic HF, owing to a higher incidence of events of other than HF such as all‐cause death and unplanned cardiovascular hospitalization. On the contrary, the incidence of HFH was lower in patients with HFpEF than in those with systolic HF. We speculated that the efficacy of CA was comparable between groups because our results showed that, first, the success rate was comparable between groups and, second, the number of HFH equally decreased between pre‐CA and post‐CA procedures in both groups. Our results suggest that CA for AF in patients with HFpEF could be a feasible option to reduce HF‐related events, similar to patients with systolic HF.
Clinical events following atrial fibrillation in heart failure with preserved ejection fraction
Several studies have addressed the efficacy of CA for AF in patients with HFpEF. The majority of the studies evaluated the efficacy by measuring surrogate markers such as symptoms, echocardiographic left ventricular diastolic function, and biomarkers between pre‐procedure and post‐procedure. 13 , 20 , 21 , 22 , 23 However, studies focusing on the comparison of clinical events after CA between HFpEF and other subtype of HF, such as the present study, are limited. Aldaas et al. evaluated the incidence of all‐cause mortality and all‐cause hospitalization following the procedure between HFpEF and systolic HF (defined as LVEF <50%, the same as our study). 24 Although the results demonstrated no differences between groups, a comparison of precise clinical events has not been conducted. In the present study, we added the information that patients with HFpEF who underwent CA for AF had a higher incidence of cardiovascular hospitalization and all‐cause death and a lower incidence of heart failure hospitalization. In addition, our result was in line with the study in that the incidence of total clinical events following CA for AF was comparable between groups. In contrast, regarding another study, Fujimoto reported that the incidence of prognostic events, which comprised all‐cause death, heart failure hospitalization, and stroke/systemic embolism, was higher in patients with HFrEF (LVEF <40%) than in those with HFpEF. 25 The disparity between their study and ours might originate from differences in therapeutic management for patients with HFrEF. The prevalence of patients who received guideline‐recommended medications, such as angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers, beta‐blockers, or mineralocorticoid receptor antagonists, was higher in our study than in Fujimoto's study. 25 In addition, the prevalence of patients who underwent implantable cardioverter defibrillator/cardiac resynchronization therapy was also two‐fold higher in our study (24% vs. 12% 25 ). Hence, the incidence of clinical events in patients with systolic HF (especially HFrEF) in our population might be reduced owing to therapeutic management other than CA. Although the results of our study and theirs 25 implied that the incidence of clinical events in patients with HFrEF could vary based on therapeutic status, reports investigating clinical events following CA for AF remained scarce. Further studies that compare the incidence of clinical events between HFpEF and HFrEF are warranted.
Clinical implications
Our study demonstrated that the number of HFHs comparably decreased in patients with HFpEF and in those with systolic HF between pre‐CA and post‐CA procedures. In addition, patients with HFpEF had a lower incidence of HFH than those with systolic HF. Our results indicated that CA would be a feasible option for patients with HFpEF, similar to systolic HF. In the current clinical setting, there are few therapeutic options for HFpEF coexisting with AF, although previous studies have described that the prognostic impact of AF was high in patients with HFpEF. 3 , 4 , 8 , 9 , 26 Our data suggest that CA for AF could be beneficial for patients with HFpEF. In particular, the incidence of HFH was lower in the period following the last session, in which the success rate was high, than that after the first session. Hence, redo procedures would be helpful to reduce HFH for patients who developed arrhythmic recurrence following the first session.
On the other hand, our results also showed that the total clinical events following the CA procedure in patients with HFpEF were comparable with those in patients with systolic HF owing to a higher incidence of all‐cause death and unplanned cardiovascular hospitalization. Our data indicate that clinicians should be cautious about the development of clinical events other than HFH. Regarding MACE in the present study, ATA recurrence, older age (≥75 years), and renal dysfunction (eGFR <45 mL/min/1.73 m2) emerged as independent predictors. Our data could be useful for identifying patients at high risk for developing clinical events following CA procedures.
Limitations
The present study had several limitations. First, the study design was a single‐centre, retrospective, observational study. We had a relatively small sample size and missed unmeasured variables regarding the population. Whether our results can be extrapolated to other populations remains unclear. Hence, further studies in other institutions are required to validate our findings. Second, our protocol combined HFrEF and HFmrEF as systolic HF, because the two aetiologies had similar characteristics in response to pharmacotherapy. 16 , 17 However, evidence that CA for AF improved the prognosis was limited in patients with HFrEF. 10 It remains uncertain whether the prognostic impact of CA for AF in HFmrEF is similar to that of HFrEF. Further studies comparing clinical events following CA for AF among the three categories of HF (HFrEF, HFmrEF, and HFpEF) would clarify this issue. Third, we diagnosed HFpEF based on the clinical symptoms. Although our criteria had higher sensitivity, the specificity was lower than that of echocardiographic criteria. It remains unclear whether our results could be applicable to populations in which HFpEF was diagnosed using echocardiographic criteria. 20 , 21 Fourth, our population comprised patients in whom attending cardiologists generally expected good efficacy with CA. This would indicate a discrepancy in patient demographics between our population and the general population of HF owing to a selection bias. Fifth, we could not perform a comparison of the incidence of clinical events in patients with HF and those without. Hence, it remains unclear what the effect of HF is on the development of clinical events in the whole patients who underwent CA for AF. Further studies performing the comparison are warranted.
Conclusions
Our results indicated that CA for AF could also be beneficial for patients with HFpEF, similar to systolic HF. However, our data also suggest that clinicians should be cautious about the development of clinical events other than HF, such as unplanned cardiovascular events and all‐cause mortality, for such patients.
Conflict of interest
None declared.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Supporting information
Table S1. A. List of patients who developed MACE in the group with HFpEF. B. List of patients who developed MACE in the group with systolic HF.
Acknowledgements
We thank the staff of the electrophysiological laboratory.
Ishiguchi, H. , Yoshiga, Y. , Shimizu, A. , Ueyama, T. , Fukuda, M. , Kato, T. , Fujii, S. , Hisaoka, M. , Uchida, T. , Omuro, T. , Okamura, T. , Kobayashi, S. , and Yano, M. (2022) Long‐term events following catheter‐ablation for atrial fibrillation in heart failure with preserved ejection fraction. ESC Heart Failure, 9: 3505–3518. 10.1002/ehf2.14079.
References
- 1. Sidney S, Go AS, Jaffe MG, Solomon MD, Ambrosy AP, Rana JS. Association between aging of the US population and heart disease mortality from 2011 to 2017. JAMA Cardiol. 2019; 4: 1280–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. Eur J Heart Fail. 2015; 17: 884–892. [DOI] [PubMed] [Google Scholar]
- 3. Tsutsui H, Isobe M, Ito H, Ito H, Okumura K, Ono M, Kitakaze M, Kinugawa K, Kihara Y, Goto Y, Komuro I, Saiki Y, Saito Y, Sakata Y, Sato N, Sawa Y, Shiose A, Shimizu W, Shimokawa H, Seino Y, Node K, Higo T, Hirayama A, Makaya M, Masuyama T, Murohara T, Momomura SI, Yano M, Yamazaki K, Yamamoto K, Yoshikawa T, Yoshimura M, Akiyama M, Anzai T, Ishihara S, Inomata T, Imamura T, Iwasaki YK, Ohtani T, Onishi K, Kasai T, Kato M, Kawai M, Kinugasa Y, Kinugawa S, Kuratani T, Kobayashi S, Sakata Y, Tanaka A, Toda K, Noda T, Nochioka K, Hatano M, Hidaka T, Fujino T, Makita S, Yamaguchi O, Ikeda U, Kimura T, Kohsaka S, Kosuge M, Yamagishi M, Yamashina A, Japanese Circulation Society and the Japanese Heart Failure Society Joint Working Group . JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure ‐digest version. Circ J. 2019; 83: 2084–2184. [DOI] [PubMed] [Google Scholar]
- 4. Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2013; 10: 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suzuki A, Shiga T, Kawashiro N, Hagiwara N, the HIJ‐HF II Investigators . Changes in characteristics and outcomes in Japanese patients with heart failure from the 2000s to the 2010s: The HIJ‐HF cohorts. J Cardiol; 2020: 132–138. [DOI] [PubMed] [Google Scholar]
- 6. Hindriks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL, ESC Scientific Document Group . ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2021; 42: 373–498. [DOI] [PubMed] [Google Scholar]
- 7. Ariyaratnam JP, Lau DH, Sanders P, Kalman JM. Atrial fibrillation and heart failure. Epidemiology, pathophysiology, prognosis, and management. Card Electrophysiol Clin. 2021; 13: 47–62. [DOI] [PubMed] [Google Scholar]
- 8. Kotecha D, Lam CSP, Van Veldhuisen DJ, Van Gelder IC, Voors AA, Rienstra M. Heart failure with preserved ejection fraction and atrial fibrillation. J Am Col Cardiol. 2016; 68: 2217–2228. [DOI] [PubMed] [Google Scholar]
- 9. Zafrir B, Lund LH, Laroche C, Ruschitzka F, Crespo‐Leiro MG, Coats AJS, Anker SD, Filippatos G, Seferovic PM, Maggioni AP, De Mora MM, Polonski L, Silva‐Cardoso J, Amir O, ESC‐HFA HF Long‐Term Registry Investigators . Prognostic implications of atrial fibrillation in heart failure with reduced, mid‐range, and preserved ejection fraction: A report from 12964 patients in the European Society of Cardiology Heart Failure Long‐Term Registry. Eur Heart J. 2018; 39: 4277–4284. [DOI] [PubMed] [Google Scholar]
- 10. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, Schunkert H, Christ H, Vogt J, Bänsch D, CASTLE‐AF Investigators . Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018; 378: 417–427. [DOI] [PubMed] [Google Scholar]
- 11. Packer DL, Piccini JP, Monahan KH, Al‐Khalidi HR, Silverstein AP, Noseworthy PA, Poole JE, Bahnson TD, Lee KL, Mark DB, CABANA Investigators . Ablation versus drug therapy for atrial fibrillation in heart failure. Results from the CABANA trial. Circulation. 2021; 143: 1377–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fukui A, Tanino T, Yamaguchi T, Hirota K, Saito S, Okada N, Akioka H, Shinohara T, Yufu K, Takahashi N. Catheter ablation of atrial fibrillation reduces heart failure rehospitalization in patients with heart failure with preserved ejection fraction. J Cardiovasc Electrophysiol. 2020; 31: 682–688. [DOI] [PubMed] [Google Scholar]
- 13. Black‐Maier E, Ren X, Steinberg BA, Green CL, Barnett AS, Rosa NS, Al‐Khatib SM, Atwater BD, Daubert JP, Frazier‐Mills C, Grant AO, Hegland DD, Jackson KP, Jackson LR, Koontz JI, Lewis RK, Sun AY, Thomas KL, Bahnson TD, Piccini JP. Catheter ablation of atrial fibrillation in patients with heart failure and preserved ejection fraction. Heart Rhythm. 2018; 15: 651–657. [DOI] [PubMed] [Google Scholar]
- 14. Chan MMY, Lam CSP. How do patients with heart failure with preserved ejection fraction die? Eur J Heart Fail. 2013; 15: 604–613. [DOI] [PubMed] [Google Scholar]
- 15. Vaduganathan M, Patel RB, Michel A, Shah SJ, Senni M, Gheorghiade M, Butler J. Mode of death in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2017; 69: 556–569. [DOI] [PubMed] [Google Scholar]
- 16. Tsutsui H, Isobe M, Ito H, Ito H, Okumura K, Ono M, Kitakaze M, Kinugawa K, Kihara Y, Goto Y, Komuro I, Saiki Y, Saito Y, Sakata Y, Sato N, Sawa Y, Shiose A, Shimizu W, Shimokawa H, Seino Y, Node K, Higo T, Hirayama A, Makaya M, Masuyama T, Murohara T, Momomura SI, Yano M, Yamazaki K, Yamamoto K, Yoshikawa T, Yoshimura M, Akiyama M, Anzai T, Ishihara S, Inomata T, Imamura T, Iwasaki YK, Ohtani T, Onishi K, Kasai T, Kato M, Kawai M, Kinugasa Y, Kinugawa S, Kuratani T, Kobayashi S, Sakata Y, Tanaka A, Toda K, Noda T, Nochioka K, Hatano M, Hidaka T, Fujino T, Makita S, Yamaguchi O, Ikeda U, Kimura T, Kohsaka S, Kosuge M, Yamagishi M, Yamashina A, Japanese Circulation Society and the Japanese Heart Failure Society Joint Working Group . JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure ‐digest version. Circ J. 2019; 83: 2084–2184. [DOI] [PubMed] [Google Scholar]
- 17. Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, Anker SD, Atherton J, Böhm M, Butler J, Drazner MH, Felker GM, Filippatos G, Fonarow GC, Fiuzat M, Gomez‐Mesa JE, Heidenreich P, Imamura T, Januzzi J, Jankowska EA, Khazanie P, Kinugawa K, Lam CSP, Matsue Y, Metra M, Ohtani T, Francesco Piepoli M, Ponikowski P, Rosano GMC, Sakata Y, SeferoviĆ P, Starling RC, Teerlink JR, Vardeny O, Yamamoto K, Yancy C, Zhang J, Zieroth S. Universal definition and classification of heart failure: A report of the heart failure society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the universal definition of heart failure. J Card Fail. 2021; 27: 387–413. [DOI] [PubMed] [Google Scholar]
- 18. Ishiguchi H, Yoshiga Y, Shimizu A, Ueyama T, Ono M, Fukuda M, Kato T, Fujii S, Hisaoka M, Uchida T, Omuro T, Shimokawa M, Okamura T, Kobayashi S, Yano M. Impact of atrial tachy‐arrhythmia recurrence on the development of long‐term adverse clinical events following catheter ablation in atrial fibrillation patients with systolic impairment: A single‐center observational study. J Am Heart Assoc. 2022; 11: e023640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miyagishima K, Hiramitsu S, Kimura H, Mori K, Ueda T, Kato S, Kato Y, Ishikawa S, Iwase M, Morimoto S, Hishida H, Ozaki Y. Long term prognosis of chronic heart failure: Reduced vs preserved left ventricular ejection fraction. Circ J. 2009; 73: 92–99. [DOI] [PubMed] [Google Scholar]
- 20. Machino‐ohtsuka T, Seo Y, Ishizu T, Sugano A, Atsumi A, Yamamoto M, Kawamura R, Machino T, Kuroki K, Yamasaki H, Igarashi M, Sekiguchi Y, Aonuma K. Efficacy, safety, and outcomes of catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction. J Am Coll Cardiol. 2013; 62: 1857–1865. [DOI] [PubMed] [Google Scholar]
- 21. Rettka M, Pott A, Kühberger A, Weinmann K, Scharnbeck D, Stephan T, Baumhardt M, Bothner C, Iturbe Orbe M, Rottbauer W, Dahme T. Restoration of sinus rhythm by pulmonary vein isolation improves heart failure with preserved ejection fraction in atrial fibrillation. Europace. 2020; 22: 1328–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamauchi R, Morishima I, Okumura K, Kanzaki Y, Morita Y, Takagi K, Nagai H, Watanabe N, Furui K, Yoshioka N, Miyazawa H, Shimojo K, Imaoka T, Sakamoto G, Murohara T. Catheter ablation for non‐paroxysmal atrial fibrillation accompanied by heart failure with preserved ejection fraction: Feasibility and benefits in functions and B‐type natriuretic peptide. Europace. 2021; 23: 1252–1261. [DOI] [PubMed] [Google Scholar]
- 23. Kawaji T, Shizuta S, Aizawa T, Yamagami S, Kato M, Yokomatsu T, Miki S, Ono K, Kimura T. Impact of catheter ablation for atrial fibrillation on cardiac disorders in patients with coexisting heart failure. ESC Heart Failure. 2021; 8: 670–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aldaas OM, Malladi CL, Mylavarapu PS, Lupercio F, Darden D, Han FT, Hoffmayer KS, Krummen D, Ho G, Raissi F, Feld GK, Hsu JC. Comparison of outcomes after ablation of atrial fibrillation in patients with heart failure with preserved versus reduced ejection fraction. Am J Cardiol. 2020; 136: 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fujimoto H, Doi Nm Okayama S, Naito M, Kobori A, Kaitani K, Inoue K, Kurotobi T, Morishima I, Yamaji H, Matsui Y, Nakazawa Y, Kusano K, Hirai K, Nakai T, Suzuki M, Yano H, Sakai S, Kimura T, Shizuta S, Saito Y, on behalf of the KPAF investigators . Long‐term prognosis of patients undergoing radiofrequency catheter ablation for atrial fibrillation: Comparison between heart failure subtypes based on left ventricular ejection fraction. Europace. 2022;24: 576–586. [DOI] [PubMed] [Google Scholar]
- 26. Kotecha D, Holmes J, Krum H, Altman DG, Manzano L, Cleland JG, Lip GY, Coats AJ, Andersson B, Kirchhof P, von Lueder TG, Wedel H, Rosano G, Shibata MC, Rigby A, Flather MD, Beta‐Blockers in Heart Failure Collaborative Group . Efficacy of beta blockers in patients with heart failure plus atrial fibrillation: An individual‐patient data meta‐analysis. Lancet. 2015; 384: 2235–2243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. A. List of patients who developed MACE in the group with HFpEF. B. List of patients who developed MACE in the group with systolic HF.


