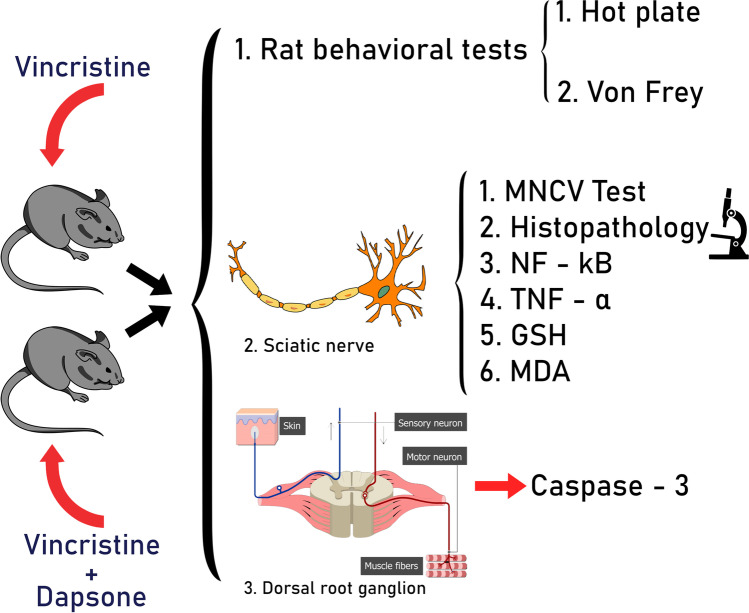
Abstract
Background
Peripheral neuropathy is a dose-limiting adverse effect of vincristine (VCR) in cancer chemotherapies. Dapsone is commonly used for the prevention of opportunistic infections following cancer therapies. Therefore, a high rate of VCR and dapsone co-administration has occurred in leukemias. Recently neuroprotective effects of dapsone have been reported in various diseases.
Objectives
Regarding the physiopathology of VCR-induced peripheral neuropathy (VIPN) and dapsone neuroprotection, this study evaluated the effect of dapsone on VIPN.
Methods
VIPN was induced by VCR injection (0.5 mg/kg IP, every other day, 1 week) in male Wistar rats. In the treatment group, dapsone(12.5 mg/kg IP, 1 week) was injected 30 min before VCR. Hot plate, Von Frey, motor neuron conduction velocity (MNCV), and histopathological tests were applied. The levels of TNF-α and NF-kB in the sciatic nerve and caspase-3 activity in dorsal root ganglion were measured by the ELISA method. The levels of malondialdehyde (MDA) and Glutathione (GSH) in the sciatic nerve were measured by spectrophotometry and colorimetric assays.
Results
VIPN was observed as araised thermal and mechanical threshold, reduced MNCV, and sciatic nerve demyelination. However, dapsone reduced the mechanical and thermal threshold and improved the MNCV. Also, dapsone reduced TNF-α, NF-kB, MDA, and Caspase-3 activity, and increased the GSH level in the sciatic nerve. Moreover, dapsone prevented VCR-induced demyelination in the sciatic nerve.
Conclusion
This research demonstrated that dapsone could be used as a protective drug against VIPN. It improves the impaired thermal and mechanical sensations by reducing inflammatory, oxidant, and apoptosis factors and preventing demyelination in the sciatic nerve.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s40199-022-00448-6.
Keywords: Vincristine, Dapsone, Peripheral neuropathy, Neuroprotective, Sciatic nerve
Introduction
Vincristine (VCR), a member of vinca alkaloids, is clinically administered in acute lymphocytic lymphoma (ALL), Hodgkin, and Non-Hodgkin lymphoma. VCR binding to tubulin inhibits the mitotic spindle formation. Despite the high efficacy of VCR in cancer treatment, VCR-induced peripheral neuropathy (VIPN) leads to the discontinuation of the treatment in 68% of the patients [1, 2]. VIPN is categorized into three classes, including sensory, motor, and autonomic neuropathy.
The peripheral nervous system includes nerves that branch out from the brain and spinal cord. Each spinal nerve is connected to the spinal cord by a dorsal root and a ventral root [3]. Sensory neuropathy is usually presented by hyperalgesia, allodynia, burning, and tingling [1]. In contrast, VIPN at higher doses of VCR is presented as numbness and mechanical and thermal hypoalgesia [4, 5].
Studies have revealed that loss of myelinated fibers of the sciatic nerve, is associated with reduced nerve conduction velocity post VCR administration [6–8]. In addition, an increase of pro-inflammatory cytokines including tumor necrosis factor-alpha (TNF-α) and interleukin-1β (IL-1β), and also the activation of the nuclear factor kappa B (NF-κB) signaling pathway in the spinal cord contributes to the development of VIPN [9]. On the other hand, increased reactive oxygen species (ROS) levels are observed following VCR-induced mitochondrial damage [10].
Dapsone, as a member of sulfonamide drugs, was first synthesized from 4,4'-Thiodianiline by Fromm and Wittman in 1908. Besides having antimicrobial effects, dapsone has been evaluated for its anti-inflammatory and neuroprotective effects [11]. It has a protective effect against spinal cord injury by increasing the expression of anti-apoptotic factors [12]. Also, decreased seizure episodes have been observed following dapsone administration which was mediated by activation of anti-oxidant pathways [13]. Besides reducing inflammatory cytokines such as TNF-α and IL-8, it has been determined that dapsone suppresses the NF-kB signaling pathway as well [14, 15].
Regarding the suggested mechanisms involved in VIPN as a severe side effect of VCR and reported neuroprotective effects of dapsone, this study aimed to evaluate the protective effects of dapsone against VIPN through three inflammatory, oxidant, and apoptotic pathways.
Methods
Animal rights
Thirty male Wistar rats (250-300 g) were provided from the animal house of the medicine department, Tehran University of Medical Sciences, Tehran, Iran. All of our procedures were conducted according to the ‘Principles of Laboratory Animal Care (NIH publication 82–23, revised in 1985 and further implemented in 1996) and legislation for the protection of animals used for scientific purposes (Directive 2010/63/EU), and institutional guidelines for animal care and use (Department of Pharmacology, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran). All animals were kept in plastic cages (four rats in each cage) under 12 h light/dark and a temperature of 20–23 °C with free access to food and water. Rats were randomly divided into four following groups; each group consisted of 6 rats: 1. Control 2. Dapsone 3. polyethylene glycol (PEG) 4. VCR 5. VCR + dapsone.
Drug administration
VCR (Sobhan, Iran) was administered 0.5 mg/kg intraperitoneal (IP by three injections on alternate days for one week [16, 17]. Dapsone (Gilaranco, Rasht, Iran) was dissolved in saline and PEG 4.5% and was administered 12.5 mg/kg IP every day for one week (similar to antimicrobial (12 mg/kg) and neuroprotective (12.5 mg/kg) doses of dapsone in previous studies) [16, 18, 19]. The time courses of drug administrations were selected regarding the half-life of VCR (85 h) and dapsone (30 h) [20, 21]. In the group receiving VCR + dapsone, dapsone was injected 30 min before VCR.
Bodyweight measurement
Animals were weighed on days 1 and 7, and their health condition, including appearance, diarrhea, movements, and mortality, were observed during the experiment.
Thermal threshold evaluation (Hot plate test)
A hot plate test was applied to measure the thermal threshold. Rats were placed on a heated plate (Borjsanat, Iran) at 54 ± 1 C˚, and first jumping or paw licking was considered a heat avoidance sign. To avoid any harmful effects, 30 s was set as the cut-off time [22].
Mechanical threshold evaluation (Von Frey test)
To evaluate the effects of VCR on sensory nerves, the Von Frey test was applied on days 1 and 7 (the last day of the experiment) regarding the previous studies [23]. For this purpose, Von Frey filaments (Bioseb, USA), varying in diameters, were applied to the rat’s plantar surface. In an ascending stimulus process, the diameters of filaments increased until reaching a positive response induced by the first filament. The mechanical threshold was documented as grams, and hypo/hyperalgesia was presented as decreased/increased mechanical thresholds.
Electrophysiological assessment
On day 7, after finishing the behavioral test, rats were anesthetized with 65 mg/kg of thiopental (IP), dissolved in saline. Motor nerve conduction velocity (MNCV) was measured in rats’ left sciatic nerve regarding the method mentioned in Ja’afer et al. paper [24]. The MNCV test was applied in both proximal and distal conditions. Proximally, the active electrode was placed at the sciatic notch and the passive electrode was applied to the dorsal aspect of the sciatic nerve between the spinous processes of the lower lumbar vertebrae and the palpable posterior surface of the femoral head on the same side. Distally, the active stimulating electrode was placed subcutaneously at the Achilles tendon and the passive electrode was placed into the thigh muscles. In both conditions, a 20 mm distance was left between the mentioned electrode [24].
Sciatic and DRG dissection
After finishing the electrophysiological assessment, rats were sacrificed with carbon dioxide. First, proximal segments of sciatic nerves (both sides) were taken from the affected hind limb of the rat [24].
For DRGs extraction, first, the spinal column (from the base of the skull to the level of the femurs) was dissected. Then the mid-line and the spinal cord were cut down and DRGs were extracted after removing the meninges [25].
Molecular assessment
To evaluate the inflammatory pathway in this research, the level of TNF-α and NF-kB in the sciatic nerve were measured by rat TNF-α and NF-kB enzyme-linked immune-sorbent assay (ELISA) Kits (ab100785, LS-F37358). Also, the level of malondialdehyde (MDA) as an oxidant factor and Glutathione (GSH) as an anti-oxidant factor in sciatic nerve were evaluated by spectrophotometry (mentioned in Buege and Aust’s paper) and Ellman GSH colorimetric assay, respectively [26, 27]. Moreover, for measuring caspase-3 activity in rat dorsal root ganglion, a caspase-3 colorimetric cell-based ELISA kit (EKC1086) was administered. All the experiments were repeated in triplicate to confirm the reproducibility of the results.
Hematoxylin and eosin (H&E) staining
For histological studies, the obtained sciatic nerves were fixed in a 4% formalin solution. H&E staining was applied on sciatic tissue samples, and degenerative neuronal changes were studied by an Olympus BX40 microscope.
Data analysis
The data analysis was performed by GraphPad Prism 6 through one-way analysis of variance (ANOVA) with posthoc Tukey tests. In this study, P < 0.05 was considered a significant difference. Also, results are presented as mean ± standard error of the mean (SEM).
Results
Effect of dapsone on VCR-induced weight loss
The results showed that there was a significant difference among the groups (P < 0.0001). The follow-up Tukey analysis revealed that during one week, while in the control group, body weight increased due to normal feeding, a significant weight loss was observed in rats receiving VCR due to decreased feeding and VCR-induced severe diarrhea (P < 0.0001). However, in the group receiving dapsone + VCR, dapsone attenuated weight loss, and diarrhea, besides increasing food intake in comparison with the VCR group (Fig. 1) (P < 0.001). No mortality was observed during this experiment.
Fig. 1.

The effect of dapsone (12.5 mg/kg IP) on VCR-induced (0.5 mg/kg IP) weight loss. Data are presented as Mean ± SEM (n = 6). (****P < 0.0001 compared to the control group) (### P < 0.001 compared to VCR)
There was no significant difference between the control and PEG groups. Therefore, the data of the PEG group was not included in the following figures.
Effect of dapsone on VCR-induced neuropathy (behavioral and MNCV tests)
Regarding Fig. 2A, there was no significant difference in the thermal threshold between groups on day one. Similarly, on day seven, there was no significant difference between the control and dapsone groups. On the other hand, VCR significantly increased the thermal threshold on day seven compared to the control group (P < 0.0001). However, in dapsone + VCR thermal threshold decreased significantly when compared to the VCR group (P < 0.01).
Fig. 2.
The effect of dapsone (12.5 mg/kg IP) on VCR-induced (0.5 mg/kg IP) neuropathy ((A)hot plate, (B)Von Frey, (C)MNCV) tests. Data are presented as Mean ± SEM (n = 6). (A) (**** P < 0.0001 compared to the control group on day 7) (## P < 0.01 compared to the VCR group on day 7). (B) (**P < 0.05 compared with the control group on day 7) (##P < 0.01 compared with VCR on day 7). (C) (****P < 0.0001 compared to the control group) (###P < 0.001 compared to VCR group)
As shown in Fig. 2B, there was no significant difference in the mechanical threshold on the first day of the experiment between groups (P = 0.873). Also, on the seventh day, no significant change was observed in the control and dapsone groups. However, there was a significant difference between VCR and the control group on the seventh day (P < 0.05). In addition, the results showed that dapsone on day seven significantly decreased the mechanical threshold when administered concurrently with VCR (P < 0.01).
The results of sciatic nerve conduction velocity on day seven, presented in Fig. 2C, demonstrated that the difference among the groups was significant (P = 0.000). The posthoc Tukey analysis revealed that VCR attenuated the nerve conduction compared to the control group (P < 0.0001). In contrast, dapsone co-administration with VCR prevented the reducing effect of VCR on nerve conduction (P < 0.001).
Molecular study results
Effect of dapsone on VCR-induced inflammation
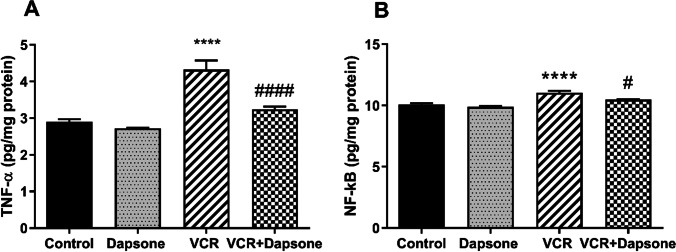
The ELISA results of this study revealed a significant difference in TNF-α levels among groups (P = 0.000). The TNF-α level was significantly elevated in the VCR group compared to the control group (P < 0.01). On the other hand, concurrent administration of dapsone with VCR significantly attenuated the TNF-α elevation in this group compared to the VCR group (P < 0.05) (Fig. 3A). Moreover, the one-way ANOVA test showed a significant difference in the NF-kB level among groups (P = 0.000). Compared to the control group, the NF-kB level rose significantly in the group receiving VCR (P < 0.05). Dapsone prevented the NF-kB elevation in the VCR + dapsone group (Fig. 3B).
Fig. 3.
The effect of dapsone (12.5 mg/kg IP) on VCR-induced (0.5 mg/kg IP) inflammation in the sciatic nerve. Data are presented as Mean ± SEM (n = 6). (A) TNF- α. (****P < 0.01 compared to the control group) (####P < 0.05 compared to VCR). (B) NF-kB. (****P < 0.05 compared to the control group) (#P < 0.05 compared to the VCR group)
Effect of dapsone on VCR-induced oxidative stress
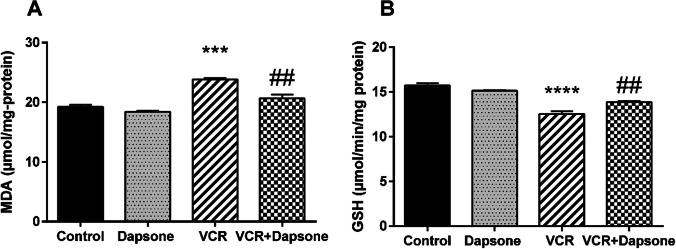
Regarding Fig. 4A, the results indicated a significant difference among groups (P = 0.000). While VCR significantly increased the MDA level compared to the control group (P < 000.1), dapsone and VCR co-administration significantly reduced the level of MDA in comparison with the VCR group (P < 0.01). In addition, results showed a significant difference in the GSH level of groups (P = 0.000). GSH levels significantly declined in the VCR group compared to the control group (P < 0.0001). However, dapsone raised the level of GSH when used simultaneously with VCR (P < 0.01) (Fig. 4B).
Fig. 4.
The effect of dapsone (12.5 mg/kg IP) on VCR-activated (0.5 mg/kg IP) oxidation in the sciatic nerve. Data are presented as Mean ± SEM (n = 6). (A) MDA. (***P < 0.001 compared to the control group) (##P < 0.01 compared to the VCR group). (B) GSH. (****P < 0.0001 compared to the control group) (##P < 0.01 compared to the VCR group)
Effect of dapsone on VCR-induced apoptosis
The apoptotic pathway studies in DRG showed a significant difference in groups’ caspase-3 activity (P = 0.000). Caspase-3 activity increased in the VCR group compared to the control group (P < 0.0001). However, a significant reduction of caspase-3 activity was observed in the VCR + dapsone group compared to the VCR group (P < 0.01) (Fig. 5).
Fig. 5.

The effect of dapsone (12.5 mg/kg IP) on VCR-induced (0.5 mg/kg IP) apoptotic pathway (caspase-3) in DRG. Data are presented as Mean ± SEM (n = 6). (****P < 0.0001 compared to the control group) (##P < 0.001 compared to the VCR group)
Histological study results
Histological study results showed severe degeneration and neuronal cell enlargement of the sciatic nerve in the VCR group compared to the control group. However, in the group receiving dapsone + VCR, the neuronal degeneration was attenuated and as shown the vacuolization was reduced (Fig. 6). There was no significant difference between dapsone and the control group.
Fig. 6.
Histopathological assessment of the effect of dapsone on VIPN in the sciatic nerve. (A-D) The H&E photographs of the sciatic nerve section: (A) The Control group showed normal nerve fibers (40x). (B) Dapsone group had no significant difference compared to the control group (40x). (C) VCR- treated group showed degeneration and vacuolization of the neuronal cells (40x). (D) In Dapsone-VCR treated group the degenerative changes were decreased, and the vacuolization was reduced (40x)
Discussion
This research was conducted to evaluate the protective effects of dapsone on VIPN. The results showed that dapsone attenuates VIPN through three critical mechanisms, including inflammatory, oxidative, and apoptotic pathways. VCR is extensively included in multi-drug chemotherapies (such as leukemias), however, its use is limited due to peripheral neuropathy [10]. On the other hand, dapsone is mainly used as preventive therapy for pneumocystis carinii pneumonia (PCP) in children with ALL [2]. Regarding the high rate of simultaneous use of both drugs in ALLs, evaluating the effect of dapsone on VIPN was of great importance [28].
In this study, VCR’s early toxic effect was observed as weight loss and diarrhea [8, 29]. However, dapsone decreased weight loss and increased food intake in rats. In addition, the results of behavioral tests (hot plate and Von Frey) showed that high dose VIPN is associated with hypoalgesia and loss of sensation. Similarly, histopathological and MNCV results confirmed the VCR-induced neuronal degeneration and reduced conduction in the sciatic nerve (observed as increased thermal and mechanical threshold) [30, 31]. Our results were in line with previous studies, which showed that VCR induces hypoalgesia in higher and cumulative doses [4, 5, 32, 33], and VCR-induced thermal hypoalgesia is accompanied by decreased nerve velocity [9, 30]. Also, altered DRG function following VCR administration may have a second role in impaired nerve conduction [34]. Our results showed that concurrent use of dapsone attenuates hypoalgesia and increases the nerve conduction velocity, by preventing axonal degeneration. Moreover, this study suggests anti-inflammatory, anti-oxidant, and anti-apoptotic effects as underlying mechanisms of dapsone neuroprotection.
For molecular evaluations, TNF-α and NF-kB levels were selected as critical molecules of VIPN. In this network, not only does TNF-α acts as a proinflammatory cytokine but also it activates the NF-kB, the main transcription factor of inflammation-related genes [35]. TNF-α directly enhances chemotaxis and activation of neutrophils, up-regulates the expression of adhesion molecules, and converts xanthine dehydrogenase into xanthine oxidase, resulting in ROS generation [36]. The elevated ROS activates ERK1/2, which induces apoptosis by increasing Bax’s and caspase-3 expression levels [37]. Also, the interaction of TNF-α with TNFR activates the (TNF receptor − associated death domain) TRADD, which in turn switches on apoptosis and raises the caspase-3 levels [38]. Regarding the mentioned interactions, this research was conducted to evaluate the role of three pathways in VIPN.
Elevated levels of pro-inflammatory cytokines in the sciatic nerve alter neuronal and glial cell function. Inflammation inhibits myelin formation by damaging Schwann cells. Consequently, injured Schwann cells are the new source of pro-inflammatory cytokines such as TNF-α and IL-1β, which cause repeated inflammation cycles following VCR injection [39]. It has been reported that antibodies against inflammatory cytokines such as TNF-α and IL-6 attenuate VIPN [40]. In this study, dapsone co-administration reduced the TNF-α level. Also, the reduction of NF-kB level showed greater effects of dapsone, preventing the initiation of oxidant and apoptotic cascades. Similar anti-inflammatory effects of dapsone were reported in spinal cord injuries and seizure attacks [41, 42].
Besides the inflammatory pathway, it has been shown that oxidant pathways are involved in VIPN. Following VCR injection the ROS elevation in DRG leads to neuronal cytotoxicity [43]. Our study showed that while VCR enhanced the level of MDA as an oxidant factor, it decreased the GSH anti-oxidant level in the sciatic nerve. Similar changes were observed in VCR-induced nephrotoxicity [44]. Besides the enhanced oxidant pathway, VCR-induced mitochondrial membrane loss causes ROS formation [45]. In this study, VCR-induced oxidation was accompanied by increased caspase-3 activity in DRG. However, dapsone exerted anti-oxidant effects by decreasing the MDA and increasing the GSH level in the sciatic nerve. Diaz-Ruiz et al. showed that dapsone reduces seizure episodes by inhibiting lipid-peroxidation, one of the main initiators of the oxidation pathway [13]. Also, dapsone’s anti-oxidant effects have been described in preventing neurodegenerative diseases [46]. On the other hand, in our study dapsone reduced the caspase-3 activity and therefore inhibited apoptosis in DRG. Inactivating apoptotic factors such as caspase-3 are similarly involved in delaying excitotoxic neuronal cell death by dapsone [47].
Taken together, the results confirmed that a network of inflammatory, oxidative, and apoptotic pathways are involved in VIPN and dapsone protects neuronal cells against VIPN by interfering in all three pathways. However, regarding the critical role of inflammation in inducing the other pathways, it is suggested that TNF-α and NF-kB reduction plays a major role in the neuroprotective effects of dapsone. Also, it should be mentioned that our findings were consistent with previous studies that demonstrated dapsone as a neuroprotective agent in neurodegenerative diseases due to its antioxidant, anti-inflammatory, and antiapoptotic effects [41, 46, 47].
Regarding the limitations, the detailed molecular pathways were not evaluated in this study. Further research can help to identify the exact role of mentioned pathways on neuronal and nonneuronal cells involved in VIPN. Also, the possible effects of dapsone on peripheral neuropathies with different etiologies can be studied.
Conclusion
In conclusion, our study showed that dapsone is effective in reducing VIPN behavioral symptoms. Also, it was found that dapsone interferes in molecular mechanisms involved in VIPN including inflammatory, oxidant, and apoptotic pathways.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We appreciate Gilaranco Co. for providing dapsone powder for this research. Special thanks go to the Mehr Laboratory for their help.
Funding
This study was supported by the National Institute for Medical Research Development [Grant No 971024] and Iran National Science Foundation (INSF).
Data availability
All data details will be available upon request.
Declarations
Conflicts of interest
The authors have no conflicts of interest to disclose.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mora E, et al. Vincristine-induced peripheral neuropathy in pediatric cancer patients. Am J Cancer Res. 2016;6(11):2416. [PMC free article] [PubMed] [Google Scholar]
- 2.Williams S, et al. Methemoglobinemia in children with acute lymphoblastic leukemia (ALL) receiving dapsone for pneumocystis carinii pneumonia (PCP) prophylaxis: a correlation with cytochrome b5 reductase (Cb5R) enzyme levels. Pediatr Blood Cancer. 2005;44(1):55–62. doi: 10.1002/pbc.20164. [DOI] [PubMed] [Google Scholar]
- 3.Li S, et al. The transcriptional landscape of dorsal root ganglia after sciatic nerve transection. Sci Rep. 2015;5(1):1–13. doi: 10.1038/srep16888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starobova H, et al. Minocycline prevents the development of mechanical allodynia in mouse models of vincristine induced peripheral neuropathy. Front Neurosci. 2019;13:653. doi: 10.3389/fnins.2019.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Authier N, et al. Pain related behaviour during vincristine-induced neuropathy in rats. NeuroReport. 1999;10(5):965–968. doi: 10.1097/00001756-199904060-00013. [DOI] [PubMed] [Google Scholar]
- 6.Boehmerle W, et al. Electrophysiological, behavioral and histological characterization of paclitaxel, cisplatin, vincristine and bortezomib-induced neuropathy in C57Bl/6 mice. Sci Rep. 2014;4:6370. doi: 10.1038/srep06370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuda Y, Li Y, Segal RA. A mechanistic understanding of axon degeneration in chemotherapy-induced peripheral neuropathy. Front Neurosci. 2017;11:481. doi: 10.3389/fnins.2017.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soliman A, et al. Study of the possible synergistic protective effects of Melatonin and Pregabalin in Vincristine induced peripheral neuropathy Wistar Albino rats. Life Sci. 2020;244:117095. doi: 10.1016/j.lfs.2019.117095. [DOI] [PubMed] [Google Scholar]
- 9.Khalilzadeh M, et al. The protective effects of sumatriptan on vincristine-induced peripheral neuropathy in a rat model. Neurotoxicology. 2018;67:279–286. doi: 10.1016/j.neuro.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Starobova H, Vetter I. Pathophysiology of chemotherapy-induced peripheral neuropathy. Front Mol Neurosci. 2017;10:174. doi: 10.3389/fnmol.2017.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang N, et al. Protective effect of dapsone on cognitive impairment induced by propofol involves hippocampal autophagy. Neurosci Lett. 2017;649:85–92. doi: 10.1016/j.neulet.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Ríos C, et al. Anti-apoptotic effects of dapsone after spinal cord injury in rats. Neurochem Res. 2015;40(6):1243–1251. doi: 10.1007/s11064-015-1588-z. [DOI] [PubMed] [Google Scholar]
- 13.Diaz-Ruiz A, et al. Antioxidant, anticonvulsive and neuroprotective effects of dapsone and phenobarbital against kainic acid-induced damage in rats. Neurochem Res. 2013;38(9):1819–1827. doi: 10.1007/s11064-013-1087-z. [DOI] [PubMed] [Google Scholar]
- 14.Dejban P, et al. Beneficial effects of dapsone on ischemia/reperfusion injury following torsion/detorsion in ipsilateral and contralateral testes in rat. Theriogenology. 2019;140:136–142. doi: 10.1016/j.theriogenology.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Rashidian A, et al. Dapsone reduced acetic acid-induced inflammatory response in rat colon tissue through inhibition of NF-kB signaling pathway. Immunopharmacol Immunotoxicol. 2019;41(6):607–613. doi: 10.1080/08923973.2019.1678635. [DOI] [PubMed] [Google Scholar]
- 16.Barzegar-Fallah A, et al. The neuroprotective effect of tropisetron on vincristine-induced neurotoxicity. Neurotoxicology. 2014;41:1–8. doi: 10.1016/j.neuro.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Vera G, et al. Involvement of cannabinoid signaling in vincristine-induced gastrointestinal dysmotility in the rat. Front Pharmacol. 2017;8:37. doi: 10.3389/fphar.2017.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ríos C, et al. Efficacy of dapsone administered alone or in combination with diazepam to inhibit status epilepticus in rats. Brain Res. 2019;1708:181–187. doi: 10.1016/j.brainres.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 19.Helton DR, et al. Pharmacokinetic profiles in rats after intravenous, oral, or dermal administration of dapsone. Drug Metab Dispos. 2000;28(8):925–929. [PubMed] [Google Scholar]
- 20.Zuidema J, Hilbers-Modderman E, Merkus F. Clinical pharmacokinetics of dapsone. Clin Pharmacokinet. 1986;11(4):299–315. doi: 10.2165/00003088-198611040-00003. [DOI] [PubMed] [Google Scholar]
- 21.Nelson RL. The comparative clinical pharmacology and pharmacokinetics of vindesine, vincristine, and vinblastine in human patients with cancer. Med Pediatr Oncol. 1982;10(2):115–127. doi: 10.1002/mpo.2950100202. [DOI] [PubMed] [Google Scholar]
- 22.Khan J, et al. Attenuation of vincristine-induced neuropathy by synthetic cyclohexenone-functionalized derivative in mice model. Neurol Sci. 2019;40:1799–1811. doi: 10.1007/s10072-019-03884-6. [DOI] [PubMed] [Google Scholar]
- 23.Farsi L, Keshavarz M, Afshari K, Javidan AN. Intravenous granulocyte colony-stimulating factor administration can attenuate neuropathic pain following spinal cord injury in male rats. Acta Med Iran. 2018;56(4):226–33. [Google Scholar]
- 24.Ja’afer FM, Hamdan FB, Mohammed FH. Vincristine-induced neuropathy in rat: electrophysiological and histological study. Exp Brain Res. 2006;173(2):334–345. doi: 10.1007/s00221-006-0499-2. [DOI] [PubMed] [Google Scholar]
- 25.Shen H, et al. An integrated cell isolation and purification method for rat dorsal root ganglion neurons. J Int Med Res. 2019;47(7):3253–3260. doi: 10.1177/0300060519855585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buege JA, Aust SD. [30] Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/S0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 27.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 28.Jain P, et al. Vincristine-induced neuropathy in childhood ALL (acute lymphoblastic leukemia) survivors: prevalence and electrophysiological characteristics. J Child Neurol. 2014;29(7):932–937. doi: 10.1177/0883073813491829. [DOI] [PubMed] [Google Scholar]
- 29.Upmanyu R, Dvivedi J, Saxena Y. Hepatotoxic effects of vincristine: an experimental study on albino rats. Indian J Physiol Pharmacol. 2009;53(3):265–270. [PubMed] [Google Scholar]
- 30.Geisler S, et al. Prevention of vincristine-induced peripheral neuropathy by genetic deletion of SARM1 in mice. Brain. 2016;139(12):3092–3108. doi: 10.1093/brain/aww251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brigo F, et al. Vincristine-related neuropathy versus acute inflammatory demyelinating polyradiculoneuropathy in children with acute lymphoblastic leukemia. J Child Neurol. 2012;27(7):867–874. doi: 10.1177/0883073811428379. [DOI] [PubMed] [Google Scholar]
- 32.Boyle FM, Wheeler HR, Shenfield GM. Glutamate ameliorates experimental vincristine neuropathy. J Pharmacol Exp Ther. 1996;279(1):410–415. [PubMed] [Google Scholar]
- 33.Cavaletti G, et al. The Total Neuropathy Score as an assessment tool for grading the course of chemotherapy-induced peripheral neurotoxicity: comparison with the National Cancer Institute-Common Toxicity Scale. J Peripher Nerv Syst. 2007;12(3):210–215. doi: 10.1111/j.1529-8027.2007.00141.x. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, et al. Sensitization of TRPV1 receptors by TNF-α orchestrates the development of vincristine-induced pain. Oncol Lett. 2018;15(4):5013–5019. doi: 10.3892/ol.2018.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang H, et al. Effect of NF-kB signaling pathway on the expression of MIF, TNF-α, IL-6 in the regulation of intervertebral disc degeneration. J Musculoskelet Neuronal Interact. 2018;18(4):551. [PMC free article] [PubMed] [Google Scholar]
- 36.Adjuto-Saccone M, et al. TNF-α induces endothelial–mesenchymal transition promoting stromal development of pancreatic adenocarcinoma. Cell Death Dis. 2021;12(7):1–15. doi: 10.1038/s41419-021-03920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death–apoptosis, autophagy and senescence. FEBS J. 2010;277(1):2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 38.Malleo G, et al. TNF-alpha as a therapeutic target in acute pancreatitis–lessons from experimental models. Sci World J. 2007;7:431–448. doi: 10.1100/tsw.2007.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Topp KS, Tanner KD, Levine JD. Damage to the cytoskeleton of large diameter sensory neurons and myelinated axons in vincristine-induced painful peripheral neuropathy in the rat. J Comp Neurol. 2000;424(4):563–576. doi: 10.1002/1096-9861(20000904)424:4<563::AID-CNE1>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 40.Kiguchi N, et al. The critical role of invading peripheral macrophage-derived interleukin-6 in vincristine-induced mechanical allodynia in mice. Eur J Pharmacol. 2008;592(1–3):87–92. doi: 10.1016/j.ejphar.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Diaz-Ruiz A, et al. Delayed administration of dapsone protects from tissue damage and improves recovery after spinal cord injury. J Neurosci Res. 2011;89(3):373–380. doi: 10.1002/jnr.22555. [DOI] [PubMed] [Google Scholar]
- 42.Afshari K, et al. Antibiotics with therapeutic effects on spinal cord injury: a review. Fundam Clin Pharmacol. 2021;35(2):277–304. doi: 10.1111/fcp.12605. [DOI] [PubMed] [Google Scholar]
- 43.Muthuraman A, et al. Ameliorative effects of amiloride and pralidoxime in chronic constriction injury and vincristine induced painful neuropathy in rats. Eur J Pharmacol. 2008;587(1–3):104–111. doi: 10.1016/j.ejphar.2008.03.042. [DOI] [PubMed] [Google Scholar]
- 44.Shati AA. Sub-chronic administration of vincristine sulfate induces renal damage and apoptosis in rats via induction of oxidative stress and activation of Raf1-MEK1/2-Erk1/2 signal transduction. Int J Morphol. 2019;37(1):273–283. doi: 10.4067/S0717-95022019000100273. [DOI] [Google Scholar]
- 45.Groninger E, et al. Vincristine induced apoptosis in acute lymphoblastic leukaemia cells: a mitochondrial controlled pathway regulated by reactive oxygen species? Int J Oncol. 2002;21(6):1339–1345. doi: 10.3892/ijo.21.6.1339. [DOI] [PubMed] [Google Scholar]
- 46.Diaz-Ruiz A, Nader-Kawachi J, Calderón-Estrella F, Mata-Bermudez A, Alvarez-Mejia L, Ríos C. Dapsone, an effective neuro, and cytoprotective drug and more. Curr Neuropharmacol. 2022;20(1):194–210. doi: 10.2174/1570159X19666210617143108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahale A, et al. Dapsone prolong delayed excitotoxic neuronal cell death by interacting with proapoptotic/survival signaling proteins. J Stroke Cerebrovasc Dis. 2020;29(8):104848. doi: 10.1016/j.jstrokecerebrovasdis.2020.104848. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data details will be available upon request.