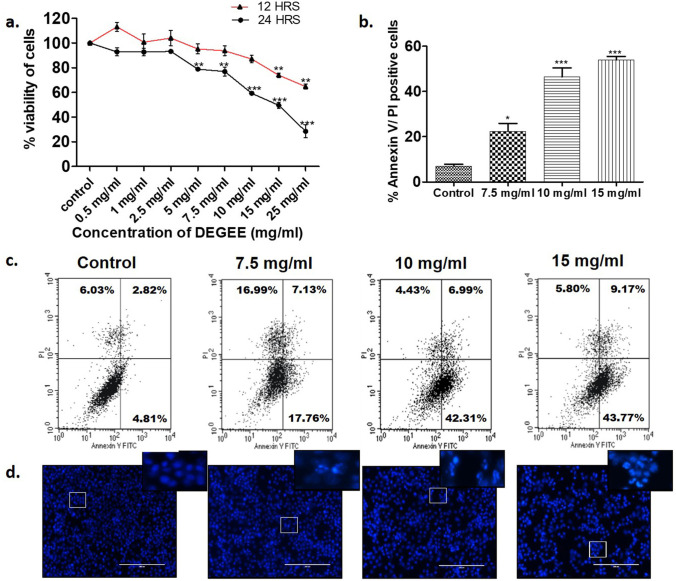
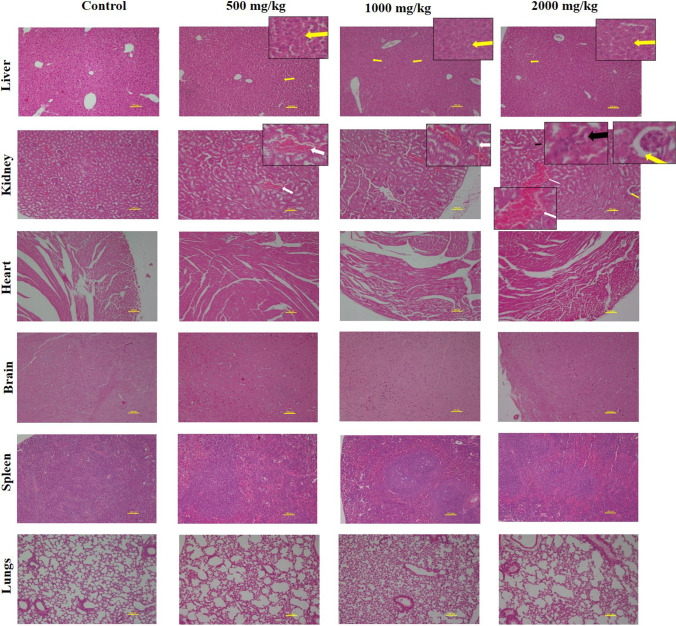
Sonal Srivastava
Sonal Srivastava
1Division of Toxicology and Experimental Medicine, CSIR- Central Drug Research Institute, Lucknow, 226031 Uttar Pradesh India
1,
Sakshi Mishra
Sakshi Mishra
1Division of Toxicology and Experimental Medicine, CSIR- Central Drug Research Institute, Lucknow, 226031 Uttar Pradesh India
1,
Jayant Dewangan
Jayant Dewangan
1Division of Toxicology and Experimental Medicine, CSIR- Central Drug Research Institute, Lucknow, 226031 Uttar Pradesh India
1,
Aman Divakar
Aman Divakar
1Division of Toxicology and Experimental Medicine, CSIR- Central Drug Research Institute, Lucknow, 226031 Uttar Pradesh India
1,
Nidhi Gupta
Nidhi Gupta
1Division of Toxicology and Experimental Medicine, CSIR- Central Drug Research Institute, Lucknow, 226031 Uttar Pradesh India
1,
Navodayam Kalleti
Navodayam Kalleti
1Division of Toxicology and Experimental Medicine, CSIR- Central Drug Research Institute, Lucknow, 226031 Uttar Pradesh India
1,
Madhav Nilakanth Mugale
Madhav Nilakanth Mugale
1Division of Toxicology and Experimental Medicine, CSIR- Central Drug Research Institute, Lucknow, 226031 Uttar Pradesh India
1,
Sadan Kumar
Sadan Kumar
1Division of Toxicology and Experimental Medicine, CSIR- Central Drug Research Institute, Lucknow, 226031 Uttar Pradesh India
1,
Sharad Sharma
Sharad Sharma
1Division of Toxicology and Experimental Medicine, CSIR- Central Drug Research Institute, Lucknow, 226031 Uttar Pradesh India
1,
Srikanta Kumar Rath
Srikanta Kumar Rath
1Division of Toxicology and Experimental Medicine, CSIR- Central Drug Research Institute, Lucknow, 226031 Uttar Pradesh India
1,✉