Abstract
Background
Recently biodegradable nanoparticles are the center of attention for the development of drug delivery systems. Molecularly imprinted polymer (MIP) is an interesting candidate for designing drug nano-carriers. MIP-based nanoparticles could be used for cancer treatment and exhibited the potential to fill gaps regarding to ligand-based nanomaterials. Also, the presence of a cross-linker can play an essential role in nanoparticle stability and physicochemical properties of nanoparticles after synthesis.
Objectives
In this research, a biodegradable drug delivery system based on MIP nanoparticles was prepared using a biodegradable cross-linker (dimethacryloyl hydroxylamine, DMHA) for methotrexate (MTX). A hydrolysable functional group CO–O-NH-CO was added to the crosslinking agent to increase the final biodegradability of the polymer.
Methods
Firstly, a biodegradable cross-linker was synthesized. Then, the non-imprinted polymers were prepared through mini-emulsion polymerization in the absence of a template; and efficient particle size distribution was determined. Finally, methotrexate was placed in imprinted polymers to achieve the desired MIP. Different types of MIPs were synthesized using different molar ratios of template, cross-linker, and functional monomer, and the optimal molar ratio was obtained at 1:4:20, respectively.
Results
HNMR successfully confirmed the chemical structure of the cross-linker. According to SEM images, nanoparticles had a spherical shape with a smooth surface. The imprinted nanoparticles showed a narrow size distribution with an average of 120 nm at a high ratio of cross-linker. The drug loading and entrapment efficiency were 6.4% and 92%, respectively. The biodegradability studies indicated that the nanoparticles prepared by DMHA had a more degradability rate than ethylene glycol dimethacrylate as a conventional cross-linker. Also, the polymer degradation rate was higher in alkaline environments. Release studies in physiological and alkaline buffer showed an initial burst release of a quarter of loaded MTX during the day and a 70% release during a week. The Korsmeyer-Peppas model described the release pattern. The cytotoxicity of MTX loaded in nanoparticles was studied on the MCF-7 cell line, and the IC50 was 3.54 μg/ml.
Conclusion
It was demonstrated that nanoparticles prepared by DMHA have the potential to be used as biodegradable drug carriers for anticancer delivery.
Graphical abstract
Synthesis schema of molecular imprinting of methotrexate in biodegradable polymer based on dimethacryloyl hydroxylamine cross-linker, for use as nanocarrier anticancer delivery to breast tumor

Keywords: Molecularly imprinted polymers, Biodegradable, Drug delivery, Anticancer, Nanomedicine
Introduction
Nanocarriers for cancer therapy are improving rapidly and can be used to solve many limitations of conventional delivery systems such as non-specific biodistribution, low water solubility, and bioavailability [1]. Nanoparticles have been fabricated in optimal size and surface characteristics to carry their loaded drugs to cancer cells selectively using the unique pathophysiology of tumors [2].
Among different materials, biodegradable polymers could be used to produce controlled delivery systems that significantly increase patient compliance and reduce side effects. Biodegradable polymers with unstable bonds like ester, amide, and anhydride bonds are susceptible to enzymatic degradation or hydrolysis [3, 4]. In this regard, the biodegradable imprinted polymers have not been widely used for anticancer delivery yet [5–7].
Molecularly imprinted polymers (MIPs) can play an essential role in developing drug delivery systems [8]. MIP-based nanoparticles can be an alternative to other nanomaterials for cancer treatment and showed the ability to fill gaps related to ligand-based nanomedicine, such as immunogenicity, stability, applicability, and economic viability [8]. MIPs are practical recognition systems that can imitate natural recovery entities such as biological receptors, precisely designed to distinguish complicated samples, such as environmental and biological fluids [9]. MIPs provide a fascinating platform for introducing definite molecular arrangements into polymeric matrices [10]. Also, MIP has been reported as a controlled release device with a narrow therapeutic index [11]. Due to their high selectivity and affinity, MIPs can be implemented widely as artificial synthetic polymers for use in molecular recognition. These systems could be prepared by polymerization of a mixture of functional monomers and target molecules (template). The interaction between functional groups of the template, functional monomers, and cross-linkers led to template removal from the crosslinked polymer matrix [12]. The generation of cavities in MIPs structure can determine the size and shape of their template and functional group placement. MIPs have demonstrated wide applications in various fields, such as analytical separation [13], solid-phase extraction [14], detection of chemicals [15], antimicrobial field, stationary chromatographic phase, and biosensor [16] to identify and separate molecules with simple structures [13].
In drug delivery, it is better to apply a cross-linker agent in biodegradable polymers to accelerate the polymer degradation and hydrolysation process [14]. Also, the presence of a cross-linker can play an essential role in nanoparticles stability and physicochemical properties after synthesis [17]. Today, a lot of efforts are made in modern pharmacy to improve the pharmacological output of drugs and reduce the side effects of the drug. Controlled release systems play an important role in delivering pharmacological and biological agents at specific and different points in the body and releasing them at a controlled and optimal rate in this respect [18].
Methotrexate (MTX) as a folic acid derivative with anticancer activity, can widely use in the clinical treatment of several solid cancers such as breast, head and neck, lung, and leukemia. MTX has been shown immunosuppressive activity, which may be a result of inhibition of lymphocyte multiplication [17, 18]. A lot of research on MTX as a target molecule in molecularly imprinted polymers has been performed so far. But, in most of these studies, the focus of researchers has been on the method of preparation and methotrexate molecularly imprinted polymer in separation and purification [19, 20]. In this regard, other novel carriers have been used to minimize side effects and control methotrexate release, such as liposomes and nano lipid carriers, but the biodegradable imprinted polymers are not being used for anticancer delivery yet [21, 22].
In this study, a new polymeric carrier, based on biodegradable MIPs with a new cross-linker was prepared for the controlled release of methotrexate. The molar ratio of template to functional monomer is the most critical factor in molecularly imprinted polymers at non-covalent imprinting, but this ratio is uncertain; thus, the optimal ratio was obtained experimentally. Also, the hydrolysable functional groups were added to the crosslinking agent to increase the biodegradability of the final polymer. Firstly, a biodegradable cross-linker was synthesized. Then, the non-imprinted polymers were prepared through mini emulsion polymerization in the absence of a template; and efficient particle size distribution was determined. The purpose of NIP synthesis in this study is to compare it with MIP and show the superiority of MIP. Finally, methotrexate was placed in imprinted polymers to achieve the desired MIP. Different types of MIPs using different molar ratios among template, functional monomer, and cross-linker were synthesized and characterized in-vitro to evaluate the biodegradable MIP in methotrexate delivery.
Materials and methods
Materials
Methacryloyl chloride (≥ 97.0%, stabilized) and hydroxylamine hydrochloride were purchased from Sigma–Aldrich (Taufkirchen, Germany). Methacrylic acid was obtained from Merck (Darmstadt, Germany) and purified by distillation under reduced pressure at 50–55 °C before use to remove the polymerization inhibitors [23]. Ethylene glycol dimethacrylate and AIBN were purchased from Sigma-Aldrich (Steinheim, Germany). Methotrexate was obtained from the Ministry of Health and Medical Education (Tehran, Iran). Sodium dodecyl sulfate (SDS), chloroform, hexadecane, hydroxylamine hydrochloride were purchased from Sigma Aldrich. Pyridine, acetonitrile, and sodium azide was provided by Merck company. 10,000 U/mL solution of penicillin and 10,000 g/mL streptomycin were obtained from Bio Whittaker Europe (Verviers, Belgium), and polysorbate 80 (Tween 80) was obtained from ICI surfactants (Eversberg, Belgium). Fetal bovine serum, trypsin, ethylene diamine tetraacetic acid (EDTA), and RPMI1640 culture medium were from Gibco.
Dialysis tubes (Sigma dialysis tubes with cut-off 12 kDa) were prepared by heating in 2 wt% solution of sodium bicarbonate and 0.05 wt% of ethylene diamine tetraacetic acid (EDTA) and then kept in 0.05 wt% of sodium azide under refrigeration until they were used. All solvents used in chromatography analysis were HPLC grade and supplied by Merck. Other reagents and solvents were used without further purification.
Synthesis of N, O-dimethacryloyl hydroxylamine as a biodegradable cross-linker
N,O-dimethacryloyl hydroxylamine was synthesized following Yin et al. [24]. Briefly, distilled methacryloyl chloride (53 °C, 130 mbar) was added to a solution of hydroxylamine hydrochloride in pyridine drop-wise and stirred at 25–30 °C for 4 h. This process is shown in Fig. 1. For the synthesis of dimethacryloyl hydroxylamine, 5.667 g of hydroxylamine hydrochloride was dissolved in 45 mL of pyridine. Then 17 mL of freshly distilled methacryloyl chloride was added dropwise to it, and the resulted mixture was stirred at 30 °C for 6 h. Then the reaction mixture was added to dichloromethane, neutralized with HCl, and finally, it was extracted 4 times with distilled water. The oily product was obtained by drying with 18 gr of MgSO4 and evaporation of dichloromethane. The reaction product was solved in diethyl ether and crystallized by successive addition of n-heptane [25].
Fig. 1.
The synthesize reaction of N,O-dimethacryloyl hydroxylamine preparation
Synthesis of biodegradable imprinted and non-imprinted nanoparticles
Biodegradable molecularly imprinted nanoparticles were fabricated by mini-emulsion polymerization [9, 10] under different conditions described in Table 1. To prepare the MTX-imprinted biodegradable polymer, the functional monomers (MAA), the biodegradable crosslinking monomer (DMHA), and hexadecane were mixed and stirred for 30 min. Afterward, MTX as a template molecule was added. The produced mixture was sonicated (Tecno-Gaz, Tecna 6, Italy) for 15 min to ensure complete dissolution of MTX and formation of monomer-template complex. AIBN was introduced to the mixture to initiate the radical polymerization reaction. The solution was gently agitated for 3 h. The organic phase was gradually injected through a syringe equipped with a 20-G Angiocatheter into 30 ml water containing SDS (1 wt%) and homogenized using a high-speed homogenizer (IKA, Ultra-Turrax, USA) at 24,000 rpm for 5 min. The resulted emulsion was purged with nitrogen for 5 min. Polymerization procedure was performed in a cylindrical laboratory reactor (100 mL) with a heating jacket and an anchor type stirrer (400 rpm) in 68 °C for 21 h. After polymerization, the obtained nanoparticles were separated by centrifugation (Sigma 3K30, Germany) for 20 min at 21,000 rpm. Nanoparticles were purified by dialysis over water, ethanol, and then ethanol containing 10% acetic acid (v/v) for five times and finally lyophilized (Christ, Alpha 2–4 LD, Germany) at -40 °C for 48 h to obtain a fine powder. The obtained nanoparticles were stored at − 20 °C before use. The extraction procedure of nanoparticles was followed by spectrometric analysis to be free from MTX. The non-imprinted polymer nanoparticles (NPs) were prepared identically according to the same polymerization procedure in the absence of MTX for control experiments [1].
Table 1.
Amounts of materials used in the preparation of biodegradable non-imprinted polymers prepared through miniemulsion polymerization
| Sample | MAA (mmol) |
AIBN (mmol) |
Cross-linker (mmol) |
Chloroform (mL) |
Hexadecane (µL) |
SDS (mL) |
|---|---|---|---|---|---|---|
| NIP1 | 1.6 | 0.093 | 1.6 | 2 | 25 | 30 |
| NIP2 | 0.8 | 0.05 | 2.4 | 2 | 25 | 30 |
| NIP3 | 0.8 | 0.05 | 2.4 | 2 | 25 | 30 |
| NIP4 | 0.8 | 0.05 | 2.4 | 1 | 12.5 | 30 |
Synthesis of non-degradable imprinted and non-imprinted nanoparticle
Imprinted NPs were prepared in the presence of MTX with EGDMA as crosslinking monomer during the polymerization process and treated under the same conditions. Non-imprinted NPs were prepared in the absence of MTX with EGDMA as a crosslinking monomer [1].
Characterization
Particle size and morphology
The particle size, size distribution, and zeta potential were measured by laser light scattering (Malvern Zeta Sizer ZS, Malvern, UK). In order to characterize the surface morphology and the shape of nanoparticles, scanning electron microscopy (SEM, LEO-1450 VP scanning electron microscope Carl Zeiss SMT AG, Oberkochen, Germany, 15 kV) was utilized. One drop of 1 mg/ml of nanoparticle dispersion was coated with gold on aluminum stubs under vacuum before the SEM measurement [26].
Differential scanning calorimetry
Thermal properties of biodegradable and non-degradable NPs were evaluated by a Mettler DSC 823 (Mettler Toledo, GmbH, Greifensee, Switzerland) equipped with a Julabo thermocryostate model FT100Y (Julabo Labortechnik GmbH, Germany). A Mettler Star software system (version 9.x) was utilized for data analysis. Indium was used to calibrate the instrument. The samples were scanned with a heating rate of 10 °C/min in a 20–400 °C temperature range [26].
HNMR and FTIR spectrum
H-NMR (Avanace 500 MHz; Bruker, Rheinstetten, Germany) was applied for chemical characterization of N,O-dimethacryloyl hydroxylamine. FTIR was employed to study the interaction between MTX and functional monomer and changes of polymer structure after template removal. A Shimadzu FT-IR 4300 spectrometer provided FT-IR spectrum with a scanning range from 4000 to 400 cm−1. Lyophilized samples were dispersed on a special disc with potassium bromide powder [26].
Drug loading and entrapment efficiency
In this step, 10 mg of freeze-dried nanoparticles were dispersed in 40 mL acetonitrile and incubated for 48 h based on previous studies [26]. Then this dispersion was centrifuged at 21,000 rpm (41,415 g) for 20 min. The supernatant was separated, and the drug concentration was measured by UV spectrophotometry at 302 nm [10].
Drug loading (DL) was the ratio of the relative amount of MTX in NPs to the whole weight of the NPs. The entrapment efficiency (EE) was obtained considering the mass ratio of entrapped MTX in NPs to the theoretical amount of MTX used in NPs synthesis. The experiments were performed in triplicate. The calculation equations have been shown below:
Binding capacity
In order to investigate the binding capacity, 50 mg washed biodegradable and non-biodegradable nanoparticles were poured into 8 ml of 40 μg/ml solution of methotrexate in acetonitrile/phosphate buffer with pH = 9 (ratio 1 to 19). Then, this mixture was dispersed for 4 min by ultrasonic and stirred gently for one hour. The particles were separated by centrifugation, and a UV spectrophotometer was used to measure the supernatant concentration of MTX. For each polymer, the amount of adsorbed drug through MIP and NIP particles has been obtained [11]. The calculation equation has been shown below:
In-vitro release experiments
Drug release from MTX-loaded nanoparticles was carried out using the dissolution method as fallows. 28 mg of biodegradable and non-biodegradable MIP nanoparticles were dispersed in 2 ml of 50 mM phosphate buffer saline solution (PBS) with pH 5.5 and 7.4 containing tween 80 (0.01 wt%). Phosphate-buffered saline (PBS) with pH = 5.5 (similar to cancer cells) and pH = 7.4 (the human blood pH) were selected as mediums. After more than 30 min stirring, nanoparticles were transferred to 12-kDa dialysis tubes that were sealed at both ends with medical clips. Next, this system was immersed in 33 ml of release medium explained above, and the outer solution of the dialysis bag was stirred at 200 rpm at 37 °C. At different time intervals, 33 ml of the external solution of dialysis bag was replaced with 33 ml of fresh PBS. The drug concentration in samples was determined by UV–Vis spectrophotometer at 302 nm wavelength [26].
Mechanism and kinetic study of drug release
Investigation of drug release kinetics has been performed for biodegradable and non-biodegradable molecularly imprinting polymers at different pHs based on four models, zero-order, first-order, Higushi, and Papas were performed. In the direct investigation of release kinetics, the obtained data were fitted to models of zero-order, first-order, Higuchi, and Pepas and based on kinetic coefficient models (ss) and the sum of the difference squares (r2) were investigated.
Degradation test
In vitro degradation test of nanoparticles was performed with 0.1% particle suspensions in 0.1 M PBS (pH 5.0, 7.4, and 9.0) in triplicate preserved by 0.02% sodium azide for the test period. Additionally, the SEM images were applied to detect the size and morphology of the prepared nanoparticles. The same procedure was carried out for non-biodegradable MIP nanoparticles. The suspensions were filled into injection vials which were sealed and incubated in a reciprocating water bath at 37 ◦C and 100 rpm. At specific time points, samples were collected, the remained MIP was rinsed with deionized water, and Z-average diameter was measured by laser light scattering. In order to determine the surface morphology of the particles and observe the degradation of the nanoparticles after 15 and 40 days, the particles were fixed, and their surface was coated with a gold layer to obtain their morphology by SEM [25]. The same procedure was done for non-degradable MIP nanoparticles [25].
Cell culture
The MTT assay was used for the evaluation of cytotoxicity of different formulations. MCF-7 (human breast cancer cell line) were cultured in DMEM cell culture medium supplemented with 10% FBS, 100 IU/mL penicillin, and 100 μg/mL streptomycin and seeded in 96-well plates (104 cells/well). The plate was incubated at 37 °C in a humidified atmosphere with 5% CO2. After 24 h, the medium was replaced with 100 μL fresh complete medium containing various concentrations (1, 10, 100, and 1000 μg/mL) of the nanoparticles without the drug [25].
After 72 h of incubation, the medium was replaced with 50 μL of MTT reagent (0.5 mg/mL in PBS) and further incubated for 4 h. The formazan crystals were dissolved in DMSO and the absorbance was read at 570 nm using a microplate reader. Cell viability was calculated by comparing the absorbance of treated cells at each concentration relative to untreated cells as a control group. The IC50 value (50% inhibitory concentration) was obtained for various treatments.
Results and discussion
Polymer synthesis
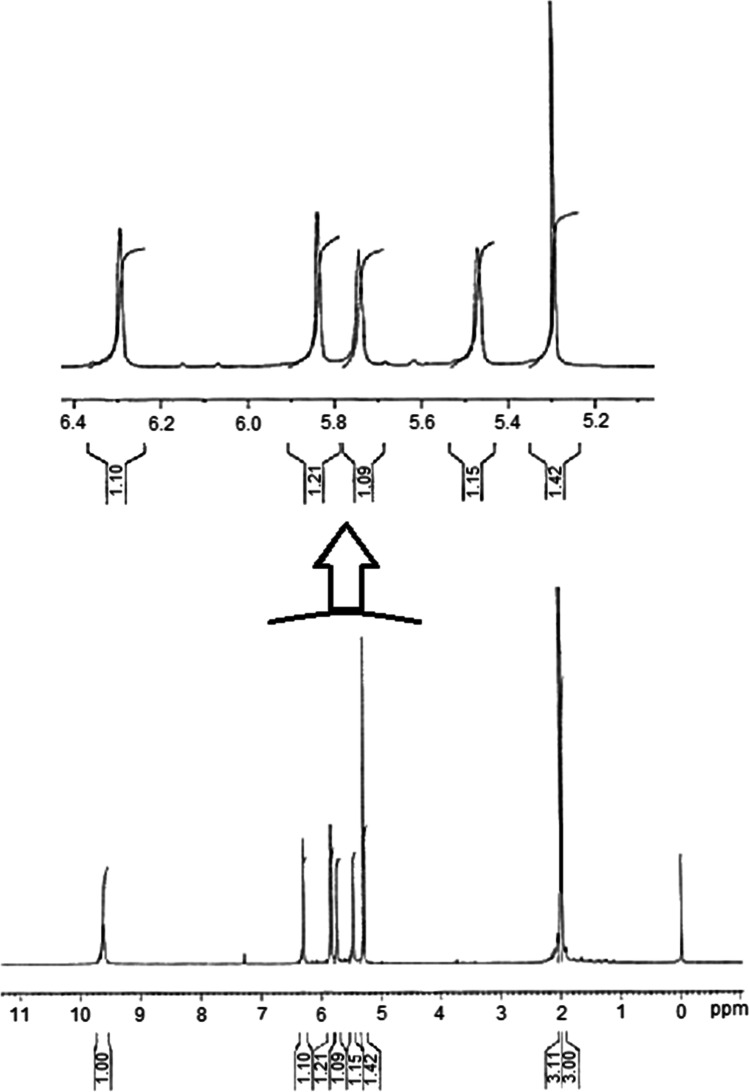
Proton NMR spectrum of synthesized N, O-dimethacryloyl hydroxylamine (DMHA) was achieved using 500 MHz NMR spectrometer.
As shown in Fig. 2, δ = 9.6 ppm is related to the proton attached to the nitrogen. Two peaks of δ = 2.007 and δ = 1.97 ppm are related to the methyl group, in which the δ = 2.007 ppm is associated with the methyl hydrogen close to nitrogen. Protons of methyl in 3H, N-CO–C (CH3) CH2 are near the oxygen atom in 1.97 ppm.
Fig. 2.
HNMR spectrum of N,O-dimethacryloyl hydroxylamine
Nanoparticle characterization
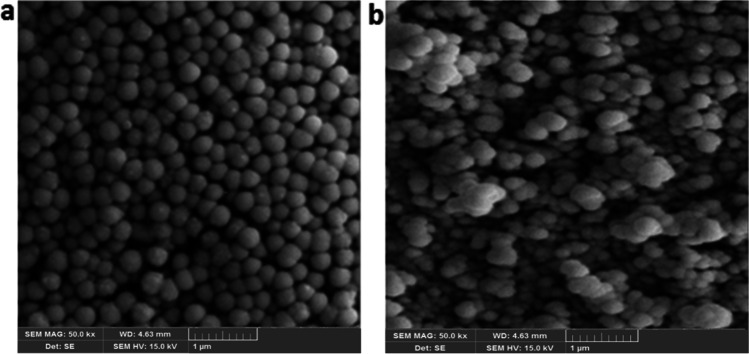
SEM method was employed to analyze the morphology of nanoparticles. As shown in Fig. 3, spherical nanoparticles with uniform size distribution were obtained by a biodegradable cross-linker.
Fig. 3.
SEM micrographs of (a, b) DMHA MIP, and (c, d) DMHA NIP nanoparticles
To study the particle size and their distribution, DLS was applied. As a result, NIP nanoparticles exhibited a smaller size than MIP nanoparticles, which could be caused by methotrexate and its hydrogen interaction in the reaction medium with the MAA functional monomer. This hydrogen interaction affects particle nucleation and growth which makes the larger MIP than NIP nanoparticles. Furthermore, reducing the amount of cross-linker or increasing the functional monomer concentration leads to a decrease in particle size. This phenomenon is consequent of the stability of particles due to electrostatic charge. The electrostatic charges produced by the carboxylic acid functional groups make the stable growing polymeric particles during the polymerization process (Repulsion forces between particles are high). The zeta potential was obtained -31.59 mV for these nanoparticles. The negative zeta potential may be attributed to carboxylic acid groups in polymeric nanoparticles. In addition, the molar ratio of the template-molecule: functional monomer: cross-linker shows a significant effect on the creation of specific sites in molecularly imprinted polymers, and the optimized molar ratio is determined depending on the template molecule spatial structure. Increasing cross-linker concentration and creation of cross-linked bonds in the polymer chain and, therefore, networking leads to an increase in the amount and strength of nanoparticles after synthesis and centrifugation. Besides, the best ratio was obtained 0.5 (MTX): 2 (MAA): 10 (DMHA).
Due to the novelty of cross-linker, the effect of functional monomer to cross-linker ratio was investigated. First, NIP1 with an approximate ratio of 1:1 (functional monomer to cross-linker) was synthesized, and after lyophilization, the nanoparticles were aggregated (Table 1). As shown in Tables 2 and 4, the particle size and polydispersity index of nanoparticles have increased. In the next step, for the preparation of NIP2, all amounts of aqueous phases were changed in the same proportion. Still, the monomer to cross-linker ratio was increased to 1:3 in order to improve the crosslinking of the polymeric chain and maintain its strong structure after lyophilization. However, the results indicated this ratio is not suitable due to particle aggregation and their inappropriate size. Mannitol could be added after synthesis and before freezing of nanoparticles to decrease particle aggregation. Mannitol with OH groups can place between crosslinks. DLS data of NIP3 showed that the particle size and polydispersity index of nanoparticles after freeze-drying is lower than NIP2. It is still higher than 0.5. Therefore, NIP4 with the same molar ratio but half content of chloroform and hexadecane were synthesized. DLS data shows that increasing the biodegradable cross-linker leads to a decrease in the particle size and polydispersity index.
Table 2.
Particle size and Polydispersity index of biodegradable non-imprinted polymers prepared through miniemulsion polymerization
| Sample | Particle size (nm) | Polydispersity index | ||
|---|---|---|---|---|
| Before freeze-drying | After freeze-drying | Before freeze drying | After freeze-drying | |
| NIP1 | 261 ± 2.82 | 899 ± 7.01 | 0.394 ± 0.005 | 0.822 ± 0.055 |
| NIP2 | 260 ± 4.50 | 377 ± 1.79 | 0.340 ± 0.007 | 0.685 ± 0.026 |
|
NIP3 (containing mannitol) |
260 ± 11.50 | 385 ± 8.79 | 0.340 ± 0.006 | 0.520 ± 0.015 |
| NIP4 | 132.88 ± 6.65 | 162 ± 5.82 | 0.200 ± 0.002 | 0.215 ± 0.004 |
Table 4.
Particle size and polydispersity index of biodegradable MTX-imprinted polymers prepared through miniemulsion polymerization
| Sample | Particle size (nm) | Polydispersity Index | ||
|---|---|---|---|---|
| Before freeze-drying | After freeze-drying | Before freeze Drying |
After freeze-drying | |
| MIP1 | 172 ± 6.57 | 1920 ± 156.50 | 0.305 ± 0.005 | 1 |
| MIP2 | 323 ± 13.35 | 661 ± 50.33 | 0.294 ± 0.003 | 0.556 ± 0.043 |
| MIP3 | 120 ± 5.05 | 497 ± 22.93 | 0.159 ± 0.003 | 0.697 ± 0.036 |
| MIP4 | 279 ± 10.03 | 661 ± 26.15 | 0.236 ± 0.009 | 0.505 ± 0.013 |
| MIP5 | 246 ± 9.43 | 516 ± 17.11 | 0.277 ± 0.006 | 0.562 ± 0.029 |
| MIP6 | 225 ± 6.93 | 377 ± 10.57 | 0.218 ± 0.006 | 0.424 ± 0.023 |
| MIP7 | 125 ± 7.11 | 323 ± 18.64 | 0.240 ± 0.007 | 0.407 ± 0.054 |
| MIP8 | 224 ± 6.86 | 153 ± 8.09 | 0.098 ± 0.001 | 0.134 ± 0.003 |
Biodegradable molecular imprinted polymer without drug and with EGDMA cross-linker in a molar ratio of 1:3 was prepared, and the nanoparticles showed an acceptable size and polydispersity index. Also, the amount of obtained sediment after centrifugation was adequate for physicochemical studies. Since methotrexate is insoluble in chloroform, DMSO was used as an organic phase solvent.
Considering the molar ratio of NIP4, imprinted polymers were synthesized in the presence of methotrexate (Tables 3 and 4). Still, the molar ratio of NIP4 was not appropriate for MIP in the presence of drug and DMSO. The cross-linker amounts were gradually increased to obtain the optimized molar ratio and reach suitable particle size and polydispersity index before and after lyophilization. Non-biodegradable MIPs with EGDMA as cross-linker were prepared according to NIP4 molecular ratios (1:3), (0.5:2:10) MIP8, and all of these showed an acceptable size and polydispersity index and the adequate sediment obtained after synthesis, centrifugation, and freezing of nanoparticles for physicochemical studies.
Table 3.
Amounts of materials used for preparation of biodegradable MTX-imprinted polymers prepared through miniemulsion polymerization
| Sample | MTX (mmol) | MAA (mmol) |
DMHA (mmol) |
AIBN (mmol) |
Hexadecane (µL) |
DMSO (mL) |
SDS (mL) |
|---|---|---|---|---|---|---|---|
| MIP1 | 0.5 | 0.8 | 2.5 | 0.064 | 12.5 | 1 | 30 |
| MIP2 | 0.5 | 2.0 | 3.5 | 0.064 | 12.5 | 1 | 30 |
| MIP3 | 0.5 | 2.0 | 4.0 | 0.064 | 12.5 | 1 | 30 |
| MIP4 | 0.5 | 2.0 | 4.5 | 0.064 | 12.5 | 1 | 30 |
| MIP5 | 0.5 | 2.0 | 5.0 | 0.064 | 12.5 | 1 | 30 |
| MIP6 | 0.5 | 2.1 | 6.0 | 0.064 | 12.5 | 1 | 30 |
| MIP7 | 0.5 | 2.1 | 8.0 | 0.076 | 12.5 | 1 | 30 |
| MIP8 | 0.5 | 2.1 | 10.0 | 0.098 | 12.5 | 1 | 30 |
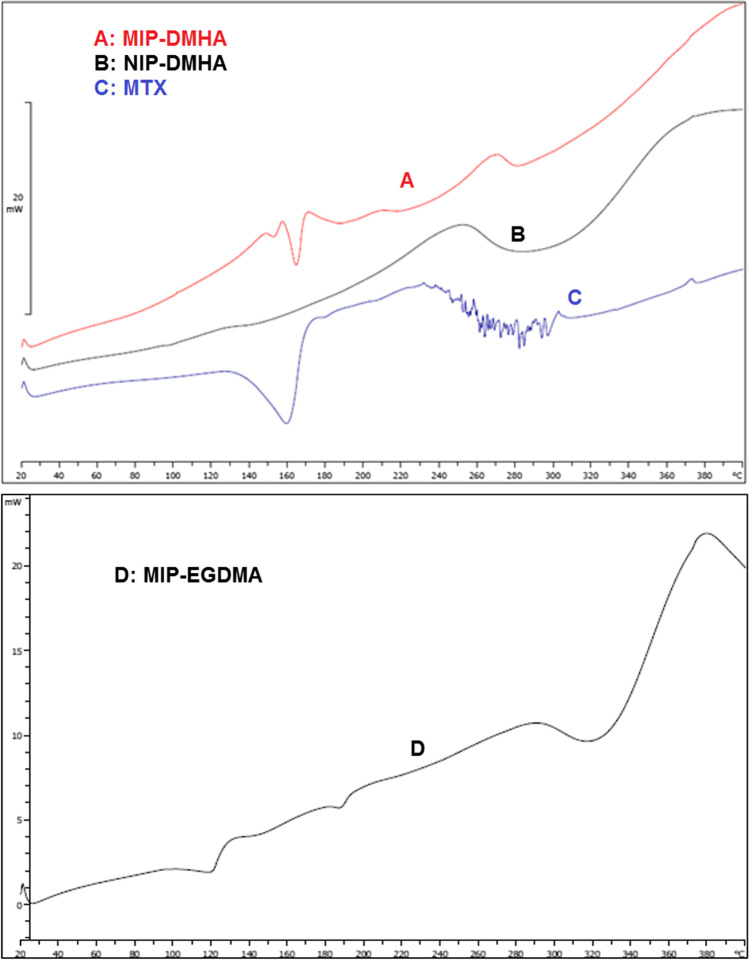
Figure 4 demonstrates the DSC thermogram of the NIP, the biodegradable MIP with DMHA cross-linker, and methotrexate. MIP and NIP polymers showed stability up to 83 °C. Their decomposition is started at 83 °C. DSC curve of MIP represents an endothermic change at 166 °C, which is related to the methotrexate present in these particles. In the 162 °C area, sharp endothermic peaks are associated with the melting point of the drug. The DSC shows that the drug was loaded to the MIP nanoparticles due to the lack of glass transition temperature and the absence of small peaks in the baseline.
Fig. 4.
DSC thermograms of a MIP nanoparticles with DMHA, b NIP nanoparticles without drug and with DMHA, c methotrexate, and d non-biodegradable MIP with EGDMA
As shown in Fig. 4d, non-biodegradable MIPs are stable at temperatures below 280 °C and start degrading from 280 °C. Therefore, MIPs prepared with EGDMA as cross-linker have very high-temperature stability versus biodegradable polymers.
The FT-IR analysis can be used to determination of functional groups in chemical compounds. In this investigation, the FT-IR spectroscopy was carried out for washed and unwashed MIP and NIP for a biodegradable polymer with a DMHA cross-linker (Fig. 5). The difference between the unwashed MIP spectrum with two other washed MIP, and NIP spectrum was seen at the 1150 cm−1 peak, and this peak is due to the stretching vibration of the C-N group in the methotrexate and the presence of the drug in unwashed MIP particles.
Fig. 5.
FT-IR spectrum of a biodegradable MIP without MTX, b washed MIP, c biodegradable MIP with MTX
The OH stretching vibration peak related to the carboxylic acid of washed MIP was found at 3530 cm−1. On the other hand, the unwashed MIP peak was detected at 3430 cm−1. The shift in OH stretching vibration is related to the hydrogen interaction of O–H in the carboxylic acid of the polymeric group with methotrexate. Also, the peaks of the carbonyl group show the same behavior. Stretching vibration in the washed MIP carbonyl group was detected at 1758 cm−1; however, due to the hydrogen interactions of carbonyl group in the unwashed MIP, this peak appears at a lower frequency of 1730 cm−1.
Drug loading, binding capacity, and release studies
To measure the loading efficiency, the standard curve for the methotrexate drug was initially drawn, and the linear equation was obtained. The percentage of the drug-loaded and the entrapment efficiency in biodegradable polymers were obtained 12.8% ± 0.4 and 92.1% ± 0.1, respectively. The reason for incubation of nanoparticles in acetonitrile at this stage is due to high penetration of acetonitrile into MIP polymers, leads to swelling, and the drug is released through penetration and swelling in a certain period of time [26, 27].
As the results show, in the molecular imprinting technique, owning to the hydrogenic interaction of the drug with the functional monomer and the formation of the complex, the drug can be encapsulated in a polymer matrix efficiently.
According to the binding capacity studies, percentage of absorbed drug was obtained 23.6% for MIP and 2.7% for NIP. The amount of drug absorbed by MIP was higher than NIP, which indicates the presence of specific sites for methotrexate in MIP.
The main purpose of NIP synthesis in this study is to compare it with MIP and indicate the superiority of MIP compared to NIP. MIPs have a higher absorption capacity than NIPs. On the other hand, the drug absorbed by MIP is the result of specific adsorption (by detection sites) and non-specific adsorption, while the drug absorbed by NIP is only the result of non-specific and superficial adsorption on NIP. This difference in uptake indicates the presence of drug-specific sites on MIP particles, which leads to higher uptake and separation of the drug by MIP-specific sites.
Figure 6 shows the release of methotrexate in phosphate buffer saline at pH = 5.5 and 7.4 in biodegradable MIPs.
Fig. 6.

The in vitro release profile of MTX in biodegradable MIP and EGDMA MIP at different times in phosphate buffer (pH = 5.5 and 7.4)
In all cases, a burst release of the drug in its early release profiles were observed. The initial burst release is attributed to the exposed and weak bonds of drug molecules. However, MIP illustrated relatively slow release and released 75% of drugs within two weeks in an acidic medium. This is related to particular MIP sites that interact strongly with the template molecule and prevent its release. In a way that requires a very long time for the phosphate buffer environment to overcome the hydrogenic interactions of drug molecules with the specific sites and dissolve the drug in itself. As seen in the acidic environment, the drug release from biodegradable polymers was faster than the physiologic environment, but there was no difference for non-degradable EGDMA polymers in both environments. In an acidic environment, the biodegradable polymers are slightly degraded from their surface.
Kinetic release models, including a zero-order and first-order model, Higuchi and Korsmeyer-Peppas equation for biodegradable and non-biodegradable MIP in different pHs were investigated by calculating R2. The corresponding variables of fitting the other kinetic models with experimental data were estimated by Exel software. Table 5 illustrate kinetic models of drug release for biodegradable MIP in pH = 7.4 and pH = 5.5, respectively. Based on the findings in Table 5, the first-order dynamic and Higuchi models demonstrated more functionality for the imprinted nanobeads (MIPs/MMIPs) because of the higher R2 values. While biodegradable MIP follows Korsmeyer-Peppas kinetics [25] and non-biodegradable MIP follows zero-order kinetics.
Table 5.
In-vitro drug release kinetic models of imprinted polymers
| Models | Parameters | pH = 5.5 | pH = 7.4 |
|---|---|---|---|
| Zero order | R2 | 0.73 | 0.90 |
| K | 0.0030 | 0.0022 | |
| First order | R2 | 0.79 | 0.93 |
| K | 0.0008 | 0.0006 | |
| Higuchi | R2 | 0.86 | 0.98 |
| K | 0.152 | 0.169 | |
| Korsmeyer–Peppas | R2 | 0.92 | 0.98 |
| K | 0.0040 | 0.0098 |
The numerical value of the diffusion number power reflects the drug release mechanism. It is affected by the ratio of the diameter to the length of the matrix (Aspect ratio). If the matrix is a low-thickness film, n = 0.45 indicates that the diffusion follows the Fick diffusion, while n ≠ 0.45 indicates non-Fick diffusion. If n = 1, the release is considered to be zero order. Korsmeyer–Peppas kinetic in MIP nanoparticles showed two values of n = 0.845 and n = 0.715 for pH = 5.5 and 7.4, respectively, which shows non-Fick diffusion.
In vitro degradation test
The particle degradation test was performed in pH (5.5, 7.4, and 9.0) at 37 °C.
Figure 7 shows SEM images taken from non-degradable polymers prepared by the EGDMA cross-linker after 15 and 45 days, illustrating EGDMA as a cross-linker non-degradable and polymers remained unchanged in pH 7.4 and 5.5 [25].
Fig. 7.
SEM micrographs of non-biodegradable MIP prepared by EGDMA a after 15 days, b after 45 days
Figures 8 and 9 illustrate SEM images taken from polymers prepared by DMHA cross-linker in three pHs of 5.5, 7.4, and 9.0. After 15 and 35 days, it has been shown that DMHA is a biodegradable cross-linker providing the hydrolysable polymer to expose ionized acids on polymer chains to form a water-soluble polymer. There are various biodegradation mechanisms of polymers (for example, ionization mechanisms and hydrolysis mechanisms). Due to the presence of hydrolysable functional group CO–O-NH-CO in the polymer network, the degradation of polymers in various pH environments was different.
Fig. 8.
SEM micrographs of biodegradable MIP degradation after 15 days in phosphate buffer a pH = 9, b pH = 7.4, and c pH = 5.5
Fig. 9.
SEM micrographs of biodegradable MIP degradation after 35 days in phosphate buffer with a pH = 9, b pH = 7.4, and c pH = 5.5
Figure 8 shows that the degradation of particles from the surface results in losing their spherical shape. The beginning of morphological changes from the surface of polymers was visible at neutral and alkaline pH. At acidic pH, minimal changes are observed on the polymer surface.
After 35 days, the polymers were degraded entirely in an alkaline environment (Fig. 9a).
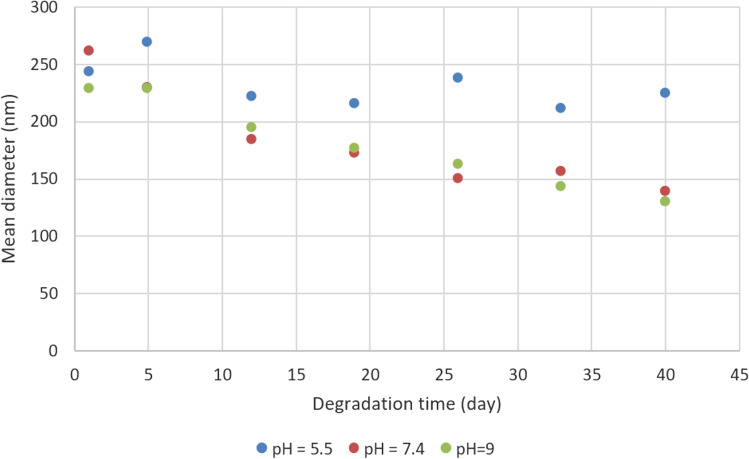
Figure 10 shows the particle size and the size distribution at different times during the degradation test in 3 environments with pH values of 5.5, 7.4, and 9.0. While time passed in the alkaline medium, the particle size decreases with hydrolysis. However, the particle size reduction in the acidic environment was smaller than in other environments.
Fig. 10.
Particle size distribution in different times of biodegradation test
Cell culture studies
The effect of MTX entrapment in formulations based on their performance in inhibiting cell growth, cell cytotoxicity of polymer, and the influence of MTX in DMSO on MCF-7 cell lines were investigated in this step. The procedure was based on the MTT colorimetric assay. Various studies have shown that non-molecularly imprinted polymer showed no toxicity on different cell lines and confirmed the biocompatibility of these drug delivery polymers.
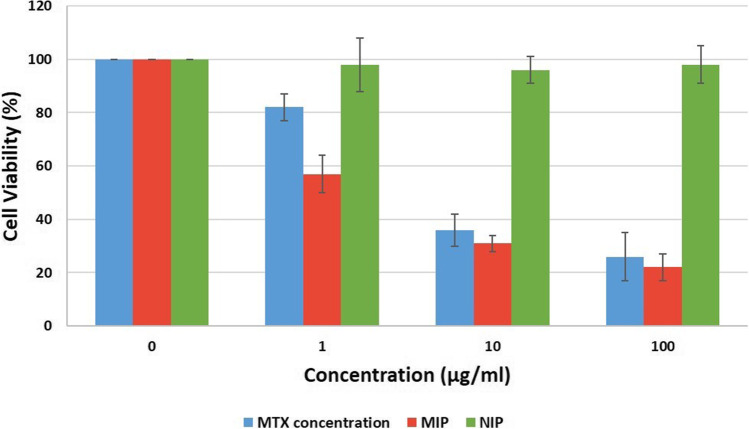
The MTX cell cytotoxicity on the MCF-7 cell line is shown in Fig. 11. The MTT test was performed at 72 h after treatment of the cells and all assays were done in triplicate. As shown in the figure, the percentage of cell viability for nanoparticles was lower than free MTX at all concentrations. In the case of NIP, the cell viabilities at all concentrations were 100%, indicating the biocompatibility of biodegradable nanoparticles. Calculation of IC50 was performed by Sigma Plot 11 software, and 3.54 μg/ml was reported for nanoparticles and 6.9 μg/ml for free MTX.
Fig. 11.
Cytotoxicity of MTX on MCF-7 cell line at 72 h
Conclusion
A biodegradable delivery system based on MIP was prepared using a new cross-linker agent for the first time for methotrexate anticancer delivery. The results showed that the cross-linker agent has a significant role in the creation of particular sites. Due to the high degree of crosslinking lattice, the nanoparticles demonstrated good stability, suitable particle size, and size distribution. The particle size decreased with increasing temperature and reducing the time and amount of initiator in mini-emulsion polymerization. Also, MIPs showed a higher absorption capacity than NIPs. On the other hand, the drug absorbed by MIP results from specific adsorption by detection sites and non-specific adsorption. This difference indicates the presence of drug-specific sites on MIP particles. MIPs exhibited the sustained release due to the presence of specific sites for the drug and decreasing the efficiency of the sites. Furthermore, in vitro degradation test was carried out in different pHs during 35 days and results showed that the MIPs were degraded in neutral and alkaline environments due to the presence of hydrolysable CO–O-NH-CO in the polymer network. In addition, the cytotoxicity was investigated on MCF7-breast cancer cells; and MIPs showed higher cytotoxicity than free anticancer. We expect that this synthesized biodegradable MIP nanoparticle is a suitable system for use in sustained drug delivery.
Declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mehdi Esfandyari-Manesh, Email: mesfandyari@sina.tums.ac.ir.
Fatemeh Ghorbani-Bidkorpeh, Email: f.ghorbani@sbmu.ac.ir.
References
- 1.Parveen S, Misra R, Sahoo SK. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed: Nanotechnol Biol Med. 2012;8(2):147–166. [DOI] [PubMed]
- 2.Han S, et al. A molecularly imprinted composite based on graphene oxide for targeted drug delivery to tumor cells. J Mater Sci. 2019;54(4):3331–3341. doi: 10.1007/s10853-018-3023-8. [DOI] [Google Scholar]
- 3.Aoki K, Saito N. Biodegradable polymers as drug delivery systems for bone regeneration. Pharmaceutics. 2020;12(2):95. doi: 10.3390/pharmaceutics12020095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Łukasiewicz S, et al. Polycaprolactone nanoparticles as promising candidates for nanocarriers in novel nanomedicines. Pharmaceutics. 2021;13(2):191. doi: 10.3390/pharmaceutics13020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi G, et al. Emerging nanomaterials with advanced drug delivery functions; focused on methotrexate delivery. Coord Chem Rev. 2018;359:32–51. doi: 10.1016/j.ccr.2018.01.007. [DOI] [Google Scholar]
- 6.Xu S, Wang L, Liu Z. Molecularly imprinted polymer nanoparticles: an emerging versatile platform for cancer therapy. Angew Chem Int Ed. 2021;60(8):3858–3869. doi: 10.1002/anie.202005309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu Z, et al. Molecularly imprinted polymer-based smart prodrug delivery system for specific targeting, prolonged retention, and tumor microenvironment-triggered release. Angew Chem Int Ed. 2021;60(5):2663–2667. doi: 10.1002/anie.202012956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaidi SA. Latest trends in molecular imprinted polymer based drug delivery systems. RSC Adv. 2016;6(91):88807–88819. doi: 10.1039/C6RA18911C. [DOI] [Google Scholar]
- 9.Zaidi SA. Molecular imprinting: a useful approach for drug delivery. Mater Sci Energy Technol. 2020;3:72–77. [Google Scholar]
- 10.Ishkuh FA, et al. Synthesis and characterization of paclitaxel-imprinted nanoparticles for recognition and controlled release of an anticancer drug. J Mater Sci. 2014;49(18):6343–6352. doi: 10.1007/s10853-014-8360-7. [DOI] [Google Scholar]
- 11.Esfandyari-Manesh M, et al. Dipyridamole recognition and controlled release by uniformly sized molecularly imprinted nanospheres. Mater Sci Eng, C. 2011;31(8):1692–1699. doi: 10.1016/j.msec.2011.07.019. [DOI] [Google Scholar]
- 12.Zhang H. Molecularly imprinted nanoparticles for biomedical applications. Adv Mater. 2020;32(3):1806328. doi: 10.1002/adma.201806328. [DOI] [PubMed] [Google Scholar]
- 13.Cheong WJ, Yang SH, Ali F. Molecular imprinted polymers for separation science: a review of reviews. J Sep Sci. 2013;36(3):609–628. doi: 10.1002/jssc.201200784. [DOI] [PubMed] [Google Scholar]
- 14.Sanagi MM, et al. Molecularly imprinted polymer solid-phase extraction for the analysis of organophosphorus pesticides in fruit samples. J Food Compos Anal. 2013;32(2):155–161. doi: 10.1016/j.jfca.2013.09.001. [DOI] [Google Scholar]
- 15.Roshan S, et al. Molecularly imprinted polymer-silica hybrid particles for biomimetic recognition of target drugs. Adv Polym Technol. 2019;2019:1–7. doi: 10.1155/2019/9432412. [DOI] [Google Scholar]
- 16.Ahmad OS, et al. Molecularly imprinted polymers in electrochemical and optical sensors. Trends Biotechnol. 2019;37(3):294–309. doi: 10.1016/j.tibtech.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Vakilinezhad MA, Alipour S, Montaseri H. Fabrication and in vitro evaluation of magnetic PLGA nanoparticles as a potential Methotrexate delivery system for breast cancer. J Drug Deliv Sci Technol. 2018;44:467–474. doi: 10.1016/j.jddst.2018.01.002. [DOI] [Google Scholar]
- 18.Rozalen M, et al. Synthesis of controlled-size silver nanoparticles for the administration of methotrexate drug and its activity in colon and lung cancer cells. RSC Adv. 2020;10(18):10646–10660. doi: 10.1039/C9RA08657A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ensafi AA, Nasr-Esfahani P, Rezaei B. Simultaneous detection of folic acid and methotrexate by an optical sensor based on molecularly imprinted polymers on dual-color CdTe quantum dots. Anal Chim Acta. 2017;996:64–73. doi: 10.1016/j.aca.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, et al. An electrochemical sensor based on a molecularly imprinted polymer for determination of anticancer drug mitoxantrone. Sens Actuators, B Chem. 2018;255:544–551. doi: 10.1016/j.snb.2017.08.023. [DOI] [Google Scholar]
- 21.Agrawal YO, et al. Methotrexate-loaded nanostructured lipid carrier gel alleviates imiquimod-induced psoriasis by moderating inflammation: formulation, optimization, characterization, in-vitro and in-vivo studies. Int J Nanomed. 2020;15:4763. doi: 10.2147/IJN.S247007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Narayani R, Rao KP. Controlled release of anticancer drug methotrexate from biodegradable gelatin microspheres. J Microencapsul. 1994;11(1):69–77. doi: 10.3109/02652049409040439. [DOI] [PubMed] [Google Scholar]
- 23.Yang P, et al. Stimuli-responsive biodegradable poly (methacrylic acid) based nanocapsules for ultrasound traced and triggered drug delivery system. Biomaterials. 2014;35(6):2079–2088. doi: 10.1016/j.biomaterials.2013.11.057. [DOI] [PubMed] [Google Scholar]
- 24.Yin W, Akala EO, Taylor RE. Design of naltrexone-loaded hydrolyzable crosslinked nanoparticles. Int J Pharm. 2002;244(1):9–19. doi: 10.1016/S0378-5173(02)00297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheler S, Kitzan M, Fahr A. Cellular uptake and degradation behaviour of biodegradable poly (ethylene glycol-graft-methyl methacrylate) nanoparticles crosslinked with dimethacryloyl hydroxylamine. Int J Pharm. 2011;403(1–2):207–218. doi: 10.1016/j.ijpharm.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Esfandyari-Manesh M, et al. Paclitaxel molecularly imprinted polymer-PEG-folate nanoparticles for targeting anticancer delivery: characterization and cellular cytotoxicity. Mater Sci Eng, C. 2016;62:626–633. doi: 10.1016/j.msec.2016.01.059. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez de Anda DA et al. Effects of solvent used for fabrication on drug loading and release kinetics of electrosprayed temozolomide‐loaded PLGA microparticles for the treatment of glioblastoma. J Biomed Mater Res Part B Appl Biomater. 2019;107(7):2317–2324. [DOI] [PMC free article] [PubMed]