Highlights
Critical strategies for converting wastes to catalysts are summarized.
Applications of waste-derived catalysts in hydrogen evolution reaction, oxygen evolution reaction, and overall water electrolysis are comprehensively reviewed.
Perspectives in the development of waste-derived catalysts are analyzed.
Keywords: Waste-derived electrocatalysts, Water splitting, Sustainable hydrogen energy, Catalyst design, Circular economy
Abstract
The sustainable production of green hydrogen via water electrolysis necessitates cost-effective electrocatalysts. By following the circular economy principle, the utilization of waste-derived catalysts significantly promotes the sustainable development of green hydrogen energy. Currently, diverse waste-derived catalysts have exhibited excellent catalytic performance toward hydrogen evolution reaction (HER), oxygen evolution reaction (OER), and overall water electrolysis (OWE). Herein, we systematically examine recent achievements in waste-derived electrocatalysts for water electrolysis. The general principles of water electrolysis and design principles of efficient electrocatalysts are discussed, followed by the illustration of current strategies for transforming wastes into electrocatalysts. Then, applications of waste-derived catalysts (i.e., carbon-based catalysts, transitional metal-based catalysts, and carbon-based heterostructure catalysts) in HER, OER, and OWE are reviewed successively. An emphasis is put on correlating the catalysts’ structure–performance relationship. Also, challenges and research directions in this booming field are finally highlighted. This review would provide useful insights into the design, synthesis, and applications of waste-derived electrocatalysts, and thus accelerate the development of the circular economy-driven green hydrogen energy scheme.
Introduction
The utilization of traditional carbon-based fuels (e.g., natural gas, coal, oil) has given rise to serious concerns about environmental pollution and climate change [1, 2]. Additionally, the ever-climbing global energy demand is essential to sustain the development of our human society. As such, it is imperative to explore sustainable and clean energy systems to meet these energy-related challenges. Featuring zero carbon footprint, earth abundance, and high gravimetric energy density, hydrogen fuel is one of the most promising candidates to revolutionize the global energy system [3–5]. The complete industrial chain of hydrogen energy contains hydrogen production, storage, transportation, and application. A prerequisite for the sustainable development of hydrogen economy is the large-scale and clean production of hydrogen gas. Currently, conventional fossil fuels are responsible for the majority of H2 production, and about 71.27% of H2 is generated from natural gas, 27.27% from coal, 0.7% from petroleum, and the remaining 0.7% from water splitting. However, fossil reformation-based hydrogen production techniques are neither renewable nor carbon neutral as the production process involves high greenhouse gas footprints [6]. Hence, water electrolysis, which only involves the conversion of hydrogen and oxygen elements has attracted broad interest in the world [7, 8]. Although water electrolysis attains a high technology readiness level (9–10), the relatively low energy efficiency (61–82%), and high levelized cost of hydrogen ($4.78 − 5.84/kg H2, alkaline water electrolyzers) remain great challenges for the large-scale industrial application of water electrolysis technique [9].
Theoretically, a low thermodynamic potential of 1.23 V (at standard conditions) is needed to drive the water-splitting process [10]. Nevertheless, a considerable overpotential (η) is generally required for practical water electrolysis due to the system hindrance and sluggish reaction kinetics [11]. To reduce energy consumption, efforts have been made to advance high-performance electrocatalysts. Although precious metal-based catalysts (e.g., IrO2, RuO2, Pt, and Pd) exhibit high catalytic activities and durability for oxygen evolution reaction (OER) and hydrogen evolution reaction (HER), their high cost profoundly restrains their industrial applications [12, 13]. Surprisingly, many well-designed earth-abundant transitional metals (e.g., Ni, Fe, Mn, Cu, Co, Mo) and carbon-based materials also show high performance for OER, HER, and overall water electrolysis (OWE) [14–18]. Electrocatalysts with diverse structural features have gained great interest, such as metal–organic frameworks [19, 20], covalent-organic frameworks [21], two-dimensional (2D) materials [22], and hierarchically structured materials [23, 24]. The implementation of these low-cost electrocatalysts would largely cut the running cost of water electrolysis systems.
Of note, the aforementioned electroactive transitional metals and carbon are rich in typical wastes, such as electronic wastes, biowastes, and wastewater. From a circular economy perspective, reutilizing these wastes in the development of new products can achieve the close-loop utilization of substances, which would not only reduce the cost of preparing new products but also benefit the waste management system [25–28]. Compared with linear and recycling economy approaches, the circular economy route could reduce resource market dependence and lowers waste disposal costs. Additionally, it is suggested that the implementation of a circular economy in all sectors can help to limit carbon emissions by 45% by 2030, and to achieve carbon neutrality by 2050 [29]. Thus, developing functional materials from wastes is a sensible way to realize the circular economy and minimize the carbon footprint of materials preparation [30–32]. Recently, synthesizing electrocatalysts from wastes has gained increasing scientific attention thanks to the huge economic and environmental benefits [33–37]. For example, Cao and coworkers employed bean sprouts to design a N, S self-doped porous carbon catalyst for HER via pyrolysis [38]. The obtained carbon catalyst exhibits an acceptable HER activity (η10 = 413 mV, Tafel slope = 98 mV dec−1) in acidic media. Aside from the pyrolysis method which is usually used for converting biowastes into carbon-based catalysts, other sophisticated methods like electrochemical synthesis [39], wet-chemical synthesis [40], and microwave synthesis [41] are also capable of constructing waste-derived electrocatalysts for water electrolysis. Generally, there are three categories of electrocatalysts derived from various wastes, namely carbon-based materials (mainly refer to pure carbon and heteroatom-doped carbon materials), transitional metal-based catalysts, and carbon-based composite catalysts.
To ameliorate the catalytic performance of waste-derived catalysts, diverse strategies have been performed to regulate the physicochemical and electronic properties of catalysts, such as heteroatom doping, nanostructure control, defect/vacancy engineering, and heterostructure construction [18, 42–44]. Many engineered waste-derived catalysts exhibit good performance for HER, OER, and OWE, and some of them even outperform noble metal-based counterparts [45–49]. Hence, waste-derived efficient electrocatalysts for water electrolysis can promote the circular economy-driven green hydrogen energy system (Scheme 1). Currently, a comprehensive review of the speedily flourishing applications of waste-derived electrocatalysts in water electrolysis is still lacking. Accordingly, it is emergency to systematically summarize remarkable breakthroughs in waste-derived water electrolysis catalysts for guiding future research.
Scheme 1.

Diagram of circular economy-driven green hydrogen energy assisted by waste-derived electrocatalysts for water electrolysis
Herein, we comprehensively summarize recent achievements in applying waste-derived electrocatalysts for water electrolysis. The general principles of water electrolysis and high-performance electrocatalyst design are analyzed. Then, we introduce the main strategies for transforming wastes into catalysts, such as pyrolysis, electrochemical synthesis, wet-chemical synthesis, as well as microwave synthesis and beyond. Consequently, the applications of waste-derived carbon-based catalysts, transitional metal-based catalysts, and carbon-based heterostructural catalysts in HER, OER, and OWE are detailed separately. The catalysts’ structure–catalytic performance relationship is emphasized. At last, perspectives in this field are also pointed out. We hope this timely review would provide guidance to the design of waste-derived high-performance electrocatalysts for water electrolysis, and stimulate further studies on the development of low-cost green hydrogen production.
General Principles of Water Electrolysis and Electrocatalyst Design
Water electrolysis involves the splitting of H2O molecules into H2 and O2 gases under potential biases (Fig. 1a). The hydrogen gas production efficiency is influenced by the electrolyzer systems, including electrolytes, catalysts/electrodes, applied potentials, etc. Herein, the general principles of water electrolysis and design principles of efficient catalysts are discussed to provide an overview of the water electrolysis system.
Fig. 1.
a Illustration of the water electrolyzer. b HER mechanisms in acidic and alkaline electrolytes. c Adsorbate evolution mechanism (AEM) for alkaline OER. d AEM for acidic OER. e Lattice oxygen participation mechanism (LOM) for alkaline OER, the dotted red circle represents the oxygen vacancy [50].
Copyright 2022, Wiley–VCH
General Principles of Water Electrolysis
Water electrolysis consists of HER at the cathode and OER at the anode. Both HER and OER follow different pathways/mechanisms in various electrolytes. Currently, HER mechanisms have been well disclosed by experimental and computational investigations. Generally, HER obeys the Volmer/Tafel or Volmer/Heyrovsky routes. In alkaline media, there are four elementary steps (i.e., H2O adsorption, H2O dissociation, OH− adsorption, and H2 generation) (Fig. 1b) [50]. Of note, the H2O adsorption and dissociation steps in alkaline HER show higher energy barriers than H3O+ adsorption in acidic HER. As a result, the activity of some catalysts (e.g., Pd, Pt) for acidic HER is theoretically much higher than that for alkaline HER [51]. It is suggested that HER catalysts with strong abilities to adsorb and dissociate H2O and bind protons would exhibit improved HER activities in alkaline media [52].
Different from the 2-electron HER process, the mechanism of the 4-electron OER is more complicated. Currently, the most acceptable OER pathways include the adsorbate evolution mechanism (AEM) and the lattice oxygen participation mechanism (LOM) [3]. As depicted in Fig. 1c, AEM for alkaline OER generally follows four steps. First, the oxidation of OH− on the electrocatalytically active site (M) forms the intermediate M–OH. Then, the M–OH becomes M–O through a proton coupling-electron transfer process. The M–O further transforms into the M–OOH intermediate via an OH− coupled with 1-electron oxidation and eventually initiates another proton-coupled electron transfer process to generate O2 molecules. Different from alkaline OER, the first step of AEM for acidic OER is the adsorption of a H2O molecule on M (Fig. 1d). Then, the dissociation of a H+ leads to the generation of M–OH, which is followed by the release of the second H+ to produce M–O. After that, M–OOH is formed after the nucleophilic attack of another H2O molecule. The final step is the desorption of the formed O2 molecule and the fourth proton coupling.
Recently, growing studies have focused on determining the origin of oxygen in O2 products, and some studies found that catalysts’ lattice oxygen participates in the OER process, namely the LOM-driven OER [53–55]. Taking LOM for alkaline OER as an illustration (Fig. 1e), OH− is first adsorbed on the oxygen vacancy (Ov)-coordinated active site (M–OH/Ov). Subsequently, the Ov site near M adsorbs an additional OH− and forms the M–OH/–OH species, which is followed by a dehydrogenation process and leads to the generation of M–OH/–O. Nevertheless, OH− is difficult to undergo further dehydrogenation directly, and an unstable transition state (M–OH) is produced, which consequently transforms into M–OO/Ov. At last, with the desorption of the formed O2 molecule and filling of OH−, the initial state M–OH/Ov is recovered. It is worth noting that the OER mechanism is highly sensitive to catalysts’ surface properties, and the in situ structural reconstruction of catalysts under OER conditions can regulate the catalysis process. To attain a better understanding of OER mechanisms, employing advanced techniques to investigate the structure self-evolution of catalysts and monitor the reaction intermediates (e.g., OH, OOH) is highly suggested [56].
Parameters for Electrocatalyst Evaluation
Rational evaluating catalysts’ activities is important for advancing the design of high-performance electrocatalysts. Hence, several parameters have been proposed, including overpotential (η), Tafel slope, Faradaic efficiency, turnover frequency (TOF), and stability.
Overpotential (η) means the extra potential which is necessitated to initiate the electrochemical reactions. In general, η at a specified current density (j, e.g., 10 mA cm−2) is employed to assess the activity of electrocatalysts [57], and a lower η represents a higher activity.
Extracted from linear sweep voltammetry (LSV) curves, Tafel plots are employed to disclose the kinetics of electrochemical reactions [24]. The linear regions of Tafel plots can be fitted with the Tafel equation (η = a + blog j, where b represents the Tafel slope). When η is zero, the corresponding j obtained from the Tafel equation is termed the exchange current density (j0). j0 shows electrocatalysts’ intrinsic activity in the equilibrium state, which is generally used for HER catalysts’ evaluation.
Faradaic efficiency unveils the utilization efficiency of electrons involved in electrochemical reactions (i.e., HER, OER). Generally, Faradaic efficiency can be gained by comparing the experimental and theoretical values of gas product amounts. The amount/volume of gas products can be obtained via the internal water displacement method or tested with gas chromatography. Also, the fluorescence-based oxygen sensing method [58] and rotating ring disk electrode voltammetry [59] have been employed to measure the amount of oxygen gas.
TOF is explicated as the number of reactants (H2O) that electrocatalysts can convert to desired products (O2 or H2) per catalytic site per time unit. Accordingly, TOF demonstrates catalysts’ intrinsic activity. The value of TOF is generally calculated with the equation, TOF = (jA)/(αFn), where j is the current density at a fixed η; A means the surface area of the electrode; α is the electron numbers of the reaction; F represents the Faraday’s constant; n means the number of moles of the active sites. Of note, not all of the sites/atoms on catalysts are catalytically active or equally accessible, and thus it is difficult to gain an accurate TOF value for electrocatalysts. However, it is still rational to compare the TOF value of similar electrocatalysts.
Stability is a principal index that governs the practicability of electrocatalysts in commercial applications [60]. Two methods are generally used to test electrocatalysts’ stability. The first one is to document chronopotentiometry or chronoamperometry curves in a long-term running. The second one is the accelerated degradation test, which measures cyclic voltammetry (CV) or LSV curves for thousands of cycles. A stable catalyst would show an insignificant shift of potential or current density after the test.
Currently, it is still challenging to provide the best values of these parameters that are required for industrial water electrolysis applications of catalysts, because the measurement of these data is different from one study to another in terms of experimental protocols and catalysts’ properties (e.g., substrate, loading amount). Nevertheless, a promising electrocatalyst should possess a low η, a high Tafel slope, a high Faradaic efficiency, a high TOF, as well as good long-term stability.
Design Principles of Efficient Catalysts for Water Electrolysis
To develop high-performance electrocatalysts for water electrolysis, four general design principles should be kept in mind. As depicted in Fig. 2, abundant active sites, high intrinsic catalytic activity, good conductivity, and long-term performance durability/stability are essentials for a high-performance electrocatalyst. To attain these essential properties, diverse methods have been applied to regulate the internal and external characteristics of catalysts, such as doping, defect engineering, and nanostructure control. In this part, the most widely used methods for engineering efficient catalysts are detailed.
Fig. 2.
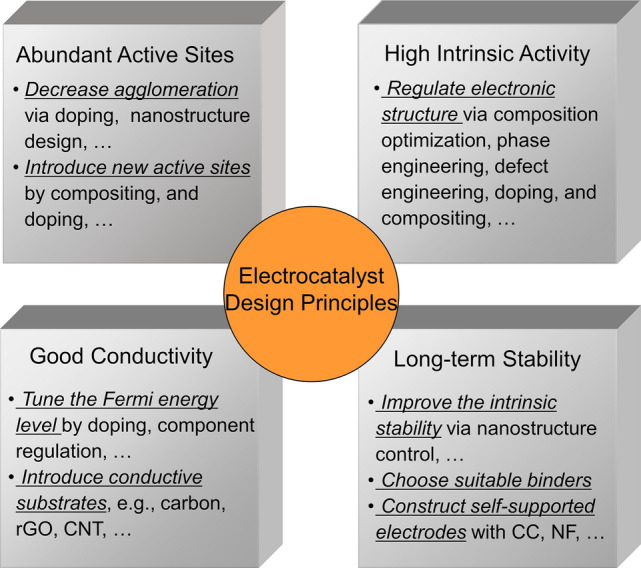
Design principles of waste-derived catalysts for water electrolysis
Abundant Electroactive Sites
Rich electroactive sites are necessary for the close contact of catalysts with electrolytes/reactants and promote electrocatalytic processes. Generally, there are four methods to construct abundant electroactive sites: (i) Dispersing catalyst particles on substrates with a high specific surface area (SSA) will reduce the agglomeration of catalysts and populate the electroactive sites; (ii) Reducing the size of catalysts by controlling the catalyst synthesis protocols allows the formation of nanosized catalyst particles. This method also can enhance the utilization efficiency of catalysts, a representative is single-atom catalysts; (iii) Previous studies also suggest that chemical doping and component regulation can tune the size of electrocatalysts and optimize the electroactive sites [61]; and (iv) Introducing electroactive dopants/materials can bring in additional catalytic sites, thus enriching surface electroactive sites.
High Intrinsic Catalytic Activity
Catalysts’ intrinsic catalytic activities largely dominate their electrocatalytic performance. Current strategies (e.g., composition optimization, heterostructure construction, doping, phase engineering, and defect engineering) for upgrading the catalysts’ intrinsic activity mainly focus on altering catalysts’ electronic structures. Typically, the d-band center and the density of state (DOS) are important electronic properties that provide meaningful information about the electron transfer behavior and reactant bonding/adsorption mechanisms on electroactive sites. Hence, applying apt strategies to modulate the electronic structure of catalysts is considered a powerful method to achieve suitable adsorption strengths/energies of reactants/intermediates (e.g., *H, *OH, *OOH) on electroactive sites, thereby high intrinsic activities.
High Electrical Conductivity
The electron transfer efficiency plays a crucial role in electrochemical reactions, and a high electrical conductivity can enhance electron transport throughout catalysts and prevent unwanted resistance at the electrolyte/catalyst interface [62]. In theory, the Fermi energy level of catalysts acts as the driving force of electron transfer [63], and better conductivity is associated with a higher electron density near the Fermi energy level. In this context, performing design strategies, like component regulation, cationic doping, and constructing heterostructures, on catalysts can attain a favorable Fermi energy level, and finally a high conductivity. Additionally, downsizing of catalyst particles and loading electroactive materials on conductive supports (e.g., nickel foam (NF), carbon papers (CP), reduced graphene oxides (rGO), and carbon nanotubes (CNT)) can also improve the electrical conductivity of entire catalysts/electrodes.
Long-term Performance Stability
To realize sustainable hydrogen fuel generation via water splitting, it is vital to maintain the performance stability of electrocatalysts in highly acidic or alkaline electrolytes. Electrochemical corrosion and detachment of electroactive materials are two major reasons for the degradation of electrodes. To overcome these barriers, component regulation, nanostructure control, and construction heterostructures can enhance the chemical and mechanical stability of catalysts under electrochemical conditions. Alternatively, developing electroactive materials on conductive and porous materials (e.g., NF, CP, porous carbon) via hydrothermal/solvothermal synthesis, electrodeposition, and electroless deposition can realize highly stable binder-free electrodes. For chemical binders-involved electrodes, the corrosion resistance property of binders to electrolytes also needs consideration, in addition to the stability of electroactive materials.
Strategies for Converting Wastes to Catalysts
Pristine wastes can hardly be used as efficient catalysts for water electrolysis. To this end, converting diverse wastes (e.g., biowastes, industrial wastes) into high-performance catalysts is required. Of note, engineering electrocatalysts from wastes can significantly decrease the catalyst preparation cost as well as the negative impacts of wastes on the environment [42]. Waste-derived catalysts’ performance is largely determined by design principles that govern the nanostructure and surface chemistry of catalysts. In this part, mainstream principles for waste-derived catalyst design are discussed, including pyrolysis, electrochemical synthesis, wet-chemical methods, microwave synthesis, and others.
Pyrolysis
Pyrolysis or carbonization is a frequently used process to design carbon-based electrocatalysts from biowastes [37, 64]. The pyrolysis/carbonization process is generally performed in a tube furnace under high temperatures, in an oxygen-free or oxygen-deficient atmosphere [65]. Catalytic properties of biowastes-derived electrocatalysts profoundly rely on parent biowastes’ properties (e.g., the ratio of heteroatoms, porous structure) and pyrolysis conditions (e.g., atmosphere, temperature, and time) [66]. Moreover, a general method to optimize the nanostructure/porosity is chemical activation during the pyrolysis process [67], and commonly used activators include KOH, K2CO3, ZnCl2, H3PO4, etc. Starting from peanut shells, Saravanan et al. developed multilayer carbon nanosheets for HER via a pyrolysis method. With KOH activation, the carbon material gains a high SSA (2338.5 m2 g−1) and uniform mesopores which improve the HER performance [68]. Aside from those one-step activation methods, several studies have proposed two-stage activation strategies to achieve a high surface area of carbon materials [69, 70]. For instance, Osman et al. used a two-stage H3PO4-KOH activation process to convert biowaste into carbon materials with a high surface of 1368 m2 g−1 and a pore volume of 0.92 cm3 g−1 [69].
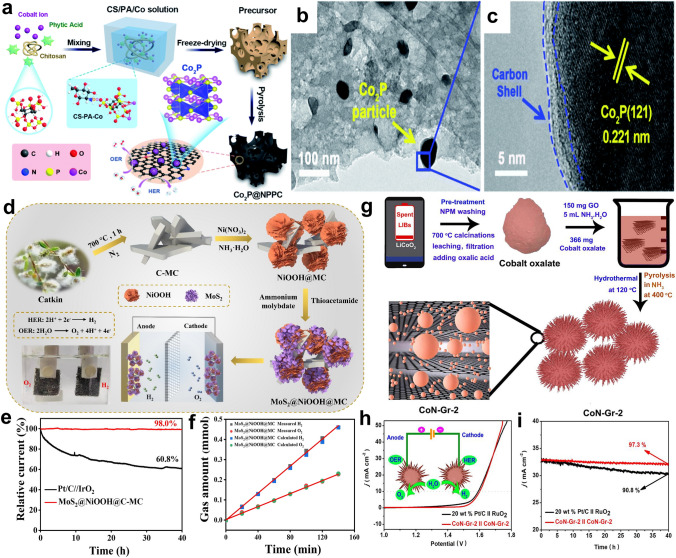
Besides using biowastes-derived materials as electrocatalysts directly, growing studies have designed carbon-based composites/heterostructures by pyrolysis [71]. The general process involves pyrolyzing the mixture of biowastes and metal salts, which could lead to the formation of metal compounds/carbon hybrids. For both HER and OER, the hybrids usually outperform the corresponding single components due to the populated electroactive sites and regulated electronic properties of metal compounds/carbon [72]. For instance, Song and co-authors designed Co and N co-doped carbon nanosheets (Co/N–CNSs) for HER from the catkins waste by a ball-milling two-stage pyrolysis process (Fig. 3a) [73]. The Co/N–CNSs show good HER activities on account of the formation of well-dispersed CoNx sites on carbon structures. Another effective method to develop waste-derived carbon-based composites is functionalizing carbon materials with electroactive nanomaterials (e.g., phosphates, oxides, sulfides) by a post-treatment (e.g., hydrothermal method, carbonization) [74, 75]. As illustrated in Fig. 3b, the Fe3O4 and NiS hybrid nanoparticles are formed on the cotton carbon (CC) via a post-carbonization treatment under a N2 atmosphere. The as-obtained Fe3O4/NiS@CC catalyst displays good OER performance with a low overpotential (η10 = 310 mV), outperforming its counterparts [74].
Fig. 3.
a Illustration of synthesis of Co/N–CNSs catalysts [73].
Copyright 2020, Royal Society of Chemistry. b Scheme of the preparation of Fe3O4/NiS@CC [74]. Copyright 2020, Elsevier. c Scanning electron microscopy (SEM) images and the scheme of NiCoMn LTHs catalysts nanostructure evolution during the electrodeposition process [39]. Copyright 2022, Royal Society of Chemistry. d Scheme of the positive-bias-driven exfoliation of LiCoO2 into CoOOH catalyst [79]. Copyright 2022, Royal Society of Chemistry
Electrochemical Synthesis
Electrochemical synthesis (e.g., electrodeposition) is powerful for engineering electrocatalysts from metal laden wastes or preparing electrocatalysts on low-cost robust substrates, such as spent stainless steel and cable wires [76–78]. The electrocatalysts’ properties are highly dependent on the property of wastes (electrolytes) and experimental protocols (e.g., deposition period, current, temperature, potential) since such parameters largely influence catalysts’ surface chemistry and nanostructure. Using the battery industrial wastewater as metal precursors, Chen et al. found that the nanostructure and elemental composition of electrodeposited NiCoMn-layered triple hydroxides (LTHs) electrodes obtained at different deposition periods were different (Fig. 3c). Specifically, the electrodeposit transforms from nanoclusters (S-1, 5 min) and nanospheres (S-2, 10 min) to nanoflowers (S-3, 20 min) and nanoplates (S-4, 30 min; S-5, 40 min). The optimal catalyst (S-3) featuring a hierarchical nanostructure, low crystallinity, and a high metal content of 67.33% possesses higher electrocatalytic activities toward both OER and HER [39].
Electrochemical transformation of solid metal-bearing wastes under a potential can also lead to high-performance electrocatalysts. Huang and coworkers developed a positive-bias-driven exfoliation method to convert spent LiCoO2 electrode materials into CoOOH which shows high OER performance (Fig. 3d) [79]. This electrochemical exfoliation process provides an eco-friendly, and high-efficiency route for constructing electrocatalysts by destroying the crystal structure of parent materials and oxidizing the electroactive elements to a high-valence state, which is suggested to benefit the OER process.
Wet-Chemical Methods
Wet-chemical methods are widely used for preparing electrocatalysts from diverse wastes, including hydrothermal/solvothermal synthesis, sol–gel process, and boriding [80–82]. All these processes involve chemical reactions in solutions, with different temperatures, pressures, and chemicals. Among them, hydrothermal/solvothermal methods are the most frequent applied one to synthesize carbon [83], metal oxides/hydroxides/sulfides/phosphides, and heterostructural catalysts [84, 85], especially carbon-based composites, such as Co2P/C [86], MoS2/C [87], Co3O4/NHPC (nitrogen-doped hierarchically porous carbon) [40]. Two processes are generally involved to construct composite electrocatalysts from biowastes with the hydrothermal method. The first one is converting biowastes into carbon via hydrothermal carbonization and metal salts are also involved, which is followed by a post-treatment (e.g., pyrolysis) to form hybrid catalysts (Fig. 4a) [88]. The second method is developing electroactive metal species on the pre-synthesized biowastes-derived carbon by a hydrothermal process (Fig. 4b) [43].
Fig. 4.

a Scheme of synthesis of cobalt phosphides decorated spirulina-derived porous N-doped carbon matrix (Co2P/NC) catalyst [88].
Copyright 2020, Wiley–VCH. b Illustration of the fabrication of N-doped carbon nanofiber/MoS2 (pBC-N/MoS2) nanocomposites [43]. Copyright 2016, American Chemical Society
The sol–gel method is generally combined with a thermochemical process to synthesize carbon-based heterostructures from biowastes. Starting from agarose biowastes, Xiao and co-authors proposed a sol–gel–calcination route to prepare Fe-Ni2P nanoparticles decorated N, P co-doped carbon catalyst (Fe-Ni2P@N, P-CNSs) [89]. Thanks to the enhanced electrical conductivity, high SSA, and rich electroactive sites, the Fe-Ni2P@N, P-CNSs catalyst shows high OER activities. Recently, researchers have developed a new boriding process to transform metal laden wastes into high-performance OER catalysts [90]. The boriding route refers to the reduction of metal species (e.g., Co, Ni, Cu, Fe, Mn, and Sn) in wastes and the generation of metal boride nanoparticles. The catalytic properties of obtained metal borides are governed by wastes’ properties (e.g., metals’ species and contents) and boriding protocols (e.g., atmosphere, temperature, reductants’ amount). In general, metal borides with small sizes, high dispersion, and a high ratio of Ni and Fe exhibit high OER performance [45].
Microwave Synthesis and Beyond
Microwave-assisted synthesis is efficient for nanocatalysts preparation because of its unique merits of short reaction time, cleanliness, and high energy utilization efficiency [91]. More importantly, different from conventional heating strategies (e.g., hydrothermal process, calcination), the microwave-assisted heating process can realize uniform heating and facilitate crystal nucleus generation/crystallization rapidly [92]. In 2018, Cova et al. proposed a microwave-assisted strategy to design Ag/Ag2S-carbon hybrid from pig bristles. The pig bristles can be efficiently decomposed with microwave heating, and the discharge of S facilitates the formation of Ag2S [41]. More recently, Miao and co-authors employed a microwave hydrothermal route to construct a NiFe-borate layered double hydroxide/biomass-derived N-doped carbon (NiFe-BLDH/NC) hybrid catalyst [93]. With multistage decentralized architecture, rich active sites, good electrical conductivity, and efficient charge/mass transfer kinetics, the NiFe-BLDH/NC shows high OER activities (η10 = 243 mV, Tafel slope = 42.7 mV dec−1). Compared with microwave synthesis, Zuliani et al. suggested that ultrasound treatment was better for the synthesis of Co/pinecones-derived carbon hybrid OER catalysts. Further analysis indicates that the ultrasound method leads to a higher number of electroactive sites than the microwave, microwave/ultrasound, and conventional heating processes [94].
Apart from the aforementioned methods, biogenic synthesis also has been employed for preparing electrocatalysts from wastes [95]. Generally, the synthesis of electrocatalysts from wastes involves a combination of different methods, representatives include hydrothermal–pyrolysis, pyrolysis–hydrothermal, sol–gel–calcination/pyrolysis, and pyrolysis–microwave processes. The rich combinations of synthesis methods allow the construction of diverse high-performance catalysts for water electrolysis.
Waste-derived Catalysts for HER
Developing cost-effective HER electrocatalysts allows sustainable and efficient hydrogen generation at the cathode part of water electrolyzers [96–98]. Currently, electroactive carbon materials, transitional metal-based catalysts, and carbon-based heterostructures synthesized from wastes have shown good HER performance in a wide pH range (Table 1). This part reviews recent advances in representative waste-derived HER electrocatalysts.
Table 1.
Summary of representative waste-derived HER electrocatalysts
| Waste | Catalyst | Electrolyte | η10 (mV) | Tafel slope (mV dec−1) |
Refs |
|---|---|---|---|---|---|
| Palm plant | Hierarchical porous carbon nanosheets | 0.5 M H2SO4 | 330 | 63 | [109] |
| Human hair ashes | Partially graphitized activated carbon nanobundles | 0.5 M H2SO4 | 16 | 51 | [67] |
| Rice husks | Corrugated graphene nanosheets | 0.5 M H2SO4 | 9 | 31 | [99] |
| Paper wastes | Co, N co-doped carbon | 0.5 M H2SO4 | 223 | 91 | [112] |
| Waste tires | Zn, S, N co-doped carbon | 1.0 M KOH | 50 | 78 | [111] |
| Waste cotton textile | O-doped biochar | 0.5 M H2SO4 | 247.6 | 120.8 | [196] |
| Polysaccharides | Defective N-doped graphene sponge | 0.5 M H2SO4 | 267 | 69.7 | [104] |
| Pistachio shells | Ni, N co-doped carbon | 1.0 M KOH | 403 | 146 | [113] |
| Plastic wastes | Holey and wrinkled graphene | 0.5 M H2SO4 | 613 | 91 | [103] |
| Peanut shells | N-doped carbon nanosheets | 0.5 M H2SO4 | 390 | 75.7 | [68] |
| Tamarindus indica shells | Graphitic carbon | 1.0 M KOH | 221 | 204 | [100] |
| Peanut root nodules | S, N co-doped carbon nanosheets | 0.5 M H2SO4 | 27 | 67.8 | [110] |
| Plastic wastes | N, O co-doped carbon | 0.5 M H2SO4 | 309 | 87 | [102] |
| Animal bones | N-, P- and Ca co-doped biochar | 0.5 M H2SO4 | 162 ± 3 | 80 | [115] |
| Cattail fibers | Porous N-doped carbon fibers | 0.5 M H2SO4 | 248 | 135 | [197] |
| Bean sprouts | N-doped carbon | 0.5 M H2SO4 | 413 | 98 | [38] |
| Plastic wastes | N-doped carbon coated Mo2C | 0.5 M H2SO4 | 186.6 | 72.9 | [124] |
| Plastic wastes | N-doped carbon supported MoS2 | 0.5 M H2SO4 | 56 | 36.6 | [131] |
| Watermelon peels | Mo2C/C | 1.0 M KOH | 133 | 71 | [198] |
| Waste-yeast cells | N, P co-doped Mo2C confined in porous carbon | 1.0 M KOH | 84 | 58.15 | [126] |
| Electronic wastes | Au@N-doped carbon | 0.5 M H2SO4 | 54.1 | 76.8 | [123] |
| Aloe waste | ZnMoO4/carbon | 1.0 M KOH | 124 | 54 | [129] |
| Fly ash | Fly ash/TiO2 | 0.1 M KOH | 125 | 115 | [120] |
| Banana waste | Pd/Fe3O4@carbon | 0.5 M H2SO4 | 293 | 227.05 | [199] |
| Wood residue | Mo2C | 0.5 M H2SO4 | 35 | 25 | [118] |
| Waste polythene | N-doped carbon-supported Mo2C | 0.5 M H2SO4 | 197.37 | 69.2 | [200] |
| Eggshell membrane | NiO/C | 1.0 M KOH | 670 | 77.8 | [80] |
| Organic liquid waste | MoS2/vertical graphene nanosheets | 0.5 M H2SO4 | 183 | 38 | [132] |
| Ion-exchange resin | Cr2O3/C | 0.5 M H2SO4 | 123 | 90 | [128] |
| Coffee waste grounds | Carbon-coated Fe nanoparticles | 0.5 M H2SO4 | 75 | 59 | [122] |
| Carbon dioxide | Ni/NiOx@C | 0.1 M KOH | 337 | – | [201] |
| Plastic waste | Mo2C/MnO2@C | 0.5 M H2SO4 | 58.3 | 36 | [46] |
| Walnut shells | Mo2C@C | 0.5 M H2SO4 | 140 | 63 | [125] |
| Sugarcane bagasse | Co-MoS2/C | 0.5 M H2SO4 | 62 | 53.86 | [133] |
| Chlorella | Co2P/N-doped carbon | 0.5 M H2SO4 | 151 | 50.21 | [202] |
| 1.0 M KOH | 252 | 70.14 | [202] | ||
| Silk cocoon | NiCo alloy/N-doped carbon | 1.0 M KOH | 179 | 40 | [203] |
| Scrap nickel | Ni2P nanoparticles | 0.5 M H2SO4 | 69 | 55 | [119] |
| 1.0 M KOH | 73 | 73 | [119] | ||
| Pig bristles | Ag/Ag2S@C | 0.5 M H2SO4 | 190 | 150 | [41] |
Waste-derived Carbon Catalysts for HER
Carbon-based electrocatalysts exhibit some features for HER, including earth abundance, easily tunable nanostructure, and high stability in broad pH conditions. To date, the development of waste-derived carbon catalysts centrally focuses on phase regulation, nanostructure control, and heteroatom doping.
The phase/crystal structure of carbon catalysts influences their catalytic properties by determining the electrical conductivity, density of electroactive sits, and intrinsic catalytic activity. Starting from human hairs, Sekar et al. designed two carbon materials with different graphitization degrees (Fig. 5a) [67]. Compared with the amorphous carbon material (HH-AC-600) prepared at a lower temperature (600 °C), the catalyst synthesized at 700 °C (HH-AC-700) shows a partial graphitization feature. Interestingly, the HH-AC-700 catalyst possesses a higher textural porosity and higher electrical conductivity than its counterpart, which contributes to better HER activities in acidic media (Fig. 5b-c). Additionally, the HH-AC-700 catalyst exhibits better stability, as evidenced by the multiple chronopotentiometry and time-dependent measurements for 1 and 10 h, respectively (Fig. 5d-e). Similar results reported by the same group also indicate that rice husks-derived graphene nanosheets prepared at a higher temperature (700 °C) with a relatively higher crystallinity exhibit better HER activities [99]. However, not all reports follow this synthesis temperature-HER activity trend. Thirumal et al. found that the activated carbon catalyst obtained at 800 °C outperformed its analogues synthesized at 700 and 900 °C due to its highest conductivity [100].
Fig. 5.
a Scheme of the preparation of human hair-derived HH-AC-700 layered nanobundles and HH-AC-600 nanobundles. b LSV curves, c Tafel plots, d multi-chronopotentiometry profiles, and e time-dependent HER stability for HH-AC-700 and HH-AC-600 catalysts [67].
Copyright 2022, MDPI. f Illustration of the HWFG preparation process, and the diagram of HWFG’s porous structure [103]. Copyright 2022, American Chemical Society. g Scheme of the fabrication process of bean sprouts carbon materials [38]. Copyright 2021, Elsevier
Engineering the nanostructure of carbon catalysts is a powerful strategy to upgrade the HER performance. Carbon catalysts with diverse morphologies/nanostructures have been developed for HER, especially nanosheets and porous architecture. These structures feature large SSA, which contributes to efficient electrolyte percolation, abundant electroactive sites, and rapid mass/charge transfer during the catalytic process [101]. Some studies have emphasized the importance of managing porous structures in carbon catalysts. In the low-temperature solvothermal dehalogenation of polyvinyl chloride (PVC) wastes, the solid-base catalyst can work as a pore-forming additive to generate hierarchically porous carbon monolith [102]. More recently, Wyss and coworkers developed a Joule heating process to convert mixed plastic wastes into holey and wrinkled flash graphene (HWFG) (Fig. 5f) [103]. The obtained graphene contains rich three-dimensional (3D) and 2D pores and displays a large surface area (874 m2 g–1). Nevertheless, the HWFG only shows a mediocre HER activity (η10 = 613 mV) in an acidic solution, which may be due to its pure carbon composition. Using hard templates is another efficient method to engineer porous structures in carbon catalysts. Niu et al. designed graphene sponges by employing SiO2 spheres as the hard template and chitosan biomass as the carbon source [104]. With a spatial structure and high surface area, the obtained defective N-doped graphene sponge shows a good HER activity (η0.5 = 203 mV) and excellent durability for about 2 h. For potential applications, the stability test should be operated for a longer period to meet the industrial demand.
Doping is powerful to upgrade carbon catalysts’ intrinsic activities [105]. Compared with the nonpolar C–C bonds in pure carbon materials, carbon atoms in heteroatom-doped carbon materials can develop polar bonds with doped heteroatoms (e.g., N, P) to impose different dipole moments depending on their difference in electronegativity and atomic size from those of carbon [106]. Accordingly, an adjustment in the DOS and charge population can be achieved on both the carbon atom and heteroatoms, which would help to improve catalytic activities in various heteroatom-doped carbon materials [107, 108]. Biowastes themselves are effective sources for in situ synthesizing heteroatoms (especially N, S, O)-doped carbon materials [109]. For instance, Cao and coworkers utilized bean sprouts as the carbon precursor to prepare carbon materials due to their self-doping characteristics under the high-temperature calcination condition (Fig. 5g) [38]. The resulting N, S co-decorated carbon catalyst shows acceptable HER activities (η10 = 413 mV, Tafel slope = 98 mV dec−1) with high durability over 2000 CV cycles in acidic media. The influence of N and S dopants on HER performance was disclosed by density functional theory (DFT) calculations. Specifically, S dopants can lead to significant changes in the electronic structures and enhance the adsorption of the H atom intermediate on catalysts, which could improve the HER activity more efficiently than single N doping [110]. Besides nonmetal doping, transitional metals also have been incorporated into carbon materials, such as Zn, S, N self-doped carbon [111], Co, N co-doped carbon [112], Ni, N co-doped carbon [113, 114], and N, P, Ca co-doped biochar [115]. The presence of metal atoms can significantly improve catalytic performance by increasing the electrical conductivity and taking the advantage of synergistic effects of different elements [115]. A special structure is metal-N–C which emerges as a promising candidate for HER [114, 116]; as suggested, the abundant Co–N electroactive sites in the Co, N co-doped carbon (Cox–N–C) contribute to enhanced HER activities [112].
Waste-derived Transitional Metal-based Catalysts for HER
Earth-abundant transitional metals, especially Fe, Cu, Ni, Co, and Mo, are extensively employed for designing high-performance HER catalysts due to their high conductivity, good electrochemical activity, as well as low cost [51, 117]. To further reduce catalysts’ fabrication cost, several studies have converted biowastes and industrial wastes into transitional metal-based HER catalysts.
Starting from the birch tree, Humagain and co-authors design a porous Mo2C catalyst for HER (Fig. 6a), the biowaste-derived biochar acts as the carbon source instead of a carbon substrate [118]. The Mo2C catalyst can efficiently catalyze water reduction in the acidic electrolyte (η10 = 35 mV, η100 = 60 mV), with high durability for 100 h. Besides metal carbides, highly conductive metal phosphides also attain great interest. In 2018, Lin et al. reported a three-step process to transform bulk scrap nickel into 3D Ni2P nanoparticle catalysts (Fig. 6b) [119]. Benefiting from its high intrinsic activity and 3D nanostructure, the obtained Ni2P catalyst exhibits high HER activities in both alkaline and acidic electrolytes with low overpotentials of 73 and 69 mV at 10 mA cm−2, respectively (Fig. 6c-d). Compared with these single component transitional metal-based catalysts, constructing hybrids from wastes can realize enhanced HER performance. Altalhi and coworkers used industrial fly ash (FA) waste with TiO2 to create a FA-TiO2 nanocomposite [120]. With a post-cathodic polarization treatment, the activated FA-TiO2 nanocomposite catalyst shows good HER activities (η10 = 125 mV, Tafel slope = 115 mV dec−1) in the alkaline electrolyte, which are comparable to those of the Pt/C catalyst. Although the FA-based composite exhibits high electrocatalytic performance, it is challenging to identify the activity origin owing to the unclear crystal structure and complicated chemical composition of FA. Since most waste-derived transitional metal-based HER electrocatalysts also show high OER activities (e.g., NiCoMn hydroxides [39]), they will be discussed in the part of waste-derived bifunctional catalysts for OWE.
Fig. 6.
a Diagram of the preparation of the Mo2C catalyst from birch tree [118].
Copyright 2018, Wiley–VCH. b Scheme of the Ni2P catalyst preparation process (PVG: photochemical vapor generation; CVD: chemical vapor deposition). c HER performance of the Ni2P catalyst in acidic and alkaline media [119]. Copyright 2018, Royal Society of Chemistry
Waste-derived Carbon-based Heterostructures for HER
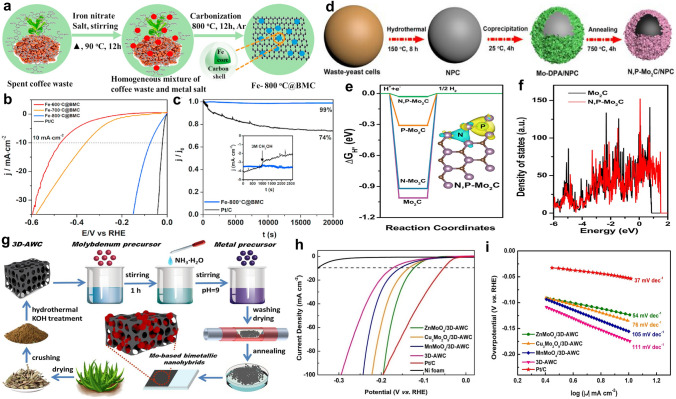
Combining carbon materials’ large surface area and high conductivity and transitional metals’ high intrinsic activity can enhance the HER performance of individual components. In this regard, hybridizing biowaste-derived carbon with transitional metal-based nanomaterials is practical to create favorable HER electrocatalysts [121]. To date, a group of waste-derived carbon-based heterostructures has been realized for HER, such as metal–carbon and metal alloys/oxides/sulfides/phosphides/carbides-carbon hybrids.
Core@shell structured metallic particle@carbon catalysts with strong carbon–metal binding and high stability can be obtained by a reduction reaction. With a carbothermal reduction process, Ahsan et al. developed an ultrathin carbon-shell (4 nm)-coated metallic Fe nanoparticles structure (Fig. 7a) [122]. LSV measurements suggest that the sample prepared at 800 °C (Fe-800 °C@BMC) delivered high HER performance (η10 = 75 mV, Tafel slope = 59 mV dec−1) in acidic media, with high durability (99% of the initial activity preserved after 20000 s) (Fig. 7b-c). Both the hierarchically porous carbon matrix and the strong electronic interaction between carbon shells and metallic Fe cores contribute to the high catalytic performance. An earlier study reported a bio-reduction and calcination route to engineer Au nanoparticles covered by N-doped carbon (Au@NC) [123]. The interface interaction and charge transport between N-doped carbon and Au core significantly benefits the HER process and leads to high activities (η10 = 54.1 mV, Tafel slope = 76.8 mV dec−1).
Fig. 7.
a Diagram of the preparation of Fe-800 °C@BMC catalyst. b LSV curves catalysts in 0.5 M H2SO4. c Chronoamperometric curves of Fe-800 °C@BMC and Pt/C catalysts at the overpotential of 350 mV, the inset shows the crossover effect of Fe-800 °C@BMC and Pt/C catalyst with the addition of 3 M methanol [122].
Copyright 2020, Elsevier. d Illustration of the reutilization of waste-yeast cells to design N, P-Mo2C/NPC catalyst. e Computational hydrogen adsorption free energy (ΔGH*) at the equilibrium potential, and the inset shows Bader charges of the N, P-Mo2C catalyst. f Calculated DOS for Mo2C and N,P-Mo2C catalysts [126]. Copyright 2022, Elsevier. g Scheme of the synthesis of Mo-based bimetallic oxides and their carbon-based hybrids. h LSV curves and i Tafel plots of catalysts in 1.0 M KOH [129]. Copyright 2020, Elsevier
Among all waste-derived carbon-based heterostructures, Mo2C/C catalysts are the most frequently studied. Traditionally, the synthesis of Mo2C needs a high temperature, which would result in severe agglomeration of particles [124]. Alternatively, encapsulating/loading Mo2C nanoparticles in/on a large surface carbon structure can enhance both catalytic efficiency and durability. Thus, biowastes have been extensively used to fabricate Mo2C/C catalysts [46, 125]. To further improve the catalytic performance, heteroatom doping is a favorable option. As shown in Fig. 7d, the N, P co-decorated Mo2C enclosed in the N, P co-doped carbon matrix (N, P-Mo2C/NPC) was prepared from waste-yeast cells [126]. The N, P-Mo2C/NPC hybrid displays a good HER activity (η10 = 84 mV) and high durability in alkaline solution. Further DFT calculations suggest that N and P dopants can significantly tune the electron density of electroactive sites on Mo2C and thus regulates the DOS of Mo2C, resulting in optimized intermediates adsorption energy (Fig. 7e, f). In the highly porous Mo2C/N-rich carbon matrix composite, the N dopant in carbon is also suggested to optimize the intrinsic activity by optimizing hydrogen adsorption strength [127].
Metal oxides/carbon heterostructures also attract enormous attention. Zhou et al. have tested the HER performance of different metal oxides/carbon catalysts prepared from spent ion-exchange resins [128]. Compared with other metal ions (i.e., Ni2+, Ag+, Pb2+, Mn2+, Cr3+, Cd2+, Zn2+ and Co2+), the Fe3+-contaminated resin-derived FeOx/C catalyst shows the best HER activity (η10 = 60 mV). Of note, the CrOx/C prepared from highly toxic metal Cr-containing ion-exchange resins can also attain a good catalytic activity (η10 = 123 mV), which provides a suitable route to reutilize hazardous wastes. Incorporating a second metal into metal oxides can enhance the catalytic performance. In some aloe waste-derived 3D carbon (3D-AWC)-supported Mo-based bimetallic oxides (ZnMoO4, MnMoO4, and Cu3Mo2O9) fabricated through a chemical precipitation route (Fig. 7g), the ZnMoO4/3D-AWC catalyst demonstrates a high HER activity in alkaline media (η10 = 124 mV, Tafel slope = 54 mV dec−1) (Fig. 7h, i) [129]. In-depth computational results indicate the good HER performance of Mo-based bimetallic oxides arises from metallic features and apt energy levels. Another efficient method to upgrade the HER performance of metal oxides/C hybrids is forming heterostructures on the carbon substrate. Upadhyay et al. have engineered a three-component Mo2C/MnO2/C heterostructure from laboratory plastic wastes [46]. In contrast to the Mo2C/C catalyst, the Mo2C/MnO2/C composite performs better toward HER. This is because the extremely fine and intertwine MnO2 nanoflakes develop a network that guarantees efficient electrons/ions transfer and enhances the structural stability over 5000 cycles of CV tests.
A group of metal sulfides/carbon hybrids also have been developed for HER recently. MoS2 is a representative HER catalyst among transitional metal dichalcogenides, due to its layer structures and abundant highly active sites [130]. Zhao et al. reported a plastic waste-derived carbon-supported MoS2 catalyst for HER [131]. The highly active MoS2 nanosheets are finely scattered on the carbon material. Interestingly, the rich pyridinic‒N in the carbon support provides additional electroactive sites, and there is a positive correlation between HER performance and the content of N dopant. The critical role of the carbon support in enhancing MoS2 catalysts’ HER performance is also identified in the organic liquid waste-derived vertical graphene nanosheets (VGNS)/MoS2 hybrid [132]. By combining VGNS with MoS2, the Schottky barrier height is reduced from 0.52 to 0.23 eV in the computational model, which is in line with the experimentally reduced overpotential by ∼ 50 mV. More recently, Ji and co-authors found that Co-doping could raise the HER performance of biowaste-derived MoS2/C [133]. The Co dopant can modulate the electronic structure of MoS2 and contribute to larger planar defect structures, which jointly ameliorate the HER performance of MoS2.
Waste-derived Catalysts for OER
As a central bottleneck of the water electrolysis system, OER with inherently sluggish kinetics requires efficient electrocatalysts to speed the catalytic process [134]. Currently, cost-effective catalysts derived from diverse wastes (e.g., biomass, spent batteries) play a key role in upgrading OER performance. Similar to HER catalysts, the waste-derived OER catalysts listed in Table 2 also can be classified into three categories, namely carbon catalysts, transitional metal-based catalysts, and carbon-based heterostructures. It can be seen that most waste-derived OER catalysts only work in alkaline media because they are likely to be corroded, dissolved, and deactivated in harsh acidic and oxidative conditions [56, 135]. In this part, OER electrocatalysts synthesized from wastes are fully discussed, and some effective catalyst design strategies are outlined.
Table 2.
Summary of representative waste-derived OER electrocatalysts
| Waste | Catalyst | Electrolyte | η10 (mV) | Tafel slope (mV dec−1) |
Refs |
|---|---|---|---|---|---|
| Lignocellulosic biowastes | N, P co-doped biochar | 0.1 M KOH | 347 | 28.8 | [139] |
| Polymer waste | N-doped carbon | 0.1 M KOH | 497 | – | [137] |
|
1.0 M HClO4 |
268 | – | [137] | ||
| Plant residues | N-doped carbon | 0.1 M KOH | 450 | – | [204] |
| Cedar tree cones | N-doped carbon | 1.0 M KOH | 106 | 191 | [205] |
| Plant leaves | N-doped carbon | 0.1 M KOH | 340 (η5) | 191 | [138] |
| Corn stalks | Fe, N co-doped carbon nanosheets | 0.1 M KOH | 309 | 127 | [141] |
| Cornstalks | Co, Fe, B, N co-doped biochar | 1.0 M KOH | 383 | 100.92 | [140] |
| Waste printed circuit boards | FeNiCuSnB | 1.0 M KOH | 199 | 53.98 | [45] |
| Spent Li-ion batteries | Ni0.5Mn0.3Co0.2(OH)2 | 1.0 M KOH | 280 | 6.79 | [157] |
| Stainless steel waste meshes | Anodized stainless steel | 1.0 M KOH | 280 | 63 | [144] |
| Spent capacitors | FeNi hydroxides | 1.0 M KOH | 303 (η20) | 80 | [156] |
| Spent Li-ion batteries | Lithium cobaltate | 1.0 M KOH | 550 | 128 | [148] |
| Waste steel alloy | Steel alloy | 1.0 M KOH | 387 | 64 | [143] |
| Waste Cu cable wires | NiFe LDHa/Cu(OH)2/Cu | 1.0 M KOH | 275 (η20) | 83 | [78] |
| Spent Li-ion batteries | NiCoMnB | 1.0 M KOH | 263 | 57.98 | [150] |
| Waste steel | Fe sheets | 1.0 M KOH | 439 | 60 | [206] |
| Spent Li-ion batteries | MnCo2O4 | 1.0 M KOH | 400 | 80 | [146] |
| Spent Zn − C batteries | Mn3O4 | 0.1 M KOH | 360 | 64 | [207] |
| Spent Li-ion batteries | De-lithiated Li0.4Ni0.5Co0.2Mn0.3O2 | 1.0 M KOH | 236 | 66 | [149] |
| Spent Li-ion batteries | Ni-incorporated LiFePO4 | 1.0 M KOH | 285 | 45 | [76] |
| Rusty stainless steel | Activated stainless steel plate | 1.0 M KOH | 260 | 32 | [145] |
| Spent Li-ion batteries | LiCoOx | 0.1 M KOH | 420 (η9.68) | 67.41 | [147] |
| Spent Li-ion batteries | CoOOH nanosheets | 1.0 M KOH | 301 | 53.8 | [79] |
| Sludge waste | ZnS/N, S co-doped carbon | 0.1 M KOH | 390 | 117 | [166] |
| Spent catalysts | Ni/CNTs/Al2O3 | 1.0 M KOH | 370 | 119 | [160] |
| Biowaste | Co/N-doped carbon | 0.1 M KOH | 390 | 72 | [159] |
| Spent adsorbents | NiCuFeB/C | 1.0 M KOH | 251 | 71.75 | [90] |
| Cotton fabric | Fe3O4/NiS@C | 1.0 M KOH | 310 | 82 | [74] |
| Food waste | FeOx/nanocarbon | 0.1 M KOH | ~ 400 | 41 | [162] |
| Peanut shells | FeNi alloy/N-doped carbon | 0.1 M KOH | 380 | 115 | [161] |
| Cocoons | FeCoNi alloy/B, N co-doped carbon | 1.0 M KOH | 321 | 42 | [158] |
| Diaper waste | NiO/C | 1.0 M KOH | 280 | 62 | [208] |
| Spent Li-ion batteries | NiMnCo-activated carbon | 1.0 M KOH | 350 | - | [165] |
| Waste paper | NiCo phosphate/C | 1.0 M KOH | 351 | 94.44 | [75] |
| Onion peels | Fe3C@N-doped carbon | 1.0 M KOH | 330 | 52 | [209] |
| Banana peels | Ba0.5Sr0.5Co0.8Fe0.2O3−δ/N-doped carbon | 0.1 M KOH | 350 | 65 | [210] |
| Mangosteen skin | NiFe-borate LDH/N-doped carbon | 1.0 M KOH | 243 | 42.7 | [93] |
| Milk powder | NiFeOx/N, P co-doped carbon | 1.0 M KOH | 320 | 59.03 | [163] |
| Millet | Co5.47 N/N-doped carbon | 0.1 M KOH | 390 | 110 | [211] |
| Polyphenylene sulfide | Fe, N, S co-doped carbon | 0.1 M KOH | 339 | 85.9 | [169] |
| Red blood cells | CoS1.097/C | 1.0 M KOH | 260 | 83 | [167] |
| Blood powder | Co3O4/C | 0.1 M KOH | 380 | 47 | [164] |
| Spent Li-ion batteries | Co3O4/C | 1.0 M KOH | 245 | 53 | [212] |
| Human hair | NiO/C | 1.0 M KOH | 320 | 49 | [213] |
| Sludge | NiFe phosphide/heteroatom-doped carbon | 1.0 M KOH | 280 | 56 | [214] |
a LDH: Layered double hydroxides
Waste-derived Carbon Catalysts for OER
Nanocarbon materials prepared from biomass and plastic wastes have shown promising OER performance, and most of them are N-doped carbon [136]. The benefit of N doping includes enhanced electrical conductivity, regulated surface electronic properties, increased structural disorder, and populated defective sites [137]. It is well accepted that the species of N dopants governs the catalytic activity of carbon catalysts. For example, the biomass (euonymus japonicus leaves)-derived N-doped porous carbon nanosheets (NPCNS) synthesized at different pyrolysis temperatures show distinct N contents (Fig. 8a, b) [138]. The sample obtained at 900 °C (NPCNS-900) contains the highest ratio of pyridinic-N, which contributes to its best OER performance compared to its analogues (Fig. 8c). The pyridinic-N shows more moderate adsorption energies toward O and OH intermediates than other N species (graphitic-N, pyrrolic-N), which is the most vital factor for the efficient OER performance of NPCNS-900.
Fig. 8.
a Scheme of synthesis of N-doped porous carbon from plant leaves. b Nitrogen contents in carbon catalysts. c LSV curves of carbon catalysts and the Pt/C catalyst [138].
Copyright 2018, Elsevier. d Diagram of the fabrication of Fe, N co-doped mesoporous and microporous carbon (Fe-MNC). e LSV curves and f corresponding Tafel plots of catalysts in 0.1 M KOH [141]. Copyright 2022, Elsevier
Introducing another electroactive element into the N-doped carbon can effectively enhance the OER performance. Ma and co-authors developed a N, P co-decorated carbon catalyst from lignocellulosic biowastes [139]. Compared with the individual N- or P-doped carbon, the N, P co-doped carbon catalysts show a better OER activity. The main reason is that N and P co-doping contributes to favorable electronic structure and a variety of electroactive defect sites. Apart from nonmetal components, designing metal-N–C structures for OER also have been realized [140]. In Luo and coworkers’ study, the Fe, N co-doped porous carbon (Fe-MNC) catalyst obtained from corn stalk soot (CSS) (Fig. 8d) can catalyze water oxidation efficiently (η10 = 309 mV) [141]. This study also investigated the importance of chemical precursors on the nanostructure and electronic properties of catalysts. Compared with Fe-NC (sample without the presence of melamine), Fe-MC (sample without the presence of 2,2-dipyridine), MC (pyrolysis the hybrid of CSS and melamine), and PMel (pyrolysis bare melamine) catalysts, Fe-MNC with the optimal porous lamellar structure, less defects, and high concentration of active Fe-Nx and Fe-Cx sites exhibits better OER performance (Fig. 8e, f).
Waste-Derived Transitional Metal-based Catalysts for OER
Transitional metal-based nanomaterials are efficient catalysts for alkaline OER [142]. Thus, many metal-rich industrial wastes have been employed to develop OER catalysts. Rich in Fe, ubiquitous steel wastes are great precursors for OER electrocatalysts. Maruthapandian et al. developed an OER electrocatalyst from high speed steel alloy by mechanical milling [143]. With major content of Fe, the steel alloy powder catalyst can act as a good pre-electrocatalyst for OER. After 50 h of the OER durability test, the waste-derived catalyst shows comparable OER activities to the RuO2 catalyst due to the formation of active metal (oxy)hydroxide phases on the catalyst surface. Starting from the industrial stainless steel 316L waste meshes, Gomaa and co-authors designed a self-supported OER catalyst (ASS-O2) via an anodization-annealing (O2 atmosphere) process [144]. Compared with catalysts prepared under other annealing atmospheres (i.e., H2, air), the ASS-O2 catalyst exhibits better OER activities owing to the formation of electroactive Fe2O3 with small amounts of FeCr alloy and NiO on the surface. A similar study also suggests that rusty stainless steel can be used as efficient free-standing OER catalysts due to the high conductivity, good mechanical stability, and especially the generated plentiful Fe/(Ni) oxyhydroxides on the catalyst surface during the electrochemical process [145].
Transitional metal oxides derived from spent batteries are a group of promising OER catalysts, and the chemical composition, surface chemistry, and nanostructure of oxides largely influence the catalytic properties. Natarajan et al. found that the OER performance of spent Li-ion batteries-derived spherical and porous spinel MnCo2O4 was better than the monometallic Co3O4 and MnO2 [146], and the reason for the better performance of MnCo2O4 has been attributed to its structural features. Lithium cobalt oxides can be easily obtained from Li-ion batteries. Chen and co-authors found that a long-time cycling treatment of LiCoOx could result in smaller particle size and activated surface, and thus contributes to enhanced OER activities [147]. Another study introduced a solvent extraction-calcination method to recover LiCoOx from Li-ion batteries [148]. The obtained oxides catalyst (calcinated at a low temperature of 600 °C) with optimal small particle size (20–100 nm) and surface area (4.8027 m2 g−1) outperforms its counterparts for OER. The synchronous reutilization of multi-metals in spent Li-ion batteries can not only shorten waste recycling procedures but also innovate mixed metal oxide catalysts. Lv et al. proposed an electric field-driven de-lithiation method to design high-performance OER catalysts from the cathode (Fig. 9a) [149]. The de-lithiated Li0.4Ni0.5Co0.2Mn0.3O2 cathode materials display a high specific surface area and a large amount of lattice oxygen, which contribute to high OER activities (η10 = 236 mV, Tafel slope = 66 mV dec−1). Sometimes, incorporating a foreign active species is necessary to enhance the catalytic properties of spent cathodes. For example, introducing a Ni promoter significantly improves the catalytic performance of spent LiFePO4 [76]. Theoretically, the insertion of Ni can effectively activate Fe sites by regulating the adsorption strength of the *OOH intermediate; also, the abundant oxygen defects promote the oxygen desorption step, which synergistically upgrade the spent LiFePO4 material’s OER performance.
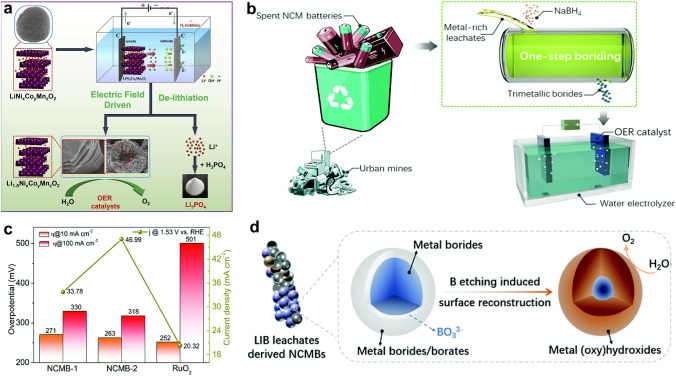
Fig. 9.
a Scheme of the electric field-driven de-lithiation process for preparing LiNixCoyMnzO2 OER catalysts [149].
Copyright 2021, Elsevier. b Illustration of the fabrication of magnetic NiCoMnB catalysts (NCMBs) from the spent batteries. c η10, η100, and the current density at 1.53 V (vs. reversible hydrogen electrode, RHE) of catalysts. d Scheme of the surface reconstruction process of spent Li-ion batteries-derived NCMBs [150]. Copyright 2021, Royal Society of Chemistry
Besides oxides, mixed metal borides synthesized from electronic wastes also have shown excellent OER catalysts [45]. As illustrated in Fig. 9b, spent Li-ion batteries can be directly converted into magnetic Ni-Co-Mn borides (NCMBs) through a fast and efficient NaBH4-mediated boriding process [150]. After the boriding reaction, the metal ion concentrations in the solution are below the emission limits of related standards, indicating the boriding process can ease the following waste effluent management process. The NCMB-2 catalyst with a larger ratio of Ni and Co content (38.4% vs. 20.3%) shows a higher OER activity (Fig. 9c), compared with the NCMB-1 analogue. In such a manner, it would be efficient to improve NCMBs’ OER performance by adjusting the composition of spent batteries precursors. In addition, the metal borides undergo surface reconstruction initiated by boron leaching and form stable metal (oxy)hydroxides on the catalyst surface (Fig. 9d). Such in situ surface/structure reconstruction processes have well been identified for various transitional metal-based catalysts [151–155], which becomes an important guideline for novel OER catalyst design.
The aforementioned studies emphasize the importance of metal (oxy)hydroxides because of their high catalytic performance and durability for alkaline OER. Consequently, it is sensible to develop metal (oxy)hydroxides directly from wastes. The NiFe hydroxides and NiCu hydroxides synthesized from upcycled capacitors [156], spent Li-ion batteries-derived Ni0.5Mn0.3Co0.2(OH)2 [157], NiFe LDH/Cu(OH)2/Cu prepared from spent Cu cable wires [78], and CoOOH obtained from spent Li-ion batteries [79] are representative efficient OER catalysts. Among these catalysts, the self-supported NiFe LDH/Cu(OH)2/Cu catalyst delivers a good OER activity (η100 = 390 mV) with excellent stability for 24 h, owing to its hierarchically heterostructural feature [78]. The multiphase heterostructure can ensure wealthy and multiple electroactive sites and endow fast mass/charge transport during OER.
Waste-Derived Carbon-based Heterostructures for OER
The high conductivity, large surface area, and redox properties of carbon materials make them good substrates to support transitional metal-based electroactive nanomaterials, intending to achieve high OER performance. Different categories of transitional metal-based materials/carbon heterostructures have been synthesized from a range of wastes, which are detailed in this part.
Metal/alloy nanoparticles feature high electrical conductivity and catalytic activities. Compositing metal/alloy with carbon is capable of mitigating the severe aggregation and growth of metal/alloy nanoparticles, thus populating electroactive sites and also enhancing the structural stability of catalysts [158]. Chen et al. prepared a hierarchically structured catalyst (Co@Co–N, S–C) from biowaste, integrating Co–N–C structures and encased Co nanoparticles [159]. The well-developed interface between hierarchical structures, the large SSA, and rich Co nanoparticles encapsulated in carbon layers jointly contribute to a high OER activity. With suitable structural and electronic properties, the spent methane decomposition catalyst (Ni/CNTs/Al2O3) can be directly used as an OER catalyst [160]. In this tricomponent hybrid, Ni nanoparticles act as electroactive sites toward OER, CNTs can facilitate low charge transfer resistance, and the Al2O3 provides porous support. Although the authors declared good stability of the Ni/CNTs/Al2O3 catalyst for 20 h, it should be cautious that the Al2O3 support may suffer from leaching/dissolution in the strong alkaline electrolyte. Besides metal particles, Yang and coworkers developed a FeNi alloy/N-doped porous carbon catalyst from peanut shells [161]. The alloy/carbon hybrid prepared at 900 °C with a higher SSA and porous size outperforms its analogues for OER owing to the enhanced mass/charge transfer and abundant active sites.
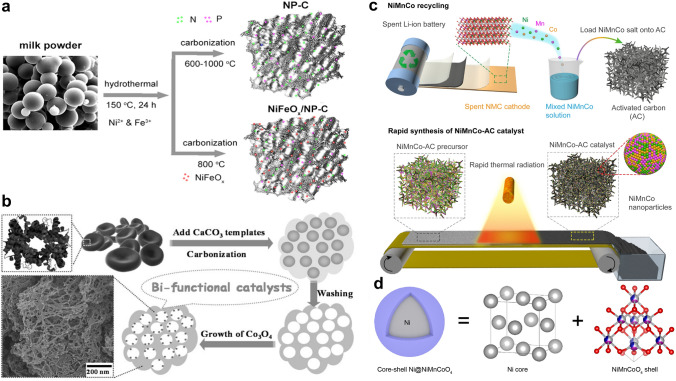
Loading metal (hydr)oxides on carbon scaffolds attracts growing interest in OER catalyst design, and the main reason is that the conductive carbon can effectively compensate for the relatively low conductivity of metal (hydr)oxides [162]. The OER performance of metal (hydr)oxides/carbon heterostructures can be optimized by regulating the external and internal properties of both metal (hydr)oxides and carbon materials. With a hydrothermal treatment-carbonization process, Chen et al. incorporated NiFeOx nanoparticles (~ 10 nm) into N, P co-decorated carbon derived from milk powder (Fig. 10a) [163]. Benefiting from porous carbon’s large surface area and NiFeOx nanoparticles’ high activities, the NiFeOx/carbon hybrid delivers a good OER activity. To regulate the nanostructure of carbon substrate, Zhang and co-authors proposed a CaCO3-involved approach to synthesize Co3O4/heteroatom-doped carbon catalysts (Fig. 10b) [164]. It is interesting to find that using CaCO3 as the template and activator leads to a unique fibrous network structure. The carbon material with a large surface area and rich heteroatom dopants can provide abundant anchoring sites for Co3O4, which significantly limit particle growth and aggregation and also improve the charge transfer process. Moreover, the intimate contact of Co3O4 and the carbon support leads to synergistic effects for OER.
Fig. 10.
a Scheme of the preparation of milk powder-derived NP-C and NiFeOx/NP-C catalysts [163].
Copyright 2018, Elsevier. b Illustration of the synthesis of Co3O4 decorated BDHC [164]. Copyright 2014, Wiley–VCH. c Scheme of the preparation of the NiMnCo-AC electrocatalyst from spent Li-ion batteries. d Core–shell model of the NiMnCo nanoparticle [165]. Copyright 2022, National Academy of Sciences, USA
Regulating metal (hydr)oxides’ properties can directly alter the catalytic performance of metal (hydr)oxides/carbon heterostructures. For instance, the intercalated borates in the hierarchical NiFe-borate LDH/N-doped carbon catalyst play a positive effect on the OER performance by improving the hydrophilicity, enlarging the surface area, populating electroactive sites, and providing abundant mass/charge transport pathways [93]. Alternatively, engineering a metal/oxide heterostructure on carbon materials is suggested to enhance the catalytic performance by the strong electronic interactions between the electroactive metals and oxides. Jiao et al. proposed a rapid thermal radiation strategy for transforming spent Li-ion batteries into a NiMnCo/carbon catalyst (NiMnCo-AC) for OER (Fig. 10c) [165]. Detailed characterizations suggest that the NiMnCo nanoparticles show a Ni@NiMnCoO4 core–shell nanostructure, including spinel NiMnCoO4 shell and fcc-structured Ni core (Fig. 10d). Further DFT calculations suggest that the charge density redistribution at the Ni/ NiMnCoO4 interface induced by Ni core and rich electroactive sites on the NiMnCoO4 shell ensure good OER performance.
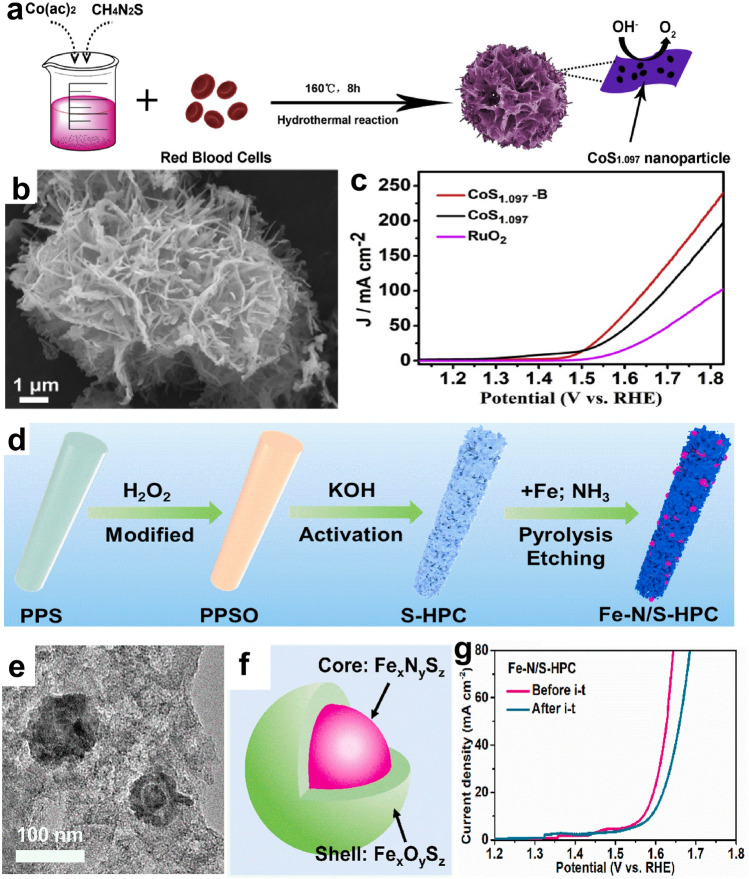
Carbon materials coupled with metal sulfides/nitrides/carbides/phosphides/borides heterostructures developed from wastes are promising OER catalysts. Compared with metal (hydr)oxides, the main merit of metal sulfides, nitrides, carbides, phosphides, and borides is their better electrical conductivity. Recent studies have emphasized the rational design of these metal compounds/carbon hybrids from wastes. Using dye sludge as the carbon source, Peng and coworkers developed a ZnS-involved N, S co-decorated carbon (ZnS/NSC) via a ZnCl2-involved pyrolysis process [166]. As suggested, the better OER performance of the ZnS/NSC catalyst prepared at a higher temperature (1000 °C) is due to its higher relative content of ZnS in the hybrid than catalysts synthesized at lower temperatures (800 and 900 °C). To improve the catalytic activity of CoS, a biowaste-derived carbon was introduced via a hydrothermal process (Fig. 11a) [167]. The obtained composite shows a flower-like (Fig. 11b) structure which enables high surface area, abundant active sites, and enhanced diffusion kinetics. It also can be seen that the nanoflower structure can provide abundant acute geometry at the nanoscale. Such features are favorable for concentrating the localized electric field at tips and providing enhanced adsorption of reaction intermediates, which would enhance the reaction kinetics [168]. Hence, the sulfide/carbon catalyst outperforms the bare sulfide and the RuO2 catalyst for OER (Fig. 11c).
Fig. 11.
a Schematic of the fabrication of CoS1.097-B catalyst. b SEM image of CoS1.097-B catalyst. c LSV curves of catalysts in 1.0 M KOH [167].
Copyright 2020, Elsevier. d Scheme of Fe–N/S-HPC preparation from polyphenylene sulfide (PPS) plastic. e Transmission electron microscopy (TEM) image of Fe–N/S-HPC. f A model of Fe-based nanoparticles in Fe–N/S-HPC. g LSV curves of Fe–N/S-HPC before and after the 15 h OER durability test [169]. Copyright 2022, Elsevier
Another method that enables upgrading sulfides/carbon catalysts’ OER performance is constructing electroactive hybrids on carbon materials. For example, Jiang et al. designed Fe3O4/NiS heterostructures on free-standing fibrous carbon (Fe3O4/NiS@C) [74]. The ternary composite shows excellent OER performance (η10 = 310 mV) and stability (no current density loss after 26 h OER operation) owing to the synergistic effect between electroactive Fe3O4 and NiS, as well as the self-standing hierarchically porous carbon structure. Using the sulfur-rich polyphenylene sulfide as the precursor, a Fe, N, S co-decorated porous carbon (Fe–N/S-HPC) was fabricated via a three-step route (Fig. 11d) [169]. The obtained Fe–N/S-HPC catalyst exhibits a honeycomb-like structure, and it is visible that rich spherical Fe particles are disseminated in the carbon matrix (Fig. 11e). Further characterizations suggest an electroactive FexNySz@FexOySz structure (Fig. 11f), which can significantly enhance the OER activity and durability. As displayed in Fig. 11g, Fe–N/S-HPC reserves a high OER activity after the electrochemical stability test for 15 h.
Waste-Derived Bifunctional Catalysts for OWE
Developing bifunctional electrocatalysts for OWE is of great significance in light of system simplification, cost reduction, and large-scale application of electrolyzers [170–172]. Encouragingly, many waste-derived catalysts deliver high activities toward both HER and OER (Table 3) and such cost-effective bifunctional electrocatalysts profoundly push the development of green hydrogen production. In this part, recent waste-derived bifunctional electrocatalysts for OWE are discussed.
Table 3.
Summary of representative waste-derived electrocatalysts for OWE
| Waste | Catalyst | Electrolyte | E10a (V) | Durability | Refs |
|---|---|---|---|---|---|
| Camellia flower | S-doped carbon | 1.0 M KOH | 1.76 | 24 h @ ~ 20 mA cm−2 | [174] |
| Corn stalks | Few-layer N-doped porous carbon | 1.0 M KOH | 1.60 | – | [173] |
| Rose flower | Ni-doped graphitic carbon | 1.0 M KOH | 1.64 | 24 h @ 1.64 V | [176] |
| Textile sludge | Fe, N co-doped carbon | 1.0 M KOH | 1.70 | 14 h @ 1.7 V | [177] |
| Battery industrial wastewater | NiCoMn LTHs | Alkaline wastewaterb | 1.58 | 24 h @ 1.6 V | [39] |
| Spent Li-ion batteries | Ni/Ni-Mn-Co–O | 1.0 M KOH | 1.62 | 25 h @ 1.58 – 1.66 V | [178] |
| Scrap copper wires | NiCoP/Cu | 1.0 M KOH | 1.59 | 24 h @ 10 mA cm−2 | [77] |
| Cornstalks | β-Mo2C/C | 1.0 M KOH | 1.65 | 30 h @ 10 mA cm−2 | [215] |
| Cotton fibers | Co/carbon tubes | 1.0 M KOH | 1.40 | 110 h @ 1.4 V | [179] |
| Miscanthus stems | Co/C | 1.0 M KOH | 1.45 | 120 h @ 50 mA cm−2 | [47] |
| Duckweed | NiFe-alloy/N, S-doped carbon | 1.0 M KOH | 1.61 | 200 h @ 2 V | [181] |
| Alfalfa | NiFe/N, P, S co-doped carbon | 1.0 M KOH | 1.60 | 50 h @ 10 mA cm−2 | [182] |
| Grapefruit peels | NiFe-alloy/ N-doped carbon | 1.0 M KOH | 1.63 | – | [180] |
| Magnolia leaves | CoP/C | 1.0 M KOH | 1.56 | 24 h @ 1.59 V | [216] |
| Polysaccharide chitin | Co2P/N, P co-doped carbon | 1.0 M KOH | 1.65 | 10 h @ ~ 20 mA cm−2 | [190] |
| Ginkgo leaves | Co2P@CoP/C | 1.0 M KOH | 1.63 | 1000 min @ 10 mA cm−2 | [189] |
| Cauliflower leaves | Ni/NiO/N-doped carbon | 0.1 M KOH | 1.688 | 20 h @ 10—30 mA cm−2 | [188] |
| Amaranth | Fe, N co-doped carbon | 1.0 M KOH | 1.53 | 30 h @ 1.53 V | [183] |
| Lotus leaves | Co/MoO2@N doped carbon | 1.0 M KOH | 1.629 | 48 h @ 10 mA cm−2 | [186] |
| Holly leaves | Co-CoO/C | 1.0 M KOH | 1.77 | – | [187] |
| Chicken feathers | Ni-Co oxides/C | 1.0 M KOH | 1.53 | 200 h @ 1.7 V | [185] |
| Willow catkins | Co3O4/N-doped hollow carbon | 1.0 M KOH | 1.74 | 20 h @ 1.74 V | [184] |
| Waste yeast | Cu8S5 decorated N, S co-doped porous carbon | 1.0 M KOH | 1.64 | 14 h @ 10 mA cm−2 | [191] |
| Tissue paper | Co9S8@Co–N/C nanorods | 1.0 M KOH | 1.61 | 70 h @ 10 mA cm−2 | [192] |
| Catkin | MoS2@NiOOH@C | 1.0 M KOH | 1.62 | 40 h @ 1.62 V | [194] |
| Willow catkins | NiFe LDH/(NiFe)Sx/hollow carbon | 1.0 M KOH | 1.53 | 100 h @ 10 mA cm−2 | [48] |
| Spent Li-ion batteries | CoN/graphene | 1.0 M KOH | 1.61 | 40 h @ 1.68 V | [195] |
aE10: Applied voltage at the current density of 10 mA cm−2
bAlkaline wastewater: Wastewater with 1 M KOH
Waste-Derived Carbon Catalysts for OWE
Engineering the nanostructure and electronic properties of some heteroatom-doped carbon materials can catalyze HER and OER synchronically. Using the mixture of corn stalks soot and melamine as the precursor, Liu and co-authors developed a N-doped porous carbon electrocatalyst (NPCSS) for OWE [173]. With large SSA, abundant electroactive sites, and rich electrochemically active pyridinic/pyrrolic N species, NPCSS acquires 10 mA cm−2 at 1.60 V in a two-electrode cell. Besides N-doped carbon, S self-doped carbon also can catalyze water splitting. Xia et al. prepared the S self-doped activated camellia (SA-Came) carbon nanospheres from camellia flowers through a hydrothermal treatment–pyrolysis route (Fig. 12a) [174]. The obtained SA-Came catalyst shows a densely interconnected spherical morphology with a small particle size of approximately 50 nm (Fig. 12b). The rough surface and rich nanopores of the catalyst contribute to increased micropores and mesopores, which further enlarge the pore volume and surface area and lead to efficient mass/charge transfer during electrochemical processes. In addition, the abundant S sites induced more polarized surface domains with highly active sites, which benefit the electrocatalytic performance. To this end, the SA-Came catalyst requires a small η (0.53 V) to attain 10 mA cm−2 (Fig. 12c) with good performance stability for 24 h (Fig. 12d).
Fig. 12.
a Scheme of the preparation of SA-Came nanospheres. b SEM image of SA-Came. c LSV curve and d Chronoamperometry analysis of the SA-Came assisted water electrolyzer [174].
Copyright 2022, Wiley–VCH
Incorporating active transitional metals into carbon materials is expected to attain enhanced catalytic performance [175]. For example, loading Ni onto the high graphitic carbon takes the advantage of graphitic carbon’s excellent electrical conductivity and the high electrocatalytic activity of Ni species [176]. In another study, Zhang et al. found that Fe species in the textile sludge facilitated the graphitization process of pyrolyzed 3D interconnected hierarchical Fe, N co-decorated carbon (TS–Fe–N–C) [177]. Combined with the high pyridinic-N content, uniformly distributed Fe-Nx and Fe3C electroactive sites, and hierarchical structure, the TS–Fe–N–C gains a high activity toward OWE (E10 = 1.70 V).
Waste-Derived Transitional Metal-based Catalysts for OWE
Transitional metals-rich wastes are highly desirable precursors for preparing bifunctional OWE electrocatalysts because of the high activity of transitional metals and low cost. For instance, Zheng et al. employed an ultrafast carbothermal shock method to transform the spent cathode of Li-ion batteries into a Ni/Ni-Mn-Co–O hybrid catalyst for OWE (E10 = 1.62 V) [178]. The co-presence of Ni-Mn-Co oxides and Ni metal ensures great conductivity and catalytic activity. Additionally, the hybrid’s small size and large electrochemically active surface area facilitate the exposure of abundant catalytic sites, promoting the mass/charge transfer process. Recently, our group has focused on the close-loop utilization of battery industrial wastewater with an electrodeposition-electrolysis route (Fig. 13a) [39]. In this process, the main metal ions (i.e., Ni, Co, Mn) have been converted into NiCoMn LTHs via electrodeposition, which shows favorable catalytic performance for OER and HER. The optimal deposit (S-3) possesses a hierarchical nanoflower structure that can act as a highly competitive electrocatalyst for post-electrodeposition (PE) wastewater electrolysis (Fig. 13b). The S-3||S-3-driven wastewater electrolyzer attains a higher hydrogen production efficiency at a much lower cost than the RuO2||Pt/C couple (Fig. 13c).
Fig. 13.
a Scheme of wastewater electrolyzed by wastewater-derived NiCoMn LTHs. b LSV curves of OWE of S-3||S-3 and RuO2||Pt/C in alkaline-deionized water and post-electrodeposition (PE) wastewater. c Comparison of hydrogen production rate and catalyst cost for the S-3||S-3 and RuO2||Pt/C PE wastewater electrolyzer systems [39].
Copyright 2022, Royal Society of Chemistry. d Schematic of the NiCoP/Cu electrode preparation. e Scheme of the OWE configuration. f LSV curves of NiCoP/Cu electrode before and after 24-h electrolysis [77]. Copyright 2018, Wiley–VCH
Besides the battery-based wastes-derived metal (hydr)oxides, the abundant waste Cu wires were used as a support to design phosphide-based bifunctional electrocatalysts for OWE [77]. Via electrodepositing highly active amorphous NiCoP films on the Cu wire, the obtained NiCoP/Cu hybrid shows high HER and OER activities. Using as a bifunctional catalyst, the NiCoP/Cu electrode attains 10 mA cm−2 at 1.59 V (Fig. 13e) and displays good stability in 24 h (Fig. 13f). These successful practices hint that it is convenient to engineer free-standing high-performance bifunctional electrocatalysts from metal-rich solid wastes/effluents.
Waste-Derived Carbon-Based Heterostructures for OWE
To ameliorate transitional metal-based materials’ catalytic properties, introducing a highly conductive carbon support is usually implemented. In consequence, exploring eco-friendly and low-cost waste-derived carbon to prepare carbon/transitional metal-based materials heterostructures for OWE is greatly attractive for advancing efficient water electrolysis systems. Encouragingly, diverse transitional metal-based materials (e.g., metals, alloys, carbides, nitrides, oxides, sulfides, and phosphides) have been successfully coupled with biowaste-derived carbon, and the formed hybrids exhibit high performance toward OWE.
Loading highly electroactive and conductive metals or alloys within the carbon matrix is suggested to attain all-around performance for OWE [47]. Recently, Jiang and co-authors developed Co particles/biomass carbon tubes (Co-BCTs) catalysts from cotton fibers [179]. Using as the bifunctional electrocatalyst for OWE, the CO-BCTs can deliver 10 mA cm−2 at an applied potential of 1.40 V. Detailed characterizations imply the tight connection of Co particles with BCTs enhances conductivity and electron transfer kinetics, while BCTs’ loosely hierarchical structure facilitates mass/charge transport and sustains high stability. Apart from metal nanoparticles, Son et al. introduced Co single atoms, nanoclusters, and nanoparticles to a waste-derived self-standing carbon material with interconnected fibrous networks [47]. The multi-sized Co species decorated carbon catalyst shows high performance for OWE (E10 = 1.45 V), and the origin of electrochemical activities for different reactions has been disclosed. Specifically, the co-presence of Co single atoms and nanoparticles mainly contributed to the OER activity, while the principal electroactive sites for HER should be Co–Nx sites. NiFe-alloys hybridized N-doped graphene-like carbon [180], N, S co-doped mesoporous carbon [181], and N, P, S tri-doped nanocarbon [182] are also active toward OWE.
Metal oxides/carbon heterostructures can benefit from carbon’s high surface area and excellent conductivity and metal oxides’ high catalytic activity, thus forming efficient OWE catalysts. For instance, FeOx [183] and Co3O4 [184] decorated N-doped hierarchical porous carbon have been reported as bifunctional electrocatalysts for OWE. To upgrade monometallic oxides’ intrinsic activity, recent studies have designed bimetallic oxides and metal/metal oxide hybrids on carbon supports. Compared with the NiCoOx/carbon hybrid, the electrochemical activities of CoOx/carbon and NiOx/carbon are lower for OWE [185]. This is because the co-presence of Ni and Co can provide more catalytically active sites than its monometallic analogues. Recently results implied that synergistic effects between metal/metal oxides could boost metal oxides/carbon hybrids’ electrochemical properties [186–188]. In an efficient bifunctional Co–CoO nanoparticles/porous carbon catalyst, multiple active sites are involved for OWE, including Co–CoO, Co–N–C, and N-doped carbon [187]. Of note, a high N content (primarily graphitic-N, pyridinic-N) in the carbon can regulate the charge distribution of adjacent carbon atom and improve the hydrophobicity of catalysts. Additionally, the rich Co–CoO nanoparticles with strong synergistic effects can provide highly active sites toward both HER and OER, thereby realizing high catalytic activities.
Metal phosphides are promising bifunctional electrocatalysts for OWE, and a feasible way to further upgrade metal phosphides’ catalytic performance is by coupling them with porous carbon materials [189]. Starting from natural polysaccharide chitin, Li et al. designed a metal phosphide-based core–shell hybrid for OWE, which is composed of Co2P core and N, P co-decorated porous carbon shell (Co2P@NPPC) (Fig. 14a) [190]. Abundant Co2P nanoparticles are well isolated and fixed in the porous carbon, which can enhance electron transfer, expose rich active sites, and keep good stability for catalytic reactions (Fig. 14b-c). In another bifunctional Co2P@CoP/N, S co-doped carbon hybrid, the role of each component in catalyzing OWE has been uncovered by Lin and coworkers [189]. Specifically, the synergistic effects of the Co2P@CoP heterostructure make a near-zero ΔGH*, resulting in a high HER activity. Also, the Co2P@CoP hybrid causes the conduction band to bend downwards, leading to high OER activity. Moreover, N and S dopants can tune the carbon support’s electronic property, and sustain the suitable electron-donating feature to enhance overall electrocatalytic properties.
Fig. 14.
a Diagram of the preparation of Co2P@NPPC. b TEM and c high resolution TEM (HRTEM) images of Co2P@NPPC [190].
Copyright 2021, Royal Society of Chemistry. d Scheme of the synthesis of the MoS2@NiOOH@C-MC composite. e Stability test of the Pt/C||IrO2 and MoS2@NiOOH@C-MC||MoS2@NiOOH@C-MC couples for OWE, at 10 mA cm−2. f The time-dependent of the experimental and theoretical H2 and O2 production amounts during OWE using the MoS2@NiOOH@C-MC catalyst [194]. Copyright 2022, Elsevier. g Scheme of the preparation of tiny CoN-coupled graphene hybrid (CoN-Gr-2). h The OWE performance of the CoN-Gr-2||CoN-Gr-2 and Pt/C||RuO2 couples. i Stability test of CoN-Gr-2 for OWE in 1.0 M KOH at 1.68 V [195]. Copyright 2021, Elsevier
Metal sulfides/carbon heterostructures have been widely designed from wastes for OWE. For instance, the textile sludge derived Cu8S5/N, S co-doped porous carbon [191], and the Co9S8/carbon nanorod framework (Co9S8@Co–N/C) fabricated from waste tissue paper towel [192] are highly efficient bifunctional catalysts. Constructing electroactive hybrids on carbon support is a universal method to boost the performance of metal sulfides/carbon heterostructures [48]. Typically, MoS2 is an active HER catalyst with low OER performance [193]. To make a bifunctional catalyst with MoS2, it is necessary to incorporate an OER active component. Liu et al. developed the MoS2@NiOOH hybrid on mesoporous carbon support synthesized from catkin (MoS2@NiOOH@C-MC) via a three-step process (Fig. 14d) [194]. By combining the high OER activity of NiOOH, HER activity of MoS2, as well as C-MC’s efficient charge transfer kinetics, the multicomponent MoS2@NiOOH@C-MC performs better than the Pt/C||IrO2 couple for OWE regarding catalytic activity and performance durability (Fig. 14e). Also, the high reaction Faraday efficiencies of 99.6% (HER) and 98.7% (OER) further evidence the excellent catalytic performances of the catalyst toward OWE (Fig. 14f). Different from most carbon-based heterostructures made from biowastes, Liu and co-authors prepared a CoN/graphene composite from spent Li-ion batteries (Fig. 14g) [195]. Benefiting from the high intrinsic conductivity and activity, sea–urchin-like nanostructure, and high surface area, the optimal sample (CoN-Gr-2) shows comparable performance to the Pt/C||IrO2 couple for OWE, with better stability for 40 h at 1.68 V (Fig. 14h, i).
Conclusions and Perspectives
Following circular economy principles, engineering-efficient electrocatalysts from wastes for water electrolysis is of great environmental and economic benefits. In this review, recent achievements in the design of waste-based catalysts for HER, OER, and OWE have been systematically analyzed. Diverse wastes (especially biowastes and electronic wastes) have been successfully converted into electrocatalysts via pyrolysis, electrochemical synthesis, wet-chemical synthesis, microwave synthesis, etc. The waste-derived carbon-based catalysts, transitional metal-based catalysts, and carbon-based heterostructures have exhibited good performance toward HER, OER, and OWE. Catalysts’ performance is highly related to their nanostructure, chemical composition, and electronic property, which can be regulated by waste precursors and synthesis methods.
Despite these exciting scientific achievements, many opportunities implore further investigations in this expanding field.
Exploring diverse wastes for the design of high-performance electrocatalysts. Currently, most studies focus on biowastes and some electronic wastes (mainly spent batteries), while other types of wastes (e.g., plastic wastes, liquid wastes) are still less explored. More attention should be paid to the reutilization of ever-growing plastic wastes due to their high carbon content, large quantity, and hazardous effect on the ecosystem. Since heteroatom-doped carbon is more active than pure carbon materials for electrochemical applications, it is better to choose biowastes/plastic wastes with a high content of non-carbon elements (e.g., N, P, S, B) as the catalyst precursors. In addition, it is a sensible way to co-treat biowastes/plastic wastes and electronic wastes to form transitional metal materials/carbon heterostructures which have shown favorable catalytic performance toward HER, OER, and OWE.
It is crucial to adopt advanced techniques to gain clear and fundamental insights into the origin of electrochemical activity. Most wastes present complicated compositions and structures, which causes many challenges in catalytic mechanism investigations and the reproducibility of research. To this end, integrating advanced analytical, electrochemical, microscopic, spectroscopic, and computational techniques to investigate the composition-structure-catalytic performance relationship would guide the design of high-efficiency electrocatalysts. Importantly, easily overlooked defects, dopants, and single-atom sites in waste-derived catalysts should be checked carefully, because these features can profoundly govern the catalytic performance.
To realize large-scale production of catalysts from wastes, facile and low-cost fabrication techniques are required. Considering the environmental impacts, catalyst preparation processes with limited carbon emissions and low energy consumption are highly suggested. Some techniques like electrodeposition, ball milling, plasma synthesis, and flash Joule heating are favorable options. Importantly, it is suggested to perform a pre-treatment process to remove hazardous and toxic substances (e.g., radioactive elements) in some typical wastes before the preparation and utilization of waste-derived catalysts. In a circular economy view, it is feasible to recover and reuse the spent waste-derived electrocatalysts for further applications.
Waste-derived electrocatalysts have shown promising performance for water electrolysis, but they are still far from satisfactory. To further improve the catalytic performance of waste-derived catalysts, advanced strategies are encouraged to improve the intrinsic catalytic activity, electroactive sites, mass/charge transfer, mechanical and electrochemical stability of catalysts. In this context, implementing sophisticated methods (e.g., heteroatom doping, nanostructure design, defect engineering, heterostructure construction, and crystallinity control) to synergistically regulate catalysts’ internal and external characteristics would meet the necessities for waste-derived electrocatalysts toward practical water electrolysis.
Considering the high redox property and low cost of waste-derived catalysts, it is of great environmental and economic value to implement waste-derived catalysts in other electrochemical reactions related to environmental remediation and energy storage/conversion, such as nitrogen/nitrate reduction, organic pollutant oxidation/reduction, oxygen reduction, carbon dioxide reduction, hydrogen oxidation, and biomass oxidation. The wide application of waste-derived catalysts would help to minimize the carbon footprint of functional materials preparation and largely facilitate waste management.
Acknowledgements
This work is supported by the Australian Research Council (ARC) Discovery Project (DP220101139). Dr. Wei Wei acknowledges the support of the Australian Research Council (ARC) through Project DE220100530. Dr. Yiwen Liu acknowledges the support of the Australian Research Council (ARC) through Project DE200100970.
Funding
Open access funding provided by Shanghai Jiao Tong University.
Contributor Information
Sining Yun, Email: yunsining@xauat.edu.cn.
Bing-Jie Ni, Email: bingjieni@gmail.com.
References
- 1.Burton N, Padilla R, Rose A, Habibullah H. Increasing the efficiency of hydrogen production from solar powered water electrolysis. Renew. Sustain. Energy Rev. 2021;135:110255. doi: 10.1016/j.rser.2020.110255. [DOI] [Google Scholar]
- 2.Huang J, Xie Y, Yan L, Wang B, Kong T, et al. Decoupled amphoteric water electrolysis and its integration with Mn–Zn battery for flexible utilization of renewables. Energy Environ. Sci. 2021;14:883–889. doi: 10.1039/D0EE03639K. [DOI] [Google Scholar]
- 3.Song J, Wei C, Huang ZF, Liu C, Zeng L, et al. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 2020;49(7):2196–2214. doi: 10.1039/C9CS00607A. [DOI] [PubMed] [Google Scholar]
- 4.Qian G, Chen J, Yu T, Luo L, Yin S. N-doped graphene-decorated NiCo alloy coupled with mesoporous NiCoMoO nano-sheet heterojunction for enhanced water electrolysis activity at high current density. Nano-Micro Lett. 2021;13:77. doi: 10.1007/s40820-021-00607-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang X, Li L, Zhao S, Tong L, Li Z, et al. MOF-like 3D graphene-based catalytic membrane fabricated by one-step laser scribing for robust water purification and green energy production. Nano-Micro Lett. 2022;14:174. doi: 10.1007/s40820-022-00923-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osman AI, Deka TJ, Baruah DC, Rooney DW. Critical challenges in biohydrogen production processes from the organic feedstocks. Biomass Conv. Bioref. 2020 doi: 10.1007/s13399-020-00965-x. [DOI] [Google Scholar]
- 7.Fu G, Kang X, Zhang Y, Yang X, Wang L, et al. Coordination effect-promoted durable Ni(OH)2 for energy-saving hydrogen evolution from water/methanol co-electrocatalysis. Nano-Micro Lett. 2022;14:200. doi: 10.1007/s40820-022-00940-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Vijayakumar P, Liu Q, Sakthivel T, Chen F, et al. Shining light on anion-mixed nanocatalysts for efficient water electrolysis: fundamentals, progress, and perspectives. Nano-Micro Lett. 2022;14:43. doi: 10.1007/s40820-021-00785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osman AI, Mehta N, Elgarahy AM, Hefny M, Al-Hinai A, et al. Hydrogen production, storage, utilisation and environmental impacts: a review. Environ. Chem. Lett. 2022;20:153–188. doi: 10.1007/s10311-021-01322-8. [DOI] [Google Scholar]
- 10.Han N, Liu P, Jiang J, Ai L, Shao Z, et al. Recent advances in nanostructured metal nitrides for water splitting. J. Mater. Chem. A. 2018;6(41):19912–19933. doi: 10.1039/C8TA06529B. [DOI] [Google Scholar]
- 11.Wang J, Gao Y, Kong H, Kim J, Choi S, et al. Non-precious-metal catalysts for alkaline water electrolysis: operando characterizations, theoretical calculations, and recent advances. Chem. Soc. Rev. 2020;49(24):9154–9196. doi: 10.1039/D0CS00575D. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Li B, Soto FA, Li X, Unocic RR, et al. Enhancing hydrogen evolution activity of monolayer molybdenum disulfide via a molecular proton mediator. ACS Catal. 2021;11(19):12159–12169. doi: 10.1021/acscatal.1c03016. [DOI] [Google Scholar]
- 13.Chen K, Wang Z, Wang L, Wu X, Hu B, et al. Boron nanosheet-supported Rh catalysts for hydrogen evolution: a new territory for the strong metal-support interaction effect. Nano-Micro Lett. 2021;13:138. doi: 10.1007/s40820-021-00662-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding H, Liu H, Chu W, Wu C, Xie Y. Structural transformation of heterogeneous materials for electrocatalytic oxygen evolution reaction. Chem. Rev. 2021;121:13174–13212. doi: 10.1021/acs.chemrev.1c00234. [DOI] [PubMed] [Google Scholar]
- 15.Liu Q, Sun S, Zhang L, Luo Y, Yang Q, et al. O-doped carbon foam as metal-free electrocatalyst for efficient hydrogen production from seawater. Nano Res. 2022;15:8922–8927. doi: 10.1007/s12274-022-4869-2. [DOI] [Google Scholar]
- 16.Niether C, Faure S, Bordet A, Deseure J, Chatenet M, et al. Improved water electrolysis using magnetic heating of FeC–Ni core–shell nanoparticles. Nat. Energy. 2018;3:476–483. doi: 10.1038/s41560-018-0132-1. [DOI] [Google Scholar]
- 17.Li R, Xu H, Yang P, Wang D, Li Y, et al. Synergistic interfacial and doping engineering of heterostructured NiCo(OH)x-CoyW as an efficient alkaline hydrogen evolution electrocatalyst. Nano-Micro Lett. 2021;13:120. doi: 10.1007/s40820-021-00639-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Z, Liu Y, Wei W, Ni BJ. Recent advances in electrocatalysts for halogenated organic pollutant degradation. Environ. Sci. Nano. 2019;6:2332–2366. doi: 10.1039/C9EN00411D. [DOI] [Google Scholar]
- 19.Pan Y, Abazari R, Wu Y, Gao J, Zhang Q. Advances in metal–organic frameworks and their derivatives for diverse electrocatalytic applications. Electrochem. Commun. 2021;126:107024. doi: 10.1016/j.elecom.2021.107024. [DOI] [Google Scholar]
- 20.Khan U, Nairan A, Gao J, Zhang Q. Current progress in 2D metal–organic frameworks for electrocatalysis. Small Struct. 2022 doi: 10.1002/sstr.202200109. [DOI] [Google Scholar]
- 21.Ruidas S, Mohanty B, Bhanja P, Erakulan E, Thapa R, et al. Metal-free triazine-based 2D Covalent organic framework for efficient H2 evolution by electrochemical water splitting. Chemsuschem. 2021;14:5057–5064. doi: 10.1002/cssc.202101663. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y, Li J, Gao X, Chu W, Gao G, et al. Recent advances in single-atom electrocatalysts supported on two-dimensional materials for the oxygen evolution reaction. J. Mater. Chem. A. 2021;9(16):9979–9999. doi: 10.1039/D1TA00154J. [DOI] [Google Scholar]
- 23.Ma Z, Yuan H, Sun J, Yang J, Tang B, et al. Versatile construction of a hierarchical porous electrode and its application in electrochemical hydrogen production: a mini review. Mater. Adv. 2021;2(4):1177–1189. doi: 10.1039/D0MA00825G. [DOI] [Google Scholar]
- 24.Li Y, Lu M, Wu Y, Ji Q, Xu H, et al. Morphology regulation of metal–organic framework-derived nanostructures for efficient oxygen evolution electrocatalysis. J. Mater. Chem. A. 2020;8(35):18215–18219. doi: 10.1039/D0TA05866A. [DOI] [Google Scholar]
- 25.Chen Z, Wei W, Chen H, Ni B. Recent advances in waste-derived functional materials for wastewater remediation. Eco-Environ. Health. 2022;1:86–104. doi: 10.1016/j.eehl.2022.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma HB, Vanapalli KR, Samal B, Cheela VRS, Dubey BK, et al. Circular economy approach in solid waste management system to achieve UN-SDGs: solutions for post-COVID recovery. Sci. Total Environ. 2021;800:149605. doi: 10.1016/j.scitotenv.2021.149605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Z, Wei W, Ni BJ, Chen H. Plastic wastes derived carbon materials for green energy and sustainable environmental applications. Environ. Funct. Mater. 2022;1:34–48. doi: 10.1016/j.efmat.2022.05.005. [DOI] [Google Scholar]
- 28.Chen L, Msigwa G, Yang M, Osman AI, Fawzy S, et al. Strategies to achieve a carbon neutral society: a review. Environ. Chem. Lett. 2022;20:2277–2310. doi: 10.1007/s10311-022-01435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang M, Chen L, Wang J, Msigwa G, Osman AI, et al. Circular economy strategies for combating climate change and other environmental issues. Environ. Chem. Lett. 2022 doi: 10.1007/s10311-022-01499-6. [DOI] [Google Scholar]
- 30.Ala'a H, Jamil F, Osman AI, Myint MTZ, Kyaw HH, et al. State-of-the-art novel catalyst synthesised from waste glassware and eggshells for cleaner fuel production. Fuel. 2022;330:125526. doi: 10.1016/j.fuel.2022.125526. [DOI] [Google Scholar]
- 31.Halawy SA, Osman AI, Mehta N, Abdelkader A, Vo DVN, et al. Adsorptive removal of some Cl-VOC's as dangerous environmental pollutants using feather-like γ-Al2O3 derived from aluminium waste with life cycle analysis. Chemosphere. 2022;295:133795. doi: 10.1016/j.chemosphere.2022.133795. [DOI] [PubMed] [Google Scholar]
- 32.Zou W, Li J, Wang R, Ma J, Chen Z, et al. Hydroxylamine mediated Fenton-like interfacial reaction dynamics on sea urchin-like catalyst derived from spent LiFePO4 battery. J. Hazard. Mater. 2022;431:128590. doi: 10.1016/j.jhazmat.2022.128590. [DOI] [PubMed] [Google Scholar]
- 33.Lee J, Kim HS, Jang JH, Lee EH, Jeong HW, et al. Atomic-scale engineered Fe single-atom electrocatalyst based on waste pig blood for high-performance AEMFCs. ACS Sustain. Chem. Eng. 2021;9(23):7863–7872. doi: 10.1021/acssuschemeng.1c01590. [DOI] [Google Scholar]
- 34.Karthik PE, Rajan H, Jothi VR, Sang BI, Yi SC. Electronic wastes: a near inexhaustible and an unimaginably wealthy resource for water splitting electrocatalysts. J. Hazard. Mater. 2022;421:126687. doi: 10.1016/j.jhazmat.2021.126687. [DOI] [PubMed] [Google Scholar]
- 35.Kaur P, Verma G, Sekhon S. Biomass derived hierarchical porous carbon materials as oxygen reduction reaction electrocatalysts in fuel cells. Prog. Mater. Sci. 2019;102:1–71. doi: 10.1016/j.pmatsci.2018.12.002. [DOI] [Google Scholar]
- 36.Rodríguez-Padrón D, Z.A. ALOthman, S.M. Osman, R. Luque Recycling electronic waste: prospects in green catalysts design. Curr. Opin. Green Sustain. Chem. 2020;25(2020):100357. doi: 10.1016/j.cogsc.2020.100357. [DOI] [Google Scholar]
- 37.Graś M, Kolanowski Ł, Chen Z, Lota K, Jurak K, et al. Partial inhibition of borohydride hydrolysis using porous activated carbon as an effective method to improve the electrocatalytic activity of the DBFC anode. Sustain. Energy Fuels. 2021;5:4401–4413. doi: 10.1039/D1SE00999K. [DOI] [Google Scholar]
- 38.Cao X, Li Z, Chen H, Zhang C, Zhang Y, et al. Synthesis of biomass porous carbon materials from bean sprouts for hydrogen evolution reaction electrocatalysis and supercapacitor electrode. Int. J. Hydrogen Energy. 2021;46:18887–18897. doi: 10.1016/j.ijhydene.2021.03.038. [DOI] [Google Scholar]
- 39.Chen H, Chen Z, Wei W, Zou W, Li J, et al. Integrating electrodeposition with electrolysis for closing loop resource utilization of battery industrial wastewater. Green Chem. 2022;24:3208–3217. doi: 10.1039/D1GC04891K. [DOI] [Google Scholar]
- 40.Guan J, Zhang Z, Ji J, Dou M, Wang F. Hydrothermal synthesis of highly dispersed Co3O4 nanoparticles on biomass-derived nitrogen-doped hierarchically porous carbon networks as an efficient bifunctional electrocatalyst for oxygen reduction and evolution reactions. ACS Appl. Mater. Interfaces. 2017;9(36):30662–30669. doi: 10.1021/acsami.7b08533. [DOI] [PubMed] [Google Scholar]
- 41.Cova CM, Zuliani A, Santiago ARP, Caballero A, Muñoz-Batista MJ, et al. Microwave-assisted preparation of Ag/Ag2S carbon hybrid structures from pig bristles as efficient HER catalysts. J. Mater. Chem. A. 2018;6(43):21516–21523. doi: 10.1039/C8TA06417B. [DOI] [Google Scholar]
- 42.Chen Z, Wei W, Chen H, Ni BJ. Eco-designed electrocatalysts for water splitting: a path toward carbon neutrality. Int. J. Hydrogen Energy. 2022 doi: 10.1016/j.ijhydene.2022.03.046. [DOI] [Google Scholar]
- 43.Lai F, Miao YE, Huang Y, Zhang Y, Liu T. Nitrogen-doped carbon nanofiber/molybdenum disulfide nanocomposites derived from bacterial cellulose for high-efficiency electrocatalytic hydrogen evolution reaction. ACS Appl. Mater. Interfaces. 2016;8(6):3558–3566. doi: 10.1021/acsami.5b06274. [DOI] [PubMed] [Google Scholar]
- 44.Li J, Pan W, Liu Q, Chen Z, Chen Z, et al. Interfacial engineering of Bi19Br3S27 nanowires promotes metallic photocatalytic CO2 reduction activity under near-infrared light irradiation. J. Am. Chem. Soc. 2021;143(17):6551–6559. doi: 10.1021/jacs.1c01109. [DOI] [PubMed] [Google Scholar]
- 45.Chen Z, Zheng R, Zou W, Wei W, Li J, et al. Integrating high-efficiency oxygen evolution catalysts featuring accelerated surface reconstruction from waste printed circuit boards via a boriding recycling strategy. Appl. Catal. B Environ. 2021;298:120583. doi: 10.1016/j.apcatb.2021.120583. [DOI] [Google Scholar]
- 46.Upadhyay S, Pandey O. Low-temperature synthesized Mo2C and novel Mo2C–MnO2 heterostructure for highly efficient hydrogen evolution reaction and high-performance capacitors. J. Power Sources. 2022;535:231450. doi: 10.1016/j.jpowsour.2022.231450. [DOI] [Google Scholar]
- 47.Son HJ, Cho YR, Park YE, Ahn SH. Flexible, compressible, versatile biomass-derived freestanding carbon monoliths as binder- and substrate-free tri-functional electrodes for solid-state zinc-air batteries and overall water splitting. Appl. Catal. B Environ. 2022;304:120977. doi: 10.1016/j.apcatb.2021.120977. [DOI] [Google Scholar]
- 48.Zou Y, Xiao B, Shi JW, Hao H, Ma D, et al. 3D hierarchical heterostructure assembled by NiFe LDH/(NiFe)Sx on biomass-derived hollow carbon microtubes as bifunctional electrocatalysts for overall water splitting. Electrochim. Acta. 2020;348:136339. doi: 10.1016/j.electacta.2020.136339. [DOI] [Google Scholar]
- 49.Saleem H, Khosravi M, Maroufi S, Sahajwalla V, O'Mullane AP. Repurposing metal containing wastes and mass-produced materials as electrocatalysts for water electrolysis. Sustain. Energy Fuels. 2022 doi: 10.1039/D2SE01068B. [DOI] [Google Scholar]
- 50.Chen Z, Yang H, Kang Z, Driess M, Menezes PW. The pivotal role of S-, P-, and F-block metals in water electrolysis: status quo and perspectives. Adv. Mater. 2022;34(18):2108432. doi: 10.1002/adma.202108432. [DOI] [PubMed] [Google Scholar]
- 51.Chen Z, Duan X, Wei W, Wang S, Ni BJ. Recent advances in transition metal-based electrocatalysts for alkaline hydrogen evolution. J. Mater. Chem. A. 2019;7(25):14971–15005. doi: 10.1039/C9TA03220G. [DOI] [Google Scholar]
- 52.Zhang L, Lei Y, Zhou D, Xiong C, Jiang Z, et al. Interfacial engineering of 3D hollow CoSe2@ ultrathin MoSe2 core@shell heterostructure for efficient pH-universal hydrogen evolution reaction. Nano Res. 2022;15:2895–2904. doi: 10.1007/s12274-021-3887-9. [DOI] [Google Scholar]
- 53.Yoo JS, Rong X, Liu Y, Kolpak AM. Role of lattice oxygen participation in understanding trends in the oxygen evolution reaction on perovskites. ACS Catal. 2018;8(5):4628–4636. doi: 10.1021/acscatal.8b00612. [DOI] [Google Scholar]
- 54.Zhang N, Chai Y. Lattice oxygen redox chemistry in solid-state electrocatalysts for water oxidation. Energy Environ. Sci. 2021;14:4647–4671. doi: 10.1039/D1EE01277K. [DOI] [Google Scholar]
- 55.Zagalskaya A, Alexandrov V. Role of defects in the interplay between adsorbate evolving and lattice oxygen mechanisms of the oxygen evolution reaction in RuO2 and IrO2. ACS Catal. 2020;10(6):3650–3657. doi: 10.1021/acscatal.9b05544. [DOI] [Google Scholar]
- 56.Chen Z, Duan X, Wei W, Wang S, Ni BJ. Electrocatalysts for acidic oxygen evolution reaction: achievements and perspectives. Nano Energy. 2020;78:105392. doi: 10.1016/j.nanoen.2020.105392. [DOI] [Google Scholar]
- 57.Li Y, Zhao T, Lu M, Wu Y, Xie Y, et al. Enhancing oxygen evolution reaction through modulating electronic structure of trimetallic electrocatalysts derived from metal–organic frameworks. Small. 2019;15(43):1901940. doi: 10.1002/smll.201901940. [DOI] [PubMed] [Google Scholar]
- 58.Han L, Dong S, Wang E. Transition-metal (Co, Ni, and Fe)-based electrocatalysts for the water oxidation reaction. Adv. Mater. 2016;28(42):9266–9291. doi: 10.1002/adma.201602270. [DOI] [PubMed] [Google Scholar]
- 59.Guo Y, Park T, Yi JW, Henzie J, Kim J, et al. Nanoarchitectonics for transition-metal-sulfide-based electrocatalysts for water splitting. Adv. Mater. 2019;31(17):e1807134. doi: 10.1002/adma.201807134. [DOI] [PubMed] [Google Scholar]
- 60.Gao J, Cong J, Wu Y, Sun L, Yao J, et al. Bimetallic Hofmann-type metal–organic framework nanoparticles for efficient electrocatalysis of oxygen evolution reaction. ACS Appl. Energy Mater. 2018;1(10):5140–5144. doi: 10.1021/acsaem.8b01229. [DOI] [Google Scholar]
- 61.Chen Z, Duan X, Wei W, Wang S, Zhang Z, et al. Boride-based electrocatalysts: emerging candidates for water splitting. Nano Res. 2020;13:293–314. doi: 10.1007/s12274-020-2618-y. [DOI] [Google Scholar]
- 62.Hu F, Wang H, Zhang Y, Shen X, Zhang G, et al. Designing highly efficient and long-term durable electrocatalyst for oxygen evolution by coupling B and P into amorphous porous NiFe-based material. Small. 2019;15(28):1901020. doi: 10.1002/smll.201901020. [DOI] [PubMed] [Google Scholar]
- 63.Meng J, Guo H, Niu C, Zhao Y, Xu L, et al. Advances in structure and property optimizations of battery electrode materials. Joule. 2017;1:522–547. doi: 10.1016/j.joule.2017.08.001. [DOI] [Google Scholar]
- 64.Yuan SJ, Dai XH. Efficient sewage sludge-derived bi-functional electrocatalyst for oxygen reduction and evolution reaction. Green Chem. 2016;18:4004–4011. doi: 10.1039/C5GC02729B. [DOI] [Google Scholar]
- 65.Chen J, Wu J, Sherrell PC, Chen J, Wang H, et al. How to build a microplastics-free environment: strategies for microplastics degradation and plastics recycling. Adv. Sci. 2022;9(6):2103764. doi: 10.1002/advs.202103764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muhyuddin M, Filippi J, Zoia L, Bonizzoni S, Lorenzi R, et al. Waste face surgical mask transformation into crude oil and nanostructured electrocatalysts for fuel cells and electrolyzers. Chemsuschem. 2022;15:202102351. doi: 10.1002/cssc.202102351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sekar S, Sim DH, Lee S. Excellent electrocatalytic hydrogen evolution reaction performances of partially graphitized activated-carbon nanobundles derived from biomass human hair wastes. Nanomaterials. 2022;12:531. doi: 10.3390/nano12030531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saravanan KA, Prabu N, Sasidharan M, Maduraiveeran G. Nitrogen-self doped activated carbon nanosheets derived from peanut shells for enhanced hydrogen evolution reaction. Appl. Surf. Sci. 2019;489:725–733. doi: 10.1016/j.apsusc.2019.06.040. [DOI] [Google Scholar]
- 69.Osman AI, Farrell C, Al-Muhtaseb AH, Harrison J, Rooney DW. The production and application of carbon nanomaterials from high alkali silicate herbaceous biomass. Sci. Rep. 2020;10:2563. doi: 10.1038/s41598-020-59481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Osman AI, O'Connor E, McSpadden G, Abu-Dahrieh JK, Farrell C, et al. Upcycling brewer's spent grain waste into activated carbon and carbon nanotubes for energy and other applications via two-stage activation. J. Chem. Technol. Biotechnol. 2020;95:183–195. doi: 10.1002/jctb.6220. [DOI] [Google Scholar]
- 71.Kang Q, Qin Y, Lu Q, Gao F. Waste leather-derived (Cr, N)-co-doped carbon cloth coupling with Mo2C nanoparticles as a self-supported electrode for highly active hydrogen evolution reaction performances. J. Power Sources. 2020;476:228706. doi: 10.1016/j.jpowsour.2020.228706. [DOI] [Google Scholar]
- 72.Yan Q, Yang X, Wei T, Zhou C, Wu W, et al. Porous-Mo2C nanoparticle clusters supported on walnut shell powders derived carbon matrix for hydrogen evolution reaction. J. Colloid Interface Sci. 2020;563:104–111. doi: 10.1016/j.jcis.2019.12.059. [DOI] [PubMed] [Google Scholar]
- 73.Song L, Chang J, Ma Y, Tan X, Xu Y, et al. Cobalt/nitrogen codoped carbon nanosheets derived from catkins as a high performance non-noble metal electrocatalyst for oxygen reduction reaction and hydrogen evolution reaction. RSC Adv. 2020;10(71):43248–43255. doi: 10.1039/D0RA08750E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang S, Shao H, Cao G, Li H, Xu W, et al. Waste cotton fabric derived porous carbon containing Fe3O4/NiS nanoparticles for electrocatalytic oxygen evolution. J. Mater. Sci. Technol. 2020;59:92–99. doi: 10.1016/j.jmst.2020.04.055. [DOI] [Google Scholar]
- 75.Cui C, Lai X, Guo R, Ren E, Qin W, et al. Waste paper-based carbon aerogel supported ZIF-67 derived hollow NiCo phosphate nanocages for electrocatalytic oxygen evolution reaction. Electrochim. Acta. 2021;393:139076. doi: 10.1016/j.electacta.2021.139076. [DOI] [Google Scholar]
- 76.Cui B, Liu C, Zhang J, Lu J, Liu S, et al. Waste to wealth: defect-rich Ni-incorporated spent LiFePO4 for efficient oxygen evolution reaction. Sci. China Mater. 2021;64:2710–2718. doi: 10.1007/s40843-021-1682-0. [DOI] [Google Scholar]
- 77.Jothi VR, Bose R, Rajan H, Jung C, Yi SC. Harvesting electronic waste for the development of highly efficient eco-design electrodes for electrocatalytic water splitting. Adv. Energy Mater. 2018;8(34):1802615. doi: 10.1002/aenm.201802615. [DOI] [Google Scholar]
- 78.Babar P, Lokhande A, Karade V, Pawar B, Gang MG, et al. Towards highly efficient and low-cost oxygen evolution reaction electrocatalysts: an effective method of electronic waste management by utilizing waste Cu cable wires. J. Colloid Interface Sci. 2019;537:43–49. doi: 10.1016/j.jcis.2018.10.079. [DOI] [PubMed] [Google Scholar]
- 79.Huang C, Lv H, Yang Z, Lian C, Du J, et al. Exfoliating spent cathode materials with robust interlayer interactions into atomic-thin nanosheets for boosting the oxygen evolution reaction. J. Mater. Chem. A. 2022;10(7):3359–3372. doi: 10.1039/D1TA08046F. [DOI] [Google Scholar]
- 80.Lu S, Hummel M, Gu Z, Gu Y, Cen Z, et al. Trash to treasure: a novel chemical route to synthesis of NiO/C for hydrogen production. Int. J. Hydrogen Energy. 2019;44:16144–16153. doi: 10.1016/j.ijhydene.2019.04.191. [DOI] [Google Scholar]
- 81.Ji Q, Yu X, Chen L, Yarley OPN, Zhou C. Facile preparation of sugarcane bagasse-derived carbon supported MoS2 nanosheets for hydrogen evolution reaction. Ind. Crops Prod. 2021;172:114064. doi: 10.1016/j.indcrop.2021.114064. [DOI] [Google Scholar]
- 82.Pegoretti VCB, Dixini PVM, Magnago L, Rocha AKS, Lelis MFF, et al. High-temperature (HT) LiCoO2 recycled from spent lithium ion batteries as catalyst for oxygen evolution reaction. Mater. Res. Bull. 2019;110:97–101. doi: 10.1016/j.materresbull.2018.10.022. [DOI] [Google Scholar]
- 83.Huang B, Liu Y, Xie Z. Biomass derived 2D carbons via a hydrothermal carbonization method as efficient bifunctional ORR/HER electrocatalysts. J. Mater. Chem. A. 2017;5(45):23481–23488. doi: 10.1039/C7TA08052B. [DOI] [Google Scholar]
- 84.Foruzin LJ, Rezvani Z, Habibi B. New ternary-component layered double hydroxide as a low-cost and efficient electrocatalyst for water oxidation: NiCaFe-LDH from eggshell bio-waste. Appl. Clay Sci. 2020;188:105511. doi: 10.1016/j.clay.2020.105511. [DOI] [Google Scholar]
- 85.Yun S, Shi J, Si Y, Sun M, Zhang Y, et al. Insight into electrocatalytic activity and mechanism of bimetal niobium-based oxides in situ embedded into biomass-derived porous carbon skeleton nanohybrids for photovoltaics and alkaline hydrogen evolution. J. Colloid Interface Sci. 2021;601:12–29. doi: 10.1016/j.jcis.2021.05.060. [DOI] [PubMed] [Google Scholar]
- 86.Zhang TQ, Liu J, Huang LB, Zhang XD, Sun YG, et al. Microbial-phosphorus-enabled synthesis of phosphide nanocomposites for efficient electrocatalysts. J. Am. Chem. Soc. 2017;139(32):11248–11253. doi: 10.1021/jacs.7b06123. [DOI] [PubMed] [Google Scholar]
- 87.Chen H, Jiang H, Cao X, Zhang Y, Zhang X, et al. Castoff derived biomass-carbon supported MoS2 nanosheets for hydrogen evolution reaction. Mater. Chem. Phys. 2020;252:123244. doi: 10.1016/j.matchemphys.2020.123244. [DOI] [Google Scholar]
- 88.Yang M, Feng F, Wang K, Li S, Huang X, et al. Synthesis of metal phosphide nanoparticles supported on porous N-doped carbon derived from spirulina for universal-pH hydrogen evolution. Chemsuschem. 2020;13(2):351–359. doi: 10.1002/cssc.201902920. [DOI] [PubMed] [Google Scholar]
- 89.Xiao Y, Deng S, Li M, Zhou Q, Xu L, et al. Immobilization of Fe-doped Ni2P particles within biomass agarose-derived porous N, P-Carbon nanosheets for efficient bifunctional oxygen electrocatalysis. Front. Chem. 2019;7:523. doi: 10.3389/fchem.2019.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Z, Zheng R, Wei W, Wei W, Zou W, et al. Recycling spent water treatment adsorbents for efficient electrocatalytic water oxidation reaction. Resour. Conserv. Recycl. 2022;178:106037. doi: 10.1016/j.resconrec.2021.106037. [DOI] [Google Scholar]
- 91.Zhang Z, Zhou D, Liao J, Bao X, Luo S. One-pot synthesis of Fe3O4/Fe/C by microwave sintering as an efficient bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. J. Alloys Compd. 2019;786:134–138. doi: 10.1016/j.jallcom.2019.01.318. [DOI] [Google Scholar]
- 92.Wang S, Zhu J, Wu X, Feng L. Microwave-assisted hydrothermal synthesis of NiMoO4 nanorods for high-performance urea electrooxidation. Chin. Chem. Lett. 2022;33:1105–1109. doi: 10.1016/j.cclet.2021.08.042. [DOI] [Google Scholar]
- 93.Miao J, Zhao X, Hu HY, Liu ZH. Hierarchical ultrathin NiFe-borate layered double hydroxide nanosheets encapsulated into biomass-derived nitrogen-doped carbon for efficient electrocatalytic oxygen evolution. Colloid Surf. A. 2022;635:128092. doi: 10.1016/j.colsurfa.2021.128092. [DOI] [Google Scholar]
- 94.Zuliani A, Cano M, Calsolaro F, Santiago ARP, Giner-Casares JJ, et al. Improving the electrocatalytic performance of sustainable Co/carbon materials for the oxygen evolution reaction by ultrasound and microwave assisted synthesis. Sustain. Energy Fuels. 2021;5:720–731. doi: 10.1039/D0SE01505A. [DOI] [Google Scholar]
- 95.Zahra T, Ahmad KS, Zequine C, Gupta RK, Thomas AG, et al. Evaluation of electrochemical properties for water splitting by NiO nano-cubes synthesized using Olea ferruginea Royle. Sustain. Energy Technol. Assess. 2020;40:100753. doi: 10.1016/j.seta.2020.100753. [DOI] [Google Scholar]
- 96.Han Z, Wang G, Zhang J, Tang Z. Direct photo-curing 3D printing of nickel-based electrocatalysts for highly-efficient hydrogen evolution. Nano Energy. 2022;102:107615. doi: 10.1016/j.nanoen.2022.107615. [DOI] [Google Scholar]
- 97.Zhang X, Zheng R, Jin M, Shi R, Ai Z, et al. NiCoSx@cobalt carbonate hydroxide obtained by surface sulfurization for efficient and stable hydrogen evolution at large current densities. ACS Appl. Mater. Interfaces. 2021;13(30):35647–35656. doi: 10.1021/acsami.1c07504. [DOI] [PubMed] [Google Scholar]
- 98.Arshad A, Yun S, Shi J, Sun M, Zafar N, et al. N-coordinated bimetallic defect-rich nanocarbons as highly efficient electrocatalysts in advanced energy conversion applications. Chem. Eng. J. 2022;435:134913. doi: 10.1016/j.cej.2022.134913. [DOI] [Google Scholar]
- 99.Sekar S, Ahmed ATA, Sim DH, Lee S. Extraordinarily high hydrogen-evolution-reaction activity of corrugated graphene nanosheets derived from biomass rice husks. Int. J. Hydrogen Energy. 2022 doi: 10.1016/j.ijhydene.2022.02.233. [DOI] [Google Scholar]
- 100.Thirumal V, Yuvakkumar R, Saravanakumar B, Ravi G, Isacfranklin M, et al. Carbonization and optimization of biomass waste for HER application. Fuel. 2022;324:124466. doi: 10.1016/j.fuel.2022.124466. [DOI] [Google Scholar]
- 101.Sun J, Wu Z, Ma C, Xu M, Luo S, et al. Biomass-derived tubular carbon materials: progress in synthesis and applications. J. Mater. Chem. A. 2021;9(24):13822–13850. doi: 10.1039/D1TA02412D. [DOI] [Google Scholar]
- 102.Wang H, Xu S, Dong W, Sun D, Zhang S, et al. Solid base assisted dual-promoted heterogeneous conversion of PVC to metal-free porous carbon catalyst. Chem. Eur. J. 2022;28:e202200124. doi: 10.1002/chem.202200124. [DOI] [PubMed] [Google Scholar]
- 103.Wyss KM, Chen W, Beckham JL, Savas PE, Tour JM. Holey and wrinkled flash graphene from mixed plastic waste. ACS Nano. 2022;16(5):7804–7815. doi: 10.1021/acsnano.2c00379. [DOI] [PubMed] [Google Scholar]
- 104.Niu J, Domenech-Carbó A, Primo A, Garcia H. Uniform nanoporous graphene sponge from natural polysaccharides as a metal-free electrocatalyst for hydrogen generation. RSC Adv. 2019;9:99–106. doi: 10.1039/C8RA08745H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen Z, Wei W, Song L, Ni BJ. Hybrid water electrolysis: a new sustainable avenue for energy-saving hydrogen production. Sustain. Horiz. 2022;1:100002. doi: 10.1016/j.horiz.2021.100002. [DOI] [Google Scholar]
- 106.Paul R, Du F, Dai L, Ding Y, Wang ZL, et al. 3D heteroatom-doped carbon nanomaterials as multifunctional metal-free catalysts for integrated energy devices. Adv. Mater. 2019;31(13):1805598. doi: 10.1002/adma.201805598. [DOI] [PubMed] [Google Scholar]
- 107.Qu K, Zheng Y, Jiao Y, Zhang X, Dai S, et al. Polydopamine-inspired, dual heteroatom-doped carbon nanotubes for highly efficient overall water splitting. Adv. Energy Mater. 2017;7(9):1602068. doi: 10.1002/aenm.201602068. [DOI] [Google Scholar]
- 108.Jiang H, Gu J, Zheng X, Liu M, Qiu X, et al. Defect-rich and ultrathin N doped carbon nanosheets as advanced trifunctional metal-free electrocatalysts for the ORR OER and HER. Energy Environ. Sci. 2019;12:322–333. doi: 10.1039/C8EE03276A. [DOI] [Google Scholar]
- 109.Prabu N, Saravanan RSA, Kesavan T, Maduraiveeran G, Sasidharan M. An efficient palm waste derived hierarchical porous carbon for electrocatalytic hydrogen evolution reaction. Carbon. 2019;152:188–197. doi: 10.1016/j.carbon.2019.06.016. [DOI] [Google Scholar]
- 110.Zhou Y, Leng Y, Zhou W, Huang J, Zhao M, et al. Sulfur and nitrogen self-doped carbon nanosheets derived from peanut root nodules as high-efficiency non-metal electrocatalyst for hydrogen evolution reaction. Nano Energy. 2015;16:357–366. doi: 10.1016/j.nanoen.2015.07.008. [DOI] [Google Scholar]
- 111.Jiang E, Song N, Hong S, She C, Li C, et al. Zn, S, N self-doped carbon material derived from waste tires for electrocatalytic hydrogen evolution. Int. J. Hydrogen Energy. 2022;47:16544–16551. doi: 10.1016/j.ijhydene.2022.03.172. [DOI] [Google Scholar]
- 112.Sun H, Xue L, Shi Y, Dong J, Wu Q, et al. Waste paper derived Co, N co-doped carbon as an efficient electrocatalyst for hydrogen evolution. React. Kinet. Mech. Catal. 2021;132:1137–1150. doi: 10.1007/s11144-021-01956-3. [DOI] [Google Scholar]
- 113.Muhyuddin M, Zocche N, Lorenzi R, Ferrara C, Poli F, et al. Valorization of the inedible pistachio shells into nanoscale transition metal and nitrogen codoped carbon-based electrocatalysts for hydrogen evolution reaction and oxygen reduction reaction. Mater. Renew. Sustain. Energy. 2022;11:131–141. doi: 10.1007/s40243-022-00212-5. [DOI] [Google Scholar]
- 114.Muhyuddin M, Filippi J, Zoia L, Bonizzoni S, Lorenzi R, et al. Waste face surgical mask transformation into crude oil and nanostructured electrocatalysts for fuel cells and electrolyzers. Chemsuschem. 2022;15:e202102351. doi: 10.1002/cssc.202102351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Deng L, Zhang Y, Wang Y, Yuan H, Chen Y, et al. In situ N-, P-and Ca-codoped biochar derived from animal bones to boost the electrocatalytic hydrogen evolution reaction. Resour. Conserv. Recycl. 2021;170:105568. doi: 10.1016/j.resconrec.2021.105568. [DOI] [Google Scholar]
- 116.Roy A, Hursán D, Artyushkova K, Atanassov P, Janáky C, et al. Nanostructured metal-NC electrocatalysts for CO2 reduction and hydrogen evolution reactions. Appl. Catal. B Environ. 2018;232:512–520. doi: 10.1016/j.apcatb.2018.03.093. [DOI] [Google Scholar]
- 117.Dang C, Yun S, Zhang Y, Dang J, Wang Y, et al. A tailored interface engineering strategy designed to enhance the electrocatalytic activity of NiFe2O4/NiTe heterogeneous structure for advanced energy conversion applications. Mater. Today Nano. 2022;20:100242. doi: 10.1016/j.mtnano.2022.100242. [DOI] [Google Scholar]
- 118.Humagain G, MacDougal K, MacInnis J, Lowe JM, Coridan RH, et al. Highly efficient, biochar-derived molybdenum carbide hydrogen evolution electrocatalyst. Adv. Energy Mater. 2018;8(29):1801461. doi: 10.1002/aenm.201801461. [DOI] [Google Scholar]
- 119.Lin Y, He L, Chen T, Zhou D, Wu L, et al. Cost-effective and environmentally friendly synthesis of 3D Ni2P from scrap nickel for highly efficient hydrogen evolution in both acidic and alkaline media. J. Mater. Chem. A. 2018;6(9):4088–4094. doi: 10.1039/C7TA09524D. [DOI] [Google Scholar]
- 120.Altalhi T, Mezni A, Ibrahim MM, Refat MS, Gobouri AA, et al. Cathodic activation of titania-fly ash cenospheres for efficient electrochemical hydrogen production: a proposed solution to treat fly ash waste. Catalysts. 2022;12:466. doi: 10.3390/catal12050466. [DOI] [Google Scholar]
- 121.Sukhbaatar B, Yoon S, Yoo B. Simple synthesis of a CoO nanoparticle-decorated nitrogen-doped carbon catalyst from spent coffee grounds for alkaline hydrogen evolution. J. Mater. Sci. 2022 doi: 10.1007/s10853-022-07436-w. [DOI] [Google Scholar]
- 122.Ahsan MA, Santiago ARP, Rodriguez A, Maturano-Rojas V, Alvarado-Tenorio B, et al. Biomass-derived ultrathin carbon-shell coated iron nanoparticles as high-performance tri-functional HER, ORR and Fenton-like catalysts. J. Cleaner Prod. 2020;275:124141. doi: 10.1016/j.jclepro.2020.124141. [DOI] [Google Scholar]
- 123.Zhou W, Xiong T, Shi C, Zhou J, Zhou K, et al. Bioreduction of precious metals by microorganism: efficient gold@N-doped carbon electrocatalysts for the hydrogen evolution reaction. Angew. Chem. Int. Ed. 2016;55(29):8416–8420. doi: 10.1002/anie.201602627. [DOI] [PubMed] [Google Scholar]
- 124.Mir RA, Kaur G, Pandey O. Facile process to utilize carbonaceous waste as a carbon source for the synthesis of low cost electrocatalyst for hydrogen production. Int. J. Hydrogen Energy. 2020;45:23908–23919. doi: 10.1016/j.ijhydene.2019.11.239. [DOI] [Google Scholar]
- 125.Yan Q, Yang X, Wei T, Zhou C, Wu W, et al. Porous β-Mo2C nanoparticle clusters supported on walnut shell powders derived carbon matrix for hydrogen evolution reaction. J. Colloid Interface Sci. 2020;563:104–111. doi: 10.1016/j.jcis.2019.12.059. [DOI] [PubMed] [Google Scholar]
- 126.Yu J, Yu W, Chang B, Li X, Jia J, et al. Waste-yeast biomass as nitrogen/phosphorus sources and carbon template: environment-friendly synthesis of N, P-Mo2C nanoparticles on porous carbon matrix for efficient hydrogen evolution. Chin. Chem. Lett. 2022;33:3231–3235. doi: 10.1016/j.cclet.2021.10.046. [DOI] [Google Scholar]
- 127.Guo T, Zhang X, Liu T, Wu Z, Wang D. N, K Co-activated biochar-derived molybdenum carbide as efficient electrocatalysts for hydrogen evolution. Appl. Surf. Sci. 2020;509:144879. doi: 10.1016/j.apsusc.2019.144879. [DOI] [Google Scholar]
- 128.Zhou Y, Zhou W, Hou D, Li G, Wan J, et al. Metal–carbon hybrid electrocatalysts derived from ion-exchange resin containing heavy metals for efficient hydrogen evolution reaction. Small. 2016;12(20):2768–2774. doi: 10.1002/smll.201503100. [DOI] [PubMed] [Google Scholar]
- 129.Han F, Yun S, Shi J, Zhang Y, Si Y, et al. Efficient dual-function catalysts for triiodide reduction reaction and hydrogen evolution reaction using unique 3D network aloe waste-derived carbon-supported molybdenum-based bimetallic oxide nanohybrids. Appl. Catal. B Environ. 2020;273:119004. doi: 10.1016/j.apcatb.2020.119004. [DOI] [Google Scholar]
- 130.Zhang X, Jia F, Song S. Recent advances in structural engineering of molybdenum disulfide for electrocatalytic hydrogen evolution reaction. Chem. Eng. J. 2020;405:127013. doi: 10.1016/j.cej.2020.127013. [DOI] [Google Scholar]
- 131.Zhao Y, Zhao J, Li Q, Gu C, Zhang B, et al. Degradation-resistant waste plastics derived carbon supported MoS2 electrocatalyst: high-nitrogen dependent activity for hydrogen evolution reaction. Electrochim. Acta. 2020;331:135436. doi: 10.1016/j.electacta.2019.135436. [DOI] [Google Scholar]
- 132.Wu A, Xu A, Yang J, Li X, Wang L, et al. Modulating Schottky barrier of MoS2 to enhance hydrogen evolution reaction activity by incorporating with vertical graphene nanosheets derived from organic liquid waste. ChemElectroChem. 2018;5:3841–3846. doi: 10.1002/celc.201801279. [DOI] [Google Scholar]
- 133.Ji Q, Yu X, Chen L, Okonkwo CE, Zhou C. Effect of cobalt doping and sugarcane bagasse carbon on the electrocatalytic performance of MoS2 nanocomposites. Fuel. 2022;324:124814. doi: 10.1016/j.fuel.2022.124814. [DOI] [Google Scholar]
- 134.Chen Z, Zheng R, Deng S, Wei W, Wei W, et al. Modular design of an efficient heterostructured FeS2/TiO2 oxygen evolution electrocatalyst via sulfidation of natural ilmenites. J. Mater. Chem. A. 2021;9(44):25032–25041. doi: 10.1039/D1TA08168C. [DOI] [Google Scholar]
- 135.Li L, Wang P, Shao Q, Huang X. Recent progress in advanced electrocatalyst design for acidic oxygen evolution reaction. Adv. Mater. 2021;33(50):2004243. doi: 10.1002/adma.202004243. [DOI] [PubMed] [Google Scholar]
- 136.Murugesan C, Senthilkumar B, Barpanda P. Biowaste-derived highly porous N-doped carbon as a low-cost bifunctional electrocatalyst for hybrid sodium–air batteries. ACS Sustain. Chem. Eng. 2022;10(28):9077–9086. doi: 10.1021/acssuschemeng.2c01300. [DOI] [Google Scholar]
- 137.J. Xuan, Z. Liu, High-performance N-doped bifunctional carbon electrocatalysts derived from polymer waste for oxygen reduction and evolution reaction. Int. J. Electrochem. Sci. 12, 10471–10483 (2017). 10.20964/2017.11.42
- 138.Huang Y, Wu D, Cao D, Cheng D. Facile preparation of biomass-derived bifunctional electrocatalysts for oxygen reduction and evolution reactions. Int. J. Hydrogen Energy. 2018;43:8611–8622. doi: 10.1016/j.ijhydene.2018.03.136. [DOI] [Google Scholar]
- 139.Ma LL, Hu X, Liu WJ, Li HC, Lam PK, et al. Constructing N, P-dually doped biochar materials from biomass wastes for high-performance bifunctional oxygen electrocatalysts. Chemosphere. 2021;278:130508. doi: 10.1016/j.chemosphere.2021.130508. [DOI] [PubMed] [Google Scholar]
- 140.Li Y, Qu Y, Liu C, Cui J, Xu K, et al. Processing agricultural cornstalks toward high-efficient stable bifunctional ORR/OER electrocatalysts. Adv. Sustain. Syst. 2022;6:2100343. doi: 10.1002/adsu.202100343. [DOI] [Google Scholar]
- 141.Luo X, Liu Z, Ma Y, Nan Y, Gu Y, et al. Biomass derived Fe, N-doped carbon material as bifunctional electrocatalysts for rechargeable Zn-air batteries. J. Alloys Compd. 2022;888:161464. doi: 10.1016/j.jallcom.2021.161464. [DOI] [Google Scholar]
- 142.Chen Z, Zheng R, Wei W, Wei W, Ni BJ, et al. Unlocking the electrocatalytic activity of natural chalcopyrite using mechanochemistry. J. Energy Chem. 2022;68:275–283. doi: 10.1016/j.jechem.2021.11.005. [DOI] [Google Scholar]
- 143.Maruthapandian V, Muralidharan S, Saraswathy V. From waste high speed steel alloy to valuable oxygen evolution reaction catalyst in alkaline medium. Electrochim. Acta. 2021;371:137848. doi: 10.1016/j.electacta.2021.137848. [DOI] [Google Scholar]
- 144.Gomaa AK, Shaheen BS, Khedr GE, Mokhtar AM, Allam NK. Facile surface treatment of industrial stainless steel waste meshes at mild conditions to produce efficient oxygen evolution catalysts. Energy Fuels. 2022;36:7025–7034. doi: 10.1021/acs.energyfuels.2c01562. [DOI] [Google Scholar]
- 145.Zhong H, Wang J, Meng F, Zhang X. In situ activating ubiquitous rust towards low-cost, efficient, free-standing, and recoverable oxygen evolution electrodes. Angew. Chem. Int. Ed. 2016;55(34):9937–9941. doi: 10.1002/anie.201604040. [DOI] [PubMed] [Google Scholar]
- 146.Natarajan S, Anantharaj S, Tayade RJ, Bajaj HC, Kundu S. Recovered spinel MnCo2O4 from spent lithium-ion batteries for enhanced electrocatalytic oxygen evolution in alkaline medium. Dalton Trans. 2017;46:14382–14392. doi: 10.1039/C7DT02613G. [DOI] [PubMed] [Google Scholar]
- 147.Chen N, Qi J, Du X, Wang Y, Zhang W, et al. Recycled LiCoO2 in spent lithium-ion battery as an oxygen evolution electrocatalyst. RSC Adv. 2016;6:103541–103545. doi: 10.1039/C6RA23483F. [DOI] [Google Scholar]
- 148.Arif A, Xu M, Rashid J, Saraj CS, Li W, et al. Efficient recovery of lithium cobaltate from spent lithium-ion batteries for oxygen evolution reaction. Nanomaterials. 2021;11:3343. doi: 10.3390/nano11123343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lv H, Huang H, Huang C, Gao Q, Yang Z, et al. Electric field driven de-lithiation: a strategy towards comprehensive and efficient recycling of electrode materials from spent lithium ion batteries. Appl. Catal. B Environ. 2021;283:119634. doi: 10.1016/j.apcatb.2020.119634. [DOI] [Google Scholar]
- 150.Chen Z, Zou W, Zheng R, Wei W, Wei W, et al. Synergistic recycling and conversion of spent Li-ion battery leachate into highly efficient oxygen evolution catalysts. Green Chem. 2021;23:6538–6547. doi: 10.1039/D1GC01578H. [DOI] [Google Scholar]
- 151.Zhao T, Shen X, Wang Y, Hocking RK, Li Y, et al. In situ reconstruction of V-doped Ni2P pre-catalysts with tunable electronic structures for water oxidation. Adv. Funct. Mater. 2021;31(25):2100614. doi: 10.1002/adfm.202100614. [DOI] [Google Scholar]
- 152.Chen Z, Zheng R, Li S, Wang R, Wei W, et al. Dual-anion etching induced in situ interfacial engineering for high-efficiency oxygen evolution. Chem. Eng. J. 2022;431:134304. doi: 10.1016/j.cej.2021.134304. [DOI] [Google Scholar]
- 153.Xu Q, Jiang H, Duan X, Jiang Z, Hu Y, et al. Fluorination-enabled reconstruction of NiFe electrocatalysts for efficient water oxidation. Nano Lett. 2021;21(1):492–499. doi: 10.1021/acs.nanolett.0c03950. [DOI] [PubMed] [Google Scholar]
- 154.Wang J, Kim SJ, Liu J, Gao Y, Choi S, et al. Redirecting dynamic surface restructuring of a layered transition metal oxide catalyst for superior water oxidation. Nat. Catal. 2021;4:212–222. doi: 10.1038/s41929-021-00578-1. [DOI] [Google Scholar]
- 155.Chen Z, Wei W, Ni BJ. Transition metal chalcogenides as emerging electrocatalysts for urea electrolysis. Curr. Opin. Electrochem. 2022;31:100888. doi: 10.1016/j.coelec.2021.100888. [DOI] [Google Scholar]
- 156.Xu J, Liu D, Lee C, Feydi P, Chapuis M, et al. Efficient electrocatalyst nanoparticles from upcycled class II capacitors. Nanomaterials. 2022;12:2697. doi: 10.3390/nano12152697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang Y, Yang H, Cao H, Wang Z, Liu C, et al. Direct preparation of efficient catalyst for oxygen evolution reaction and high-purity Li2CO3 from spent LiNi0.5Mn0.3Co0.2O2 batteries. J. Cleaner Prod. 2019;236:117–576. doi: 10.1016/j.jclepro.2019.07.051. [DOI] [Google Scholar]
- 158.Lu R, Sam DK, Wang W, Gong S, Liu J, et al. Boron, nitrogen co-doped biomass-derived carbon aerogel embedded nickel-cobalt-iron nanoparticles as a promising electrocatalyst for oxygen evolution reaction. J. Colloid Interface Sci. 2022;613:126–135. doi: 10.1016/j.jcis.2022.01.029. [DOI] [PubMed] [Google Scholar]
- 159.Chen J, Yang S, Zhang Z, Wang F. Biomass-derived cobalt/carbon hierarchically structured composites for efficient oxygen electrocatalysis and zinc–air batteries. Catal. Sci. Technol. 2022;12:4365–4371. doi: 10.1039/D2CY00720G. [DOI] [Google Scholar]
- 160.Sathiskumar C, Meesala L, Kumar P, Rao BR, John NS, et al. Waste to wealth: spent catalyst as an efficient and stable bifunctional oxygen electrocatalyst for zinc–air batteries. Sustain. Energy Fuels. 2021;5:1406–1414. doi: 10.1039/D1SE00007A. [DOI] [Google Scholar]
- 161.Yang L, Zeng X, Wang D, Cao D. Biomass-derived FeNi alloy and nitrogen-codoped porous carbons as highly efficient oxygen reduction and evolution bifunctional electrocatalysts for rechargeable Zn-air battery. Energy Storage Mater. 2018;12:277–283. doi: 10.1016/j.ensm.2018.02.011. [DOI] [Google Scholar]
- 162.Hof F, Boni A, Valenti G, Huang K, Paolucci F, et al. From food waste to efficient bifunctional nonprecious electrocatalyst. Chem. Eur. J. 2017;23:15283–15288. doi: 10.1002/chem.201704041. [DOI] [PubMed] [Google Scholar]
- 163.Chen X, Wei L, Wang Y, Zhai S, Chen Z, et al. Milk powder-derived bifunctional oxygen electrocatalysts for rechargeable Zn-air battery. Energy Storage Mater. 2018;11:134–143. doi: 10.1016/j.ensm.2017.10.011. [DOI] [Google Scholar]
- 164.Zhang C, Antonietti M, Fellinger TP. Blood ties: Co3O4 decorated blood derived carbon as a superior bifunctional electrocatalyst. Adv. Funct. Mater. 2014;24(48):7655–7665. doi: 10.1002/adfm.201402770. [DOI] [Google Scholar]
- 165.Jiao M, Zhang Q, Ye C, Liu Z, Zhong X, et al. Recycling spent LiNi1-x-yMnxCoyO2 cathodes to bifunctional NiMnCo catalysts for zinc-air batteries. PNAS. 2022;119:e2202202119. doi: 10.1073/pnas.2202202119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Peng Y, Zhang F, Zhang Y, Luo X, Chen L, et al. ZnS modified N, S dual-doped interconnected porous carbon derived from dye sludge waste as high-efficient ORR/OER catalyst for rechargeable zinc-air battery. J. Colloid Interface Sci. 2022;616:659–667. doi: 10.1016/j.jcis.2022.02.102. [DOI] [PubMed] [Google Scholar]
- 167.Wang H, Chang F, Gu J, Xie X, Chen H, et al. Highly efficient catalytic CoS1.097 embedded in biomass nanosheets for oxygen evolution reaction. Int. J. Hydrogen Energy. 2020;45:2765–2773. doi: 10.1016/j.ijhydene.2019.11.170. [DOI] [Google Scholar]
- 168.Nairan A, Liang C, Chiang SW, Wu Y, Zou P, et al. Proton selective adsorption on Pt–Ni nano-thorn array electrodes for superior hydrogen evolution activity. Energy Environ. Sci. 2021;14:1594–1601. doi: 10.1039/D1EE00106J. [DOI] [Google Scholar]
- 169.Wang M, Du X, Zhang M, Su K, Li Z. From S-rich polyphenylene sulfide to honeycomb-like porous carbon with ultrahigh specific surface area as bifunctional electrocatalysts for rechargeable Zn-air batteries. Carbon. 2022;198:264–274. doi: 10.1016/j.carbon.2022.07.042. [DOI] [Google Scholar]
- 170.Chen Z, Duan X, Wei W, Wang S, Ni BJ. Iridium-based nanomaterials for electrochemical water splitting. Nano Energy. 2020;78:105270. doi: 10.1016/j.nanoen.2020.105270. [DOI] [Google Scholar]
- 171.Xin W, Jiang WJ, Lian Y, Li H, Hong S, et al. NiS2 nanodotted carnation-like CoS2 for enhanced electrocatalytic water splitting. Chem. Commun. 2019;55:3781–3784. doi: 10.1039/C9CC01235D. [DOI] [PubMed] [Google Scholar]
- 172.Chen Z, Ibrahim I, Hao D, Liu X, Wu L, et al. Controllable design of nanoworm-like nickel sulfides for efficient electrochemical water splitting in alkaline media. Mater. Today Energy. 2020;18:100573. doi: 10.1016/j.mtener.2020.100573. [DOI] [Google Scholar]
- 173.Liu Z, Zhou Q, Zhao B, Li S, Xiong Y, et al. Few-layer N-doped porous carbon nanosheets derived from corn stalks as a bifunctional electrocatalyst for overall water splitting. Fuel. 2020;280:118567. doi: 10.1016/j.fuel.2020.118567. [DOI] [Google Scholar]
- 174.Xia C, Surendran S, Ji S, Kim D, Chae Y, et al. A sulfur self-doped multifunctional biochar catalyst for overall water splitting and a supercapacitor from camellia japonica flowers. Carbon Energy. 2022;4:491–505. doi: 10.1002/cey2.207. [DOI] [Google Scholar]
- 175.Chen Z, Wei W, Ni BJ. Cost-effective catalysts for renewable hydrogen production via electrochemical water splitting: recent advances. Curr. Opin. Green Sustain. Chem. 2021;27:100398. doi: 10.1016/j.cogsc.2020.100398. [DOI] [Google Scholar]
- 176.Zhou B, Zhang M, He W, Wang H, Jian M, et al. Blue rose-inspired approach towards highly graphitic carbons for efficient electrocatalytic water splitting. Carbon. 2019;150:21–26. doi: 10.1016/j.carbon.2019.05.009. [DOI] [Google Scholar]
- 177.Zhang Y, Shi Y, Yan B, Zhang F, Wang S, et al. A novel iron-based composite flocculant for enhanced wastewater treatment and upcycling hazardous sludge into trifunctional electrocatalyst. Appl. Surf. Sci. 2021;569:151034. doi: 10.1016/j.apsusc.2021.151034. [DOI] [Google Scholar]
- 178.Zheng X, Zhao X, Lu J, Li J, Miao Z, et al. Regeneration of spent cathodes of Li-ion batteries into multifunctional electrodes for overall water splitting and rechargeable Zn-air batteries by ultrafast carbothermal shock. Sci. China Mater. 2022;65:2393–2400. doi: 10.1007/s40843-021-1984-8. [DOI] [Google Scholar]
- 179.Jiang E, Song N, Hong S, Xiao M, Zhu D, et al. Cobalt supported on biomass carbon tubes derived from cotton fibers towards high-efficient electrocatalytic overall water-splitting. Electrochim. Acta. 2022;407:139895. doi: 10.1016/j.electacta.2022.139895. [DOI] [Google Scholar]
- 180.He F, Han Y, Tong Y, Zhong M, Wang Q, et al. NiFe Alloys@N-doped graphene-like carbon anchored on n-doped graphitized carbon as a highly efficient bifunctional electrocatalyst for oxygen and hydrogen evolution reactions. ACS Sustain. Chem. Eng. 2022;10(18):6094–6105. doi: 10.1021/acssuschemeng.2c01381. [DOI] [Google Scholar]
- 181.Kumar A, Chaudhary DK, Parvin S, Bhattacharyya S. High performance duckweed-derived carbon support to anchor NiFe electrocatalysts for efficient solar energy driven water splitting. J. Mater. Chem. A. 2018;6(39):18948–18959. doi: 10.1039/C8TA06946H. [DOI] [Google Scholar]
- 182.Wang J, Gao Y, You TL, Ciucci F. Bimetal-decorated nanocarbon as a superior electrocatalyst for overall water splitting. J. Power Sources. 2018;401:312–321. doi: 10.1016/j.jpowsour.2018.09.011. [DOI] [Google Scholar]
- 183.Yang Z, Yan C, Xiang M, Shi Y, Ding M, et al. Biomass carbon dual-doped with iron and nitrogen for high-performance electrocatalyst in water splitting. Int. J. Energy Res. 2021;45:8474–8483. doi: 10.1002/er.6381. [DOI] [Google Scholar]
- 184.Wang B, Xu L, Liu G, Zhang P, Zhu W, et al. Biomass willow catkin-derived Co3O4/N-doped hollow hierarchical porous carbon microtubes as an effective tri-functional electrocatalyst. J. Mater. Chem. A. 2017;5(38):20170–20179. doi: 10.1039/C7TA05002J. [DOI] [Google Scholar]
- 185.Abdolahi B, Gholivand MB, Shamsipur M, Amiri M. Engineering of nickel-cobalt oxide nanostructures based on biomass material for high performance supercapacitor and catalytic water splitting. Int. J. Energy Res. 2021;45:12879–12897. doi: 10.1002/er.6618. [DOI] [Google Scholar]
- 186.Yaseen W, Xie M, Yusuf BA, Xu Y, Rafiq M, et al. Hierarchical Co/MoO2@N-doped carbon nanosheets derived from waste lotus leaves for electrocatalytic water splitting. Int. J. Hydrogen Energy. 2022;47:15673–15686. doi: 10.1016/j.ijhydene.2022.03.037. [DOI] [Google Scholar]
- 187.Yang M, Wu D, Cheng D. Biomass-derived porous carbon supported Co-CoO yolk-shell nanoparticles as enhanced multifunctional electrocatalysts. Int. J. Hydrogen Energy. 2019;44:6525–6534. doi: 10.1016/j.ijhydene.2019.01.155. [DOI] [Google Scholar]
- 188.Hoang VC, Dinh KN, Gomes VG. Hybrid Ni/NiO composite with N-doped activated carbon from waste cauliflower leaves: a sustainable bifunctional electrocatalyst for efficient water splitting. Carbon. 2020;157:515–524. doi: 10.1016/j.carbon.2019.09.080. [DOI] [Google Scholar]
- 189.Lin C, Kang M, Zheng L, Fu X, Wang S. Ammonium nitrate-assisted low-temperature synthesis of Co, Co2P@CoP embedded in biomass-derived carbons as efficient electrocatalysts for hydrogen and oxygen evolution reaction. ChemistrySelect. 2020;5:7338–7346. doi: 10.1002/slct.202001810. [DOI] [Google Scholar]
- 190.Li D, Li Z, Ma J, Peng X, Liu C. One-step construction of Co2P nanoparticles encapsulated in N, P co-doped biomass-based porous carbon as bifunctional efficient electrocatalysts for overall water splitting. Sustain. Energy Fuels. 2021;5:2477–2485. doi: 10.1039/D1SE00062D. [DOI] [Google Scholar]
- 191.Zhang Y, Chen L, Yan B, Zhang F, Shi Y, et al. Regeneration of textile sludge into Cu8S5 decorated N, S self-doped interconnected porous carbon as an advanced bifunctional electrocatalyst for overall water splitting. Chem. Eng. J. 2023;451:138497. doi: 10.1016/j.cej.2022.138497. [DOI] [Google Scholar]
- 192.Amiinu IS, Pu Z, He D, Monestel HGR, Mu S. Scalable cellulose-sponsored functionalized carbon nanorods induced by cobalt for efficient overall water splitting. Carbon. 2018;137:274–281. doi: 10.1016/j.carbon.2018.05.025. [DOI] [Google Scholar]
- 193.Vedhanarayanan B, Shi J, Lin JY, Yun S, Lin TW. Enhanced activity and stability of MoS2 through enriching 1T-phase by covalent functionalization for energy conversion applications. Chem. Eng. J. 2021;403:126318. doi: 10.1016/j.cej.2020.126318. [DOI] [Google Scholar]
- 194.Liu J, Zhao S, Wang C, Ma Y, He L, et al. Catkin-derived mesoporous carbon-supported molybdenum disulfide and nickelhydroxyloxide hybrid as a bifunctional electrocatalyst for driving overall water splitting. J. Colloid Interface Sci. 2022;608:1627–1637. doi: 10.1016/j.jcis.2021.10.069. [DOI] [PubMed] [Google Scholar]
- 195.Liu T, Cai S, Zhao G, Gao Z, Liu S, et al. Recycling valuable cobalt from spent lithium ion batteries for controllably designing a novel sea-urchin-like cobalt nitride-graphene hybrid catalyst: towards efficient overall water splitting. J. Energy Chem. 2021;62:440–450. doi: 10.1016/j.jechem.2021.03.052. [DOI] [Google Scholar]
- 196.Zhao Y, Chen W, Liu F, Zhao P. Hydrothermal pretreatment of cotton textile wastes: biofuel characteristics and biochar electrocatalytic performance. Fuel. 2022;316:123327. doi: 10.1016/j.fuel.2022.123327. [DOI] [Google Scholar]
- 197.Han G, Hu M, Liu Y, Gao J, Han L, et al. Efficient carbon-based catalyst derived from natural cattail fiber for hydrogen evolution reaction. J. Solid State Chem. 2019;274:207–214. doi: 10.1016/j.jssc.2019.03.027. [DOI] [Google Scholar]
- 198.Fakayode OA, Yusuf BA, Zhou C, Xu Y, Ji Q, et al. Simplistic two-step fabrication of porous carbon-based biomass-derived electrocatalyst for efficient hydrogen evolution reaction. Energy Convers. Manage. 2021;227:113628. doi: 10.1016/j.enconman.2020.113628. [DOI] [Google Scholar]
- 199.Kandathil V, Moolakkil A, Kulkarni P, Veetil AK, Kempasiddaiah M, et al. Pd/Fe3O4 supported on bio-waste derived cellulosic-carbon as a nanocatalyst for CC coupling and electrocatalytic application. Front. Chem. Sci. Eng. 2022 doi: 10.1007/s11705-022-2158-y. [DOI] [Google Scholar]
- 200.Mir RA, Pandey O. An ecofriendly route to synthesize C-Mo2C and C/N-Mo2C utilizing waste polyethene for efficient hydrogen evolution reaction (HER) activity and high performance capacitors. Sustain. Energy Fuels. 2020;4:655–669. doi: 10.1039/C9SE00516A. [DOI] [Google Scholar]
- 201.Remmel AL, Ratso S, Divitini G, Danilson M, Mikli V, et al. Nickel and nitrogen-doped bifunctional ORR and HER electrocatalysts derived from CO2. ACS Sustain. Chem. Eng. 2022;10(1):134–145. doi: 10.1021/acssuschemeng.1c05250. [DOI] [Google Scholar]
- 202.Mu X, Gong L, Yang G, Xiong Y, Wan J, et al. Biomass-based transition metal phosphides supported on carbon matrix as efficient and stable electrocatalyst for hydrogen evolution reaction. Int. J. Energy Res. 2022;46:3502–3511. doi: 10.1002/er.7400. [DOI] [Google Scholar]
- 203.Sam DK, Wang W, Gong S, Sam EK, Lv X, et al. CO2 assisted synthesis of silk fibroin driven robust N-doped carbon aerogels coupled with nickel–cobalt particles as highly active electrocatalysts for HER. Int. J. Hydrogen Energy. 2021;46:21525–21533. doi: 10.1016/j.ijhydene.2021.03.243. [DOI] [Google Scholar]
- 204.Li Q, He T, Zhang YQ, Wu H, Liu J, et al. Biomass waste-derived 3D metal-free porous carbon as a bifunctional electrocatalyst for rechargeable zinc–air batteries. ACS Sustain. Chem. Eng. 2019;7(20):17039–17046. doi: 10.1021/acssuschemeng.9b02964. [DOI] [Google Scholar]
- 205.Murugan N, Thangarasu S, Choi GB, Choi J, Oh TH, et al. Interconnected hierarchical nanoarchitectonics of porous carbon nanosheets derived from renewable biomass for efficient oxygen evolution reaction. J. Alloys Compd. 2022;923:166321. doi: 10.1016/j.jallcom.2022.166321. [DOI] [Google Scholar]
- 206.Devi M, Ojha K, Ganguli A, Jha M. Transformation of waste tin-plated steel to iron nanosheets and their application in generation of oxygen. Int. J. Environ. Sci. Technol. 2019;16:3669–3678. doi: 10.1007/s13762-018-1778-8. [DOI] [Google Scholar]
- 207.Farzana R, Sayeed MA, Joseph J, Ostrikov K, O'Mullane AP, et al. Manganese oxide derived from a spent Zn–C battery as a catalyst for the oxygen evolution reaction. ChemElectroChem. 2020;7:2073–2080. doi: 10.1002/celc.202000422. [DOI] [Google Scholar]
- 208.Atchudan R, Selvam NCS, Edison TNJI, Perumal S, Vinodh R, et al. Synthetic disposable material derived-carbon supported NiO: efficient hybrid electrocatalyst for water oxidation process. Fuel. 2021;294:120558. doi: 10.1016/j.fuel.2021.120558. [DOI] [Google Scholar]
- 209.Bandal HA, Pawar AA, Kim H. Transformation of waste onion peels into core-shell Fe3C@N-doped carbon as a robust electrocatalyst for oxygen evolution reaction. Electrochim. Acta. 2022;422:140545. doi: 10.1016/j.electacta.2022.140545. [DOI] [Google Scholar]
- 210.Wang J, Zhao H, Gao Y, Chen D, Chen C, et al. Ba0.5Sr0.5Co0.8Fe0.2O3−δ on N-doped mesoporous carbon derived from organic waste as a bi-functional oxygen catalyst. Int. J. Hydrogen Energy. 2016;41:10744–10754. doi: 10.1016/j.ijhydene.2016.04.049. [DOI] [Google Scholar]
- 211.Rong Z, Dong C, Zhang S, Dong W, Huang F. Co5.47N loaded N-doped carbon as an efficient bifunctional oxygen electrocatalyst for a Zn–air battery. Nanoscale. 2020;12:6089–6095. doi: 10.1039/D0NR00731E. [DOI] [PubMed] [Google Scholar]
- 212.Yang C, Jin Z, Zhang X, Zheng X, He X. Ultrafast regenerating spent LiCoO2 lithium-ion batteries into bifunctional electrodes for rechargeable Zn-air batteries. ChemElectroChem. 2022;9:e202101494. doi: 10.1002/celc.202101494. [DOI] [Google Scholar]
- 213.Sekar S, Kim DY, Lee S. Excellent oxygen evolution reaction of activated carbon-anchored NiO nanotablets prepared by green routes. Nanomaterials. 2020;10:1382. doi: 10.3390/nano10071382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Li G, Zhu T, Ye Y, Liang S, Duan W, et al. Transforming Ni-coagulated polyferriertic sulfate sludge into porous heteroatom-doped carbon-supported transition metal phosphide: an efficient catalyst for oxygen evolution reaction. Energy Technol. 2020;8:1900995. doi: 10.1002/ente.201900995. [DOI] [Google Scholar]
- 215.Xing J, Li Y, Guo S, Jin T, Li H, et al. Molybdenum carbide in-situ embedded into carbon nanosheets as efficient bifunctional electrocatalysts for overall water splitting. Electrochim. Acta. 2019;298:305–312. doi: 10.1016/j.electacta.2018.12.091. [DOI] [Google Scholar]
- 216.Li X, Qian X, Xu Y, Duan F, Yu Q, et al. Electrodeposited cobalt phosphides with hierarchical nanostructure on biomass carbon for bifunctional water splitting in alkaline solution. J. Alloys Compd. 2020;829:154535. doi: 10.1016/j.jallcom.2020.154535. [DOI] [Google Scholar]