Graphical abstract

Keywords: Antifertility potential, GCMS, Estrous cycle, Uterine histology, Persicaria hydropiper, Total cholesterol
Abstract
People from some parts of the world traditionally depend on different herbal medicines for fertility regulation. The Mishing women of Assam, India have been using the dry root powder of Persicaria hydropiper for years as a birth control medicine. The present study was designed to investigate the chemical composition of methanolic extract from the dry roots of P. hydropiper as well as to study its anti-implantation effect. P. hydropiper roots were collected from paddy fields and the methanolic extract was prepared using dry powdered roots. Gas chromatography-mass spectrometry (GCMS) analysis of the methanolic root extract was performed for phytochemical analysis. The estrous cycle of the female mice was monitored by observation of the cells in the vaginal smear. The estrogenic and anti-implantation effect was observed using routine histological procedures with Haematoxylin & Eosin staining performed in mice. Total serum cholesterol level was also measured. The GCMS analysis revealed the presence of stigmasterol and 3-deoxyestradiol, which are known to possess antifertility properties. The extract (1000 mg/kg bodyweight dose) altered the duration and sequence of the estrous cycle of cyclic females with a prolonged metestrous of 2 days, followed by an early estrous. There was hyperplasia in the endometrial epithelium and even shedding of the same on high duration treatment on day 6. There was a significant (p < 0.05) rise in total cholesterol levels in the treated groups. The highest rise was observed in the day 1 group (from 67.91 ± 1.98 to 147.53 ± 3.20 mg/dl) while the lowest change was there in the day 2 group (from 78.76 ± 2.04 to 103.26 ± 2.34 mg/dl). The presence of compounds like stigmasterol and 3-deoxyestradiol with profound antifertility properties possibly has an influence on the molecular pathway for embryo implantation. The changes in uterine histoarchitecture in the form of uterine hyperplasia on treatment with the extract point out towards the effect of the estrogenic compounds. Such implantation preventing results provides support to the traditional belief and opens the door for new drug discovery for reproduction regulation. A detailed molecular study is necessary in this regard.
1. Introduction
The dependency of the animals on plants for food, oxygen and shelter started with the beginning of higher life forms. With the beginning of civilization, human beings learned to recognize the plants that are meant for meeting the necessities of their life. The use of plant extracts for medicinal purposes can be traced to myths and traditions (Mamodev, 2012). Almost all the folk healing methods around the globe use herbs as a part of their tradition (Anttila et al., 2002). The evolution of such folk healing methods led to the emergence of well-known traditional medicine systems, such as the Ayurvedic and Unani of the Indian subcontinent, the Chinese and Tibetan, the Native American of North America, the Amazonian of South America, several local systems within Africa and traditional Arabic and Islamic medicines (Mamodev, 2012, El-Seedi et al., 2019). According to the WHO statistics, about 80 % of the world population depends on the traditional plant-based medicinal systems (Singh et al., 2018). There is an estimation that 10 % of about 75,000 higher plant species around the world are used in traditional medicine; only 1–5 % of them have been studied scientifically and are known to have therapeutic potential (Rao and Gurjar, 1990). The incorporation of natural products in medicines is based on the selection criteria like pharmacognosy, ethnopharmacology and traditional medicine (Lautié et al., 2020). Recent history states that the use of plant-based medicines involved the isolation of the active compounds, which started with the isolation of Morphin from opium in the 19th century; this process of isolation and characterization of pharmacologically active compounds are still continued (Balunas and Kinghorn, 2005). Such natural products have played a crucial role in drug discovery for various diseases like cancer, infectious diseases, therapeutic diseases, multiple sclerosis, etc. (Atanasov et al., 2021). The process of drug discovery involves the selection of a target protein and finding of a small drug-like molecule or biological therapeutic (called a developmental candidate) that eventually progresses to preclinical and clinical (if successful in the preclinical stage) stages and finally production of a marketed medicine (Hughes et al., 2011). The strategy of discovering drugs from natural products is the most successful one as they offer some advantages over the conventional mode of synthesis-based drug discovery like safety, low cost, minimum side effects, better ADME compliance, and most importantly confront against drug resistance developed by the pathogens (Rout et al., 2009).
Persicaria hydropiper (formerly Polygonum hydropiper), commonly known as marsh paper smartweed, marsh- pepper knotweed, smartweed or water pepper, is an annual plant of 40–70 cm height, belonging to the family of Polygonaceae (Huq et al., 2014, Nasir et al., 2021). The genus Persicaria has been segregated from the older genus Polygonum in the recent consensus because of the differences in the form of synapomorphies like the presence of tepals having three principal nerves, distinct nectaries in trichome and papillae form and non-dilated filaments in Persicaria (Sanchez et al., 2011). The name Persicaria hydropiper has been contributed by authors Delarbre and Antoine and was published in Flore d’Auvergne ed. 2: 518. 1800. (Fl. Auvergne) (tropicos.org). This plant is distributed in the Northern hemisphere (Seimandi et al., 2021). Apart from using as spice, food flavour, and garnish for a variety of traditional dishes, this plant has a wide range of uses for medicinal purposes such as in diarrhaea, dyspepsia, cancer, uterine disorders, gastric, pulmonary disorders, etc. (Huq et al., 2014). In Bangladesh, this plant is used for the treatment of gastrointestinal disorders, helminthiasis, anxiety, insomnia, convulsion, inflammatory dyspepsia, diarrhoea, menorrhagia, hemorrhoids, helminthiasis, CNS disorders, stomach pain (Mollik et al., 2010, Mahmud and Lina, 2017). The leaf juice of this plant is used in pain, headache, toothache, dysentery, gastric ulcer, dysmenorrhoea and anorexia. The root juice is a remedy for skin diseases and also is a CNS depressant and wound healer and the seeds are used in burning treatment, bladder pain, stomach pain and erysipelas, and are diuretics (Mahmud and Lina, 2017). Vietnamese use the stems of this plant for treating snake-bite (Loi, 2000). In India, people use this plant for treating general pain, colic pain and headache (Choudhury and Srivastava, 2006, Das et al., 2006, Choudhary et al., 2010). The extract of this plant is known to be used to cure menstrual irregularities (Blatter et al., 1998). The decoction of the whole plant is used to treat excessive menstrual bleeding (Chevallier, 1996). The women of the Garo tribe of Bangladesh consume leaf juice to reduce menstrual pain (Rahmatullah et al., 2009). The antifertility property of P. hydropiper is also reported (Hazarika and Sarma, 2006a). In Europe, this plant is used to induce premature abortion (Uddin et al., 2014). The plant is used to prevent ovulation and termination of pregnancy in traditional Chinese medicine (Xiao and Wong, 1991). The folk women of Assam, a province of northeast India traditionally use the powdered dry root of P. hydropiper for the prevention of unwanted pregnancy (Hazarika and Sarma, 2006a).
The plant has shown lots of pharmacological properties in different experiments. anti-adipogenic, neuroprotective, antibacterial, antifungal, anthelmintic, antifertility, antiglycation, anti-hypertension, anti-inflammatory, anti-neoplasmic, anti-nociceptive, antioxidant, anti-pyretic, antiulcer, cytotoxic, estrogenic, thrombolytic, membrane stabilizing and gastrointestinal motility activities of the plant are recorded from many literatures (Bairagi et al., 2022). The essential oil from leaf of the plant showed decline in anxiety and improvement in exploratory behaviour, motor and coordination abilities primarily because of its anti-radical and cholinesterase inhibitory properties (Tong et al., 2021).
To understand the exact mechanism of the plant extract action, it is necessary to analyze the phytochemical constituents. The constituents of the aerial parts (stem, leaves and flowers) of P. hydropiper have already been done to test their different activities (Ayaz et al., 2016). Various extracts of the parts of this plant such as methanol, ethanol, petroleum ether, chloroform and n-hexane revealed the presence of alkaloids, flavonoids, phenols, saponins, triterpenes, tannins, steroids and carbohydrates (Mahmud and Lina, 2017, Rahman et al., 2002; Ayaz et al., 2014; Sharif et al., 2014). Compounds like persicarin, quercetin 3-sulfate, quercetin, polygodial, polygonal, waburganal, etc. are reported to be present in the aerial parts of the plants (Ayaz et al., 2020). However, sufficient information on the constituents of the root is still lacking. Since the present study emphasizes on the fertility regulatory property of the root extract of P. hydropiper, the analysis of the possible relationship between the compounds and antifertility activity is also necessary. Thus, the present study aims to investigate the bioactive compounds present in the dry root powder of P. hydropiper and to study the alterations in the estrous cycle and endometrial histology due to the application of the extract that leads to loss of pregnancy.
2. Materials and methods
2.1. Material used
The roots of P. hydropiper were collected from the paddy fields of Gohpur (26.8790°N, 93.6058°E) and Nagaon (26.3464°N, 92.6840°E) from the month of February to May 2018. A sample of the plant was identified by the scientists of the Department of Botany, Chaiduar College, Gohpur, Assam and subsequently checked the name in https://www.theplantlist.org (access number: kew-2575083). The voucher specimen has been deposited to the Department of Botany, Chaiduar College, Gohpur, Assam (Voucher No. CDC/BOT/2018–0167).
2.2. Preparation of root extract
The collected roots were washed properly and shade dried. Dried roots were chopped into small pieces and ground into powder in a mixer-grinder to 60 mesh size. The root powder was soaked in methanol for 72 h at room temperature (25 ± 2 °C) and subsequently filtered. The filtrate was concentrated under vacuum at 27 ± 2 °C temperature and stored at −20 °C until use.
2.3. GC–MS analysis
The GC–MS analysis of the phytocompounds from the methanolic extract of P. hydropiper was done using Clarus 680 GC & Clarus 600 CMS, Perkin Elmer, USA. Spectroscopic detection by GC–MS involved an electron ionization system. Inert helium gas was used as a carrier gas. The injection temperature was 280 °C while the transfer temperature and source temperature were 200 °C and 180 °C respectively. The oven temperature was programmed from 60 °C for 3 min, to 200 °C at 5 °C/min, held for 3 min and then to 300 °C at 6 °C/min and held for 10 min. Diluted samples (1/100, v/v, in methanol) of 1 µL were injected manually.
Identification of chemical compounds of the extract was based on retention time on the capillary column and matching of the spectra with computer software (Turbomass NIST 2008) data of standards, attached to the GCMS instrument. The retention index (Kovat’s index) was calculated based on the retention time of each compound using the following formula:
Where,
RIx → Retention index of x
z → n-alkane with z-carbon atoms eluting before x
tRx → Retention time of x
tRz → Retention time of z.
tR(z + 1) → Retention time of n-alkane with z + 1 carbon atom.
eluting after x.
2.4. Experimental animals
Adult cyclic female albino mice (30 ± 5 gm body weight) were used in the present investigation. The animals were purchased from the College of Veterinary Science, Khanapara, Guwahati, Assam, India. Animals were kept under uniform husbandry conditions with natural light and temperature in the Animal house of Chaiduar College, Gohpur. Mice were fed with a routine diet (Bengal gram) and water ad libitum. The estrous cycle of the adult females was studied by observation of cell types in the vaginal smears prior to the experiment. The females showing only the normal estrous cycle (95–105 h) were selected for the in vivo study. All the experiments involving animals were carried out according to the NRC Guide for the Care and Use of Laboratory Animals, 2011.
2.5. Monitoring of estrous cycle
The estrous cycle of both control and treated mice was monitored through the observation of cell types in the vaginal smear following the method Montes and Luque (1988). Vaginal fluid was collected with the help of a smooth dropper filled with distilled water. Collected fluid was placed on a slide to prepare a smear and allowed for air dry. Dried slides were gently placed in methanol for 4–5 min. Following the methanol treatment, the slides were put in May- Grunwald Stain for 10 min and then kept in Giemsa stain for 15 min for staining of the cell types of vaginal smear. Finally, the slides were washed in tap water and dehydrated in 95 % and 100 % alcohol. Dehydrated slides were treated with xylene and finally mounted with DPX for permanent preparation.
2.6. Ovariectomy
Adult cyclic female mice were ovariectomized (OVX) according to the method proposed by Hogan et al. (1986). The intact cyclic mice were injected intraperitoneally with pentobarbital. Ovariectomy was performed by two dorso-lateral incisions of approximately 1 cm length just above the ovaries. The skin was cut almost together with the dorsal muscles using sharp dissecting scissors and thus, the peritoneal cavity was accessed. No suturing was required for the muscle incision. The wounds in the skin were closed bilaterally with the help of one single catgut suture. The OVX females were allowed to recover for a minimum period of three weeks before the commencement of the experiment.
2.7. Detection of pregnancy
Normal cyclic females were selected and allowed to mate with males with proven fertility at a ratio of 2:1 (2 females:1 male). Mice in mating were monitored every morning between 7 AM–8 AM. Detection of a copulatory plug confirmed pregnancy. The day when the plug was observed was considered day 1 of pregnancy.
2.8. Administration of crude root extract
The methanolic crude root extract (CRE) of P. hydropiper was administered to the adult female mice through the oral route. The crude extract was suspended in water and administered to the female mice in different doses (250 mg, 500 mg, 750 mg and 1000 mg/kg body weight). The extract was fed to both the ovary-intact and OVX female mice. Administration of the root extract was made for the early gestational period, i.e., up to day 6 of pregnancy in the case of ovary-intact females. The administration of the CRE in the OVX females was done for a similar period. The extract was administered during the morning hours between 8.00 and 9.00 AM to all the groups. The control ovary-intact and OVX females were treated with water as a vehicle in the same manner and for a similar period.
2.9. Determination of threshold dose
The threshold dose of crude root extract was determined by the administration of four different doses to the adult cyclic female mice during the early gestational period. Each group of females was treated with different doses of CRE. After one complete gestation period, total litter size was considered as the number of total implantation sites. The optimal dose was determined by observing the complete suppression of pregnancy. The percentage of suppression was calculated as the average percentage of the difference between the number of implantation sites in the control and that of the treated divided by the total number of females mated.
2.10. Collection of tissues
Experimental animals were sacrificed by anaesthetizing with pentobarbital intraperitoneally. The uterus was dissected out and fixed in a 10 % formalin solution for histological studies. Blood samples were collected by cardiac puncture and allowed to stand until clotted. After clot formation, the samples were centrifuged and serum samples were collected for determination of total cholesterol.
2.11. Histological studies
Routine histological study of uterine tissues was done following the method of Culling (1974). The tissue was fixed in 10 % formalin for histological observations. Tissues were washed in running tap water to remove excess fixative and dehydrated in graded series of ethanol (30 %-50 %-70 %-90 %-100 %), cleared in xylene and embedded in paraffin wax (60 °C). Paraffin blocks were sectioned at 5 μm thickness using rotary microtome. Dried slides containing fully stretched tissues were stained using routine HE staining and mounted in DPX.
2.12. Determination of total cholesterol
The quantitative in vitro determination of total cholesterol was carried out according to Roeschlau’s method (1974) with some modifications, using the ERBA Total Cholesterol kit (Code No.: 120194, Transasia Biomedicals ltd., India). All the prepared solutions were incubated at 37 °C inside a hot air oven for 10 min. Blank solution followed by the standard and test solutions was aspirated in a Biochemical Analyzer (ERBA diagnostics Mannheim GmbH). The absorbance of the standard and test samples was observed at 505 nm using the ERBA Biochemical Analyzer.
2.13. Statistical analysis
The data were expressed in mean values and standard error. They were statistically analyzed using the SPSS software (SPSS 16.0). Students’ t-test was used to detect differences between control (vehicle) and CRE treated groups. A difference was considered significant if the double-tailed (paired) probability (P) was < 0.05.
3. Results
3.1. GCMS analysis
The bioactive compounds present in the methanolic extract obtained from P. hydropiper dry root powder are shown in Table 1. Their identification and characterization were based on their elution order in the MS column. The molecular formula and molecular weight of the compounds are also presented in the table. The GC chromatogram of the extract is presented in Fig. 1.
Table 1.
List of compounds present in the methanolic root extract of P. hydropiper.
| Sl. No. | Compound Name | Molecular Formula | Molecular Weight (g/mol) | Peak Area (%) | Retention Index |
|---|---|---|---|---|---|
| 1 | (1R,2S,4R,5S)-1,2,4,5-Tetraethylcyclohexane | C14H28 | 196.378 | 1.019 | 1930 |
| 2 | 1,1,1,3,5,5,5-Heptamethyltrisiloxane | C7H21O2Si3 | 221.498 | 0.825 | 1827 |
| 3 | 1,1,3,3,5,5,7,7,9,9-Decamethylpentasiloxane | C10H30O4Si5 | 354.771 | 1.312 | 1810 |
| 4 | 1,2,4,5-Tetraethylcyclohexane | C14H28 | 196.378 | 1.019 | 1930 |
| 5 | 1,4-Bis(trimethylsilyl)benzene | C12H22Si2 | 222.478 | 1.312 | 1810 |
| 6 | 1,5-Diisopropenyl-2,3-dimethylcyclohexane | C12H24 | 192.346 | 4.138 | 1876 |
| 7 | 1,16-Dichlorohexadecane | C16H32Cl2 | 295.332 | 0.352 | 1875 |
| 8 | 1-Chlorohexadecane | C16H33Cl | 260.89 | 1.019 | 1930 |
| 9 | 1-Methyl-1-(2-methylpropyl)-2-nonylcyclopropane | C17H34 | 238.459 | 0.352 | 1875 |
| 10 | 1-Tridecanethiol | C13H28S | 216.427 | 1.019 | 1930 |
| 11 | 2-(4-Methylcyclohexyl)prop-2-en-1-ol | C10H18O | 154.253 | 4.138 | 1876 |
| 12 | 2,2-Dibromocholestan-26-al | C27H44Br2O | 544.456 | 4.138 | 1876 |
| 13 | 2,5-Dimethyl-2-undecene | C13H26 | 182.351 | 0.417 | 1914 |
| 14 | 2-Butyl-1,1,3-trimethyl-cyclohexane | C13H26 | 182.351 | 0.417 | 1914 |
| 15 | 2-Methyldodec-1-ene | C13H26 | 182.351 | 1.019 | 1930 |
| 16 | 3-Deoxyestradiol | C18H24O | 256.389 | 0.356 | 1884 |
| 17 | 3-Hexylthiane 1,1-dioxide | C11H22O2S | 218.355 | 0.199 | 1916 |
| 18 | 4,8,13-Duvatriene-1,3-diol | C20H34O2 | 306.49 | 4.138 | 1876 |
| 19 | 4-Nonene, 5-butyl- | C13H26 | 182.351 | 0.199 | 1916 |
| 20 | 5-Chlorostigmastan-3-yl acetate | C31H53ClO2 | 493.213 | 2.934 | 1802 |
| 21 | 5-Isopropenyl-2,7-dimethyl-1,8-nonadiene | C14H24 | 192.346 | 4.138 | 1876 |
| 22 | 9-Methoxy-9-borabicyclo[3.3.1]nonane | C9H17BO | 152.044 | 1.019 | 1930 |
| 23 | 11-Chloroundecoxy(trimethyl)silane | C14H31ClOSi | 278.936 | 0.352 | 1875 |
| 24 | 11-Methylidenetricosane | C24H48 | 336.648 | 1.019 | 1980 |
| 25 | Arachidic acid | C20H40O2 | 312.538 | 8.055 | 1897 |
| 26 | Behenyl behenate | C44H88O2 | 649.186 | 0.356 | 1884 |
| 27 | Benzyl icosanoate | C27H46O2 | 402.663 | 0.356 | 1884 |
| 28 | Benzyl stearate | C25H42O2 | 374.609 | 0.352 | 1875 |
| 29 | beta-Sitosterol trimethylsilyl ether | C32H58OSi | 486.9 | 2.934 | 1802 |
| 30 | beta-Sitosteryl acetate | C31H52O2 | 456.755 | 2.934 | 1802 |
| 31 | Cholest-5-en-3-beta-yl cinnamate | C36H52O2 | 516.81 | 2.934 | 1802 |
| 32 | Cholesta-3,5-diene | C27H44 | 368.649 | 2.934 | 1802 |
| 33 | Cholesterol chloroformate | C28H45ClO2 | 449.116 | 2.934 | 1802 |
| 34 | Cholesteryl benzoate | C34H50O2 | 490.772 | 2.934 | 1802 |
| 35 | Cholesteryl myristate | C41H72O2 | 597.025 | 2.934 | 1802 |
| 36 | Citronellyl isovalerate | C15H28O2 | 240.387 | 0.535 | 1915 |
| 37 | Citronellyl propionate | C13H24O2 | 212.33 | 0.535 | 1915 |
| 38 | Crinosterol | C28H46O | 398.675 | 2.934 | 1802 |
| 39 | Cyclododecanol | C12H24O | 184.323 | 0.535 | 1915 |
| 40 | Cyclopentadecanol | C15H30O | 226.404 | 0.535 | 1915 |
| 41 | Dodecamethylpentasiloxane | C12H36O4Si5 | 384.841 | 0.825 | 1827 |
| 42 | Ergocalciferol | C28H44O | 396.659 | 0.271 | 1860 |
| 43 | Heptadecanoic acid | C17H34O2 | 270.457 | 8.055 | 1897 |
| 44 | (22E)-26,27-Dinorergosta-5,22-dien-3beta-ol acetate | C28H44O2 | 412.658 | 2.934 | 1802 |
| 45 | Isopropyl palmitate | C19H38O2 | 298.511 | 8.055 | 1897 |
| 46 | (3beta,22Z)-Chola-5,22-dien-3-ol | C24H38O | 342.567 | 2.934 | 1802 |
| 47 | l-Ascorbic acid, dihexadecanoate | C38H68O8 | 652.954 | 8.055 | 1897 |
| 48 | Lauric acid | C12H24O2 | 200.322 | 8.055 | 1897 |
| 49 | Ledane | C15H26 | 206.373 | 4.138 | 1876 |
| 50 | Lignoceric acid | C24H48O2 | 368.646 | 8.055 | 1897 |
| 51 | Methyl 13-methylpentadecanoate | C17H34O2 | 270.457 | 0.616 | 1903 |
| 52 | Methyl 14-methylpentadecanoate | C17H34O2 | 270.457 | 0.616 | 1903 |
| 53 | Methyl elaidate | C19H36O2 | 296.495 | 0.356 | 1884 |
| 54 | Methyl oleate | C19H36O2 | 296.495 | 0.356 | 1884 |
| 55 | Methyl palmitate | C17H34O2 | 270.457 | 0.616 | 1903 |
| 56 | Methyl trimethicone | C10H30O3Si4 | 310.687 | 0.825 | 1827 |
| 57 | Myristic acid | C14H28O2 | 228.376 | 8.055 | 1897 |
| 58 | Myristyl chloride | C14H29Cl | 232.836 | 0.352 | 1875 |
| 59 | 3alpha,11beta-Bis(trimethylsiloxy)-5beta-androstan-17-one O-benzyl oxime | C32H53NO3Si2 | 555.95 | 1.312 | 1810 |
| 60 | Octadecane, 1-chloro- | C18H37Cl | 288.944 | 0.352 | 1875 |
| 61 | Palmitic acid | C16H32O2 | 256.43 | 8.055 | 1897 |
| 62 | Pentadecanoic acid | C15H30O2 | 242.403 | 8.055 | 1897 |
| 63 | 5alpha-Ergost-25-ene-3beta,5,6beta,12beta-tetrol | C28H48O4 | 448.688 | 4.138 | 1876 |
| 64 | 1,2-Epoxyoctadecane | C18H36O | 268.485 | 0.535 | 1915 |
| 65 | Stearic acid | C18H36O2 | 284.484 | 8.055 | 1897 |
| 66 | Stigmasterol | C29H48O | 412.702 | 0.271 | 1860 |
| 67 | Stigmasterol TMS ether | C32H56OSi | 484.884 | 0.271 | 1860 |
| 68 | Tetracos-15-enoate | C24H45O2- | 365.622 | 0.535 | 1915 |
| 69 | Thymol-TMS | C13H22OSi | 222.403 | 0.825 | 1827 |
| 70 | Tridecanoic acid | C13H26O2 | 214.349 | 8.055 | 1897 |
| 71 | 3alpha-(Trimethylsiloxy)-17-(phenylmethoxyimino)-5alpha-androstan-11-one | C29H43NO3Si | 481.752 | 1.312 | 1810 |
| 72 | Vitamin D2 | C28H44O | 396.659 | 0.271 | 1860 |
| 73 | (1R,6S)-4alpha,7,7-Trimethylbicyclo[4.1.0]heptan-3beta-ol | C10H18O | 154.253 | 4.138 | 1876 |
| 74 | (1S,3S,4S,6R)-4,7,7-trimethylbicyclo[4.1.0]heptan-3-ol | C10H18O | 154.253 | 4.138 | 1876 |
| 75 | (1alpha,3beta,4alpha,6alpha)-4,7,7-Trimethylbicyclo[4.1.0]heptan-3-ol | C10H18O | 154.253 | 4.138 | 1876 |
| 76 | (1R,2Z,4S,7Z,11Z)-1,7,11-trimethyl-4-propan-2-ylcyclotetradeca-2,7,11-trien-1-ol | C20H34O | 290.491 | 4.138 | 1876 |
| 77 | (22E)-Stigmasta-5,22-dien-3beta-ol trifluoroacetate | C31H47F3O2 | 508.71 | 4.138 | 1808 |
| 78 | 2,3-bis(trimethylsilyloxy)propyl (9E,12E)-octadeca-9,12-dienoate | C27H54O4Si2 | 498.895 | 4.138 | 1808 |
Fig. 1.
GC chromatogram of the methanolic root extract of Persicaria hydropiper.
Analysis of the 20 prominent peaks of the chromatogram led to the identification of 78 compounds in the methanolic extract. Compounds like 1, 25-dihydroxy vitamin D3, 3-Deoxyestradiol, cyclodecanol, tridecanoic acid, tetradecanoic acid, pentadecanoic acid, n- hexadecanoic acid, heptadecanoic acid, octadecanoic acid, dodecanoic acid, isopropyl palmitate, thunbergol, 22-stigmasten-3–one, 3α-(trimethysiloxy) cholest-5-ene, vitamin A aldehyde, stigmasterol, 5. alpha-ergost-8(14)-ene, lithocholic acid, dihydrotachysterol, stigmasterol trimethylsilyl ether, 22, 23-dibromostigmasterol acetate, ergocalciferol, hexestrol di-TMS, tetrasiloxane, decamethyl-1,2-bis (trimethylsilyl) benzene, β-sitosterol acetate, stigmast-5-en-3-ol oleate, cholesterol chloroformate, stigmastan-3,5-diene, ergosta-5,22-diene-3-ol, (3.β., 22E,24S)-, chola-5,22-dien-3-ol, (3.β.,22Z)-, etc. were found in the analysis.
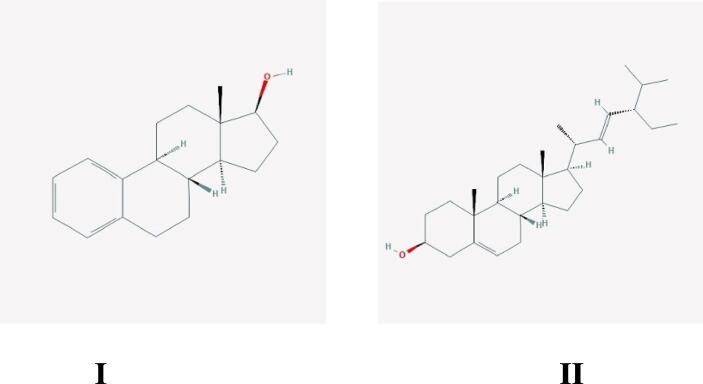
Analysis revealed some sterols and their related compounds like dihydrotachysterol, stigmasterol, crinosterol, β-sitosterol acetate, stigmast-5-en-3-ol, oleate, cholest-5-en-3-ol (3.β)-, carbonochloridate, stigmastan-3,5-diene, 22,23-dibromostigmasterol acetate, ergosta-5,22-dien- 3-ol, (3.β., 22E, 24S)-, chola-5,22-dien-3-ol, (3.β., 22Z)-, cholesta-3,5-diene, 3-deoxyestradiol, Cholesterol chloroformate, ergosta-4, 6, 22-triene, cholesterol pentafluoropropionate, β-sitosterol trimethylsilyl ether, stigmasterol trimethylsilyl ether, 22, 23-dibromostigmasterol acetate, etc. Among them, stigmasterol and 3-deoxyestradiol were found to contain antifertility activity (Fig. 2).
Fig. 2.
Structures of some compounds having antifertility property- I. 3-deoxyestradiol (PubChem, CID = 228944, 2018), II. Stigmasterol (PubChem, CID = 5280794, 2018).
3.2. Threshold dose determination
Among all the doses of the crude root extract of P. hydropiper, 1000 mg/Kg body weight dose was found to be 100 % effective in the prevention of pregnancy in the female mice with positive mating (Table. 2). The dose of 1000 mg/Kg body weight was selected for the study of the antifertility property of the P. hydropiper crude root extract.
Table 2.
Determination of threshold dose for P. hydropiper methanolic root extract.
| Treatment | Dose (mg/Kg body weight) | No. of Mated Female |
No. of Pregnant Female |
Percentage of Pregnancy |
No. of Implantation sites | Percentage of Suppression |
|---|---|---|---|---|---|---|
| Control | Water | 10 | 10 | 100 % | 9.40 ± 0.52 | 0 % |
| CRE | 250 | 10 | 10 | 100 % | 6.80 ± 0.29 | 27.66 % |
| CRE | 500 | 10 | 6 | 60 % | 4.60 ± 0.42 | 51.06 % |
| CRE | 750 | 10 | 3 | 30 % | 1.67 ± 0.33 | 82.23 % |
| CRE | 1000 | 10 | 0 | 0 % | 0.00 ± 0.00 | 100 % |
* Values are expressed as mean ± SEM.
*Values are significant at 95 % confidence level (p < 0.05).
3.3. Estrous cycle monitoring and effect of CRE on estrous cycle
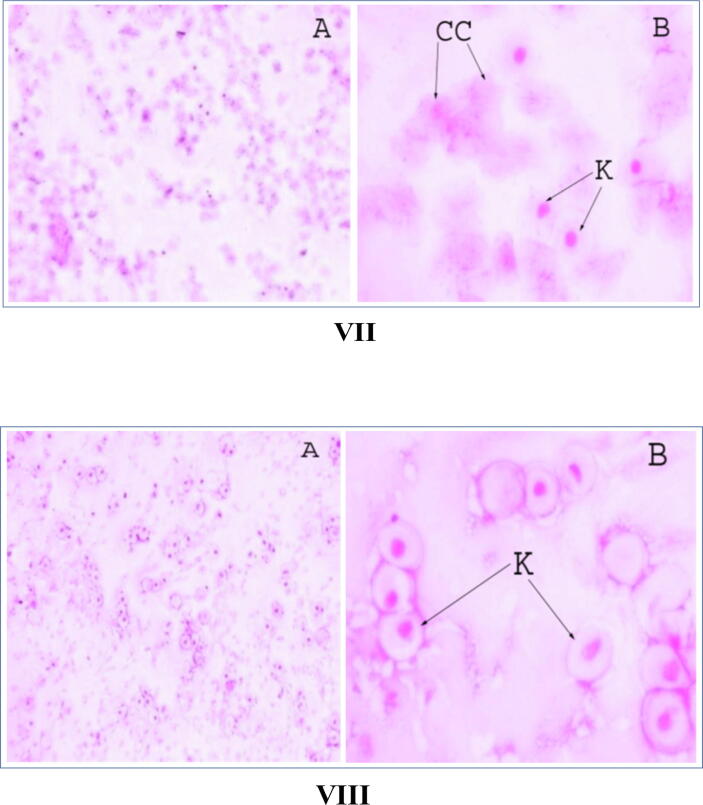
The estrous cycle of mice is characterized by four major phases: proestrous, estrous, metestorus and diestrous (Fig. 3: I and II). Cyclic control mice showed nucleated epithelial cells also called karyopyknotic cells (K) either singly or in sheet during the proestrous phase. The estrous phase was characterized by exfoliated cytology of cornified cells (CC). The period of metestrous followed estrous and occurs after ovulation. The cytology of the vaginal smear was characterized by many leucocytes among a few cornified cells. The vaginal smear during diestrous consists mainly of leucocytes and occasionally a few parabasal cells. Cyclic female mice were subjected to the CRE treatment for two consecutive estrous cycles, i. e., for 8 days. The first cycle in every case followed the usual sequence of stages with a diminished number of cells in the vaginal smear (Fig. 4: I to IV). In the second cycle under study, the sequence was greatly disrupted with a prolonged metestrous stage, followed by an early estrous stage (Fig. 4: V to VII). That stage was followed by proestrous (Fig. 4: VIII). There was a noticeable decrease in total cell number in the smear.
Fig. 3.
Estrous cycle of cyclic control mice (I. = A, B, C, D × 10x and II. = A, B, C, D × 40x). A = Proestrous, B = Estrous, C = Metestrous, D = Diestrous. K = Karyopyknotic cells, CC = Cornified cells, L = leucocytes.
Fig. 4.
Different stages of estrous cycle of treated cyclic mice (A × 10x, B × 40x). K = Karyopyknotic cells, CC = Cornified cells, L = leucocytes. I. Day 1 treatment- Proestrous stage, II. Day 2 treatment- Early estrous stage, III. Day 3 treatment- Metestrous stage, IV. Day 4 treatment- Diestrous stage, V. Day 5 treatment- Metestrous stage, VI. Day 6 treatment-Metestrous stage, VII. Day 7 treatment- Early estrous stage, VIII. Day 8 treatment- Day 1 treatment.
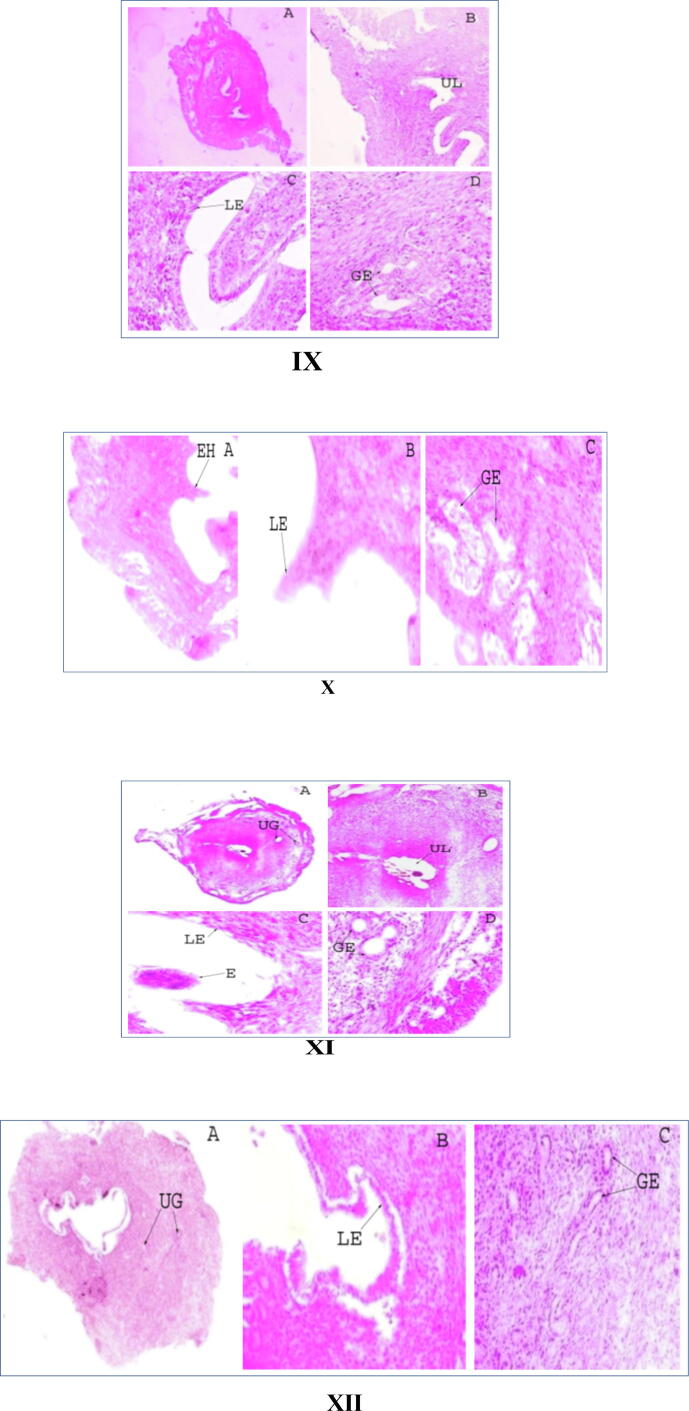
3.4. Study of uterine histoarchitecture
All the uterine sections of Day 1 to Day 6 pregnant mice showed the presence of all the uterine layers, i. e., endometrium, myometrium and perimetrium. The stroma of the uterine layers was seen compact. A distinct endometrial lumen was seen in each section. The epithelial layer surrounding the lumen was smooth. Multiple layers of endometrial glands were seen, but they gradually became less abundant with progressive days of pregnancy. The glandular epithelium was thin and the epithelial cells contained a deeply stained nucleus (Fig. 5: I, III, V, VII, IX and XI). However, the thickness increased on Day 5 and Day 6 of pregnancy (Fig. 5: IX and XI). Section of Day 6 pregnant uterus showed the presence of an embryo in the uterine lumen (Fig. 5: XI).
Fig. 5.
T.S. of control and treated uteri of 1–6 days of pregnancy: I. Day 1 Control, II. Day 1 Treated, III. Day 2 Control, IV. Day 2 Treated, V. Day 3 Control, VI. Day 3 Treated, VII. Day 4 Control, VIII. Day 4 Treated, IX. Day 5 Control, X. Day 5 Treated, XI. Day 6 Control, XII. Day 6 Treated (A × 4x, B × 10x, C, D × 40x; for Day 5 and Day 6 treated uteri, A × 10x, B, C × 40x). LE = Luminal Epithelium, UG = Uterine Glands, UL = Uterine lumen, GE = Glandular epithelium, EH = Endometrial hyperplasia, E = Embryo.
The CRE-treated uterine sections showed similar compartmentalization as that of the control ones. However, luminal epithelium showed a higher rate of mitosis resulting in endometrial hyperplasia. Large numbers of endometrial glands were seen in sections of Day 1-Day 3 CRE treatment (Fig. 5: II, IV and VI). In Day 4-Day 6 sections, endometrial glands are less numerous (Fig. 5: VIII, X and XII). Glandular epithelia of all the sections were thinner with deeply stained nuclei. The uterus of the Day 6 treated mouse showed shedding of the luminal epithelium (Fig. 5: XII).
The uterine section of the control OVX mouse showed the presence of all the three uterine layers-endometrium, myometrium and perimetrium. The cells of all the layers were least proliferated. The endometrium was very thin with minimal glands. There were few uterine glands seen in the section (Fig. 6: I). On the contrary, the uterine histology of the CRE-treated OVX mouse showed proliferation of cells in all the three layers as compared to the control one (Fig. 6: II). Uterine glands with thin glandular epithelium were observed in the OVX CRE-treated mouse (Fig. 6: II).
Fig. 6.
T.S. of uteri of control and extract treated ovariectomized mouse: I. OVX Control, II. OVX Treated (A × 10x, B × 40x). UL = Uterine lumen, LE = Luminal epithelium, UG = Uterine glands, GE = Glandular epithelium.
3.5. Determination of total cholesterol level
The serum cholesterol level showed a significant increase in the treated groups as compared to the control ones in OVX as well as pregnant females (Fig. 7). The cholesterol level was found to be highest in the treated group on Day 1 of pregnancy. However, all the values of serum cholesterol levels were found within the normal range.
Fig. 7.
Comparison of serum cholesterol levels among the control and CRE treated groups of pregnant and ovariectomized mice.
4. Discussion
Medicinal plants serve as the resources of different types of drugs that contribute various ingredients to fight against a wide range of diseases. Analysis and extraction of those ingredients are important steps in the formulation of a new drug (Gomathi et al., 2015). Therefore, the present study was designed to study the phytocompounds present in the dry root extract of P. hydropiper and to investigate the estrogenic compounds which may have possible antifertility properties. Moreover, the study also included the effect of the CRE on the estrous cycle and uterine histoarchitecture which have a possible correlation with the presence of some specific phytocompounds in the extract.
P. hydropiper is a well-known medicinal plant, possessing lots of medicinal properties. Previous investigations on the phytochemical contents of leaves, stem, whole plant and essential oil of this plant detected the presence of chemicals like (+)-catechin, hyperin, (−)-epicatechin, isoquercitrin, isorhamnetin, quercetin, quercitrin, kaempferol, rhamnazin, rutin, 3-β-angeloyloxy-7-epifutronolide, futronolide, changweikangic acid A, (+)-winterin, hydropiperosides A and B, vanicosides A, B and E, caffeic acid, ρ-coumaric acid, apigenin-7-O-glucoside, galloyl quercetin-3-O-glucoside, isorhamnetin, isorhamnetin-3,7-disulphate, kaempferol rutinoside, quercetin-3-O-glucoside, quercetin-3-O-rhamnoside, 7,4ʹ-dimethylquercetin, drimenol, isodrimeninol, 11-ethoxycinnamolide, confertifolin, ethyl benzene, etc. (Huq et al., 2014). The essential oil of the plant was reported to have 141 compounds, among which the most abundant ones were α-bulnesene, β-caryophyllene epoxide, cis-geranylacetone, decahydronaphthalene, dihydro-alpha-ionone and nerolidol. Some of the compounds found in the plant essential oil like 4-Thujanol, α-bulnesene, α-muurolene, β-elemene, β-ocimene, bornyl acetate, campherenone, caprylic acid, fenchol, fixol, isocaryophyllene, limonene, myrcene, nerolidol, octylcyclopropane, sativene, β-caryophyllene epoxide, and terpineol could bind to α-amylase and α-glucosidase, thus have a potential to prevent diabetes mellitus (Mahnashi et al., 2022a, Mahnashi et al., 2022b). Six compounds Ph-1, Ph-2, Ph-3, Ph-4, Ph-5 and Ph-6 from the ethyl acetate fraction of aerial parts of P. hydropiper showed radical scavenging and α-amylase and α-glucosidase inhibitory activity (Ayaz et al., 2022). Crude, chloroform, hexane, ethyl acetate, butanol, aquous and saponin extract of the plant also showed potent inhibition against α-amylase and α-glucosidase enzymes. Moreover, the 24 compounds identified so far by HPLC-DAD phenolics analysis, such as gallic acid, caffeic acid, quercetin, hydroxybenzoic acid, etc. showed in-silico inhibition of α-amylase and α-glucosidase (Mahnashi et al., 2022a, Mahnashi et al., 2022b). 4-methyl-5-oxo-tetrahydrofuran-3-yl acetate and methyl 4-hydroxy-3-methoxybenzoate isolated from P. hydropiper showed cytotoxicity against cervical cancer cells (HeLa cells), breast cancer cells (MCF-7) and NIH/3T3 fibroblast cells. The compounds also showed anti-angiogenic and anti-tumour activities (Mahnashi et al., 2021).
The dry root extract of this plant is traditionally used as a contraceptive measure among the Mishing women of Assam (Hazarika and Sarma, 2006a). The estrogenic property of P. hydropiper dry root extract has been established earlier (Hazarika and Sarma, 2006b, Hazarika and Sarma, 2007). In the present study, GCMS analysis of the methanolic root extract of P. hydropiper revealed the presence of various compounds which have been mentioned in the result section. Different studies show these compounds as bioactive ones with numerous healing properties (Mathieu and Adorini, 2002, Odhiambo et al., 2017, Abidizadegan et al., 2021). There are reports regarding the presence of hydropiperoside, gallic acid, ellagic acid 3,3′-dimethyl ether, anthraquinone, polygonolide in the P. hydropiper root extract from previous studies (Ayaz et al., 2020), but these compounds have not been detected in the present study. This difference in phytochemical contents may be because of the differences in geographical location, environmental factors and climatic conditions of the habitats from which the samples were collected (Liu et al., 2016, Kumar et al., 2017a, Kumar et al., 2017b, AL-Hmadi et all., 2021).
Among the detected compounds in the present investigation, some are estrogenic substances. The estrogenic substances are known for their effects on the maintenance of the state of pregnancy by affecting the equilibrium of reproductive hormones that regulate the hypothalamo- hypophyseal-gonadal axis (Havranex et al., 1973, Iguchi and Sato, 2000). They show effects on fertility in sheep and cattle with low birth rates, uterine prolapse, hydrops uteri and pyomethron. The effect may be temporary or permanent, as temporary infertility occurs in ewes consuming estrogenic pastures during mating time, while long-term consumption causes permanent infertility (Burton and Wells, 2002). Neonatal exposure to phytoestrogens during early postnatal days renders permanent acyclicity in rats (Whitten et al., 1993). Consumption of these estrogenic compounds may cause infertility by shortening the time of the transport of egg, disrupting the estrous cycle, lowering the plasma progesterone and decreasing pregnanediol which finally stops the development of endometrium (Shibeshi et al., 2006). Maintenance of the proper synchronization between the synthesis of both estrogen and progesterone is important for implantation (Zhang et al., 2013). The antifertility effect of the plant extract can be believed to be due to the mimicking action of the phytoestrogens.
Among the compounds detected so far by GCMS, stigmasterol is claimed to possess the estrogenic activity and it was found to be responsible for the antifertility activity in immature mice (Pal et al., 2012). This compound has an affinity for the estrogen receptors and thus leads to infertility in animals (Dande and Patil, 2012, Suryawanshi, 2011). It is also identified as a precursor of semisynthetic progesterone, which plays an important role in the regulatory and tissue rebuilding mechanisms related to estrogenic effects and also acts as an intermediate in the biosynthesis of androgens, estrogens and corticoids (Alamgir, 2018). An elevated level of progesterone inhibits the secretion of FSH and LH, thus preventing ovulation (Apgar and Greenberg, 2000). Likewise, stigmastan-3, 5-diol is also estrogenic in nature (Odhiambo et al, 2017). The compound stigmasterol was also isolated from the chloroform fraction of P. hydropiper and this compound could bind to beta amyloid cleaving enzyme 1 (BACE 1) in in silico studies. Inhibition of BACE 1 can be considered as a management tool for Alzheimer’s disease (Ayaz et al., 2021).
Another compound, 3-deoxyestradiol is a classical estrogen having an affinity for estrogen receptors (Borgona and Scali, 1991). Estrogen receptor agonists have been used as drugs for contraception (Li and Chen, 2006). This compound is also known to possess female contraceptive activity (Kincl and Dorfman, 1965). Being an estradiol, it may exert its estrogenic effects primarily via the estrogenic receptors α and β, receptor α binding predominating in the uterus, mammary gland, pituitary, adipose tissue and bone and receptor β mediating signaling in the ovary, prostate, lung and cardiovascular and central nervous tissue (Yaşar et al., 2017).
The presence of these components having antifertility properties sheds light upon the traditional belief of the plant being a remedy for contraception. The result of the GCMS analysis also supports the previous findings about the estrogenic and implantation inhibitory activities of the plant root extract. However, for validation of these activities in animals, certain experiments were conducted on female albino mice. The threshold dose for complete suppression of pregnancy was found to be 1000 mg/kg body weight of the experimental mice. This effective dose was also confirmed by previous literatures (Hazarika and Sarma, 2006a, Hazarika and Sarma, 2006b).
The estrous cycle is a rhythmical reproductive cycle occurring in female mammals; loss of cyclicity indicates disruption of estrogen and progesterone balance of the ovaries. Treatment with some substances can cause alteration in the estrous cycle, characterized by a prolonged estrous or a persistent diestrous or any other irregular pattern with extended duration (Monima et al., 2019). The present study showed a prolonged metestrous stage upon treatment with the CRE, followed by an early estrous, with prthe evalence of cornified cells. As cornification of cells is brought about by the action of estrogen, the prolonged metestrous and early occurrence of estrous may be due to the estrogenic effect of the crude extract (Sharanabasappa et al., 2002). Such prolonged estrous and metestrous was also seen upon treatment with the benzene extract of Hibiscus rosa sinensis flowers (Murthy et al., 1997) and with root extract of Metroxylon sago (Haryono et al., 2013). Moreover, there was a complete absence of the diestrous phase. The presence of steroids and terpenoids in the extract may lead to resistance of the diestrous and elongation of the estrous (Haryono et al., 2013). Similar extended metestrous and decreased diestrous were noticed in normal cyclic rats when progesterone was administered between the estrous and metestrous stage, delaying ovulation (He et al., 2017). However, there may be some differences in the pathway of action between the continuous treatment of the CRE from the proestrous stage and the administration of exogenous progesterone between estrous and metestrous. The alteration in the estrous cycle takes place as a result of high steroid exposure that may be caused by the consumption of phytoestrogens (Hazarika, 2006).
The implantation of the embryo is considered as one of the crucial events for the establishment of pregnancy that depends upon the interaction of the implanting embryo and the receptive endometrium (Messaoudi et al., 2019). Endometrial receptivity is defined as the period of maturation during which the trophectodermal cells of the blastocyst can attach to the endometrium and subsequently invade the endometrial stroma as well as vasculature; histological study of the uterine endometrium has always been considered as a tool for endometrial receptivity (Lessey and Young, 2019). The thickness of the endometrium acts as an indicator of receptivity (Shaodi et al., 2020). A thickened endometrium developed during the reproductive cycle provides a site for attachment of the embryo (Richter et al., 2007). However, a highly thick endometrium beyond the optimum range of thickness leads to decreased rate of pregnancy or even zero pregnancy (Eftekhar et al., 2019).
In the present study, it was found that the CRE-treated endometrium of the pregnant mice showed extensive mitosis of the endometrium and development of endometrial hyperplasia. Moreover, there was a gradual decrease in the number of endometrial glands in the treated females. Endometrial glands synthesize and secrete lots of proteins required for endometrial receptivity and embryo implantation during the pre-implantation and early pregnancy period (Carson et al., 2000, Gray et al., 2001). Loss of the endometrial glands is associated with implantation defects and infertility, because such a uterus cannot accommodate a developing embryo (Monima et al., 2019).
Endometrial hyperplasia is characterized by excessive proliferation of the endometrial cells or the inner uterine lining, often caused by high levels of estrogen, with an insufficient amount of progesterone-like hormones (Horn et al., 2007). It is considered as one of the major reasons for repeated implantation failure (Valdes et al., 2017). Therefore, in the present study, the development of hyperplasia of the endometrium may be regarded as the cause for the termination of pregnancy of the treated mice. The inhibition of pregnancy may be due to insufficient progesterone concentration, as progestin treatment in women with endometrial hyperplasia resulted in successful pregnancy (Li et al., 2017). Moreover, there was an extensive proliferation of the uterine tissue, especially of endometrium in the CRE-treated OVX mice. In places, endometrial hyperplasia was also observed. Estrogen possesses a well-established role in endometrial cell proliferation (Hazarika and Sarma, 2006a). The uterus is very sensitive to estrogen and whenever it is exposed to a little dose of estrogen, it increases the volume and accumulates fluid inside it (Barbosa et al., 2015). As the CRE contains a few bioactive estrogenic compounds, this proliferation may be attributed to them. In the treated pregnant group, shedding of the inner endometrial lining was observed on the Day 6 of treatment. Such shedding of endometrium was seen in menstrual-like mice model due to inhibition of the FSH and LH release in the serum as well as significantly decreased level of serum progesterone (Wang et al., 2013, Wang et al., 2019). Therefore, a possible decrease of those hormones may be a factor in endometrial shedding of the mice of Day 6.
Cholesterol is a precursor for steroid hormones, bile salts and vitamin D; its level increases during the pregnancy period to aid in the fetal development by means of cell membrane formation and maintaining membrane integrity and membrane-associated signaling cascade (Grimes and Wild, 2018). In the present study, there was a progressive increase in the total cholesterol level with progressive days of pregnancy in the control group. This rise may be due to the elevation in the concentration of maternal estrogen that causes a rise in total cholesterolevelsel (Bartels and O’Donoghue, 2011). But there was a sudden rise in cholesterol levels in the CRE-treated mice on first day of pregnancy, followed by a decrease on the second day of treatment. With progressing treatment days, the total cholesterol level showed a minimal rise, but all the values were within the normal range. An earlier study suggests that the use of hormonal contraceptives (both progesterone only and estrogen and progesterone containing) increases total cholesterol in human females (Asare et al., 2014). In the present investigation, a sudden rise in total cholesterol level in the treated pregnant group is thought to be the result of the combined action of the elevated maternal estrogen level as well as estrogenic compounds in the CRE. As the first day of pregnancy occurred during the estrous phase of the estrous cycle, the maternal estrogen level was considered to be the highest (Wood et al., 2007), which probably led to the highest rise in the total cholesterol level. Moreover, in the CRE, there were a few cholesterol-based compounds that may have a possible role in the rise of total cholesterol level in the treated group.
5. Conclusion
In the present study, GCMS analysis of the methanolic dry root extract of P. hydropiper reveals the presence of 78 phytocompounds, three of which are estrogenic. Two of the estrogenic compounds, stigmasterol and 3-deoxyestradiol are known to possess potent antifertility properties. This extract was also found to disrupt the estrous cycle, and to induce an anti- implantation effect by forming endometrial hyperplasia. The estrogenic compounds stigmasterol and 3-deoxyestradiol possibly have altered the molecular pathway of implantation by means of formation of hyperplasia. Such results are somehow supportive of the traditional belief. The methanolic root extract has increased the total cholesterol level too. A systematic study in this aspect is in progress. Keeping in view about the presence of certain compounds with antifertility properties and the effect of the extract as a whole for pregnancy suppression, this plant may be regarded as a potential source of new compounds of therapeutic value as well as for the development of the new contraceptive drug.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgment
The authors are grateful to the Department of Biotechnology, Government of India and the Advanced Institutional Level Biotech Hub, Chaiduar College, Gohpur for providing financial support (Grant No.-102/I.F. D/SAN/2420/2018-19 Dated 20.09.2018) and necessary facilities to carry on the present work.
Ethical approval
Approval was obtained from the Institutional Animal Ethical Committee of Gauhati University (Ethical Approval No.-IEAC/2021-22/03). All the experiments with animals were carried on according to the international rules on the use and care of the laboratory animals.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abidizadegan M., Peltomaa E., Blomster J. The Potential of Cryptophyte Algae in Biomedical and Pharmaceutical Applications. Front. Pharmacol. 2021;11 doi: 10.3389/fphar.2020.618836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alamgir N.M. Therapeutic Use of Medicinal Plants and their Extracts: Volume 2, Progress in Drug Research 74. Springer International Publishing AG, part of Springer Nature; 2018. Secondary Metabolites: Secondary Metabolic Products Consisting of C and H; C, H, and O; N, S, and P Elements; and O/N Heterocycles; pp. 165–309. [Google Scholar]
- Al-Hmadi H., El Mokni R., Joshi R.K., Mohamed Ashour L., Hammami S. The Impact of Geographical Location on the Chemical Compositions of Pimpinella lutea Desf, Growing in Tunisia. Appl. Sci. 2021;11:7739. [Google Scholar]
- Anttila, A., Bhat, R.V., Bond, J.A., Borghoff, S.J., et al., 2002. Some traditional herbal medicines some mycotoxins, naphthalene and styrene. IARC monographs on the evaluation of carcinogenic risks to humans. Lyon, France. [PMC free article] [PubMed]
- Apgar B.S., Greenberg G. Using progestins in clinical practice. Am. Fam. Physician. 2000;62(8):1839–1846. [PubMed] [Google Scholar]
- Asare G.A., Santa S., Ngala R.A., Asiedu B., Afriyie D., Amoah A.G.B. Effect of hormonal contraceptives on lipid profile and the risk indices for cardiovascular disease in a Ghanaian community. Int. J. Women’s Health. 2014;6:597–603. doi: 10.2147/IJWH.S59852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atanasov A.G., Zotchev S.B., Dirsch V.M., Supuran C.T., the International Natural Product Sciences Taskforce Natural products in drug discovery: advances and opportunities. Nature Rev. Drug Discov. 2021;20:200–216. doi: 10.1038/s41573-020-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz M., Ahmad I., Sadiq A., Ullah F., Ovais M., Khalil A.T., Devkota H.P. Persicaria hydropiper (L.) Delarbre: A review on traditional uses, bioactive chemical constituents and pharmacological and toxicological activities. J. Ethnopharmacol. 2020;251 doi: 10.1016/j.jep.2019.112516. [DOI] [PubMed] [Google Scholar]
- Ayaz M., Wadood A., Sadiq A.F.U., Anichkina O., Ghufran M. In-silico evaluations of the isolated phytosterols from Polygonum hydropiper L against BACE1 and MAO drug targets. J. Biomol. Struct. Dynam. 2021 doi: 10.1080/07391102.2021.1940286. [DOI] [PubMed] [Google Scholar]
- Ayaz M., Sadiq A., Mosa O.F., Zafar T.A., Hamdoon A.A.E., Elkhalifa E.M., Elawad M.A., Ahmed A., Ullah F., Ghufran M., Kabra A. Antioxidant, Enzyme Inhibitory, and Molecular Docking Approaches to the Antidiabetic Potentials of Bioactive Compounds from Persicaria hydropiper L. Evidence-Based Complement. Alternat. Med. 2022:6705810. doi: 10.1155/2022/6705810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz M., Junaid M., Ullah F., Sadiq A., Ovais M., Ahmed W., Ahmad S., Anwar Z. Chemical profiling, antimicrobial and insecticidal evaluations of Polygonum hydropiper L. BMC Complement. Alternat. Med. 2016;16:502. doi: 10.1186/s12906-016-1491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bairagi J., Saikia P.J., Boro F., Hazarika A. A review on the ethnopharmacology, phytochemistry and pharmacology of Polygonum hydropiper Linn. J. Pharm. Pharmacol. 2022;74:619–645. doi: 10.1093/jpp/rgab175. [DOI] [PubMed] [Google Scholar]
- Balunas M.J., Kinghorn A.D. Drug discovery from medicinal plants. Life Sci. 2005;78:431–441. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Barbosa S.D., Guerra O.J.M., Neto B.M., et al. Effects of ethanol extract of Cenostigma macrophyllum Tul. (caneleiro) on reproductive parameters of female rats. Rev Cubana Plant Med. 2015;20(3):265–276. [Google Scholar]
- Bartels A., O’Donoghue K. Cholesterol in pregnancy: a review of knowns and unknowns. Obst. Med. 2011;4:147–151. doi: 10.1258/om.2011.110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter E., Caius J.F., Mhask K.S. Periodical experts Book Agency; Vivek Vihar, India: 1998. Indian Medicinal Plants. [Google Scholar]
- Borgona J.-L., Scali J. Differential interactions of estrogens and antiestrogens at the 17β- hydroxy or counterpart function with the estrogen receptor. Eur. J. Biochem. 1991;199:575–585. doi: 10.1111/j.1432-1033.1991.tb16157.x. [DOI] [PubMed] [Google Scholar]
- Burton J.L., Wells M. The effect of phytoestrogens on the female genital tract. J. Clin. Pathol. 2002;55:401–407. doi: 10.1136/jcp.55.6.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson D.D., Bagchi I., Dey S.K., Enders A.C., Fazleabas A.T., Lessey B.A., Yoshinaga K. Embryo implantation. Dev. Biol. 2000;223:217–237. doi: 10.1006/dbio.2000.9767. [DOI] [PubMed] [Google Scholar]
- Chevallier A. Dorling Kindersley; London UK: 1996. The Encyclopedia of Medicinal Plants. [Google Scholar]
- Choudhary R.K., Oh S., Lee J. An ethnomedicinal inventory of knotweeds of Indian Himalaya. J. Med. Plant Res. 2010;5:2095–2103. [Google Scholar]
- Choudhury R.K., Srivastava R.C. IPR and traditional knowledge of Adi, Memba and Khamba tribes of upper Siang district, Arunachal Pradesh. Intellect. Prop. Rights: Plant Variet Genome Conser. 2006;10:151–158. [Google Scholar]
- Culling C.F.A. 3rd Edn. Bufferworths; London: 1974. Hand book of histopathological and histochemical techniques. [Google Scholar]
- Dande P., Patil S. Evaluation of saponin from Trigonella foenum Graecum seed for its anti- fertility activity. Asian J. Pharm. Clin. Res. 2012;5(3):154–157. [Google Scholar]
- Das N.J., Saikia S.P., Sarkar S., Devi K. Medicinal plants of North-Kamrup district of Assam used in primary healthcare system. Indian J. Tradit Knowl. 2006;5:489–493. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Database; CID=228944, https://pubchem.ncbi.nlm.nih.gov/compound/228944 (accessed Dec 8, 2018).
- National Center for Biotechnology Information. PubChem Compound Database; CID=5280794, https://pubchem.ncbi.nlm.nih.gov/compound/5280794 (accessed Dec 8, 2018).
- Eftekhar M., Mehrjardi S.Z., Molaei B., Taheri F., Mangoli E. The correlation between endometrial thickness and pregnancy outcomes in fresh ART cycles with different age groups: a retrospective study. Middle East Fert. Soc. J. 2019;24:10. [Google Scholar]
- El-Seedi H.R., Khalifa S.A.M., Yosri N., Khatib A., Lei C., Aamer S., Thomas E., Verpoorte R. Plants mentioned in the Islamic Scriptures (Holy Qur'ân and Ahadith): Traditional uses and medicinal importance in contemporary times. J. Ethnopharmacol. 2019;243 doi: 10.1016/j.jep.2019.112007. [DOI] [PubMed] [Google Scholar]
- Garber J.C., Waynee B.R., Bielitzki J.T., Clayton L.A., Donovan J.C. eight ed. The National Academies Press; Washington, D.C: 2011. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- Gomathi D., Manokaran K., Ravikumar G., Devaki K., Uma C. GC-MS analysis of bioactive compounds from the whole plant ethanolic extract of Evolvulus alsinoides (L.) J. Food Sci. Technol. 2015;52(2):1212–1217. doi: 10.1007/s13197-013-1105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C.A., Frank F.B., Becky J.T., Anne A.W., Greg A.J., Fuller W.B., Thomas E.S. Developmental Biology of Uterine Glands. Biol. Reprod. 2001;65:1311–1323. doi: 10.1095/biolreprod65.5.1311. [DOI] [PubMed] [Google Scholar]
- Grimes S.B., Wild R. In: Endotext (Internet) Feingold K.R., Anawalt B., Boyce A., editors. MDText.com, Inc; South Dartmouth (MA): 2018. Effect of Pregnancy on Lipid Metabolism and Lipoprotein Levels. [Google Scholar]
- Haryono A., Gunawan Y.E., Suatma S.S., Rahmadur M. Anti- fertility Effect of Various Plants at Dayak Tribe to Swiss Webster Mice. J. Trop. Life Sci. 2013;3(2):108–112. [Google Scholar]
- Havranex F., Stroufová A., Kozlová J., Herzmann J., Hejda J. On the mechanism of contraceptive action of estrogens administered after ovulation. Ceska Gynekol. 1973;38:617–619. [PubMed] [Google Scholar]
- Hazarika A. Rajiv Gandhi University; 2006. Study of Polygonum hydropiper Linn. Root Extract on Fertility Regulation in Female Albino Rat. Thesis. [Google Scholar]
- Hazarika A., Sarma H.N. The estrogenic effects of Polygonum hydropiper root extract induce follicular recruitment and endometrial hyperplasia in female albino rats. Contraception. 2006;74:426–434. doi: 10.1016/j.contraception.2006.05.073. [DOI] [PubMed] [Google Scholar]
- Hazarika A., Sarma H.N. Polygonum hydropiper crude root extract mimics estrogenic properties in females: Evidence of uterine protein profiles studied by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Reprod. Med. Biol. 2006;5:155–160. doi: 10.1111/j.1447-0578.2006.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazarika A., Sarma H.N. Effects of crude root extract of Polygonum hydropiper on estrous cycle and induction of reversible sterility in female albino rat. J. Endocrinol. Reprod. 2007;11(1):36–40. [Google Scholar]
- He W., Li X., Adekunbi D., Liu Y., Long H., Wang L.i., Lyu Q., Kuang Y., O’Byrne K.T. Hypothalamic effects of progesterone on regulation of the pulsatile and surge release of luteinising hormone in female rats. Sci. Rep. 2017;7:8096. doi: 10.1038/s41598-017-08805-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B., Constantini F., Lacy E. Cold Spring Harbour press, USA; A laboratory manual: 1986. Manipulating the mouse embryo. [Google Scholar]
- Horn L.-C., Meinel A., Handzel R., Einenkel J. Histopathology of endometrial hyperplasia and endometrial carcinoma An update. Ann. Diagnost. Pathol. 2007;11:297–311. doi: 10.1016/j.anndiagpath.2007.05.002. [DOI] [PubMed] [Google Scholar]
- https://www.tropicos.org/name/100349011/ (accessed 24 July 2022).
- Hughes J.P., Rees S., Kalindjian S.B., Philpott K.L. Br. J. Pharmacol. 2011;162:1239–1249. doi: 10.1111/j.1476-5381.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq A.K.M., Jamal J.A., Stanlas J. Ethnobotanical, Phytochemical, Pharmacological and Toxicological Aspects of Persicaria hydropiper (L.) Delabre. Evidence-Based Complement. Alternat. Med. 2014;782830 doi: 10.1155/2014/782830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi I., Sato T. Endocrine disruption and developmental abnormalities of female reproduction. Am. Zool. 2000;40:402–411. [Google Scholar]
- Kincl F.A., Dorfman R.I. Antifertlity activity of various steroids in the female rat. J. Reprod. Fertil. 1965;10:105–113. doi: 10.1530/jrf.0.0100105. [DOI] [PubMed] [Google Scholar]
- Kumar S., Yadav M., Yadav A., Yadav J.P. Impact of spatial and climatic conditions on phytochemical diversity and in vitro antioxidant activity of Indian Aloe vera (L.) Burm.f. S. Afr. J. Bot. 2017;111:50–59. [Google Scholar]
- Kumar S., Yadav A., Yadav M., Yadav J.P. Effect of climate change on phytochemical diversity, total phenolic content and in vitro antioxidant activity of Aloe vera (L.) Burm.f. BMC Res. Notes. 2017;10:60. doi: 10.1186/s13104-017-2385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautié E., Russo O., Ducrot P., Boutin J.A. Unraveling Plant Natural Chemical Diversity for Drug Discovery Purposes. Front. Pharmacol. 2020;11:397. doi: 10.3389/fphar.2020.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessey B.A., Young S.L. What exactly is endometrial receptivity? Fertil. Steril. 2019;111(4):611–617. doi: 10.1016/j.fertnstert.2019.02.009. [DOI] [PubMed] [Google Scholar]
- Li H., Chen Y.Z. Prediction of estrogen receptor agonists and characterization of associated molecular descriptors by statistical learning methods. J. Mol. Graph. Model. 2006;25(3):313–323. doi: 10.1016/j.jmgm.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Li M., Song J.-L., Zhao Y., Wu S.-L., Liu H.-B., Tang R., Yan L. Fertility outcomes in infertile women with complex hyperplasia or complex atypical hyperplasia who received progestin therapy and in vitro fertilization*. J. Zhejiang Univ.-SCIENCE B (Biomed. Biotechnol.) 2017;18(11):1022–1025. doi: 10.1631/jzus.B1600523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Yin D., Li N., Hou X., Wang D., Li D., Liu J. Influence of Environmental Factors on the Active Substance Production and Antioxidant Activity in Potentilla fruticosa L. and Its Quality Assessment. Sci. Rep. 2016;6:28591. doi: 10.1038/srep28591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi D.T. Hanoi Medicine Publishing House; Hanoi, Vietnam: 2000. The Glossary of Vietnamese Medicinal Plants and Items. [Google Scholar]
- Mahmud M.S.A., Lina S.a.M.M. Evaluation of sedative and anxiolytic activities of methanol extract of leaves of Persicaria hydropiper in mice. Clin. Phytosci. 2017;3 doi: 10.1186/s40816-017-0056-5. [DOI] [Google Scholar]
- Mahnashi M.H., Alqahtani Y.S., Alyami B.A., Alqarni A.O., Ullah F., Wadood A., Sadiq A., Shareef A., Ayaz M. Cytotoxicity, anti-angiogenic, anti-tumor and molecular docking studies on phytochemicals isolated from Polygonum hydropiper L. BMC Complement Med. Ther. 2021;21:239. doi: 10.1186/s12906-021-03411-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahnashi M.H., Alqahtani Y.S., Alyami B.A., Alqarni A.O., Alqahl S.A., Ullah F., Sadiq A., Zeb A., Ghufran M., Kuraev A., Nawaz A., Ayaz M. HPLC-DAD phenolics analysis, α-glucosidase, α-amylase inhibitory, molecular docking and nutritional profiles of Persicaria hydropiper L. BMC Complement. Med. Therap. 2022;22:26. doi: 10.1186/s12906-022-03510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahnashi M.H., Alqahtani Y.S., Alyami B.A., Alqarni Ali O., Ayaz Muhammad, Ghufran Mehreen, Ullah Farhat, Sadiq Abdul, Ullah Ihsan, Haq Ikram Ul, Khalid Mohammad, Murthy H.C., Ananda Phytochemical Analysis, α-Glucosidase and Amylase Inhibitory, and Molecular Docking Studies on Persicaria hydropiper L. Leaves Essential Oils. Evidence-Based Complement. Alternat. Med. 2022;7924171 doi: 10.1155/2022/7924171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamodev N. Medicinal plants studies: History, challenges and prospective. Med. Aromatic Plants. 2012;1:8. [Google Scholar]
- Mathieu C., Adorini L. The coming of age of 1,25-dihydroxyvitamin D3 analogs as immunomodulatory agents. Trends Mol. Med. 2002;8(4):174–179. doi: 10.1016/s1471-4914(02)02294-3. [DOI] [PubMed] [Google Scholar]
- Messaoudi S., Kasmi I.E.L., Bourdiec A., Crespo K., Bissonnette L., Le Saint C., Bissonnette F., Kadoch I.-J. 15 years of transcriptomic analysis on endometrial receptivity: what have we learnt? Fert. Res. Pract. 2019;5:9. doi: 10.1186/s40738-019-0059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollik M.A.H., Hossan M.S., Paul A.K., et al. A comparative analysis of medicinal plants used by folk medicinal healers in three districts of Bangladesh and inquiry as to mode of selection of medicinal plants. Ethnobot. Res. Appl. 2010;8:195–218. [Google Scholar]
- Monima L.A., Buhari M., Lawal S., Isaac E., Fred S., Elna O., Edmund B., Diaz M.E., Fernandez B., Victor A., Ikwap K. Effect of Cleome gynandra leaf extract on the Estrous cycle and histology of the ovary and Uterus of wistar albino rats. Anatomy J. Africa. 2019;8(1):1385–1394. [Google Scholar]
- Montes G.S., Luque E.H. Effects of steroids on vaginal smears in the rat. Acta. Anat. 1988;133:192–199. doi: 10.1159/000146639. [DOI] [PubMed] [Google Scholar]
- Murthy D.R., Krishna R., Madhusudana C., Patil B.S. Effect of Benzene Extract of Hibiscus Rosa Sinensis on the Estrous Cycle and Ovarian Activity in Albino Mice. Biol. Pharm. Bull. 1997;20(7):756–758. doi: 10.1248/bpb.20.756. [DOI] [PubMed] [Google Scholar]
- Nasir A., Khalil Atif A.K., Bhatti M.Z., Rehman A.U., Li J., Zahida P. Review on Pharmacological and Phytochemical Prospects of Traditional Medicinal Plant: Persicaria hydropiper (Smartweed) Curr. Top. Med. Chem. 2021;21(12):1027–1036. doi: 10.2174/1568026621666210303145045. [DOI] [PubMed] [Google Scholar]
- Odhiambo R.S., Kareru P.G., Mwangi E.K., Onyango D.W. Contraceptive and Phytochemical Profile of Lime- Yellow Pea (Macrotyloma axillare E. Mey) Verdc: A Tropical Climber. EJMP. 2017;19(2):1–13. [Google Scholar]
- Pal D., Mazumdar U.K., Gupta M. Fractionation of stigmasterol derivative and study of the effects of Celsia coromandelina aerial parts petroleum ether extract on appearance of puberty and ovarian steroidogenesis in immature mice. Pharm. Biol. 2012;50(6):747–753. doi: 10.3109/13880209.2011.628321. [DOI] [PubMed] [Google Scholar]
- Rahman E., Goni S.A., Rahman M.T., Ahmed M. Antinociceptive activity of Polygonum hydropiper. Fitoterapia. 2002;73:704–706. doi: 10.1016/s0367-326x(02)00239-3. [DOI] [PubMed] [Google Scholar]
- Rahmatullah M., Mukti I.J., Haque A.K.M., Fahmidul H., Mollik M.d., Parvin A., Jahan K., Chowdhury R., Majeedul H., Rahman T. An ethnobotanical survey and pharmacological evaluation of medicinal plants used by the Garo tribal community living in Netrokona district, Bangladesh. Adv. Nat. Appl. Sci. 2009;3(3):402–418. [Google Scholar]
- Rao A.V.R., Gurjar M.K. Drugs from plant resources: An overview. Pharmatimes. 1990;22(5):19–20. [Google Scholar]
- Richter K.S., Bugge K.R., Bromer J.G., Levy M.J. Relationship between endometrial thickness and embryo implantation, based on 1,294 cycles of in vitro fertilization with transfer of two blastocyst-stage embryos. Fertil. Steril. 2007;87(1):53–59. doi: 10.1016/j.fertnstert.2006.05.064. [DOI] [PubMed] [Google Scholar]
- Roeschlau P., Bernt E., Gruber W. Enzymatic determination of total cholesterol in serum. Z Klin Chem. Klin Biochem. 1974;12(5):226. PMID: 4440114. [PubMed] [Google Scholar]
- Rout S.P., Choudary K.A., Kar D.M., Das L., Jain A. Plants in traditional medicinal system—future source of new drugs. Int. J. Pharm. Pharm. Sci. 2009;1:1–23. [Google Scholar]
- Sanchez A., Schuster T.M., Burke J.M., Kron K. A taxonomy of Polygonoideae (Polygonaceae): a new tribal classification. Taxon. 2011;60:151–160. [Google Scholar]
- Seimandi G., Álvarez N., Stegmayer M.I., Fernández L., Ruiz V., Favaro M.A., Derita M. An Update on Phytochemicals and Pharmacological Activities of the Genus Persicaria and Polygonum. Molecules. 2021;26:5956. doi: 10.3390/molecules26195956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaodi Z., Qiuyuan L., Yisha Y., Cuilian Z. The effect of endometrial thickness on pregnancy outcomes of frozen-thawed embryo transfer cycles which underwent hormone replacement therapy. PLoS ONE. 2020;15(9):e0239120. doi: 10.1371/journal.pone.0239120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharanabasappa A., Vijayakumar B., Patil S.B. Effect of Momordica charantia Seed Extracts on Ovarian and Uterine Activities in Albino Rats. Pharm. Biol. 2002;40(7):501–507. [Google Scholar]
- Sharif S., Ahmed T., Haque M.A., et al. Phytochemical screenings, thrombolytic activity, membrane stabilizing activity and cytotoxic properties of Polygonum hydropiper. Res. J. Med. Plant. 2014;8:92–98. [Google Scholar]
- Shibeshi W., Makonnen E., Debella A., Zerihun L. Phytochemical, contraceptive efficacy and safety evaluations of the methanolic leaves extract of Achyranthes aspera L. in rats. Pharmacol. Online. 2006;3:217–224. [Google Scholar]
- Singh S., Singh D.B., Singh S., Shukla R., Ramteke P.W., Misra K. Exploring Medicinal Plant Legacy for Drug Discovery in Postgenomic Era. Natl. Acad. Sci., India, Sect. B Biol. Sci. Proc. 2018 doi: 10.1007/s40011-018-1013-x. [DOI] [Google Scholar]
- Suryawanshi J.A.S. Neem- natural contraceptive for male and female- an overview. Int. J. Biomol. Biomed. 2011;1:1–6. [Google Scholar]
- Tong X., Li X., Ayaz M., Ullah F., Sadiq A., Ovais M., Shahid M., Khayrullin M., Hazrat A. Neuroprotective Studies on Polygonum hydropiper L. Essential Oils Using Transgenic Animal Models. Front. Pharmacol. 2021;11 doi: 10.3389/fphar.2020.580069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin K., Mahbubur A.H.M., Islam R.A.K.M. Taxonomy and traditional medicine practices of Polygonaceae (Smartweed) family at Rajshahi, Bangladesh. Int. J. Adv. Res. 2014;2:459–469. [Google Scholar]
- Valdes C.T., Schut A., Simon C. Implantation failure of endometrial origin: it is not pathology, but our failure to synchronize the developing embryo with a receptive endometrium. Fertil. Steril. 2017;108(1):15–18. doi: 10.1016/j.fertnstert.2017.05.033. [DOI] [PubMed] [Google Scholar]
- Wang S.-F., Chen X.-H., He B., Yin D.-D., Gao H.-J., Zhao H.-Q., Nan N., Guo S.-G., Liu J.-B., Wu B., Xu X.B. Acute restraint stress triggers progesterone withdrawal and endometrial breakdown and shedding through corticosterone stimulation in mouse menstrual-like model. Reproduction. 2019;157(2):149–161. doi: 10.1530/REP-18-0163. [DOI] [PubMed] [Google Scholar]
- Wang Q., Xu X., He B., Li Y., Chen X., Wang J. A critical period of progesterone withdrawal precedes endometrial breakdown and shedding in mouse menstrual-like model. Reprod. Endocrinol. 2013;28(6):1670–1678. doi: 10.1093/humrep/det052. [DOI] [PubMed] [Google Scholar]
- Whitten P.L., Lewis C., Naftolin F. A phytoestrogen diet induces the premature anovulatory syndrome in lactationally exposed female rats. Biol. Reprod. 1993;49:1117–1121. doi: 10.1095/biolreprod49.5.1117. [DOI] [PubMed] [Google Scholar]
- Wood G.A., Fata J.E., Watson K.L.M., Khokha R. Circulating hormones and estrous stage predict cellular and stromal remodeling in murine uterus. Reproduction. 2007;133:1035–1044. doi: 10.1530/REP-06-0302. [DOI] [PubMed] [Google Scholar]
- Xiao P.G., Wong W.N. Can ethnopharmacology contribute to the development of anfertility drugs? J. Ethnopharmacol. 1991;32:167–177. doi: 10.1016/0378-8741(91)90114-s. [DOI] [PubMed] [Google Scholar]
- Yaşar P., Ayaz G., User S.D., Güpür G., Muyan M. Molecular mechanism of estrogen–estrogen receptor signaling. Reprod. Med. Biol. 2017;16:4–20. doi: 10.1002/rmb2.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Lin H., Kong S., Wang S., Wang H., Wang H., Armant D.R. Physiological and molecular determinants of embryo implantation. Mol. Aspects Med. 2013;34(5):939–980. doi: 10.1016/j.mam.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]