Abstract
Ethnopharmacological relevance
Xuanfei Baidu Decoction (XFBD), one of the “three medicines and three prescriptions” for the clinically effective treatment of COVID-19 in China, plays an important role in the treatment of mild and/or common patients with dampness-toxin obstructing lung syndrome.
Aim of the study
The present work aims to elucidate the protective effects and the possible mechanism of XFBD against the acute inflammation and pulmonary fibrosis.
Methods
We use TGF-β1 induced fibroblast activation model and LPS/IL-4 induced macrophage inflammation model as in vitro cell models. The mice model of lung fibrosis was induced by BLM via endotracheal drip, and then XFBD (4.6 g/kg, 9.2 g/kg) were administered orally respectively. The efficacy and molecular mechanisms in the presence or absence of XFBD were investigated.
Results
The results proved that XFBD can effectively inhibit fibroblast collagen deposition, down-regulate the level of α-SMA and inhibit the migration of fibroblasts. IL-4 induced macrophage polarization was also inhibited and the secretions of the inflammatory factors including IL6, iNOS were down-regulated. In vivo experiments, the results proved that XFBD improved the weight loss and survival rate of the mice. The XFBD high-dose administration group had a significant effect in inhibiting collagen deposition and the expression of α-SMA in the lungs of mice. XFBD can reduce bleomycin-induced pulmonary fibrosis by inhibiting IL-6/STAT3 activation and related macrophage infiltration.
Conclusions
Xuanfei Baidu Decoction protects against macrophages induced inflammation and pulmonary fibrosis via inhibiting IL-6/STAT3 signaling pathway.
Keywords: COVID-19, Xuanfei baidu decoction, Pulmonary fibrosis, Macrophage polarization, IL-6/STAT3
Abbreviations: IPF, Idiopathic pulmonary fibrosis; COVID-19, coronavirus disease 2019; XFBD, Xuanfei Baidu Decoction; LPS, lipopolysaccharide; BLM, bleomycin; TNF-α, tumor necrosis factor-alpha; IL-6, Interleukin 6; IL-1β, Interleukin 1β; IL-10, Interleukin 10; iNOS, inducible nitric oxide synthase; TGF-β, transforming growth factor-β; TCM, Traditional Chinese Medicine; Arg-1, Arginase 1; μCT, Micro CT; α-SMA, α-smooth muscle actin; Dex, Dexamethasone; FBS, Fetal bovine serum; F4/80, Mouse EGF-like module-containing mucin-like hormone receptor-like 1; STAT3, Signal transducer and activator of transcription 3; EMT, Epithelial-mesenchymal transition
Graphical abstract
1. Introduction
In December 2019, there was an outbreak of new coronavirus pneumonia (Coronavirus disease 2019, COVID-19) caused by a new type of coronavirus (SARS-CoV-2). There is no clinically targeted specific medicine. In addition to direct viral damage, hyperinflammatory responses also known as cytokine storms is a major reason of disease severity and death. Pro-inflammatory response can solve the virus infection in most cases, however, if inflammation persists and immune cells dysfunction, this recovery response cannot be completed, which may lead to further accumulation of immune cells in the lungs, forming an inflammatory storm and damaging the lung infrastructure (Tay et al., 2020).
There are numerous medications in clinical trials or practice against COVID-19, such as a monoclonal neutralizing antibody binding IL-6 receptors tocilizumab (Xu et al., 2020), an anti-Ebola drug remdesivir (Beigel et al., 2020) and so on. Among these drugs, only dexamethasone has a confirmed clinical benefit reducing 28-day mortality rate by 17% compared to that in the remdesivir-treated group (Ledford, 2020). In China, Traditional Chinese Medicine (TCM) has been proven effective for COVID-19 treatment (Liu et al., 2020a). According to the report of State Council of the People's Republic of China (2020), more than 74,000 patients were treated with TCM, and clinical observation shows that the overall effective rate of TCM, (2020) reached above 90%. The National Administration of TCM recommended “three medicines and three prescriptions” for clinically treatment of COVID-19. “Three medicines” are Jinhua Qinggan Granule, Lianhua Qingwen Capsule and Xuebijing Injection, “Three prescriptions” refer to Qingfei Paidu Decoction, HuaShi BaiDu Formula, and XuanFei BaiDu Granule (Huang et al., 2020). An empirical study from Wuhan showed that Qingfei Baidu decoction contributed to the recovery of various disease progresses in COVID-19 patients (Luo et al., 2020). Furthermore, XFBD can significantly improve the clinical symptoms of COVID-19 patients, decrease the number of white blood cells and lymphocytes, and play an anti-inflammatory effect by significantly reducing C-reactive protein and erythrocyte sedimentation rate (Xiong et al., 2020). Furthermore, the 7th edition of the “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia of P.R. China” mentioned that Xuanfei Baidu Decoction (XFBD) is one of the three clinically used TCM remedies for treating common COVID-19 patients, it showed very promising clinic outcomes. XFBD integrates 13 classical herbs including Ephedrae Herba from Ephedra sinica Stapf, Ephedra intermedia Schrenk & C.A.Mey. and Ephedra equisetina Bunge, Armeniacae Semen Amarum from Prunus sibirica L., Prunus armeniaca L. and Prunus mandshurica (Maxim.) Koehne, Coicis Semen from Coix lacryma-jobi L.var.ma-yuen (Roman.) Stapf, Polygoni Cuspidati Rhizoma et Radix from Polygonum cuspidatum Siebold & Zucc., Gypsum Fibrosum, Atractylodis Rhizoma from Atractylodes lancea (Thunb.) DC., Pogostemonis Herba from Pogostemon cablin (Blanco) Benth., Artemisia Annua Herba from Artemisia annua L., Verbenae Herba from Verbena officinalis L., Phragmitis Rhizoma from Phragmites australis subsp. australis, Descurainiae Semen Lepidii Semen from Descurainia sophia(L.)Webb. ex Prantl. and Lepidium apetalum Willd., Citri Grandis Exocarpium from Citrus maxima (Burm.) Merr., Glycyrrhizae Radix et Rhizoma from Glycyrrhiza uralensis Fisch. ex DC., Glycyrrhiza inflata Batalin., Glycyrrhiza glabra L. and Gypsum Fibrosum. It is reported that multiple herbs of XFBD and their main components have an effect in balancing immune inflammatory response, resisting viral infection and viral protein transcription, and restoring the balance of liver and gallbladder metabolism and energy metabolism in the body by regulating the biological processes of viral infection, immune inflammation, liver and gallbladder metabolism and energy metabolism (Wang et al., 2020). However, the underlying mechanism regulating immune responses by XFBD still remains unknown.
Based on the published data, COVID-19 patients can lead to pulmonary fibrosis, and the prognosis of this serious complication is also worthy of our attention (Zhang et al., 2021). The morphological features of pulmonary fibrosis are thickened alveolar septum, collagen deposition and fibroblast proliferation, and diffuse inflammation (Chanda et al., 2019; Craig et al., 2015). It is generally believed that the development of fibrotic diseases includes two stages: inflammatory response and fibrosis stage (Li et al., 2016; Martinez et al., 2017). In the early stage of lung injury, the body undergoes inflammatory response. Inflammatory cells such as neutrophils and macrophages infiltrate and secrete large number of inflammatory factors to promote the repair of lung injury, such as: tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interleukin-1β (IL-1β), etc.(Heukels et al., 2019). In the fibrosis stage, there will be excessive proliferation of fibroblasts and myofibroblasts and excessive accumulation of extracellular matrix (ECM), which will further lead to impaired lung function. Accordingly, the control of inflammation in the early stage of pulmonary fibrosis can effectively slow down the occurrence and development of pulmonary fibrosis.
At present, there have been a lot of studies on molecular signal transduction pathways that regulate the different stages of pulmonary fibrosis development. Multi-signaling pathways such as transforming growth factor-β (TGF-β)/PI3K/AKT, Wnt-β-catenin, hedgehog and Notch pathways have a relation with lung development (Chanda et al., 2019). For example, the activation of the PI3K/AKT signaling pathway induced by TGF-β1 can lead to EMT, fibroblast proliferation and collagen accumulation, which is a key step in the development of fibrosis (Liu et al., 2016a). Wnt-β-catenin signal induces the activation of lung fibroblasts into a fibrotic phenotype, promotes the proliferation of fibroblasts, and the differentiation of myofibroblasts (King et al., 2011). IL-6 mediates many complications in the lungs, and its release imbalance is related to the pathogenesis of various respiratory diseases. IL-6 promotes the phosphorylation and translocation of STAT3 (Pulivendala et al., 2020). STAT3 is associated with activation, proliferation of fibroblasts and ECM deposition, which in turn contributes to fibrotic disease progression.
In this study, we focuse on exploring the potential anti-fibrosis role of XFBD, using fibroblasts and macrophages cell models, as well as a bleomycin-induced mouse lung fibrosis model. The anti-fibrosis pharmacodynamics and mechanism of XFBD have been studied, hoping to provide more choices and basis for the applications of traditional Chinese medicine in the treatment of IPF.
2. Materials and methods
2.1. Chemicals and reagents
XFBD was provided by TianJin Modern TCM Innovation Center (TRT, 200302). Reference standards including amygdalin, sinapine, verbenalin, hastatoside, liquiritin, glycyrrhizic acid, acteoside and naringin were bought from Chengdu Desite Bio-Technology Co., Ltd. (Chengdu, China), and ephedrine and polydatin were purchased from National Institutes for Food and Drug Control (Beijing, China). HPLC-grade methanol (MeOH) and acetonitrile were obtained from Fisher (Leicestershire, UK). LC-MS/HPLC-grade formic acid (FA) was obtained from Anaqua Chemicals Supply (Wilmington, USA). LPS was purchased from Sigma-Aldrich (Shanghai) trading company Ltd. Bleomycin hydrochloride was purchased from Dalian Meilun Biotech (Dalian, China). Enzyme linked immunosorbent assay (ELISA) kits of IL-6, TNF-α, IL-10 and Arg-1 were purchased from Shanghai ZCi BiO Science & Technology Co., Ltd. F4/80, and α-SMA primary antibodies were purchased from Abcam (Cambridge, MA, USA). MTT cell proliferation and cytotoxicity detection kit were purchased from Beijing Solarbio Science & Technology Co., Ltd.
2.2. Gene networks analysis
Prediction of targets of XFBD was performed by BATMAN-TCM (a Bioinformatics Analysis Tool for Molecular Mechanism of Traditional Chinese Medicine) (Liu et al., 2016b). BATMAN-TCM is the first online bioinformatics analysis tool dedicated to analyzing and predicting the molecular mechanism of TCM. In the BATMAN-TCM, the compounds of the herbs in XFBD were obtained from TCMID database (http://119.3.41.228:8000/tcmid/). And all these compounds’ data was curated from literatures. The online tools such as Gene Cards (http://www.genecards.org/) were used to predict potential therapeutic targets for XFBD in the treatment of IPF (Stelzer et al., 2016). The Kyoto Encyclopedia of Genes and Genomics (KEGG) pathway analysis in the DAVID database were used for investigating gene function. We constructed a PPI (protein–protein interaction) network to clarify the molecular mechanisms of anti-inflammation effects of XFBD by using the Cytoscape software (version 3.7.2; http://www.cytoscape.org) (Kohl et al., 2011) and the STRING website (version 11.0, http://www.string-db.org/) with a required confidence >0.4 (Szklarczyk, 2017). Next, we used Cytoscape software (version 3.7.2) to analyze the degree of connectivity in the PPI network and obtained the hub genes.
2.3. UHPLC analysis
The freeze-dried powder of XFBD (0.4000 g) was extracted with ultrapure water (1:25, g/mL) in an ultrasonic water bath for 30 min. The solution was diluted with 50% methanol at the ratio of 1:1 and vortex-mixed for 5 min. Then the solution was centrifuged at 14,000 rpm for 10 min before filtered with a 0.22 μm filter membrane. Aliquot (2 μL) of the supernatant solution was injected into UHPLC-PDA for analysis. A Waters Acquity UHPLC System (Waters Co., Milford, MA) equipped with a photodiode array detector (PDA) was used to separate the multiple components in Xuanfei Baidu Decoction. All separations were performed a ZORBAX RRHD Eclipse XDB-C18 column (2.1 × 100 mm, 1.8 μm, Agilent Technologies). The flow rate was 0.3 mL/min. The column temperature was 40 °C.
2.4. Cell culture
Mouse macrophage cell line (RAW264.7) and mouse fibroblast cell line (NIH-3T3) were purchased from ATCC. RAW264.7 macrophages and Murine embryo fibroblast (NIH-3T3 cell line) were maintained in Dulbecco's Modified Eagle's Medium (DMEM, Gibco) supplemented with 10% heat-inactivated FBS, penicillin (100 units/mL), streptomycin (100 mg/mL), followed by incubation at 37 °C in a humidified atmosphere containing 5% CO2 and 95% air.
2.5. MTT assay
The RAW264.7 cells were seeded into 96-well plates at a density of 4 × 104 cells per well at 37 °C in a humidified atmosphere containing 5% CO2 and 95% air. After inoculation, the cells are then treated with the different concentrations of XFBD (0, 10, 25, 50, 100, 200 μg/mL) for 24 h. NIH-3T3 cells were seeded into 96-well plates at a density of 4 × 103 cells per well. After 24 h, cells were incubated for 48 h in the presence of different concentrations of XFBD (0, 1, 2.5, 5, 10, 25, 50 μg/mL).
Cell viability was detected using MTT (methylthiazole diphenyl-tamazole). After discarding the supernatant, adding MTT (10%) and incubated 4 h. The MTT solution was removed, then the precipitation dissolved in the DMSO solution. Measure the absorbance value at 490 nm using the enzyme-labeled instrument (Tecan, Austria).
2.6. Imunofluorescence assay
The cells were treated with IL-4 (20 ng/mL), TGF-β (5 ng/mL) and XFBD for 48 h. After fixed with paraformaldehyde (4%) for 30 min, the cells were treated with BSA (2%) for 1.5 h at 37 °C and followed by incubation with the α-SMA primary antibodies (1:500, Abcam, #ab5694) overnight at 4 °C. the cells were stained with the corresponding secondary antibodies after washed 3 times in PBS. Nuclei were stained with Hoechst 33342 for 10 min. Visual images and quantitative analysis were obtained with Operetta High Content Analysis (HCA) System (PerkinElmer, Boston, MA, USA). We selected three multiple wells in each group for the experiment, each well selected three fields for quantification, and each field selected 10 cells for fluorescence intensity quantification, and finally took the average of the fluorescence intensity of each well as the fluorescence data of the entire well.
2.7. Flowcytometry assay
A suspension containing 5 × 105 cells was incubated with an APC-anti-CD206 antibody (Biolegend Co., 2.5 μg/106 cells) for 30 min at 4 °C in the dark. Un-administered cells staining with APC-anti-CD206 was used as a negative control. Then, cells were washed with PBS and analyzed by Attune® NxT, Acoustic Focusing Cytometer (Invitrogen).
2.8. Enzyme linked immunosorbent assay (ELISA)
The level of IL-6 and Arg-1 is determined by ELISA. Cytokine concentration is calculated using standard curve. Specific operations according to the manual provided by the manufacturer.
2.9. Collagen deposition detection by picro-sirius red (PSR) assay
Total collagen content was determined by PSR assay. A total of 100 μL NIH-3T3 cells (5000 cells/well) were seeded into 96-well plates. Cells were then treated with XFBD or SB431542 (5 μmol/L) with or without TGF-β1 (5 ng/mL) for 48 h at 37 °C. The medium was removed from 96 wells and fixation was conducted by iced methanol overnight at −20 °C. After washing 3 times with 200 μL PBS each well, 100 μL Sirius red reagent (Shanghai yuanye Bio-Technology, Shanghai, China) was added each well and incubated for 4 h. Then free Sirius red was removed and cells were washed with 0.01% acetic acid for 3 times. After that, 200 μL 0.1 M NaOH was added. After 4 h, the absorbance at 540 nm was determined by multiplate reader (Tecan, Austria).
2.10. Animal experiments
SPF healthy male C57BL/6 mice (22–25 g) were provided by SPF (Beijing) Biotechnology Co., LTD. The research was conducted with the Guidelines for Animal Experiments of Tianjin University of Traditional Chinese Medicine for laboratory animal use. Mice were raised in Tianjin International Biopharmaceutical Joint Research Institute, and the temperature was maintained at 20–25 °C, and the relative humidity was 40–60%. 5 mice in each cage, they were given a regular feed and free water. After 1 week of adaptive feeding, the mice were randomly divided into control group (Control), model group (BLM), XFBD low dose group (XFBD-L) and XFBD high group (XFBD-H) (n = 6). The control group was intragastrically administered with clear water after sham operation; the model group was intragastrically administered with clear water after BLM tracheal instillation; the XFBD-L group was intragastrically administered with XFBD solution (4.6 g/kg) after BLM tracheal instillation, and the XFBD-H group was intragastrically administered with XFBD solution (9.2 g/kg) after BLM tracheal instillation. The dosage of XFBD used in mice was converted from clinical dosage. Mice in the model group received bleomycin hydrochloride (1.5 mg/kg) 50 μL by intratracheal instillation, and mice in the control group received the same volume of saline.
2.11. Micro CT scanning (μCT)
Mice were scanned with a μCT scanner (Micro-CT QuantumFX μCT Software, PerkinElmer). Each mouse was accepted a CT scanning and then euthanized. The mice treated by BLM were scanned on day 10 and the control group mice were scanned. The mice are positioned in the CT scanner and use the following parameters to obtain the impact of the chest for anatomical positioning and attenuation correction: 90 kV, 160 mA and 40 mm. The X-ray system uses a 5 mm micro focusing tube with a spot size and a conical beam geometry to generate X-rays. The maximum width of the image field is 68 mm and the voxel size is 35 × 35 × 35 mm. In this section, detailed methods for quantitative measurement of pulmonary fibrosis in mice were described. Mice with a fibrosis score greater than 3 were successfully modeled.
2.12. Tissue sampling and histopathological observation
After anesthesia by tribromoethanol, the whole blood, alveolar lavage fluid, lung and other major organs of mice were collected. Lung tissues were washed with iced saline. Part of lung tissues were degassed with paraformaldehyde and fixed in 4% paraformaldehyde. Pathological sections and staining were performed after 72 h. The remaining lung tissue was quickly frozen in liquid nitrogen and transferred to refrigerator at −80 °C until use.
The lung tissues that fixed in 4% paraformaldehyde fixing solution were dehydrated, embedded in paraffin and sliced into 5 μm sections. The sections were stained with hematoxylin and eosin (H&E) reagent and visualized under a light microscope. The whole pathological changes were observed at 100× and photographed under 200× by Leica Microsystems CMS GmbH Ernst-Leitz-Str.17–7 (Leica, Germany). A semi-quantitative histological score was used to assess the severity of IPF with double-blind method. Inflammation was scored from 0 (normal) to 5 (extremely severe damage) according to the degree of lung injury, including alveolar inflammation, congestion or bleeding of alveolar wall, proliferation of lymphocytes, emphysema, and degeneration or necrosis of bronchial epithelial cells. Extravascular area, vascular area, vascular outer perimeter, and ascular inner perimeter of arteries with a diameter < 50 μm were also measured. The following formula was used for calculation: the thickness of vascular wall (μm) = outer diameter of the pulmonary arterioles − inner diameter of the pulmonary arterioles of the pulmonary arterioles); the ratio of vascular wall area (WA%) = 100% × (transection area of the walls of pulmonary arterioles)/ (cross-sectional area of pulmonary arterioles). Masson staining kit was utilized to detect collagen deposits according to the manufacturer's instructions.
2.13. Immunohistochemistry (IHC)/immunofluorescence (IF) staining
For IHC analysis, the prepared paraffin sections are deparaffinized and hydrated in organic reagents such as xylene. Sodium citrate buffer was used for antigen retrieval, and the slices were treated with 3% H2O2 in order to eliminate endogenous peroxidase activity. Incubate the sections with 10% FBS to block the binding of non-specific antibodies, after which slides were immunostained using the α-SMA primary antibody (1:100; Abcam, #ab5694), the CD206 primary antibody (1:150; Abcam, #ab64693), the IL-6 primary antibody (1:200; Abcam, #ab208113) and the STAT3 (1:150; Abcam, #ab68153) primary antibody. Incubate horseradish peroxidase (HRP) conjugated goat anti-rabbit secondary antibody, and use DAB substrate kit (Boster Biological Technology Co., Ltd; #AR1022) for color development of positive results. Then the slides were counter-stained with Mayer's hematoxylin staining solution and differentiated with 1% hydrochloric acid and ethanol. Finally, the slides are dehydrated and sealed. Image J software was utilized to analyze the photos. The intensity of each stained slide contains at least the average of five non-overlapping fields.
For IF analyses, there was no need to quench endogenous peroxidase activity. After incubating slides with 10% FBS, slides were immunostained using the F4/80 primary antibody (1:200; Abcam, #ab6640). Alexa Fluor®488-conjugated goat anti-Rat secondary antibody was utilized for detected and visualized of antibody-antigen complexes. (1:200; Abcam, #150157). The nuclei were stained using Hoechst 33,342, after that slides were sealed.
2.14. Real-time quantitative PCR (RT-qPCR) analysis
After 10 days of continuous administration, the lung tissues of each group of mice were collected, quick-frozen in liquid nitrogen and stored at −80 °C. According to the manufacturer's instructions, TRIzol® reagent (Invitrogen, Waltham, MA, USA) was utilized to extract total RNA samples from lung tissue. The RNA sample was reverse transcribed into complementary DNA (cDNA) use the Transcriptor First Strand cDNA Synthesis Kit (Roche, Germany). Using Bestar® SybrGreen qPCR mastermix (DBI®Bioscience, Germany) for RT-qPCR. The mRNA expression of IL-6 and STAT3 was verified by reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR). Gyceraldehyde 3-phosphate dehydrogenase (GAPDH) was utilized as an endogenous reference gene to quantify the expression levels of key genes. Gene-specific primers were synthesized by Sangon Biotech (Shanghai, China). Using LightCycler®480 Software Version 1.5.0.39 (Roche, Germany) for 45 cycles amplification and analysis. After normalization with the GAPDH gene, the relative mRNA expression level of the target gene was calculated using the 2−ΔΔCT method. The sequences of primers for qPCR are shown in Table 1 .
Table 1.
Primer sequences for RT-qPCR.
| Primer name | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| qMouse STAT3 | AATCTCAACTTCAGACCCGCCAAC | GCTCCACGATCCTCTCCTCCAG |
| qMouse Il6 | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| qMouse Gapdh | TGGTGAAGCAGGCATCTGAG | TGCTGTTGAAGTCGCAGGAG |
2.15. Statistical analysis
All experiments were performed in triplicate. GraphPad (GraphPad Prism 5, San Diego, California, USA) was used for data statistics and analysis. All data were presented as the mean ± SD. Statistical analysis was performed by one-way analysis of variance (ANOVA) test with post hoc Tukey's test. A value of P < 0.05 was considered statistically significant.
3. Results
3.1. Identification and enrichment analysis of candidate targets for XFBD against idiopathic pulmonary fibrosis
We conducted a virtual study to explored the underlying mechanism both involved in the anti-fibrosis activity of XFBD by using the online BATMAN-TCM server and Gene Cards databases. XFBD is composed of 13 traditional Chinese medicines, among these medicines and related components, 1939 potential targets of 188 compounds were predicted using BATMAN-TCM server and a total of 3054 idiopathic pulmonary fibrosis (IPF) targets were retrieved from the Gene Cards databases. Venn Diagram (http://bioinformatics.psb.ugent.be/webtools/Venn/) was used to find genes that were both the potential therapeutic targets of XFBD and the IPF-related targets. Total of 763 potential therapeutic targets of XFBD on IPF were obtained.
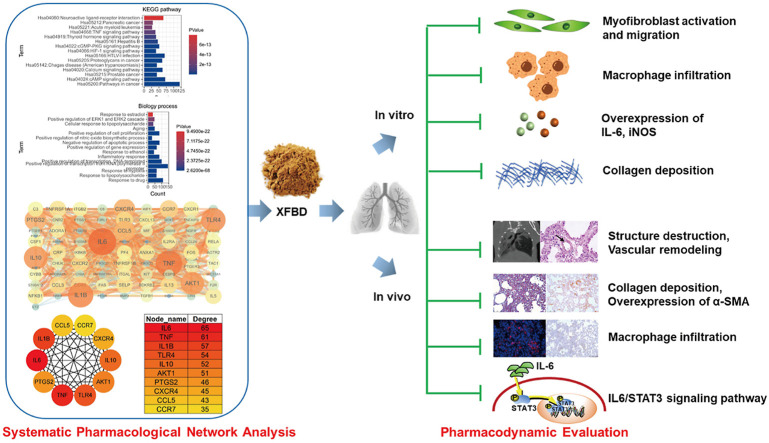
The biological classification of the 763 potential targets of XFBD in the treatment of IPF was analyzed by using the functional enrichment analysis of the DAVID website. Subsequently, KEGG analysis confirmed that 763 genes were enriched in 167 pathways. Here we used FDR corrected p < 0.05 as the enrichment screening criterion and obtained the top 15 enriched functional clusters (Fig. 1 A). The results showed that the 763 genes mainly enriched in tumor, virus infection and immune pathways. Furthermore, gene ontology (GO) analysis of biological processes showed significant enrichment of biological process related to inflammation and 82 genes were involved in the biological process of inflammatory response (Fig. 1B). It was suggested that XFBD might alleviate IPF by regulating inflammatory response. Since the protein-protein interaction (PPI) networks are relevant to visualize the role of various key proteins in disease, a visual PPI network of the 82 genes was subsequently constructed using the Cytoscape software (Fig. 1C). And the 10 genes with the highest degree of nodes were identified as hub genes, including IL6, TNF, IL1B, TLR4, IL10, AKT1, PTGS2, CXCR4, CCL5 and CCR7 (Fig. 1D and E). The gene with highest node degrees was IL6, which was 65.
Fig. 1.
Network Pharmacology analysis on the potential mechanisms of XFBD against Idiopathic Pulmonary fibrosis (IPF). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis (A) and gene ontology (GO) analysis of biological processes (B) were investigated using the DAVID website. PPI interactions and hub genes of XFBD targets related to inflammatory response were analyzed by Cytoscape software (the larger the node, the higher the degree) (C–E).
3.2. Identification of active ingredients in XFBD
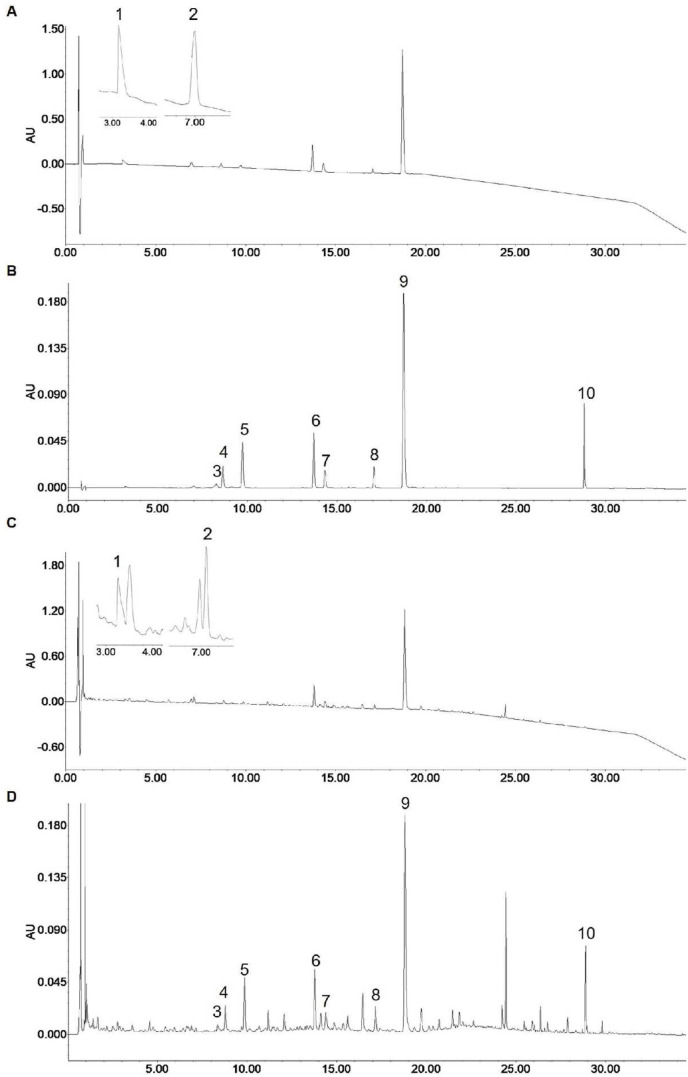
The contents of the ten ingredients were analyzed by using the UHPLC-PDA method. There are shown representative chromatograms of the mixed ten standards and XFBD extracted solution (Fig. 2 ). The contents of the investigated analyses are as follows: 1.19 mg/g for ephedrine, 4.97 mg/g for amygdalin, 3.63 mg/g for sinapine, 5.04 mg/g for hastatoside, 4.49 mg/g for verbenalin, 8.45 mg/g for polydatin, 3.40 mg/g for liquiritin, 3.46 mg/g for acteoside, 54.91 mg/g for naringin and 4.80 mg/g for glycyrrhizic acid in the freeze-dried powder of XFBD (Table 2 ).
Fig. 2.
Identification of active ingredients in Xuanfei Baidu Decoction. The typical chromatograms of standard compounds of 210 nm (A), 254 nm (B) and sample of 210 nm (C), 254 nm (D). (1) ephedrine, (2) amygdalin, (3) sinapine, (4) hastatoside, (5) verbenalin, (6) polydatin, (7) liquiritin, (8) acteoside, (9) naringin and (10) glycyrrhizic acid.
Table 2.
Linear regression and contents of 10 compounds (n = 6).
| Peak No. | Rt (min) | Compounds | Regression equation | R2 | Linearity range (μg/mL) | Content (mg/g) | RSD (%) |
|---|---|---|---|---|---|---|---|
| 1 | 3.3 | ephedrine | y = 131 94x+37.738 | 0.999 7 | 2.00–50.0 | 1.19 | 1.64 |
| 2 | 7.1 | amygdalin | y = 669 3.6x-50.185 | 0.999 2 | 2.50–100 | 4.97 | 0.70 |
| 3 | 8.4 | sinapine | y = 133 0x+107.5 | 0.999 8 | 1.25–50.0 | 3.63 | 3.09 |
| 4 | 8.7 | hastatoside | y = 259 6.2x+94.754 | 0.999 9 | 1.00–100 | 5.04 | 3.47 |
| 5 | 9.8 | verbenalin | y = 554 2.7x+5.5247 | 0.999 9 | 1.00–100 | 4.49 | 3.89 |
| 6 | 13.7 | polydatin | y = 362 5.7x+1160 | 0.999 8 | 1.50–150 | 8.45 | 3.43 |
| 7 | 14.4 | liquiritin | y = 444 1.4x+119.17 | 0.999 8 | 0.50–50.0 | 3.40 | 3.15 |
| 8 | 17.1 | acteoside | y = 381 9.9x+317.12 | 0.999 9 | 0.50–50.0 | 3.46 | 2.13 |
| 9 | 18.8 | naringin | y = 233 5.8x+4000.1 | 0.999 9 | 10.0–1000 | 54.91 | 1.15 |
| 10 | 28.8 | glycyrrhizic acid | y = 444 2x+1227.1 | 0.999 9 | 1.00–100 | 4.80 | 1.42 |
3.3. XFBD inhibits the activation and migration of fibroblasts
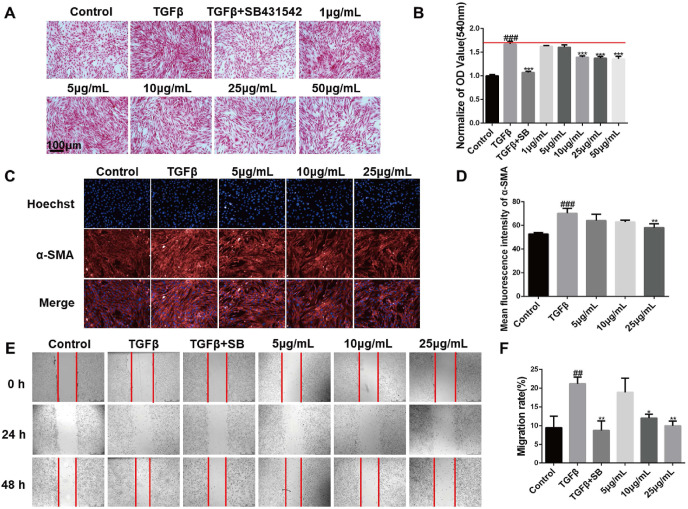
Fibroblasts can be induced into myofibroblasts by transforming growth factor-β1 (TGF-β1), which will cause excessive collagen production and overexpression of α-Smooth muscle actin (α-SMA) (Liu et al., 2019). Therefore, inhibiting its activation is one of the targets of pulmonary fibrosis treatment (Liu et al., 2019; Lu et al., 2018). First, mouse embryonic fibroblasts NIH-3T3 cells were treated with a series of concentrations (1, 2.5, 5, 10, 25, 50 μg/mL) of XFBD, and the results proved that cells were well tolerance with 50 μg/mL XFBD (Fig. S1). In the TGF-β1 induced fibroblast activation experiment, collagen deposition results showed that the amount of collagen deposition induced by TGF-β1 was increased around 1.7 times (1 ± 0.31 vs 1.67 ± 0.06, p < 0.001),compared with the blank control group, TGF-β1 inhibitor SB431542 can effectively reduce collagen deposition to normal level (1.67 ± 0.06 vs 1.07 ± 0.24, p < 0.001),XFBD can effectively inhibit collagen deposition of fibroblasts at a concentration of 10 μg/mL (1.67 ± 0.06 vs 1.39 ± 0.03, p < 0.001) (Fig. 3 A and B). Immunofluorescence analysis results further suggested that XFBD can inhibit the expression of a-SMA, which is the biomarker of the fibroblasts activation at the concentration of 25 μg/mL (70.31 ± 4.09 vs 58.07 ± 3.36, p < 0.01) (Fig. 3C and D). Similarly, the migration assay showed that 10 μg/mL drug already has a good inhibitory effect on activated fibroblasts (12.05 ± 1.05, p < 0.01), and the inhibitory effect is more significant at 25 μg/mL (9.99 ± 1.26, p < 0.01) (Fig. 3E and F).
Fig. 3.
XFBD inhibits the activation and migration of fibroblasts. (A, B) The inhibitory effect of XFBD on TGF-β1-induced fibroblast activation (magnification × 200). (C, D) The effect of XFBD on TGF-β1-induced α-SMA expression was evaluated by immunofluorescence analysis. (E, F) Inhibition of XFBD on the migration of activated fibroblasts. #p < 0.05, ##p < 0.01, ###p < 0.001, vs. control group, *p < 0.05, **p < 0.01, ***p < 0.001, vs. Model group. Scale bar = 100 μm.
3.4. XFBD inhibits macrophages produced inflammation
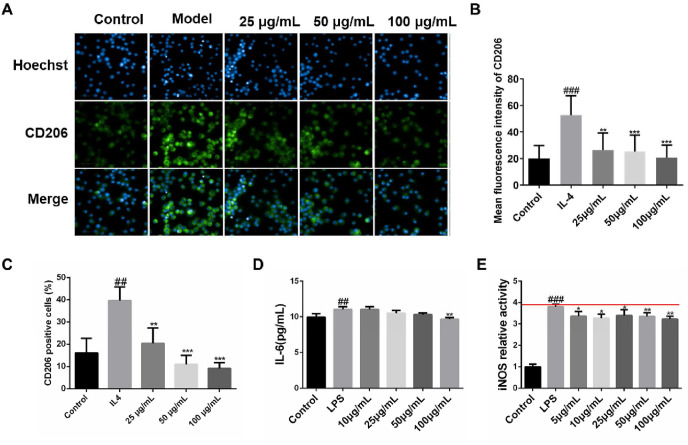
Macrophages cause the secretion of inflammatory factors and pro-fibrosis factors, which accelerate the development of fibrosis (Li et al., 2019; Mari and Crestani, 2019). Particularly, phenotypic changes of macrophages can affect the process of lung fibrosis (Chandrasekaran et al., 2019; Dong and Ma, 2018). We furtherly investigated its effects on the polarized macrophages and the expression of CD206+ of macrophages after treating XFBD, which is the mannose receptor, a high specificity M2 macrophage marker. As shown in Fig. 4 A and B, XFBD at a concentration of 25 μg/mL could effectively down-regulate the fluorescence expression of CD206+ of M2 polarized macrophages (28.39 ± 14.32, p < 0.001). Flow cytometry analysis also demonstrated that XFBD can reduce the proportion of CD206 positive cells in RAW264.7 cells ( Fig. 4 C & Fig. S2 ).
Fig. 4.
XFBD inhibits the inflammatory response of macrophages (A, B) XFBD inhibits IL-4 induced polarization of M2 macrophages in vitro. (C) Flowcytometry result of the proportion of CD206 positive cells (D) The inhibitory effect of XFBD on LPS-induced IL-6 expression. (E) The inhibitory effect of XFBD on LPS-induced iNOS expression. #p < 0.05, ##p < 0.01, ###p < 0.001, vs. control group, *p < 0.05, **p < 0.01, ***p < 0.001, vs. Model group. Scale bar = 100 μm.
To investigate the mechanism of XFBD against the development of lung injury, murine macrophage RAW264.7 were treated with LPS (100 ng/mL) and different concentrations of XFBD. Then the expression of proinflammatory factor IL-6 was tested. The ELISA results showed that XFBD could effectively inhibit the upregulation of IL-6, when compared with the model group which treated with LPS (11.06 ± 0.36 vs 9.69 ± 0.22, p < 0.01) (Fig. 4D). Similarly, the activity of iNOS was also tested after LPS inducement. When incubated with XFBD at a dose of 5 μg/mL, iNOS activity can be inhibited effective (3.80 ± 0.11 vs 3.36 ± 0.22, p < 0.05) (Fig. 4E). Altogether, these results suggest that XFBD could inhibit the proinflammatory cytokines produced by macrophages.
3.5. XFBD has protective effect against bleomycin-induced pulmonary fibrosis in mice
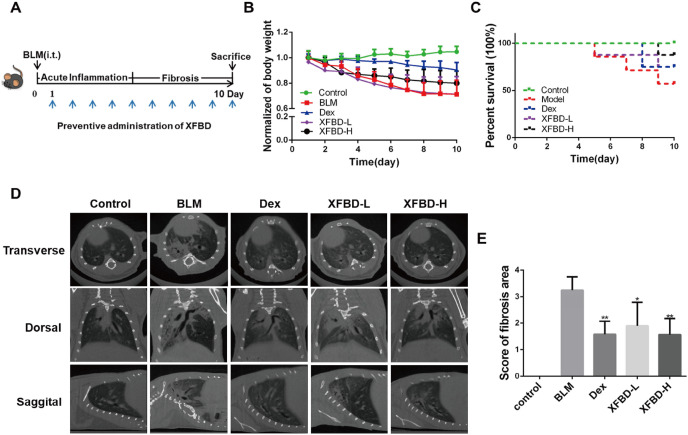
The bleomycin model of in vivo lung injure is a well-described model, which can reflect the pathological characteristics of human pulmonary fibrosis (Della Latta et al., 2015; Tashiro et al., 2017). Here, we established a bleomycin-induced pulmonary fibrosis model in mice, mimicking the pre-inflammatory stage for the purpose of suppressing the occurrence of inflammation. On the 10th day after BLM tracheal instillation, the mice were killed by overdose anesthesia (Fig. 5 A). In the BLM-induced pulmonary fibrosis model, XFBD at high dosage (XFBD-H group) improved weight loss and survival rate of pulmonary fibrosis mice, compared with the model and XFBD-L administration groups (Fig. 5B and C). Additionally, μCT scanning results showed that patch-shaped ground-glass shadows appeared in the middle and lower lungs of the model group, presenting extensive, double-lung, symmetrical gridded changes, and honeycomb changes at the 10th day, while XFBD-H can significantly inhibit the occurrence and development of pulmonary fibrosis (Fig. 5D). A four-point ranking scale was used to semi-quantitatively evaluate the quality of μCT images, and the scoring data further confirmed the effectiveness of XFBD (Fig. 5E).
Fig. 5.
XFBD has protective effect on mice treated with bleomycin. (A) Timeline of XFBD administration. (B) Body weight changes of mice in each group. (C) Survival rates of mice after different treatment. (D) Scanning of pulmonary fibrosis after BLM treatment at 10th day. (E) μCT score of pulmonary fibrosis. *p < 0.05, **p < 0.01, ***p < 0.001, vs. Model group. Scale bar = 100 μm.
3.6. XFBD ameliorated the pathologenesis of pulmonary fibrosis
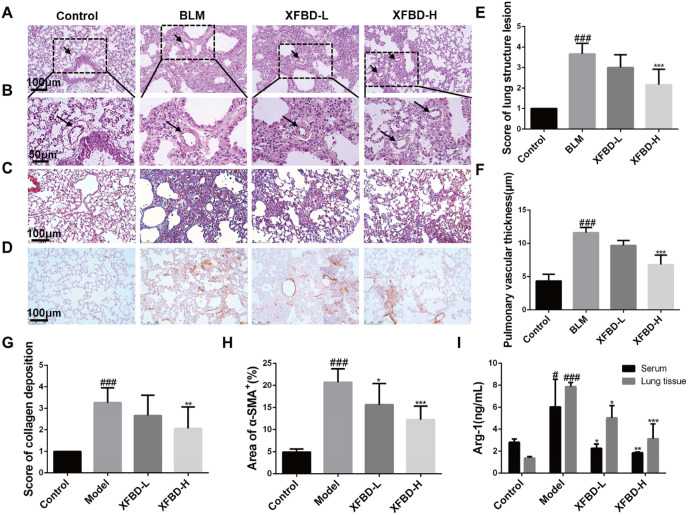
After the administration of XFBD in the early inflammatory stage, the occurrence and development of pulmonary fibrosis was significantly inhibited, and the alveolar cavity of the lung tissue of the normal control group mice was clearly visible, with few inflammatory cell infiltrating. H&E staining of lung tissue in the model group showed that alveolar structure was disordered and collapsed, with prominent inflammatory cell infiltration. The damage degree of lung tissue in mice treated with XFBD-H (2.17 ± 0.75, p < 0.001) was significantly reduced compared with that in the model group (3.67 ± 0.52, p < 0.001) (Fig. 6 A&6 E). We also found that the occurrence of pulmonary fibrosis will result in thickening of the pulmonary blood vessel wall. The pulmonary vascular thickness in the model group (11.6 ± 0.78, p < 0.001) increased by 3 times compared with the control group, and XFBD-H (6.84 ± 1.41, p < 0.001) has a significant inhibitory effect on the increase of pulmonary vascular thickness (Fig. 6B and F). To test the effect of XFBD on collagen deposition, Masson's trichrome staining was carried out. The data demonstrated that XFBD at low or high dosage (XFBD-L and XFBD-H) significant inhibited the deposition of collagen in the IPF mice. Compared with the model group, the XFBD-H (3.26 ± 0.68 vs 2.67 ± 0.94, p < 0.01) group has a significant improvement effect (Fig. 6C and G). Through immunohistochemical staining of fibroblast activation marker α-SMA, we found that compared with the control group, the BLM-induced model group showed a large amount of positive expression of α-SMA in the lungs, and the XFBD administration group at different doses had a significant effect on collagen deposition. The results showed that XFBD-H (20.75 ± 3.02 vs 12.28 ± 3.06, p < 0.001) can significantly down-regulate the level of α-SMA compared with the model group (Fig. 6D and H). In addition, we tested the expression of arginine-1 (Arg-1) in mouse serum and lung tissue homogenate (Fig. 6I). Compared with the control group, the expression level of Arg-1 in the lung tissue of the model group increased by about 4 times (7.86 ± 0.41 vs 1.93 ± 1.11, p < 0.001), after treatment with XFBD-H, the level of Arg-1 was significantly decreased (7.86 ± 0.41 vs 3.13 ± 1.36, p < 0.001), which further presented the anti-fibrosis effect of XFBD.
Fig. 6.
XFBD inhibits pathological changes of pulmonary fibrosis. (A) The histopathological changes of lung tissues were examined using H&E staining (magnification × 200) and (B) Alveolar structural changes of pulmonary vascular thickness and the value changes (magnification × 400). (C) Masson staining to assess the deposition of collagen in the lungs (Magnification 200 × ). The detect (D) and quantify (H) of α-SMA by Immunohistochemical staining. (E) The morphological damage score for the lung tissues. (F) Improvement of XFBD on vascular remodeling in pulmonary fibrosis mice. (G) The collagen deposition. (I) The level of Arg-1 in lung tissue. #p < 0.05, ##p < 0.01, ###p < 0.001, vs. control group, *p < 0.05, **p < 0.01, ***p < 0.001, vs. Model group. Scale bar = 100 μm.
3.7. XFBD inhibits pulmonary fibrosis by down-regulating M2 polarization and IL-6/STAT3 pathway
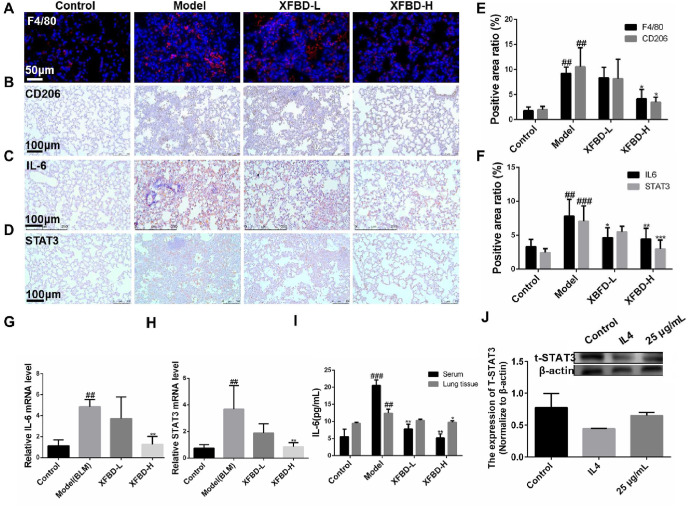
On the 10th day after BLM tracheal instillation, the mice were killed by overdose anesthesia, the left lung in the mice was prepared for further investigation. Observed by immunofluorescence staining of left lung sections of pulmonary fibrosis mice consults showed that the XFBD high-dose group reduced the infiltration of total macrophages and M2 macrophages in the lungs (Fig. 7A, B & 7 E). Here, the total macrophages were labeled with F4/80 (9.24 ± 1.24 vs 4.16 ± 1.89, p < 0.05), CD206+ was used to mark the M2 macrophages (10.56 ± 3.81 vs 3.5 ± 1.01, p < 0.05). Immunohistochemical staining experiments were performed to observe the expression of IL-6 and STAT3 protein in the lungs of mice. Compared with the control group, the positive expression of IL-6 and STAT3 in the lung tissue of the model group induced by BLM was significantly up-regulated (Fig. 7C and D). Quantitative results showed that XFBD-H (7.83 ± 2.48 vs 4.45 ± 1.57, p < 0.01) group inhibited IL-6 upregulation better than XFBD-L (7.83 ± 2.48 vs 5.82 ± 2.6, p < 0.05), and XFBD-H also showed a significant inhibitory effect on the expression of STAT3 (7.08 ± 2.25 vs 2.98 ± 1.32, p < 0.001) ( Fig. 7 F).
Fig. 7.
XFBD inhibits pulmonary fibrosis by down-regulating M2 polarization and IL-6/STAT3 pathway. (A, B, E) XFBD inhibits fibrosis-induced macrophage infiltration and M2 polarization in mouse lungs in vivo. (C, D, F) Representative microphotographs of immunohistochemical analysis for expression of IL-6 and STAT3 in lung tissue sections showing reduced immunopositivity upon XFBD treatment as compared to BLM alone treated lung sections (Magnification: 20 × ). (G, H) Quantitative analyses of mRNA of the IL-6 and STAT3 genes in mice. (I) Quantitative analyses of cytokine content of IL-6 by ELISA in mice serum and lung tissue. (J) Western blot result of the expression of total STAT3 in polarized macrophages group and XFBD group. #p < 0.05, ##p < 0.01, ###p < 0.001, vs. control group, *p < 0.05, **p < 0.01, ***p < 0.001, vs. Model group. Scale bar = 100 μm.
We prepared mice lung tissue homogenate and further investigated the mechanism via evaluating the gene expression of IL-6 and STAT3. Compared with the control group, the expression of IL-6 and STAT3 genes in the lung tissue of the model group induced by BLM was significantly up-regulated. The XFBD-H group inhibited the expression of IL-6 (4.86 ± 0.69 vs 1.27 ± 0.75, p < 0.01) ( Fig. 7 G) and STAT3 (3.68 ± 1.77 vs 0.86 ± 0.31, p < 0.01) ( Fig. 7 H). The results showed that XFBD could down-regulate the expression of IL-6 and STAT3 in mice at the gene level. In addition, the IL-6 protein expression in mouse serum and lung tissue was detected, and both XFBD-L and XFBD-H groups could significantly inhibit the upregulation of IL-6. It is worth noting that in mouse serum, compared to the model group, XFBD-H (5.93 ± 1.70 vs 18.55 ± 4.16, p < 0.01) groups showed more significant regulation ( Fig. 7 I). Western blot results also showed that polarized M2 macrophage cells with decreased total STAT3, while after XFBD treatment, was back to normal expression level (Fig. 7J).
4. Discussion
Pulmonary fibrosis caused by SARS-CoV was an important clinical feature which seriously affected the quality of SARS-CoV patients' life. Pathological examination results showed that there were more fibrotic components in the patient's alveolar cavity edema fluid, and fibroblast proliferation in the patient's alveolar septum, which led to pulmonary interstitial fibrosis (Zhang et al., 2021).
The clinical trial data of XFBD showed that it satisfactorily shortens the duration for virus clearance as well as the hospitalization period compared to the control group only treated with anti-viral drugs (Li et al., 2021). It is reported that, multiple herbs of XFBD and their main components have an effect in balancing immune inflammatory response, resisting viral infection and viral protein transcription, and restoring the balance of liver and gallbladder metabolism and energy metabolism in the body by regulating the biological processes of viral infection, immune inflammation, liver and gallbladder metabolism and energy metabolism (Wang et al., 2020).
This study is based on the significant clinical antiviral pneumonia efficacy of XFBD. First, this traditional Chinese medicine compound was analyzed by the network pharmacological pharmacodynamic material basis analysis and their ingredients were tested, and then the efficacy of XFBD against fibroblast activation and macrophage inflammation in vitro and in vivo were discussed. Last, the pharmacodynamic test and the mechanism were explored.
Some of the compounds quantified by UHPLC were reported to have anti-inflammatory or anti-fibrotic effects. For example, glycyrrhizic acid inhibited the proliferation of 3T3 fibroblasts and down-regulated the expression of IL-6 in LPS-induced RAW264.7 macrophages; glycyrrhizic acid also reduced the inflammation in BLM-induced pulmonary fibrosis rats in vivo via inhibiting the activation of TGF-β signaling pathway (Gao et al., 2015; Wu et al., 2015). Polydatin could significantly reduce the levels of IL-6 and TNF-α in pulmonary fibrosis tissue (Liu et al., 2020b). We established a TGF-β1-induced fibroblast activation model and interleukin 4 (IL-4)/Lipopolysaccharide (LPS)-induced macrophage inflammation models in vitro. The results proved that XFBD can effectively inhibit fibroblast collagen deposition, down-regulate the level of α-SMA and inhibit the migration of fibroblasts. In vivo experiments, the results proved that XFBD improved the weight loss and survival rate of the mice. The XFBD high-dose administration group had a significant effect in inhibiting collagen deposition and the expression of α-SMA in the lungs of mice.
Macrophages are a key factor affecting pulmonary fibrosis. M2 type macrophages are thought to cause the activation of TGF-β/Smad and IL-6/STAT3 signaling pathways. In this study, we found that the anti-fibrotic effect of XFBD was achieved by regulating the inflammatory response of macrophages. In a macrophage inflammation model, XFBD could down-regulate the expression of IL-4-induced type 2 macrophage marker protein CD206+, as well as LPS-induced macrophage IL-6 and iNOS. Similarly, we proved that XFBD could down-regulate the expression of macrophages and M2 macrophages in vivo, which proved that XFBD could inhibit the type 2 polarization of macrophages and down-regulate the secretion of inflammatory factors and proteins.
In addition, PPI interactions and hub genes of XFBD targets related to inflammatory response were analyzed by Cytoscape software (the larger the node, the higher the degree), Among these genes, IL-6 demonstrated the highest node degrees, which was 65. IL-6 is a pleiotropic cytokine that sends inflammatory signals throughout the body from local lesions, it stimulates immune cell recruitment including monocyte/macrophage, in turn, these immune cells produce more inflammatory factor to recruit more macrophage cells, driving the inflammatory cascade reaction, which cause persistent tissue damage and finally lead to pathological fibrosis development (Tanaka et al., 2016; Wynn and Vannella, 2016). The experimental results proved that XFBD effectively down-regulated the gene expression of IL-6 and STAT3 and the protein expression of both. STAT3 can mediate gene transcription in response to the IL-6 cytokine family (Shieh et al., 2019; Waters et al., 2019). Thus, targeting IL-6/STAT3 would be an effective approach to regulate fibroblast activation and differentiation.
Studies have shown that the IL-6/STAT3 signaling pathway was activated in M2 macrophages (Yin et al., 2018). IL-6 secreted by trophoblast cells activates the STAT3 pathway to promote the polarization of M2 macrophages. In addition, activated M2 macrophages promote the invasion and migration of trophoblast cells in a feedback-regulated manner (Ding et al., 2021). Therefore, in this study, the effect of XFBD on the polarization of M2 macrophages may be through inhibiting the activation of the IL-6/STAT3 pathway.
5. Ethics statement
This study was conducted in accordance with the recommendations of the “Guidelines for Animal Experiments of Tianjin University of Traditional Chinese Medicine”. The program has been approved by the Institutional Animal Care and Use Committee of Tianjin International Joint Academy of Biotechnology and Medicine (TJAB- JY-2011-002).
Funding
This work was supported by National Key R&D Program of China [2020YFA0708000]; and the grant from National Natural Science Foundation of China (No. 82074032).
Author contributions
J.Y., Y.X.C. and H.Z. designed the experiments. X.Q.S., Y.Y.W. and H.L.Q. performed the biological experiments of pulmonary fibrosis model in vitro and in vivo. R.S. performed network pharmacology analysis. X.H.C. performed UHPLC analysis. L.L. and Y.W. participates in sample preparation. Y.Z., B.L.Z. and X.M.G. provided guidance to use Xuanfei Baidu prescription and participated in the discussion, Y.Y.W., R.S., X.Q.S., X.H.C. and Z.F.X. drafted the manuscript. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jep.2021.114701.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M.D., Ruiz-Palacios G.M., Benfield T., Fatkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C., Members A.-S.G. Remdesivir for the treatment of covid-19 - preliminary report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda D., Otoupalova E., Smith S.R., Volckaert T., De Langhe S.P., Thannickal V.J. Developmental pathways in the pathogenesis of lung fibrosis. Mol. Aspect. Med. 2019;65:56–69. doi: 10.1016/j.mam.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran P., Izadjoo S., Stimely J., Palaniyandi S., Zhu X., Tafuri W., Mosser D.M. Regulatory macrophages inhibit alternative macrophage activation and attenuate pathology associated with fibrosis. J. Immunol. 2019;203:2130–2140. doi: 10.4049/jimmunol.1900270. [DOI] [PubMed] [Google Scholar]
- Craig V.J., Zhang L., Hagood J.S., Owen C.A. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2015;53:585–600. doi: 10.1165/rcmb.2015-0020TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Latta V., Cecchettini A., Del Ry S., Morales M.A. Bleomycin in the setting of lung fibrosis induction: from biological mechanisms to counteractions. Pharmacol. Res. 2015;97:122–130. doi: 10.1016/j.phrs.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Ding J., Yang C., Cheng Y., Wang J., Zhang S., Yan S., He F., Yin T., Yang J. Trophoblast-derived IL-6 serves as an important factor for normal pregnancy by activating Stat3-mediated M2 macrophages polarization. Int. Immunopharm. 2021;90:106788. doi: 10.1016/j.intimp.2020.106788. [DOI] [PubMed] [Google Scholar]
- Dong J., Ma Q. Macrophage polarization and activation at the interface of multi-walled carbon nanotube-induced pulmonary inflammation and fibrosis. Nanotoxicology. 2018;12:153–168. doi: 10.1080/17435390.2018.1425501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Tang H., He H., Liu J., Mao J., Ji H., Lin H., Wu T. Glycyrrhizic acid alleviates bleomycin-induced pulmonary fibrosis in rats. Front. Pharmacol. 2015;6:215. doi: 10.3389/fphar.2015.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heukels P., Moor C.C., von der Thusen J.H., Wijsenbeek M.S., Kool M. Inflammation and immunity in IPF pathogenesis and treatment. Respir. Med. 2019;147:79–91. doi: 10.1016/j.rmed.2018.12.015. [DOI] [PubMed] [Google Scholar]
- Huang Y.F., Bai C., He F., Xie Y., Zhou H. Review on the potential action mechanisms of Chinese medicines in treating Coronavirus Disease 2019 (COVID-19) Pharmacol. Res. 2020;158:104939. doi: 10.1016/j.phrs.2020.104939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T.E.J., Pardo A., Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–1961. doi: 10.1016/s0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- Kohl M., Wiese S., Warscheid B. Cytoscape: software for visualization and analysis of biological networks. Methods Mol. Biol. 2011;696:291–303. doi: 10.1007/978-1-60761-987-1_18. [DOI] [PubMed] [Google Scholar]
- Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. 2020;582:469. doi: 10.1038/d41586-020-01824-5. [DOI] [PubMed] [Google Scholar]
- Li M., Luan F., Zhao Y., Hao H., Zhou Y., Han W., Fu X. Epithelial-mesenchymal transition: an emerging target in tissue fibrosis. Exp. Biol. Med. 2016;241:1–13. doi: 10.1177/1535370215597194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Jin F., Du J., He Q., Yang B., Luo P. Macrophage-secreted TSLP and MMP9 promote bleomycin-induced pulmonary fibrosis. Toxicol. Appl. Pharmacol. 2019;366:10–16. doi: 10.1016/j.taap.2019.01.011. [DOI] [PubMed] [Google Scholar]
- Li F., Li Y., Zhang J., Li S., Mao A., Zhao C., Wang W., Li F. The therapeutic efficacy of Xuanfei Baidu Formula combined with conventional drug in the treatment of coronavirus disease 2019: a protocol for systematic review and meta-analysis. Medicine (Baltim.) 2021;100 doi: 10.1097/md.0000000000024129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Chu H., Ma Y., Wu T., Qian F., Ren X., Tu W., Zhou X., Jin L., Wu W., Wang J. Salvianolic acid B attenuates experimental pulmonary fibrosis through inhibition of the TGF-β signaling pathway. Sci. Rep. 2016;6:27610. doi: 10.1038/srep27610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Guo F., Wang Y., Li C., Zhang X., Li H., Diao L., Gu J., Wang W., Li D., He F. BATMAN-TCM: a bioinformatics analysis tool for molecular mechANism of traditional Chinese medicine. Sci. Rep. 2016;6:21146. doi: 10.1038/srep21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.S., Lv X.X., Liu C., Qi J., Li Y.X., Wei X.P., Li K., Hua F., Cui B., Zhang X.W., Yu J.J., Yu J.M., Wang F., Shang S., Zhao C.X., Hou X.Y., Yao Z.G., Li P.P., Li X., Huang B., Hu Z.W. Targeting degradation of the transcription factor C/EBPbeta reduces lung fibrosis by restoring activity of the ubiquitin-editing enzyme A20 in macrophages. Immunity. 2019;51:522–534. doi: 10.1016/j.immuni.2019.06.014. e527. [DOI] [PubMed] [Google Scholar]
- Liu M., Gao Y., Yuan Y., Yang K., Shi S., Zhang J., Tian J. Efficacy and safety of integrated traditional Chinese and western medicine for corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. Pharmacol. Res. 2020;158:104896. doi: 10.1016/j.phrs.2020.104896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Chen B., Nie J., Zhao G., Zhuo J., Yuan J., Li Y., Wang L., Chen Z. Polydatin prevents bleomycin-induced pulmonary fibrosis by inhibiting the TGF-β/Smad/ERK signaling pathway. Exp Ther Med. 2020;20:62. doi: 10.3892/etm.2020.9190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Wu L., Liu L., Ruan Q., Zhang X., Hong W., Wu S., Jin G., Bai Y. Quercetin ameliorates kidney injury and fibrosis by modulating M1/M2 macrophage polarization. Biochem. Pharmacol. 2018;154:203–212. doi: 10.1016/j.bcp.2018.05.007. [DOI] [PubMed] [Google Scholar]
- Luo E., Zhang D., Luo H., Liu B., Zhao K., Zhao Y., Bian Y., Wang Y. Treatment efficacy analysis of traditional Chinese medicine for novel coronavirus pneumonia (COVID-19): an empirical study from Wuhan, Hubei Province, China. Chin. Med. 2020;15:34. doi: 10.1186/s13020-020-00317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari B., Crestani B. Dysregulated balance of lung macrophage populations in idiopathic pulmonary fibrosis revealed by single-cell RNA seq: an unstable "menage-a-trois. Eur. Respir. J. 2019;54 doi: 10.1183/13993003.01229-2019. [DOI] [PubMed] [Google Scholar]
- Martinez F.J., Collard H.R., Pardo A., Raghu G., Richeldi L., Selman M., Swigris J.J., Taniguchi H., Wells A.U. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. 2017;3:17074. doi: 10.1038/nrdp.2017.74. [DOI] [PubMed] [Google Scholar]
- Pulivendala G., Bale S., Godugu C. Honokiol: a polyphenol neolignan ameliorates pulmonary fibrosis by inhibiting TGF-beta/Smad signaling, matrix proteins and IL-6/CD44/STAT3 axis both in vitro and in vivo. Toxicol. Appl. Pharmacol. 2020;391:114913. doi: 10.1016/j.taap.2020.114913. [DOI] [PubMed] [Google Scholar]
- Shieh J.M., Tseng H.Y., Jung F., Yang S.H., Lin J.C. Elevation of IL-6 and IL-33 levels in serum associated with lung fibrosis and skeletal muscle wasting in a bleomycin-induced lung injury mouse model. Mediat. Inflamm. 2019:1–12. doi: 10.1155/2019/7947596. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer G., Rosen N., Plaschkes I., Zimmerman S., Twik M., Fishilevich S., Stein T.I., Nudel R., Lieder I., Mazor Y., Kaplan S., Dahary D., Warshawsky D., Guan-Golan Y., Kohn A., Rappaport N., Safran M., Lancet D. The GeneCards suite: from gene data mining to disease genome sequence analyses. Curr. Protoc. Bioinformatics. 2016;54:1301–13033. doi: 10.1002/cpbi.5. [DOI] [PubMed] [Google Scholar]
- Szklarczyk D., Morris J.H., Cook H., Kuhn M., Wyder S., Simonovic M., Santos A., Doncheva N.T., Roth A., Bork P., Jensen L.J., von Mering C. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Narazaki M., Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8:959–970. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- Tashiro J., Rubio G.A., Limper A.H., Williams K., Elliot S.J., Ninou I., Aidinis V., Tzouvelekis A., Glassberg M.K. Exploring animal models that resemble idiopathic pulmonary fibrosis. Front. Med. 2017;4:118. doi: 10.3389/fmed.2017.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TCM . 2020. treatment Effective on over 90% of COVID-19 Patients on Mainland. [cited 2020 17 December];Availablefrom: http://english.www.gov.cn/news/topnews/202003/23/content_WS5e787a9dc6d0c201c2cbf3b4.html. [Google Scholar]
- Wang H., Song H.X., Wang D.F., Ma X.R., Zou D.X., Miao J.X., Wang Y.L., Yang W.P. The molecular mechanism of Xuanfei Baidu Formula in the treatment of COVID-19 antiviral effect based on network pharmacology and molecular docking. Journal of Hainan Medical University. 2020;26:1361–1372. doi: 10.13210/j.cnki.jhmu.20200617.003. [DOI] [Google Scholar]
- Waters D.W., C Blokland K.E., Pathinayake P.S., Wei L., Schuliga M., Jaffar J., Westall G.P., Hansbro P.M., Prele C.M., Mutsaers S.E., Bartlett N.W., Burgess J.K., Grainge C.L., Knight D.A. STAT3 regulates the onset of oxidant-induced senescence in lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 2019;61:61–73. doi: 10.1165/rcmb.2018-0328OC. [DOI] [PubMed] [Google Scholar]
- Wu C., He L., Guo H., Tian X., Liu Q., Sun H. Inhibition effect of glycyrrhizin in lipopolysaccharide-induced high-mobility group box 1 releasing and expression from RAW264.7 cells. Shock. 2015;43:412–421. doi: 10.1097/SHK.0000000000000309. [DOI] [PubMed] [Google Scholar]
- Wynn T.A., Vannella K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W.Z., Wang G., Du J., Ai W. Efficacy of herbal medicine (Xuanfei Baidu decoction) combined with conventional drug in treating COVID-19:A pilot randomized clinical trial. Integr Med Res. 2020;9:100489. doi: 10.1016/j.imr.2020.100489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X., Pan A., Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. U. S. A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z., Ma T., Lin Y., Lu X., Zhang C., Chen S., Jian Z. IL-6/STAT3 pathway intermediates M1/M2 macrophage polarization during the development of hepatocellular carcinoma. J. Cell. Biochem. 2018;119:9419–9432. doi: 10.1002/jcb.27259. [DOI] [PubMed] [Google Scholar]
- Zhang C., Wu Z., Li J.W., Tan K., Yang W., Zhao H., Wang G.Q. Discharge may not be the end of treatment: pay attention to pulmonary fibrosis caused by severe COVID-19. J. Med. Virol. 2021;93:1378–1386. doi: 10.1002/jmv.26634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.