Abstract
Previous research has indicated that racial-ethnic minority communities lack a wide variety of health-related organizations. We examine how this relates to the early COVID-19 vaccine rollout. In a series of spatial error and linear growth models, we analyze how racial-ethnic residential segregation is associated with the distribution of vaccine sites and vaccine doses across ZIP codes in the five largest urban counties in Texas. We find that Black and Latino clustered ZIP codes are less likely to have vaccine distribution sites and that this disparity is partially explained by the lack of hospitals and physicians’ offices in these areas. Moreover, Black clustering is also negatively related to the number of allocated vaccine doses, and again, this is largely explained by the unequal distribution of health care resources. These results suggest that extant disparities in service provision are key to understanding racial-ethnic inequality in an acute crisis like the COVID-19 pandemic.
Keywords: COVID-19, health care, race-ethnicity, residential segregation, vaccines
The COVID-19 pandemic has been one of the most disruptive world events in modern times in terms of health and fundamentally reshaping the economy and people’s social lives. Moreover, this impact has not been equally shared. Some people, because of existing social and economic resources, have been better able to shield themselves from transmission of the disease and the various negative consequences reverberating from the pandemic. In particular, Black, Latino, and Native American populations in the United States have experienced higher rates of infection and, when infected, have been more likely to experience severe disease and death (Millett et al. 2020; Peek et al. 2021; Ramos and Zamudio 2020). Other work has shown how the effects of the pandemic are also spaced and placed, with poor and minority neighborhoods suffering disproportionately from the disease and having limited access to care sites and testing locations (McMinn et al. 2020; Yang, Choi, and Sun 2021). In this analysis, we focus on racial-ethnic disparities in access to the vaccine in the United States specifically as it relates to racial-ethnic residential segregation.
On December 11, 2020, the U.S. Food and Drug Administration issued the first emergency use authorization for the Pfizer-BioNTech vaccine for COVID-19 in individuals 16 years of age and older in the United States. Eight days later, a second vaccine, Moderna, was approved for use in individuals ages 18 and older. However, distribution of the vaccine has been a logistical hurdle necessitating extremely low temperature cold storage and personnel needs, all while having to maintain COVID-19 safety protocols. For example, unpunctured vials of the vaccines must be kept between −90°C and −60°C for the Pfizer-BioNTech vaccine and between −50°C and −15°C for the Moderna vaccine, well below the temperature of a standard freezer. An obvious question that has emerged is how to do this equitably, especially when vaccine supply is low. Roughly following guidelines from the Centers for Disease Control and Prevention (CDC; 2020a), in the case of Texas, which is focus of this analysis, Phase 1A of the rollout was reserved for frontline health care workers, residents of long-term nursing facilities for the elderly, and workers in those same facilities. Phase 1B included people ages 65 and above and people ages 16 and above with at least one comorbidity defined by the state as being particularly vulnerable for the disease, such as cancer, type 2 diabetes, obesity, chronic heart disease, and so on. Although the goal was to equitably distribute vaccine doses to reach the vulnerable, the question remains whether this goal was met.
This is the overarching question that we tackle with this analysis. We focus only on the Phase 1A and 1B periods to examine who got early access while vaccine supply and eligibility were still limited. Obviously, many considerations are at play for how any individual can get the vaccine, and early media reports indicate that access to reliable Internet and transportation play a major role in people’s ability to find an appropriate appointment time and travel to the location (Menchaca and Agnew 2021; Prescott and Prescott 2021; Stone 2021). Here, we focus on the structural element of this story to examine where these vaccine allocations went and whether certain types of neighborhoods were advantaged or disadvantaged in the rollout. We ask: What neighborhood characteristics are related to the distribution of COVID-19 vaccines? Specifically, are racial-ethnic minority communities less likely to have vaccine supply? Furthermore, how do these patterns relate to extant inequalities in health care provision? We posit that racial-ethnic minority areas will be less likely to receive vaccine doses and that this will be explained away or mediated by existing disparities in service provision across neighborhoods given the difficult infrastructure requirements of distributing the two vaccines.
Background
Segregation and Access to Health Care
Research has long documented important health and health care outcomes related to racial-ethnic residential segregation for Black and Latino communities in the United States. For instance, analysts have documented a relationship between segregation and racial differences in mortality for several causes of death (Collins 1999; Collins and Williams 1999; Hart et al. 1998), including infant mortality (Grady 2006; McFarland and Smith 2011). In addition, other studies have examined overall physical health, mental health, and functional disability and found that minority residents of racially segregated neighborhoods are more likely to report experiencing overall poorer physical and mental health and disability (Acevedo-Garcia 2000; Acevedo-Garcia et al. 2003; Anderson and Fullerton 2014; Lee 2009). Finally, a few studies on access to health care demonstrate an association between racial-ethnic segregation and diminished access to health care coverage, having a personal physician, and health care utilization (Anderson and Fullerton 2014; Gaskin et al. 2009; Rodriguez et al. 2007).
Although there is robust literature demonstrating the association between segregation and several health and health care outcomes, less attention has been given to understanding and testing the mechanisms that could link these two. A notable exception is Williams and Collins’s (2001) piece in which they describe racial-ethnic minority segregation as a “fundamental cause” of health and detail how this form of segregation can be fundamentally linked to health outcomes. They suggest that racial-ethnic minority segregation, with its intimate ties to socioeconomic status and overall life chances, can be thought of as a fundamental cause of poor health/health care outcomes in these communities (Williams and Collins 2001). Williams and Collins (2001) describe several specific mechanisms by which racial-ethnic minority segregation may limit life opportunities that impact health/health care outcomes. One such mechanism argues that racial-ethnic minority residential segregation may be related to health outcomes because it can constrain access to a variety of key community resources, including daily necessities such as food, recreation, and public services, and critical resources, such as health care. This perspective focuses on the unequal distribution of resources throughout urban areas and how that inequity may lead to diminished health outcomes for these residents. This is the approach utilized in the study presented here.
Recently, there has been an explosion of interest in this mechanism, and such work has shown how different neighborhood characteristics, mainly differentiated by race and class, relate to service providers and community organizations. The bulk of this research has focused on food resources, known as the “food deserts” or “food swamps” literature (Beaulac, Kristjansson, and Cummins 2009; Cooksey-Stowers, Schwartz, and Brownell 2017). This literature has demonstrated that disproportionately poor and minority neighborhoods lack food resources and have demonstrated important differences in quality and price across neighborhoods (Beaulac et al. 2009; Cooksey-Stowers et al. 2017; Moore and Diez Roux 2006; Walker, Keane, and Burke 2010). Although food is the focus of much of this literature, other work has shown a similar pattern for physical fitness centers, park and green space, retail establishments, nonprofit associations, and social services (Allard 2009; Anderson 2017; Gordon-Larsen et al. 2006; Marwell and Gullickson 2013; Small and McDermott 2006).
Whereas the work on food and retail has been well studied, there are relatively few sociological studies on the case of health care specifically, which is most relevant to our present study on the COVID-19 vaccine distribution. The studies that exist on health care have generally shown a similar pattern in that Black and Latino segregated areas are less likely to have a wide variety of health care establishments in terms of density or distance to facilities (Anderson 2017; Dai 2010; Dinwiddie et al. 2013; Gaskin et al. 2012; Ko et al. 2014; Rodriguez et al. 2007). Specifically, this work has demonstrated that racial-ethnic minority segregation and socioeconomic variables are related to a lower incidence of physicians’ offices, primary care providers, mental health practitioners, urgent care facilities, auxiliary health care practitioners, surgical centers, and dialysis facilities (Anderson 2017; Dai 2010; Dinwiddie et al. 2013; Gaskin et al. 2012; Ko et al. 2014; Rodriguez et al. 2007).
Some limited work has also linked the distribution of health care locations to health and health care outcomes. For example, Dai (2010), in a study of Detroit area neighborhoods, found that Black residents of segregated neighborhoods had fewer facilities that provided mammography services and consequently had higher rates of late-stage breast cancer diagnosis. Another study found that racial-ethnic residential segregation was linked to a greater likelihood of patients seeing nonpsychiatrists for mental health needs (Dinwiddie et al. 2013). A study of the Phoenix area found that the lack of health care service provision in Latino-segregated neighborhoods was linked to a lower likelihood of seeing a personal physician for pediatric care and greater use of clinics (Anderson 2020). Chan et al. (2012) also found that although Black and Latinos living in highly segregated areas had adequate spatial access to facilities, they were less likely to receive certain services, especially specialist care.
This work provides some initial evidence for a disparity by racial-ethnic residential segregation in health care service provision. In this analysis, we aim to extend this literature to examine how this lack of adequate health care provision relates to the COVID-19 pandemic and the early rollout of the vaccine. There may be myriad reasons that affect where and how people choose to procure provider services, with physical proximity/convenience being only one of them that may be important to people of limited means, who lack of access to transportation or are elderly/homebound and may find travel difficult. Distance from providers does not create an impenetrable barrier to access (in this case, vaccine access), but it can increase the “friction of distance,” which may be difficult to overcome absent other kinds of individual or household resources (Tobler 1970). Therefore, with this analysis, we are not assuming that people can only or will only seek vaccine access within their local communities but, rather, that having access in close proximity would facilitate their ability to get it, especially for those who are more vulnerable.
Segregation and the COVID-19 Pandemic
At this stage, limited work has been conducted in a systematic fashion on the COVID-19 pandemic and, more specifically, the vaccine rollout. However, the extant work published over the past year, including media reporting, has demonstrated vast inequalities by race-ethnicity throughout the pandemic. This work has shown that racial-ethnic minority populations have been more vulnerable to COVID-19 disease in terms of rates of infection and mortality (Millett et al. 2020; Ramos and Zamudio 2020). As it relates to segregation specifically, new work has shown that areas with higher numbers of racial-ethnic minorities were more likely to have higher rates of infections and that this pattern was further exacerbated where residential segregation was also high (Yang et al. 2021). Various news reports have also indicated that minority-segregated areas lack vital resources to contend with the spread of the virus, such as testing facilities and health care resources (Garnam and Cai 2021; Godoy and Wood 2020; Martinez 2021; McMinn et al. 2020).
Moreover, what is happening now is clearly part of a broader pattern of inequality in infectious disease as it relates to racial-ethnic residential segregation. Previous work has shown important disparities by racial-ethnic residential segregation in terms of infectious disease, such as tuberculosis and sexually transmitted diseases, among others (Acevedo-Garcia 2000, 2001; Biello et al. 2012; Strully 2011). The limited literature that examines past pandemics and social inequality has focused primarily on race-ethnicity and socioeconomic status (Økland and Mamelund 2019; Roberts and Tehrani 2020; Strully 2011).
From this limited early work on the topic at hand and the more extensive literature on broader inequalities in health care by segregation, we expect these inequalities by segregation to carry over to the early COVID-19 vaccine rollout. Throughout fall 2020, when the possibility of a vaccine was becoming a reality, public health and medical practice journals published numerous editorials with recommendations for how to effectively and equitably roll out the vaccine (Persad, Peek, and Emanuel 2020). However, states must work within the existing public health and health care infrastructure to provide the vaccine in a manner that fulfills more stringent requirements than most vaccines. Given the existing unequal infrastructure, it would be difficult to imagine that the vaccine distribution could be done equitably. The first weeks of vaccine distribution saw reporting from media outlets questioning the equity of allocation locations (Harper 2021; Oladipo 2021). This remains an empirical question, though, because no systematic work has been conducted on this topic concerning the COVID-19 pandemic.
Conceptual Framework and Hypotheses
From the theory and extant work, we thus have several hypotheses for this analysis. Given the documented inequalities in community establishments, we expect that the gap in service provision in segregated communities will extend to the case of the COVID-19 vaccine rollout. We examine this in two ways—the number of vaccination sites and the allocation of doses of vaccine. We also examine this at the neighborhood level by examining the geographic clustering of groups in space (details in the following). Thus, we have the following first hypothesis:
Hypothesis 1: A higher degree of racial-ethnic minority clustering across urban areas will be related to fewer vaccination sites and fewer vaccine doses.
However, we also expect that this will be related to the extant lack of health care provision within these neighborhoods. Thus, we expect that the density of existing health care organizations within urban neighborhoods will attenuate this association. This leads us to our second hypothesis:
Hypothesis 2: The density of health care service providers across urban areas will attenuate the association between the racial-ethnic minority clustering and vaccine provision.
We test these two hypotheses in a study of early vaccine allocations over a 9-week period in the five largest urban counties in Texas.
Data and Methods
Data
To examine the association between racial-ethnic minority clustering and the vaccine rollout, we combined several sources of area-level data measured at the ZIP (Zone Improvement Plan) area unit of analysis (N = 431). First, for data on vaccine allocations, we used data from the Texas Department of State Health Services. Since the first shipment of the Pfizer-BioNTech vaccine on December 14, 2020, the state of Texas has released information on a weekly basis on the location and quantity of doses allocated across the state by county. We collected these data each week for the first 10 weeks of the vaccine rollout (December 14, 2020, to February 15, 2021). However, we decided to exclude the first week from our analysis because these allocations went only to major hospital sites and were exclusively distributed to first-tier health care workers under Texas’s Phase 1A of the vaccine distribution. We chose these weeks for several reasons. We wanted to focus on the early vaccine rollout when supplies were limited and access was difficult to examine which areas got early access to this privilege. Second, we chose this specific set of weeks due to several circumstances in the weeks that followed. In the subsequent week (Week 11 of the rollout), most of the state of Texas experienced a catastrophic winter storm that affected vaccine distributions both in terms of the ability to make shipments to vaccine sites and the ability to keep those doses properly chilled because of widespread power outages. Following this event, in March, the state began to open up eligibility, and the federal government allocated more funding to the vaccine rollout that dramatically increased vaccine supplies across the state.
To analyze the spatial distribution of these sites, an address location was provided for each site, and we geocoded these to a point-level location in ESRI’s ArcMap. Furthermore, because we were primarily interested in how these inequalities relate to urban dynamics, not differences between urban and rural locales, we focused on the five largest counties in the state, which are also the core counties of the five largest cities in the state. These included Bexar County (San Antonio), Dallas County (Dallas), Harris County (Houston), Tarrant County (Fort Worth), and Travis County (Austin). We used counties rather than cities or metropolitan areas because we paired the vaccine data with data on infection rates by ZIP code, which were provided by county health departments.
We recognize that other states have provided similar data about their vaccine allocations. However, we chose to focus on a single state because each state has implemented its own criteria for how vaccines should be allocated and who should be eligible to receive them. Texas, in particular, allocated vaccines to sites based on two pieces of information: an advisory panel, which made recommendations for allocations, and a registration system for vaccine providers to determine eligibility and feasibility. Texas appointed a team of subject matter experts onto an Expert Vaccine Advisory Panel to develop vaccine allocation strategies as recommendations to the Texas Commissioner of Health. Information from the CDC and an appointed Advisory Committee on Immunization Practices helped develop these strategies. Vaccine equity was one of the stated goals of this panel, but the exact algorithm used by the panel is not publicly available. On the supply side of this question, vaccine provider registration data aided in determining the physical locations and quantities for distribution. According to the CDC provider agreement, registration data included licensure information, patient population numbers, and other logistical details required to ensure each facility’s ability to store and administer the vaccines.
Although we limited our analysis to this one state, we argue that Texas serves as a good test case. Part of this rationale is practical—Texas provided detailed information on the location and quantities of vaccines in a publicly available format. It is also the only state with that many major U.S. cities (5 in the top 20 largest cities in the United States) and that also has a high degree of racial-ethnic diversity across all three of the largest racial-ethnic groups in the United States. Although Texas cities do not have the highest rates of residential segregation, certain Texas cities are fairly segregated, and there is quite a bit of variation across cities to allow for comparison. Full descriptive statistics for variables used in our analysis and the racial-ethnic breakdown for each county (divided by county) can be found in Appendix A in the online version of the journal for reference.
We combined these data with sociodemographic data and data on establishments from two census products. First, for sociodemographic data, including our measures of racial-ethnic clustering, we used the 2014 to 2018 American Community Survey 5-year estimates at the ZIP code tabulation area level. The Census Bureau only provides data at this small geographic unit using 5-year aggregates because the data are not representative for small units of analysis like the ZIP code for a single year. We also combined this with data on establishments from the 2016 County Business Patterns (CBP) ZIP Code Industry Detail File. The CPB uses IRS tax records to provide counts of establishments by ZIP code and by industry type using the North American Industry Classification System (NAICS). We included several industry codes related to health care, which we hypothesized may be related to higher vaccine allocations (details in the following). Although 2016 is not the most recent year of data publicly available (2018), starting in 2017, the Census Bureau stopped releasing counts of establishments where the count for the ZIP code was less than three to further deidentify the information. Because we included several industry classifications that are relatively rare, such as hospitals, we opted to use the older version of the data to get better estimates of the available resources in neighborhoods.1
Across all models, we also included a control variable for the cumulative number of infections in the ZIP code for all five of our counties. These came from publicly available sources from each county’s public health department. These were collected in each of the five counties on February 9, 2021, which was the second to last week of our vaccine rollout time frame such that it could have affected allocation decisions. We included this as a covariate in the event that state health officials were using the infection rate as the basis by which vaccines were being allocated by factoring in local vulnerability.
We included two dependent variables, each meant to capture a different facet of the vaccine rollout across urban areas in the state. First, we examined the number of vaccine sites per 100,000 people in a ZIP code. This came from the geocoded locations for vaccine allocations and reflected a simple count of the locations providing COVID-19 vaccines. We also divided this number by the population size and multiplied by 100,000 to derive a rate per 100,000 people. Beyond the mere availability of a vaccine site, we also examined the distribution of vaccine doses per 10,000 people to each site for each week of the 9-week period. The state provided data on the week-by-week allocations to each of the geocoded locations. To derive a rate, we divided this number by the population and multiplied by 10,000.
Our main independent variables in this analysis included a set of variables for racial-ethnic clustering. Although much of the literature on racial-ethnic segregation is focused on measuring global segregation across a large area, such as the county or metropolitan area (Massey and Denton 1988), in this study, we were interested in coding for which areas within the county have disproportionately high numbers of certain groups. Typically, studies that examine racial-ethnic concentration at a smaller geographic unit of analysis use composition scores, which reflect simply the percentage of a group over a certain area.2 However, this approach is aspatial and ignores the role of geographic clustering across space and how adjacent areas may influence each other (Reardon and O’Sullivan 2004; Roberto 2018). Thus, for this analysis, we used a geographic clustering score that considered two pieces of information: the concentration of a group in an area (ZIP codes) and the extent to which that group is geographically clustered. To be more precise, we refer to this as clustering rather than segregation throughout the discussion of this analysis, and we conceptualized this as a neighborhood-level measure of segregation. We used the following formula:
where is the variable for ZIP code i, is the variable for ZIP code j, and is the spatial weight between ZIP codes i and j (Anderson 2017). The measure is essentially the product of the percentage of a certain group in a ZIP code and the spatial weight of its neighborhoods (row standardized). We used a queen contiguity matrix to calculate the spatial weight. This produces a theoretical range of 0 to 10,000. For example, a ZIP code could have a score of 10,000 if that ZIP code contained 100% of its residents from a certain group and all adjacent neighborhoods also had a population composition of 100% of the same group. In practice, no ZIP code in Texas has this high of a score for any group, and the scores vary considerably depending on the county and group in question. For this reason, we also group mean centered (to the county) each of these scores to make them relative to the population sizes of the county. Otherwise, these measures might reflect differences in the relative sizes of these groups across areas rather than differences within a particular county in terms of how these groups are spatially patterned. For example, San Antonio is 64.2% Latino, whereas Austin is only 33.9% Latino. In each of these contexts, what might be considered a disproportionately Latino community would be different, and group mean centering would contextualize this difference. This is an approach used in previous work examining neighborhoods (Sampson, Raudenbush, and Earls 1997). For this study, we included three scores: clustering measure for percentage Black (non-Latino), clustering measure for percentage Latino (of any race), and clustering measure for percentage Asian (non-Latino).3
To examine whether these patterns relate to existing disparities in resource distribution, we also included four variables for counts of organizations that are more likely to receive vaccine allocations. These variables came from the CBP data set, which classifies establishments by industry code using NAICS codes. In this analysis, we included counts by ZIP code of general hospitals (622///), physicians’ offices (6211//), pharmacies (446110), and retirement and assisted living communities for the elderly (6233//).4
We also included several control variables to account for populations that are eligible to receive the vaccine earlier on the priority list, vulnerability to the disease, and other sociodemographic factors. These included population density, percentage age 65 and above, percentage of people employed in service occupations, median family income, percentage of people with a bachelor’s degree or higher, percentage of households with no private vehicle, and cumulative number of infections in a ZIP code. Descriptive statistics for all variables can be found in Table 1.
Table 1.
Descriptive Statistics for Variables Used in Statistical Models.
| Variable Name | Mean | SD | Range | Description |
|---|---|---|---|---|
| Dependent variables | ||||
| Vaccine sites | 7.92 | 17.95 | 0 to 169.71 | Number of vaccine sites per 100,000 people in a ZIP code |
| Vaccine doses | 1,199.01 | 8,295.32 | 0 to 238,415.58 | Number of vaccine doses allocated to ZIP code per 10,000 people |
| Independent variables | ||||
| Black clustering | 0 | 648.99 | −700.47 to 4,150.46 | Clustering measure of % Black |
| Latino clustering | 0 | 1,611.93 | −2,986.80 to 5,851.91 | Clustering measure of % Latino |
| Asian clustering | 0 | 84.01 | −73.08 to 812.42 | Clustering measure of % Asian |
| Population density | 3,291.17 | 2,359.18 | 6.83 to 16,811.43 | Population density per square mile |
| Population age 65+ | 10.94 | 4.13 | 0 to 36.60 | % of population age 65 and above |
| % Service work | 17.01 | 6.43 | 0 to 43.40 | % of employed population in service occupations |
| Household income | 68,094.12 | 31,467.35 | 17,798 to 240,417 | Median household income |
| % Bachelor’s degree | 32.96 | 21.30 | 2.60 to 90.00 | % of population over 25 with at least a bachelor’s degree |
| % No vehicle | 6.10 | 5.38 | 0 to 39 | % of households with no private vehicle |
| Infections | 2,127.59 | 1,652.52 | 0 to 9,906 | Number of positive SARS-CoV-2 infections |
| Hospitals | .61 | 1.46 | 0 to 15 | Number of general hospitals |
| Physicians’ offices | 26.84 | 41.99 | 0 to 294 | Number of physicians’ offices |
| Pharmacies | 3.89 | 3.39 | 0 to 18 | Number of pharmacies |
| Retirement communities | 1.41 | 1.88 | 0 to 14 | Number of retirement and assisted living facilities for the elderly |
Note: N = 431. Data come from the Texas Department of State Health Services, the 2014–2018 American Community Survey, and the 2016 County Business Patterns.
Methods
To model these two different dependent variables, we present two sets of models, one for each outcome. First, for the number of vaccine distribution sites, we estimated a series of spatial error models. We calculated univariate global Moran’s I statistics for our key independent variables that suggested that spatial autocorrelation was a problem and would therefore not meet the assumptions of ordineary least squares (OLS) regression. Furthermore, the LaGrange multiplier statistics indicated that the spatial error model was the most appropriate method to contend with this autocorrelation (Anselin, Florax, and Rey 2004). Specifically, we used a queen spatial weight matrix because this was found to best maximize global Moran’s I for each of the key variables used in the models (Anselin 1995; Anselin, Florax, and Rey 2004). We also included Kelejian and Prucha (2010) robust standard errors to account for significant heteroscedasticity. However, of note, the term for lambda is not significant in the models presented in Table 2, suggesting that correlated errors in omitted variables may not be particularly a problem here. The OLS results were also virtually identical to what is presented here in terms of the sign, significance, and relative effect sizes for each of our variables. Because spatial autocorrelation was significant in our preliminary analyses, though, we chose to present the results as spatial error models. These results can be found in Table 2.
Table 2.
Coefficients and Z-Ratios from Spatial Error Models of Vaccination Sites per 100,000 People.
| Variable Name | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 |
|---|---|---|---|---|---|
| Black clusteringa | −.535*** (−3.299) |
−.503** (−3.044) |
−.461** (−2.819) |
−.516** (−3.091) |
−.547*** (−3.326) |
| Latino clusteringa | −.137* (−2.185) |
−.101 (−1.530) |
−.104 (−1.625) |
−.120† (−1.793) |
−.149* (−2.315) |
| Asian clusteringa | .474 (.383) |
.198 (.226) |
.100 (.097) |
.230 (.187) |
.565 (.446) |
| Population density | −.001** (−2.729) |
−.001** (−2.687) |
−.002** (−3.033) |
−.002** (−2.845) |
−.001** (−2.680) |
| Population age 65 and up | .099 (.353) |
.173 (.594) |
.085 (.291) |
.105 (.377) |
.204 (.691) |
| % Service work | −.426† (−1.767) |
−.391† (−1.697) |
−.406† (−1.711) |
−.418† (−1.723) |
−.413† (−1.713) |
| Median household incomea | −.073 (−1.380) |
−.029 (−.629) |
−.053 (−1.137) |
−.068 (−1.318) |
−.081 (−1.482) |
| % Bachelor’s degree | .172† (1.826) |
.102 (1.206) |
.100 (1.069) |
.164† (1.757) |
.187† (1.897) |
| % No vehicle | 2.193*** (4.175) |
2.014*** (3.726) |
2.040*** (3.887) |
2.180*** (4.122) |
2.153*** (4.132) |
| Infectionsa | .004 (.100) |
−.015 (−.386) |
−.024 (−.598) |
−.030 (−.754) |
.022 (.533) |
| Hospitals | 3.290** (2.866) |
||||
| Physicians’ offices | .097* (2.383) |
||||
| Pharmacies | .419† (1.677) |
||||
| Retirement communities | −.719* (−2.387) |
||||
| Lambda | −.935 (−.765) |
−1.263 (−1.000) |
−.740 (−.603) |
−.775 (−.640) |
−.660 (−.540) |
| Pseudo R2 | .345 | .408 | .384 | .349 | .350 |
For the second set of models for the week-by-week allocation of vaccine doses, we present a series of hierarchical linear growth models (Raudenbush and Bryk 2002; Singer and Willett 2003) with a correction for spatial dependency using a queen contiguity spatial weight matrix (Savitz and Raudenbush 2009). Given the nested structure of the data, weekly allocations per ZIP code, we used this approach to model the cumulative change over time in the number of vaccine doses per ZIP code. However, because the data were still organized by physically adjacent spatial units of analysis at Level 2, we used Savitz and Raudenbush’s (2009) routine to account for spatial dependency using HLM 8.1, which is an approach used in similar health research (O’Connell 2015). These results can be found in Table 3.
Table 3.
Coefficients and T-Ratios from Linear Growth Models of Weekly Vaccination Allocations per 10,000 People.
| Variable Name | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 |
|---|---|---|---|---|---|
| Fixed effects | |||||
| Week | 312.927*** (8.553) |
312.927*** (8.553) |
312.927*** (8.553) |
312.927*** (8.553) |
312.927*** (8.553) |
| Black clustering | −1.014* (−1.984) |
−.820† (−1.785) |
−.593 (−1.215) |
−.907† –1.771) |
−1.036* (−2.029) |
| Latino clustering | −.424† (−1.657) |
−.204 (−.886) |
−.250 (−1.026) |
−.345 (−1.337) |
−.465† (−1.810) |
| Asian clustering | 4.731 (1.256) |
3.212 (.949) |
2.669 (.745) |
3.597 (.947) |
4.956 (1.317) |
| Population density | −.376* (−2.355) |
−.347* (−2.415) |
−.467** (−3.072) |
−.437** (−2.687) |
−.352* (−2.190) |
| Population age 65 and up | −141.990† (−1.841) |
−90.095 (−1.298) |
−150.614* (−2.060) |
−140.289† (−1.825) |
−109.474 (−1.364) |
| % Service work | −154.071† (−1.941) |
−138.248† (−1.939) |
−138.666† (−1.842) |
−148.326† –1.873) |
−147.523† (−1.858) |
| Median household income | −.038* (−2.109) |
−.012 (−.752) |
−.027 (−1.608) |
−.035* (−1.970) |
−.040* (−2.250) |
| % Bachelor’s degree | 42.995 (1.348) |
2.743 (.095) |
6.362 (.207) |
39.659 (1.245) |
48.578 (1.513) |
| % No vehicle | 485.960*** (6.382) |
384.743*** (5.568) |
401.661*** (5.487) |
478.015*** (6.288) |
471.639*** (6.149) |
| Infections | −.223 (−1.132) |
−.323† (−1.826) |
−.354† (−1.891) |
−.371† (−1.759) |
−.156 (−.774) |
| Hospitals | 1855.981*** (10.099) |
||||
| Physicians’ offices | 50.823*** (6.954) |
||||
| Pharmacies | 192.471† (1.908) |
||||
| Retirement communities | −241.504 (−1.440) |
||||
| Random effects | |||||
| Level 2 error variance | 30,132,845.834 | 23,546,583.226 | 26,689,874.927 | 29,920,526.251 | 30,046,250.421 |
| Level pseudo R2 | .146 | .333 | .244 | .152 | .149 |
Note: Level 1 N = 3,879. Level 2 N = 431. Data come from the Texas Department of State Health Services, the 2014–2018 American Community Survey, and the 2016 County Business Patterns. The Level 2 pseudo R2 is calculated from the proportional reduction in error variance from a model with no Level 2 variables (τ = 35,296,518.409).
p < .1, *p < .05, **p < .01, ***p < .001 (for two-tailed test).
For each set of models, we first present a model with only the clustering scores and ZIP-code-level control variables included (Model 1). Then, we add the health care resource variables one by one in the model (Models 2–5) to avoid the problems of multicollinearity and to isolate the effect of each organizational type as health care organizations tend to agglomerate. To our knowledge, no formal mediation test exists that can account for the spatial dependencies in the models and the multilevel structure of the second set of models. Therefore, we used an informal approach and examine change in the effect sizes with the inclusion of certain variables.
Results
From the results in Table 2, we can see that the racial-ethnic clustering scores have a significant relationship to the density of vaccine sites. Specifically, for Black and Latino clustering, these scores are significant and negative, meaning that as the clustering of these two groups increases, the number of vaccine sites per 100,000 people decreases. Essentially, the higher the concentration and clustering of these two groups, the lower the number of vaccine sites. These effect sizes are also large. We discuss these coefficients in terms of standard deviation changes because the scale of each variable is so different, although the original coefficients are available in the tables. In the case of Black clustering, a 1 SD (648.99) increase in Black clustering relates to 3.47 decrease in the number of vaccine sites per 100,000 people. Given that the average number of sites per 100,000 people is only 7.92, this is a notable change. For Latino clustering, a 1 SD (1,611.93) increase in Latino clustering is related to a 1.59 decrease in the number of vaccine sites per 100,000 people. These are both sizable coefficients and indicate that Black- and Latino-clustered areas are less likely to have vaccine sites.
Several of the other area-level coefficients are significant as well. Population density is significant and negative, the percentage of households with no car is significant and positive, and two others, percentage college educated and percentage in service work, are significant at the .1 level. The largest effect comes from the percentage of households with no car, where a 1 SD increase (5.38) relates to an increase of 11.8 vaccine sites. This seems to run counter to what we would expect from the literature because private vehicle ownership may relate to socioeconomic status, but it may also be an indication of central city location where residents may perceive less of a need to own a vehicle. Taken together, from the pseudo R2 value, these sociodemographic characteristics of ZIP codes explain 34.5% of the variation in vaccine sites per 100,000 people, which is sizable.
In Models 2 to 5, when we add the health care establishment variables, this pattern changes somewhat. First, when we include two such health care establishments, the number of hospitals and the number of physicians’ offices, these are both significant and positive. This indicates that having more health care establishments in a ZIP code relates to a greater number of vaccine distribution sites. This is a notable increase as well, where each additional hospital is related to an increase of 3.29 vaccination sites and each additional physician’s office is related to a .10 increase in sites. This is expected because hospitals and clinics are where most of the vaccine doses were allocated.
The addition of these two variables also reduces the size of the coefficients for the clustering variables, suggesting that the lower number of vaccine sites in these areas is a function of their lack of health care resources. In the case of Latino clustering, the coefficients drop to nonsignificance with the inclusion of hospitals and physicians’ offices, and these changes relate to a 26.48% and a 23.99% reduction, respectively, in the size of the coefficients for Latino clustering. This percentage change in the coefficient size is somewhat smaller for Black clustering at 9.09% and 13.77%, respectively. This is reflected in their pseudo R2 values as well, where hospitals alone explain an additional 6.33% of the variation in vaccine sites and physicians’ offices explain 3.96%. This suggests that existing health care resources in areas may explain some part of the negative association between minority clustering in ZIP codes and the number of vaccine sites. However, these findings are only significant at the .1 level for pharmacies and significant and negative for retirement communities. Therefore, the bulk of this effect seems to be driven by hospitals and physicians’ offices rather than other kinds of facilities that may offer the vaccine.
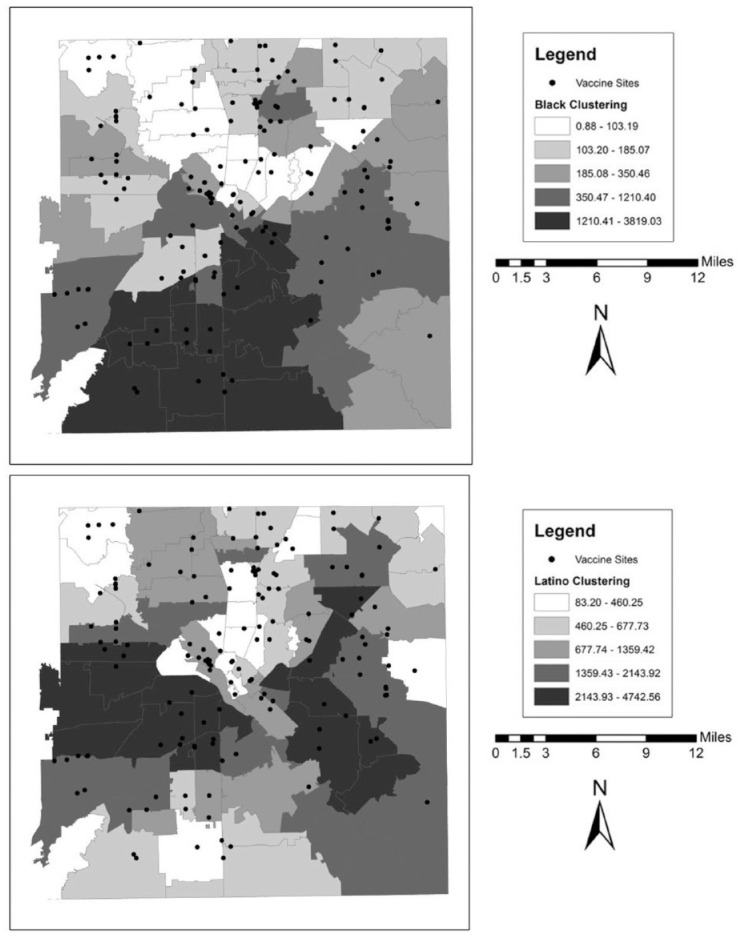
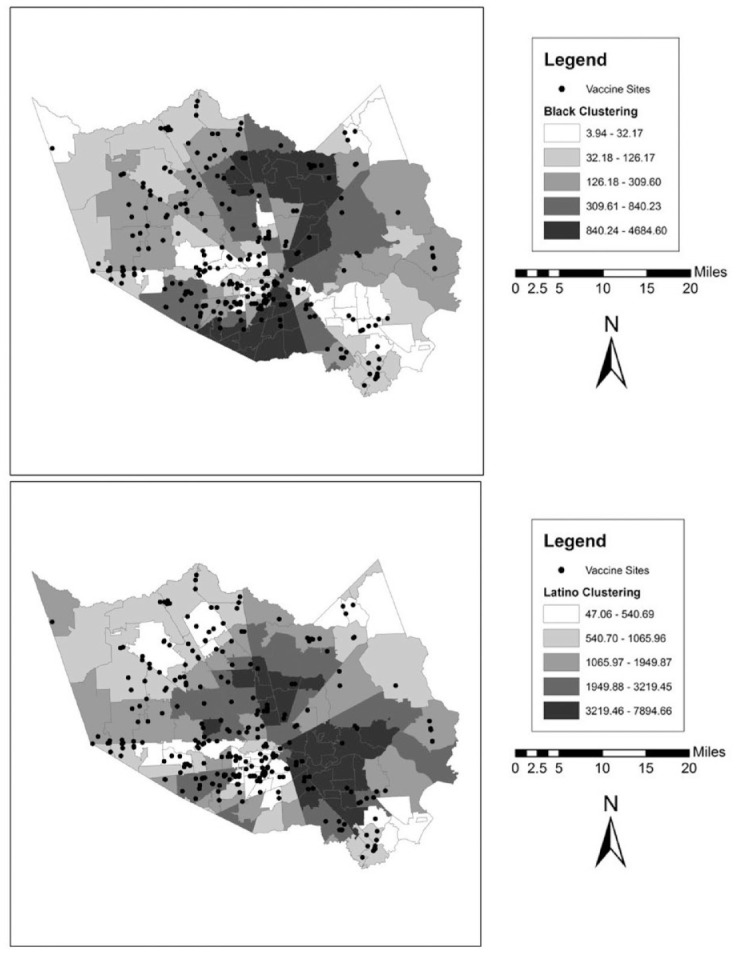
We also illustrate these patterns graphically in a series of maps for each county. These can be found in Figures 1 to 5. In the background of each map is a choropleth quintile map of the two clustering scores with the location of the vaccine sites overlaid on top. Choropleth maps use shading to map patterns across a polygonal area. Here, we use a quintile map, meaning that the range of mapped values in the polygons include an equal number of ZIP codes for each shade. Note that because these are evenly distributed quintile maps, the scale is different for each group and location. From these maps, it is clear that minority clustered areas are less likely to have vaccine sites, but these patterns differ somewhat by county, with more stark patterns in certain counties over others and depending on the group in question. For example, Harris and Travis counties each have a clearer clustering pattern to them where the vaccine sites appear to be less likely to be located in racial-ethnic minority areas. By contrast, Tarrant County (Fort Worth) has a more scattered pattern to the location of the facilities.
Figure 1.
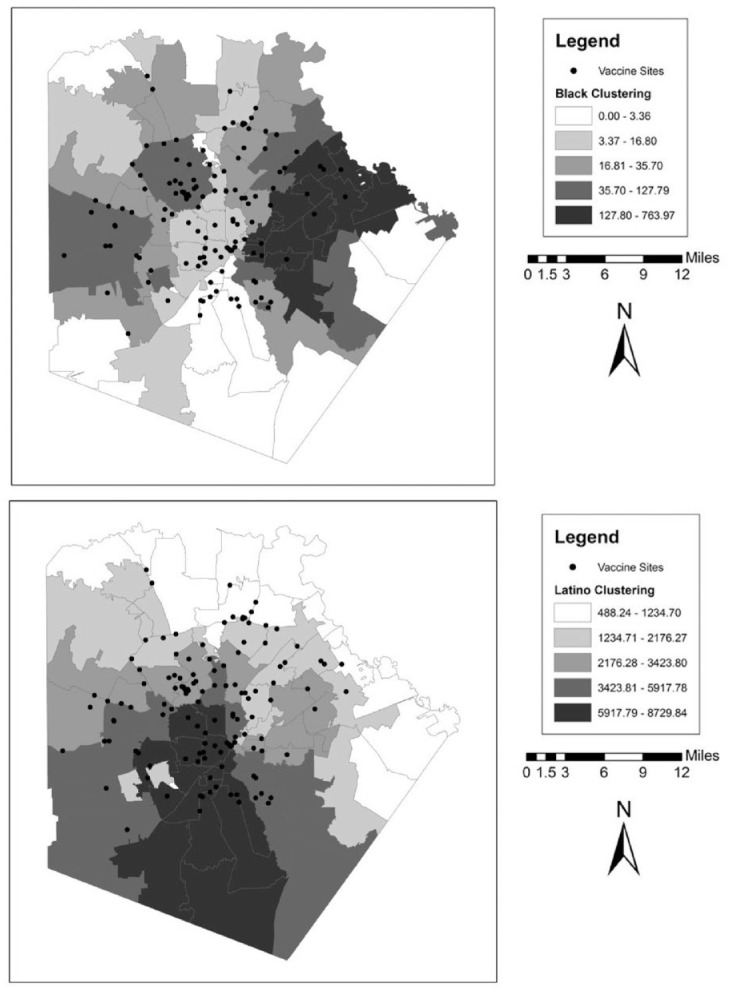
Vaccine Distribution Sites over Racial-Ethnic Clustering Scores in Bexar County (San Antonio), Texas.
Note: Data come from the Texas Department of State Health Services and the 2014–2018 American Community Survey.
Figure 5.
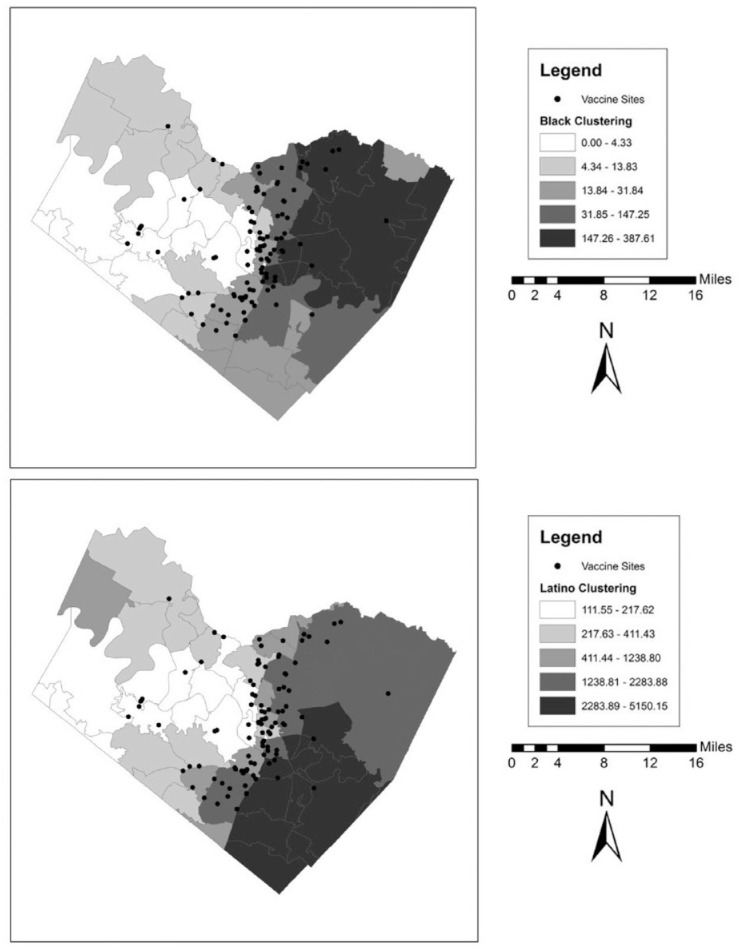
Vaccine Distribution Sites over Racial-Ethnic Clustering Scores in Travis County (Austin), Texas.
Note: Data come from the Texas Department of State Health Services and the 2014–2018 American Community Survey.
Figure 2.
Vaccine Distribution Sites over Racial-Ethnic Clustering Scores in Dallas County (Dallas), Texas.
Note: Data come from the Texas Department of State Health Services and the 2014–2018 American Community Survey.
Figure 3.
Vaccine Distribution Sites over Racial-Ethnic Clustering Scores in Harris County (Houston), Texas.
Note: Data come from the Texas Department of State Health Services and the 2014–2018 American Community Survey.
Figure 4.
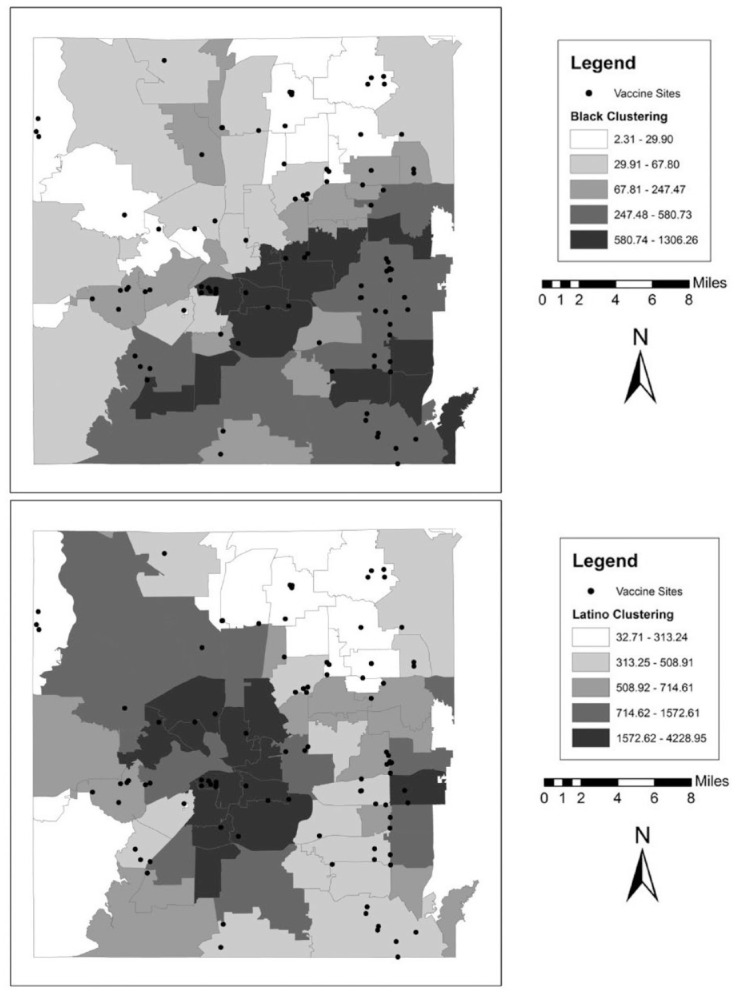
Vaccine Distribution Sites over Racial-Ethnic Clustering Scores in Tarrant County (Fort Worth), Texas.
Note: Data come from the Texas Department of State Health Services and the 2014–2018 American Community Survey.
In Table 3, we present the results for the number of vaccine allocations over the 9-week period using a series of linear growth models. The results with this dependent variable reflect a similar pattern to the one described previously with some notable differences. Again, in Model 1, the coefficient for Black clustering is significant and negative, indicating that the higher the degree of concentration and clustering of Blacks across these five counties, the smaller the vaccine allocations week to week. Specifically, every 1 SD increase in Black clustering (648.99) relates to 658.08 fewer vaccine doses per 10,000 people in a ZIP code. The same figure for a 1 SD change in Latino clustering (1,611.93) is 492.66 vaccine doses per 10,000 people, which is not an inconsequential amount, although this coefficient is only significant at the .1 level for Latino clustering. In all subsequent models, the results for Latino clustering are not significant. Concerning the control variables, the results are similar to previous models except that median household income is significant and negative here.
When we add the different types of health care establishments to the baseline model, the results are similar in pattern to the results previously described. Once again, the distribution of hospitals and physicians’ offices is significant and positive, meaning that their presence in an area is related to a higher allocation of vaccine doses per 10,000 people. Moreover, the inclusion of these variables weakens the association between the clustering scores and the number of vaccine doses per 10,000 people, especially in the case of Black clustering. Black clustering is only significant in Model 1 and Model 5, and the inclusion of hospitals and physicians’ offices rather substantially reduces the size of those coefficients. These reductions are percentage changes of 19.13%, 41.52%, and 10.55% for hospitals, physicians’ offices, and pharmacies, respectively. Hospitals and physicians’ offices appear to explain away a substantial portion of the differential allocations across areas related to Black clustering. We also see changes in the size of the coefficients for Latino clustering, but this score was only significant at the .1 level in Model 1.
Discussion
In this study, we aim to understand how neighborhood sociodemographic characteristics relate to the early COVID-19 vaccine rollout. We hypothesize that racial-ethnic minority clustered areas will be less likely to have vaccination sites, and we surmise that this is primarily a function of a lack of key health care sites prior to the pandemic. We test these hypotheses across the five largest urban counties in the state of Texas during the first 10 weeks of the vaccine rollout in Texas (excluding the first week). Moreover, we test this using two ways of capturing access to the vaccine for neighborhoods—the number of vaccine sites and the number of doses allocated to each site.
For the first outcome, examining the number of vaccine sites across these urban counties, we find that a higher concentration and clustering of Black and Latino residents in ZIP codes is associated with fewer vaccine sites per 100,000 people. These results provide support for Hypothesis 1 for Black and Latino clustering (not Asian clustering). Moreover, this negative association is partially explained by the existing distribution of health care resources across communities, especially hospitals and physicians’ offices. The presence of these establishments is associated with a higher number of vaccine sites, so the lack of these sites in segregated communities means fewer vaccine sites for these areas. However, we do not observe the same association for pharmacies and retirement communities. Indeed, the association for retirement communities is actually significant and negative and appears to have a suppression effect with the racial-ethnic clustering scores. We speculate that this may be because these groups are less likely to use retirement homes for elderly relatives as other work suggests (Dilworth-Anderson, Williams, and Gibson 2002), although we cannot directly test this assertion with the data here. These findings provide partial support for Hypothesis 2, with stronger evidence for the case of Latino clustering.
For the second outcome, we find a similar pattern, although with somewhat weaker effects. Black clustering is again significant and negatively associated with the number of vaccine doses per 10,000 people in a ZIP code over a 9-week period (although this is only significant for Latino clustering at the .1 level), meaning that minority segregated communities were less likely to receive doses of vaccine over this period. This provides further partial support for Hypothesis 1 in the case of Black clustering. And, again, these results are somewhat attenuated with the inclusion of the number of hospitals and physicians’ offices. Both these types of establishments are strongly associated with a higher number of vaccine doses, and they reduce the size of the coefficient for Black clustering. Thus, we find partial support for Hypothesis 2.
These findings are fitting with previous literature on segregation and the distribution of resources more broadly that demonstrates poor access to a wide variety of community establishments that would support well-being (Anderson 2017; Dinwiddie et al. 2013; Gaskin et al. 2012; Ko and Ponce 2013). We find this for Black and Latino clustering but not for Asian clustering. However, the existing work on Asian segregation is limited and has not found the same inequalities as Black and Latino segregations (Anderson 2017). The findings presented here also point to a much stronger race story than one about socioeconomic dynamics, which is a prominent theme in much of the previous literature on resource allocation across neighborhoods (Beaulac et al. 2009; Ko et al. 2014). In our study, area-level median income was not significant in the first analysis, and in the second analysis, it was significant and negative, meaning that higher income areas were less likely to receive doses. Thus, the story seems to be more about race specifically rather than class, which limited research has also shown (Anderson 2017; Small and McDermott 2006).
Given the lack of detail provided by the state in terms of how specifically the vaccine allocation algorithm was designed, we are not necessarily suggesting that this is an act of blatant and purposive racism on the part of the advisory board responsible for vaccine allocation decision-making. Rather, because the infrastructure in terms of health care resources already disadvantages racial-ethnic minority communities, this represents a case of structural discrimination. Thus, we extend this literature by demonstrating yet another empirical instance of a racial disparity, especially for a hugely impactful event like the current COVID-19 crisis. Some limited findings on the COVID-19 pandemic, including media sources, has revealed important disparities by race-ethnicity in infection rates and access to medical services, such as testing sites and ICU facilities (CDC 2020b; McMinn et al. 2020; Miller, Peek, and Parker 2020; Millett et al. 2020; Ross 2021). The vaccine rollout appears to fit within this pattern. This is relevant to the current inequities we have seen throughout the pandemic and has implications for the next crisis.
Despite these advances to the literature, the study has several limitations. First, the study only relates the sociodemographic characteristics of neighborhoods with the distribution of vaccine sites and doses and not who is being vaccinated at these sites. Media reporting on access to the vaccine has highlighted how differential access to the Internet to search for vaccine appointments, time to spend searching for open appointments, and adequate transportation have led to important disparities in early access to the vaccine, even for eligible populations (Garnam and Cai 2021; Harper 2021; Menchaca and Agnew 2021; Oladipo 2021; Ross 2021; Stone 2021). This study only factors in disparities in the built environment in terms of the location of vaccine sites without accounting for who is going to these sites. Second, the data from the Texas Department of Health Services only includes address locations for where vaccine doses were shipped. Although the vast majority of shipping locations were the same as where the vaccines were administered, this approach fails to consider mobile units that some city and county health departments have utilized to reach special populations, like mobility-challenged patients in elder care facilities. These types of activities were not possible to systematically track over time. Future work should consider these limitations.
With this study, we contribute to the literature on the unequal distribution of resources across neighborhoods, particularly by racial-ethnic minority clustering. Moreover, we demonstrate that these patterns are not a neutral fact. Not having health care infrastructure in place means that when confronted with a public health catastrophe, the existing inequalities in our health care system are deepened. The COVID-19 pandemic is, for many people, one of the most disruptive and challenging public health events of our lifetimes. Vaccination in this context represents a lifeline to spare further human suffering and loss of life and a potential return to normalcy. However, this valuable resource was not distributed evenly across urban areas, with limited access to populations already at risk for complications from the virus. Although state public health officials implemented eligibility systems that prioritized health care workers, the elderly, and those with medical comorbidities, the geographic component of the vaccine rollout and allocation of the vaccine over time has not been equal. This highlights the necessity of creating more equitable access to care broadly so that in crisis times, the infrastructure is available to equitably meet the needs of the affected communities.
Supplemental Material
Supplemental material, sj-docx-1-hsb-10.1177_00221465221074915 for Racial-Ethnic Residential Clustering and Early COVID-19 Vaccine Allocations in Five Urban Texas Counties by Kathryn Freeman Anderson and Darra Ray-Warren in Journal of Health and Social Behavior
Author Biographies
Kathryn Freeman Anderson is an associate professor in the Department of Sociology at the University of Houston. Her research focuses on understanding the social sources of health disparities in the United States. In particular, she examines the role of race-ethnicity and urban neighborhood dynamics to analyze how these factors may affect individual health. Her recent work has been published in Social Problems, City & Community, and Race and Social Problems.
Darra Ray-Warren is a graduate student in the Department of Sociology at the University of Houston. Her research interests are in the socioeconomic determinants affecting mental and physical health, social status, and the public policies that may impact human flourishing. Her current research focuses on criminal law and punishment rationale, emerging progressive prosecutorial and indigent defense styles, and the social factors that drive criminal case outcomes.
We also ran the same models using the 2018 version of the data, which is the most recent version available but likely has undercounts of establishments in many areas. The results generally followed the same pattern as what is presented here but with different effect sizes in some cases. As an additional sensitivity check, we also checked previous waves of the data to see how correlated the counts are over time to justify the use of an earlier date. Using the 2012 CBP file (being four years before our data, which is the same distance from 2016 to 2020), we found that all four of our organizational types were highly correlated. From 2012 to 2016, hospitals, physicians’ offices, pharmacies, and retirement communities had a correlation of .93, .98, .86, and .86, respectively, indicating that these organizational resources are relatively stable over time, especially health care provision.
Our analysis focused on these clustering scores as a more geographically informed way of examining the problem. To relate these findings to the broader literature that typically uses composition scores, we ran the same models using percentage non-Latino Black, percentage Latino, percentage non-Latino Asian, and another version of these models’ variables that also included their spatial W lags (which is the other term in the clustering equation). The results were similar to what we found in the present study, with some differences in the effect sizes (results available on request). We chose to present the results with the clustering score as indicated by the formula because we think this best captures the spatial dynamics of neighborhood-level segregation in a manner that accounts for both the composition of groups in an area and the extent to which they are spatially clustered, which is one of the main ways that segregation is theorized and conceptualized in the literature (Massey and Denton 1988).
The distribution of Whites also plays a role in the segregation level of an area. However, due to multicollinearity, we could not include all four clustering scores for each of these groups in a single model. Instead, we focused on the distribution of racial-ethnic minority populations across these counties. As a check on this choice of approach, we also ran the same models using the clustering score for percentage White. This score for Whites was not significant in any of the models, suggesting that the distribution of Whites across areas is unrelated to the distribution of vaccine sites and allocations.
We also tested several other organizational types that might have received vaccine allocations, including general stores (452///), supermarkets (4451//), freestanding ambulatory care facilities (621493), and other (nonelderly) nursing and residential care facilities (623///). We only present the results for those that were most relevant and had significant results.
Footnotes
ORCID iD: Kathryn Freeman Anderson  https://orcid.org/0000-0002-7425-8923
https://orcid.org/0000-0002-7425-8923
Supplemental Material: Appendix A is available in the online version of the article.
References
- Acevedo-Garcia Dolores. 2000. “Residential Segregation and the Epidemiology of Infectious Diseases.” Social Science & Medicine 51(8):1143–61. [DOI] [PubMed] [Google Scholar]
- Acevedo-Garcia Dolores. 2001. “ZIP Code-Level Risk Factors for Tuberculosis: Neighborhood Environment and Residential Segregation in New Jersey, 1985–1992.” American Journal of Public Health 91(5):734–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo-Garcia Dolores, Lochner Kimberly, Osypuk Theresa, Subramanian S. V.2003. “Future Directions in Residential Segregation and Health Research: A Multilevel Approach.” American Journal of Public Health 93(2):215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard Scott W.2009. Out of Reach: Place, Poverty, and the New American Welfare State. New Haven, CT: Yale University Press. [Google Scholar]
- Anderson Kathryn Freeman. 2017. “Racial Residential Segregation and the Distribution of Health-Related Organizations in Urban Neighborhoods.” Social Problems 64(2):256–76. [Google Scholar]
- Anderson Kathryn Freeman. 2020. “Residential Segregation, Neighborhood Health Care Organizations, and Children’s Health Care Utilization: The Case of the Phoenix Urbanized Area.” City & Community 19(3):771–801. [Google Scholar]
- Anderson Kathryn Freeman, Fullerton Andrew. 2014. “Residential Segregation, Health, and Health Care: Answering the Latino Question.” Race and Social Problems 6(3):262–79. [Google Scholar]
- Anselin Luc. 1995. “Local Indicators of Spatial Association—LISA.” Geographical Analysis 27(2):93–115. [Google Scholar]
- Anselin Luc, Florax Raymond, Rey Sergio. 2004. Advances in Spatial Econometrics: Methodology, Tools and Applications. Berlin: Springer-Verlag. [Google Scholar]
- Beaulac Julie, Kristjansson Elizabeth, Cummins Steven. 2009. “A Systematic Review of Food Deserts, 1966–2007.” Preventing Chronic Disease 6(3):1–10. [PMC free article] [PubMed] [Google Scholar]
- Biello Katie, Kershaw Trace, Nelson Robert, Hogben Matthew, Ickovics Jeannette, Niccolai Linda. 2012. “Racial Residential Segregation and Rates of Gonorrhea in the United States, 2003–2007.” American Journal of Public Health 102(7):1370–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2020. a. “CDC’s COVID-19 Vaccine Rollout Recommendations.” https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations.html. [PubMed]
- Centers for Disease Control and Prevention. 2020. b. “COVID-19 in Racial and Ethnic Minority Groups.” https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/racial-ethnic-minorities.html.
- Chan Kitty, Gaskin Darrell, Dinwiddie Gniesha, McCleary Rachael. 2012. “Do Diabetic Patients Living in Racially Segregated Neighborhoods Experience Different Access and Quality of Care?” Medical Care 50(8):692–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins Chiquita. 1999. “Racism and Health: Segregation and Causes of Death Amenable to Medical Intervention in Major U.S. Cities.” Annals of the New York Academy of Sciences 896:396–98. [DOI] [PubMed] [Google Scholar]
- Collins Chiquita, Williams David. 1999. “Segregation and Mortality: The Deadly Effects of Racism?” Sociological Forum 14(3):495–523. [Google Scholar]
- Cooksey-Stowers Kristen, Schwartz Marlene, Brownell Kelly. 2017. “Food Swamps Predict Obesity Rates Better Than Food Deserts in the United States.” International Journal of Environmental Research and Public Health 14(11):1366–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Dajun. 2010. “Black Residential Segregation, Disparities in Spatial Access to Health Care Facilities, and Late-Stage Breast Cancer Diagnosis in Metropolitan Detroit.” Health & Place 16(5):1038–52. [DOI] [PubMed] [Google Scholar]
- Dilworth-Anderson Peggye, Williams Ishan Canty, Gibson Brent. 2002. “Issues of Race, Ethnicity, and Culture in Caregiving Research: A 20-Year Review (1980–2000).” The Gerontologist 42(2):237–72. [DOI] [PubMed] [Google Scholar]
- Dinwiddie Gniesha, Gaskin Darrell, Chan Kitty, Norrington Janette, McCleary Rachel. 2013. “Residential Segregation, Geographic Proximity and Type of Services Used: Evidence for Racial-Ethnic Disparities in Mental Health.” Social Science & Medicine 80:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnam Juan Pablo, Cai Mandi. 2021. “Advocates Worry Vaccines Will Be out of Reach for Black and Hispanic Neighborhoods Devastated by COVID-19.” The Texas Tribune, January9. https://www.texastribune.org/2021/01/09/texas-coronavirus-vaccine-racial-inequality/.
- Gaskin Darrell J., Dinwiddie Gniesha, Chan Kitty, McCleary Rachael. 2012. “Residential Segregation and the Availability of Primary Care Physicians.” Health Services Research 47(6):2353–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin Darrell J., Price Adrian, Brandon Dwayne, LaVeist Thomas. 2009. “Segregation and Disparities in Health Services Use.” Medical Care Research and Review 66(5):578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godoy Maria, Wood Daniel. 2020. “What Do Coronavirus Racial Disparities Look like State by State?” National Public Radio, May30. https://www.npr.org/sections/health-shots/2020/05/30/865413079/what-do-coronavirus-racial-disparities-look-like-state-by-state.
- Gordon-Larsen Penny, Nelson Melissa, Page Phil, Popkin Barry. 2006. “Inequality in the Built Environment Underlies Key Health Disparities in Physical Activity and Obesity.” Pediatrics 117(2):417–24. [DOI] [PubMed] [Google Scholar]
- Grady Sue. 2006. “Racial Disparities in Low Birthweight and the Contribution of Residential Segregation: A Multilevel Analysis.” Social Science & Medicine 63(12):3013–29. [DOI] [PubMed] [Google Scholar]
- Harper Karen Brooks. 2021. “As Vaccine Eligibility Widens, Some Vulnerable Texans Are Still Fighting for Access.” The Texas Tribune, March31. https://www.texastribune.org/2021/03/31/vaccine-eligibility-texas/.
- Hart Kevin, Kunitz Stephen, Sell Ralph, Mukamel Dana. 1998. “Metropolitan Governance, Residential Segregation, and Mortality among African Americans.” American Journal of Public Health 88(3):434–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelejian Harry, Prucha Ingmar. 2010. “Specification and Estimation of Spatial Autoregressive Models with Autoregressive and Heteroskedastic Disturbances.” Journal of Econometrics 157(1):53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko Michelle, Needleman Jack, Derose Kathryn Pitkin, Laugesen Miriam, Ponce Ninez. 2014. “Residential Segregation and the Survival of U.S. Urban Public Hospitals.” Medical Care Research and Review 71(3):243–60. [DOI] [PubMed] [Google Scholar]
- Ko Michelle, Ponce Ninez. 2013. “Community Residential Segregation and the Local Supply of Federally Qualified Health Centers.” Health Services Research 48(1):253–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Min-Ah A.2009. “Neighborhood Residential Segregation and Mental Health: A Multilevel Analysis on Hispanic Americans in Chicago.” Social Science & Medicine 68(11):1975–84. [DOI] [PubMed] [Google Scholar]
- Martinez A.2021. “The Racial Disparities, Systemic Racism Behind Who Has Received Vaccines.” National Public Radio, March18. https://www.npr.org/2021/03/18/978496045/the-racial-disparities-systemic-racism-behind-who-has-received-vaccines.
- Marwell Nicole, Gullickson Aaron. 2013. “Inequality in the Spatial Allocation of Social Services: Government Contracts to Nonprofit Organizations in New York City.” Social Service Review 87(2):319–53. [Google Scholar]
- Massey Douglas, Denton Nancy. 1988. “The Dimensions of Residential Segregation.” Social Forces 67(2):281–315. [Google Scholar]
- McFarland Michael, Smith Cheryl. 2011. “Segregation, Race, and Infant Well-Being.” Population Research and Policy Review 30(3):467–93. [Google Scholar]
- McMinn Sean, Carlsen Audrey, Jaspers Bret, Talbot Ruth, Adeline Stephanie. 2020. “In Large Texas Cities, Access to Coronavirus Testing May Depend on Where You Live.” National Public Radio, May27. https://www.npr.org/sections/health-shots/2020/05/27/862215848/across-texas-black-and-hispanic-neighborhoods-have-fewer-coronavirus-testing-sit.
- Menchaca Megan, Agnew Duncan. 2021. “Texas’ Decentralized Internet Reliant System for Vaccine Appointments Leaves Many Eligible People Unable to Access a Shot.” The Texas Tribune, March20. https://www.texastribune.org/2021/03/20/texas-coronavirus-vaccine-difficult/.
- Miller William, Peek Monica, Parker William. 2020. “Scarce Resource Allocation Scores Threaten to Exacerbate Racial Disparities in Health Care.” CHEST 158(4):1332–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millett Gregorio, Jones Austin, Benkeser David, Baral Stefan, Mercer Laina, Beyrer Chris, Honermann Brian, et al. 2020. “Assessing Differential Impacts of COVID-19 on Black Communities.” Annals of Epidemiology 47:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore Latetia, Diez Roux Ana. 2006. “Associations of Neighborhood Characteristics with the Location and Type of Food Stores.” American Journal of Public Health 96(2):325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell Heather. 2015. “Where There’s Smoke: Cigarette Use, Social Acceptability, and Spatial Approaches to Multilevel Modeling.” Social Science & Medicine 140:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oladipo Gloria. 2021. “How Chicago’s Vaccine Rollout Is Inhibited by Longstanding Inequality.” The Guardian, February5. https://www.theguardian.com/us-news/2021/feb/05/chicago-blacks-latinos-vaccine-distribution?utm_term=2bd88cc02b2faffa886b221bdd480dc4&utm_campaign=GuardianTodayUS&utm_source=esp&utm_medium=Email&CMP=GTUS_email.
- Økland Helene, Mamelund Svenn-Erik. 2019. “Race and 1918 Influenza Pandemic in the United States: A Review of the Literature.” International Journal of Environmental Research and Public Health 16(14):2487–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek Monica, Simons Russell, Parker William, Ansell David, Rogers Selwyn, Edmonds Brownsyne Tucker. 2021. “COVID-19 among African Americans: An Action Plan for Mitigating Disparities.” American Journal of Public Health 111(2):286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persad Govind, Peek Monica, Emanuel Ezekiel. 2020. “Fairly Prioritizing Groups for Access to COVID-19 Vaccines.” JAMA 324(16):1601–1602. [DOI] [PubMed] [Google Scholar]
- Prescott Gina Marie, Prescott William Allan. 2021. “Health Information Technology Utilization and Impact on COVID-19 Vaccination.” Journal of the American Pharmacists Association 61(40):E230–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos Elliot, Zamudio María Inés. 2020. “In Chicago, 70% of COVID-19 Deaths Are Black.” WBEZ Chicago, April5. https://www.wbez.org/stories/in-chicago-70-of-covid-19-deaths-are-black/dd3f295f-445e-4e38-b37f-a1503782b507.
- Raudenbush Stephen, Bryk Anthony. 2002. Hierarchical Linear Models: Applications and Data Analysis Methods. Thousand Oaks, CA: SAGE Publications. [Google Scholar]
- Reardon Sean, O’Sullivan David. 2004. “Measures of Spatial Segregation.” Sociological Methodology 34:121–62. [Google Scholar]
- Roberto Elizabeth. 2018. “The Spatial Proximity and Connectivity Method for Measuring and Analyzing Residential Segregation.” Sociological Methodology 48(1):182–224. [Google Scholar]
- Roberts Jennifer, Tehrani Shadi. 2020. “Environments, Behaviors, and Inequalities: Reflecting on the Impacts of the Influenza and Coronavirus Pandemics in the United States.” International Journal of Environmental Research and Public Health 17(12):4484–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Rudolph, Sen Saunak, Mehta Kala, Moody-Ayers Sandra, Bacchetti Peter, O’Hare Ann. 2007. “Geography Matters: Relationships among Urban Residential Segregation, Dialysis Facilities, and Patient Outcomes.” Annals of Internal Medicine 146(7):493–501. [DOI] [PubMed] [Google Scholar]
- Ross Janell. 2021. “When a Texas County Tried to Ensure Racial Equity in COVID-19 Vaccinations, It Didn’t Go as Planned.” Time Magazine, March2. https://time.com/5942884/covid-19-vaccine-racial-inequity-dallas/.
- Sampson Robert, Raudenbush Stephen, Earls Felton. 1997. “Neighborhoods and Violent Crime: A Multilevel Study of Collective Efficacy.” Science 277(5328):918–24. [DOI] [PubMed] [Google Scholar]
- Savitz Natalya Verbitsky, Raudenbush Stephen. 2009. “Exploiting Spatial Dependence to Improve Measurement of Neighborhood Social Processes.” Sociological Methodology 39(1):151–83. [Google Scholar]
- Singer Judith, Willett John. 2003. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York, NY: Oxford University Press. [Google Scholar]
- Small Mario, McDermott Monica. 2006. “The Presence of Organizational Resources in Poor Urban Neighborhoods: An Analysis of Average and Contextual Effects.” Social Forces 84(3):1697–724. [Google Scholar]
- Stone Will. 2021. “‘Just Cruel’: Digital Race For COVID-19 Vaccines Leaves Many Seniors Behind.” National Public Radio, February4. https://www.npr.org/sections/health-shots/2021/02/04/963758458/digital-race-for-covid-19-vaccines-leaves-many-seniors-behind.
- Strully Kate. 2011. “Health Care Segregation and Race Disparities in Infectious Disease: The Case of Nursing Homes and Seasonal Influenza Vaccinations.” Journal of Health and Social Behavior 52(4):510–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler Waldo. 1970. “A Computer Movie Simulating Urban Growth in the Detroit Region.” Economic Geography 46(Suppl. 1):234–40. [Google Scholar]
- Walker Renee, Keane Christopher, Burke Jessica. 2010. “Disparities and Access to Healthy Food in the United States: A Review of Food Deserts Literature.” Health & Place 16(5):876–84. [DOI] [PubMed] [Google Scholar]
- Williams David, Collins Chiquita. 2001. “Racial Residential Segregation: A Fundamental Cause of Racial Disparities in Health.” Public Health Reports 116(5):404–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Tse-Chuan, Choi Seung-won Emily, Sun Feinuo. 2021. “COVID-19 Cases in U.S. Counties: Roles of Racial-Ethnic Density and Residential Segregation.” Ethnicity & Health 26(1):11–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-hsb-10.1177_00221465221074915 for Racial-Ethnic Residential Clustering and Early COVID-19 Vaccine Allocations in Five Urban Texas Counties by Kathryn Freeman Anderson and Darra Ray-Warren in Journal of Health and Social Behavior


