Abstract
Mucin 1 (MUC1) was the first discovered transmembrane protein of the mucin family; it normally covers epithelial cells of the mucous membrane, providing lubrication and protection. However, aberrant expression of MUC1 is involved in cancer development, invasion and metastasis. It has been reported that MUC1 upregulation is highly associated with the progression of different epithelial cancer types, such as lung, liver, pancreatic and breast cancer. Therefore, MUC1 can be used as a specific marker and a target for immunotherapy in clinical applications, and the detection of MUC1 expression levels can be used to diagnose the occurrence, metastasis, prognosis and recurrence of cancer. The present review summarizes the abnormal expression of MUC1 in different tumours and discusses its clinical significance, thereby highlighting the potential diagnostic and therapeutic significance of MUC1 in cancer.
Keywords: MUC1, tumour antigen, immunotherapy
1. Introduction
Mucins are a family of high-molecular weight glycoproteins. In recent years, accumulating evidence has demonstrated that mucins play an important role in the initiation and progression of tumours (1). Therefore, research on the members of the mucin family has become a hot spot in the field of tumour immunotherapy. Among them, Mucin 1 (MUC1) is widely studied for its role in the pathogenesis of various cancer types, and it is the most intensively studied transmembrane protein of the mucin family. In general, abnormal expression of the MUC1 oncogene is associated with the progression of malignant tumours. For example, studies have found that the high expression of MUC1 is associated with the survival and prognosis of patients with lung, gastric, colorectal and pancreatic cancer (2-4). Thus, MUC1 is potentially a valid marker for the clinical diagnosis of tumours and an important antigen for targeted therapy (5).
2. Distribution, structure and biological characteristics of MUC1
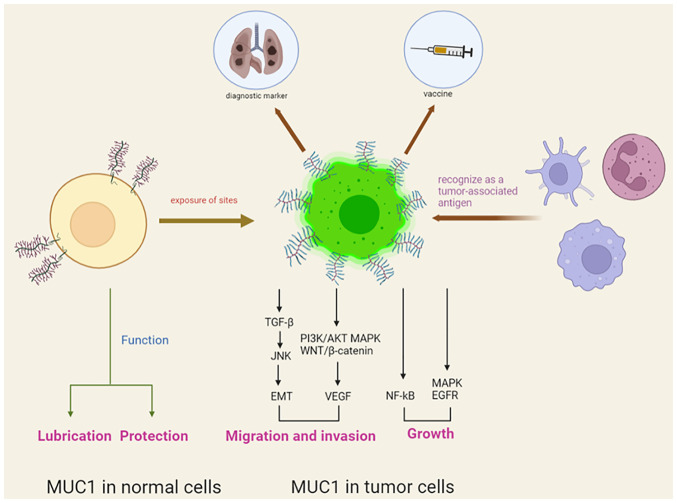
In healthy tissues, MUC1 is expressed on the proximal luminal surface or proximal glandular surface of glandular epithelial cells, and its expression is characterized by apical expression, polar distribution and complete glycosylation. In diseased tissues, the upregulation of MUC1 together with loss of polarization and exposure of sites originally covered by glycan chains leads to the recognition of MUC1 as a tumour-associated antigen (TAA) by the immune system, indicating that it is a target for immunotherapy (6,7).
MUC1 is a high-molecular weight transmembrane glycoprotein, with a molecular weight of 300-600 kD, that is composed of two subunits, including the extracellular amino-terminal subunit (MUC1-N) and the transmembrane carboxy-terminal subunit (MUC1-C) (8). These two subunits are linked by non-covalent bonds to form a heterodimeric complex in the cell membrane and can also be spontaneously hydrolysed into two subunits. MUC1-N consists of 20-amino acid variable number tandem repeats, which are rich in amino acid residues and can be highly glycosylated. MUC1-C is a transmembrane subunit containing extracellular, transmembrane and intracellular domains (9). The cytoplasmic tail of MUC1 (MUC1-CT) consists of an extracellular domain of 58 amino acids, a transmembrane domain of 28 amino acids and an intracellular domain of 72 amino acids. MUC1-CT is highly conserved among different species and plays an important role in numerous processes, such as signal transduction and intercellular interactions (10).
MUC1 has a lubricating and protective function in normal mucosal epithelial cells. However, upregulation of MUC1 promotes tumour progression by affecting multiple signalling pathways, as well as by regulating the proliferation and epithelial mesenchymal transition of tumourcells. Therefore, MUC1 is considered a vital oncogene that regulates the developmental processes of cancer. It has been shown that MUC1 promotes cell growth and the proliferation of cancer by regulating PI3K/AKT, MEK/ERK, p53, nuclear factor-κB, epidermal growth factor receptor, WNT/β-catenin and JNK/TGF-β (3,11,12). MUC1 also regulates tumour cell invasion and metastasis by interacting with E-calmodulin and intercellular adhesion molecules (13,14). In addition, MUC1 promotes tumour angiogenesis and accelerates the invasion and metastasis of tumours by stimulating the expression of proangiogenic factors, such as vascular endothelial growth factor (4,15). Taken together, these results show that the MUC1 oncogene plays an important role in cancer development (Fig. 1).
Figure 1.
Different functions of MUC1 in normal or tumour cells. MUC1, mucin 1; NF-κΒ, nuclear factor κB; EMT, epithelial-mesenchymal transition; EGFR, epidermal growth factor receptor; VEGF, vascular endothelial growth factor.
3. MUC1 expression in different tumours and its clinical significance
It has been reported that the MUC1 oncogene is commonly overexpressed in various epithelial adenocarcinomas, such as lung, liver, pancreatic, breast and ovarian cancer (10). The expression level of MUC1 in different cancer types is a key factor for the application of MUC1 in clinical diagnosis and treatment. The present review assesses the clinical significance of MUC1 by assembling cutting-edge data about the expression of MUC1 in different tumours.
Expression of MUC1 in lung cancer
Lung cancer has become the most common cause of cancer death worldwide. It has been reported that the mucin family, especially MUC1, plays an important role in the progression of lung cancer, and various vaccines for lung cancer targeting MUC1 are in clinical trials (16,17).
Approximately 85% of patients with lung cancer have the same histological subtype, namely, non-small cell lung cancer (NSCLC) (18). MUC1 is apically expressed and polar in normal tissues. However, in malignant tumours, the distribution of MUC1 loses its polarity and shows abnormal depolarization expression on the surface of the entire tumour cell, which is often associated with a poor prognosis (6,19). A clinical study reported that among 126 patients with non-small cell lung cancer, the 5-year survival rates of patients in the MUC1 depolarized expression group (percentage of tumour cells with depolarized MUC1 expression >10%), the low-grade polarized expression group (polarized MUC1 expression <50% and depolarized MUC1 expression <10%) and the high-grade polarized expression group (polarized MUC1 expression >50% and depolarized MUC1 expression <10%) were 43.9, 61.5 and 79.4%, respectively (19). These findings suggest that the depolarized expression of MUC1 in tumour cells is significantly associated with poor outcome in patients with lung cancer.
Lung adenocarcinoma and lung squamous cell carcinoma are the most common types of NSCLC. In a previous study that included 178 patients with stage IB NSCLC, the percentages of high MUC1 expression in patients with lung adenocarcinoma and lung squamous carcinoma were 86.3 and 39.1%, respectively (20). These findings suggest that MUC1 plays an important role in the progression of lung adenocarcinoma. MUC1-Tn antigen is an oversimplified mucin-1 O-glycan that is overexpressed in different cancer types. For example, the study found that MUC1-Tn was abnormally expressed in breast, lung and gastric cancer, among others, and may therefore be a target for cancer diagnosis (21). Therefore, the expression of MUC1-Tn in cancer tissues should be screened. In a previous study, the results of immunohistochemical analysis of 175 lung adenocarcinoma tissues showed that high MUC1-Tn (MUC1 glycoconjugate antigen) expression was observed in 44 (25.1%) specimens and was associated with patient sex (male patients, 33.3%; female patients, 17.6%), smoking history (ex-smoker, 31.8%; non-smoker, 12.5%), tumour stage (T1a-c stage, 16.2%; T2a-b+T3+T4 stage, 40.6%) and pleural invasion (positive, 40%; negative, 20%) (22). Another study suggested that the abnormal expression of MUC1 was correlated with the poor prognosis of patients with lung adenocarcinoma (23). Results indicate the potential utility of MUC1 as a clinical diagnostic marker and therapeutic target for lung adenocarcinoma through the contributing role in pathological features, such as development and invasive metastasis (24).
As it is difficult to obtain tumour tissues for clinical analysis from patients with lung cancer who have contraindications to surgery, blood markers are needed to predict the degree of cancer disease progression and chemotherapy efficacy in patients with advanced disease. It has been suggested that MUC1 can be used as a marker of circulating tumour cells (CTCs) in the peripheral blood of patients with NSCLC (25). A previous study on 66 patients with advanced NSCLC showed that MUC1 mRNA expression in the peripheral blood of patients treated for 4 weeks (relative expression, 4.46) was significantly lower than that in the patients before treatment (relative expression, 5.95) (26). In this previous study, the researchers also set 4.2 as the threshold of positivity for MUC1 mRNA in the peripheral blood of patients with NSCLC on the basis of the association between the level of MUC1 mRNA and pathological features. According to this, the positive expression of MUC1 in patients before and after 4 weeks of gefitinib treatment was 75.8% (50/66) and 45.5% (30/66), respectively, and the follow-up revealed that the survival time of MUC1-positive patients was significantly shorter than that of MUC1-negative patients. These findings suggest that peripheral blood MUC1 mRNA can be used to assess the therapeutic efficacy of gefitinib for patients with NSCLC. Another study also found that MUC1 could be used as a marker of CTCs in patients with NSCLC (27). Therefore, MUC1 mRNA in peripheral blood is expected to provide significant guidance for adjusting treatment regimens for patients with advanced disease.
Expression of MUC1 in breast cancer
Breast cancer is the most common cancer in women. The early diagnosis and treatment of patients with breast cancer is often poor, as the pathogenesis of the disease is still not clear.
Triple-negative breast cancer (TNBC) is a subtype of breast tumour lacking oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 expression. TNBC is difficult to treat and has a high mortality rate due to a lack of therapeutic target molecules (28). A previous clinical study showed that 49 out of 52 (94.2%) cancer tissues of patients with TNBC were positive for MUC1 expression (29). These findings suggest that MUC1 is an ideal target of tumour immunotherapy for TNBC. MUC1 promotes the expression of programmed death ligand 1 in TNBC cells, resulting in increased immune escape function and invasiveness of tumour cells (30). MUC1has been suggested to be a potential target to inhibit the development and progression of TNBC (31).
In addition, MUC1 has great potential for the rapid diagnosis of breast cancer. A previous clinical study showed that the expression levels of serum and salivary IgG anti-MUC1 in patients with breast cancer were higher than those in healthy women (P<0.001) (32), suggesting that the levels of autoantibodies against MUC1 in the serum and saliva of patients with breast cancer may provide references in cancer screening. Researchers have used quantitative (q)PCR to detect the mRNA level of MUC1 in the blood from healthy volunteers mixed with breast cancer cells (MCF-7) to simulate the CTCs of breast cancer, which indicated that this method had a sensitivity of detecting 1 CTC among 1x106-107 white blood cells and a high specificity of 96.8% (detected 1 MUC1-positive individual among 30 healthy volunteers) (33). The qPCR method also found that after the first cycle of chemotherapy, the treatment efficiency in patients lacking MUC1 expression (60%; 6/10) was higher than that in MUC1-positive patients (12.5%; 3/24) (33). These results suggest that MUC1 can provide clinical significance in the diagnosis of CTCs and the prediction of chemotherapy efficacy for patients with breast cancer. Other studies have indicated that upregulation of the MUC1 gene is associated with a poor prognosis in patients with breast cancer (34,35). MUC1 may also be a valid target for predicting and improving the prognosis of patients.
Expression of MUC1 in ovarian cancer
Ovarian cancer is one of the most lethal gynaecological malignancies, and the prognosis of affected patients is often poor due to its inconspicuous early symptoms and its tendency to invade surrounding organs (36). The MUC1 oncogene is involved in the progression, metastasis and drug resistance of ovarian cancer cells (37).
The immunohistochemical results of a clinical study revealed that all tumour specimens were MUC1-positive (19/19; 100%), with 47.4% (9/19) of these cases expressing high MUC1 levels (+++) in more than one-half of the cancer tissues (positive staining of cancer tissue >50%), and another 52.6% (10/19) of these cases expressing very high MUC1 levels (++++) (38). Another study on 60 primary ovarian cancer paraffin-embedded and sectioned tissue specimens showed that the positive expression rate of MUC1 was 95.0% (57/60), and the high expression rate of MUC1 was associated with tumour stage and postoperative residual tumour tissue (39). It has been suggested that MUC1 is involved in the progression of ovarian tumours and the poor prognosis of patients. Therefore, the MUC1 gene has great potential in the clinical diagnosis and treatment of patients with ovarian cancer (40). The intracellular segment of MUC1 (MUC1-CT) has also been reported as a potential site for ovarian cancer immunotherapy (41).
Expression of MUC1 in cholangiocarcinoma
The clinical outcome of patients with cholangiocarcinoma is generally poor, as cholangiocarcinoma cells are highly invasive and prone to lymph node and vascular metastasis (42).
In a previous study, an immunohistochemical staining analysis was performed to assay cancer tissues from 85 patients with cholangiocarcinoma. The study reported that the positive staining rate of MUC1 was 65.9% (56/85), and the positive expression rate of MUC1 was associated with tumour differentiation (poorly differentiated, 91%; moderately differentiated, 84%; highly differentiated, 43%), tumour stage (T1 stage, 50%; ≥T2 stage, 80%), neurological invasion (positive, 83%; negative, 57%) and patient survival time (median survival time of MUC1-positive patients, 29.21 months; median survival time of MUC1-negative patients, 56.48 months) (43). The results of another study showed that the positive immunohistochemical staining rate of MUC1 in the cholangiocarcinoma tissues of 25 patients was 44% (11/25) (44). Thus, these findings suggested that the upregulation of MUC1 plays an important role in the progression of cholangiocarcinoma and is expected to be a valid marker for the diagnosis of affected patients.
Cholangiocarcinoma can be divided into intrahepatic cholangiocarcinoma and extrahepatic cholangiocarcinoma depending on the site. One study showed that the positive expression rate of MUC1 was 25, 57 and 80 in patients with three staging types of intrahepatic cholangiocarcinoma, namely, intraductal, peri-biliary infiltrative and mass type, respectively (43). These results suggest that the abnormal expression of MUC1 has clinical value in the diagnosis of intrahepatic cholangiocarcinoma of different stages. Survival analysis of 61 patients with intrahepatic cholangiocarcinoma revealed that the median survival time of the MUC1 low expression group (qPCR: MUC1/GAPDH <0.056) was significantly higher (55.06 months) than that of the MUC1 high expression group (17.25 months); the overall survival rates at 1, 3 and 5 years were 73, 57 and 45%, respectively, for patients in the MUC1 low expression group, whereas the overall survival rates for the same periods in the MUC1 high expression group were 35, 26 and 20%, respectively, with a significantly higher recurrence rate in the MUC1 high expression group (54.8%) than in the low expression group (30.0%) (45). Another study found that, in 50 tissues of mass-type intrahepatic cholangiocarcinoma, the positive expression rate of MUC1 was 76.0% (38/50), and the abnormal expression of MUC1 was a prognosis-related risk factor for the disease (P=0.0011) (46). These findings suggest that MUC1 is associated with a low survival time and high recurrence rate in patients with intrahepatic cholangiocarcinoma, which may be a valid predictor of prognostic status for these patients.
Another study demonstrated that the positive expression rate of MUC1 in the intraductal and peribiliary infiltrative types of extrahepatic cholangiocarcinoma was 47 and 85% (P=0.006), respectively (43), suggesting that MUC1 is also differentially expressed in different stages of extrahepatic cholangiocarcinoma, which is expected to assist in the diagnosis, prognosis prediction and treatment of cholangiocarcinoma in clinical practice (47).
Expression of MUC1 in gallbladder cancer
Gallbladder cancer has insidious symptoms in the early stage and is prone to invade other surrounding organs; therefore, patients are often in the middle and late stages when they are diagnosed, resulting in poor treatment outcomes (48).
MUC1 has been found to be strongly positively expressed in primary cancer cells derived from the ascites of patients with gallbladder cancer (49). A study collected 629 specimens from patients with gallbladder cancer for analysis, and the results showed that the positive expression rate of MUC1 in gallbladder cancer tissue specimens was 85.71% (18/21), which was significantly higher than its expression level in 605 patients with non-neoplastic gallbladder disease (5.29%; 32/605) (50).
Gallbladder adenocarcinoma is the most common type of gallbladder cancer in clinical practice, accounting for 85% of all gallbladder cancer cases. One study showed that the positive expression of MUC1 in the cancerous tissues of 108 patients with gallbladder adenocarcinoma (57.4%; 62/108) was significantly higher than its expression in paraneoplastic tissues (21.7%; 10/46), chronic cholecystitis (5.7%, 2/35) and adenomatous polyps (20.0%; 3/15) (51). These results suggest that MUC1 has a high specificity and accuracy in the diagnosis of gallbladder cancer, especially gallbladder adenocarcinoma, and that it can be used as a marker for clinical diagnosis. Another study also indicated that the positive expression rate of MUC1 was correlated with tumour size (tumour maximum diameter <2 cm, 38.7%; maximum diameter ≥2 cm, 64.9%; P<0.05), tumour stage (T1 stage, 28.6%; T2 stage, 42.9%; T3 stage, 59.5%; T4 stage, 77.3%; P<0.01) and lymph node metastasis (with lymph node metastasis, 69.5%; without metastasis, 42.9%; P<0.01) (51). These results further suggest that the abnormal expression of MUC1 is involved in promoting the progression and metastasis of gallbladder cancer, and that it may be of clinical significance in the diagnosis and prediction of clinical stage and metastasis for patients with gallbladder cancer.
Expression of MUC1 in bladder cancer
MUC1 plays a role in maintaining mucosal integrity and inhibiting urinary bacterial invasion in the normal urinary epithelium, and its abnormal expression is involved in promoting the progression and metastasis of bladder cancer in cancerous tissues (52).
A previous study showed that the positive expression rate of MUC1 was 61.8% (333/539) in tissue specimens from patients with bladder cancer, and its positive expression rate was associated with patient sex (female, 24.3%; male, 75.7%; P=0.044) and tumour pathological grade (high grade, 50.8%; low grade, 49.2%; P=0.013) (53). A study on 97 bladder cancer tissue specimens found that the positive expression rate of MUC1 was 89.7% (87/97); of these specimens, 57 had >50% tumour cells positive for MUC1, indicating strong positive expression of MUC1(54). Similarly, another study found an increasing trend of MUC1 expression in normal bladder mucosa, benign bladder disease and bladder tumours (55). It is suggested that MUC1 is involved in the development and drug resistance of bladder cancer (56). Thus, MUC1 may be an important clinical marker and targeted therapeutic molecule due to its high accuracy in the diagnosis of bladder cancer.
Expression of MUC1 in hepatocellular carcinoma
Hepatocellular carcinoma is one of the most common malignancies in clinical practice. The high aggressiveness and recurrence rate of this disease are important reasons for the low survival rate of patients (57).
One study showed that the positive expression rate of MUC1 in 96 patients with primary hepatocellular carcinoma was 77.1% (74/96), 68 specimens of which had strong positive immunohistochemical staining (++; positive cells >25%); MUC1 was only weakly positively expressed (+; positive cells <25%) in 20.0% (4/20) of patients with cirrhosis and there was no MUC1 expression in 10 normal liver tissue samples (58). In the aforementioned specimens of liver cancer, the positive expression rate of MUC1 was correlated with the degree of tumour differentiation (positive expression rate of MUC1: 53% highly differentiated, 85% moderately differentiated and 92% hypofractionated; strong positive expression rate: 47% highly differentiated, 80% moderately differentiated and 85% hypofractionated) and lymph node metastasis (positive, 90%; negative, 61%), suggesting that MUC1 is involved in the development and metastasis of hepatocellular carcinoma.
Another study found that the positive expression rate of MUC1 in 186 hepatocellular carcinoma specimens was 45.7% (85/186), and its positive expression rate was correlated with tumour differentiation (P=0.0001) and lymph node metastasis (P=0.0419) (59). A similar study showed that MUC1 was differentially expressed in hepatocellular carcinoma and paraneoplastic tissues, and that its expression rate was correlated with low patient survival rates (60). These findings suggest that MUC1 may be a diagnostic marker and a potential therapeutic target for hepatocellular carcinoma.
Expression of MUC1 in thyroid cancer
A previous report showed that MUC1 was positively expressed in 75.8% (219/289) of 289 patients with thyroid cancer, which was significantly higher than its expression level in non-malignant thyroid tissue (28/121; 23.1%) (61). Another study showed that the positive expression of MUC1 was significantly higher in thyroid cancer tissues (78.3%) than in paraneoplastic (24%) and normal (10%) tissues, and that its expression was associated with tumour stage (stage III+IV, 92.9%; stage I+II, 65.6%) and lymph node metastasis (positive, 90.5%; negative, 50%) (62). A previous study also showed that the positive expression rate of MUC1 in thyroid cancer was 77.6%, and its expression was associated with peripheral tumour invasion (P=0.035) and lymph node metastasis (P=0.013) (63). These results suggest that MUC1 is involved in the development and metastatic invasion of thyroid cancer, indicating that it is a potential specific marker for clinical diagnosis.
Thyroid cancer is classified into papillary, follicular, undifferentiated and medullary carcinoma (64). RT-PCR analysis revealed that the expression of MUC1 in papillary thyroid cancer tissues was significantly higher than that in follicular carcinoma (P<0.05) (65), suggesting that there may be variability in the expression of MUC1 in different types of thyroid carcinoma. Additional clinical data are required to support these findings.
Expression of MUC1 in colorectal cancer
Colorectal cancer is one of the most common malignancies in China, and is prone to occur in the sigmoid colon and rectum. In recent years, a number of reports have confirmed that MUC1 may be a dominant antigen for the diagnosis and targeted therapy for colorectal cancer (66,67).
A previous study showed that the positive expression rate of MUC1 was 55.6% (25/45) in the cancer tissues of 45 patients with colorectal cancer, but that this rate was 0.0% in paracancerous tissues (0/20); the expression rate in cancer tissues was significantly correlated with lymph node metastasis (with metastasis, 84.2%; without metastasis, 34.6%) (68). Another study reported that the levels of MUC1 mRNA in colorectal cancer tissues were significantly higher than those in normal tissues (P=0.004) (69). These results suggest that MUC1 is involved in colorectal carcinogenesis and metastasis, indicating that MUC1 has a high specificity and accuracy in the diagnosis and prediction of colorectal cancer.
Another study showed that the positive expression rate of MUC1 in 202 colorectal cancer specimens (43.6%; 88/202) was significantly higher than its positive expression rate in 202 normal colorectal mucosa specimens (8.9%; 18/202), and the positive expression rate of MUC1 showed a significant increasing trend in tumour stages I (20%), II (35%), III (54%) and IV (60%) (P=0.006) (70). In addition, the expression rate of MUC1 was also associated with lymph node metastasis in patients (N0, 31%; N1, 61%; N2, 47%; N3, 100%) (70). These studies suggest that the abnormal expression of MUC1 is involved in the occurrence, development and invasive metastasis of colorectal cancer (71), indicating that MUC1 may aid in the clinical diagnosis of progression and postoperative metastasis. In addition, MUC1 may also serve as a specific target for colorectal cancer drugs or vaccines (72).
Expression of MUC1 in cervical cancer
Cervical cancer is a malignant disease caused by human papillomavirus infection, and it is divided into two main histological types, namely, cervical adenocarcinoma and cervical squamous carcinoma. The incidence of cervical adenocarcinoma is higher than that of cervical squamous carcinoma and the prognosis of patients with cervical adenocarcinoma is even worse than that of patients with cervical squamous carcinoma (73).
Among 52 patients with cervical adenocarcinoma, a study showed that the positive expression rate of MUC1 was 59.6% (31/52), which was associated with cervical cancer stage (FIGO stage 1a, 33%; FIGO stage 1b1, 50%; FIGO stage 1b2, 67%; FIGO stage 2a, 63%; FIGO stage 2b, 100%), lymph node metastasis (positive, 91%; negative, 51%) and ovarian metastasis (positive, 100%; negative, 55%) (74). Thus, MUC1 is involved in the malignant process of cervical adenocarcinoma and may be a target molecule for diagnosis and treatment.
As cervical cancer cells are prone to metastasis to other organs (75), it is important to detect early metastases of cervical cancer for clinical treatment. qPCR analysis of 179 lymph nodes from 21 patients with primary cervical cancer revealed that the MUC1 mRNA levels in lymph nodes with a positive histological diagnosis of metastasis were significantly higher than those in lymph nodes with negative histology (P<0.001); the specificity and sensitivity of MUC1 for the diagnosis of cervical cancer lymph node metastasis was 78% (percentage of negative qPCR tests in 162 negative lymph nodes) and 76% (percentage of positive qPCR tests in 17 positive lymph nodes), respectively (76). These results suggest that MUC1 level indicates lymph node metastasis in cervical cancer and provides a reference for the extent of surgical lymph node dissection.
Expression of MUC1 in pancreatic cancer
Pancreatic cancer is one of the most rapidly progressing malignancies with the worst prognosis. Most pancreatic cancer patients have poor treatment outcomes due to missing the optimal time for surgery (77).
A previous study showed that abnormal upregulation of MUC1 can be detected in >60% of pancreatic cancer cases, which correlates with the poor prognosis of the patients. It has been suggested that MUC1 is involved in the development and progression of pancreatic cancer, and that it may be an important marker in the clinical diagnosis of the disease (78). In addition, the pro-cancer mechanism of MUC1 may be associated with its promotion of glucose metabolism in pancreatic cancer cells (79).
A previous study showed that among 101 patients with pancreatic cancer, the median survival time of the patients in the low MUC1 expression group (39.7 months) was significantly higher than that of patients in the high MUC1 expression group (13.4 months) (80). These findings indicated that MUC1 is associated with a poor prognosis and low survival time in patients with pancreatic cancer, suggesting that it may be an important target molecule to determine and improve the prognosis of patients.
4. Progress and perspectives
In summary, the MUC1 oncogene is aberrantly expressed in a variety of tumours, and its expression is mostly associated with tumour progression (Table I). Therefore, MUC1 may play an important role in the clinical diagnosis of tumours. Some researchers have attempted to use radiolabelled MUC1 antibodies or aptamers for targeted tumour imaging, which is expected to achieve a rapid diagnosis with high specificity when used in combination with traditional pathological biopsy methods (81). Studies have found that targeting the MUC1 antigen can be used for the radiographic diagnosis of breast cancer, which can be used to diagnose 90% of breast cancer cases, including TNBC (82,83). As CTCs also carry the MUC1 oncogene, the detection of MUC1 levels in the peripheral blood can also be applied in the clinical diagnosis of patients with cancer. The present review illustrates that the level of MUC1 in the peripheral blood of patients with NSCLC and breast cancer is associated with the treatment outcome and prognosis of patients, and that it is expected to be widely used in clinical practice in the future for the in vitro diagnosis of cancer, for chemotherapy efficacy assessment, and for postoperative recurrence and metastasis monitoring, due to its advantages of being an easy and non-invasive sampling method (26). Although, to the best of our knowledge, there are few related reports to date, the level of MUC1 mRNA in the patient serum is a significant reference for the initial diagnosis of tumours (84).
Table I.
Expression of MUC1 in different tumours.
| Cancer type | Positive expression rate of MUC1, % (n/total n) (Ref.) | Positive expression rate of MUC1 in patients with different stages, (%) (Ref.) | Overall survival time, months |
|---|---|---|---|
| Lung adenocarcinoma | 86.3 (44/51) (20) | T1a-c, 16.2; T2a-b+T3+T4, 40.6(22) | |
| Breast cancer | 94.2 (49/52) (29) | ||
| Ovarian cancer | 100.0 (19/19) (38); 95.0 (57/60) (39) | ||
| Cholangiocarcinoma | 65.9 (56/85) (43) | Poorly differentiated, 91; moderately differentiated, 84; highly differentiated, 43; T1, 50; ≥T2, 80(43) | MUC1 positive, 29.21; MUC1 negative, 56.48(43) |
| Gallbladder carcinoma | 57.4 (62/108) (51) | T1, 28.6; T2, 42.9; T3, 59.5; T4, 77.3(51) | |
| Liver cancer | 77.1 (74/96) (58) | Poorly differentiated, 92; moderately differentiated, 85; highly differentiated, 53(58) | |
| Thyroid carcinoma | 78.3 (47/60) (62) | I+II, 65.6; III+IV, 92.9(62) | |
| Colorectal cancer | 43.6 (88/202) (70) | I, 20; II, 35; III, 54; IV, 60(70) | |
| Cervical carcinoma | 59.6 (31/52) (74) | FIGO 1a, 33; FIGO 1b1, 50; FIGO 1b2, 67; FIGO 2a, 63; FIGO 2b, 100(74) |
In tumour immunotherapy, the exposed glycosylation sites can be recognized by the immune system as TAAs. Therefore, MUC1 has been an important target for tumour vaccine design and development in recent years. Tumour vaccines based on MUC1 effectively prevent cancer progression and metastasis. At present, MUC1-based tumour vaccines mainly include DNA vaccines, dendritic cell (DC) vaccines, virus vaccines and subunit vaccines. A variety of MUC1 DNA vaccines have been developed to date. Studies have confirmed that the MUC1 DNA vaccine induces a specific immune response against MUC1, produces an obvious tumour suppression effect and prolongs patient survival time (85-87). In phase I/II clinical trials, MUC1-loaded DC vaccines in the combination therapy of patients with advanced pancreatic cancer enhanced the disease suppression rate and effectively extended the survival period (88,89). TG4010, which is a viral vaccine expressing MUC1 has attracted much attention in recent years. In a phase II clinical trial, the vaccine extended the survival period of patients with NSCLC and no serious adverse reactions were found. At present, TG4010 has entered phase III clinical trials and is expected to enhance the efficacy of radiotherapy and chemotherapy for patients with cancer (16,90). Studies have confirmed that MUC1 subunit vaccines enhance specific immune responses, and these vaccines are expected to enter clinical trials in the future (91,92). In addition, studies have confirmed that chimeric antigen receptor-T cells targeting MUC1 have good antitumour function in tumour models in vivo and in vitro, which may provide new strategies for tumour treatment (93-95). Although MUC1-based tumour vaccines have not been successfully applied to clinical treatment at present, MUC1 has great potential in tumour treatment. In the process of vaccine development, it is very important to design vaccines according to the expression characteristics of MUC1 in different tumor tissues. In addition, the safety and effectiveness of tumour vaccines still need to be further verified, especially for patients with advanced cancer whose T-cell functions are severely damaged. MUC1 is expected to generate new hope for the clinical diagnosis and treatment of a number of tumors in the near future.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
YL drafted the initial manuscript, and edited and critically revised the manuscript. WN contributed substantially in drafting the manuscript, and in editing and critically revising the manuscript for intellectual content. GT put forward the concept, critically revised the article for intellectual content, and was responsible for the organization, revision and submission of the manuscript. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wi DH, Cha JH, Jung YS. Mucin in cancer: A stealth cloak for cancer cells. BMB Rep. 2021;54:344–355. doi: 10.5483/BMBRep.2021.54.7.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu F, Liu F, Zhao H, An G, Feng G. Prognostic significance of mucin antigen MUC1 in various human epithelial cancers: A meta-analysis. Medicine (Baltimore) 2015;94(e2286) doi: 10.1097/MD.0000000000002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bose M, Grover P, Sanders AJ, Zhou R, Ahmad M, Shwartz S, Lala P, Nath S, Yazdanifar M, Brouwer C, Mukherjee P. Overexpression of MUC1 induces non-canonical TGF-β signaling in pancreatic ductal adenocarcinoma. Front Cell Dev Biol. 2022;10(821875) doi: 10.3389/fcell.2022.821875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khodabakhsh F, Merikhian P, Eisavand MR, Farahmand L. Crosstalk between MUC1 and VEGF in angiogenesis and metastasis: A review highlighting roles of the MUC1 with an emphasis on metastatic and angiogenic signaling. Cancer Cell Int. 2021;21(200) doi: 10.1186/s12935-021-01899-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Supruniuk K, Radziejewska I. MUC1 is an oncoprotein with a significant role in apoptosis (Review) Int J Oncol. 2021;59(68) doi: 10.3892/ijo.2021.5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W, Zhang Z, Zhang S, Zhu P, Ko JK, Yung KK. MUC1: Structure, function, and clinic application in epithelial cancers. Int J Mol Sci. 2021;22(6567) doi: 10.3390/ijms22126567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beatty PL, Finn OJ. Preventing cancer by targeting abnormally expressed self-antigens: MUC1 vaccines for prevention of epithelial adenocarcinomas. Ann N Y Acad Sci. 2013;1284:52–56. doi: 10.1111/nyas.12108. [DOI] [PubMed] [Google Scholar]
- 8.Guo M, You C, Dou J. Role of transmembrane glycoprotein mucin 1 (MUC1) in various types of colorectal cancer and therapies: Current research status and updates. Biomed Pharmacother. 2018;107:1318–1325. doi: 10.1016/j.biopha.2018.08.109. [DOI] [PubMed] [Google Scholar]
- 9.Cascio S, Finn OJ. Intra- and extra-cellular events related to altered glycosylation of MUC1 promote chronic inflammation, tumor progression, invasion, and metastasis. Biomolecules. 2016;6(39) doi: 10.3390/biom6040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao T, Cen Q, Lei H. A review on development of MUC1-based cancer vaccine. Biomed Pharmacother. 2020;132(110888) doi: 10.1016/j.biopha.2020.110888. [DOI] [PubMed] [Google Scholar]
- 11.Li W, Han Y, Sun C, Li X, Zheng J, Che J, Yao X, Kufe D. Novel insights into the roles and therapeutic implications of MUC1 oncoprotein via regulating proteins and non-coding RNAs in cancer. Theranostics. 2022;12:999–1011. doi: 10.7150/thno.63654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagiwara M, Fushimi A, Bhattacharya A, Yamashita N, Morimoto Y, Oya M, Withers HG, Hu Q, Liu T, Liu S, et al. MUC1-C integrates type II interferon and chromatin remodeling pathways in immunosuppression of prostate cancer. Oncoimmunology. 2022;11(2029298) doi: 10.1080/2162402X.2022.2029298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yasumizu Y, Rajabi H, Jin C, Hata T, Pitroda S, Long MD, Hagiwara M, Li W, Hu Q, Liu S, et al. MUC1-C regulates lineage plasticity driving progression to neuroendocrine prostate cancer. Nat Commun. 2020;11(338) doi: 10.1038/s41467-019-14219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosseinzadeh A, Merikhian P, Naseri N, Eisavand MR, Farahmand L. MUC1 is a potential target to overcome trastuzumab resistance in breast cancer therapy. Cancer Cell Int. 2022;22(110) doi: 10.1186/s12935-022-02523-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Utispan K, Koontongkaew S. Mucin 1 regulates the hypoxia response in head and neck cancer cells. J Pharmacol Sci. 2021;147:331–339. doi: 10.1016/j.jphs.2021.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Quoix E, Lena H, Losonczy G, Forget F, Chouaid C, Papai Z, Gervais R, Ottensmeier C, Szczesna A, Kazarnowicz A, et al. TG4010 immunotherapy and first-line chemotherapy for advanced non-small-cell lung cancer (TIME): Results from the phase 2b part of a randomised, double-blind, placebo-controlled, phase 2b/3 trial. Lancet Oncol. 2016;17:212–223. doi: 10.1016/S1470-2045(15)00483-0. [DOI] [PubMed] [Google Scholar]
- 17.Ning Y, Zheng H, Zhan Y, Liu S, Yang Y, Zang H, Luo J, Wen Q, Fan S. Comprehensive analysis of the mechanism and treatment significance of Mucins in lung cancer. J Exp Clin Cancer Res. 2020;39(162) doi: 10.1186/s13046-020-01662-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwan TY, Chowdhury EH. Clinical outcomes of chemotherapeutic molecules as single and multiple agents in advanced non-small-cell lung carcinoma (NSCLC) patients. Medicina (Kaunas) 2021;57(1252) doi: 10.3390/medicina57111252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaira K, Nakagawa K, Ohde Y, Okumura T, Takahashi T, Murakami H, Endo M, Kondo H, Nakajima T, Yamamoto N. Depolarized MUC1 expression is closely associated with hypoxic markers and poor outcome in resected non-small cell lung cancer. Int J Surg Pathol. 2012;20:223–232. doi: 10.1177/1066896911429296. [DOI] [PubMed] [Google Scholar]
- 20.Situ D, Wang J, Ma Y, Zhu Z, Hu Y, Long H, Rong T. Expression and prognostic relevance of MUC1 in stage IB non-small cell lung cancer. Med Oncol. 2011;28 (Suppl 1):S596–S604. doi: 10.1007/s12032-010-9752-4. [DOI] [PubMed] [Google Scholar]
- 21.Palladino P, Papi F, Minunni M, Nativi C, Scarano S. Structurally constrained MUC1-tn mimetic antigen as template for molecularly imprinted polymers (MIPs): A promising tool for cancer diagnostics. Chempluschem. 2022;87(e202200068) doi: 10.1002/cplu.202200068. [DOI] [PubMed] [Google Scholar]
- 22.Kato T, Ujiie H, Hatanaka KC, Nange A, Okumura A, Tsubame K, Naruchi K, Sato M, Kaga K, Matsuno Y, et al. A novel Tn antigen epitope-recognizing antibody for MUC1 predicts clinical outcome in patients with primary lung adenocarcinoma. Oncol Lett. 2021;21(202) doi: 10.3892/ol.2021.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie Q, Zhao S, Liu W, Cui Y, Li F, Li Z, Guo T, Yu W, Guo W, Deng W, Gu C. YBX1 enhances metastasis and stemness by transcriptionally regulating MUC1 in lung adenocarcinoma. Front Oncol. 2021;11(702491) doi: 10.3389/fonc.2021.702491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu S, Zhang X, Jiang Q, Liang T. Detection of circulating natural antibodies against CD25, MUC1, and VEGFR1 for early diagnosis of non-small cell lung cancer. FEBS Open Bio. 2020;10:1288–1294. doi: 10.1002/2211-5463.12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warawdekar UM, Sirajuddin MM, Pramesh CS, Mistry RC. An approach of selecting appropriate markers from the primary tumor to enable detection of circulating tumor cells in patients with non-small cell lung cancer. J BUON. 2015;20:782–790. [PubMed] [Google Scholar]
- 26.Li J, Hu YM, Du YJ, Zhu LR, Qian H, Wu Y, Shi WL. Expressions of MUC1 and vascular endothelial growth factor mRNA in blood are biomarkers for predicting efficacy of gefitinib treatment in non-small cell lung cancer. BMC Cancer. 2014;14(848) doi: 10.1186/1471-2407-14-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savarese-Brenner B, Heugl M, Rath B, Schweizer C, Obermayr E, Stickler S, Hamilton G. MUC1 and CD147 are promising markers for the detection of circulating tumor cells in small cell lung cancer. Anticancer Res. 2022;42:429–439. doi: 10.21873/anticanres.15501. [DOI] [PubMed] [Google Scholar]
- 28.Borri F, Granaglia A. Pathology of triple negative breast cancer. Semin Cancer Biol. 2021;72:136–145. doi: 10.1016/j.semcancer.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Siroy A, Abdul-Karim FW, Miedler J, Fong N, Fu P, Gilmore H, Baar J. MUC1 is expressed at high frequency in early-stage basal-like triple-negative breast cancer. Hum Pathol. 2013;44:2159–2166. doi: 10.1016/j.humpath.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maeda T, Hiraki M, Jin C, Rajabi H, Tagde A, Alam M, Bouillez A, Hu X, Suzuki Y, Miyo M, et al. MUC1-C induces PD-L1 and immune evasion in triple-negative breast cancer. Cancer Res. 2018;78:205–215. doi: 10.1158/0008-5472.CAN-17-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamashita N, Long M, Fushimi A, Yamamoto M, Hata T, Hagiwara M, Bhattacharya A, Hu Q, Wong KK, Liu S, Kufe D. MUC1-C integrates activation of the IFN-gamma pathway with suppression of the tumor immune microenvironment in triple-negative breast cancer. J Immunother Cancer. 2021;9(e002115) doi: 10.1136/jitc-2020-002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laidi F, Bouziane A, Errachid A, Zaoui F. Usefulness of salivary and serum auto-antibodies against tumor biomarkers HER2 and MUC1 in breast cancer screening. Asian Pac J Cancer Prev. 2016;17:335–339. doi: 10.7314/apjcp.2016.17.1.335. [DOI] [PubMed] [Google Scholar]
- 33.Cheng JP, Yan Y, Wang XY, Lu YL, Yuan YH, Jia J, Ren J. MUC1-positive circulating tumor cells and MUC1 protein predict chemotherapeutic efficacy in the treatment of metastatic breast cancer. Chin J Cancer. 2011;30:54–61. doi: 10.5732/cjc.010.10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jing X, Liang H, Hao C, Yang X, Cui X. Overexpression of MUC1 predicts poor prognosis in patients with breast cancer. Oncol Rep. 2019;41:801–810. doi: 10.3892/or.2018.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Liu L, Feng Z, Wang X, Huang Y, Dai H, Zhang L, Song F, Wang D, Zhang P, et al. Tumor markers CA15-3, CA125, CEA and breast cancer survival by molecular subtype: A cohort study. Breast Cancer. 2020;27:621–630. doi: 10.1007/s12282-020-01058-3. [DOI] [PubMed] [Google Scholar]
- 36.Gaitskell K, Hermon C, Barnes I, Pirie K, Floud S, Green J, Beral V, Reeves GK. Ovarian cancer survival by stage, histotype, and pre-diagnostic lifestyle factors, in the prospective UK million women study. Cancer Epidemiol. 2022;76(102074) doi: 10.1016/j.canep.2021.102074. Million Women Study Collaborators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma Q, Song J, Wang S, He N. MUC1 regulates AKT signaling pathway by upregulating EGFR expression in ovarian cancer cells. Pathol Res Pract. 2021;224(153509) doi: 10.1016/j.prp.2021.153509. [DOI] [PubMed] [Google Scholar]
- 38.Budiu RA, Mantia-Smaldone G, Elishaev E, Chu T, Thaller J, McCabe K, Lenzner D, Edwards RP, Vlad AM. Soluble MUC1 and serum MUC1-specific antibodies are potential prognostic biomarkers for platinum-resistant ovarian cancer. Cancer Immunol Immunother. 2011;60:975–984. doi: 10.1007/s00262-011-1010-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L, Ma J, Liu F, Yu Q, Chu G, Perkins AC, Li Y. Expression of MUC1 in primary and metastatic human epithelial ovarian cancer and its therapeutic significance. Gynecol Oncol. 2007;105:695–702. doi: 10.1016/j.ygyno.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Barani M, Bilal M, Sabir F, Rahdar A, Kyzas GZ. Nanotechnology in ovarian cancer: Diagnosis and treatment. Life Sci. 2021;266(118914) doi: 10.1016/j.lfs.2020.118914. [DOI] [PubMed] [Google Scholar]
- 41.Hu XF, Yang E, Li J, Xing PX. MUC1 cytoplasmic tail: A potential therapeutic target for ovarian carcinoma. Expert Rev Anticancer Ther. 2006;6:1261–1271. doi: 10.1586/14737140.6.8.1261. [DOI] [PubMed] [Google Scholar]
- 42.Elvevi A, Laffusa A, Scaravaglio M, Rossi RE, Longarini R, Stagno AM, Cristoferi L, Ciaccio A, Cortinovis DL, Invernizzi P, Massironi S. Clinical treatment of cholangiocarcinoma: An updated comprehensive review. Ann Hepatol. 2022;27(100737) doi: 10.1016/j.aohep.2022.100737. [DOI] [PubMed] [Google Scholar]
- 43.Park SY, Roh SJ, Kim YN, Kim SZ, Park HS, Jang KY, Chung MJ, Kang MJ, Lee DG, Moon WS. Expression of MUC1, MUC2, MUC5AC and MUC6 in cholangiocarcinoma: Prognostic impact. Oncol Rep. 2009;22:649–657. doi: 10.3892/or_00000485. [DOI] [PubMed] [Google Scholar]
- 44.Mall AS, Tyler MG, Ho SB, Krige JEJ, Kahn D, Spearman W, Myer L, Govender D. The expression of MUC mucin in cholangiocarcinoma. Pathol Res Pract. 2010;206:805–809. doi: 10.1016/j.prp.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 45.Chen FY, Zhou C, Zhang XY, Zhou KQ, Peng YF, Yu L, Fan J, Zhou J, Hu J, Wang Z. Integrated Bioinformatics analysis and clinical validation reveals that high expression of mucin 1 in intrahepatic cholangiocarcinoma predicts recurrence after curative resection. Exp Ther Med. 2020;20(50) doi: 10.3892/etm.2020.9178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsumura N, Yamamoto M, Aruga A, Takasaki K, Nakano M. Correlation between expression of MUC1 core protein and outcome after surgery in mass-forming intrahepatic cholangiocarcinoma. Cancer. 2002;94:1770–1776. doi: 10.1002/cncr.10398. [DOI] [PubMed] [Google Scholar]
- 47.Supimon K, Sangsuwannukul T, Sujjitjoon J, Phanthaphol N, Chieochansin T, Poungvarin N, Wongkham S, Junking M, Yenchitsomanus PT. Anti-mucin 1 chimeric antigen receptor T cells for adoptive T cell therapy of cholangiocarcinoma. Sci Rep. 2021;11(6276) doi: 10.1038/s41598-021-85747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bosch DE, Salipante SJ, Schmidt RA, Swanson PE, Bryan A, SenGupta DJ, Truong CD, Yeh MM. Neutrophilic inflammation in gallbladder carcinoma correlates with patient survival: A case-control study. Ann Diagn Pathol. 2022;56(151845) doi: 10.1016/j.anndiagpath.2021.151845. [DOI] [PubMed] [Google Scholar]
- 49.Garcia P, Bizama C, Rosa L, Espinoza JA, Weber H, Cerda-Infante J, Sánchez M, Montecinos VP, Lorenzo-Bermejo J, Boekstegers F, et al. Functional and genomic characterization of three novel cell lines derived from a metastatic gallbladder cancer tumor. Biol Res. 2020;53(13) doi: 10.1186/s40659-020-00282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhoge A, Khandeparkar SGS, Joshi AR, Gogate B, Kulkarni MM, Bhayekar P. Immunohistochemical study of MUC1 and MUC5AC expression in gall bladder lesions. J Clin Diagn Res. 2017;11:EC12–EC16. doi: 10.7860/JCDR/2017/26537.10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiong L, Yang Z, Yang L, Liu J, Miao X. Expressive levels of MUC1 and MUC5AC and their clinicopathologic significances in the benign and malignant lesions of gallbladder. J Surg Oncol. 2012;105:97–103. doi: 10.1002/jso.22055. [DOI] [PubMed] [Google Scholar]
- 52.Kaur S, Momi N, Chakraborty S, Wagner DG, Horn AJ, Lele SM, Theodorescu D, Batra SK. Altered expression of transmembrane mucins, MUC1 and MUC4, in bladder cancer: Pathological implications in diagnosis. PLoS One. 2014;9(e92742) doi: 10.1371/journal.pone.0092742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stojnev S, Ristic-Petrovic A, Velickovic LJ, Krstic M, Bogdanovic D, Khanh DT, Ristic A, Conic I, Stefanovic V. Prognostic significance of mucin expression in urothelial bladder cancer. Int J Clin Exp Pathol. 2014;7:4945–4958. [PMC free article] [PubMed] [Google Scholar]
- 54.Gonul II, Cakir A, Sozen S. Immunohistochemical expression profiles of MUC1 and MUC2 mucins in urothelial tumors of bladder. Indian J Pathol Microbiol. 2018;61:350–355. doi: 10.4103/IJPM.IJPM_12_18. [DOI] [PubMed] [Google Scholar]
- 55.Tao TT, Chen J, Hu Q, Huang XZ, Fu J, Lv BD, Duan Y. Urothelial carcinoma of the bladder with abundant myxoid stroma: A case report and literature review. Medicine (Baltimore) 2020;99(e21204) doi: 10.1097/MD.0000000000021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shigeta K, Hasegawa M, Kikuchi E, Yasumizu Y, Kosaka T, Mizuno R, Mikami S, Miyajima A, Kufe D, Oya M. Role of the MUC1-C oncoprotein in the acquisition of cisplatin resistance by urothelial carcinoma. Cancer Sci. 2020;111:3639–3652. doi: 10.1111/cas.14574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873(188314) doi: 10.1016/j.bbcan.2019.188314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan SF, Li KZ, Wang L, Dou KF, Yan Z, Han W, Zhang YQ. Expression of MUC1 and its significance in hepatocellular and cholangiocarcinoma tissue. World J Gastroenterol. 2005;11:4661–4666. doi: 10.3748/wjg.v11.i30.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ichikawa T, Yamamoto T, Uenishi T, Tanaka H, Takemura S, Ogawa M, Tanaka S, Suehiro S, Hirohashi K, Kubo S. Clinicopathological implications of immunohistochemically demonstrated mucin core protein expression in hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2006;13:245–251. doi: 10.1007/s00534-005-1070-4. [DOI] [PubMed] [Google Scholar]
- 60.Jiang QL, Feng SJ, Yang ZY, Xu Q, Wang SZ. CircHECTD1 up-regulates mucin 1 expression to accelerate hepatocellular carcinoma development by targeting microRNA-485-5p via a competing endogenous RNA mechanism. Chin Med J (Engl) 2020;133:1774–1785. doi: 10.1097/CM9.0000000000000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morari EC, Silva JR, Guilhen AC, Cunha LL, Marcello MA, Soares FA, Vassallo J, Ward LS. Muc-1 expression may help characterize thyroid nodules but does not predict patients' outcome. Endocr Pathol. 2010;21:242–249. doi: 10.1007/s12022-010-9137-4. [DOI] [PubMed] [Google Scholar]
- 62.Zhan XX, Zhao B, Diao C, Cao Y, Cheng RC. Expression of MUC1 and CD176 (Thomsen-Friedenreich antigen) in papillary thyroid carcinomas. Endocr Pathol. 2015;26:21–26. doi: 10.1007/s12022-015-9356-9. [DOI] [PubMed] [Google Scholar]
- 63.Hu YJ, Luo XY, Yang Y, Chen CY, Zhang ZY, Guo X. Characterization and significance of MUC1 and c-myc expression in elderly patients with papillary thyroid carcinoma. Genet Mol Res. 2015;14:15325–15330. doi: 10.4238/2015.November.30.9. [DOI] [PubMed] [Google Scholar]
- 64.Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, LiVolsi VA, Papotti MG, Sobrinho-Simões M, Tallini G, Mete O. Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol. 2022;33:27–63. doi: 10.1007/s12022-022-09707-3. [DOI] [PubMed] [Google Scholar]
- 65.Baek SK, Woo JS, Kwon SY, Lee SH, Chae YS, Jung KY. Prognostic significance of the MUC1 and MUC4 expressions in thyroid papillary carcinoma. Laryngoscope. 2007;117:911–916. doi: 10.1097/MLG.0b013e31803d1720. [DOI] [PubMed] [Google Scholar]
- 66.Guo M, Luo B, Pan M, Li M, Xu H, Zhao F, Dou J. Colorectal cancer stem cell vaccine with high expression of MUC1 serves as a novel prophylactic vaccine for colorectal cancer. Int Immunopharmacol. 2020;88(106850) doi: 10.1016/j.intimp.2020.106850. [DOI] [PubMed] [Google Scholar]
- 67.Guo M, You C, Dong W, Luo B, Wu Y, Chen Y, Li J, Pan M, Li M, Zhao F, Dou J. The surface dominant antigen MUC1 is required for colorectal cancer stem cell vaccine to exert anti-tumor efficacy. Biomed Pharmacother. 2020;132(110804) doi: 10.1016/j.biopha.2020.110804. [DOI] [PubMed] [Google Scholar]
- 68.Wang HS, Wang LH. The expression and significance of Gal-3 and MUC1 in colorectal cancer and colon cancer. Onco Targets Ther. 2015;8:1893–1898. doi: 10.2147/OTT.S83502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kasprzak A, Siodla E, Andrzejewska M, Szmeja J, Seraszek-Jaros A, Cofta S, Szaflarski W. Differential expression of mucin 1 and mucin 2 in colorectal cancer. World J Gastroenterol. 2018;24:4164–4177. doi: 10.3748/wjg.v24.i36.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hazgui M, Weslati M, Boughriba R, Ounissi D, Bacha D, Bouraoui S. MUC1 and MUC5AC implication in Tunisian colorectal cancer patients. Turk J Med Sci. 2021;51:309–318. doi: 10.3906/sag-2003-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Niv Y, Rokkas T. Mucin expression in colorectal cancer (CRC): Systematic review and meta-analysis. J Clin Gastroenterol. 2019;53:434–440. doi: 10.1097/MCG.0000000000001050. [DOI] [PubMed] [Google Scholar]
- 72.Schimanski CC, Kasper S, Hegewisch-Becker S, Schröder J, Overkamp F, Kullmann F, Bechstein WO, Vöhringer M, Öllinger R, Lordick F, et al. Adjuvant MUC vaccination with tecemotide after resection of colorectal liver metastases: A randomized, double-blind, placebo-controlled, multicenter AIO phase II trial (LICC) Oncoimmunology. 2020;9(1806680) doi: 10.1080/2162402X.2020.1806680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shimada M, Tsuji K, Shigeta S, Nagai T, Watanabe Z, Tokunaga H, Kigawa J, Yaegashi N. Rethinking the significance of surgery for uterine cervical cancer. J Obstet Gynaecol Res. 2022;48:576–586. doi: 10.1111/jog.15112. [DOI] [PubMed] [Google Scholar]
- 74.Togami S, Nomoto M, Higashi M, Goto M, Yonezawa S, Tsuji T, Batra SK, Douchi T. Expression of mucin antigens (MUC1 and MUC16) as a prognostic factor for mucinous adenocarcinoma of the uterine cervix. J Obstet Gynaecol Res. 2010;36:588–597. doi: 10.1111/j.1447-0756.2010.01221.x. [DOI] [PubMed] [Google Scholar]
- 75.Gardner AB, Charo LM, Mann AK, Kapp DS, Eskander RN, Chan JK. Ovarian, uterine, and cervical cancer patients with distant metastases at diagnosis: Most common locations and outcomes. Clin Exp Metastasis. 2020;37:107–113. doi: 10.1007/s10585-019-10007-0. [DOI] [PubMed] [Google Scholar]
- 76.Samouëlian V, Mechtouf N, Leblanc E, Cardin GB, Lhotellier V, Querleu D, Révillion F, Rodier F. Sensitive molecular detection of small nodal metastasis in uterine cervical cancer using HPV16-E6/CK19/MUC1 cancer biomarkers. Oncotarget. 2018;9:21641–21654. doi: 10.18632/oncotarget.24956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cai J, Chen H, Lu M, Zhang Y, Lu B, You L, Zhang T, Dai M, Zhao Y. Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis. Cancer Lett. 2021;520:1–11. doi: 10.1016/j.canlet.2021.06.027. [DOI] [PubMed] [Google Scholar]
- 78.Wang S, You L, Dai M, Zhao Y. Mucins in pancreatic cancer: A well-established but promising family for diagnosis, prognosis and therapy. J Cell Mol Med. 2020;24:10279–10289. doi: 10.1111/jcmm.15684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fu X, Tang N, Xie WQ, Mao L, Qiu YD. MUC1 promotes glycolysis through inhibiting BRCA1 expression in pancreatic cancer. Chin J Nat Med. 2020;18:178–185. doi: 10.1016/S1875-5364(20)30019-4. [DOI] [PubMed] [Google Scholar]
- 80.Sierzega M, Mlynarski D, Tomaszewska R, Kulig J. Semiquantitative immunohistochemistry for mucin (MUC1, MUC2, MUC3, MUC4, MUC5AC, and MUC6) profiling of pancreatic ductal cell adenocarcinoma improves diagnostic and prognostic performance. Histopathology. 2016;69:582–591. doi: 10.1111/his.12994. [DOI] [PubMed] [Google Scholar]
- 81.Maleki F, Rezazadeh F, Varmira K. MUC1-targeted radiopharmaceuticals in cancer imaging and therapy. Mol Pharm. 2021;18:1842–1861. doi: 10.1021/acs.molpharmaceut.0c01249. [DOI] [PubMed] [Google Scholar]
- 82.Alirezapour B, Ashkezari MD, Fini MM, Rasaee MJ, Mohammadnejad J, Paknejad M, Maadi E, Yousefnia H, Zolghadri S. Preparation and preclinical characterization of (111)In-DTPA-Anti-MUC1 as a radioimmunoconjugate for diagnosis of breast cancer by single-photon emission computed tomography. J Cancer Res Ther. 2022;18:158–167. doi: 10.4103/jcrt.JCRT_730_20. [DOI] [PubMed] [Google Scholar]
- 83.Stergiou N, Nagel J, Pektor S, Heimes AS, Jäkel J, Brenner W, Schmidt M, Miederer M, Kunz H, Roesch F, Schmitt E. Evaluation of a novel monoclonal antibody against tumor-sassociated MUC1 for diagnosis and prognosis of breast cancer. Int J Med Sci. 2019;16:1188–1198. doi: 10.7150/ijms.35452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pierga JY, Bidard FC, Denis MG, de Cremoux P. Prognostic value of peripheral blood double detection of CK19 and MUC1 mRNA positive cells detected by RT-quantitative PCR in 94 breast cancer patients with a follow up of 9 years. Mol Oncol. 2007;1:267–268. doi: 10.1016/j.molonc.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu C, Xie Y, Sun B, Geng F, Zhang F, Guo Q, Wu H, Yu B, Wu J, Yu X, et al. MUC1- and survivin-based DNA vaccine combining immunoadjuvants CpG and interleukin-2 in a bicistronic expression plasmid generates specific immune responses and antitumour effects in a murine colorectal carcinoma model. Scand J Immunol. 2018;87:63–72. doi: 10.1111/sji.12633. [DOI] [PubMed] [Google Scholar]
- 86.Ruan J, Duan Y, Li F, Wang Z. Enhanced synergistic anti-Lewis lung carcinoma effect of a DNA vaccine harboring a MUC1-VEGFR2 fusion gene used with GM-CSF as an adjuvant. Clin Exp Pharmacol Physiol. 2017;44:71–78. doi: 10.1111/1440-1681.12654. [DOI] [PubMed] [Google Scholar]
- 87.Gong YF, Zhou QB, Liao YD, Mai C, Chen TJ, Tang YQ, Chen RF. Optimized construction of MUC1-VNTRn DNA vaccine and its anti-pancreatic cancer efficacy. Oncol Lett. 2017;13:2198–2206. doi: 10.3892/ol.2017.5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ogasawara M, Miyashita M, Yamagishi Y, Ota S. Dendritic cell vaccination combined with a conventional chemotherapy for patients with relapsed or advanced pancreatic ductal adenocarcinoma: A single-center phase I/II trial. Ther Apher Dial. 2021;25:415–424. doi: 10.1111/1744-9987.13659. [DOI] [PubMed] [Google Scholar]
- 89.Ota S, Miyashita M, Yamagishi Y, Ogasawara M. Baseline immunity predicts prognosis of pancreatic cancer patients treated with WT1 and/or MUC1 peptide-loaded dendritic cell vaccination and a standard chemotherapy. Hum Vaccin Immunother. 2021;17:5563–5572. doi: 10.1080/21645515.2021.2003645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tosch C, Bastien B, Barraud L, Grellier B, Nourtier V, Gantzer M, Limacher JM, Quemeneur E, Bendjama K, Préville X. Viral based vaccine TG4010 induces broadening of specific immune response and improves outcome in advanced NSCLC. J Immunother Cancer. 2017;5(70) doi: 10.1186/s40425-017-0274-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Glaffig M, Stergiou N, Hartmann S, Schmitt E, Kunz H. A synthetic MUC1 anticancer vaccine containing mannose ligands for targeting macrophages and dendritic cells. ChemMedChem. 2018;13:25–29. doi: 10.1002/cmdc.201700646. [DOI] [PubMed] [Google Scholar]
- 92.Hu B, Wang J, Guo Y, Chen T, Ni W, Yuan H, Zhang N, Xie F, Tai G. Pre-clinical toxicity and immunogenicity evaluation of a MUC1-MBP/BCG anti-tumor vaccine. Int Immunopharmacol. 2016;33:108–118. doi: 10.1016/j.intimp.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 93.Zhang H, Zhao H, He X, Xi F, Liu J. JAK-STAT domain enhanced MUC1-CAR-T cells induced esophageal cancer elimination. Cancer Manag Res. 2020;12:9813–9824. doi: 10.2147/CMAR.S264358. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 94.Mei Z, Zhang K, Lam AK, Huang J, Qiu F, Qiao B, Zhang Y. MUC1 as a target for CAR-T therapy in head and neck squamous cell carinoma. Cancer Med. 2020;9:640–652. doi: 10.1002/cam4.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou R, Yazdanifar M, Roy LD, Whilding LM, Gavrill A, Maher J, Mukherjee P. CAR T cells targeting the tumor MUC1 glycoprotein reduce triple-negative breast cancer growth. Front Immunol. 2019;10(1149) doi: 10.3389/fimmu.2019.01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pang L, Wang J, Fan Y, Xu R, Bai Y, Bai L. Correlations of TNM staging and lymph node metastasis of gastric cancer with MRI features and VEGF expression. Cancer Biomark. 2018;23:53–59. doi: 10.3233/CBM-181287. [DOI] [PubMed] [Google Scholar]
- 97.Jeong O, Jung MR, Kang JH. Prognostic value of the anatomic region of metastatic lymph nodes in the current TNM staging of gastric cancer. J Gastric Cancer. 2021;21:236–245. doi: 10.5230/jgc.2021.21.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.