Abstract
The functionality of polymorphonuclear leukocytes (PMNs) once they migrate into the digestive lumen is still ill defined. More specifically, phagocytic function and bactericidal action of PMNs after transepithelial migration have not received much attention. The aim of the present study is to compare PMN behavior before and after transepithelial migration, in particular (i) phagocytosis and bactericidal activity; (ii) expression of surface molecules, particularly those involved in phagocytosis; and (iii) apoptosis. Cultured human intestinal epithelial T84 cell monolayers were used. The effect of transepithelial migration on phagocytosis was evaluated by immunofluorescence and electron microscopy and by flow cytometric assessment of the engulfment of a strain of Escherichia coli transfected with the green fluorescent protein. Superoxide production by PMNs was investigated by luminol-mediated chemiluminescence. Expression of various surface molecules on PMNs was evaluated by flow cytometry, while PMN apoptosis was assayed by morphologic changes and DNA fragmentation. E. coli phagocytosis by the PMNs was markedly increased after transepithelial migration without modification of superoxide production. CD11b/CD18 and CD47 expression was increased upon PMN transmigration, whereas CD16 expression was decreased and CD29, CD46, CD49e, CD49f, CD55, CD59, CD61, CD95 levels remained unchanged. Apoptosis in transmigrated PMNs was slightly advanced and was observed after 12 h compared to 16 h for nontransmigrated PMNs. In conclusion, the phagocytic capacity of the PMNs is augmented after transepithelial migration, with a dramatic increase in the level of CD11b/CD18 and preservation of the superoxide production. These results suggest a higher bactericidal activity of the PMNs once they have translocated into the digestive lumen.
During an active bacterial disease in the alimentary tract, polymorphonuclear leukocytes (PMNs) have to cross the endothelium, migrate through the lamina propria, and finally transmigrate across the epithelial barrier. Numerous bacteria colonize the surface epithelium and/or invade the subepithelial space, whereas some pathogens are still present in the luminal space, where they multiply. Diarrhea is an efficient way to eliminate pathogens from the intestinal tract. The involvement of neutrophils in triggering the extrusion of water and chloride is well documented (20). Neutrophils are also known to provide an innate host defense against pathogens present in the gastrointestinal tract through their phagocytic function. Surprisingly, the effect of transepithelial migration on the phagocytic capacity of neutrophils has not received much attention.
Phagocytosis induces cytoplasmic superoxide oxygen production in PMNs, which correlates with the induction of apoptosis (34, 37). The likely consequence of this apoptosis is a reduced liberation of proteolytic enzymes and other PMN metabolites that could contribute to the induction of an acute inflammatory process (6).
The aim of this work was to investigate the physiological status of the PMNs after their transepithelial migration. We have compared the phagocytosis of the PMNs before and after migration, by assessing the engulfment of an Escherichia coli strain expressing the green fluorescent protein (3, 4, 9). This comparison was assessed by measuring the fluorescent intensity by flow cytometry and by using immunofluorescence and electron microscopy to count the bacteria observed inside the PMNs. Phagocytosis-mediated production of reactive oxygen intermediates (ROI) was assessed by chemiluminescence. The mechanisms involved in the regulation of the phagocytic function were addressed by comparing the levels of expression of molecules known to participate in PMN phagocytosis, such as CD11b, CD18, CD16, CD29, CD47, CD49e, CD49f, and CD61. Finally, apoptosis of the transmigrated PMNs was compared to that of control PMNs, i.e., nontransmigrated PMNs, by using morphologic and DNA fragmentation studies. In addition, the level of some antigens which have been implicated in apoptosis, such as CD95, CD55, CD46, and CD59 (16), were evaluated by flow cytometry before and after migration.
MATERIALS AND METHODS
Tissue culture and electrophysiology.
T84 cells (passages 65 to 90), a human colonic carcinoma cell line, were obtained from the American Type Culture Collection and grown and maintained as confluent monolayers on collagen-coated permeable supports with detailed modifications (18, 19). The cells were grown as monolayers in a 1:1 mixture of Dulbecco-Vogt modified Eagle medium and Ham F-12 medium supplemented with 15 mM HEPES buffer (pH 7.5), 14 mM NaHCO3, 40 mg of penicillin per ml, 90 mg of streptomycin per ml, 8 mg of ampicillin per ml, and 5% newborn calf serum. The monolayers were grown on 0.33-cm2 ring-supported polycarbonate filters (Costar, Cambridge, Mass.) and used 6 to 14 days after being plated. Steady-state resistance was reached in 4 to 6 days, with variability being related largely to the cell passage number. The monolayers received a weekly feeding following initial plating. Confluent monolayers on permeable supports were constructed to permit a basolateral-to-apical migration of PMN (“inverted inserts”) as previously described (19). To assess currents, transepithelial potentials, and resistance, a commercial voltage clamp (Bioengineering Department, University of Iowa) was used and interfaced with an equilibrated pair of calomel electrodes along with a pair of Ag|AgCl electrodes submerged in Hanks balanced salt solution (HBSS). Agar bridges were used to interface the electrode with the solutions on either side of the monolayers (one calomel and one Ag|AgCl electrode in each well), and resistance was measured as detailed elsewhere (19). Transepithelial resistances were not altered by preincubation of the T84 monolayers with Escherichia coli HMS174 (DE3) (Novagen, Abingdon, United Kingdom) in the lower reservoirs, and the morphological features of these cells did not show any modification in comparison with control cells, providing evidence that the epithelial barrier was intact before starting the transmigration (reference 27 and data not shown).
Neutrophil transmigration.
Human neutrophils were isolated from whole blood by a gelatin sedimentation technique (19). Briefly, whole blood anticoagulated with citrate-dextrose was centrifuged at 300 × g for 20 min at 20°C. The plasma and buffy coat were removed, and the gelatin-cell mixture was incubated at 37°C for 30 min to remove contaminating erythrocytes. Residual erythrocytes were then lysed with isotonic ammonium chloride. After being washed in HBSS without Ca2+ or Mg2+, the cells were counted and resuspended at 5 × 107 PMNs/ml. PMNs (95% pure) with 98% viability by trypan blue exclusion were used for experiments within 1 h after isolation. The physiologically (basolateral-to-apical) directed PMN transepithelial migration assay has been described previously (19). Neutrophil transmigration experiments were performed at 37°C on 0.33-cm2 inverts, using low-attachment Costar plates. Once isolated, the PMNs were suspended in modified HBSS (without Ca2+ and Mg2+) with 10 mM HEPES (pH 7.4) (Sigma Chemical Co.) at a concentration of 5 × 107/ml before being added to the inverts (106 cells/well). Transmigration of PMNs was initiated by applying 0.1 μM N-formyl-l-methionyl-leucyl-l-phenylalanine (fMLP) (Sigma) to the lower reservoir and incubating it for 15 min to allow a transepithelial chemotactic gradient to form prior to the addition of PMNs. In some experiments, T84 monolayers were preincubated with 30 μM NBD phallacidin (Sigma) overnight to induce a rigidification of the actin cytoskeleton (12). Control acellular filters were used for each experiments.
Phagocytosis capacity of PMNs and superoxide production.
The phagocytosis capacity of the control and transmigrated PMNs was monitored by measuring the engulfment of E. coli HMS174 (DE3) expressing the green fluorescent protein (GFP), as previously described (4). The GFP was amplified by PCR from the pEGFP-1 vector (Clontech, Palo Alto, Calif.) ligated in plasmid pET28a(+) (Novagen) and hence inducible by isopropylthio-β-d-galactopyranoside (IPTG) (ICN, Irvine, Calif.). IPTG (0.5 mM) was added for expression of GFP. The number of bacteria incubated with PMNs was determined by measurement of optical density considering that an optical density at 600 nm of 1 corresponds to 109 bacteria. Nontransmigrated PMNs were incubated for 120 min with bacteria in HBSS without calcium and fMLP or with calcium and fMLP (10−7 M). For posttransmigration phagocytosis, E. coli was directly incubated in the lower reservoir. In some experiments, PMN transmigration was assessed in the presence of both E. coli and anti-CD11b antibody (antibody 44a; diluted 1:100) (American Type Culture Collection) or anti-CD47 antibody (antibody BRIC126; diluted 1:50) (International Blood Group Reference Laboratory, Bristol, United Kingdom) in the lower reservoir. A ratio of 1:10, i.e., 104 PMNs for 105 E. coli cells, was used for all conditions tested. The number of phagocytosed E. coli cells in PMNs was estimated by fluorescence microscopy. The amount of phagocytosed E. coli in PMNs was also measured by flow cytometry with a FACScan apparatus (Beckton Dickinson).
NADPH oxidase-catalyzed superoxide generation was assayed by luminol-dependent chemiluminescence (35). Control PMNs were resuspended at 2.5 × 105 cells/ml in 96-well microtiter plates (Dynatech Laboratories, Guyancourt, France). Transmigrated PMNs (2.5 × 105 cells/ml) were added to other wells. fMLP (10−7 M), opsonized zymosan (OPZ; 0.05 ml of a 108-particle/ml suspension), or various amounts of E. coli were used to trigger ROI production before the addition of 80 μM luminol (Sigma) in the dark. The chemiluminescence resulting from the reaction of luminol with oxygen radicals was measured at 37°C with a luminometer (ML 3000 Microtiter Plate luminometer; Dynatech Laboratories).
Electron microscopy studies.
Control PMNs were observed before and after 2 h of transmigration. The number of E. coli cells phagocytosed by PMNs was compared in PMNs incubated for 120 min in HBSS with calcium and 10−7M fMLP and in transmigrated PMNs. During the transmigration, E. coli was incubated in the lower reservoirs. In some experiments, phagocytosis of transmigrated PMNs was assessed in the presence of both E. coli and anti-CD11b antibody or E. coli and anti-CD47 antibody in the lower reservoirs. Control T84 monolayers were examined to verify the absence of morphological modification due to the incubation with E. coli, in particular at the tight-junction level. PMNs were fixed with 2% freshly prepared formaldehyde in 0.1 M sodium cacodylate (pH 7.4) for 1 h at 4°C. Pellets were rinsed in cacodylate buffer, postfixed in 1% OsO4 for 1 h, dehydrated through graded alcohols, and embedded in epoxy resin. Oriented 1-mm sections were obtained with diamond knives, and multiple areas were thinly sectioned, mounted on copper mesh grids, and stained with uranyl acetate and lead citrate. Ultrathin sections were examined on a JEOL 1200 XII electron microscope.
Flow cytometric assay.
The flow cytometric assay was performed before and after a 2-h transmigration at 37°C. PMNs that had transmigrated across the acellular filter or through the epithelial monolayer from 12 Costar plates were pooled for flow cytometric analysis, as were control PMNs in HBSS with and without 10−7 M f-MLP (incubation, 2 h). Neutrophils in HBSS were fixed in 1% formalin for 30 min at room temperature. The cells were then washed once in HBSS and incubated with polyclonal goat immunoglobulin (Ig) for 20 min. The neutrophils were washed again in HBSS and treated with monoclonal antibody K20 (anti-CD29) (INSERM U343, Nice, France), GB24 (anti-CD46), GB36 (anti-CD49f), H19 (anti-CD59) (all from INSERM U364), Bear 1 (anti-CD11b), 3G8 (anti-CD16), 7E4 (anti-CD18), SAM1 (anti-CD49e), JS11KSC2.3 (anti-CD55), SZ21 (anti-CD61), ZB4 (anti-CD95) (all from Immunotech, Marseille, France), BRIC126 (anti-CD47) (International Blood Group Reference Laboratory), an isotype-matched control, or HBSS for 20 min at room temperature and then washed twice. The cells were then exposed to FITC-conjugated goat anti-mouse Ig (Sigma) for 20 min at room temperature in the dark and then washed and resuspended in 500 μl of HBSS. Analysis was performed on a FACScan apparatus (Becton Dickinson), with the channel number (log scale) representing the mean fluorescence intensity for 10,000 cells.
PMN apoptosis.
Apoptosis was monitored by fragmentation of DNA. DNA was extracted from 107 PMNs after various periods of transmigration (8, 12, and 16 h) and from control PMNs in HBSS and fMLP 10−7 M at 37°C at the same time points. Control PMNs at 8 h were exposed to tumor necrosis factor alpha (TNF-α) (10 U/ml) (Sigma) plus cycloheximide (10 mg/ml) (Research Organics, Cleveland, Ohio). PMN DNA fragmentation was assessed by the procedure for assaying DNA fragmentation in extracted total genomic DNA (21). In brief, the cells were lysed with TES buffer (20 mM Tris HCl, 200 mM EDTA, 1% sodium dodecyl sulfate)–RNase (20 μg/ml) (Boehringer Mannheim, Indianapolis, Ind.) at 37°C for 1 h. Proteins were degraded by incubation with proteinase K (1 mg/ml; Boerhinger Mannheim) at 55°C for 3 h. Residual proteins were removed by phenol extraction. The DNA was then precipitated with alcohol overnight at −20°C. The next day, the DNA was rinsed with alcohol, mixed with loading buffer, and then electrophoresed in a 2% agarose gel containing 10 μg of ethidium bromide per ml. The gel was examined and photographed under UV light for revealing the internucleosomal fragmentation of DNA (laddering) characteristic of apoptosis.
The morphological changes of apoptosis were examined by light and electron microscopy. Briefly, control and transmigrated PMNs at 12 h were fixed with methanol and stained with Wright-Giemsa and the slides were examined by light microscopy (original magnification, ×630). At least 400 cells of each preparation in various fields were counted. For electron microscopy, control and transmigrated PMNs at 12 h were processed as described above. Apoptotic cells were easily distinguishable on the basis of their reduced volume, chromatin condensation, and nuclear fragmentation.
Data presentation.
Results are expressed as the mean ± standard deviation. Interreader variability was analyzed by analysis of variance. Means of groups were analyzed by the two-tailed Student t test.
RESULTS
Transmigrated neutrophils exhibit an increased phagocytosis capacity without modification in the production of ROI.
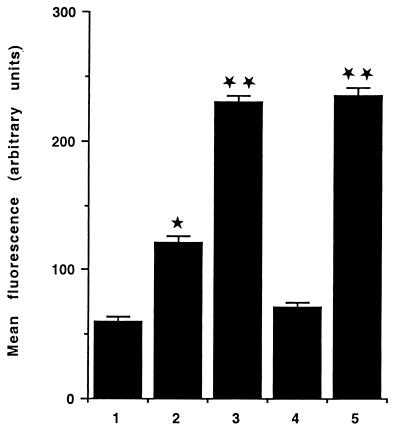
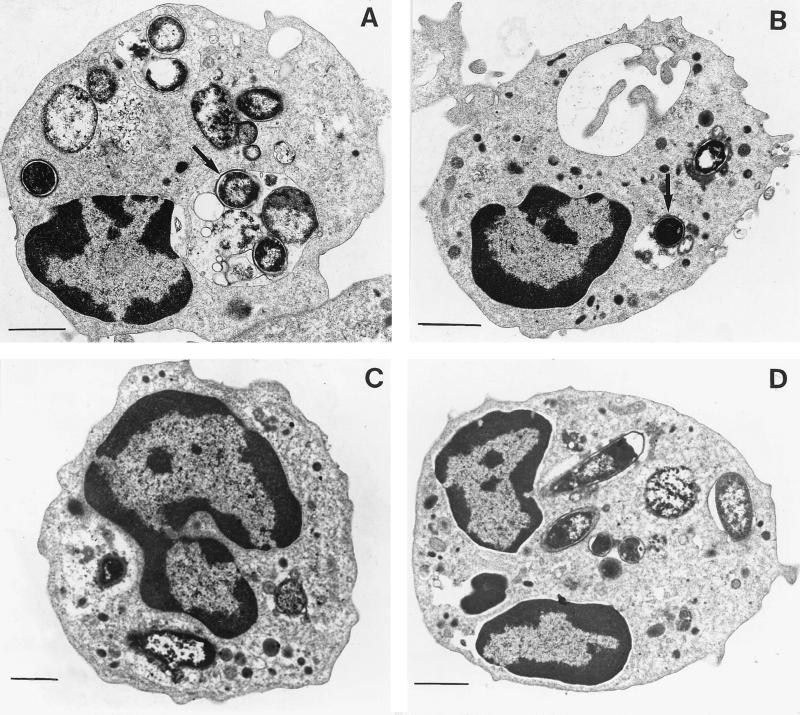
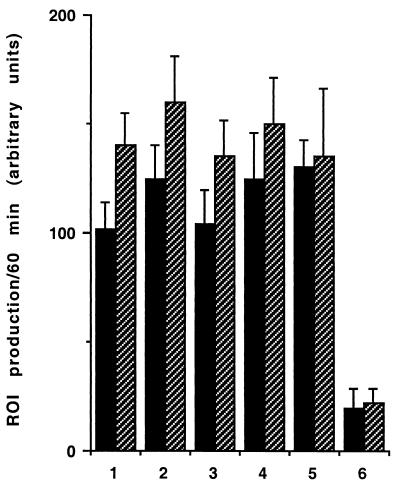
As shown in Fig. 1, PMNs that had transmigrated across a monolayer of T84 cells exhibited an increased level of fluorescence intensity when challenged with GFP-labeled E. coli in comparison with control PMNs. This was visible when control cells were maintained in HBSS without calcium (230.62 ± 2.99 and 60.39 ± 1.45 arbitrary units [AU] for transmigrated PMNs and PMNs in HBSS without calcium, respectively) or in the presence of calcium and 10−7M fMLP (230.62 ± 2.99 and 120.93 ± 2.67 AU for transmigrated PMNs and PMNs in HBSS with calcium and 10−7 M fMLP, respectively) (Fig. 1). The increased capacity of transmigrated PMNs to engulf bacteria was abolished when the cells were incubated with an anti-CD11b antibody (230.62 ± 2.99 and 71.21 ± 1.51 AU for transmigrated PMNs and transmigrated PMNs incubated with an anti-CD11b antibody, respectively) but was maintained when the cells were incubated with an anti-CD47 antibody (230.62 ± 2.99 and 235.13 ± 3.11 AU for transmigrated PMNs and transmigrated PMNs incubated with an anti-CD47 antibody, respectively) (Fig. 1). The number of fluorescent E. coli associated with transmigrated PMNs (with or without incubation with an anti-CD47 antibody) as assessed by light microscopy was larger (5 ± 4 and 2 ± 2 E. coli organisms per cell for transmigrated PMNs and control PMNs in 10−7 M fMLP, respectively) than for any of the above-mentioned control conditions (results not shown). However, it was difficult by this technique to differentiate bacteria bound at the PMN surface from bacteria having undergone a phagocytosis process. To better assess the number of E. coli really phagocytosed by PMNs, electron microscopy experiments were performed. This showed that the number of E. coli per cell observed in a thin section increased from 2 ± 1 in control PMNs in 10−7 M fMLP to 8 ± 2 E. coli per cell in transmigrated PMNs (P < 0.01) (Fig. 2A and B). This increased number of bacteria inside the transmigrated PMNs was diminished when cells were incubated with an anti-CD11b antibody (3 ± 2 E. coli organisms per cell observed in thin section) but was maintained when cells were incubated with an anti-CD47 antibody (7 ± 2 E. coli organisms per cell observed in thin section) (P < 0.01) (Fig. 2C and D). ROI production by PMNs was triggered by various amounts of E. coli, by opsonised zymosan, or by 10−7 M fMLP alone (Fig. 3) and assessed by photon emission with luminol, an oxygen radical-trapping agent. ROI production gradually increased to reach a maximal value within 10 min. As shown in Fig. 3, the extent of phagocytosis-mediated ROI production was not significantly different in transmigrated PMNs from that in control PMNs. No significant differences were observed between transmigrated and control PMNs under the conditions tested. The maximal rate of ROI production was achieved within 10 min for all conditions tested.
FIG. 1.
The amount of phagocytosed E. coli quantified by flow cytometry is increased in transmigrated PMNs. 1, PMNs in HBSS without calcium; 2, PMNs in HBSS with calcium and 10−7 M fMLP; 3, transmigrated PMNs; 4, transmigrated PMNs in the presence of anti-CD11b antibody; 5, transmigrated PMNs in the presence of anti-CD47 antibody. Results are presented as means and standard errors of the means for 6 to 12 monolayers (∗, P < 0.05; ∗∗, P < 0.01).
FIG. 2.
Increased number of E. coli seen in the cytoplasm of transmigrated PMNs under electron microscopy. (A) Transmigrated PMNs. Numerous bacteria are noted in the cytoplasm of PMNs (arrow). (B) Control PMNs. A few bacteria are observed in the cytoplasm of PMNs incubated for 120 min with bacteria in HBSS with calcium and 10−7 M fMLP (arrow). (C) Transmigrated PMNs in the presence of anti-CD11b antibody. (D) Transmigrated PMNs in the presence of anti-CD47 antibody. Bars, 10 μm.
FIG. 3.
Transepithelial migration of PMN across T84 cells does not alter ROI production by the PMNs. Different concentrations of bacteria, OPZ, or fMLP were used to trigger ROI production in control PMNs (solid bars) and transmigrated PMNs (hatched bars). 1, 5 bacteria/PMN; 2, 10 bacteria/PMN; 3, 15 bacteria/PMN; 4, 25 bacteria/PMN; 5, OPZ (0.05 ml of a 108-particle/ml suspension); 6, 10−7 M fMLP. The kinetics of ROI production was measured by a luminol-dependent chemiluminescence assay. Statistical analyses were performed by the unpaired Student t test.
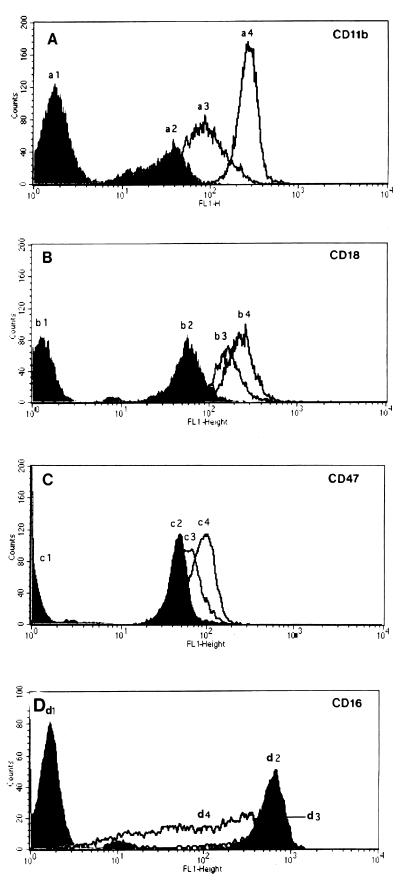
Transmigration across the T84 cell monolayers increases the expression of CD11b, CD18, and CD47 antigens at the surface of PMNs.
When resuspended human neutrophils were exposed to 10−7 M fMLP, they exhibited a marked increase in surface expression of CD11b, CD18, and CD47 (Fig. 4). The level of these antigens was enhanced when they were subjected to a gradient of fMLP through an acellular filter (Fig. 4). Surface expression of CD11b, CD18, and CD47 was even higher when the PMNs transmigrated through an epithelium monolayer (Fig. 4A to C). Under this latter condition, the most remarkable difference was observed for CD11b, whose level increased about threefold compared to that obtained after migration through an acellular filter (P < 0.05). Preincubation of T84 cells with phallacidin diminished the increase in the level of CD11b, CD18, and CD47 expression on transmigrated neutrophils, suggesting that expression of these antigens was partially dependent on epithelial cytoskeleton remodeling (results not shown). In contrast, transmigration of neutrophils across a T84 monolayer decreased the expression of CD16 in comparison with control neutrophils (Fig. 4D). We verified that a series of other surface antigens such as CD29, CD95, CD49d, CD49e, CD49f, CD55, CD46, CD59, and CD61 (results not shown) was not significantly affected by the transmigration process.
FIG. 4.
Flow cytometry histogram of log fluorescence intensity of CD11b (A), CD18 (B), CD47 (C), and CD16 (D) on human neutrophils. In each series of experiments (n = 3), untreated cells (a1, b1, c1, and d1), 10 μM fMLP-treated cells (a2, b2, c2, and d2), cells that transmigrated across acellular filters due to 10 μM fMLP (a3, b3, c3, and d3), or cells that transmigrated across epithelial monolayers due to 10 μM f-MLP (a4, b4, c4, and d4) were examined for adhesion molecule expression.
The onset of apoptosis is slightly accelerated in transmigrated PMNs.
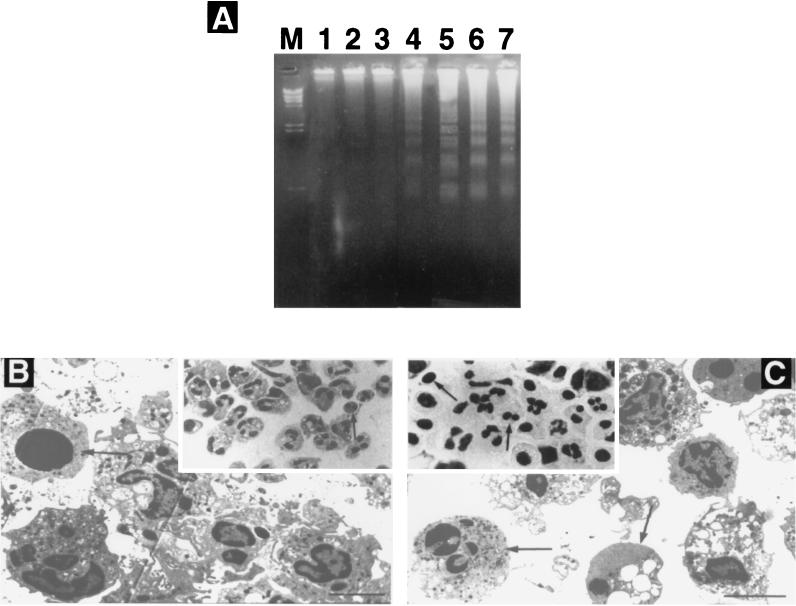
Electrophoresis of DNA isolated from posttransmigrated PMNs showed internucleosomal DNA fragmentation (laddering, bands at 200-bp intervals) characteristic of apoptosis as early as 12 h (Fig. 5A, lane 4), whereas this apoptosis hallmark was not visible before 16 h in control PMNs (lanes 3 and 5). Posttransmigrated PMNs showed marked signs of apoptosis at 16 h (lane 6). Control PMNs and posttransmigrated PMNs did not show any sign of apoptosis at 8 h (lanes 1 and 2). Control PMNs at 8 h exposed for 3 h to TNF-α plus cycloheximide showed marked apoptosis (lane 7). Phagocytosis of E. coli induced apoptosis in a similar manner both in transmigrated PMNs and in resuspended PMNs exposed to 10−7 M fMLP (data not shown).
FIG. 5.
Analysis of neutrophil apoptosis. (A) DNA fragmentation analysis. DNAs obtained from control PMNs and from transmigrated PMNs were separated on a 2% agarose gel containing ethidium bromide. Lanes: M, a standard 123-bp DNA ladder; 1, 3, and 5, DNA from control PMNs at 8, 12, and 16 h of culture, respectively; 2, 4, and 6, DNA from transmigrated PMNs at the same time points; 7, DNA from control PMNs at 8 h of culture including 3 h of treatment with TNF-α and cycloheximide. (B and C) Morphological features of neutrophil apoptosis. Control cells at 12 h in culture show rare nuclear condensation (arrow) (inset in panel B). Numerous apoptotic bodies and pyknotic nuclei (arrows) can be identified at 12 h for the transmigrated PMNs (inset in panel C) (Wright-Giemsa stain). Magnification, ×630. Transmission electron microscopy of control PMNs after 12 h in culture (B) and transmigrated PMNs after 12 h (C) shows apoptotic cells (arrows). Bars, 30 μm.
Assessment of PMN apoptosis by DNA gel electrophoresis correlated significantly with the rate of PMN apoptosis as assessed by counting PMNs presenting characteristic morphologic changes. By light microscopy, control PMNs showed 12% ± 5% apoptotic cells after 12 h whereas this proportion was 48% ± 9% in transmigrated PMNs (P < 0.001) (Fig. 5B and C, insets). Observation by transmission electron microscopy showed that cells undergoing apoptosis underwent cytoplasmic shrinkage, vacuole, formation, and nuclear condensation (Fig. 5B and C). For some neutrophils, the nuclear chromatin stained uniformly rather than having heterogenous staining due to the normal distribution of euchromatin and heterochromatin. By 12 h, a much larger proportion of transmigrated PMNs than of control cells were apoptotic, corroborating the slightly accelerated onset of apoptosis observed by other means.
DISCUSSION
Neutrophils are recruited abundantly into the intestinal lumen in response to pathogenic infections. This recruitment is triggered by the attachment of an array of bacterial pathogens to the epithelial surface. Contact between bacterial pathogens and epithelial-cell apical membranes elicits a variety of epithelial responses which do not occur with members of the commensal flora. Such interactions result in the polarized secretion of the potent chemotactic peptide interleukin-8 from the epithelial cell (22). In addition to their action on induction of water and electrolyte movements in epithelial cells, PMNs are known to participate in the elimination of pathogens via phagocytosis and associated ROI production. We found that in comparison with the nontransmigrated PMNs, transepithelially migrated PMNs showed a two- to threefold-higher capacity to phagocytose E. coli, with an equal capacity to produce ROI in response to bacteria or zymosan ingestion, thus strongly suggesting that they exhibited an enhanced bactericidal potential. This was not correlated with a change in superoxide production following ingestion of the bacteria.
The phagocytic function of the PMNs is mediated mainly by the complement receptor type III (CR3) or by the Fc receptors (7, 28). A crucial role of CR3 (CD11b/CD18) is to mediate the uptake of infectious microorganisms. This conclusion comes from observations of patients who are genetically deficient in CR3 (1). These patients suffer recurrent, life-threatening infections with a variety of organisms. Very late antigen protein (VLA) integrins such as VLA5 and VLA6 have also been reported to be involved in phagocytosis (15, 23, 29).
Migration of the PMNs through a monolayer of T84 cells was accompanied by a dramatic increase in the cell surface expression of CD11b and CD18 molecules, even though exposure to fMLP or to a gradient of fMLP through an acellular filter also enhanced, but to a lesser extent, the expression of these integrin subunits. In neutrophils, CD11b/CD18 is necessary for the adherence of the PMNs to the intestinal epithelium (25). Hence, upregulation of CD11b/CD18 may facilitate migration across the epithelial barrier. In this situation, it is difficult to conclude whether transmigration per se was responsible for the overexpression of CD11b/CD18 or whether a subpopulation of neutrophils expressing higher levels of CD11b/CD18 was allowed to migrate more rapidly in the lower reservoirs. It is worth noting that the increased CD11b/CD18 expression in transmigrated PMNs fits reasonably well with the increase of phagocytosis observed in this population. Indeed, the integrin CD11b/CD18 (CR3) is implicated in phagocytosis (7), since antibodies against CR3 not only inhibit the binding of C3bi-coated particles to phagocytes but also inhibit the binding of microbes such as Streptococcus and E. coli (34). Because binding of these microbes was observed in absence of a source of complement, it was proposed that CR3 could also directly interact with the bacterial surface. This is supported by the observation that cells deficient in the CD18 antigen exhibit defective recognition of several microbes (11). For instance, normal PMNs avidly bind unopsonized E. coli whereas PMNs from patients with leukocyte adhesion deficiency do not (38), indicating an important role of the CD18 antigen in this nonopsonic recognition event. PMNs bind to E. coli lipopolysaccharide structures, the most prevalent molecule at the bacterial surface (38). The binding site on CR3 for lipopolysaccharides appears to be distinct from the binding site for C3bi or fibrinogen on the same molecule (14, 33, 39). Therefore, one must conclude that CD11b/CD18 participates in the phagocytosis of both complement-opsonized and nonopsonized bacteria. In our work, under conditions where bacteria were free of complement coating, the upregulation of CD11b/CD18 in the transmigrated PMNs was correlated with an increased phagocytic function. This was confirmed by incubation of bacteria with anti-CD11b antibodies in the lower reservoir. Under these conditions, no increased phagocytosis by the transmigrated PMNs was observed. The origin of the CD11b/CD18 molecules that appeared at the surface of the transmigrated neutrophils remains to be elucidated. However, it is worth noting that PMNs contain intracellular pools of membrane receptors, including CR1 and CR3, that may translocate to the plasma surface during activation (2).
We have shown that the level of CD47 at the surface of PMNs was also increased following transepithelial migration. This molecule has been previously demonstrated to be involved in the migration of the PMNs (26). This increase could facilitate the paracellular passage of the PMNs between two epithelial cells. It has been previously shown that CD47-deficient mice have increased numbers of bacterial infections (17). Under our conditions, no modification of the number of bacteria inside the transmigrated PMNs was detected after preincubation with anti-CD47 antibodies in the lower reservoir, suggesting that the increased expression of CD47 might facilitate PMN transmigration but cannot account for the postmigration increased phagocytosis.
Expression of CD16 (FcγRIII) was diminished at the surface of PMNs upon transmigration. It has been previously shown that this molecule, which binds human IgG1 and IgG3, is involved in phagocytosis of opsonized particles (28). In our study, the decrease in CD16 could be due to the presence of lipopolysaccharide secreted by the bacteria or by the activation by agents such as fMLP, which have been reported to induce shedding of FcγRIIIB (13). However, it is difficult to draw any conclusion from these data, since neutrophil FcγRIIIB was shown to be sensitive to digestion by leukocyte elastase and pronase (32), suggesting that at a site of inflammation, the function of FcγRIIIB may be largely diminished.
In contrast to what we observed for CD11b/CD18, CD47, and CD16, surface expression of the antigens CD29, CD49e, and CD49f was not modified on transmigrated PMNs. These molecules have been described to behave as receptors for Yersinia (15). Moreover, these antigens have been involved in the upregulation of CD11b after interaction of PMNs with the matrix proteins of the basal lamina such as laminin or fibronectin (31). The receptor that mediates responses to fibronectin has been identified as being an integrin of the β3 family (10). However, in our model this mode of upregulation of CD11b is unlikely, because a very small amount of matrix is present at the basal side of the cultured monolayers.
PMNs are the principal circulating phagocytes, with a 5- to 6-h half-life in the circulation before they undergo apoptosis (30). It has been proposed that PMN apoptosis can be modulated by different effectors, but the mechanisms underlying these regulations remain incompletely understood. PMN apoptosis is associated with an impairment of functional activity in response to soluble mediators, as demonstrated for shape change, phagocytosis, degranulation and the respiratory burst (36). Apoptotic PMNs show diminished expression of CD16 on the cell surface (8). During the apoptotic process, the plasma membrane of apoptotic PMNs remains intact, thus preventing the release of the toxic contents of these cells. Although the lifetime of PMNs is short, it can be modulated by various mediators. Granulocyte-macrophage colony-stimulating factor is known to prolong the lifetime of PMNs by inhibiting apoptosis (5). In contrast, TNF-α accelerates the initiation of programmed cell death of human PMNs (24). TNF-α-mediated apoptosis of human PMNs seems to involve the β2 integrin (CD11/CD18) (34). In this regard, it is noteworthy that activated PMNs, which have transmigrated through the epithelial monolayer, exhibited a decreased CD16 expression and an increased CD11b/CD18 expression associated with a slightly accelerated apoptotic program. In fact, apoptosis of transmigrated PMNs began after 12 h, whereas the first sign of DNA fragmentation in nontransmigrated PMNs appeared only after 16 h. The role of this migration-mediated acceleration of the apoptotic program might be to attenuate the inflammation in the intestinal lumen, since in our model the NADPH-driven oxygen radical production in postmigrated PMNs remained unaffected.
We have previously shown that the actin cytoskeleton of the epithelial cells constrains the passage of the PMNs from the lumen to the chorion and retains the PMNs in the lumen (12). While we cannot draw conclusions about the origin of the augmentation of CD11b/CD18 in transmigrated neutrophils (migration-induced translocation of intracellular pools versus selection of a faster-migrating CD11b/CD18 overexpression subpopulation), our study provides evidence that transepithelial migration is accompanied by potentialization of the phagocytic function of PMNs, probably via an increase of CD11b/CD18 expression. This helps in our understanding of the mechanism through which neutrophils intervene in the defense against bacteria within the intestinal lumen.
ACKNOWLEDGMENTS
We are grateful to Mireille Mari and Dominique Sadoulet for their technical assistance.
REFERENCES
- 1.Anderson D C, Springer T A. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150, 95 glycoproteins. Annu Rev Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- 2.Berger M, O'Shea J, Crous A S, Folks T M, Chused T M, Brown E J, Frank M M. Human neutrophils increase expression of C3bi as well as C3b receptors upon activation. J Clin Investig. 1984;74:1566–1571. doi: 10.1172/JCI111572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalfie M, Euskirchen G, Ward W, Prasher D C. Green fluorescent protein as a marker for gene expression. Science. 1994;263:303–305. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 4.Cormack B P, Valvivia R F, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- 5.Cox G, Gauldie J, Jordana M. Bronchial epithelial cell-derived cytokines (G-CSF and GM-CSF) promote the survival of peripheral blood neutrophils in vitro. Am J Respir Cell Mol Biol. 1992;7:507–513. doi: 10.1165/ajrcmb/7.5.507. [DOI] [PubMed] [Google Scholar]
- 6.Coxon A, Rieu P, Barkalow F J, Askari S, Sharpe A H, Von Adrian U H, Arnaout M A, Mayadas T N. A novel role for the β2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity. 1996;5:653–666. doi: 10.1016/s1074-7613(00)80278-2. [DOI] [PubMed] [Google Scholar]
- 7.Detmers P A, Wright S D, Olsen E, Kimball B, Cohn Z A. Aggregation of complement receptors on human neutrophils in the absence of ligands. J Cell Biol. 1987;105:1137–1145. doi: 10.1083/jcb.105.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dransfield I, Buckle A M, Savill J S, McDowall A, Haslett C, Hogg N. Neutrophil apoptosis is associated with a reduction in CD16 (FcgRIII) expression. J Immunol. 1994;153:1254–1263. [PubMed] [Google Scholar]
- 9.Gerdes H, Kaether C. Green fluorescent protein: applications in cell biology. FEBS Lett. 1996;389:44–47. doi: 10.1016/0014-5793(96)00586-8. [DOI] [PubMed] [Google Scholar]
- 10.Gresham H D, Goodwin J L, Allen P M, Anderson D C, Brown E J. Characterization of the Arg-Gly-Asp binding proteins of human monocytes and polymorphonuclear leukocytes. J Exp Med. 1988;167:777–793. doi: 10.1084/jem.167.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gresham H D, Graham I L, Anderson D C, Brown E J. Leukocyte adhesion-deficient neutrophils fail to amplify phagocytic function in response to stimulation. J Clin Investig. 1991;88:588–597. doi: 10.1172/JCI115343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofman P, D'Andrea L, Carnes D, Colgan S P, Madara J L. Intestinal epithelial cytoskeleton selectively constrains lumen-to-tissue migration of neutrophils. Am J Physiol. 1996;271:312–320. doi: 10.1152/ajpcell.1996.271.1.C312. [DOI] [PubMed] [Google Scholar]
- 13.Huizinga T W, van der Schoot C E, Jost C. The PI-linked receptor FcRIII is released on stimulation of neutrophils. Nature. 1988;333:667–669. doi: 10.1038/333667a0. [DOI] [PubMed] [Google Scholar]
- 14.Ingalls R R, Arnaoult M A, Delude R L, Flaherty S, Savedra R, Golenbock D T. The CD11/CD18 integrins: characterization of three novel LPS signaling receptors. Prog Clin Biol Res. 1998;397:107–117. [PubMed] [Google Scholar]
- 15.Isberg R, Leong J M. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 16.Jones J, Morgan B P. Apoptosis is associated with reduced expression of complement regulatory molecules, adhesion molecules and other receptors on polymorphonuclear leucocytes: functional relevance and role in inflammation. Immunology. 1995;86:651–660. [PMC free article] [PubMed] [Google Scholar]
- 17.Lindberg F P, Bullard D C, Caver T E, Gresham H D, Beaudet A L, Brown E J. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science. 1996;274:795–798. doi: 10.1126/science.274.5288.795. [DOI] [PubMed] [Google Scholar]
- 18.Madara J L, Stafford J, Dharmasathaphorn K, Carlson S. Structural analysis of a human intestinal epithelial cell line. Gastroenterology. 1987;92:1133–1145. doi: 10.1016/s0016-5085(87)91069-9. [DOI] [PubMed] [Google Scholar]
- 19.Madara J L, Colgan S, Nusrat A, Delp C, Parkos C. A simple approach to measurement of electrical parameters of cultured epithelial monolayers: use in assessing neutrophil-epithelial monolayers. J Tiss Cult Meth. 1992;14:209–216. [Google Scholar]
- 20.Madara J L, Parkos C, Nash S, Matthews J, Delp C, Wayne L. Chloride secretion in a model intestinal epithelium induced by a neutrophil derived secretagogue. J Clin Investig. 1992;89:1938–1944. doi: 10.1172/JCI115800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin S J, Lennon S V, Bonham A M, Cotter T G. Induction of apoptosis (programmed cell death) in human leukemic HL-60 cells by inhibition of RNA or protein synthesis. J Immunol. 1990;145:1859–1867. [PubMed] [Google Scholar]
- 22.McCormick B A, Hofman P M, Kim J, Carnes D K, Miller S I, Madara J L. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J Cell Biol. 1993;131:1599–1608. doi: 10.1083/jcb.131.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCormick B A, Nusrat A, Parkos C A, D'Andrea L, Hofman P M, Carnes D, Liang T W, Madara J L. Unmasking of intestinal lateral membrane β1 integrin consequent to transepithelial neutrophil migration in vitro facilitates inv-mediated invasion by Yersinia pseudotuberculosis. Infect Immun. 1997;65:1414–1421. doi: 10.1128/iai.65.4.1414-1421.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohata H, Yatomi Y, Sweeney E A, Hakomori S, Igarashi Y. A possible role of sphingosine in induction of apoptosis by tumor necrosis factor-α in human neutrophils. FEBS Lett. 1994;355:267–270. doi: 10.1016/0014-5793(94)01218-0. [DOI] [PubMed] [Google Scholar]
- 25.Parkos C A, Delp C, Arnaout M A, Madara J L. Neutrophil migration across a cultured intestinal epithelium. Dependence on a CD11b/CD18 mediated event and enhanced efficiency in physiological direction. J Clin Investig. 1991;88:1605–1612. doi: 10.1172/JCI115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parkos C A, Colgan S P, Liang T W, Nusrat A, Bacarra A E, Carnes D K, Madara J L. CD47 mediates post-adhesive events required for neutrophil migration across polarized intestinal epithelia. J Cell Biol. 1996;132:437–450. doi: 10.1083/jcb.132.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Philpott D J, McKay D M, Sherman P M, Perdue M H. Infection of T84 cells with enteropathogenic Escherichia coli alters barrier and transport functions. Am J Physiol. 1996;270:634–645. doi: 10.1152/ajpgi.1996.270.4.G634. [DOI] [PubMed] [Google Scholar]
- 28.Revetch J V, Kinet J P. Fc receptors. Annu Rev Immunol. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 29.Rieu P, Lesavre P, Halbwachs-Mecarelli A. Evidence for integrins other than β2 on polymorphonuclear neutrophils: expression of α6β1 heterodimer. J Leukoc Biol. 1993;53:576–582. doi: 10.1002/jlb.53.5.576. [DOI] [PubMed] [Google Scholar]
- 30.Savill J S, Wyllie A H, Henson J E, Walport M J, Henson P M, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation: programmed cell death in the neutrophil leads to recognition by macrophages. J Clin Investig. 1989;83:865. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simms H, D'Amico R. Regulation of polymorphonuclear neutrophil CD16 and CD11b/CD18 expression by matrix proteins during hypoxia is VLA-5, VLA-6 dependent. J Immunol. 1995;155:4979–4990. [PubMed] [Google Scholar]
- 32.Tosi M F, Berger M F. Functional differences between the 40 kDa and 50-70 kDa IgG Fc receptors on human neutrophils revealed by elastase treatment and anti-receptor antibodies. J Immunol. 1988;141:2097–2106. [PubMed] [Google Scholar]
- 33.Troelstra A, de Graaf-Miltenburg L A, van Bommel T, Verhoef J, van Kessel K P, van Strijp J A. Lipopolysaccharide-coated erythrocytes activate human neutrophils via CD14 while subsequent binding is through CD11b/CD18. J Immunol. 1999;162:4220–4225. [PubMed] [Google Scholar]
- 34.Walzog B, Jeblonski F, Zakrzewicz A, Gaehtgens P. β2 integrins (CD11/CD18) promote apoptosis of human neutrophils. FASEB J. 1997;11:1177–1186. doi: 10.1096/fasebj.11.13.9367353. [DOI] [PubMed] [Google Scholar]
- 35.Watson F, Robinson J, Edward S W. Protein Kinase C-dependent and -independent activation of the NADPH oxidase of human neutrophils. J Biol Chem. 1991;266:7432–7439. [PubMed] [Google Scholar]
- 36.Whyte M K B, Meagher L C, MacDermont J, Haslett C. Impairment of function in aging neutrophils is associated with apoptosis. J Immunol. 1993;150:5124–5134. [PubMed] [Google Scholar]
- 37.Winterbourn C C. Neutrophil oxidants: production and reactions. In: Das D K, Essman W B, editors. Oxygen radicals: systemic events and disease processes. S. Basel, Switzerland: Karger; 1990. pp. 31–70. [Google Scholar]
- 38.Wright S D, Jong M T C. Adhesion-promoting receptors on human macrophages recognize E. coli by binding to lipopolysaccharide. J Exp Med. 1986;164:1876–1888. doi: 10.1084/jem.164.6.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wright S D, Levin S M, Jong M T C, Chad Z, Kabbash L G. CR3 (CD11b/CD18) expresses one binding site for Arg-Gly-Asp-containing peptides, and a second site for bacterial lipopolysaccharide. J Exp Med. 1989;169:175–183. doi: 10.1084/jem.169.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]