Abstract
Objective
Robot-assisted coronary artery bypass (RCAB) is typically not offered to higher risk patients with reduced cardiopulmonary function, critical coronary artery disease, and challenging chest wall anatomy. In this study, we report the novel use of nonemergency intraoperative peripheral extracorporeal membrane oxygenation as partial cardiopulmonary support during RCAB for patients who were considered high-risk candidates for conventional CAB and at the same time not eligible for RCAB without cardiopulmonary support.
Methods
Forty-five high risk patients (mean age, 68 years; Society of Thoracic Surgeons score, 6.27%; ejection fraction, 45%) underwent RCAB with nonemergency peripheral extracorporeal membrane oxygenation support for the following indications: inability to tolerate single-lung ventilation (n = 17; 38%), low ejection fraction <35% (n = 17; 38%), inadequate exposure of internal thoracic artery (n = 24; 53%), critical coronary artery disease (n = 16; 36%), and hemodynamic instability after anesthesia induction (n = 3; 7%). Following robotic internal thoracic artery takedown, all patients had beating heart minimally invasive direct CAB through a 2-inch minithoracotomy.
Results
Up to 30 days, there were no strokes (0%), myocardial infarctions (0%), or access vessel complications (0%). One noncardiac related mortality (2.2%) was related to hemodialysis access issues in a patient with preexisting end-stage renal disease. One redo-CAB (2.2%) patient required sternotomy to locate the target vessel. Thirty-four (75.6%) patients were extubated within 6 hours of surgery.
Conclusions
Our results examine the feasibility of using peripheral extracorporeal membrane oxygenation during RCAB for high-risk patients who otherwise had limited options. The use of peripheral extracorporeal membrane oxygenation in RCAB can potentially expand the surgical treatment options in high-risk coronary artery disease patients.
Key Words: minimally invasive cardiac surgery, robotic coronary artery bypass, MIDCAB, ECMO
Abbreviations and Acronyms: CAB, coronary artery bypass; CTA, computed tomography angiogram; ECMO, extracorporeal membrane oxygenation; ITA, internal thoracic artery; LAD, left anterior descending; LITA, left internal thoracic artery; MIDCAB, minimally invasive direct coronary artery bypass; RCAB, robot-assisted coronary artery bypass; RITA, right internal thoracic artery; STS, Society of Thoracic Surgeons; TEE, transthoracic echocardiogram
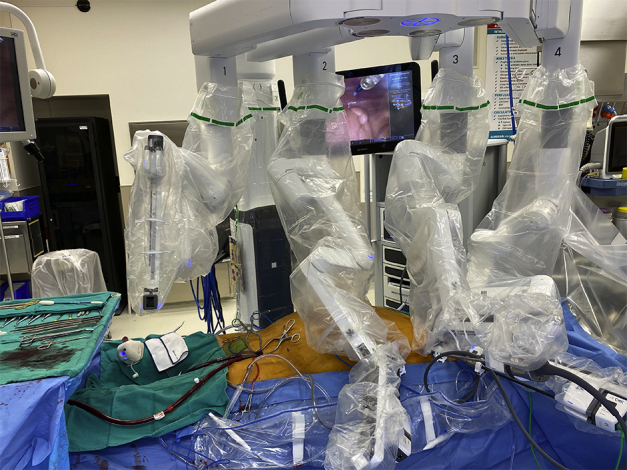
RCAB with peripheral ECMO.
Central Message.
The use of peripheral ECMO expands the application of RCAB to higher-risk patients.
Perspective.
RCAB with ECMO support is a novel revascularization strategy that may redefine the indications for minimally invasive CAB.
The rationale for minimally invasive coronary artery bypass (CAB) surgery is based on the principle that the left internal thoracic artery (LITA) to left anterior descending artery (LAD) anastomosis confers the greatest survival advantage compared to other bypasses.1, 2, 3 The benefit of a minimally invasive approach is the reduced risk of stroke (no aortic manipulation), faster recovery, and reduced need for transfusions while maintaining the same graft patency rate as conventional CAB surgery.1 Minimally invasive direct CAB (MIDCAB) has a 96.8% 10-year LITA-LAD patency rate.4 Davierwala and colleagues5 published the largest MIDCAB experience with the longest follow-up to date, noting excellent 10-year (77%) and 20-year (55%) survival. Disadvantages of MIDCAB include limited applicability (lower-risk patients with preserved ventricular function and primarily single-vessel disease),4,5 the need for single-lung ventilation, limited dissection of ITA, pain from the chest wall retractor, and the learning curve for off-pump procedures. All of these factors have hindered wider adoption of minimally invasive CAB surgery.
Robotic technology, which was initially envisioned for cardiac surgery but popularized through abdominopelvic surgery, is being utilized once again in cardiac surgery. The 2 robotic approaches for coronary surgery are totally endoscopic CAB and robot-assisted CAB (RCAB). RCAB involves robotic thoracic artery takedown followed by a conventional MIDCAB anastomosis through a left minithoracotomy. Robotic technology provides a significant advantage from MIDCAB because it allows greater visualization, a thorough proximal and distal dissection of the ITA, identification of the target vessel before minithoracotomy, and the ability to harvest the right internal thoracic artery (RITA) in total arterial revascularization. Ultimately, this translates to greater accuracy, a more limited minithoracotomy, less pain, and faster recovery compared with MIDCAB. RCAB outcomes are equivalent to MIDCAB with a 93.4% graft patency rate at 8 years,6 0.6% to 1.6% mortality at 30 days,7 0.3% to 1.5% stroke rate at 30 days,7 and 1% to 11% rate of repeat vascularization.8 Consistent with the challenges of MIDCAB, RCAB is generally not applicable to higher-risk patients who cannot tolerate single-lung ventilation due to poor pulmonary reserve, reduced cardiac function, prior cardiac surgery, challenging chest wall anatomy, and critical coronary artery disease.
In this study, we report the novel use of nonemergency intraoperative peripheral extracorporeal membrane oxygenation (ECMO) as partial cardiopulmonary support during RCAB for patients who were considered high-risk candidates for conventional CAB and at the same time not eligible for RCAB without cardiopulmonary support. The utilization of peripheral ECMO expands the indications of RCAB to higher-risk patients with reduced cardiopulmonary function, critical coronary artery disease, and challenging chest wall anatomy (ie, previous chest radiation, sternotomy, cardiomegaly, elevated hemidiaphragm, and obesity). Additionally, performing RCAB with ECMO support increases intraprocedural safety, reduces conversion to sternotomy, and provides a model for the adoption of minimally invasive techniques.
Methods
We performed a retrospective review of 137 RCAB procedures performed at Baylor St Luke's Medical Center in Houston, Tex, from April 2019 to October 2021. A total of 67.2% low-risk patients underwent RCAB without ECMO. In these cases, peripheral sheaths were placed as a contingency measure for ECMO. Forty-five (32.8%) high-risk patients (mean Society of Thoracic Surgeons [STS] score, 6.27% ± 7.25%) underwent RCAB with intraoperative ECMO support, which was usually determined preoperatively based on risk factors and imaging. The high-risk patients included in the study were not eligible for conventional minimally invasive bypass techniques due to their inability to tolerate single-lung ventilation, critical coronary artery anatomy, low ejection fraction, or challenging chest wall anatomy (ie, previous chest radiation, sternotomy, cardiomegaly, elevated hemidiaphragm, and obesity). Additionally, due to their comorbidities and poor functional status, they were also deemed high risk for sternotomy. Because most patients had disease not amenable to percutaneous coronary intervention, these no-option patients were considered for RCAB with ECMO support as a bailout procedure. Exclusion criteria included no options for femoral or axillary cannulation; decompensated cirrhosis; active infection; evolving stroke; or any disease condition, including malignancy; with a expected life expectancy <2 years.
Patient demographic characteristics and 30-day outcomes are reported as mean values with standard deviation where applicable. Institutional review board approval (Baylor College of Medicine IRB No. H-43621) was obtained for this study. Patient consent was obtained for publishing intraoperative photographs and videos, consistent with institutional policy.
Preoperative Evaluation
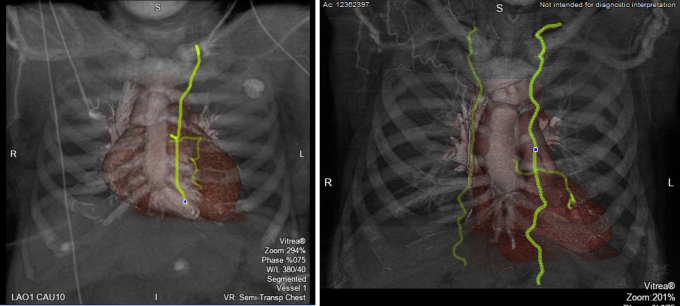
All cases were performed by a single cardiac surgeon experienced in minimally invasive techniques. Preoperative evaluation included coronary angiography, computed tomography angiogram (CTA) of the chest/abdomen/pelvis, transthoracic echocardiogram (TEE), and carotid duplex ultrasonography. The CTA was used to evaluate conduit adequacy and length, the depth of the target vessel (intramyocardial vs epicardial), and suitability of peripheral vessels (femoral or axillary) for cannulation. When feasible, 3-dimensional reconstruction of the conduit and target vessels may also be performed (Figure 1). The location of the incision; that is, correct interspace for the minithoracotomy, was also confirmed with CT imaging. Additional anatomic factors such as cardiomegaly that would preclude adequate visualization without ECMO were also noted.
Figure 1.
Preoperative computerized tomography angiography (CTA). CTA with 3-dimensional reconstruction allows evaluation of the target and conduit for optimal preoperative planning.
Operative Technique
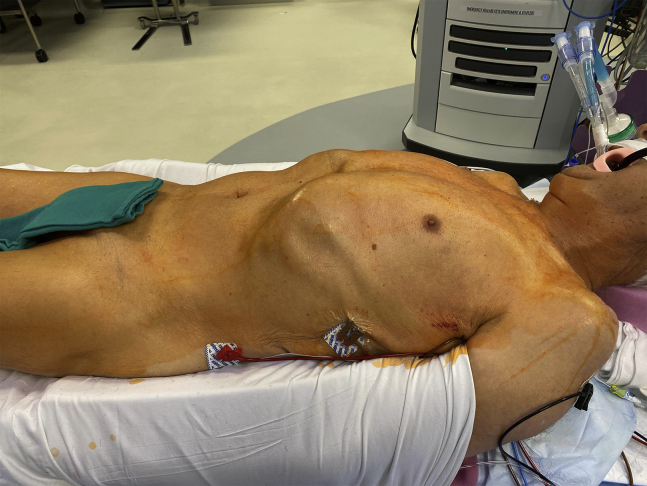
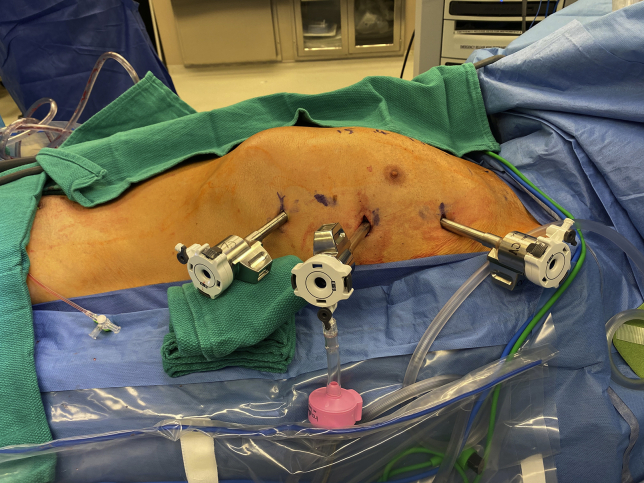
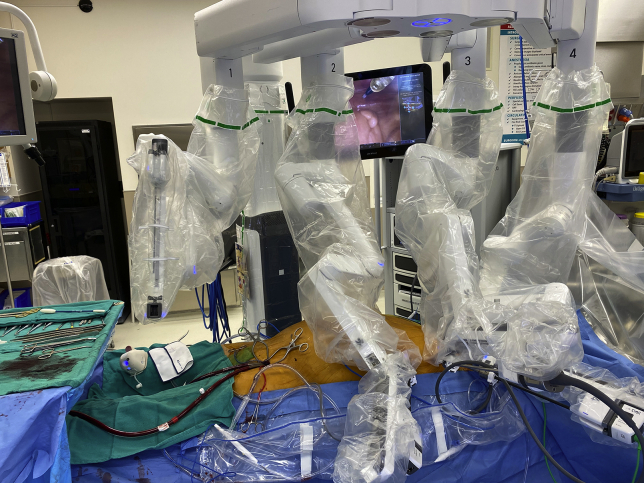
Our approach to RCAB, which consists of robotic ITA takedown, minithoracotomy and MIDCAB anastomosis, has been previously described in detail.9 Video 1 highlights the key intraoperative steps. Following general anesthesia with a double-lumen endotracheal tube, patients received a peripheral erector spinae nerve block with ropivacaine (Naropin; AstraZeneca) when feasible. The left chest was elevated with a bump and the left arm tucked (Figure 2). Ports were typically placed in a straight line through the third, fifth, and seventh interspace (or occasionally the sixth interspace) as shown in Figures 3 and 4. The Da Vinci Xi (Intuitive Surgical Inc) was used to take down the LITA and, when indicated, the RITA. The pericardium was opened to confirm the target location and verify the appropriate interspace for the minithoracotomy. A standard MIDCAB anastomosis was performed after snaring the proximal and distal target vessel with silastic bands. Intraoperative TEE was used in every case to monitor for wall motion abnormalities during coronary occlusion. A coronary shunt was used to assist with the anastomosis. A Doppler probe was used to confirm flow after removing the snares. Through a coordinated strategy with anesthesia, which includes minimization of narcotics and the use of regional nerve blocks when feasible, patients were fast-tracked for early extubation.
Figure 2.
Positioning for robot-assisted coronary artery bypass. Following double-lumen endotracheal tube placement, a bump is placed under the left chest and both arms are tucked. This exposes the anterior axillary line for port placement.
Figure 3.
Port placement for robot-assisted coronary artery bypass. Ports are placed in a straight line along the anterior axillary line, within the third, fifth, and seventh interspaces.
Figure 4.
Set-up for robot-assisted coronary artery bypass with peripheral extracorporeal membrane oxygenation (ECMO). Peripheral cannulation for ECMO provides partial cardiopulmonary support for internal thoracic artery (ITA) harvest and the ITA to coronary artery anastomosis.
Intraoperative ECMO
The indications for ECMO support included the inability to tolerate single-lung ventilation due to chronic lung disease, critical coronary artery anatomy (eg, left main disease with significant concomitant right coronary disease, or left dominant coronary circulation precluding safe proximal occlusion), cardiogenic shock or low ejection fraction (<35%), inadequate exposure of the ITA conduit (ie, chest radiation, previous sternotomy, cardiomegaly, elevated diaphragm, or obesity), and hemodynamic instability or arrhythmia after anesthesia induction. In almost all cases, the decision to use intraoperative ECMO was based on preoperative CT and echocardiograph imaging, as well as the patient's ability to tolerate single-lung ventilation after anesthesia. Peripheral cannulation was performed via the femoral vessels and occasionally, over the axillary vessels if severe peripheral arterial disease was present. Before cannulation, heparin was administered to target an activated clotting time of 180 to 300 seconds. Following TEE guidance to confirm wire placement, a 17 to 21 Fr arterial cannula and a 25 Fr multistage venous cannula were placed. ECMO flow was maintained at 3 to 5 L/minute. During the anastomosis, an additional dose of heparin was given to target an activated clotting time of ≥300 seconds. Following decannulation, the vessels were primarily repaired and protamine administered. When ECMO was not necessary, percutaneous femoral sheaths were placed as a precaution, in case emergent peripheral cannulation was required.
Results
Patient Demographic Characteristics
Of the 137 RCAB cases performed between 2019 and 2021, 45 (32.8%) high-risk patients underwent RCAB with nonemergency intraoperative ECMO via peripheral cannulation. As indicated in Table 1, the high-risk cohort of patients (mean STS score, 6.27% ± 7.25%) had multiple comorbidities, including chronic kidney disease (n = 15; 33.3%), chronic obstructive pulmonary disease (n = 15; 33.3%), stroke (n = 9; 20.0%), prior percutaneous coronary intervention (n = 18; 40.0%), previous sternotomy (n = 4; 8.9%) and an average ejection fraction of 45% ± 14%. Comorbidities not well accounted for in the STS score were cirrhosis (n = 7; 15.6%) and malignancy (n = 4; 8.9%) with a life expectancy >2 years.
Table 1.
Demographic characteristics of patients who underwent robot-assisted coronary artery bypass with intraoperative extracorporeal membrane oxygenation support
| Metric | n | Result |
|---|---|---|
| STS estimated mortality (%) | 6.27 ± 7.25 | |
| Age (y) | 67.7 ± 9.2 | |
| Male sex | 29 | 64.4 |
| DM | 23 | 51.1 |
| HTN | 44 | 97.8 |
| CKD | 15 | 33.3 |
| COPD | 15 | 33.3 |
| PAD | 15 | 33.3 |
| History of stroke | 9 | 20.0 |
| History of MI | 27 | 60.0 |
| Preoperative ejection fraction (%) | 45.0 ± 13.5 | |
| Previous sternotomy | 4 | 8.9 |
| Prior PCI | 18 | 40.0 |
Values are presented as mean ± standard deviation or n (%). STS, Society of Thoracic Surgeons; DM, diabetes mellitus, HTN, hypertension; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; PAD, pulmonary artery disease; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Procedure
The indications for the implementation of intraoperative ECMO support during RCAB are summarized in Table 2. Most patients had more than 1 indication with the combination of chronic lung disease (n = 17; 38%), critical coronary anatomy (n = 16; 36%), and low ejection fraction (n = 17; 38%) being the most common. Rarely, hemodynamic instability or arrhythmia after anesthesia induction (n = 3; 7%) necessitated the use of ECMO. All 45 patients underwent revascularization with a LITA-LAD bypass, whereas 10 patients (22.2%) underwent an additional RITA bypass to the ramus, obtuse marginal, diagonal, or right coronary artery. Twenty patients (44.4%) were selected for a hybrid approach with percutaneous coronary intervention, which was typically performed after the RCAB to avoid the risk of bleeding with P2Y12 inhibitors.
Table 2.
Indications for the implementation of elective intraoperative extracorporeal membrane oxygenation support during robot-assisted coronary artery bypass
| Indication | n | % |
|---|---|---|
| Chronic lung disease or inability to tolerate single lung vent | 17 | 38 |
| Critical coronary anatomy | 16 | 36 |
| Preoperative cardiogenic shock or low ejection fraction | 17 | 38 |
| Inadequate exposure of the internal thoracic artery∗ | 24 | 53 |
| Postinduction hemodynamic instability/arrhythmia | 3 | 7 |
Reasons for inadequate exposure included adhesions from previous chest radiation or sternotomy, cardiomegaly, elevated hemidiaphragm, and obesity.
Outcomes of RCAB With ECMO Support
In this high-risk group of patients, there were no strokes, access vessel complications, or myocardial infarctions at 30 days (Table 3). One noncardiac mortality (2.2%) occurred in a patient with preexisting end-stage renal disease who developed an arrhythmia and cardiac arrest following a delay in hemodialysis due to access issues. The patient had a coronary angiogram demonstrating a patent LITA-LAD graft. One redo case (2.2%) required conversion to sternotomy because of difficulty exposing an intramyocardial target vessel. One patient (2.2%), who presented in cardiogenic shock, was not weaned off ECMO support in the operating room and was decannulated on postoperative day 2. The mean ECMO time was 260.4 ± 237.1 minutes, and the mean operating room time was 325.9 ± 89.0 minutes. The blood transfusion rate was 37.8% (n = 17). Transient renal insufficiency not requiring hemodialysis occurred in 11.1% (n = 5) of patients. The incidence of pneumonia was 8.9% (n = 4), whereas groin infections were noted in 4.4% (n = 3) of patients.
Table 3.
Thirty-day outcomes of patients who underwent robot-assisted coronary artery bypass with intraoperative extracorporeal membrane oxygenation (ECMO) support
| Metric | n | % |
|---|---|---|
| Mortality | 1 | 2.2 |
| CVA | 0 | 0.0 |
| Myocardial infarction | 0 | 0.0 |
| Conversion to open sternotomy | 1 | 2.2 |
| Failure to wean intraoperative ECMO support | 1 | 2.2 |
| Access vessel complication | 0 | 0.0 |
| Blood transfusion | 17 | 37.8 |
| Extubated within 6 h | 34 | 75.6 |
| Extubated after 6 h | 11 | 24.4 |
| Transient renal insufficiency | 5 | 11.1 |
| Pneumonia | 4 | 8.9 |
| Surgical Site Infection | 3 | 4.4 |
| Mean operating room time∗ (min) | – | 325.9 ± 89.0 |
| Mean ECMO time∗ (min) | – | 260.4 ± 237.1 |
| Average length of stay∗ (d) | – | 8.8 ± 9.5 |
| Discharge to home | 38 | 86.4 |
| Discharge to facility | 6 | 13.6 |
CVA, Cardiovascular accident.
Values are presented as mean ± standard deviation.
Ten patients (22.2%) were extubated in the operating room, whereas a total of 34 patients (75.6%) were extubated within 6 hours of surgery, and ultimately, 41 (91.2%) within 24 hours (Table 4). Four patients (8.9%) had a prolonged intubation, >24 hours. The average length of stay was 8.8 ± 9.5 days. 86% (n = 38) of patients were discharged home and 14% (n = 6) to a facility (eg, rehab, long-term acute care, or skilled nursing facility).
Table 4.
Extubation times of patients undergoing robot-assisted coronary artery bypass with extracorporeal membrane oxygenation support
| Extubation time/site | n | % |
|---|---|---|
| On table | 10 | 22.2 |
| Within 6 h in the ICU | 24 | 53.3 |
| Within 24 h in the ICU | 7 | 15.6 |
| >24 h | 4 | 8.9 |
ICU, Intensive care unit.
Discussion
The patient population requiring CAB surgery is increasingly elderly with a growing number of comorbidities. Unfortunately, as minimally invasive and RCAB techniques have evolved, the indications for surgery have never been clearly defined. Paradoxically, most of the patients selected for a minimally invasive approach tend to be lower risk with preserved ventricular function and primarily single-vessel disease.4,5 This strategy unfortunately hinders higher-risk patients who would benefit the most from a minimally invasive approach with a reduced stroke rate (no aortic manipulation), faster recovery, decreased risk of infection, and reduced rate of transfusion.1
In this study, we report our experience with the novel use of intraoperative peripheral ECMO during RCAB because partial cardiopulmonary support for very-high-risk patients who otherwise had no other revascularization options. This strategy allowed us to expand the application of RCAB to patients with reduced cardiac and pulmonary function, critical coronary artery disease/anatomy, and challenging chest wall anatomy (eg, previous chest radiation, sternotomy, cardiomegaly, elevated hemidiaphragm, or obesity). There were no strokes, access vessel complications, or myocardial infarctions at 30 days. Ninety-eight percent of patients were weaned off ECMO support in the operating room. Utilizing a fast-track anesthesia strategy based on established enhanced recovery after cardiac surgery, 75.6% were extubated within 6 hours.10 Most of the patients from this high-risk cohort (86%) were discharged home, albeit at a length of stay of 8.8 ± 9.5 days. Taken together, our experience examined the feasibility of RCAB with ECMO for higher-risk patients with coronary artery disease.
We propose that RCAB with intraoperative ECMO support serve as a model for next-generation minimally invasive CAB. It represents the evolution of the technological advances in robotic surgery and cardiopulmonary support. Allowing greater visualization, higher accuracy, and better hemodynamics compared with MIDCAB, RCAB with ECMO support confers significant advantages over the MIDCAB approach. Furthermore, the added hemodynamic support by ECMO provides a margin of safety in the operating room by potentially reducing the risk of conversion to sternotomy. It is well established that conversion to sternotomy in off-pump cases is associated with a significantly higher morbidity and mortality.11,12 By establishing RCAB with ECMO as a safe revascularization strategy, we also hope to redefine the indications for minimally invasive CAB. Historically, the ideal candidates for such approaches were younger, healthier patients with preserved ventricular function and primarily single-vessel disease. Arguably, these patients would have also tolerated a sternotomy quite well. In our opinion, the candidates most likely to benefit from a minimally invasive approach; that is, RCAB with ECMO, are elderly and frail, with poor mobility, reduced rehab capability, and a higher number of comorbidities.
We favor the use of intraoperative short-term ECMO over conventional cardiopulmonary bypass for multiple reasons. ECMO requires a lower dose of heparin, maintains pulsatile flow and may produce less inflammation because there is less hemodilution, hypothermia, and exposure to the air–blood interface.13 Further basic science research comparing the inflammatory response during RCAB with ECMO vs conventional CAB is ongoing from our group. For cases where cardiotomy and cardiac arrest are not required, short-term ECMO is emerging as a suitable alternative to cardiopulmonary bypass. The use of intraoperative ECMO for cardiopulmonary support is already a well-established technique in lung transplantation, which has been associated with reduced morbidity compared with conventional cardiopulmonary bypass.14,15
With this approach, multivessel revascularization may be accomplished with bilateral ITA or a hybrid approach with percutaneous coronary intervention for non-LAD targets. Decompressing the heart with ECMO facilitates bilateral ITA takedown through the same left thoracoscopic ports. In 10 patients (22.2%), we utilized the RITA to bypass the ramus, obtuse marginal, diagonal, or right coronary artery. Repositioning of the heart for these additional targets is, of course, well tolerated with ECMO support. A hybrid revascularization approach with postoperative percutaneous coronary intervention was planned for 20 (44.4%) patients. For higher-risk patients, a hybrid approach offers the durability and survival advantage of the LITA with the reduced adverse events associated with percutaneous coronary intervention (ie, reduced length of stay, risk of transfusion, and need for prolonged ventilation).16 Although randomized data for hybrid revascularization has not been reported, it has emerged as an alternative option for higher-risk patients.1,16 Given the increased number of patients with previous percutaneous coronary intervention (40% in our series), there is certainly a higher demand for a hybrid revascularization approach.
Conclusions
Our primary objective was to illustrate the feasibility of RCAB with ECMO support and report short-term outcomes. To our knowledge, no such approach has been reported in the literature. As with any other novel procedure, several limitations must be noted. Longer-term results, including graft patency and reintervention rate, are important in establishing the durability of the technique. Routine angiography was not performed for any patients in this series, especially because there were no electrocardiograph changes or troponin level elevations consistent with myocardial infarction. As we identify more patients who would benefit from this approach, we plan to perform a CTA at 1 year to evaluate graft patency rates. Another noteworthy observation is the sample size of 45 patients (RCAB with ECMO) out of 137 total patients who underwent RCAB. Although this may appear to be a small sample, it is important to note that a relatively small number of patients qualify for this novel approach. As outlined previously, this approach is an option for patients who are at prohibitively high risk for CAB and at the same time not eligible for RCAB without cardiopulmonary support. Given that the average robotic cardiac program performs <10 cases per year, our center represents a higher-volume center, performing an average of 80 robotic coronary operations per year.17,18 Accruing a higher sample size would certainly multi-institutional adoption, which we hope to inspire. Furthermore, our average length of stay is higher at 8.8 ± 9.5 days, but it is important to note that this high-risk cohort of patients (with an average STS score of 6.27%) would have arguably had an ever higher length of stay if they underwent conventional CAB. RCAB and conventional CAB do have equivalent costs but the addition of percutaneous coronary intervention for hybrid cases is associated with an increased cost.19,20 The addition of ECMO would further increase the cost of the procedure, but this may be offset by the overall reduced morbidity and hospitalization.
Taken together, we provide a novel revascularization strategy, RCAB with nonemergency intraoperative ECMO, as an option for patients who are not eligible for established minimally invasive coronary bypass techniques and at the same time, deemed too high risk for conventional CAB. RCAB with ECMO is a feasible technique with excellent 30-day outcomes (eg, no strokes, access vessel complications, or myocardial infarctions). By expanding the application of RCAB to higher-risk patients, we hope to challenge the dogma that minimally invasive CAB is only for lower-risk patients.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to:https://aats.blob.core.windows.net/media/21%20AM/AM21_A37/AM21_A37_04.mp4.

Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Supplementary Data
Robot-assisted coronary artery bypass preoperative evaluation and surgical technique. The preoperative evaluation, set-up, and intraoperative steps are summarized in this video. Images were obtained with permission from reference 9. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00178-X/fulltext.
References
- 1.Gaudino M., Bakaeen F., Davierwala P., Di Franco A., Fremes S.E., Patel N., et al. New strategies for surgical myocardial revascularization. Circulation. 2018;138:2160–2168. doi: 10.1161/CIRCULATIONAHA.118.035956. [DOI] [PubMed] [Google Scholar]
- 2.Cameron A., Davis K.B., Green G., Schaff H.V. Coronary bypass surgery with internal-thoracic-artery grafts—effects on survival over a 15-year period. N Engl J Med. 1996;334:216–219. doi: 10.1056/NEJM199601253340402. [DOI] [PubMed] [Google Scholar]
- 3.Loop F.D., Lytle B.W., Cosgrove D.M., Stewart R.W., Goormastic M., Williams G.W., et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314:1–6. doi: 10.1056/NEJM198601023140101. [DOI] [PubMed] [Google Scholar]
- 4.Repossini A., Di Bacco L., Nicoli F., Passaretti B., Stara A., Jonida B., et al. Minimally invasive coronary artery bypass: twenty-year experience. J Thorac Cardiovasc Surg. 2019;158:127–138. doi: 10.1016/j.jtcvs.2018.11.149. [DOI] [PubMed] [Google Scholar]
- 5.Davierwala P.M., Verevkin A., Bergien L., von Aspern K., Deo S.V., Misfeld M., et al. Twenty-year outcomes of minimally invasive direct coronary artery bypass surgery: the Leipzig experience. J Thorac Cardiovasc Surg. February 16, 2021 doi: 10.1016/j.jtcvs.2020.12.149. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Currie M.E., Romsa J., Fox S.A., Vezina W.C., Akincioglu C., Warrington J.C., et al. Long-term angiographic follow-up of robotic-assisted coronary artery revascularization. Ann Thorac Surg. 2012;93:1426–1431. doi: 10.1016/j.athoracsur.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 7.Cao C., Indraratna P., Doyle M., Tian D.H., Liou K., Mukholm-Larsen S., et al. A systematic review on robotic coronary artery bypass graft surgery. Ann Cardiothorac Surg. 2016;5:530–543. doi: 10.21037/acs.2016.11.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravikumar N., George V., Shirke M.M., Ashry A., Harky A. Robotic coronary artery surgery: outcomes and pitfalls. J Card Surg. 2020;35:3108–3115. doi: 10.1111/jocs.14988. [DOI] [PubMed] [Google Scholar]
- 9.Liao K.K. Robotic coronary artery bypass grafting. Op Techn Thorac Cardiovasc Surg. 2020;15:194–205. [Google Scholar]
- 10.Lu S.Y., Lai Y., Dalia A.A. Implementing a cardiac enhanced recovery after surgery protocol: nuts and bolts. J Cardiothor Vasc Anesth. 2020;34:3104–3112. doi: 10.1053/j.jvca.2019.12.022. [DOI] [PubMed] [Google Scholar]
- 11.Chakravarthy M., Prabhakumar D., Patil T.A., George A., Jawali V. Conversion during off-pump coronary artery bypass graft surgery: a case-control study. Ann Card Anaesth. 2019;22:18–23. doi: 10.4103/aca.ACA_227_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevens L., Noiseux N., Avezum A., Ayapati D.R., Chen X., Lucchese F.A., et al. Conversion after off-pump coronary artery bypass grafting: the CORONARY trial experience. Eur J Cardiothorac Surg. 2017;51:539–546. doi: 10.1093/ejcts/ezw361. [DOI] [PubMed] [Google Scholar]
- 13.Miller J.E., Fanning J.P., McDonald C.I., McAuley D.F., Fraser J.F. The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit Care. 2016;20:1–10. doi: 10.1186/s13054-016-1570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoetzenecker K., Benazzo A., Stork T., Sinn K., Schwarz S., Schweiger T., et al. Bilateral lung transplantation on intraoperative extracorporeal membrane oxygenator: an observational study. J Thorac Cardiovasc Surg. 2020;160:320–327. doi: 10.1016/j.jtcvs.2019.10.155. [DOI] [PubMed] [Google Scholar]
- 15.Hoetzenecker K., Schwarz S., Muckenhuber M., Benazzo A., Frommlet F., Schweiger T., et al. Intraoperative extracorporeal membrane oxygenation and the possibility of postoperative prolongation improve survival in bilateral lung transplantation. J Thorac Cardiovasc Surg. 2018;155:2193–2206. doi: 10.1016/j.jtcvs.2017.10.144. [DOI] [PubMed] [Google Scholar]
- 16.Moreno P.R., Stone G.W., Gonzalez-Lengua C.A., Puskas J.D., et al. The hybrid coronary approach for optimal revascularization. J Am Coll Cardiol. 2020;76:321–333. doi: 10.1016/j.jacc.2020.04.078. [DOI] [PubMed] [Google Scholar]
- 17.Whellan D.J., McCarey M.M., Taylor B.S., Rosengart T.K., Wallace A.S., Shroyer A.L.W., et al. Trends in robotic-assisted coronary artery bypass grafts: a study of the Society of Thoracic Surgeons adult cardiac Surgery database, 2006 to 2012. Ann Thor Surg. 2016;102:140–146. doi: 10.1016/j.athoracsur.2015.12.059. [DOI] [PubMed] [Google Scholar]
- 18.Harskamp R.E., Brennan J.M., Xian Y., Halkos M.E., Puskas J.D., Thourani V.H., et al. Practice patterns and clinical outcomes after hybrid coronary revascularization in the United States: an analysis from the Society of Thoracic Surgeons adult cardiac database. Circulation. 2014;130:872–879. doi: 10.1161/CIRCULATIONAHA.114.009479. [DOI] [PubMed] [Google Scholar]
- 19.Leyvi G., Schechter C.B., Sehgal S., Greenberg M.A., Snyder M., Forest S., et al. Comparison of index hospitalization costs between robotic CABG and conventional CABG: implications for hybrid coronary revascularization. J Cardiothorac Vasc Anesth. 2016;30:12–18. doi: 10.1053/j.jvca.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 20.Poston R.S., Tran R., Collins M., Reynolds M., Connerney I., Reicher B., et al. Comparison of economic and patient outcomes with minimally invasive versus traditional off-pump coronary artery bypass grafting techniques. Ann Surg. 2008;284:638–646. doi: 10.1097/SLA.0b013e31818a15b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Robot-assisted coronary artery bypass preoperative evaluation and surgical technique. The preoperative evaluation, set-up, and intraoperative steps are summarized in this video. Images were obtained with permission from reference 9. Video available at: https://www.jtcvs.org/article/S2666-2507(22)00178-X/fulltext.