This subgroup analysis of patients with HER2-positive breast cancer with a history of brain metastases from DESTINY-Breast01 demonstrated the strong clinical activity of trastuzumab deruxtecan (T-DXd) in this patient population, warranting continued investigation.
Abstract
DESTINY-Breast01 (NCT03248492) evaluated trastuzumab deruxtecan (T-DXd; DS-8201) in patients with heavily pretreated HER2-positive metastatic breast cancer (mBC). We present a subgroup of 24 patients with a history of treated brain metastases (BM), a population with limited treatment options. In patients with BMs, the confirmed objective response rate (cORR) was 58.3% [95% confidence interval (CI), 36.6%–77.9%], and the median progression-free survival (mPFS) was 18.1 months (95% CI, 6.7–18.1 months). In patients without BMs (n = 160), cORR was 61.3% and mPFS was 16.4 months. Eight patients (47.1%) experienced a best overall intracranial response of partial response or complete response. Seven patients (41.2%) had a best percentage change in brain lesion diameter from baseline consistent with stable disease. Two patients (8.3%) with BMs and two (1.3%) without BMs experienced progression in the brain. The safety profile of T-DXd was consistent with previous studies. The durable clinical activity of T-DXd in this population warrants further investigation.
Significance:
Advances in treating HER2-positive metastatic breast cancer have greatly improved patient outcomes, but intracranial progression remains an important risk for which few therapeutic options are currently available. T-DXd demonstrated durable efficacy in patients with stable, treated BMs.
This article is highlighted in the In This Issue feature, p. 2711
INTRODUCTION
Recent advances in treatment have improved the overall survival (OS) of patients with HER2-positive metastatic breast cancer (mBC; refs. 1–4). However, approximately 22% to 50% of patients with advanced disease will develop brain metastases (BM; refs. 1–4). This relatively high incidence may be a result of improved OS after the initial breast cancer diagnosis due to better control of extracranial disease by HER2-targeted treatments, a biological predilection for HER2-positive breast cancer to metastasize to the brain, and greater detection because of more advanced imaging techniques (5). Patients with mBC with BMs typically have a poorer prognosis and decreased quality of life compared with patients without BMs (1). Initial treatment of BMs in these patients typically involves locally directed therapies, including resection, together with postoperative radiation, stereotactic radiosurgery (SRS), and/or whole-brain radiotherapy (6). Despite the prevalence of central nervous system (CNS) metastases in patients with HER2-positive mBC, clinical trials of HER2-directed therapies have often excluded patients with progressive BMs (7).
For the underlying systemic disease, HER2-directed treatment is administered according to the locally prescribed regimens for HER2-positive mBC (i.e., trastuzumab plus pertuzumab and chemotherapy in the first-line setting; ref. 1). However, the limited ability of some systemic treatments to cross the blood–brain barrier (BBB) and the unique brain tumor microenvironment, characterized by distinct cell types and metabolic limitations, may contribute to poor control of such lesions (1, 8). Thus, additional treatment options are needed to target the BMs frequently observed in patients with HER2-positive mBC.
The tumor microenvironment may cause enhanced vascular permeability, allowing for the extravasation of large molecules, such as antibody–drug conjugates (ADC), through the BBB. In a preclinical study of the HER2-targeting ADC trastuzumab emtansine (T-DM1), it was proposed that the enhanced vascular permeability of the BBB may lead to adequate concentrations of T-DM1 in BMs to elicit a clinical benefit (9). Trastuzumab deruxtecan (T-DXd) is an ADC that comprises a humanized, monoclonal, anti-HER2 antibody with a topoisomerase I inhibitor payload attached via a plasma-stable, tumor-selective, cleavable, tetrapeptide-based linker (10, 11). Based on the DESTINY-Breast01 (NCT03248492) and DESTINY-Breast03 (NCT03529110) studies (12, 13), T-DXd was approved for the treatment of patients with unresectable or metastatic HER2-positive breast cancer after a previous (United States) or ≥2 previous (various countries worldwide) anti-HER2–based regimens (14, 15). In mature results from DESTINY-Breast01, T-DXd demonstrated a confirmed objective response rate (cORR) by independent central review (ICR) of 62.0% (114/184) and a median progression-free survival (mPFS) of 19.4 months in patients with HER2-positive unresectable or mBC previously treated with T-DM1 at a data cutoff of March 26, 2021 (12, 16). In the phase III DESTINY-Breast03 trial, which included patients with HER2-positive mBC previously treated with trastuzumab and a taxane, mPFS was not yet reached [95% confidence interval (CI), 18.5–nonevaluable (NE)] in the T-DXd arm versus 6.8 months (95% CI, 5.6–8.2) in the T-DM1 arm [hazard ratio (HR), 0.28 (95% CI, 0.22–0.37); ref. 13].
Here we describe a subgroup analysis from DESTINY-Breast01, reporting safety and efficacy of T-DXd 5.4 mg/kg in patients with a history of BMs and with stable BMs visible on baseline imaging.
RESULTS
Patient Characteristics
A total of 184 patients received T-DXd 5.4 mg/kg (12), 24 of whom (13.0%) had a history of BMs. Demographic and clinical characteristics of patients in the BM subgroup were generally similar to those of patients in the non-BM subgroup (Supplementary Table S1), although a greater proportion of patients in the BM subgroup had an Eastern Cooperative Oncology Group performance status of 0 (62.5% vs. 54.4%) or had hormone receptor–negative (58.3% vs. 43.1%) breast cancer compared with patients in the non-BM subgroup. A higher proportion of patients in the BM subgroup previously received a HER2 tyrosine kinase inhibitor (TKI; 62.5% vs. 48.8%) or radiotherapy (any location; 83.3% vs. 68.8%) compared with patients in the non-BM subgroup. In the BM subgroup, 58.3% (14/24) of patients had radiotherapy of their CNS lesion (including SRS and whole-brain radiation); 12.5% (3/24) of patients had radiotherapy plus surgery; 4.2% (1/24) of patients had radiotherapy plus surgery and capecitabine; 4.2% (1/24) of patients had surgery only, defined as craniotomy, metastasectomy, or resection/removal of the brain lesion; and 20.8% (5/24) of patients had no reported prior CNS therapy. Patients in both subgroups were heavily pretreated, with a median of six prior therapies in the metastatic setting.
At the time of data cutoff (August 1, 2019), 45.8% (11/24) of patients in the BM subgroup and 42.5% (68/160) of patients in the non-BM subgroup were still receiving T-DXd. Patients in both subgroups primarily discontinued treatment because of progressive disease (PD; 25.0% in the BM subgroup; 29.4% in the non-BM subgroup) and adverse events (12.5% and 15.6%, respectively). Median treatment duration was 11.0 months (range, 0.7–20.2) for the BM subgroup and 9.9 months (range, 0.7–20.5) for the non-BM subgroup.
Efficacy
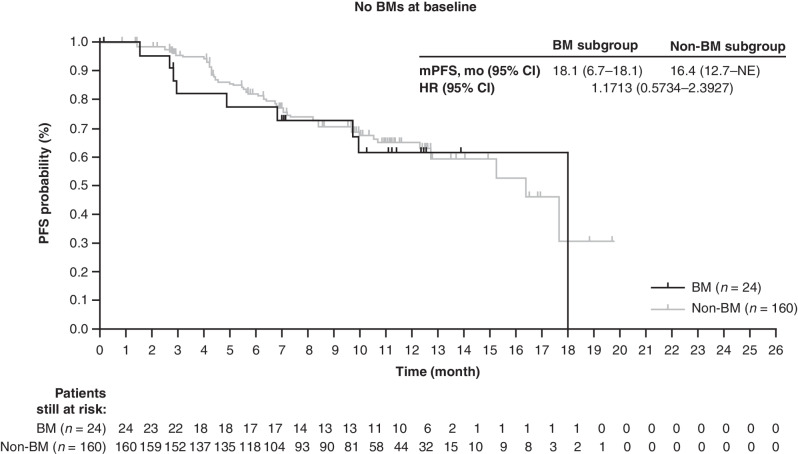
The systemic efficacy of T-DXd 5.4 mg/kg in the BM and non-BM subgroups was consistent with the efficacy observed in patients in the overall study population (Table 1). The primary endpoint, cORR by ICR, was 58.3% (95% CI, 36.6–77.9) in the BM subgroup, with one (4.2%) of these patients achieving a complete response (CR) and 13 (54.2%) a partial response (PR). Of the 10 remaining patients in the BM subgroup, eight (33.3%) had stable disease (SD), resulting in a disease control rate (DCR; CR + PR + SD) of 91.7% (22/24). In the non-BM subgroup, cORR was 61.3% (95% CI, 53.2–68.8), with 10 patients (6.3%) achieving CR and 88 (55.0%) achieving a PR. SD was achieved by 59 patients (36.9%), and DCR was 98.1% (157/160). The median follow-up was 11.0 months (range, 0.7–19.6) for patients with BMs and 11.1 months (range, 2.4–19.9) for patients in the non-BM subgroup. The median time to response with confirmation was 2.8 months (95% CI, 1.3–4.1) for the BM subgroup and 1.5 months (95% CI, 1.4–2.6) for the non-BM subgroup. mPFS was 18.1 months (95% CI, 6.7–18.1) for the BM subgroup and 16.4 months (95% CI, 12.7–NE) for the non-BM subgroup (Fig. 1 and Table 1).
Table 1.
Systemic efficacy of T-DXd 5.4 mg/kg
| BM subgroup (n = 24) | Non-BM subgroup (n = 160) | All patients (N = 184) | |
|---|---|---|---|
| Confirmed ORR (CR + PR) by ICR, n (%)a | 14 (58.3) 95% CI, 36.6–77.9 | 98 (61.3) 95% CI, 53.2–68.8 | 112 (60.9) 95% CI, 53.4–68.0 |
| CR | 1 (4.2) | 10 (6.3) | 11 (6.0) |
| PR | 13 (54.2) | 88 (55.0) | 101 (54.9) |
| SD | 8 (33.3) | 59 (36.9) | 67 (36.4) |
| PD | 1 (4.2) | 2 (1.3) | 2 (1.1) |
| NE | 1 (4.2) | 1 (0.6) | 3 (1.6) |
| BOR in the brain (CR + PR), n (%) b | 8 (47.1) | ||
| DOR (CR or PR), median mo (95% CI) | 16.9 (5.7–16.9) | 14.8 (13.8–NE) | 14.8 (13.8–16.9) |
| PFS, median mo (95% CI) | 18.1 (6.7–18.1) | 16.4 (12.7–NE) | 16.4 (12.7–NE) |
Abbreviations: CR, complete response; BOR, best overall response; DOR, duration of response; PR, partial response; SD, stable disease.
aData are for all randomly assigned patients who received ≥1 dose of T-DXd 5.4 mg/kg and had measurable tumors based on ICR at baseline (N = 184).
bBy investigator assessment. Percentage expressed out of the number of patients with baseline BM measurements as provided by the investigator (n = 17).
Figure 1.
PFS. Kaplan–Meier analyses in the BM and non-BM subgroups. The tick marks in each panel indicate patient data that were censored.
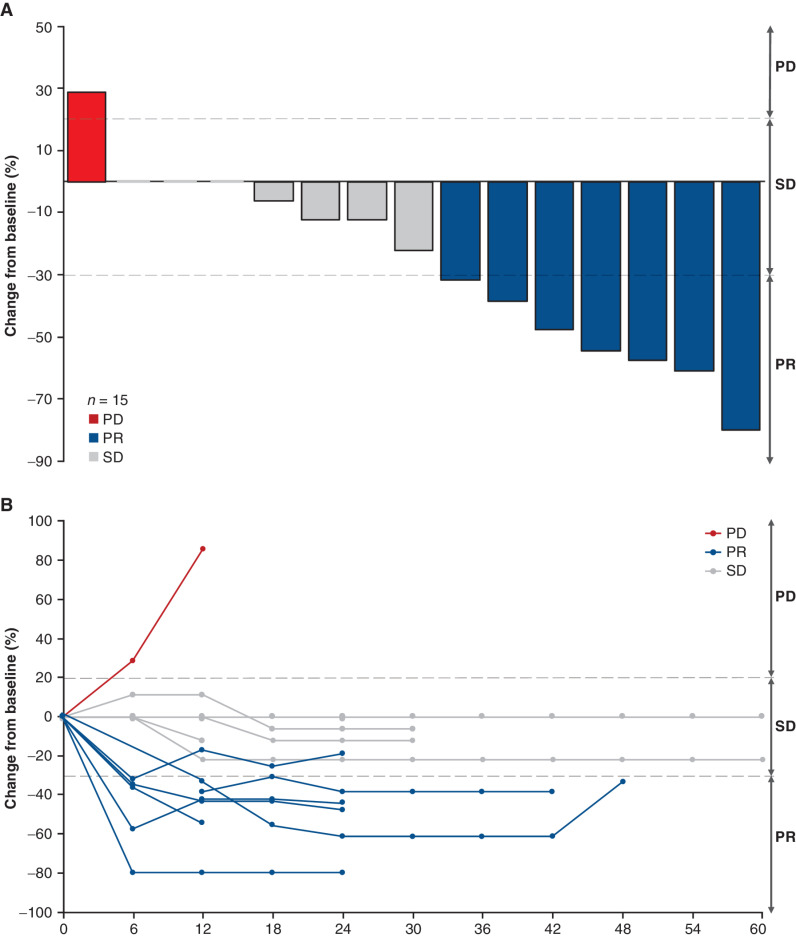
Of the 24 patients in the BM subgroup, 17 had reported tumor measurements for brain lesions at baseline (Table 2). Fifteen of 17 patients had data available to evaluate responses in the brain. Of the patients with available data, 14 had completed radiotherapy ≥60 days before treatment and one had not received any prior radiotherapy. Per investigator assessment, seven of 17 patients (41.2%) had a best percentage change in brain lesion diameters consistent with a response of PR (≥30% decrease) in the brain, and seven of 17 patients (41.2%) had a best percentage change in brain lesion diameters consistent with SD (Fig. 2A). The reductions in brain lesions were sustained over time (Fig. 2B).
Table 2.
Prior treatment and brain lesion measurements over time for patients in the BM subgroup
| Longest diameter of lesion (mm) at baseline and time following treatment | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Prior BM treatmenta | Imaging methodb | Baseline | Week 6 | Week 12 | Week 18 | Week 24 | Week 30 | Week 36 | Week 42 | Week 48 | Week 54 | Week 60 | BOR in brain lesionc | BOR |
| 1 | RT | MRI | 9 | 9 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | SD | PR |
| 2 | Surgery, RT, capecitabine | CT | 43 | 25.1 | 15.9 | 12.6 | 12.0 | 10.6 | 10.9 | 8.0 | 7.9 | 10.2 | 10.0 | PR | PR |
| 3 | Surgery, RT | MRI | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | SD | PR |
| 4 | NR | CT | 46 | 38 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | SD | PR | |
| 5 | RT | CT | 12 | NR | CR | CR | CR | CR | CR | CR | PD | PR | PR | ||
| CT | 18 | NR | 12 | 8 | 7 | 7 | 7 | 7 | 12 | ||||||
| CT | 10 | NR | CR | CR | CR | CR | CR | CR | PD | ||||||
| 6 | RT | MRI | 13 | 8 | 8 | 9 | 8 | 8 | 8 | 8 | PR | SD | |||
| 7 | NR | CT | 8 | 8 | 8 | 7 | 7 | 7 | SD | SD | |||||
| 8 | NR | MRI | 17.1 | 19 | 19 | 16 | 16 | 16 | SD | PR | |||||
| 9 | RT | MRI | 5.2 | 2.2 | 3.0 | 3.0 | PR | PR | |||||||
| 10 | Surgery, RT | MRI | 4.7 | 3.2 | 3.9 | 3.5 | PR | SD | |||||||
| 11 | RT | MRI | 23 | 15 | 13 | 13 | PR | PR | |||||||
| 12 | RT | MRI | 25 | 5 | 5 | 5 | PR | PR | |||||||
| 13 | RT | MRI | 5 | 5 | 5 | 5 | SD | SD | |||||||
| 14 | RT | MRI | 8 | 8 | 7 | SD | PR | ||||||||
| 15 | RT | CT | 10.8 | 6.9 | 4.8 | PR | SD | ||||||||
| 16 | RT | MRI | 7 | 9 | 13 | PD | SD | ||||||||
| 17 | RT | MRI | 5 | 5 | SD | SD | |||||||||
| 18 | Surgery, RT | None | N/A | N/A | PR | ||||||||||
| 19 | RT | None | N/A | N/A | PR | ||||||||||
| 20 | RT | SD | |||||||||||||
| 21 | RT | None | N/A | N/A | PR | ||||||||||
| 22 | NR | None | N/A | N/A | NE | ||||||||||
| 23 | Surgery | None | N/A | N/A | PD | ||||||||||
| 24 | NR | MRI | Data not available for this patient | CR | |||||||||||
Note: “RT” includes whole-brain RT, brain-directed stereotactic RT, and brain-directed radiosurgery. “Surgery” includes any brain-directed surgery (craniotomy, metastasectomy in brain, resection, or removal of brain lesion).
Abbreviations: BOR, best overall response; N/A, not applicable (no brain lesion present at baseline); NR, nothing reported; RT, radiotherapy (brain-specific).
aIncludes BM-specific treatment for past BMs and any at baseline. These data were collected retrospectively.
bImaging was performed per protocol every 6 weeks for subjects with baseline BMs. Measurements were not required per protocol but were performed by some sites and collected retrospectively. For subjects without measurements, imaging was performed, but measurements were either not taken or not provided by the study site.
cProvided by the investigator retrospectively.
Figure 2.
CNS response. A, Best response in brain lesions in patients with stable BMs in the BM subgroup. B, Brain lesion measurements over time in patients with stable BMs in the BM subgroup. Three patients with reported baseline measurements had no change over time. Two patients with BMs at baseline did not have sufficient data to evaluate response in the brain and are not shown.
In total, 48 patients experienced systemic PD, including eight patients with a history of BMs. Sites of progression were generally similar between patients in the BM and non-BM subgroups (Supplementary Table S2), although the rate of progression in the brain was greater in patients with a history of BMs relative to those without (8.3% and 1.3%, respectively) at time of data cutoff. Overall, four patients, two of whom had a history of BMs, had disease progression occurring in the brain. These progression events occurred earlier in the two BM subgroup patients (days 75 and 85) than in the two non-BM patients (days 323 and 498).
Safety
All patients in the BM subgroup and 99.4% of non-BM patients experienced ≥1 treatment-emergent adverse event (TEAE; Supplementary Table S3). As in the overall study population, any-grade TEAEs in the BM subgroup were predominantly gastrointestinal or hematologic. The percentage of grade ≥3 TEAEs was 54.2% in the BM subgroup and 57.5% in the non-BM subgroup. In the BM subgroup, the most prevalent grade ≥3 TEAEs were decreased neutrophil count (25.0%), decreased lymphocyte count (16.7%), fatigue (12.5%), and diarrhea (8.3%; Supplementary Table S4). Drug-related TEAEs associated with discontinuation occurred in two patients (8.3%) with a history of BMs (grade 1 and 2 pneumonitis) and 25 (15.6%) in those without. Pneumonitis (n = 11) and interstitial lung disease (ILD; n = 5) were the predominant causes for discontinuation among the non-BM patients. There were nine TEAEs associated with deaths, two of which were in the BM subgroup and resulted from disease progression and respiratory failure (each, n = 1; adjudicated as drug-related ILD/pneumonitis per an independent ILD/pneumonitis adjudication committee). TEAEs in the non-BM subgroup associated with death included one case each of acute respiratory failure, general physical health deterioration, lymphangitis, pneumonia, pneumonitis, and shock hemorrhagic. One patient had two TEAEs associated with death (acute kidney injury and acute hepatic failure; Supplementary Table S3). ILD/pneumonitis, an important risk associated with T-DXd, adjudicated by an independent committee as related to study treatment, occurred in four patients (16.7%) in the BM subgroup [grade 1, n = 3 (12.5%); grade 5, n = 1 (4.2%)] and 21 patients (13.1%) in the non-BM subgroup [grade 1, n = 6 (3.8%); grade 2, n = 10 (6.3%); grade 3, n = 2 (1.3%), grade 5, n = 3 (1.9%)]. There were 25 adjudicated ILD/pneumonitis cases in the overall study population [13.6%; grade 1/2, n = 19 (10.3%); grade 3, n = 2 (1.1%); grade 4, n = 0; grade 5, n = 4 (2.2%); ref. 12].
Patient Case Study
One patient treated with T-DXd 5.4 mg/kg every 3 weeks in the DESTINY-Breast01 study who had baseline BMs was a 48-year-old patient with HER2-positive (IHC 3+)/hormone receptor–negative mBC. Before enrollment, the patient had received 16 lines of treatment, including T-DM1, pertuzumab, trastuzumab, and lapatinib. The patient also underwent whole-brain radiotherapy 5 years before study inclusion and SRS 3 years before study inclusion to treat brain lesions. Lesions were observed at the patient's baseline scan in the brain, lymph nodes, retroperitoneum, lung, pancreas, bone, and axillary lymph node.
At baseline, the diameter of the brain lesion was 10.6 mm and was reduced by 35% at the 6-week scan. The patient's overall status improved, allowing for reduction in pain medication. PR was achieved in all target lesions and non-CR/non-PD response in the nontarget lesions.
By the subsequent scan at 12 weeks, the brain lesion measured 4.6 mm—a 57% reduction from baseline. Although a PR continued to be observed for all target lesions and a non-CR/non-PD response was observed for all nontarget lesions, new lesions were observed in the chest wall. The overall response at 12 weeks was PD, leading to the patient's discontinuation from study treatment per the protocol.
DISCUSSION
Among DESTINY-Breast01 patients with HER2-positive mBC, T-DXd 5.4 mg/kg demonstrated durable activity in patients with and without a history of BMs, similar to that observed in the overall population. The cORR and mPFS in the BM and non-BM subgroups were similar to the overall population. The cORR was 58.3% for patients with BMs and 61.3% for patients without BMs, and mPFS was 18.1 months and 16.4 months, respectively. Additionally, the safety profile of T-DXd in patients with and without a history of BMs was similar to the overall population. Because additional treatment options are needed specifically for patients with HER2-positive mBC and BMs, these data are encouraging and warrant further investigation.
An important concern in treating BMs has been that large, systemically administered molecules may not penetrate the BBB to achieve an effective concentration in the tumor bed. Because of their smaller size, chemical inhibitors, such as TKIs, have been considered to have better penetration of the BBB than larger antibody-based treatments (e.g., trastuzumab, T-DM1, and T-DXd; ref. 4). However, clinical and preclinical studies have detected accumulation of trastuzumab and T-DM1 in brain lesions, possibly as the result of disruption of the BBB from prior local surgery and/or radiation, or because of a blood–tumor barrier with varying permeability or an altered blood–tumor barrier after penetration of the BBB by BMs (5, 9). Such evidence suggests that the brain's status as a “sanctuary site” in patients with HER2-positive mBC is not completely explained by the lack of treatment accessibility. The immune privilege status of the brain may also restrict trastuzumab-mediated antibody-dependent cell-mediated cytotoxicity. Consequently, ADCs such as T-DXd or T-DM1 may be better suited than monoclonal antibodies for treating BMs, since they can cause tumor cell death via delivery of a cytotoxic drug.
The efficacy of anti-HER2 agents in treating BMs has recently been reported. T-DM1 was examined in the second-line or later setting in a subgroup analysis of the single-arm KAMILLA trial in patients with HER2-positive mBC who had treated or asymptomatic, untreated BMs at baseline (17). For this subgroup, the best overall response was 21.4% (27/126; 95% CI, 14.6–29.6; CR, n = 3; PR, n = 24), and a ≥30% reduction in the sum of the largest diameters of target brain lesions was observed in 42.9% of patients (54/126). The mPFS was 5.5 months compared with 7.7 months in patients without baseline BMs (17). The HER2CLIMB trial was one of the first trials to enroll a large proportion of patients with BMs, including patients with previously untreated, treated stable, or treated and progressing BMs (18). Based on HER2CLIMB, tucatinib in combination with trastuzumab and capecitabine was recently approved for patients with HER2-positive mBC, including those with BMs, after ≥1 prior (United States) or ≥2 prior (Europe) lines of HER2-targeted therapy (18, 19). Patients with BMs at baseline or a history of BMs were included in this trial and achieved a mPFS of 7.6 months with tucatinib combination therapy (19). The ORR for all patients in HER2CLIMB (N = 511) was 40.6% (95% CI, 35.3–46.0), and the intracranial ORR for patients with active BMs and measurable intracranial disease at baseline (n = 75) was 47.3% (95% CI, 33.7–61.2; refs. 18, 19). In the overall population, median OS was 24.7 months (95% CI, 21.6–28.9) for tucatinib plus trastuzumab and capecitabine versus 19.2 months (95% CI, 16.4–21.4) for placebo plus trastuzumab and capecitabine (HR, 0.73; 95% CI, 0.59–0.90; P = 0.004; ref. 20).
Although cross-trial comparisons are difficult to interpret, the response rate and mPFS for patients with a history of BMs in DESTINY-Breast01 were numerically higher compared with patients with BMs at baseline from the KAMILLA and HER2CLIMB trials (cORR, 58.3%; mPFS, 18.1 months), but there were differences between the study populations. Patients in the BM subgroup in DESTINY-Breast01 had stable BMs and required a washout period of ≥60 days before randomization if they had any history of radiation, surgery, or other therapy, including steroids or anticonvulsants to control symptoms from BMs. The number of patients with BMs was also relatively low (12). In the KAMILLA trial, patients with BMs were included if they had untreated, asymptomatic BMs or controlled brain disease treated with radiotherapy >14 days before enrollment (17). In the HER2CLIMB trial, patients with BMs were included unless they needed immediate local intervention. HER2CLIMB also enrolled 291 patients with treated and stable, treated and progressing, and untreated BMs. Additionally, patients with untreated BMs >2 cm in diameter could be enrolled in HER2CLIMB provided they received approval from the medical monitor (19).
Intracranial efficacy of T-DXd has also been reported in preclinical and clinical studies. Kabraji and colleagues reported prolonged survival with T-DXd in HER2-positive and HER2-low breast cancer BM–derived murine models compared with control (21). Additionally, a clinical benefit rate of 75% (12/16) was observed in a retrospective cohort of breast cancer patients with BMs (21). In the phase II TUXEDO-1 trial (NCT04752059), T-DXd demonstrated efficacy and safety in patients with HER2-positive breast cancer with newly diagnosed BMs or BMs with radiologic progression after prior local therapy. Intracranial response was observed in 11/15 patients (73.3%; ref. 22). In the phase III DESTINY-Breast03 trial, 23/36 (63.9%) patients with stable BMs at baseline experienced intracranial CR or PR (23). In the ongoing phase II DEBBRAH trial (NCT04420598), T-DXd demonstrated preliminary efficacy in patients with HER2-positive advanced breast cancer with stable, asymptomatic untreated, or progressing BMs after radiotherapy and/or surgery. Intracranial ORR was reported in two patients (50.0%; 95% CI, 6.7–93.2) with asymptomatic untreated BMs and four patients (44.4%; 95% CI, 13.7–78.8) with treated and progressing BMs (24). The ongoing DESTINY-Breast07 (NCT04538742), DESTINY-Breast09 (NCT04784715), DESTINY-Breast12 (NCT04739761), and HER2CLIMB-04 (NCT04539938) trials will continue to assess the efficacy of T-DXd in patients with HER2-expressing breast cancer and BMs, including active BMs.
Patients with HER2-negative mBC comprise approximately 85% of the mBC population, and approximately 60% of tumors from patients with HER2-negative mBC express low levels of HER2 (25, 26). T-DXd was approved in the United States for the treatment of patients with unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/in situ hybridization−) breast cancer after prior chemotherapy in the metastatic setting based on results from DESTINY-Breast04 (NCT03734029; ref. 27). Therefore, the data reported here suggest that a large subset of patients with mBC and BMs may experience clinical benefit with T-DXd. The ongoing DESTINY-Breast06 (NCT04494425) and DESTINY-Breast08 (NCT04556773) studies, and a cohort from DEBBRAH, are continuing to assess HER2-low patients with treated/stable BMs.
DESTINY-Breast01 did not include patients with active BMs, and 19 of 24 patients in the BM subgroup had received prior surgery or radiation to the brain. BMs were to be listed as nontarget lesions; although BMs were monitored by imaging every 6 weeks (Q6W), diameter measurements were not required. In addition, our subgroup analysis included only 24 patients, of whom 17 had brain lesion measurements available for analysis. Tumor measurements were obtained retrospectively by contacting the treating investigators, and the date of data cutoff could not be independently verified. However, the responses noted in the brain generally occurred within the first two on-treatment scans, and so the impact of data cutoff on ORR was likely minimal. Additionally, per Response Evaluation Criteria in Solid Tumors, tumor lesions located in a previously irradiated area are usually not considered target lesions (28). Thus, proper response assessments were not possible for the patients in the BM subgroup with prior irradiation.
In conclusion, T-DXd demonstrated strong clinical activity in both the overall population of patients with HER2-positive mBC and the subgroup of those with a history of BMs in DESTINY-Breast01. These positive findings lay the groundwork for further investigation of T-DXd in this substantial patient subset, including those with active BMs, for which treatment options remain limited.
METHODS
Study Design and Patients
DESTINY-Breast01 is a two-part, multicenter, open-label (unblinded) phase II trial of T-DXd in adult patients with unresectable or metastatic HER2-positive breast cancer previously treated with T-DM1. The overall design, study protocol, and primary results of this study have been previously published (12).
Briefly, patients were adults (≥20 years of age in Japan and Korea; ≥18 years of age in all other study sites) with unresectable or metastatic HER2-positive (IHC 3+ or in situ hybridization+ as centrally confirmed on archival tissue) breast cancer that progressed on T-DM1 or who had discontinued T-DM1 for reasons other than PD. Patients previously treated with HER2-targeted TKIs or capecitabine were eligible. Additionally, patients with baseline BMs that were treated, asymptomatic, or did not require therapy to control symptoms were eligible. All treatment to control symptoms, including radiation, surgery, or other therapy (including steroids or anticonvulsants), had to be completed >60 days before randomization. Radiotherapy had to have been completed ≥60 days before treatment initiation to decrease the likelihood that prior radiotherapy contributed to tumor evaluation on study. The planned number of patients with BMs or a history of BMs to be included in the study was approximately 20% of the ≈150 patients expected to receive the recommended part 2 dose (T-DXd 5.4 mg/kg every 3 weeks); additional patients with any current or past history of BMs were excluded. Tumors were assessed by imaging Q6W (±7 days); a collection of MRI scans for central analysis of the brain Q6W was only required for patients with BMs at baseline. Brain lesion measurements were assessed per investigator but were considered nontarget lesions, and thus collection of diameter measurements was not required. Patients with BMs had to have a target lesion elsewhere, which was measured for the analysis of sum of the longest diameters. Patients were also excluded if they had current or suspected noninfectious ILD/pneumonitis or a history of ILD/pneumonitis that required treatment with glucocorticoids. Complete eligibility criteria can be found in the previously published analysis (12).
Study Oversight
Daiichi Sankyo and AstraZeneca collaborated on this study, which was sponsored and designed by Daiichi Sankyo and approved by the institutional review board at each participating site. All patients provided written informed consent. Data were analyzed and interpreted by the sponsor and authors. All authors reviewed the manuscript and confirmed the accuracy of the data, its analysis, and adherence to the DESTINY-Breast01 protocol, available in the Supplementary Materials.
Endpoints
The data cutoff for this analysis was August 1, 2019. The primary endpoint was cORR (CR + PR) per ICR using Response Evaluation Criteria in Solid Tumors, version 1.1. Secondary endpoints were investigator assessed and included duration of response, PFS, OS, DCR (CR + PR + SD), safety, and best percentage change in the sum of the longest diameters of measurable tumors. The primary and secondary endpoints were determined for all patients who received the recommended dose of T-DXd 5.4 mg/kg (previously described; ref. 12) and are presented here for patients with and without a history of BMs.
Safety
TEAEs were categorized in accordance with the Medical Dictionary for Regulatory Activities, version 20.1, and graded using the NCI Common Terminology Criteria for Adverse Events, version 4.03.
An independent adjudication committee was established to evaluate all potential incidents of ILD/pneumonitis (12).
Statistical Analysis
The statistical analyses for evaluating the endpoints in all patients who received the recommended part 2 dose were previously described (12) and were also applied to the BM subgroup. Briefly, the Clopper–Pearson method was used to calculate the two-sided 95% CI for the response rate and the Kaplan–Meier method was used to estimate the distribution of time-to-event endpoints of duration of response and PFS. The corresponding two-sided 95% CI for the median time-to-event endpoints was calculated with the Brookmeyer and Crowley methods.
Data Availability
Anonymized individual participant data and applicable supporting clinical trial documents may be available upon request at https://vivli.org. In cases in which clinical trial data and supporting documents are provided pursuant to company policies and procedures, Daiichi Sankyo Companies will continue to protect the privacy of the company and its clinical study subjects. Details on data sharing criteria and the procedure for requesting access can be found at https://vivli.org/ourmember/daiichi-sankyo. Data will be provided upon request.
Supplementary Material
Acknowledgments
Editorial assistance was provided by Jenna M. Gaska, PhD (ArticulateScience, LLC), Jill Seabrook, PhD, and Laura Halvorson, PhD (ApotheCom), and financially supported by Daiichi Sankyo and AstraZeneca.
Footnotes
Note: Supplementary data for this article are available at Cancer Discovery Online (http://cancerdiscovery.aacrjournals.org/).
Authors’ Disclosures
G. Jerusalem reports personal fees from Daiichi Sankyo during the conduct of the study, as well as grants from Novartis, Roche, and Pfizer, personal fees (consulting) from Novartis, Amgen, Roche, Pfizer, Bristol Myers Squibb, Lilly, AstraZeneca, Daiichi Sankyo, and AbbVie, personal fees (lectures, presentations, speakers bureaus, manuscript writing, or educational events) from Novartis, Roche, Amgen, Pfizer, Bristol Myers Squibb, Lilly, and AstraZeneca, personal fees (meetings/travel) from Novartis, Roche, Pfizer, Lilly, Amgen, Bristol Myers Squibb, and AstraZeneca, personal fees (advisory board) from Novartis, Roche, Amgen, Pfizer, Bristol Myers Squibb, Lilly, AstraZeneca, Daiichi Sankyo, and AbbVie, and personal fees (medical writing) from Novartis, Roche, Lilly, Amgen, Bristol Myers Squibb, AstraZeneca, MedImmune, and Merck outside the submitted work. Y.H. Park reports other support from Daiichi Sankyo during the conduct of the study, as well as personal fees and nonfinancial support from AstraZeneca, Novartis, and Eisai, grants, personal fees, and nonfinancial support from Pfizer, nonfinancial support from MSD, personal fees from Lilly and Daiichi Sankyo, grants from Roche, and nonfinancial support from Boryung outside the submitted work. T. Yamashita reports grants and other support from Chugai, Taiho, Kyowa Kirin, and Nippon Kayaku and other support from Eisai, Pfizer, Daiichi Sankyo, AstraZeneca, Novartis, and Eli Lilly outside the submitted work. S.A. Hurvitz reports grants from Daiichi Sankyo during the conduct of the study, as well as grants from Ambrx, Amgen, Arvinas, AstraZeneca, Bayer, CytomX, Daiichi Sankyo, Dantari, Dignitana, Genentech/Roche, G1-Therapeutics, Gilead, GSK, Immunomedics, Eli Lilly, Macrogenics, Novartis, OBI Pharma, Orinove, Pfizer, Phoenix Molecular Designs, Pieris, PUMA, Radius, Samumed, Sanofi, Seattle Genetics/Seagen, and Zymeworks and personal fees and nonfinancial support from Daiichi Sankyo outside the submitted work. S. Modi reports personal fees from Daiichi Sankyo, AstraZeneca, Seagen, Macrogenics, and Genentech during the conduct of the study, as well as personal fees from Daiichi Sankyo, AstraZeneca, Genentech, Macrogenics, and Seagen outside the submitted work. F. Andre reports grants from Roche, Novartis, Pfizer, Lilly, Daiichi Sankyo, and AstraZeneca outside the submitted work. I.E. Krop reports nonfinancial support from AstraZeneca during the conduct of the study, as well as personal fees from AstraZeneca, Daiichi Sankyo, Seagen, Genentech, Macrogenics, Merck, and Novartis, and grants from Genentech and Pfizer outside the submitted work. X. Gonzàlez Farré reports honoraria from Roche and Pierre Fabre, and travel support from Roche. B. You reports other support from Daiichi Sankyo outside the submitted work. C. Saura reports personal fees from AstraZeneca, Gilead, Daiichi Sankyo, Seagen, Eisai Europe Ltd., Phillips Health Works, Pfizer, Solti, Puma Biotechnology, Pierre Fabre, Roche Farma, SA, and Novartis and nonfinancial support from Lilly SAU, Pierre Fabre, AstraZeneca, Exeter Pharma LLC, AX's Consulting SARL, and MediTech outside the submitted work. S.-B. Kim reports grants from Novartis and Sanofi-Aventis, and grants and nonfinancial support from DongKook Pharm. Co., Novartis, AstraZeneca, Lilly, DaeHaw Pharmaceutical Co., ISU Abxis, OBI Pharma, Beigene, and Daiichi Sankyo outside the submitted work, as well as holds stock in Genopeaks and NeogeneTC. C.R. Osborne reports personal fees from Daiichi Sankyo/AstraZeneca outside the submitted work. R.K. Murthy reports grants and personal fees from Daiichi Sankyo and personal fees from AstraZeneca during the conduct of the study, as well as personal fees from Sanofi, Puma, and Novartis, grants and personal fees from Pfizer, Genentech, and Seattle Genetics, and grants from EMD Serono outside the submitted work. L. Gianni reports nonfinancial support from Daiichi Sankyo during the conduct of the study, as well as personal fees from AstraZeneca and Seagen, and nonfinancial support from Novartis, Pfizer, Roche, Ipsen, and Daiichi Sankyo/AstraZeneca outside the submitted work. T. Takano reports grants, personal fees, and nonfinancial support from Daiichi Sankyo during the conduct of the study, as well as grants and personal fees from Daiichi Sankyo and Chugai, grants from Ono, MSD, and Eisai, and personal fees from Kyowa Kirin, Pfizer, Eli Lilly, and Celltrion Healthcare outside the submitted work. Y. Liu is an employee of Daiichi Sankyo. J. Cathcart is an employee of Daiichi Sankyo. C. Lee reports other support (employment) from Daiichi Sankyo during the conduct of the study, as well as other support (employment) from Daiichi Sankyo outside the submitted work. C. Perrin reports personal fees from a regional advisory board organized by Daiichi Sankyo outside the submitted work. No disclosures were reported by the other authors.
Authors’ Contributions
G. Jerusalem: Data curation, investigation, writing–review and editing. Y.H. Park: Data curation, investigation, writing–review and editing. T. Yamashita: Data curation, investigation, writing–review and editing. S.A. Hurvitz: Data curation, investigation, writing–review and editing. S. Modi: Data curation, investigation, writing–review and editing. F. Andre: Data curation, investigation, writing–review and editing. I.E. Krop: Data curation, investigation, writing–review and editing. X. Gonzàlez Farré: Data curation, investigation, writing–review and editing. B. You: Data curation, investigation, writing–review and editing. C. Saura: Data curation, investigation, writing–review and editing. S.-B. Kim: Data curation, investigation, writing–review and editing. C.R. Osborne: Data curation, investigation, writing–review and editing. R.K. Murthy: Data curation, investigation, writing–review and editing. L. Gianni: Data curation, investigation, writing–review and editing. T. Takano: Data curation, investigation, writing–review and editing. Y. Liu: Data curation, formal analysis, supervision, methodology, writing–review and editing. J. Cathcart: Data curation, formal analysis, supervision, methodology, writing–review and editing. C. Lee: Data curation, formal analysis, supervision, methodology, writing–review and editing. C. Perrin: Data curation, investigation, writing–review and editing.
References
- 1. Hurvitz SA, O'Shaughnessy J, Mason G, Yardley DA, Jahanzeb M, Brufsky A, et al. Central nervous system metastasis in patients with HER2-positive metastatic breast cancer: patient characteristics, treatment, and survival from SystHERs. Clin Cancer Res 2019;25:2433–41. [DOI] [PubMed] [Google Scholar]
- 2. Olson EM, Najita JS, Sohl J, Arnaout A, Burstein HJ, Winer EP, et al. Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era. Breast 2013;22:525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, Kuter I, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 2003;97:2972–7. [DOI] [PubMed] [Google Scholar]
- 4. Clayton AJ, Danson S, Jolly S, Ryder WD, Burt PA, Stewart AL, et al. Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer 2004;91:639–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Venur VA, Leone JP. Targeted therapies for brain metastases from breast cancer. Int J Mol Sci 2016;17:1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramakrishna N, Anders CK, Lin NU, Morikawa A, Temin S, Chandarlapaty S, et al. Management of advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: ASCO guideline update. J Clin Oncol 2022;40:2636–55. [DOI] [PubMed] [Google Scholar]
- 7. Costa R, Gill N, Rademaker AW, Carneiro BA, Chae YK, Kumthekar P, et al. Systematic analysis of early phase clinical studies for patients with breast cancer: inclusion of patients with brain metastasis. Cancer Treat Rev 2017;55:10–5. [DOI] [PubMed] [Google Scholar]
- 8. Hosonaga M, Saya H, Arima Y. Molecular and cellular mechanisms underlying brain metastasis of breast cancer. Cancer Metastasis Rev 2020;39:711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Askoxylakis V, Ferraro GB, Kodack DP, Badeaux M, Shankaraiah RC, Seano G, et al. Preclinical efficacy of ado-trastuzumab emtansine in the brain microenvironment. J Natl Cancer Inst 2016;108:djv313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res 2016;22:5097–108. [DOI] [PubMed] [Google Scholar]
- 11. Lüftner D, Peipp M. New therapeutic strategies in advanced nonoperable or metastatic HER2-positive breast cancer. Geburtshilfe Frauenheilkd 2021;81:666–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med 2020;382:610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cortés J, Kim SB, Chung WP, Im SA, Park YH, Hegg R, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med 2022;386:1143–54. [DOI] [PubMed] [Google Scholar]
- 14. Enhertu 100 mg powder for concentrate for solution for infusion. Summary of product characteristics. Munich (Germany): Daiichi Sankyo Europe GmbH; 2021. Available from: https://www.ema.europa.eu/en/documents/product-information/enhertu-epar-product-information_en.pdf. [Google Scholar]
- 15. Enhertu (fam-trastuzumab deruxtecan-nxki) for injection, for intravenous use. Prescribing information. Basking Ridge (NJ): Daiichi Sankyo, Inc.; 2022. Available from: https://daiichisankyo.us/prescribing-information-portlet/getPIContent?productName=Enhertu&inline=true. [Google Scholar]
- 16. Saura Manich C, Modi S, Krop I, Park YH, Kim SB, Tamura K, et al. Trastuzumab deruxtecan (T-DXd) in patients with HER2-positive metastatic breast cancer (MBC): updated survival results from a phase II trial (DESTINY-Breast01). Ann Oncol 2021;32Suppl 5:S485–S6. Abstract nr 279P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Montemurro F, Delaloge S, Barrios CH, Wuerstlein R, Anton A, Brain E, et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial. Ann Oncol 2020;31:1350–8. [DOI] [PubMed] [Google Scholar]
- 18. Lin NU, Borges V, Anders C, Murthy RK, Paplomata E, Hamilton E, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB Trial. J Clin Oncol 2020;38:2610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med 2020;382:597–609. [DOI] [PubMed] [Google Scholar]
- 20. Curigliano G, Mueller V, Borges V, Hamilton E, Hurvitz S, Loi S, et al. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): final overall survival analysis. Ann Oncol 2022;33:321–9. [DOI] [PubMed] [Google Scholar]
- 21. Kabraji S, Ni J, Sammons S, LiT,Van Swearingen AED, Wang Y, et al. Preclinical and clinical efficacy of trastuzumab deruxtecan in breast cancer brain metastases. Clin Cancer Res 2022 Sep 30 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bartsch R, Berghoff AS, Furtner J, Marhold M, Bergen ES, Roider-Schur S, et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med 2022;28:1840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hurvitz S, Kim SB, Chung WP, Im SA, Park YH, Hegg R, et al. Trastuzumab deruxtecan (T-DXd; DS-8201a) vs. trastuzumab emtansine (T-DM1) in patients (pts) with HER2+ metastatic breast cancer (mBC): subgroup analyses from the randomized phase 3 study DESTINY-Breast03 [abstract]. In: Proceedings of the 2021 San Antonio Breast Cancer Symposium; 2021 Dec 7–10; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 2022;82( 4 Suppl): Abstract nr GS3-01. [Google Scholar]
- 24. Pérez-García JM, Batista MV, Cortez P, Ruiz-Borrego M, Cejalvo JM, de la Haba-Rodriguez J, et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: the DEBBRAH trial. Neuro Oncol 2022May 26 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tarantino P, Hamilton E, Tolaney SM, Cortes J, Morganti S, Ferraro E, et al. HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol 2020;38:1951–62. [DOI] [PubMed] [Google Scholar]
- 26. Schettini F, Chic N, Brasó-Maristany F, Paré L, Pascual T, Conte B, et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 2021;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med 2022;387:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized individual participant data and applicable supporting clinical trial documents may be available upon request at https://vivli.org. In cases in which clinical trial data and supporting documents are provided pursuant to company policies and procedures, Daiichi Sankyo Companies will continue to protect the privacy of the company and its clinical study subjects. Details on data sharing criteria and the procedure for requesting access can be found at https://vivli.org/ourmember/daiichi-sankyo. Data will be provided upon request.