Abstract
The sialic acid–binding immunoglobulin-like lectin (Siglec)–sialic acid immune axis is an evolutionarily conserved immunoregulatory pathway that provides a mechanism for establishing self-recognition and combatting invasive pathogens. Perturbations in the pathway lead to many immune dysregulated diseases, including autoimmunity, neurodegeneration, allergic conditions, and cancer. The purpose of this review is to provide a brief overview of the relationship between Siglecs and sialic acid as they relate to human health and disease, to consider current Siglec-based therapeutics, and to discuss new therapeutic approaches targeting the Siglec–sialic acid immune axis, with a focus on cancer.
Introduction
Sialic acid–binding immunoglobulin-like lectin (Siglec) receptors are key proteins involved in surveying surface glycans in vertebrates, and they are broadly expressed on innate and adaptive immune cells (1, 2). These proteins evolved concurrently with the recombination-activating genes RAG1 and RAG2, which are responsible for generating the diverse array of antigen receptors in the immune system. Siglecs, therefore, likely represent a critical mechanism for tempering immune responses and minimizing autoimmunity from the highly potent adaptive immune response system (3). The Siglec family consists of 15 receptors in humans, most commonly expressed on innate immune cells, although some members are expressed on lymphoid cells [e.g., Siglec-2 (also known as CD22) on B cells, and Siglec-5, -7, and -9 on activated T cells] or stromal cells such as astrocytes [e.g., Siglec-4 (also known as MAG-4)] (2).
Siglecs have been studied as therapeutic targets in cancer for many years (4). This is primarily because Siglec family members are lineage markers on malignant immune cells and thus can be used to target a cytotoxic payload to the malignant cells (5). The two most prominent examples of this are Siglec-2 (CD22) on B-cell malignancies and Siglec-3 (CD33) on myeloid leukemias. Considering the increased Siglec expression in some cancers, the development of Siglec-targeting therapeutics has focused on eliminating Siglec-expressing cells. Recently, the clinical successes of immune checkpoint inhibitors have highlighted the potency and durability of immunomodulatory therapies in cancer (6). Although the antitumor activity of checkpoint inhibitors can be remarkable, some patients do not benefit, and it is likely that negative regulatory elements, including inhibitory Siglecs, contribute to these failures (5). With a deeper understanding of Siglecs and their role in inhibiting anticancer immune responses, therapies are now being developed to disrupt the Siglec–sialic acid immune axis (6). Together, the potential to utilize Siglecs to deliver a cytotoxic payload and to modulate the immune–cancer axis strongly suggest that the Siglec family is an attractive target class for generating new anticancer therapeutics (5, 6). More detailed reviews on the function and biology of Siglec receptors have been published previously (7–9). In this review, we focus on the therapeutic targeting of Siglec receptors for cancer therapy.
Functional Roles of Siglec Interactions with the Sialic Acid Glycocalyx
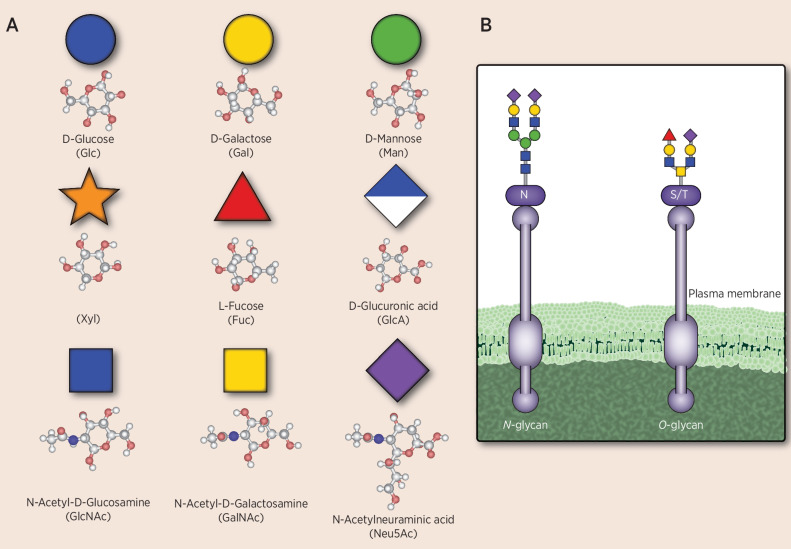
The glycocalyx is a functional system composed of proteoglycans, glycosphingolipids, and glycoproteins; it surrounds all eukaryotic cells, is involved in cell-to-cell communication and recognition of self, provides a protective barrier against pathogens, and modulates inflammation and repair (5, 10). Within the glycocalyx, the sugar codes are diverse and biosynthesis of glycans is distinct from that of proteins, primarily in that it is not template based and depends on gene expression of relevant enzymes, availability of substrates, and structure of the protein to which the glycans are attached (11–13). All jawed vertebrates have cell-surface glycans that terminate with sialic acid residues. Although approximately 50 different sialic acid residues have been identified in nature, N-acetylneuraminic acid (Neu5Ac) is the most abundant in humans and serves as a key marker of self in glycan-coding sequences (6, 14). Specific types of sialic acids are found, such as N-glycolylneuraminic acid (Neu5Gc), which is derived from food sources, and 9-O-acetylated-Neu5Ac (11). In addition to sialic acid, eight other specific sugar moieties are used to generate the cell-surface immune markers in humans (Fig. 1; ref. 15). Unlike DNA and proteins that use linear sequences to encode unique structures, glycan structures take on increased complexity through branching due to differences in composition, anomeric form, linkage, and substitution of monosaccharides, with the terminal sialic acid residue often playing a large role in cell-to-cell interactions (15).
Figure 1.
Sugar building blocks of human cell-surface immune markers. The sugar moieties shown in A are common carbohydrate components of cell-surface molecules, including sialoglycans, in humans, some of which serve as immune markers. The composition, branching anomeric form, and linkage lead to a diversity of molecules, with Neu5Ac often being the sialic acid moiety in the outermost position and playing a key role in the interaction between cells. B shows an example of an N-linked glycan in which glycosylation occurs at an asparagine residue (N) and an O-glycan in which glycosylation occurs at a serine or threonine residue (S/T). Sialic acids other than Neu5Ac including O-acetylated-Neu5Ac or Neu5Gc can be sometimes found in cancer.
One of the functions of sialic acid–containing glycans, or sialoglycans, is to serve as self-associated molecular patterns (SAMP), which help to differentiate self from nonself and appropriately dampen the immune system (5, 16–18). In contrast to SAMPs, pathogen-associated molecular patterns and danger-associated molecular patterns trigger increased immune activity (18). However, under abnormal circumstances, aberrant glycosylation can negatively impact immune regulation, and alterations in glycosylation have been associated with cancer and inflammatory diseases such as immunoglobulin A (IgA) nephropathy, systemic lupus erythematosus, and inflammatory bowel disease (19). Changes in sialic acid glycosylation can be mediated through various mechanisms, including availability of glycan substrates and expression of sialyltransferases. These changes can be a sign of malignant transformation of cells, with hypersialylation common in cancer (20).
Siglecs are cell-surface receptors resembling the Ig superfamily. They contain an extracellular domain with a variable number of C2-set Ig-like domains, which is similar to the constant regions of antibodies, and an amino terminal domain with a V-set variable region, which is similar to the variable domain of antibodies. The V-set domain contains a carbohydrate-recognition domain that facilitates binding to sialic acid–containing ligands; it is the variability within this domain that provides additional specificity across the spectrum of sialic acid codes that are represented on cell surfaces (7, 16, 21, 22). There is evidence that in addition to binding sialic acid ligands, Siglecs may also be able to bind protein ligands, including endogenous and exogenous proteins from pathogens (5).
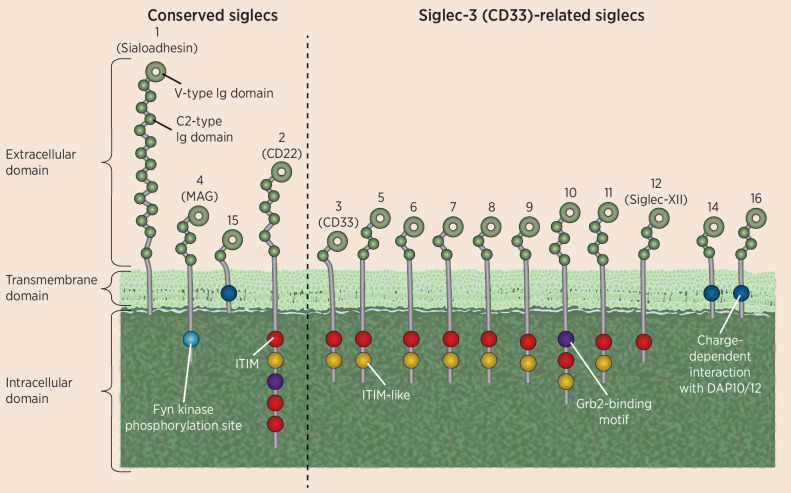
The 15 human Siglecs can be divided into two main subgroups based on their sequence similarity and evolutionary conservation (Fig. 2). The conserved Siglec group contains Siglec-1 (sialoadhesin), which is primarily involved in monocyte/macrophage interactions with other cell types; Siglec-2 (CD22), which is involved in B-cell receptor antagonism in B cells; Siglec-4, which is involved in preservation of myelination by oligodendrocytes and Schwann cells; and Siglec-15, which is an important receptor in bone formation and immune regulation. The other group encompasses all CD33-related Siglecs (Siglec-3, -5 through -12, -14, and -16), which function primarily in immunomodulation and inhibitory regulation of cell growth and proliferation (1). The genetics of Siglecs shed light on their evolutionary history and function. In humans, SIGLEC1 is located on chromosome 20, SIGLEC2 and SIGLEC4 are near each other on chromosome 19, and the majority of the CD33-related Siglecs are clustered within an approximately 500-kb region of chromosome 19q13.3–13.4 (1, 12, 23, 24). The CD33-related Siglecs are an expanded group associated with mammalian evolution, and are less conserved across mammalian species, sharing approximately 50% to 99% identity. They are quickly evolving through multiple processes, including gene duplication, exon shuffling, exon loss, and gene conversion (1, 2, 7, 16, 21, 25–27). During recent evolution, Siglec-13 and Siglec-17 became inactive—similar pseudogenes can be found in the genomes of early hominoids such as the Neanderthal and Denisovan (28). Siglec-12, expressed on epithelial cells and cancer cells, is often referred to as Siglec-XII because of the loss of its capacity to bind to sialic acid ligands in humans (28). However, most of the CD33-related Siglecs are mainly expressed on immune cells and can modulate immune cell functions such as cell survival, growth, and cytokine production (10, 16).
Figure 2.
Illustration of the structure and diversity of Siglecs. There are two main groups of Siglecs, those which are highly conserved, as shown on the left, and a more diverse group of CD33-related Siglecs, as shown on the right. All Siglecs have an extracellular V-type Ig domain and at least one C2-type Ig domain. Many Siglecs also contain at least one cytoplasmic ITIM domain, involved in immunosuppressive signaling.
Siglecs may be immune activating or inhibitory depending on receptor signaling domains found in the C-terminus. In humans, three Siglecs have a positively charged amino acid within their transmembrane domain: Siglec-14, Siglec-15, and Siglec-16. When activated, these Siglecs can bind to the adaptor DAP12, which contains an immunoreceptor tyrosine-based activation motif (ITAM), and recruit SYK, which generally leads to immune activation (7, 10, 16, 27). In contrast, 10 of the 15 Siglecs function to inhibit the immune system through an immunoreceptor tyrosine-based inhibition motif (ITIM). Signaling through the ITIM results in recruitment of tyrosine phosphatases, such as SHP1 and SHP2, which contain SH2 domains and ultimately dampen the immune response (7, 10, 16, 27, 29). The physiologic role of inhibitory Siglecs is to protect against excess stimulation of the immune response through their interaction with sialoglycans, which are displayed on mammalian cells but not on pathogens. In support of this hypothesis, glycans that terminate with sialic acid residues, which are therefore more likely to engage Siglecs, induce lower levels of immunogenicity than other glycans in healthy individuals (30). In addition to sialoglycans, Siglec receptors can also bind to protein ligands in some instances (31–33).
Siglec–sialic acid signaling can occur in a cis or trans manner, and signaling may be modulated depending on whether binding sites are available or masked. Specifically, this means that sialic acid–decorated glycans may engage Siglecs through cell–cell interactions, that is, in trans, or through binding Siglecs on the immune cell expressing the sialic acid–decorated ligands, that is, in cis. This latter phenomenon of Siglec binding through cis interactions may be a mechanism for immune cell autoregulation, complementing the trans interactions that occur during immune surveillance of other cells. As with other glycan-binding receptors, effective interactions require clustering of the receptors and ligands (2, 16). Activating CD33-related Siglec receptors (i.e., Siglec-14 and Siglec-16) most likely act as paired receptors on immune cells together with their inhibitory counterparts. Siglec-5 and Siglec-14, as well as Siglec-11 and Siglec-16, have very high sequence homology in the first two domains and have an exact overlap of sialoglycan binding (34). It is likely that activating receptors developed to counteract bacteria that exploit inhibitory Siglec receptors.
Siglecs are the key immune receptors that bind to cell-surface sialic acids, with interactions that ultimately lead to modulation of the immune system (10, 16, 18, 25, 35–37). Siglec receptors are broadly expressed on innate and adaptive immune cells. Malignant cells, through hypersialylation, have increased binding to Siglecs, and it is the interaction with inhibitory Siglecs that dampens the immune response and enables metastasis (21, 38–42). Transformation into a malignant cell often coincides with expression changes in genes that code for glycosyltransferases, glycosidases, and other genes involved in glycan synthesis. Genetic and epigenetic changes, transcription factor activity, environmental cues, and metabolic changes may trigger the changes in glycan synthesis that lead to hypersialylation on tumor cells (13). In addition, changes in lysosomal and Golgi transporters, as well as in sialidases and sialyltransferases, appear to be involved in hypersialylation of malignant cells (5).
The evolution of Siglecs has been shaped by continuous pressure from pathogens and tumor cells that have developed mechanisms to evade immune detection (17, 36, 43). Of importance, a balance must be maintained between activation and inhibition of an immune response, as an overactive immune response can lead to autoimmune disease (17).
Importance of Sialoglycan–Siglec Interactions in Human Biology and Disease
In the setting of CD33-related Siglecs and potential for immunomodulatory dysregulation, genetic association studies have demonstrated relationships between a number of these Siglecs and various clinical inflammatory disease states. For example, the association between informative SNPs in SIGLEC3 and Alzheimer's disease, which is not considered an inflammatory disorder, arose from one of the first genome-wide association studies (44–46). Further genetic associations between CD33-related Siglecs and inflammatory diseases include systemic lupus erythematosus (Siglec-6), asthma (Siglec-8), and cancer (Siglec-3, -9, -12; ref. 36). With immune responses playing a role in many diseases, including cancer, targeting the immunomodulatory function of sialoglycan–Siglec interactions has therapeutic potential (6).
Some Siglecs are expressed on specific immune cell types, which provide a basis for understanding disease associations and highlights the potential for targeted delivery of Siglec-based therapeutics. For example, Siglec-8, which is expressed only on eosinophils and mast cells, has been targeted with antibodies with remarkable promise in eosinophilic esophagitis and gastritis (47). Siglec-15 is expressed on myeloid cells, including antigen-presenting cells and osteoclasts, and it may be possible to target for development of therapeutics for osteoporosis (2, 48). In the bone, Siglec-15 on osteoclast precursor cells can interact with sialylated CD44 and modulate receptor activator of nuclear factor κ B (RANK)–mediated signaling (49).
Siglec-7 is constitutively expressed on natural killer (NK) cells and has been identified as a potential target to improve NK cell–mediated antitumor immunity (41, 50, 51). Siglec-9, which has been linked to both cancer and infections, has broad expression on immune cells, including monocytes, neutrophils, dendritic cells, macrophages, and some NK- and T-cell subsets (27, 42, 52), and therefore serves as a key Siglec target for immune modulation in cancer. Siglec-10 is enriched on macrophages, and through its binding to sialylated CD24, it may function in parallel with the signal regulatory protein α (SIRPα)–CD47 axis to inhibit macrophage-mediated phagocytosis (27, 53). Siglec-10 and its murine counterpart Siglec-G are expressed on B1 cells and regulate antibody production and tolerance to antigens (54). Siglec-10/G binding to a CD24 complex with high mobility group box 1 (HMGB1) has been shown to dampen the immune response to danger signals (55).
Hypersialylation on tumor cells has been associated with multiple cancers, including lung, pancreatic, and breast cancers, Wilms tumor, rhabdosarcoma, glioma, and neuroblastoma (56, 57). Interaction of Siglec receptors with cancer-associated sialoglycans was reported to influence the prognosis in a lung cancer cohort: A variant of Siglec-9 with lower binding affinity to sialoglycans was associated with improved outcomes in patients with non–small cell lung carcinoma (42). Several experimental preclinical investigations further support the hypothesis that interrupting Siglec–sialoglycan binding in cancer could help avoid immune evasion by tumor cells and, conversely, that the inhibitory signaling of Siglecs could be used to reduce aberrant immune response in autoinflammatory diseases, making sialoglycan–Siglec interactions a therapeutic target for multiple diseases (6).
Targeting Siglecs and Sialoglycan–Siglec Immunoregulatory Interactions
The consistent and specific expression of Siglecs on subsets of immune cells provides a substantial opportunity to target Siglecs and the sialic acid glycocalyx for cancer treatment; broadly, two approaches are being pursued. The first approach uses agents that target Siglecs on immune cells that have transformed into malignant cells to localize a cytotoxic payload to a particular immune cell type. Agents that use this approach have already been approved (e.g., gemtuzumab ozogamicin; Table 1), yet significant therapeutic opportunity remains. The second approach uses agents that target the immunoregulatory interaction between Siglecs and their sialic acid ligands to reprogram immune cells for an immunologic attack.
Table 1.
Agents approved or in clinical development that target Siglecs as tumor-associated markers.
| Siglec/Sialic acid target | Drug name(s) | Status | Description |
|---|---|---|---|
| Siglec-2 (CD22) | |||
| UCART22 (Cellectis) | Phase I | Allogeneic anti-CD22 CAR T | |
| JCAR-018 (BMS) | Phase I | Anti-CD22 CAR T | |
| Senl H19×22P (AvalonGloboCare) | Phase I | Anti-CD22/CD19 CAR T | |
| MendCART (Hrain Biotechnology) | Phase I | Anti-CD22 CAR-T; NCT02721407 | |
| YT-19/22 (China Immunotech) | Phase I | Anti-CD19/anti-CD22 synthetic T-cell antigen receptor (STAR-T) | |
| AUTO1/22 (Autolus Therapeutics) | Phase I | Anti-CD19/anti-CD22 CAR T | |
| CTA-101 (Nanjing Bioheng Biotech) | Phase I | Anti-CD19/anti-CD22 CAR T | |
| LB-1909 (Nanjing Legend Biotech) | Phase I | Anti-CD19/anti-CD20/anti-CD22 CAR T | |
| GC-022 (Gracell Biotechnology) | Phase I | Anti-CD19/anti-CD22 CAR T; NCT04303247 | |
| TRPH-222 | Phase I (anticipated phase II to start in 2022) | Anti-CD22 ADC | |
| JNJ-75348780 (J&J) | Phase I | Anti-CD3/CD22 BiTE | |
| Inotuzumab ozogamicin (Besponsa, Pfizer) | Approved for use in acute lymphoblastic leukemia (ALL) | Anti-CD22 ADC | |
| Moxetumomab pasudotox (Lumoxiti, AstraZeneca) | Approved for use in hairy cell leukemia (HCL) | Anti-CD22 ADC | |
| Siglec-3 (CD33) a | |||
| Gemtuzumab ozogamicin (Mylotarg, Pfizer) | Approved for use in acute myeloid leukemia (AML) | ADC | |
| VOR33 (Vor BioPharma) | Phase I | Anti-CD33 CAR T being assessed in AML | |
| PRGN-3006 (Precigen) | Phase I | Autologous CAR T targeting CD33 in phase I for myeloid disorders | |
| ICG-136 (iCell Gene Therapeutics) | Phase I | Anti-CD33 CAR T being assessed in AML | |
| Eluvixtamab (AMG 330; Amgen) | Phase I | Anti-CD33/CD3 bispecific | |
| GEM333 (GEMoaB) | Phase I | Anti-CD33/CD3 bispecific | |
| JNJ-67571244 (J&J) | Phase I | Anti-CD33/CD3 bispecific | |
| CD33 NKE (BMS) | Phase I | NK-cell engager | |
aMany preclinical studies are ongoing. This table lists only clinical studies that appear to be active.
Therapeutics Targeting the Siglecs as Tumor-Associated Markers
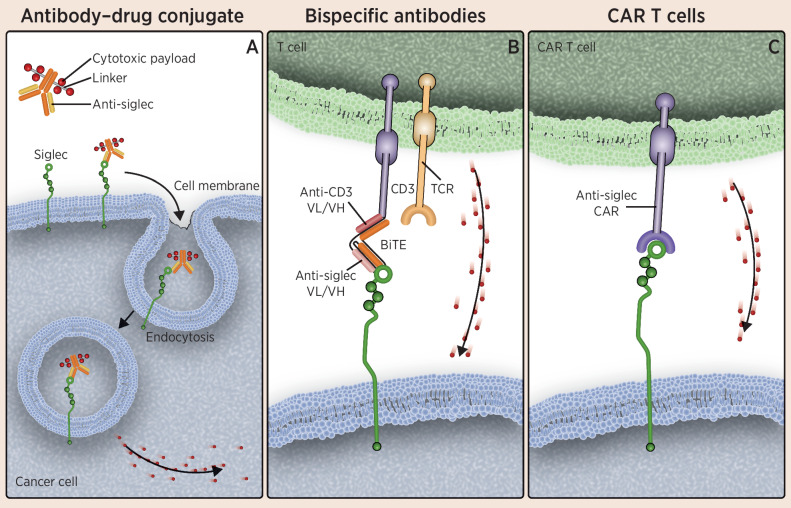
Because Siglecs are cell surface receptors, antibody-based therapeutics represent an effective approach to target malignant immune cells retaining Siglec expression as lineage markers. Antibody-tethered cytotoxic function can take many forms, including antibody–drug conjugates (ADC), anti-Siglec bispecific T-cell engagers (BiTE), and chimeric-antigen receptor (CAR) T-cell therapies (Table 1; Fig. 3; refs. 58–62). Although antibody-dependent cellular cytotoxicity (ADCC) may be considered favorable because of the potential for increased safety over ADCs, naked Siglec-targeted antibodies have not demonstrated sufficient activity in the cancer setting (63).
Figure 3.
Therapies targeting Siglecs on malignant myeloid and lymphoid cells. A, Illustrates ADCs composed of an anti-Siglec conjugated to a cytotoxic small-molecule payload. The antibody portion of the drug targets Siglecs, which are displayed on the surface of cancer cells, leading to internalization of the antibody and the drug and subsequent release of the cytotoxic payload within the cancer cell. B, Depicts the use of anti-Siglec BiTEs to link cytotoxic T cells to cancer cells, resulting in destruction of the cancer cell. As shown in C, CAR T-cell therapies have been developed that target Siglecs displayed on the surface of cancer cells, leading to cytotoxicity in those cells. Cytotoxic granules are depicted as red dots. TCR, T-cell receptor; VH, variable heavy chain; VL, variable light chain.
ADCs
Siglecs are endocytosed after binding to a ligand, with internalization of tethered molecules, making them excellent targets for ADC therapies, especially in cases where the toxin must be delivered within specific subsets of immune cells (2, 61, 62). In general, Siglec-3 is highly expressed, with relative specificity on myeloid cells, and can serve as a lineage marker for myeloid cells. It also is enriched on acute myeloid leukemia (AML) cells (2, 27, 64). The first ADC to gain FDA approval was gemtuzumab ozogamicin (Mylotarg), which targets Siglec-3 (CD33). It is indicated for the treatment of adults and children who have CD33-positive AML.
Siglec-2 is expressed primarily on B cells. Thus, anti–Siglec-2 ADCs have been used for B-cell leukemias and lymphomas (27). Inotuzumab ozogamicin (Besponsa) is an ADC comprising an anti–Siglec-2 antibody linked to a small-molecule toxin, calicheamicin, which induces DNA damage, it is indicated for the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (65). Moxetumomab pasudotox (Lumoxiti) is not an ADC, but a protein construct that is a fusion between an anti–Siglec-2 monobody and PE38, a fragment of a Pseudomonas toxin. It is approved for the treatment of hairy cell leukemia, a rare type of slow-growing leukemia that arises in B cells (2, 66). The demonstration of effective agents targeting Siglecs has solidified their relevance as targets for cancer treatment.
BiTEs and CAR T-cell therapy
On the basis of the precedent of targeting Siglec-2 (CD22) and Siglec-3 (CD33) with ADCs and related therapeutics, clinical development has been progressing for Siglec-targting agents that employ BiTE or CAR T-cell technology (2, 58–60). BiTEs represent a mechanism for efficiently engaging cytotoxic T cells to kill cancer cells. They are composed of two single-chain variable fragments designed to target two antigens, with a flexible linker in between. One fragment targets a marker on the surface of T cells, CD3, and the other targets a tumor-associated antigen. When simultaneous binding occurs, it allows the T cell to come into contact and kill tumor cells (67). The first BiTE to gain FDA approval was blinatumomab (Blincyto), a CD3/CD19 BiTE, which is indicated for Philadelphia negative relapsed or refractory B-cell progenitor acute lymphoblastic leukemia. Since then, many other BiTEs have been developed and are being evaluated for the treatment for hematologic malignancies (59). For example, AMG330, a CD3/CD33 BiTE that is being evaluated as a potential treatment for AML. In mouse models, AMG330 treatment reduces tumor growth, but additional preclinical studies are needed to optimize effector-to-target ratio as the data showed there was insufficient target cell lysis in samples that had low initial effector-to-target ratios (68).
CAR T cells provide highly potent and specific targeting, albeit with systemic immune toxicity requiring careful management. Clinical studies of Siglec-targeting CAR T cells are all in early phases and data are currently insufficient to establish whether enhanced efficacy or an improved therapeutic index can be achieved compared with the ADC strategy. In nonclinical studies, the efficacy of Siglec-2–targeting CAR T cells may be influenced by the epitope targeted by the CAR; however, the mechanism for this effect is not fully understood (2, 69–72). As with Siglec-2 targeting, CAR T cells created against Siglec-3 have shown activity in preclinical studies, but because Siglec-3 is expressed on myeloid precursor cells more broadly, treatment also led to hematopoietic toxicity (2). One option to potentially mitigate this liability could be to create modified CAR T-cell therapies that can be switched off to prevent long-term, life-threatening immune suppression (2, 58). One of the more recently developed CAR T-cell therapies targets Siglec-6, which is commonly expressed on AML cell lines but not on normal hematopoietic stem and progenitor cells (HSPC). In preclinical and in vitro studies, the approach has shown specific antileukemia activity and does not appear to affect viability or lineage differentiation of HSPCs, which may suggest the possibility of treating AML without the need for allogeneic hematopoietic stem cell transplantation (73). Bispecific CAR T-cell therapies such as those targeting CD19 and CD22 may address issues of antigen loss and CAR T-cell resistance. Cytokine production appeared to be an important indicator for potency in a phase I trial of CD19/CD22 bispecific CAR T-cell therapy (72).
Immunomodulators of Sialoglycan–Siglec Interactions
The untapped future of Siglec-targeted therapy depends on improved functional understanding of Siglecs and identifying ways to exploit this new knowledge to enable an antitumor immune responses. This will be challenging given the diversity of Siglec function as well as the diversity of sialic acid synthesis and binding characteristics (15). There are several potential approaches to unleashing an antitumor response via modulation of the Siglec–sialic acid axis, including blocking the immune-suppressive effects of inhibitory Siglecs, driving immune-activating Siglecs, and altering synthesis and expression of the sialic acid glycocalyx. Currently, several agents are in preclinical studies utilizing any of these approaches, and some therapeutics, including NC318 and E-602, are being evaluated in clinical trials; (Table 2). However, based on cumulative evidence of the immunomodulatory effects of the Siglec–sialic acid interactions and the complexity of this system, a broad approach to exploring this pathway is warranted.
Table 2.
Agents in preclinical or clinical development targeting Siglecs with the intent of immune modulation.
| Siglec/Sialic acid target | Drug name(s) | Status |
|---|---|---|
| Siglec-7 | Anti–Siglec-7 (Palleon) | Preclinical |
| Siglec-9 | Anti–Siglec-9 (Palleon) | Preclinical |
| Anti–Siglec-9 (Innate Pharma) | Preclinical | |
| Anti–Siglec-9 (Memo Therapeutics AG) | Preclinical | |
| Anti–Siglec-9 (Verseau Therapeutics) | Preclinical | |
| Siglec-10 | Anti-CD24 (Siglec-10 ligand; Pheast Therapeutics) | Preclinical |
| Siglec-15 | NC318 (NextCure) | Phase II |
| EPB-001 (Elpis Biopharmaceuticals) | Preclinical | |
| Anti–Siglec-15 (OncoResponse) | Preclinical | |
| MIL104 (Mab Works) | Preclinical | |
| Pan-Siglec | E-602 (Palleon) | Phase 1/2 |
| AL009 (Alector) | Preclinical | |
| PD-L1–sialidase (Palleon) | Preclinical | |
| HER2-sialidase (Palleon) | Preclinical |
Abbreviations: HER2, human epidermal growth factor receptor 2; IND, investigational new drug; PD-L1, programmed death-ligand 1.
Releasing immunosuppression with anti-Siglec blockers
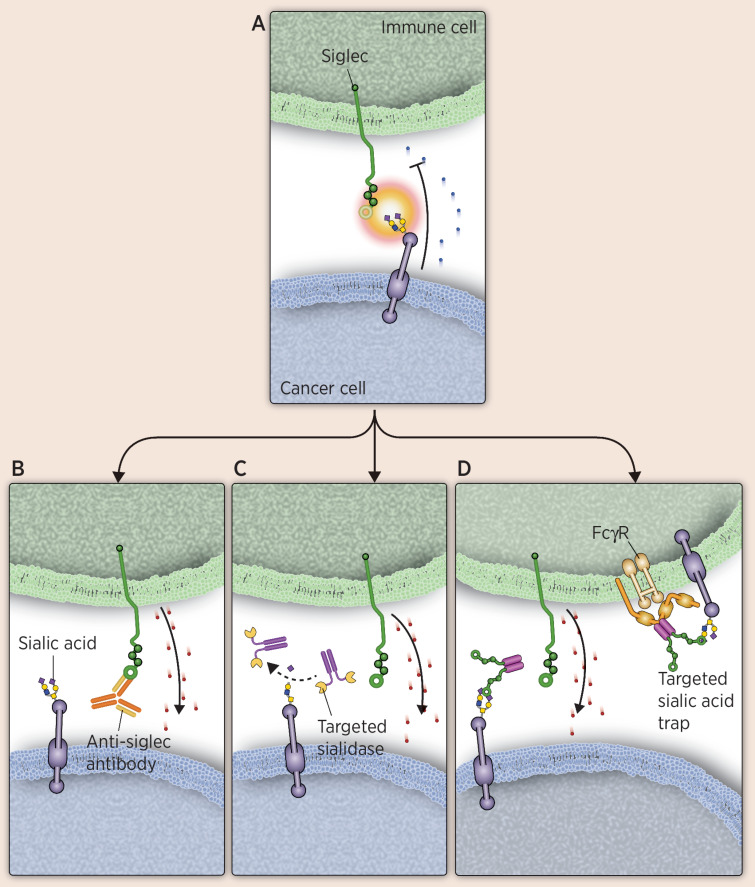
Antibody-based therapies that specifically target inhibitory Siglec function have the potential to enhance immune responses by counteracting immune-suppressive signaling from tumor cells overexpressing sialic acids (Fig. 4). Because innate immune responsiveness is an initiator and modulator of the adaptive immune response, modulating Siglecs has broad therapeutic potential. The anti–Siglec-15 NC318 is furthest along in clinical development (74). Although typically expressed on osteoclasts, Siglec-15 is also abundant on solid tumor cells such as colon cancer, endometrial cancer, and thyroid cancer, as well as on tumor-associated macrophages (2). Siglec-15 signaling leads to suppressed T-cell function, despite the presence of an ITAM in the Siglec-15 intracellular domain. The ligand of Siglec-15 has not yet been elucidated, but high levels of Siglec-15 have been associated with increased tumor growth and decreased T-cell infiltration (27). Interestingly, Siglec-15 may inhibit antitumor activity by acting as a ligand itself (26). Preclinical studies have shown that NC318 blocks Siglec-15 function and leads to reversal of T-cell suppression, halting of tumor growth, and prevention of metastasis to the lungs in mouse models that constitutively express Siglec-15 (26). Initial clinical data for this molecule have demonstrated antitumor responses in solid tumors (74). Of interest, derepression of immune activation appears validated, based on two reported cases of vitiligo that were considered an immune-related adverse event related to treatment (74). Antibodies targeting Siglec-7 and -9 are also being investigated for their therapeutic potential. Siglec-7 and -9 are associated with inhibition of an endogenous antitumor immune response. Preliminary data in mouse models have shown that using antibodies to block Siglec-7 and -9 signaling reduces tumor burden significantly in vivo (75).
Figure 4.
Therapies targeting Siglecs as immune modulators. To overcome immune evasion mechanisms of cancer cells, several approaches are being evaluated, including Siglec-blocking antibodies, targeted sialidases, and targeted sialic acid traps. A, Illustrates an interaction between an inhibitory Siglec on an immune cell and a sialylated glycan on a cancer cell, leading to immunosuppression. B, Illustrates an anti-Siglec binding to a Siglec to block binding, thus preventing their ability to suppress an immune response. C, Shows a sialidase conjugated to an antibody that targets it to cancer cells. Once bound, it desialylates the ligand, preventing immune suppression from Siglec–sialic acid interactions. D, Depicts a Siglec-Fc fusion that functions as a sialic acid trap. The Fc portion allows localization to immune cells, whereas the Siglec portion can bind sialic acid on tumor or immune cells, blocking inhibitory immune signaling. Cytotoxic granules are depicted as red dots.
Targeting sialic acid immunosuppressive signaling ligands on tumor cells
Interference with the synthesis of the sialic acid–based ligands of Siglecs or the ligand protein anchor that carries the sialic acid ligands is one strategy for interrupting immune-inhibitory interactions between Siglecs and sialic acid residues. Enzymes involved in sialic acid biosynthesis that could be targeted include UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase (GNE) and cytidine monophosphate sialic acid synthase (76, 77). GNE is the rate-limiting enzyme for sialic acid biosynthesis (76). Alternatively, sialyltransferases and sialic acid transporters such as ST6GAL-1, ST6GalNAc-I, or C1galt1c1 could be targeted, although preliminary data on efficacy of this approach are conflicting (38, 78, 79). Interestingly, a recent study showed that complete elimination of sialylated structures on a murine colorectal cancer cell line resulted in significant increases in tumor growth in vivo. Therefore, complete desialylation may be detrimental to the antitumor immune response, and additional studies are needed (80).
With regard to anchor proteins, CD24, the ligand for Siglec-10 is known to be highly expressed on tumor cells. Siglec-10 is also highly expressed on tumor-associated macrophages, and its interaction with CD24 is involved in inhibiting phagocytosis. Blocking CD24 with anti-CD24 in preclinical studies has been shown to increase tumor phagocytosis, macrophage-dependent reduction in tumor growth, and extension of survival (53). Both of these mechanisms could be effective options; however, these mechanisms are not currently being explored in ongoing clinical development programs.
The sialic acid–targeting approach furthest along in clinical development for involves degradation of overexpressed sialic acid moieties on tumor cells with a technology termed “EAGLE” (Enzyme-Antibody Glycan-Ligand Editing, https://palleonpharma.com/pipeline). This approach leverages two key features of tumor cells: overexpression of targeted tumor antigens and amplification of sialic acid expression to block immune response. EAGLE allows for the fusion of human sialidases with human mAbs. The resulting fusion is antibody-like and selectively removes terminal sialic acid residues on sialoglycans of tumor cells (Fig. 4). The technology was originally developed by combining an anti-HER2 to a sialidase conjugate that selectively removed diverse sialoglycans from breast cancer cells, leading to enhanced immune cell infiltration and activation, as well as prolonged survival, in mouse models (81).
The lead developmental therapeutic in this class, E-602, is a bi-sialidase joined with an antibody Fc fragment. This entity is designed to desialylate both immune cells and tumor cells, and is currently in phase I clinical trials. One potential limitation of this construct is the lack of specificity in targeting the sialidase activity; however, the EAGLE platform has alternative modular structures that allow for tumor-associated antigen targeting with modular inclusion of antibody-antigen–binding fragment regions as a means for focused activity. For example, one therapeutic is composed of trastuzumab (an anti-HER2) linked to a bacterial sialidase from Vibrio cholerae. This conjugate functions to specifically remove Siglec ligands on HER2-positive breast cancer cells and results in the presentation of Fc chains to stimulate NK cell–mediated ADCC (27, 82, 83). In addition to targeting sialoglycan–Siglec interactions, sialidases also have other effects on immune cells and the tumor microenvironment. Recently, an interaction of CD28, which is expressed on T cells, with sialoglycans was described. Sialidases could therefore improve interactions between antigen-presenting cells with adaptive immune cells (84).
Another strategy that has shown promising effects is injection of a sialic acid mimetic directly into solid tumors to locally block sialic acid expression. In multiple tumor models, growth was suppressed and immune cell composition shifted. Populations of tumor-infiltrating NK cells and CD8+ T cells were increased, whereas regulatory T cells and myeloid regulatory cells were reduced, leading to an increase in CD8+ T cell–mediated killing and reduced tumor growth (85).
Disrupting immunosuppressive signaling with a sialic acid trap
AL009 is a multi-Siglec inhibitor being investigated for its ability to enhance innate and adaptive immunity. It is an engineered human Siglec-9–Fc fusion protein and functions by blocking a critical glycan checkpoint pathway that drives immune inhibition (Fig. 4). The Siglec portion of AL009 binds the sialic acid ligands recognized by Siglec-9 and increases immune function by preventing Siglec–sialic acid signaling. Human Siglec-9 has been shown by glycan arrays to broadly engage various sialoglycans (86). The engineered Fc portion binds to a subset of Fc receptors on myeloid cells, thereby targeting AL009 to the myeloid-cell compartment (87). In vitro, AL009 was shown to repolarize suppressive macrophages and prevent T-cell suppression. In syngeneic mouse tumor models, a variation of AL009, which has a mouse Fc instead of human, reduced tumor volume and enhanced immune activation, particularly when used in combination with anti–PD-1 treatment (87).
Conclusion
Combining glycobiology and cancer immunology to create therapies targeting sialoglycan–Siglec interactions holds potential for a new generation of therapeutics to treat a range of diseases, including cancer, neurodegenerative diseases, autoinflammatory conditions, and allergies. Large molecules targeting Siglecs to localize cytotoxic therapeutics to a particular cell type have been successful in the treatment of blood cancers. As we gain an increased understanding of the immunologic function of sialoglycan–Siglec interactions, new approaches have significant potential to disrupt the core innate immunosuppressive signaling axis and reinvigorate robust and durable antitumor immune responses.
Acknowledgments
H. Läubli received financial support from Swiss National Science Foundation (SNSF Nr. 310030_184720/1, to H. Läubli).
Authors' Disclosures
H. Läubli reports grants from Palleon Pharmaceuticals during the conduct of the study; grants and personal fees from GlycoEra; grants from Novartis and Bristol-Myers Squibb; personal fees from Alector and Merck Sharp Dome outside the submitted work; in addition, H. Läubli has a patent for WO2021094545A1 issued. S.C. Nalle reports a patent for WO 2021/091885 A2 pending. S.C. Nalle and D. Maslyar report employment and shareholder status of Alector, a publicly traded biotechnology company developing AL009 for clinical use. No other disclosures were reported.
References
- 1. Bornhofft KF, Goldammer T, Rebl A, Galuska SP. Siglecs: a journey through the evolution of sialic acid-binding immunoglobulin-type lectins. Dev Comp Immunol 2018;86:219–31. [DOI] [PubMed] [Google Scholar]
- 2. Lenza MP, Atxabal U, Oyenarte I, Jimenez-Barbero J, Ereno-Orbea J. Current status on therapeutic molecules targeting Siglec receptors. Cells 2020;9:2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nishana M, Raghavan SC. Role of recombination activating genes in the generation of antigen receptor diversity and beyond. Immunology 2012;137:271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leung SO, Goldenberg DM, Dion AS, Pellegrini MC, Shevitz J, Shih LB, et al. Construction and characterization of a humanized, internalizing, B-cell (CD22)-specific, leukemia/lymphoma antibody, LL2. Mol Immunol 1995;32:1413–27. [DOI] [PubMed] [Google Scholar]
- 5. Laubli H, Varki A. Sialic acid-binding immunoglobulin-like lectins (Siglecs) detect self-associated molecular patterns to regulate immune responses. Cell Mol Life Sci 2020;77:593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murugesan G, Weigle B, Crocker PR. Siglec and anti-Siglec therapies. Curr Opin Chem Biol 2021;62:34–42. [DOI] [PubMed] [Google Scholar]
- 7. Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol 2014;14:653–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duan S, Paulson JC. Siglecs as immune cell checkpoints in disease. Annu Rev Immunol 2020;38:365–95. [DOI] [PubMed] [Google Scholar]
- 9. van Houtum EJH, Bull C, Cornelissen LAM, Adema GJ. Siglec signaling in the tumor microenvironment. Front Immunol 2021;12:790317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol 2007;7:255–66. [DOI] [PubMed] [Google Scholar]
- 11. Pearce OM, Laubli H. Sialic acids in cancer biology and immunity. Glycobiology 2016;26:111–28. [DOI] [PubMed] [Google Scholar]
- 12. Adams OJ, Stanczak MA, von Gunten S, Laubli H. Targeting sialic acid-Siglec interactions to reverse immune suppression in cancer. Glycobiology 2018;28:640–7. [DOI] [PubMed] [Google Scholar]
- 13. RodrIguez E, Schetters STT, van Kooyk Y. The tumour glyco-code as a novel immune checkpoint for immunotherapy. Nat Rev Immunol 2018;18:204–11. [DOI] [PubMed] [Google Scholar]
- 14. Varki A, Schnaar RL, Schauer R. Sialic acids and other nonulosonic acids. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, et al., editors. Essentials of glycobiology. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2015. p 179–95. [PubMed] [Google Scholar]
- 15. Varki A, Gagneux P. Multifarious roles of sialic acids in immunity. Ann N Y Acad Sci 2012;1253:16–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barenwaldt A, Laubli H. The sialoglycan-Siglec glyco-immune checkpoint - a target for improving innate and adaptive anti-cancer immunity. Expert Opin Ther Targets 2019;23:839–53. [DOI] [PubMed] [Google Scholar]
- 17. Varki A. Are humans prone to autoimmunity? Implications from evolutionary changes in hominin sialic acid biology. J Autoimmun 2017;83:134–42. [DOI] [PubMed] [Google Scholar]
- 18. Varki A. Since there are PAMPs and DAMPs, there must be SAMPs? Glycan "self-associated molecular patterns" dampen innate immunity, but pathogens can mimic them. Glycobiology 2011;21:1121–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reily C, Stewart TJ, Renfrow MB, Novak J. Glycosylation in health and disease. Nat Rev Nephrol 2019;15:346–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cagnoni AJ, Perez Saez JM, Rabinovich GA, Marino KV. Turning-off signaling by siglecs, selectins, and galectins: chemical inhibition of glycan-dependent interactions in cancer. Front Oncol 2016;6:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jandus C, Simon HU, von Gunten S. Targeting siglecs–a novel pharmacological strategy for immuno- and glycotherapy. Biochem Pharmacol 2011;82:323–32. [DOI] [PubMed] [Google Scholar]
- 22. O'Reilly MK, Paulson JC. Siglecs as targets for therapy in immune-cell-mediated disease. Trends Pharmacol Sci 2009;30:240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mucklow S, Hartnell A, Mattei MG, Gordon S, Crocker PR. Sialoadhesin (Sn) maps to mouse chromosome 2 and human chromosome 20 and is not linked to the other members of the sialoadhesin family, CD22, MAG, and CD33. Genomics 1995;28:344–6. [DOI] [PubMed] [Google Scholar]
- 24. Schwarz F, Fong JJ, Varki A. Human-specific evolutionary changes in the biology of siglecs. Adv Exp Med Biol 2015;842:1–16. [DOI] [PubMed] [Google Scholar]
- 25. Varki A, Angata T. Siglecs–the major subfamily of I-type lectins. Glycobiology 2006;16:1R–27R. [DOI] [PubMed] [Google Scholar]
- 26. Wang J, Sun J, Liu LN, Flies DB, Nie X, Toki M, et al. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat Med 2019;25:656–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith BAH, Bertozzi CR. The clinical impact of glycobiology: targeting selectins, Siglecs and mammalian glycans. Nat Rev Drug Discov 2021;20:217–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang X, Mitra N, Secundino I, Banda K, Cruz P, Padler-Karavani V, et al. Specific inactivation of two immunomodulatory SIGLEC genes during human evolution. Proc Natl Acad Sci U S A 2012;109:9935–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Avril T, Freeman SD, Attrill H, Clarke RG, Crocker PR. Siglec-5 (CD170) can mediate inhibitory signaling in the absence of immunoreceptor tyrosine-based inhibitory motif phosphorylation. J Biol Chem 2005;280:19843–51. [DOI] [PubMed] [Google Scholar]
- 30. Schneider C, Smith DF, Cummings RD, Boligan KF, Hamilton RG, Bochner BS, et al. The human IgG anti-carbohydrate repertoire exhibits a universal architecture and contains specificity for microbial attachment sites. Sci Transl Med 2015;7:269ra1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carlin AF, Chang YC, Areschoug T, Lindahl G, Hurtado-Ziola N, King CC, et al. Group B Streptococcus suppression of phagocyte functions by protein-mediated engagement of human Siglec-5. J Exp Med 2009;206:1691–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fong JJ, Tsai CM, Saha S, Nizet V, Varki A, Bui JD. Siglec-7 engagement by GBS beta-protein suppresses pyroptotic cell death of natural killer cells. Proc Natl Acad Sci U S A 2018;115:10410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aalto K, Autio A, Kiss EA, Elima K, Nymalm Y, Veres TZ, et al. Siglec-9 is a novel leukocyte ligand for vascular adhesion protein-1 and can be used in PET imaging of inflammation and cancer. Blood 2011;118:3725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwarz F, Landig CS, Siddiqui S, Secundino I, Olson J, Varki N, et al. Paired Siglec receptors generate opposite inflammatory responses to a human-specific pathogen. EMBO J 2017;36:751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pillai S, Netravali IA, Cariappa A, Mattoo H. Siglecs and immune regulation. Annu Rev Immunol 2012;30:357–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Angata T. Associations of genetic polymorphisms of Siglecs with human diseases. Glycobiology 2014;24:785–93. [DOI] [PubMed] [Google Scholar]
- 37. Varki A, Schnaar RL, Crocker PR. I-type lectins. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, et al., editors. Essentials of glycobiology. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2015. p 453–67. [Google Scholar]
- 38. Chiang CH, Wang CH, Chang HC, More SV, Li WS, Hung WC. A novel sialyltransferase inhibitor AL10 suppresses invasion and metastasis of lung cancer cells by inhibiting integrin-mediated signaling. J Cell Physiol 2010;223:492–9. [DOI] [PubMed] [Google Scholar]
- 39. Bull C, Boltje TJ, van Dinther EA, Peters T, de Graaf AM, Leusen JH, et al. Targeted delivery of a sialic acid-blocking glycomimetic to cancer cells inhibits metastatic spread. ACS Nano 2015;9:733–45. [DOI] [PubMed] [Google Scholar]
- 40. Beatson R, Tajadura-Ortega V, Achkova D, Picco G, Tsourouktsoglou TD, Klausing S, et al. The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9. Nat Immunol 2016;17:1273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hudak JE, Canham SM, Bertozzi CR. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nat Chem Biol 2014;10:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Laubli H, Pearce OM, Schwarz F, Siddiqui SS, Deng L, Stanczak MA, et al. Engagement of myelomonocytic Siglecs by tumor-associated ligands modulates the innate immune response to cancer. Proc Natl Acad Sci U S A 2014;111:14211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gagneux P, Varki A. Evolutionary considerations in relating oligosaccharide diversity to biological function. Glycobiology 1999;9:747–55. [DOI] [PubMed] [Google Scholar]
- 44. Heneka MT, Carson MJ, El Khoury J, Landreth GE, B F, Feinstein DL, et al. Neuroinflammation in Alzheimer's disease. Lancet Neurol 2015;14:388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat Genet 2011;43:429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nat Genet 2011;43:436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dellon ES, Peterson KA, Murray JA, Falk GW, Gonsalves N, Chehade M, et al. Anti-Siglec-8 antibody for eosinophilic gastritis and duodenitis. N Engl J Med 2020;383:1624–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sun J, Lu Q, Sanmamed MF, Wang J. Siglec-15 as an emerging target for next-generation cancer immunotherapy. Clin Cancer Res 2021;27:680–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Angata T. Siglec-15: a potential regulator of osteoporosis, cancer, and infectious diseases. J Biomed Sci 2020;27:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jandus C, Boligan KF, Chijioke O, Liu H, Dahlhaus M, Demoulins T, et al. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. J Clin Invest 2014;124:1810–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Daly J, Sarkar S, Natoni A, Stark JC, Riley NM, Bertozzi CR, et al. Targeting hypersialylation in multiple myeloma represents a novel approach to enhance NK cell-mediated tumor responses. Blood Adv 2022;6:3352–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Haas Q, Boligan KF, Jandus C, Schneider C, Simillion C, Stanczak MA, et al. Siglec-9 regulates an effector memory CD8(+) T-cell subset that congregates in the melanoma tumor microenvironment. Cancer Immunol Res 2019;7:707–18. [DOI] [PubMed] [Google Scholar]
- 53. Barkal AA, Brewer RE, Markovic M, Kowarsky M, Barkal SA, Zaro BW, et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 2019;572:392–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen GY, Brown NK, Zheng P, Liu Y. Siglec-G/10 in self-nonself discrimination of innate and adaptive immunity. Glycobiology 2014;24:800–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science 2009;323:1722–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang X, Li X, Zeng YN, He F, Yang XM, Guan F. Enhanced expression of polysialic acid correlates with malignant phenotype in breast cancer cell lines and clinical tissue samples. Int J Mol Med 2016;37:197–206. [DOI] [PubMed] [Google Scholar]
- 57. Bhide GP, Colley KJ. Sialylation of N-glycans: mechanism, cellular compartmentalization and function. Histochem Cell Biol 2017;147:149–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J 2021;11:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tian Z, Liu M, Zhang Y, Wang X. Bispecific T cell engagers: an emerging therapy for management of hematologic malignancies. J Hematol Oncol 2021;14:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Einsele H, Borghaei H, Orlowski RZ, Subklewe M, Roboz GJ, Zugmaier G, et al. The BiTE (bispecific T-cell engager) platform: development and future potential of a targeted immuno-oncology therapy across tumor types. Cancer 2020;126:3192–201. [DOI] [PubMed] [Google Scholar]
- 61. Khongorzul P, Ling CJ, Khan FU, Ihsan AU, Zhang J. Antibody-drug conjugates: a comprehensive review. Mol Cancer Res 2020;18:3–19. [DOI] [PubMed] [Google Scholar]
- 62. Drago JZ, Modi S, Chandarlapaty S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat Rev Clin Oncol 2021;18:327–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Feldman EJ, Brandwein J, Stone R, Kalaycio M, Moore J, O'Connor J, et al. Phase III randomized multicenter study of a humanized anti-CD33 monoclonal antibody, lintuzumab, in combination with chemotherapy, versus chemotherapy alone in patients with refractory or first-relapsed acute myeloid leukemia. J Clin Oncol 2005;23:4110–6. [DOI] [PubMed] [Google Scholar]
- 64. Godwin CD, Gale RP, Walter RB. Gemtuzumab ozogamicin in acute myeloid leukemia. Leukemia 2017;31:1855–68. [DOI] [PubMed] [Google Scholar]
- 65. Besponsa Package Insert; 2017.
- 66. Kreitman RJ, Tallman MS, Robak T, Coutre S, Wilson WH, Stetler-Stevenson M, et al. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol 2012;30:1822–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Slaney CY, Wang P, Darcy PK, Kershaw MH. CARs versus BiTEs: a comparison between T cell-redirection strategies for cancer treatment. Cancer Discov 2018;8:924–34. [DOI] [PubMed] [Google Scholar]
- 68. Aigner M, Feulner J, Schaffer S, Kischel R, Kufer P, Schneider K, et al. T lymphocytes can be effectively recruited for ex vivo and in vivo lysis of AML blasts by a novel CD33/CD3-bispecific BiTE antibody construct. Leukemia 2013;27:1107–15. [DOI] [PubMed] [Google Scholar]
- 69. Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med 2018;24:20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shah NN, Highfill SL, Shalabi H, Yates B, Jin J, Wolters PL, et al. CD4/CD8 T-cell selection affects chimeric antigen receptor (CAR) T-cell potency and toxicity: updated results from a phase I anti-CD22 CAR T-cell trial. J Clin Oncol 2020;38:1938–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Dai H, Wu Z, Jia H, Tong C, Guo Y, Ti D, et al. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. J Hematol Oncol 2020;13:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Spiegel JY, Patel S, Muffly L, Hossain NM, Oak J, Baird JH, et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat Med 2021;27:1419–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jetani H, Navarro-Bailon A, Maucher M, Frenz S, Verbruggen C, Yeguas A, et al. Siglec-6 is a novel target for CAR T-cell therapy in acute myeloid leukemia. Blood 2021;138:1830–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tolcher A, Hamid O, Weber JS, LoRusso P, Shantz K, Heller KN, et al. Single agent anti-tumor activity in PD-1 refractory NSCLC: phase 1 data from the first-in-human trial of NC318, a Siglec-15-targeted antibody. November 6-–10, 2019; National Harbor, Maryland. [Google Scholar]
- 75. Ibarlucea-Benitez I, Weitzenfeld P, Smith P, Ravetch JV. Siglecs-7/9 function as inhibitory immune checkpoints in vivo and can be targeted to enhance therapeutic antitumor immunity. Proc Natl Acad Sci U S A 2021;118:e2107424118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Stanczak MA, Siddiqui SS, Trefny MP, Thommen DS, Boligan KF, von Gunten S, et al. Self-associated molecular patterns mediate cancer immune evasion by engaging Siglecs on T cells. J Clin Invest 2018;128:4912–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Teoh ST, Ogrodzinski MP, Ross C, Hunter KW, Lunt SY. Sialic acid metabolism: a key player in breast cancer metastasis revealed by metabolomics. Front Oncol 2018;8:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cornelissen LAM, Blanas A, Zaal A, van der Horst JC, Kruijssen LJW, O'Toole T, et al. Tn antigen expression contributes to an immune suppressive microenvironment and drives tumor growth in colorectal cancer. Front Oncol 2020;10:1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Perdicchio M, Cornelissen LA, Streng-Ouwehand I, Engels S, Verstege MI, Boon L, et al. Tumor sialylation impedes T cell mediated anti-tumor responses while promoting tumor associated-regulatory T cells. Oncotarget 2016;7:8771–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cornelissen LAM, Blanas A, van der Horst JC, Kruijssen L, Zaal A, O'Toole T, et al. Disruption of sialic acid metabolism drives tumor growth by augmenting CD8(+) T cell apoptosis. Int J Cancer 2019;144:2290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gray MA, Stanczak MA, Mantuano NR, Xiao H, Pijnenborg JFA, Malaker SA, et al. Targeted glycan degradation potentiates the anticancer immune response in vivo. Nat Chem Biol 2020;16:1376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Daly J, Carlsten M, O'Dwyer M. Sugar free: novel immunotherapeutic approaches targeting siglecs and sialic acids to enhance natural killer cell cytotoxicity against cancer. Front Immunol 2019;10:1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Xiao H, Woods EC, Vukojicic P, Bertozzi CR. Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc Natl Acad Sci U S A 2016;113:10304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Edgar LJ, Thompson AJ, Vartabedian VF, Kikuchi C, Woehl JL, Teijaro JR, et al. Sialic acid ligands of CD28 suppress costimulation of T cells. ACS Cent Sci 2021;7:1508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bull C, Boltje TJ, Balneger N, Weischer SM, Wassink M, van Gemst JJ, et al. Sialic acid blockade suppresses tumor growth by enhancing T-cell-mediated tumor immunity. Cancer Res 2018;78:3574–88. [DOI] [PubMed] [Google Scholar]
- 86. Padler-Karavani V, Hurtado-Ziola N, Chang YC, Sonnenburg JL, Ronaghy A, Yu H, et al. Rapid evolution of binding specificities and expression patterns of inhibitory CD33-related Siglecs in primates. FASEB J 2014;28:1280–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nalle S, Lam H, Leung L, Liang S, Maslyar D. AL009, a fusion protein and multi-Siglec inhibitor, repolarizes suppressive myeloid cells and potentiates anticancer effects. November 10–14, 2021; Washington D.C. [Google Scholar]