Abstract
We systematically reviewed existing literature regarding lower extremity neuromuscular rate of force development (RFD), maximal muscle strength (Fmax), and physical function in neurodegenerative populations, and to what extent these outcomes are affected and/or associated. Following PRISMA guidelines, 4 databases (Pubmed, Embase, SPORTDiscus, Web of Science) were searched. Across aging, Parkinson Disease (PD), Alzheimer’s Disease (AD), Multiple Sclerosis (MS), or Stroke, included studies should report (Part 1) deficits in lower extremity RFD, Fmax, and physical function (~ individuals having inferior vs. superior physical function), and/or (Part 2) associations between RFD (or Fmax) and physical function. A total of N=32 studies (n=1087 participants) were included. Part 1: deficits in RFD (-31%, mean; N=22) were comparable to deficits in physical function (-26%; N=7), yet both deficits exceeded that of Fmax (-21%; N=20). Part 2: associations between RFD and physical function (r2=0.13, mean; N=16) were comparable to associations between Fmax and physical function (r2=0.15; N=12). Lower extremity RFD is (1) particularly sensitive (i.e. adapts earlier and/or more extensively) towards neurodegeneration, and more so than Fmax, and (2) of importance for physical function but apparently not superior to Fmax. RFD could serve as a useful indicator/biomarker of changes in neuromuscular function elicited by neurodegeneration.
Keywords: Aging, Neurodegeneration, Neurological Disorders, Neuromuscular Function
Introduction
Neurological disorders of the central nervous system (CNS), more specifically neurodegenerative disorders, affect millions of people worldwide[1-4]. These disorders afflict people across all ages, yet particularly at advanced age, with the number of neurodegenerative patients estimated to increase considerably in the decades to come[2]. The deleterious changes of the central nervous system that accompany neurodegenerative disorders – a phenomenon also observed with aging[5] – altogether constitute major causes of disability[2-4,6,7].
Some of the most common and prevalent neurodegenerative disorders comprise Parkinson Disease (PD), Alzheimer’s Disease (AD), Multiple Sclerosis (MS), and Stroke[2-4,8]. These disorders induce marked structural and functional changes of the central nervous system (e.g. loss of neurons, glial cells, and myelin along with reductions in motor unit recruitment and firing frequency responsible for muscle activation/control)[5,9-11]. PD is characterized by loss of dopaminergic neurons within the Substantia Nigra[12,13]. AD is characterized by amyloid plaques and neurofibrillary tangles causing macroscopic atrophy from synaptic and neuronal loss[14]. MS is characterized by demyelination and axonal loss within the CNS[15]. Stroke differs as it is characterized by an acute episode of focal dysfunction of the brain, retina or spinal cord[16], yet it has been argued to be accompanied by secondary neurodegeneration over time, thus resembling some of the processes observed with PD, AD, and MS[11]. With aging, neurodegenerative processes also occur and resemble those observed with PD, AD, and MS – albeit less pronounced – comprising loss of neurons, glial cells, myelin etc[5,7,13].
A common consequence of the structural and functional CNS changes described above is that neuromuscular function (i.e. the nerve-muscle interaction responsible for motor function) deteriorates, initiating a cascade of deleterious events. Specifically, the outlined neurodegenerative populations are accompanied by impairments in neuromuscular function (i.e. ability to generate and control muscle force), often preferentially affecting the lower extremities[17-20], that eventually causes limitations in physical function (i.e. ability to perform activities of daily living such as walking) and ultimately disability[17,21-30]. To counteract this, it is of paramount importance to identify and understand relevant and modifiable neuromuscular predictors of physical function / disability.
The most examined feature of neuromuscular function is maximal muscle strength, defined as the ability of a muscle or muscle group to generate maximal force (or torque) during a voluntary contraction (termed force max; Fmax), with numerous studies having reported moderate-to-strong associations between lower extremity Fmax and physical function (e.g. walking) in the outlined neurodegenerative populations[31-37]. In contrast, the rate by which muscle strength can be developed, defined as the ability to increase force (or torque) as rapidly as possible during a voluntary contraction (termed rate of force development; RFD)[38], may contribute independently to physical performance[39], particularly in tasks requiring fast body movements. Previous studies have even argued RFD to be more sensitive to detect acute and/or chronic adaptations in neuromuscular function compared to Fmax[38,40,41]. The proposed reason for this is that RFD is particularly reliant on a well-functioning CNS[38,42]. In aging and neurodegenerative disorders, it is thus likely that RFD is preferentially prone to changes due to the substantial neurodegeneration observed in these populations. Also, in line with the aforementioned information emphasizing the importance of Fmax, some studies (yet fewer in number than with Fmax) have reported moderate-to-strong associations between lower extremity RFD and physical function (e.g. between knee extension RFD and walking) in aging[39,43] as well as in neurodegenerative disorders such as PD[44], MS[33], and Stroke[45]. Nonetheless, we are unaware of any systematic reviews evaluating whether RFD (compared to Fmax) is preferentially affected and associated with physical function in the outlined neurodegenerative populations. Therefore, the aim of this study was to carry out a systematic review summarizing existing evidence from studies investigating lower extremity RFD alongside physical function in aging and common neurodegenerative disorders (PD, AD, MS and Stroke). Hopefully, such information can help advance our understanding of RFD and its implications for physical function, and to clarify whether RFD can serve as a useful indicator (or biomarker) associated with neurodegeneration.
Design and Methods
Literature search
This review is based on a systematic literature search of Pubmed, Embase, SPORTDiscus, and Web of Science that was performed to identify scientific articles investigating lower extremity RFD alongside physical function in populations preferentially undergoing neurodegeneration, i.e. aging, PD, AD, MS, and Stroke. The literature search included articles published before April 24th 2020. Using the Boolean operators ‘and’ and ‘or’, five search strategies concerning aging, Parkinson Disease, Alzheimer’s Disease, Multiple Sclerosis, and Stroke were performed combined with the following search terms: ‘rate of force development’, ‘rate of torque development’, ‘rate of strength development’, ‘strength development rate’ and ‘explosive muscle strength’. In addition to a free text search, each neurodegenerative condition was also identified using Mesh-terms or Emtree-terms in Pubmed and Embase, respectively. Filters were applied on Pubmed and Embase to encompass study populations only comprised of humans. Regarding aging, the filter was limited to ages 65+ years. For the exact search strategies and terms used in the various databases, please see Supplementary Table 1. Finally, reference lists of the included studies were checked for relevant articles. This systematic review is composed in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA)[46]. Furthermore, Cochrane’s Covidence served as a tool for screening and identification of duplicates.
Supplementary Table 1.
Search Strategies. Table showing the exact search strategies in different databases.
| Database | Number | Search terms |
|---|---|---|
| Pubmed | ||
| Aging | 465 | (((((((aging[MeSH Terms]) OR “aging”) OR “ageing”) OR “aged”) OR old*) OR elder*)) AND (((((“rate of force development”) OR “rate of torque development”) OR “rate of strength development”) OR “strength development rate”) OR “explosive muscle strength”) Filters: Humans; Aged: 65+ years |
| Parkinson Disease | 17 | (((parkinson disease[MeSH Terms]) OR parkinson*)) AND (((((“rate of force development”) OR “rate of torque development”) OR “rate of strength development”) OR “strength development rate”) OR “explosive muscle strength”) Filters: Humans |
| Multiple Sclerosis | 7 | (((multiple sclerosis[MeSH Terms]) OR “multiple sclerosis”)) AND (((((“rate of force development”) OR “rate of torque development”) OR “rate of strength development”) OR “strength development rate”) OR “explosive muscle strength”) Filters: Humans |
| Stroke | 63 | (((stroke[MeSH Terms]) OR stroke*)) AND (((((“rate of force development”) OR “rate of torque development”) OR “rate of strength development”) OR “strength development rate”) OR “explosive muscle strength”) Filters: Humans |
| Alzheimer’s Disease | 9 | ((((Dementia[MeSH Terms]) OR “dementia”) OR alzheimer*)) AND (((((“rate of force development”) OR “rate of torque development”) OR “rate of strength development”) OR “strength development rate”) OR “explosive muscle strength”) Filters: Humans |
| Embase | ||
| Aging | 229 | (‘aging’/exp OR ‘aging’ OR ‘ageing’ OR ‘aged’ OR old* OR elder*) AND (‘rate of force development’ OR ‘rate of torque development’ OR ‘rate of strength development’ OR ‘strength development rate’ OR ‘explosive muscle strength’) AND [aged]/lim AND [humans]/lim |
| Parkinson Disease | 16 | (‘parkinson disease’/exp OR parkinson*) AND (‘rate of force development’ OR ‘rate of torque development’ OR ‘rate of strength development’ OR ‘strength development rate’ OR ‘explosive muscle strength’) AND [humans]/lim |
| Multiple Sclerosis | 7 | (‘multiple sclerosis’/exp OR ‘multiple sclerosis’) AND (‘rate of force development’ OR ‘rate of torque development’ OR ‘rate of strength development’ OR ‘strength development rate’ OR ‘explosive muscle strength’) AND [humans]/lim |
| Stroke | 25 | (‘cerebrovascular accident’/exp OR stroke*) AND (‘rate of force development’ OR ‘rate of torque development’ OR ‘rate of strength development’ OR ‘strength development rate’ OR ‘explosive muscle strength’) AND [humans]/lim |
| Alzheimer’s Disease | 2 | (‘dementia’/exp OR ‘dementia’ OR alzheimer*) AND (‘rate of force development’ OR ‘rate of torque development’ OR ‘rate of strength development’ OR ‘strength development rate’ OR ‘explosive muscle strength’) AND [humans]/lim |
| SPORTDiscus | ||
| Aging | 157 | ( “aging” OR “ageing” OR “aged” OR old* OR elder* ) AND ( “rate of force development” OR “rate of torque development” OR “rate of strength development” OR “strength development rate” OR “explosive muscle strength” ) |
| Parkinson Disease | 6 | parkinson* AND ( ”rate of force development” OR ”rate of torque development” OR ”rate of strength development” OR ”strength development rate” OR ”explosive muscle strength” ) |
| Multiple Sclerosis | 1 | ”multiple sclerosis” AND ( ”rate of force development” OR ”rate of torque development” OR ”rate of strength development” OR ”strength development rate” OR ”explosive muscle strength” ) |
| Stroke | 25 | stroke* AND ( “rate of force development” OR “rate of torque development” OR “rate of strength development” OR “strength development rate” OR “explosive muscle strength” ) |
| Alzheimer’s Disease | 0 | ( “dementia” OR alzheimer* ) AND ( “rate of force development” OR “rate of torque development” OR “rate of strength development” OR “strength development rate” OR “explosive muscle strength” ) |
| Web of Science | ||
| Aging | 304 | TOPIC: (“aging” OR “ageing” OR “aged” OR old* OR elder*) AND TOPIC: (“rate of force development” OR “rate of torque development” OR “rate of strength development” OR “strength development rate” OR “explosive muscle strength”) |
| Parkinson Disease | 19 | TOPIC: (parkinson*) AND TOPIC: (“rate of force development” OR “rate of torque development” OR “rate of strength development” OR “strength development rate” OR “explosive muscle strength”) |
| Multiple Sclerosis | 8 | TOPIC: (“multiple sclerosis”) AND TOPIC: (“rate of force development” OR “rate of torque development” OR “rate of strength development” OR “strength development rate” OR “explosive muscle strength”) |
| Stroke | 38 | TOPIC: (stroke*) AND TOPIC: (“rate of force development” OR “rate of torque development” OR “rate of strength development” OR “strength development rate” OR “explosive muscle strength”) |
| Alzheimer’s Disease | 1 | TOPIC: (“dementia” OR alzheimer*) AND TOPIC: (“rate of force development” OR “rate of torque development” OR “rate of strength development” OR “strength development rate” OR “explosive muscle strength”) |
Inclusion and exclusion criteria
Only studies including older individuals (mean age ≥65 years) or subjects with definite PD, AD, MS or Stroke were included in this review, with additional inclusion criteria being 1) available in English, Danish or German; 2) human studies; and 3) assessment of lower extremity RFD and physical function (see definitions below). The exclusion criteria were 1) reviews; 2) case reports including ≤4 participants; 3) abstracts only; and 4) interventions not reporting baseline data. One investigator (SDL) performed the initial assessment of eligibility identifying potentially relevant studies to include, while another investigator (LGH) performed assessment of eligibility of those potentially relevant studies. Consensus was subsequently reached between the two investigators.
Definition of outcomes and coding of studies
The terms Fmax (defined as the ability of a muscle or muscle group to generate maximal force or torque during a voluntary contraction) and RFD (defined as the ability to increase force or torque as rapidly as possible during a voluntary contraction) were both used throughout this review, derived from a force-time (or torque-time) curve. As RFD can be derived and expressed in many different ways (e.g. as slopes from the onset of contraction to specific time points or as specific regions of the rising phase of a muscle contraction)[38], all measures were included. Furthermore, we defined early phase RFD (comprising RFD derived from the onset of contraction to time points ≤100 ms) and late phase RFD (comprising RFD derived from the onset of contraction to time points 150-300 ms). While both early and late phase RFD rely on a well-functioning CNS, this has been argued to be especially pronounced in early phase RFD[38,42], providing a rationale for examining this in-depth in the outlined neurodegenerative populations.
The following classification of lower extremity physical function was used: Short walk tests, comprising walking tests ≤10 m; Long walk tests, comprising 400 m walk test, 6 minute walk test (6MWT), 2 minute walk test (2MWT); Chair rise tests, comprising sit-to-stand tests (STS), chair rise/stand; Time-up-and-go tests (TUG), comprising traditional TUG as well as Expanded Timed Get-up-and-go (ETGUG), 8-Feet Up-and-Go; Short Physical Performance Battery (SPPB); Stair climb tests; and Balance tests, comprising static/dynamic sway/posturagraphy analysis (also including perturbations), balance score, stability index, Flamingo Balance Test, Fullerton Advanced Balance scale etc. History of falls were also used, representing a dichotomous classification of individuals having inferior vs. superior lower extremity physical function, respectively.
Data extraction and analysis
Characteristics of the participants (number of participants, age, gender, time since diagnosis (patients only), disease stage (patients only)) along with mean or median of the study outcomes (i.e. measures of lower extremity Fmax, RFD, Function) were extracted. Data was included according to two types of analyses: Part 1) parallel observations of baseline deficits in lower extremity Fmax, RFD, and physical function (along with associations between these deficits, subsequently calculated by the present study investigators) and Part 2) reported associations between lower extremity RFD and physical function (denoted RFD-Function) as well as Fmax and physical function (denoted Fmax-Function). Of note, we allowed that Part 1 could contain deficit data from studies comparing individuals having inferior vs. superior physical function, such as patients vs. healthy controls, older vs. young adults, and older fallers vs. non-fallers etc. Regarding Part 2, associations were considered weak if r2<0.16, moderate if 0.16≤r2<0.49, strong if 0.49≤r2<0.81, and very strong if r2≥0.81[47].
Recollecting that RFD can be derived and expressed in several different ways, all reported measures were included yet summarized to represent one lower extremity RFD value from each study. Also, if studies reported data on more than one muscle group or action (such as flexion and extension), data on Fmax and RFD was summarized to represent one value, respectively, from each study. The same approach was done for early and late phase RFD, respectively. Table 1 and Supplementary Table 2 contain detailed characteristics of the identified studies including all extracted outcomes of Fmax, RFD, and physical function (including specific muscle actions as well as the approach used to derive/calculate RFD). WebPlotDigitizer (https://apps.automeris.io/wpd/) was used to extract numerical data in studies where only graphical plots were published.
Table 1.
Deficits (Part 1).
| Study | Deficit (%) | Muscle actions | Assessment details | |||||
|---|---|---|---|---|---|---|---|---|
| Fmax | RFD | Function | ||||||
| Older non-fallers vs. fallers | Bento et al. 2010# | Non-fallers Fallers |
n=13 n=18 |
-17.6 | -17.2 | Dorsi+Plantar flex, Hip ext+flex+abd+add, Knee ext+flex |
RFD20-80% | |
| Crozara et al. 2013 | Non-fallers Fallers |
n=22 n=21 |
-11.1 | -10.3 | Dorsi+Plantar flex, Knee ext+flex | RFD50ms | ||
| Kamo et al. 2019 | Non-fallers Fallers |
n=34 n=88 |
-2.4 | -13.8 | Knee ext | RFD200ms | ||
| LaRoche et al. 2010 | Non-fallers Fallers | n=12 n=11 |
-18.5 | -12.8 | Dorsi+Plantar flex, Knee ext+flex |
RFD200ms | ||
| Morcelli et al. 2016a,b | Non-fallers Fallers |
n=24 n=20 |
-10.7 | -24.1 | Hip ext+flex+abd+add | RFD50ms, RFD100ms, RFD150ms, RFD200ms | ||
| Palmer et al. 2015# | Non-fallers Fallers |
n=9 n=6 |
-35.3 | -37.3 | Hip ext | RFD50ms, RFD100-200ms | ||
| Pijnappels et al. 2008 | Non-fallers Fallers |
n=10 n=7 |
-26.1 | -39.3 | Knee ext, Leg press, Plantar flex | RFD100ms | ||
| Weighted mean 95% CI [lower: upper] | Participants Studies | n=295 N=7 | -10.8 [-12.2 : -9.4] | -17.8 [-25.5 : -10.0] | ||||
| Older lower vs. higher functioning | Clark et al. 2013# | Fast walkers Slow walkers |
n=12 n=8 |
-40.7 | -14.7 | Plantar flex | RFDmax
Long walkmax, Short walkusual+max, SPPB |
|
| LaRoche et al. 2011 | Normal strength Low strength |
n=11 n= 13 |
-23.7 | -28.0 | -11.0 | Dorsi+Plantar flex Knee ext+flex |
RFD200ms
Chair rise, Short walkusual, SPPB |
|
| Palmer et al. 2016# | Higher function Lower function |
n=9 n=6 |
-19.2 | -36.1 | Knee ext+flex | RFD50ms, RFD200ms | ||
| Weighted mean 95% CI [lower: upper] | Participants Studies | n=59 N=3 | -22.0 [-31.9 : -12.0] | -34.4 [-40.8 : -27.9] | -12.7 [-13.7 : -11.7] | |||
| Older vs. younger adults | Crozara et al. 2013 | Young Old |
n=18 n= 43 |
-39.0 | -59.3 | Dorsi+Plantar flex Knee ext+flex |
RFD50ms | |
| Inacio et al. 2019# | Young Old |
n=15 n=15 | -34.4 | -49.8 | Hip abd+add | RFDpeak | ||
| Izquierdo et al. 1999 | Young Old |
n=12 n=10 | -46.6 | -64.9 | Squat | RFDpeak | ||
| Mackey et al. 2006 | Young Old |
n=25 n=25 | -4.3 | -15.6 | Plantar flex | RFD0-85% | ||
| Morcelli et al. 2016a,b | Young Old |
n=18 n=44 | -46.5 | -62.6 | Hip ext+flex+abd+add | RFD50ms, RFD100ms, RFD150ms, RFD200ms | ||
| Palmer et al. 2017# | Young Old |
n=11 n=11 | -17.6 | -24.3 | Hip ext | RFD50ms, RFD100ms, RFD150ms, RFD200ms | ||
| Sundstrup et al. 2010 | Young Old | n=49 n=18 | -21.7 | -21.8 | Knee ext | RFD100ms, RFD200ms | ||
| Unhjem et al. 2019# | Young Old |
n=9 n=32 | -4.8 | -31.2 | -23.4 | Leg press (dyn) | RFD30ms, RFD50ms, RFD100ms, RFD150ms, RFD200ms
Chair rise, Short walkusual, Stair climb |
|
|
Weighted mean 95% CI [lower: upper] |
Participants Studies | n=355 N=8 | -27.0 [-41.6 : -12.3] | -40.8 [-59.0 : -22.5] | -23.4 | |||
| PD patients vs. healthy controls | Malling et al. 2016 | Healthy controls PD patients |
n=17 n=13 | -25.4 | -33.0 | STS (up+down) | RFD0-70%
Chair rise |
|
| Noorvee et al. 2006# | Healthy controls PD patients | n=12 n=12 | -6.1 | -26.2 | -13.2 | Knee ext | RFD200ms
Short walkusual |
|
| Pääsuke et al. 2002# | Healthy controls PD patients | n=12 n=14 | -7.3 | -35.5 | -49.3 | Knee ext (unilat.), STS (up) | RFDmax
Chair rise |
|
| Pääsuke et al. 2004# | Healthy controls PD patients | n=16 n=12 | -19.5 | -32.6 | -31.7 | Knee ext (bilat.), STS (up) | RFDmax
Chair rise |
|
| Weighted mean 95% CI [lower : upper] | Participants Studies | n=108 N=4 | -11.3 [-19.1 : -3.5] | -30.4 [-36.1 : -24.7] | -30.7 [-47.3 : -14.1] | |||
| All | Weighted mean 95% CI [lower: upper] | Participants Studies | n=817 N=22 | -20.5 [-29.4 : -11.7] | -30.6 [-40.8 : -20.4] | -25.9 [-36.1 : -15.6] | ||
STS: sit to stand. SPPB: short physical performance battery. #: Fmax and RFD outcomes that were initially reported as absolute values, but subsequently normalised by body mass reported by the study. In studies reporting data from more than one muscle action, mean deficit values of Fmax and RFD (based on a mean RFD value in studies reporting data on more than one RFD measure), respectively, were calculated and presented. In studies reporting data on more than one measure of physical function, a mean deficit value of Function was calculated and presented.
Supplementary Table 2.
Description of all identified studies.
| Study | Study type | Groups (n) (Women:Men) Age | Times since diagnosis/ Disease stage | MVC | RFD | Functional Outcome(s) |
|---|---|---|---|---|---|---|
| Cross-sectional studies | ||||||
| Aging | ||||||
|
Altubasi et al. 2015
Elderly |
Cross-sectional | Elderly (n=21) (13:8) 71.3±4.6 |
NA | KE: 163.6±57.7 Nm | RFDmaxKE: 108.7±64.3 Nm/s |
Stairclimbing: 4.7±0.9 s Ramp up walk: 2.2±0.3 s TUG: 7.1±1.1 s 4 m walking time: 3.1±0.6 s |
| Data presented as mean±SD | Equipment: Biodex Dynamometer | |||||
|
Bento et al. 2010
Elderly with no fall history vs. one fall vs. ≥2 falls |
Cross-sectional |
≥2 falls
(n=10) (10:0) 67.8±8.8 |
NA | HAd: 90.96±52.66 Nm HAb: 104.25±59.58 Nm HF: 70.21±44.68 Nm HE: 129.55±66.19 Nm KF: 28.11±10.32 Nm KE: 70.27±29.1 Nm DF: 20.59±19.59 Nm PF: 23.31±13.22 Nm |
RFD(20-80%MVC): HAd: 0.85±0.63 Nm/s HAb: 0.72±0.58 Nm/s HF: 0.76±0.55 Nm/s HE: 1.49±1.09 Nm/s KF: 0.23±0.13 Nm/s KE: 0.50±0.33 Nm/s DF: 0.09±0.09 Nm/s PF: 0.21±0.17 Nm/s |
≥2 falls within the last 12 mo |
|
1 fall
(n=8) (8:0) 66.0±4.9 |
NA | HAd: 74.47±44.68 Nm HAb: 87.23±28.19 Nm HF: 61.70±8.51 Nm HE: 112.23±51.6 Nm KF: 27.53±10.61 Nm KE: 65.68±20.36 Nm DF: 15.51±6.35 Nm PF: 21.95±7.71 Nm |
RFD(20-80%MVC): HAd: 0.80±0.59 Nm/s HAb: 0.76±0.28 Nm/s HF: 0.72±0.19 Nm/s HE: 1.21±0.74 Nm/s KF: 0.25±0.09 Nm/s KE: 0.59±0.19 Nm/s DF: 0.14±0.03 Nm/s PF: 0.24±0.09 Nm/s |
1 fall within the last 12 mo | ||
|
Non-fallers
(n=13) (13:0) 67.6±7.5 |
NA | HAd: 110.11±45.21 Nm HAb: 106.38±48.94 Nm HF: 70.21±36.7 Nm HE: 157.45±111.7 Nm KF: 44.46±27.53 Nm KE: 76.58±43.31 Nm DF: 18.98±13.31 Nm PF: 27.03±16.53 Nm |
RFD(20-80%MVC): HAd: 1.02±0.63 Nm/s HAb: 0.89±0.5 Nm/s HF: 0.74±0.57 Nm/s HE: 1.55±1.0 Nm/s KF: 0.44±0.29 Nm/s* KE: 0.71±0.47 Nm/s DF: 0.13±0.11 Nm/s PF: 0.24±0.20 Nm/s |
No fall history | ||
| Data presented as mean±SD Strength data extracted from figure | *Larger than the two other groups of fallers | Equipment: Load cell | ||||
|
Clark et al. 2013
Elderly faster vs. slower walkers |
Cross-sectional |
Faster
(n=12) (6:6) 70.8±4.5 |
NA | NR | VGRFDPF, dyn: 3218±442 N/s* | Δusual-max speed > 0.6 m/s 10 m usual speed: 1.37±0.15 m/s 10 m max speed: 2.17±0.20 m/s* 400 m usual speed: 1.31±0.15 m/s* SPPB: 11.8±0.6* BBT: 54.7±1.3 |
|
Slower
(n=8) (4:4) 71.4±5.0 |
NA | NR | VGRFDPF, dyn: 2010±1112 N/s | Δusual-max speed: > 0.6 m/s 10 m usual speed: 1.24±0.15 m/s 10 m max speed: 1.76±0.16 m/s 400 m usual speed: 1.1±0.11 m/s SPPB: 10.1±1.2 BBT: 55.1±1.1 |
||
| Data presented as mean±SD | *Different from Slower | Equipment: Bertec force plate | ||||
|
Crockett et al. 2013
Elderly |
Cross-sectional |
Elderly
(n=29) (17:12) 67.3±5.5 |
NA | KE Con90°/s: 144.7±42.9 Nm Con180°/s: 70.6±21.5 Nm Ecc: 183.1±54.9 Nm |
RFDmaxKE: Con90°/s: 393.1±159.7 Nm/s Con180°/s: 396.7±135.2 Nm/s Ecc: 287.0±92.2 Nm/s |
30s STS: 14.0±3.6 |
| Data presented as mean±SD | Equipment: Humac Norm dynamometer | |||||
| Crozara et al. 2013 Elderly fallers and non-fallers vs. healthy young females |
Cross-sectional | Elderly fallers (n=21) (21:0) 69.62±7.16 |
NA | KE: 1.32±0.27 Nm/kg KF: 0.54±0.07 Nm/kg PF: 0.74±0.29 Nm/kg DF: 0.34±0.08 Nm/kg |
RFD50KE: 2.51± 1.23 Nm/s/kg KF: 0.94±0.47 Nm/s/kg PF: 1.18±0.65 Nm/s/kg DF: 0.83±1.20 Nm/s/kg |
Falls within the last year |
| Elderly non-fallers (n=22) (22:0) 66.14±6.1 |
NA | KE: 1.52±0.28 Nm/kg* KF: 0.64±0.18 Nm/kg* PF: 0.76±0.21 Nm/kg DF: 0.39±0.08 Nm/kg |
RFD50KE: 2.87±1.16 Nm/s/kg KF: 1.16±0.58 Nm/s/kg PF: 1.29±0.5 Nm/s/kg DF: 0.84±0.41 Nm/s/kg |
No falls within the last year | ||
| Young (n=18) (18:0) 21.79±2.12 |
NA | KE: 2.61±0.45 Nm/kg*§
KF: 1.26±0.26 Nm/kg*§ PF: 1.36±0.29 Nm/kg*§ DF: 0.48±0.11 Nm/kg*§ |
RFD50KE: 7.23±3.15 Nm/s/kg*§
KF: 3.63±1.38 Nm/s/kg*§ PF: 2.90±1.55 Nm/s/kg*§ DF: 1.55±0.44 Nm/s/kg*§ |
__ | ||
| Data presented as mean±SD Strength data extracted from figure |
*Different from fallers. §Different from non-fallers |
Equipment: Biodex dynamometer | ||||
| Hester et al. 2020 >Elderly |
Cross-sectional | Elderly (n=26) (19:7) 73.73±4.9 |
NA | HG: 29.04±10.6 kg | VGRFDPF,dyn: NR | 5-Chair rise: NR 30 s Chair rise: NR Preferred walking speed: NR Maximal walking speed: NR TUG |
| Data presented as mean±SD | Equipment: Force plate | |||||
| Inacio et al. 2019 Young vs. old |
Cross-sectional | Elderly (n=15) (6:9) 71.3±0.9 |
NA | HAb: 0.039±0.002 Nm/m×kg* HAd: 0.061±0.001 Nm/m×kg* VGRFLateral stepping: 50% BW:0.105±0.004 N/m×kg* 65% BW:0.116±0.003 N/m×kg* 80% BW: 0.139±0.005 N/m×kg |
RFDpeakHAb: 73.3±6.3 Nm/s* HAd: 145.3±12.5 Nm/s VGRFDmaxLateral stepping: 50% BW: 1777.3±101.4 N/s* 65% BW: 1598.3±106.5 N/s* 80% BW: 1245.7±86.8 N/s* |
Lateral balance perturbations: Incidens of lateral stepping: 50% BW: 73.3±8.0% 65% BW: 65.3±7.5% 80% BW: 43.4±8.6% |
| Young (n=15) (8:7) 29.1±1.1 |
NA | HAb: 0.063±0.001 Nm/m×kg HAd: 0.088±0.002 Nm/m×kg VGRFLateral stepping: 50% BW: 0.06±0.002 N/m×kg 65% BW: 0.09±0.002 N/m×kg 80% BW: 0.14±0.04 N/m×kg |
RFDpeakHAb: 213.7±20.5 Nm/s HAd: 179.5±16.8 Nm/s VGRFDmaxLateral stepping: 50% BW: 642.1±38.6 N/s 65% BW: 619.5±32.6 N/s 80% BW: 732.6±37.1 N/s |
Lateral balance perturbations: Incidens of lateral stepping: 50% BW: 92.5±3.2%* 65% BW: 94.0±2.9%* 80% BW: 85.4±4.0%* |
||
| Data presented as mean±SE | *Different from Young | Equipment: Biodex dynamometer & AMTI force platform | ||||
| Izquierdo et al. 1999 Young vs. middle-aged vs. old | Cross-sectional |
Young (~20 y) (n=12) (0:12) 21±1 |
NA | Squat, static: 1381±81 N | VGRFDpeaksquat, static: 8474.03±616.8 N/s |
Balance: Time of transition: 1.72±0.27 s Time inside center: 81.92±2.39 s Straightness of trajectory: 75±2% Distance center of pressure: 5814±387 mm* Balance area: 4926±215 mm2* |
|
Middle-aged (~40 y) (n=10) (0:10) 40±2 |
NA | Squat, static: 1039±92 N§ | VGRFDpeaksquat, statis: 8246.75±1071.4 N/s |
Balance: Time of transition: 2.01±0.6 s Time inside center: 75.38±2.61 s Straightness of trajectory: 74±3% Distance center of pressure: 10707±2372 mm Balance area: 5546±857 mm2 |
||
|
Elderly (~70 y) (n=10) (0:10) 71±5 |
NA | Squat, static: 747±63 N*§ | VGRFDpeaksquat, statis: 3019.80±389.6 N/s*§ |
Balance: Time of transition: 2.51±0.71 s*§ Time inside center: 55.77±6.54 s*§ Straightness of trajectory: 76±5% Distance center of pressure: 9463±2079 mm Balance area: 6305±627 mm2 |
||
| Characteristics of subjects presented as mean±SD Strength and functional data presented as mean±SE RFD data extracted from figure |
*Different from Middle-aged
§Different from Young |
Equipment: Dinascan force platform | ||||
|
Kamo et al. 2019
Elderly with no fall history vs. one fall vs. ≥2 falls |
Cross-sectional |
≥ 2 falls
(n=10) (6:4) 71.4±2.9 |
NA | KE: 2.0±0.3 Nm/kg | RFD200KE: 3.5±2.0 Nm/s/kg | ≥ 2 falls within the last year 30 s Chair stand: 19.6±7.6 Usual gait speed: 1.37±0.36 m/s One leg standing test: 80.1±51.6 s |
|
1 fall
(n=24) (11:13) 71.2±3.7 |
NA | KE: 2.1±0.7 Nm/kg | RFD200KE: 6.5±3.6 Nm/s/kg* | 1 fall within the last year 30 s chair stand: 22.6±6.6 Usual gait speed:1.31±0.18 m/s One leg standing test: 93.0±41.3 s |
||
|
Non-fallers
(n=88) (43:45) 71.3±4.7 |
NA | KE: 2.1±0.6 Nm/kg | RFD200KE: 5.8±2.7 Nm/s/kg* | No history of falls 30 s chair stand: 22.1±7.5 Usual gait speed:1.37±0.19 m/s One leg standing test: 82.4±41.8 s |
||
| Data presented as mean±SD | *Different from multiple falls groups | Equipment: Mobie hand-held dynamometer | ||||
| LaRoche et al. 2010 Elderly fallers vs. non-fallers | Cross-sectional |
Fallers
(n=11) (11:0) 71.3±5.4 |
NA | KE: 1.49±0.46 Nm/kg KF: 0.59±0.73 Nm/kg DF: 0.32±0.06 Nm/kg PF: 0.76±0.14 Nm/kg *(Combined measure) |
RFD200KE: 6.97±2.9 Nm/s/kg KF: 4.02±2.17 Nm/s/kg DF: 1.57±0.36 Nm/s/kg PF: 3.18±1.14 Nm/s/kg |
≥3 falls within the last year |
|
Non-fallers
(n=12) (12:0) 71.2±6.2 |
NA | KE: 1.72±0.56 Nm/kg KF: 0.72±0.25 Nm/kg DF: 0.38±0.09 Nm/kg PF: 1.04±0.27 Nm/kg |
RFD200KE: 6.90±3.86 Nm/s/kg KF: 4.50±2.67 Nm/s/kg DF: 1.93±0.55 Nm/s/kg PF: 4.12±1.89 Nm/s/kg |
No history of unexplained falls | ||
| Data presented as mean±SD | *Difference in composite Z-score between groups | Equipment: Humac Norm dynamometer | ||||
|
LaRoche et al. 2011
Low vs. normal strength in elderly |
Cross-sectional |
Low strength
(n=13) (13:0) 71.2±4.3 |
NA | KE: 1.16±0.16 Nm/kg* KF: 0.69±0.18 Nm/kg PF: 0.55±0.25 Nm/kg* DF: 0.34±0.06 Nm/kg |
RFD200KE: 6.25±2.62 Nm/kg/s* KF: 3.32±1.44 Nm/kg/s* PF: 2.84±1.88 Nm/kg/s DF: 1.43±0.59 Nm/kg/s |
KE torque <1.5 Nm/kg SPPB: 10.7±1.3 Balance score: 3.8±0.6 Habitual gait speed: 1.12±0.19 m/s* Chair rise: 11.5±1.15 s |
|
Normal strength
(n=11) (11:0) 72.1±5.2 |
NA | KE: 1.65±0.13 Nm/kg KF: 0.83±0.14 Nm/kg PF: 0.85±0.24 Nm/kg DF: 0.39±0.11 Nm/kg |
RFD200KE: 9.51±3.29 Nm/kg/s KF: 4.77±1.19 Nm/kg/s PF: 3.64±1.41 Nm/kg/s DF: 1.92±0.59 Nm/kg/s |
KE torque >1.5 Nm/kg SPPB: 11.4±0.7 Balance score: 3.9±0.3 Habitual gait speed: 1.32±0.25 m/s Chair rise: 10.3±1.4 s |
||
| Data presented as mean±SD | *Different from Normal strength | Equipment: Humac Norm dynamometer | ||||
|
Lopez et al. 2017
Elderly |
Cross-sectional |
Elderly sedentary men
(n=50) (0:50) 66±5.4 |
NA | NR | RFD50KE: 1.24±0.62 Nm/s RFD100KE: 1.01±0.40 Nm/s | 30s STS: 16.7±2.6 |
| Data presented as mean±SD | Equipment: Cybex Norm dynamometer | |||||
|
Mackey et al. 2006
Elderly vs. young |
Cross-sectional |
Elderly
(n=25) (25:0) 78±7 |
NA | VGRFPF, static: 0.89±0.12 Nm/kg×m PF, dynamic: 0.96±0.16 Nm/kg×m* |
VGRFD(0-85%MVC)PF, dynamic: 5.57±1.98 Nm/s×kg×m | Balance recovery trials: Static recovery angle: 13.1±2.4°* Dynamic recovery angle: 4.6±1.8°* |
|
Young
(n=25) (25:0) 25±4 |
NA | VGRFPF, static: 0.93±0.06 Nm/kg×m PF, dynamic: 1.04±0.15 Nm/kg×m NB! Strength data is measured during balance recovery trials. |
VGRFD(0-85%MVC)PF Dynamic: 6.60±2.49 Nm/s×kg×m | Balance recovery trials: Static recovery angle: 16.3±1.5° Dynamic recovery angle: 7.2±1.2° |
||
| Data presented as mean±SD | *Different from Young | Equipment: Bertec force plate | ||||
|
Morcelli et al. 2016a+b
Elderly fallers vs. non-fallers vs. healthy young females |
Cross-sectional |
Fallers
(n=20) (20:0) 68.9±6.5 |
NA | HE: 1.23±0.46 Nm/kg HF: 0.69±0.17 Nm/kg HAb: 0.72±0.22 Nm/kg HAd: 0.52±0.19 Nm/kg |
RFD50HE: 1.68±0.82 Nm/s/kg HF: 1.71±0.90 Nm/s/kg HAb: 1.52±0.75 Nm/s/kg HAd: 0.90±0.59 Nm/s/kg RFD100HE: 1.91±0.95 Nm/s/kg HF: 1.75±0.88 Nm/s/kg HAb: 1.74±0.87 Nm/s/kg HAd: 0.99±0.62 Nm/s/kg RFD150HE: 1.89±0.92 Nm/s/kg HF: 1.43±0.72 Nm/s/kg HAb: 1.64±0.80 Nm/s/kg HAd: 0.86±0.55 Nm/s/kg RFD200HE: 1.23±0.46 Nm/s/kg HF: 1.06±0.44 Nm/s/kg HAb: 1.27±0.67 Nm/s/kg HAd: 0.68±0.36 Nm/s/kg |
Fall(s) in the year before evaluation |
|
Non-fallers
(n=24) (24:0) 65.5±6.16 |
NA | HE: 1.41±0.35 Nm/kg HF: 0.76±0.1 Nm/kg HAb: 0.82±0.15 Nm/kg HAd: 0.57±0.14 Nm/kg |
RFD50HE: 2.36±0.96 Nm/s/kg HF: 1.80±0.56 Nm/s/kg HAb: 1.85±0.70 Nm/s/kg HAd: 1.22±0.61 Nm/s/kg RFD100HE: 2.58±1.01Nm/s/kg HF: 1.97±0.61 Nm/s/kg HAb: 2.32±0.80 Nm/s/kg HAd: 1.37±0.67 Nm/s/kg RFD150HE: 2.38±0.87 Nm/s/kg HF: 1.77±0.52 Nm/s/kg HAb: 2.37±0.74 Nm/s/kg§ HAd: 1.22±0.53 Nm/s/kg RFD200HE: 1.95±0.63 Nm/s/kg HF: 1.36±0.41 Nm/s/kg HAb: 1.94±0.66 Nm/s/kg§ HAd: 0.89±0.35 Nm/s/kg |
No falls in the year before evaluation | ||
|
Young
(n=18) (18:0) 21.8±2.1 |
NA | HE: 2.61±0.58 Nm/kg*§
HF: 1.24±0.15 Nm/kg*§ HAb: 1.37±0.26 Nm/kg*§ HAd: 1.14±0.32 Nm/kg*§ |
RFD50HE: 6.53±2.13 Nm/s/kg*§
HF: 4.70±1.53 Nm/s/kg*§ HAb: 5.64±1.82 Nm/s/kg*§ HAd: 3.99±1.78 Nm/s/kg*§ RFD100 HE: 7.16±2.38 Nm/s/kg*§ HF: 4.93±1.36 Nm/s/kg*§ HAb: 6.32±1.80 Nm/s/kg*§ HAd: 4.33±1.78 Nm/s/kg*§ RFD150HE: 6.33±2.07 Nm/s/kg*§ HF: 3.83±0.85 Nm/s/kg*§ HAb: 5.07±1.27 Nm/s/kg*§ HAd: 3.55±1.29 Nm/s/kg*§ RFD200HE: 4.75±1.53 Nm/s/kg*§ HF: 2.25±0.87 Nm/s/kg*§ HAb: 2.30±1.20 Nm/s/kg§ HAd: 2.01±0.77 Nm/s/kg*§ |
__ | ||
| Data presented as mean±SD | *Difference compared to Elderly non-fallers
§Difference compared to Elderly fallers |
Equipment: Biodex dynamometer | ||||
|
Palmer et al. 2015
Elderly fallers vs. non-fallers |
Cross-sectional |
Fallers
(n=6) (6:0) 72.67±6.89 |
NA | HE: 7.96±3.04 Nm | RFD50HE: 37.43±23.95 Nm/s RFD100-200HE: 28.73±17.70 Nm/s | >1 fall in the last 12 mo |
|
Non-fallers
(n=9) (9:0) 71.44±6.95 |
NA | HE: 11.16±4.59 Nm | RFD50HE: 80.86±48.12 Nm/s* RFD100-200HE: 34.28±18.56 Nm/s | No falls in the last 12 mo | ||
| Data presented as mean±SD | *Difference compared to fallers | Equipment: Load cell | ||||
|
Palmer et al. 2016
Elderly higher vs. lower functioning |
Cross sectional |
Higher functioning
(n=9) (6:3) 87.1±6.0 |
NA | KE: 51.2±26.0 Nm KF: 35.1±13.2 Nm |
RFD50KE: 241.5±111.5 Nm/s KF: 238.4±117.1 Nm/s * (collapsed across muscle) RFD200KE: 121.3±49.1 Nm/s KF: 109.3±47.9 Nm/s |
Able to successfully rise from chair |
|
Lower functioning
(n=6) (3:3) 89.2±6.0 |
NA | KE: 39.3±16.3 Nm KF: 32.4±15.1 Nm |
RFD50KE: 110.9±56.8 Nm/s KF: 129.8±62.1 Nm/s RFD200KE: 89.5±56.9 Nm/s KF: 101.5±68.0 Nm/s |
Unable to successfully rise from chair | ||
| Data presented as mean±SD | *Different compared with Lower functioning when collapsed across muscle | Equipment: Load cell | ||||
|
Palmer et al. 2017
Young vs. old |
Cross-sectional |
Elderly
(n=11) (11:0) 67±8 |
NA | HE: 70.28±31.02 Nm* | RFD50HE: 254.30±105.87 Nm/s* RFD200HE: 180.16±69.0 Nm/s* |
OSI: 0.69±0.19* |
|
Young
(n=11) (11:0) 26±8 |
NA | HE: 94.89±23.95 Nm | RFD50HE: 364.14±121.74 Nm/s RFD200HE: 274.29±82.17 Nm/s | OSI: 0.48±0.16 | ||
| Data presented as mean±SD | *Different from Young females | Equipment: Load cell | ||||
|
Pijnappels et al. 2008
Elderly fallers vs. non-fallers |
Cross-sectional |
Fallers
(n=7) (7:0) 71±4.5 |
NA | KE: 1.44±0.41 Nm/kg* PF: 1.41±0.39 Nm/kg* Leg press: 11.74±1.5 N/kg* |
RFD100KE: 5.02±1.71 Nm/s/kg* PF: 3.81±1.29 Nm/s/kg* Leg press: 31.16±17.52 N/s/kg |
Fully supported by safety harness in more than half of the tripping trials. |
|
Non-fallers
(n=10) (3:7) 71±4.5 |
NA | KE: 2.07±0.45 Nm/kg PF: 1.83±0.36 Nm/kg Leg press: 15.61±2.06 N/kg |
RFD100KE: 8.62±3.12 Nm/s/kg PF: 6.01±2.1 Nm/s/kg Leg press: 51.47±28.53 N/s/kg |
Never fully supported by the safety harness. | ||
| Data presented as mean±SD Strength data extracted from figure |
*Different from Non-fallers | Equipment: Cybex Norm dynamometer | ||||
|
Rech et al. 2014
Elderly |
Cross-sectional |
Active old women
(n=45) (45:0) 70.28±6.2 |
NA | KE: 108.09±28.7 Nm | RFD50KE: 0.47±0.25 Nm/ms RFD100KE: 0.45±0.24 Nm/ms RFD250KE: 0.29±0.11 Nm/ms RFD300KE: 0.25±0.09 Nm/ms |
30s STS: 12.9±2.3 Usual gait speed: 1.3±0.2 m/s |
| Data presented as mean±SD | Equipment: Cybex Norm dynamometer | |||||
| Seynnes et al. 2005 Elderly | Cross-sectional | Elderly (n=19) (19:0) 77.9±1.2 | NA | KE: 108.1±5.1 Nm | RFDmaxKE: 126.0±18.7 Nm/s | Chair-rise: 0.64±0.02 s Stairclimbing power: 224.71±9.78 W 6MWT: 348.6±8.2 m |
| Data presented as mean±SD | Equipment: Biodex dynamometer | |||||
|
Sundstrup et al. 2010
Elderly trained vs. untrained vs. young untrained |
Cross-sectional |
Football-trained elderly
(n=10) (0:10) 69.6±1.4 |
NA | KE: 2.32±0.12 Nm/kg | RFD30KE: 10.27±0.77 Nm/s/kg* RFD100KE: 13.34±0.68 Nm/s/kg* RFD200KE: 9.26±0.54 Nm/s/kg* |
Flamingo balance test: 15.5±1.0* |
|
Untrained elderly
(n=8) (0:8) 70.5±1.0 |
NA | KE: 2.21±0.18 Nm/kg | RFD30KE: 6.88±1.15 Nm/s/kg RFD100KE: 8.08±1.30 Nm/s/kg RFD200KE: 6.68±0.87 Nm/s/kg |
Flamingo balance test: 33.2±1.9 | ||
|
Untrained young
(n=49) (0:49) 32.4±0.9 |
NA | KE: 2.90±0.07 Nm/kg*§ | RFD30KE: 11.32±0.43 Nm/s/kg* RFD100KE: 14.29±0.43 Nm/s/kg* RFD200KE: 10.11±0.32 Nm/s/kg* |
Flamingo balance test: 15.0±0.6* | ||
| Data presented as mean±SE | *Different from Untrained elderly . §Different from Football-trained elderly | Equipment: KinCom dynamometer | ||||
|
Thompson et al. 2018
Young vs. old |
Cross-sectional |
Elderly
(n=18) (10:8) 71.1±5.9 |
NA | Squat: 432.7±47.46 Nm* KE+KF: 150.8±62.98 Nm* | RFD50Squat: 590.2±193.35 Nm/s* KE+KF: 225.8±188.95 Nm/s RFD200Squat: 1073.5±653.4 Nm/s* KE+KF: 529.2±261 Nm/s* |
NR 10 m walk 400 m walk Chair stand |
|
Young
(n=20) (10:10) 21.9±2.6 |
NA | Squat: 635.3±169.78 Nm KE+KF: 209.4±151.52 Nm | RFD50Squat: 1076.6±681.11 Nm/s KE+KF: 275.3±355.94 Nm/s RFD200Squat: 1872.1±674.8 Nm/s KE+KF: 728.9±243.4 Nm/s |
NR 10 m walk 400 m walk Chair stand |
||
| Data presented as mean±SD | *Different from young | Equipment: Biodex dynamometer | ||||
|
Unhjem et al. 2019
Young vs. old |
Cross-sectional |
Sedentary elderly
(n=10) (0:10) 71±4 |
NA | Leg press, dyn: 106±3 kg* | RFD30Leg pres, dyn: 1610 N/s RFD50Leg pres, dyn: 1946 N/s RFD100Leg pres, dyn: 2389 N/s RFD150Leg pres, dyn: 2554 N/s RFD200Leg pres, dyn: 2495 N/s |
Habitual walking speed: 1.26 m/s* Chair rise: 9.7 s* Stairclimbing: 498 W* One leg-standing: Without multitasking: 7.07 cm/s* With multitasking: 9.43 cm/s* |
|
Active elderly
(n=11) (0:11) 73±6 |
NA | Leg press, dyn: 128±4 kg*§ | RFD30Leg pres, dyn: 2035 N/s RFD50Leg pres, dyn: 2690 N/s RFD100Leg pres, dyn: 3433 N/s RFD150Leg pres, dyn: 3478 N/s RFD200Leg pres, dyn: 3097 N/s |
Habitual walking speed: 1.56 m/s§
Chair rise: 8.6 s* Stairclimbing: 554 W* One leg-standing: Without multitasking: 5.37 cm/s* With multitasking: 7.21 cm/s* |
||
|
Old master athletes
(n=11) (0:11) 71±4 |
NA | Leg press, dyn: 186±10 kg*#§ | RFD30Leg pres, dyn: 4389 N/s RFD50Leg pres, dyn: 5451 N/s RFD100Leg pres, dyn: 5999 N/s RFD150Leg pres, dyn: 5636 N/s RFD200Leg pres, dyn: 4961 N/s |
Habitual walking speed: 1.49 m/s§
Chair rise: 6.2 s#§ Stairclimbing: 701 W*#§ One leg-standing: Without multitasking: 6.46 cm/s* With multitasking: 8.12 cm/s* |
||
|
Young
(n=9) (0:9) 22±2 |
NA | Leg press, dyn: 147±7 kg | RFD30Leg pres, dyn: 3292 N/s RFD50Leg pres, dyn: 4053 N/s RFD100Leg pres, dyn: 5008 N/s RFD150Leg pres, dyn: 4961 N/s RFD200Leg pres, dyn: 4491 N/s |
Habitual walking speed: 1.62 m/s Chair rise: 6.5 s Stairclimbing: 879W One leg-standing: Without multitasking: 4.02 cm/S With multitasking: 4.45 cm/s |
||
| Data presented as mean±SD | *Different from young. #Different from active older adults. §Different from sedentary older adults | Equipment: Force plate | ||||
| Parkinson Disease | ||||||
|
Malling et al. 2016
PD patients vs. healthy controls |
Intervention (controlled) 8 w control period followed by motor ‘reactive’ training + aerobic training (8 w, 24 sess.) |
PD patients
(n=13) (0:13) 63 |
Hoehn & Yahr staging: 2.1 | NR | VGRFD(30-70%MVC)STS, up: pre: 10.66±0.93 ×BW/s post4w: 11.33±0.96 ×BW/s post8w: 13.67±2.25×BW/s ∆: 3.01 ×BW/s (28.24%) VGRFD(30-70%MVC)STS, down: pre: 7.94±0.8 ×BW/s post4w: 8.85±0.75 ×BW/s* post8w: 9.7±0.88 ×BW/s* ∆: 1.76 ×BW/s (23%) VGRFD(0-70%MVC)DPB, lat: precontrol period: 2.06±0.3 ×BW/s pre: 2.02±0.37 ×BW/s post8w: 2.36±0.36 ×BW/s* ∆8w: 0.34 ×BW/s (16%) VGRFD(0-70%MVC)DPB, med: precontrol period: 1.85±0.35 ×BW/s pre: 1.71±0.33 ×BW/s post8w: 2.04±0.36 ×BW/s* ∆8w: 0.33 ×BW/s (19%) |
5STS: Completion time: pre: 9.93±1.08 s post4w: 8.14±0.82 s* post8w: 7.51±0.88 s* ∆8w: -2.42 s (-24%) Standing-time: pre: 0.73±0.11 s post4w: 0.58±0.06 s* post8w: 0.54±0.05 s* ∆8w: -0.19 s (-27%) Sitting-time: pre: 0.51±0.06 s post4w: 0.37±0.05 s* post8w: 0.32±0.05 s* ∆8w: -0.19 s (-36%) Dynamic postural balance: Completion time: precontrol period: 24.35±4.03 s pre: 20.94±3.41 s post8w: 19.32±3.26 s ∆8w: -1.62 s (-7.73%) Flatness: precontrol period: 25.23±5.43 N pre: 23.06±4.01 N post8w: 23.14±3.51 N ∆8w: 0.08 s (0.35%) |
|
Healthy controls
(n=17) (0:17) 58 |
NA | NR | VGRFD(30-70%MVC)STS, up: pre: 14.83±0.41×BW/s * VGRFD(30-70%MVC)STS, down: pre: 10.26±0.35×BW/s * VGRFD(0-70%MVC)DPB, lat: precontrol period: 3.78±0.3 ×BW/s§ VGRFD(0-70%MVC)DPB, med: precontrol period: 3.28±0.27×BW/s§ |
5STS: Completion time: pre: 7.33±0.36 s* Standing-time: pre: 0.55±0.02 s* Sitting-time: pre: 0.39±0.03 s Dynamic postural balance: Completion time: precontrol period: 12.32±1.13 s§ Flatness: rowspan="2"precontrol period: 40.60±5.35 N§ |
||
| Data presented as mean±SE RFD and functional data extracted from figure | *Different compared to pre-values after control period for PD patients §Different compared to pre-values before control period for PD patients |
Equipment: AMTI force plate | ||||
|
Noorvee et al. 2006
PD patients vs. healthy controls |
Cross-sectional |
PD patients
(n=12) (5:7) 67.4±1.2 |
Hoehn & Yahr staging: II-III | KE: 292.77±25.3 N | RFD200KE: 832.84±109.97 N/s* | Postural sway test: Eyes open: 7.25±1.76 Eyes closed: 6.68±1.2 6 m walk: 0.99±0.06 m/s* UPDRS motor: NR |
|
Healthy controls
(n=12) (5:7) 66.8±1.1 |
NA | KE: 317.17±23.49 N | RFD200KE: 1129.03±105.57 N/s | Postural sway test: Eyes open: 5.92±0.37 Eyes closed: 5.15±10.66 6 m walk: 1.14±0.03 m/s UPDRS motor: NR |
||
| Data presented as mean±SE Strength data extracted from figure |
*Different from Healthy controls | Equipment: Custom-made dynamometric chair | ||||
|
Pääsuke et al. 2002
PD patients vs. healthy controls |
Cross-sectional |
PD patients
(n=14) (14:0) 72.6±2.2 |
10.3±1.2 y Hoehn & Yahr staging: I-III | KE Absolute Right: 224.32±14.87 N* Left: 208.11±13.51 N* Normalized (relative to BW) Right: 39.22±2.29% Left: 35.15±2.3% VGRFSTS: Right: 371.74±20.83 N Left: 344.98±12.64 N |
RFDpeakKE: Right: 867.83±103.5 N/s* Left: 835.99±79.62 N/s* VGRFDmaxSTS: Right: 874.25±125.75 N/s* Left: 838.32±179.64 N/s* |
Chair rise: 3.42±0.14 s* |
|
Healthy controls
(n=12) (12:0) 72.8±0.8 |
NA | KE Absolute Right: 267.57±18.92 N Left: 263.51±13.52 N Normalized (relative to BW) Right: 42.40±2.65% Left: 41.69±1.94% VGRFSTS: Right: 364.31±29.74 N Left: 358.36±22.31 N |
RFDpeakKE: Right: 1504.78±175.16 N/s Left: 1449.04±167.2 N/s VGRFDmaxSTS: Right: 1305.39±215.57 N/s Left: 1239.52±227.55 N/s |
Chair rise: 2.29±0.14 s | ||
| Data presented as mean±SE Strength data extracted from figure | *Different from Healthy controls | Equipment: Dynamometer & VISTI force plates | ||||
|
Pääsuke et al. 2004
PD patients vs. healthy controls |
Cross-sectional |
PD patients
(n=12) (12:0) 74.3±6.9 |
10.7±4.5 y Hoehn & Yahr staging: I-III | Bilateral KE Absolute: 711.94±290.4 N* Normalized: 11.35±4.96 N/kg* Unilateral KE More affected: 483.87±169.35 N Less affected: 548.38±169.36 N VGRFSTS: 705.1±91.43 N |
RFDpeakKE bilateral: 2625±1521.15 N/s* VGRFDmaxSTS: 2391.13±818.55 N/s* |
Chair rise: 2.45±0.24 s* |
|
Healthy controls
(n=16) (16:0) 71.7±4.4 |
NA | Bilateral KE Absolut: 1147.54±515.22 N Normalized: 17.45±5.55 N/kg Unilateral KE Dominant: 802.42±330.64 N Non-dominant: 733.87±358.9 N VGRFSTS: 735.03±92.3 N |
RFDpeakKE bilateral: 4092.31±1709.61 N/s VGRFDmaxSTS: 3411.29±975.81 N/s | Chair rise: 1.86±0.3 s | ||
| Data presented as mean±SD Strength data extracted from figure |
*Different from Healthy controls | Equipment: Dynamometer & VISTI force plates | ||||
| Multiple Sclerosis | ||||||
|
Kjølhede et al. 2015a
MS patients |
Cross-sectional |
MS patients
(n=25) (26:8) 43.3±8.2 |
EDSS: 2.9 | Stronger leg: KE: 2.30±0.53 Nm/kg KF: 0.97±0.29 Nm/kg Weaker leg: KE: 1.99±0.49 Nm/kg* KF: 0.81±0.29 Nm/kg* |
Stronger leg: RFDmaxKE: 13.43±3.46 Nm/kg/s RFDmaxKF: 8.42±1.35 Nm/kg/s RFD200KE: 7.35±2.49 Nm/kg/s RFD200KF: 2.98±0.99 Nm/kg/s Weaker leg: RFDmaxKE: 12.65±3.32 Nm/kg/s* RFDmaxKF: 8.09±1.38 Nm/kg/s* RFD200KE: 6.56±2.20 Nm/kg/s* RFD200KF: 2.39±0.92 Nm/kg/s* |
T25FWT: 1.72±0.31 m/s 2MWT: 1.64±0.33 m/s 5STS: 9.48±2.70 s Stairclimbing: 10.64 s |
| Data presented as mean±SD | *Different from stronger leg | Equipment: Humac Norm dynamometer | ||||
| Stroke | ||||||
|
Nadeau et al. 1997
Stroke patients |
Cross-sectional |
Hemiparetic stroke patients
(n=16) (4:12) 47.9±15.6 |
43.9±36.5 mo Fugl-Meyer Assessment: 184.3 |
PF: 50.8±24.4 Nm | RFDpeakPF: 110.5±56.7 Nm/s | TUG: 9.20±2.40 s Comfortable walking speed: 0.76±0.27 m/s Maximal safe walking speed: 1.08±0.33 m/s |
| Data presented as mean±SD | Equipment: Biodex dynamometer | |||||
|
Pohl et al. 2002
Stroke patients |
Cross-sectional |
Stroke patients
(n=83) (39:44) 70.3±9.8 |
78.6±27.4 d Fugl-Meyer Assessment: 23.7±3.7 |
Affected knee: KE: 53.55±24.27 Nm Less affected knee: KE: 52.58±25.62 Nm |
Affected knee: RFD150KE: 66.02±69.42 Nm/s Less affected knee: RFD150KE: 81.76±77.01 Nm/s |
10 m walking speed: 63.2±25.9 cm/s |
| Data presented as mean±SD | Equipment: Cybex dynamometer | |||||
|
Takeda et al. 2018
Stroke patients |
Cross-sectional |
Chronic stroke patients
(n=20) (3:17) 63.6±10.1 |
5.7 y Fugl-Meyer Assessment: 17.9±6.5 |
NR | Affected limb: RFD50KE: 6.48±4.62 N/kg/s* RFD100KE: 8.82±5.75 N/kg/s* RFD200KE: 6.93±3.99 N/kg/s* RFD300KE: 6.08±3.39 N/kg/s* Non-affected limb: RFD50KE: 14.96±13.81 N/kg/s RFD100KE: 15.23±9.10 N/kg/s RFD200KE: 13.14±7.74 N/kg/s RFD300KE: 11.70±5.97 N/kg/s |
Walking speed: Maximum: 0.75 m/s Comfortable: 0.58 m/s |
| Data presented as mean±SD | *Different from non-affected limb | Equipment: Handheld dynamometer | ||||
NA: not applicable; NR: not reported; SD: standard deviation; IQR: interquartile range; MS: Multiple Sclerosis; PD: Parkinson Disease; EDSS: Expanded Disability Status Scale; UPDRS: Unified Parkinson’s Disease Rating Scale; RCT: Randomized controlled trial; QF: Quadriceps Femoris; KE: Knee extension; KF: Knee flexion; DF: Ankle dorsiflexion; PF: Plantarflexion; HE: Hip extension; HF: Hip flexion; Ham: Hamstrings; HAd: Hip adductor; HAb: Hip abductor; LE: leg extension; Con: concentric; Ecc: eccentric; Dyn: dynamic; BW: body weight; RFD: rate of moment development; VGRF: Vertical ground reaction force; VGRFD: Vertical ground reaction force development; T25FWT: Timed 25 Foot Walk Test; 2MWT: Two-minute Walk Test; 6MWT: Six-minute Walk Test; 30s STS: Sit-to-stand movement; BBT: Berg Balance Test; BBS: Berg Balance Scale, OSI: overall stability index; TUG: Time up-and-go; SPPB: Short Physical Performance Battery; COP: center of pressure; LCOP: total length of the COP trajectory; ACOP: area of the rectangle circumscribing the COP trajectory; ETGUG: expandend timed get up-and-go; FAB scale: Fullerton Advanced Balance scale; FAC: Functional Ambulation Category; AP: anteroposterior direction; ML: mediolateral direction; CTSIB: Clinical test of Sensory Interaction on Balance Test; DPB: Dynamic postural balance test. Unless else is noted, all strength data was measured during isometric contractions. Italic values indicates that the value was calculated by the investigator. Red text denote excluded outcomes or studies from the main analysis.
In addition to the qualitative analysis (summary of identified studies and their data), we also performed quantitative analysis by calculating sample size weighted averages across selected studies. These data are presented as mean±CI95%. Also, within- and between-outcome analyses were carried out by using linear mixed model, with study set as random effect and outcome (Fmax, RFD, Function) as fixed effect. Simple unadjusted regression analyses were carried out to examine associations between parallel observations of deficits (Part 1). All statistical analyses were carried out using STATA (STATA/IC 14.2, StataCorp, College Station, Texas, USA), while graphical illustrations were created using GraphPad Prism 7.0 (GraphPad Software, La Jolla, California, USA, www.graphpad.com).
Results
General study characteristics
The literature search yielded N=1399 potential articles of which N=32 articles (n=1087 study participants) were ultimately included (after removal of duplicates, screening of title and abstract, full text reading, check of reference lists) (Figure 1). Across the different populations, N=24 articles (n=830 subjects) evaluated aging[48-71], N=4 articles (n=108 subjects) evaluated PD[30,44,72,73], N=1 article (n=34 subjects) evaluated MS[33], and N=3 articles (n=115 subjects) evaluated Stroke[45,74,75]. No articles investigating AD met the inclusion criteria. Except for one study[56], large-scale studies having >100 participants were lacking as sample sizes ranged from 15 to 83 subjects. All studies reported data as mean and standard deviations or standard errors. Fmax was presented as Nm, Nm/kg, Nm/m/kg, N, N/kg, or kg, and RFD values as Nm/s, Nm/s/kg, N/s, N/s/kg, Nm/s/kg/m, N/ms, Nm/ms, or ×BW/s. Regarding the analysis of deficits (Part 1), all strength data was normalised to body weight, except for strength outcomes measured as vertical ground reaction force or vertical ground reaction RFD. Supplementary Table 2 contain detailed information of the identified studies including all extracted outcomes of Fmax, RFD, and physical function.
Figure 1.
Study flowchart.
Large heterogeneity was identified in the assessment of physical function and lower extremity Fmax and RFD (in particular) of the included studies. Specifically, a wide range of muscle actions was used to generate lower extremity Fmax and RFD, but most often involving knee extension/flexion, hip flexion/extension/abduction/adduction, and ankle plantar/dorsi flexion (Supplementary Table 2, Table 1, Table 2). While RFD was derived from isometric Fmax (also termed maximal voluntary isometric contraction, MVC) in most studies, some also derived it from dynamic Fmax during plantar flexion[50], leg press[71], balance trials[59,73], sit-to-stand movements[30,72,73], lateral stepping[54], squat jump , and isokinetic (60°/s), concentric (90°/s and 180°/s), and eccentric contractions[51]. Measures of RFD were quite divergent, but most often presented as RFD50ms, RFD100ms, RFD200ms, and RFDmax (Supplementary Table 2). Also, a wide range of different tests of physical function was applied, with data being summarized according to the classification outlined in Methods (i.e. comprising short and long walking tests, chair rise test tests, stair climbing, TUG, SPPB, retrospective fall history) (Supplementary Table 2). As for the different balance tests, these were deemed too divergent that it left us unable to summarize data. Consequently, balance data was excluded from further data analysis (for transparency, specific information on these tests along with data are reported in Supplementary Table 2).
Table 2.
Associations (Part 2).
| Study | Associations (r2-values) | Muscle actions | Assessment details | ||||
|---|---|---|---|---|---|---|---|
| Fmax-Function | RFD-Function | ||||||
| Older adults | Altubasi et al. 2015 | n=21 | 0.05 | 0.12 | Knee ext | RFDmax Short walkusual+max, Stair climb, TUG |
|
| Bento et al. 2010 | n=31 | 0.02 | Knee flex | RFD20-80% Number of falls | |||
| Crocket et al. 2015 | n=29 | 0.08 | Knee ext | RFDmax Chair rise | |||
| Hester et al. 2019 | n=26 | 0.25 | Plantar flex (dyn) | VGRFD Chair rise, Short walkusual+max, TUG |
|||
| LaRoche et al. 2011 | n=24 | 0.25 | 0.22 | Dorsi+Plantar flex Knee ext+flex | RFD200ms Long walkusual+max | ||
| Lopez et al. 2017 | n=50 | 0.16 | Knee ext | RFD50ms, RFD100ms Chair rise | |||
| Rech et al. 2014 | n=45 | 0.15 | 0.11 | Knee ext | RFD50ms, RFD100ms, RFD250ms, RFD300ms Chair rise, Short walkusual | ||
| Seynnes et al. 2005 | n=19 | 0.20 | 0.05 | Knee ext | RFDmax
Chair rise, Long walkmax, Stair climb |
||
| Thompson et al. 2018 | n=18 | 0.29 | 0.19 | Knee ext+flex, Squat | RFD50ms, RFD200ms
Chair rise, Long walkmax, Short walkmax |
||
| Unhjem et al. 2019 | n=41 | 0.30 | 0.27 | Leg press (dyn) | RFD30ms, RFD50ms, RFD100ms, RFD150ms, RFD200ms Chair rise | ||
| Weighted mean 95% CI [lower : upper] | Participants Studies |
n=295 N=10 |
0.19 [0.12 : 0.27] |
0.14 [0.09 : 0.19] |
|||
| PD patients | Pääsuke et al. 2002 | n=14 | 0.19 | 0.09 | Knee ext | RFDmax Chair rise | |
| Pääsuke et al. 2004 | n=12 | 0.22 | 0.09 | Knee ext (bilat.) | RFDmax Number of falls | ||
| Weighted mean 95% CI [lower : upper] | Participants Studies | n=26 N=2 |
0.20 [0.20 : 0.21] | 0.09 [0.08 : 0.10] | |||
| MS patients | Kjølhede et al. 2015a | n=34 | 0.13 | 0.11 | Knee ext+flex | RFD200ms, RFDmax Chair rise, Long walkmax, Short walkmax, Stair climb |
|
| Stroke patients | Nadeau et al. 1997 | n=12 | 0.06 | 0.09 | Plantar flex | RFDpeak
Short walkusual+max, TUG |
|
| Pohl et al. 2002 | n=83 | 0.04 | 0.09 | Knee ext | RFD150ms
Short walkusual |
||
| Takeda et al. 2018 | n=20 | 0.20 | 0.24 | Knee ext | RFD50ms, RFD100ms, RFD200ms, RFD300ms Short walkusual+max | ||
| Weighted mean 95% CI [lower : upper] | Participants Studies | n=115 N=3 | 0.07 [0.03 : 0.11] | 0.11 [0.04 : 0.19] | |||
| All | Weighted mean 95% CI [lower : upper] | Participants Studies | n=470 N=16 | 0.15 [0.10 : 0.20] | 0.13 [0.09 : 0.17] | ||
TUG: timed up and go. VGRFD: vertical ground reaction RFD (derived from force plate). In studies reporting data on more than one association between Fmax or RFD (based on a mean RFD value in studies reporting data on more than one RFD measure) and physical function, mean association values of Fmax-Function and RFD-Function, respectively, were calculated.
Part 1 – Parallel observations of deficits in Fmax, RFD, and physical function
Data extraction identified studies reporting parallel observations of Fmax and RFD deficits in older fallers vs. non-fallers, older lower functioning vs. higher functioning, older vs. young, and PD patients vs. healthy controls. Deficits were observed in Fmax (ranging from -11% to -27%, p<0.001), RFD (ranging from -18% to -41%, p<0.001) and Function (ranging from -13% to -31%, p<0.001) in each separate study population (specific results are displayed in Table 1 and Figure 2A).
Figure 2A.
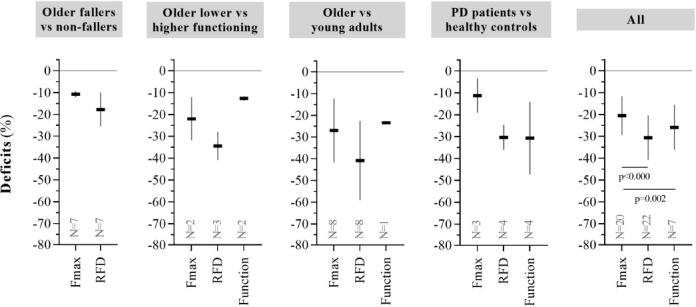
Parallel observations of deficits in Fmax, RFD, and physical function (Part 1). Sample size weighted average deficits in Fmax, RFD, and physical function across all identified studies. Data are presented as mean±CI95%. See Table 1 for further data details.
Across all study populations (comprising N=20 studies/n=706 participants in Fmax, N=22/n=817 in RFD, and N=7/n=193 in Function), deficits were greater in RFD vs. Fmax (-31% [-41:-20] vs. -21% [-29:-12], respectively; difference=10.9% point [5.5:16.3], p<0.000) (Table 1, Figure 2A) and greater in Function vs. Fmax (-26% [-36:-16] vs. -21% [-29:-12], respectively; difference=14.3% point [5.5:23.1], p=0.002), but comparable between RFD and Function (-31% [-41:-20] vs. -26% [-36:-16], respectively; difference=3.3% point [-5.1:11.8], p=0.441) (Table 1, Figure 2A). Across these studies, no calculated associations were observed between deficits in Fmax and Function (r2=0.09 [-0.63:0.87], p=0.630, N=5) or between deficits in RFD and Function (r2=0.34 [-0.09:0.86], p=0.167, N=7). A calculated association was, however, observed between deficits in Fmax and RFD (r2=0.81 [0.57:0.92], p<0.000, N=20).
Deficits in Fmax and RFD across separate muscle groups and across separate contraction modes (isometric and dynamic; early and late RFD) are shown in Figure 2B. Deficits were greater in RFD (N=4) vs. Fmax (N=5) during knee+hip extensor muscle actions (i.e. comprising leg press, squat and chair rise) (difference=21.8% point [-27.6:-16.1], p<0.000), but not in any other of these outcomes (Figure 2B left). Also, deficits were greater in RFD (N=19) vs. Fmax (N=18) during isometric muscle contractions (difference=7.5% point [-11.8:-3.2], p=0.001), and greater in RFD (N=4) vs. Fmax (N=6) during dynamic muscle contractions (difference=22.9% point [-25.2:-20.5], p<0.000) (Figure 2B middle). However, derived from isometric muscle contractions (if reported), deficits did not differ between early RFD (N=10) and late RFD (N=11) (-38% [-58:-18] vs. -28% [-43:-13], respectively; difference=2.8% point [-3.5:9.0], p=0.385) (Figure 2B right).
Figure 2B.
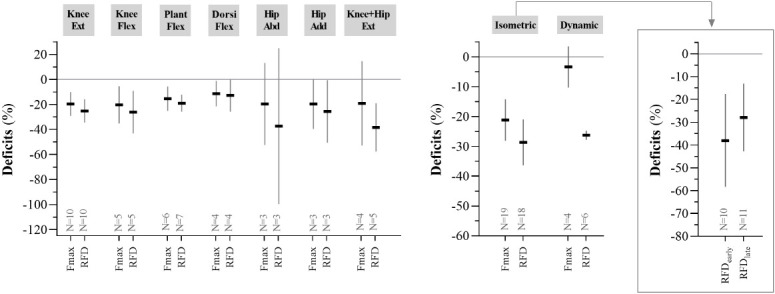
Parallel observations of deficits in Fmax and RFD across muscle groups and contraction mode. Sample size weighted average deficits in Fmax and RFD across muscle groups as well as contraction mode (including early and late RFD derived from isometric contractions) from all identified studies. “Hip + Knee Ext” is comprised of leg press, squat, and chair rise. The presented data support and elaborate Figure 2A and Table 1. Data are presented as mean±CI95%.
Part 2 – Associations between RFD or Fmax and physical function
Data extraction identified studies reporting associations between Fmax or RFD and physical function in older adults as well as in PD, MS, and Stroke patients. Reported associations were observed for Fmax-Function (ranging from r2=0.07 to r2=0.20) and RFD-Function (ranging from r2=0.09 to r2=0.14) in each separate study population (specific results are displayed in Table 2 and Figure 2C).
Figure 2C.
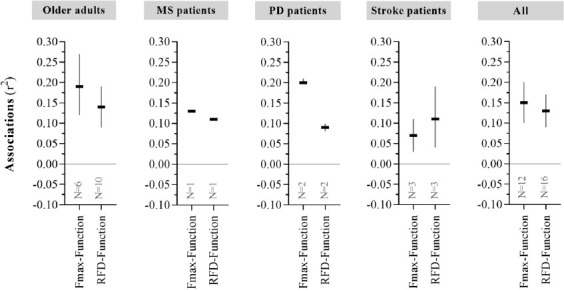
Associations between RFD or Fmax and Physical function (Part 2). Sample size weighted average associations between Fmax or RFD and physical function (denoted Fmax-Function and RFD-Function, respectively) being reported across all selected studies. Data are presented as mean±CI95%. See Table 2 for further data details.
Across all studies (comprising N=12/n=334 in Fmax-Function and N=16/n=470 in RFD-Function), reported associations between Fmax-Function and RFD-Function were comparable (r2=0.15 [0.10:0.20] vs. r2=0.13 [0.09:0.17]; difference=0.03 ‘r2 points’ [-0.01:0.06], p=0.158) (Table 2, Figure 2C). Furthermore, associations between early RFD-Function (N=4) and late RFD-Function (N=6) were comparable (r2=0.14 [0.09:0.20] vs. r2=0.14 [0.12:0.16], respectively; difference=0.01 ‘r2 points’ [-0.01:0.03], p=0.209) (data not shown).
Discussion
This systematic review is the first to summarize existing evidence on the importance of lower extremity RFD (as well as Fmax) for physical function in neurodegenerative populations, comprising aging and common neurodegenerative disorders (PD, MS, and Stroke). Overall, large heterogeneity was observed regarding deriving/calculating RFD from force-time curves as well as assessment of physical function (balance in particular). Also, most of the identified studies had sample sizes of n ≤40, and only N=8 out of N=32 investigated neurodegenerative patients with none in AD.
The analysis of deficits (Part 1) identified a preferentially impaired ability to generate RFD, compared to Fmax, in aging (individuals having inferior vs. superior physical function) and PD (patients vs. healthy controls). While this was accompanied by limitations in physical function, no associations were observed between these deficits. The analysis of reported associations (Part 2) revealed that Fmax and RFD were almost identical in explaining physical function (15% and 13%, respectively, corresponding to weak associations) in aging, PD, MS, and Stroke.
Deficits in RFD, Fmax and physical function (Part 1)
The background for the present review stems from the statements emphasizing that RFD is a particularly sensitive outcome being able to detect acute and/or chronic adaptations in neuromuscular function (i.e. by adapting earlier and/or more extensively than Fmax) and is of particular importance for physical function[38,40,41]. Indeed, the ability to produce a rapid rise in force seems vital as several daily activities such as preventing a fall after postural perturbation[76], walking fast[77], or stairclimbing[78] are characterized by a limited time to develop force (approximately 30-250 ms), prompting less time than it takes to achieve maximal strength (approximately 300-600 ms)[77,79]. Despite the apparent theoretical importance of lower extremity RFD for physical function, strong supporting evidence has previously been lacking with individual studies revealing divergent findings (see Table 2). To exemplify, in older fallers vs. non-fallers, Pijnappels and colleagues[66] reported deficits in RFD and Fmax of a similar magnitude, Kamo and colleagues (the largest identified study)[56] reported deficits in RFD but not Fmax, Bento and colleagues[49] reported no deficits in neither RFD nor Fmax, whereas Palmer and colleagues[64] reported deficits in early RFD but not in late RFD or Fmax. Study findings on the distinction between early and late RFD – with the former suggested to be specifically important for certain functional tasks requiring very short muscle response time (≤100 ms)[64,66] – are nevertheless also divergent and currently inconclusive[57,49,60,64].
The findings of the present review overall support that RFD is particularly sensitive towards neurodegeneration by adapting earlier and/or more extensively compared to Fmax, as indicated by the deficit point estimates and confidence intervals (RFD vs. Fmax difference=10.9% point [5.5:16.3]; Figure 2A, Table 1). This was based on studies involving older individuals and PD patients, with a preferentially impaired ability to generate RFD (and Fmax) in individuals having inferior vs. superior physical function (i.e. older fallers vs. non-fallers, older low functioning vs. high functioning, old vs. young, PD patients vs. healthy controls). A similar pattern was observed across different muscle groups as well as contraction mode, yet most robustly from knee extensor and isometric contractions (Figure 2B). Moreover, while the numerical differences indicated that early phase RFD (compared to late phase RFD) is also sensitive towards neurodegeneration, the point estimates and confidence intervals did not support this conclusion (early vs. late phase RFD difference=2.8% point [-3.5:9.0]).
As individuals having inferior physical function expectedly have a higher degree of neurodegeneration (PD patients by definition), the observed preferential impairments (i.e. deficits) in RFD appear meaningful. Although few studies have examined the existence of such a “neurodegeneration-related” link, Cruickshank and colleagues[80] reported that striatum (brain area involved in inhibiting and facilitating movement) was degenerated in prodromal Huntington’s disease patients compared to healthy controls, and was strongly associated with plantar flexion RFD200ms. Also, Yamada and colleagues[81] reported that older fallers were characterised by more global brain atrophy compared to non-fallers. Lastly, Hammond and colleagues[82] reported large deficits in voluntary RFD (but not in Fmax) in PD patients compared to healthy controls, yet not in evoked ‘involuntary’ RFD induced by octet/tetanic electrical muscle stimulation, indicating CNS dysfunction as the main underlying contributing factor. Interestingly, it has been shown that at least 50% of dopaminergic neurons in the Substantia Nigra of PD patients are compromised prior to the appearance of traditional functional symptoms[13]. These different study findings should nevertheless be cautiously interpreted as lifestyle factors (physical activity/exercise participation in particular), may potentially influence the extent of neurodegeneration, impairments in neuromuscular function, and limitations in physical function[83-85].
The present review emphasizes the structural and functional CNS changes known to occur in the outlined neurodegenerative populations[5,9-11] as main determinants of RFD, thus expanding our understanding of RFD being particularly reliant on a well-functioning CNS[38,42]. However, other determinants may also have contributed to the observed deficits in RFD such as reduced muscle size, altered muscle architecture, fast-to-slow transition in muscle fiber type composition, reduced intrinsic muscle fiber contractility, and reduced mechanical properties of tendons and aponeuroses[38]. These changes are all commonly observed with advanced age[86-89] (including PD and Stroke patients due to their often-advanced age), but to some extent also in the younger neurodegenerative patient populations (MS[17] and potentially also Stroke) especially with advanced disease progression. However, as previously proposed in narrative reviews[90-92], neurodegeneration often precede and potentially drive these muscular/tendinous changes of the outlined neurodegenerative populations. While this reassures us that neurodegeneration was the main determinant of the observed deficits in RFD, we cannot exclude additional contributions from these other determinants. Moreover, impairments in cognitive function – also commonly observed in the outlined neurodegenerative populations[93-96] – may indirectly influence RFD. This was examined in an aging study, reporting a gender-dependent association between RFD and some domains of cognitive function (men: memory; women: executive function, attention, language)[97]. The link between cognitive function and RFD should nevertheless be interpreted with caution due to the scarcity of studies.
Associations between RFD or Fmax and physical function (Part 2)
Across all studies, the reported associations between RFD and physical function as well as between Fmax and physical function, were of a comparable magnitude (explaining 13% and 15%, respectively) (Figure 2C, Table 2), corresponding to weak associations. While this means that factors other than RFD (along with Fmax) contributed to determining physical function in the outlined neurodegenerative populations, these associations may also have been ‘contaminated’ by high variability in the extracted outcome measures of neuromuscular function (RFD in particular) and physical function.
The present findings are corroborated by a recent impressive large-scale study (n=1089, age range 26-96 years) from Osawa and colleagues[39]. They investigated the association between RFD or Fmax and physical function (walking, chair rise, performance batteries), and reported r2-values ranging from 0.18-0.51 for RFD-Function and from 0.18-0.54 for Fmax-Function. The greater magnitude of these associations compared to that observed in the present study are likely explained by the very large sample size spanning the entire adult lifespan, that further enabled them to adjust for age, race, and BMI. Despite the apparent comparable magnitude of RFD and Fmax being associated with physical function, the RFD-Function association remained almost unaffected after adjusting for Fmax. This provide novel evidence revealing that RFD and Fmax impact physical function independently of each other[39]. We strongly emphasize to keep this notion in mind, when interpreting the findings of the present study and discussing the importance of RFD vs. Fmax in a clinical context.
Due to the large heterogeneity in lower extremity muscle actions and functional tests across the included studies of the present review, we presented deficits in RFD and Fmax across specific muscles groups and contraction modes only, but did not go further into their associations with physical function. A major challenge of interpreting such associations, is that some tests of physical function preferentially rely on specific muscle groups and/or on specific muscle response times (relating to early RFD, late RFD, or Fmax, respectively). This is furthermore complicated by the involvement and contribution of balance/coordination in determining physical function.
Methodological considerations
The present review has a number of limitations that should be kept in mind when interpreting the findings and using this to design future studies. First, large heterogeneity was present in the approach used to derive/calculate RFD in the identified studies, in line with recent observations by Blazevich and colleagues[98]. This contrasts the fact that recommendations on methodological procedures have been put forward[38]. In order to report this divergent data, we chose to summarize and report one value of lower extremity RFD from each study assuming that this overall represent the rising phase of the force-time curve. Second, some heterogeneity was seen in relation to muscle groups (including single- vs. multi-joint) and contraction modes (dynamic vs. isometric) used to derive RFD and Fmax, yet we deemed it too speculative to go further into details on how this could implicate the transfer to functional performance outcomes. To exemplify, isometric testing appear superior since RFD will not be influenced by changes in the force-length relationship of the muscle during shortening[57,99], whereas dynamic testing - especially when this involve multi-joint movements - may better reflect the dynamic movement required during tasks such as locomotion[70]. In relation to the latter, specific tests of physical function often preferentially rely on specific muscle groups and/or on specific muscle response times (relating to early RFD, late RFD, or Fmax, respectively), but also on the involvement and contribution of balance/coordination. This challenges the interpretation of the present findings but especially our general understanding of associations between RFD (of Fmax) and physical function. Fourth, modest sample sizes characterised the included studies, yielding limited statistical power from individual studies. Fifth, our quantitative analysis included sample size weighted averages only, but not variance. This was chosen since we aimed to report deficit (percentage) values for Part 1 and 2, and since no variance was reported for Part 2. Sixth, as we identified, included, and summarized data across different types of observational and intervention studies (see Table 1 and Supplementary Table 2), we chose not to carry out risk of bias and quality assessment. Mainly since no single tool exists that can embrace such diversity of study types. Seventh, the present study findings were summarized across different study populations known to undergo different types of neurodegeneration, which may have impacted our findings. Figure 2A and 2C nevertheless help elucidate any potential population-specific influence.
Clinical implications and perspectives
Identification of modifiable neuromuscular predictors of physical function/disability is of major relevance to the outlined neurodegenerative populations, particularly as this can help optimize counteractive strategies (e.g. rehabilitation and physical exercise). The fact that lower extremity RFD appeared particularly sensitive towards neurodegeneration, indicate that RFD could serve as a useful indicator of changes in the nerve-muscle interaction. Whether RFD (and Fmax) can also be viewed as a biomarker per se is debatable[100], although some areas within aging research are currently presenting muscle strength as a biomarker of deterioration in physical function and progression in disability[101-103].
As lower extremity RFD and MVC were associated with physical function of a comparable magnitude in the outlined neurodegenerative populations, this may imply that RFD is somewhat redundant to assess in a clinical context. Counteractive strategies should altogether focus on and target Fmax (and perhaps also RFD if being meaningful), along with additional important aspects such as balance/coordination as well as aerobic capacity and endurance. On the other hand, the intriguing study findings by Osawa and colleagues do however support to also assess RFD, as it was shown to impact physical function independently of Fmax[39]. To expand our knowledge on the potential independent impact of RFD and Fmax, respectively, future studies should investigate whether some tests of physical function or specific phases thereof preferentially rely on specific muscle groups and/or on specific muscle response times (relating to early RFD, late RFD, or Fmax, respectively).
Conclusion
The present systematic review provided novel summarized data on lower extremity RFD along with its importance for physical function in neurodegenerative populations (i.e. aging and common neurodegenerative disorders). Overall, the findings reveal that lower extremity RFD is (1) particularly sensitive (i.e. adapts earlier and/or more extensively) towards neurodegeneration, and more so than Fmax, and (2) of importance for physical function but apparently not superior to Fmax. Altogether, RFD could serve as a useful indicator (or biomarker) of changes in neuromuscular function elicited by neurodegeneration.
Authors’ Contributions
SDL, UD, and LGH contributed to the conception of the study and to the development of the search strategy. SDL and LGH conducted the systematic search and completed the acquisition of data, performed the data analysis, and took the lead in writing the manuscript. All the authors discussed the results and contributed to the final manuscript.
Funding
Open access funding provided by Department of Public Health, Aarhus University.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.World Health Organization. What are neurological disorders?[online] Available at:https://www.who.int/features/qa/55/en/
- 2.World Health Organization. Neurological disorders –public health challenges. Switzerland: WHO; 2006. [Google Scholar]
- 3.Feigin VL, Vos T, Alahdab F, et al. Burden of Neurological Disorders Across the US From 1990-2017:A Global Burden of Disease Study. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deuschl G, Beghi E, Fazekas F, et al. The burden of neurological diseases in Europe:an analysis for the Global Burden of Disease Study 2017. Lancet Public Health. 2020;5:e551–e567. doi: 10.1016/S2468-2667(20)30190-0. [DOI] [PubMed] [Google Scholar]
- 5.Borzuola R, Giombini A, Torre G, et al. Central and Peripheral Neuromuscular Adaptations to Ageing. J Clin Med. 2020;9 doi: 10.3390/jcm9030741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wyss-Coray T. Ageing, neurodegeneration and brain rejuvenation. Nature. 2016;539:180. doi: 10.1038/nature20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniele S, Giacomelli C, Martini C. Brain ageing and neurodegenerative disease:The role of cellular waste management. Biochemical Pharmacology. 2018;158:207–216. doi: 10.1016/j.bcp.2018.10.030. [DOI] [PubMed] [Google Scholar]
- 8.Pringsheim T, Fiest K, Jette N. Global Perspectives. American Academy of Neurology. 2015;83:1661–1664. doi: 10.1212/WNL.0000000000000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ljubisavljevic MR, Ismail FY, Filipovic S. Transcranial magnetic stimulation of degenerating brain:a comparison of normal aging, Alzheimer's, Parkinson's and Huntington's disease. Curr Alzheimer Res. 2013;10:578–596. doi: 10.2174/15672050113109990133. [DOI] [PubMed] [Google Scholar]
- 10.Mamoei S, Hvid LG, Boye Jensen H, Zijdewind I, Stenager E, Dalgas U. Neurophysiological impairments in multiple sclerosis-Central and peripheral motor pathways. Acta Neurol Scand. 2020 doi: 10.1111/ane.13289. [DOI] [PubMed] [Google Scholar]
- 11.Ong LK, Walker FR, Nilsson M. Is Stroke a Neurodegenerative Condition?A Critical Review of Secondary Neurodegeneration and Amyloid-beta Accumulation after Stroke. AIMS Medical Science. 2017;4:1–16. [Google Scholar]
- 12.Stoker TB, Greenland JC. Parkinson's Disease –Pathogenesis and Clinical Aspects. Brisbane Codon Publications. 2018 [PubMed] [Google Scholar]
- 13.Fearnley JM, Lees AJ. Ageing and Parkinson's Disease:Substantia Nigra Regional Selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 14.Lane CA, Hardy J, Schott M. Alzheimer's disease. Eur J Neurol. 2017;25:59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 15.Compston A, Coles A. Multiple Sclerosis. The Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 16.Hankey GJ. Stroke. The Lancet. 2017;389:641–654. doi: 10.1016/S0140-6736(16)30962-X. [DOI] [PubMed] [Google Scholar]
- 17.Jorgensen M, Dalgas U, Wens I, Hvid LG. Muscle strength and power in persons with multiple sclerosis - A systematic review and meta-analysis. J Neurol Sci. 2017;376:225–241. doi: 10.1016/j.jns.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Candow DG, Chilibeck PD. Differences in size, strength, and power of upper and lower body muscle groups in young and older men. J Gerontol A Biol Sci Med Sci. 2005;60:148–156. doi: 10.1093/gerona/60.2.148. [DOI] [PubMed] [Google Scholar]
- 19.Pang MY, Mak MK. Muscle strength is significantly associated with hip bone mineral density in women with Parkinson's disease:a cross-sectional study. J Rehabil Med. 2009;41:223–230. doi: 10.2340/16501977-0311. [DOI] [PubMed] [Google Scholar]
- 20.Pang MY, Mak MK. Trunk muscle strength, but not trunk rigidity, is independently associated with bone mineral density of the lumbar spine in patients with Parkinson's disease. Mov Disord. 2009;24:1176–1182. doi: 10.1002/mds.22531. [DOI] [PubMed] [Google Scholar]
- 21.Zidan M, Acoverde C, De Araujo NB, et al. Motor and functional changes in different stages of Alzheimer's disease. Rev Psig Clin. 2012;39:161–165. [Google Scholar]
- 22.Opara JA, Malecki A, Socha T. Motor assessment in Parkinson's disease. Annals of Agricultural and Environmental Medicine. 2017;24:411–415. doi: 10.5604/12321966.1232774. [DOI] [PubMed] [Google Scholar]
- 23.Bean JF, Leveille SG, Kiely Dk, Bandinelli S, Guralnik JM, Ferrucci L. A Comparison of Leg Power and Leg Strength Within the InCHIANTI Study:Which Influences Mobility More? Journal of gerontology. 2003;58(A):728–733. doi: 10.1093/gerona/58.8.m728. [DOI] [PubMed] [Google Scholar]
- 24.Byrne C, Faure C, Keene DJ, Lamb SE. Ageing, Muscle Power and Physical Function:A Systematic Review and Implications for Pragmatic Training Interventions. CrossMark. (Sport Μed) 2016 doi: 10.1007/s40279-016-0489-x. [DOI] [PubMed] [Google Scholar]
- 25.Mayson DJ, Kiely DK, LaRose SI, Bean JF. Leg Strength or Velocity of Movement Which Is More Influential on the Balance of Mobility Limited Elders? Am K Phys Med Rehabil. 2008;87:969–976. doi: 10.1097/PHM.0b013e31818dfee5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Recovery of Walking Function in Stroke Patients:The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:27–32. doi: 10.1016/s0003-9993(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 27.Hvid LG, Feys P, Baert I, Kalron A, Dalgas U. Accelerated Trajectories of Walking Capacity Across the Adult Life Span in Persons With Multiple Sclerosis:An Underrecognized Challenge. Neurorehabil Neural Repair. 2020;34:360–369. doi: 10.1177/1545968320907074. [DOI] [PubMed] [Google Scholar]
- 28.Gerrits KH, Beltman MJ, Koppe PA, et al. Isometric muscle function of knee extensors and the relation with functional performance in patients with stroke. Arch Phys Med Rehabil. 2009;90:480–487. doi: 10.1016/j.apmr.2008.09.562. [DOI] [PubMed] [Google Scholar]
- 29.Ogawa Y, Kaneko Y, Sato T, Shimizu S, Kanetaka H, Hanyu H. Sarcopenia and Muscle Functions at Various Stages of Alzheimer Disease. Front Neurol. 2018;9:710. doi: 10.3389/fneur.2018.00710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pääsuke M, Ereline J, Gapeyeva H, Joost K, Mottus K, Taba P. Leg-extension strength and chair-rise performance in elderly women with Parkinson's disease. J Aging Phys Act. 2004;12:511–524. doi: 10.1123/japa.12.4.511. [DOI] [PubMed] [Google Scholar]
- 31.Ferrucci L, Guralnik JM, Buchner D, et al. Departures from linearity in the relationship between measures of muscular strength and physical performance of the lower extremities:The women's health and aging study. Journal of gerontology. 1997;52(A):M275–M285. doi: 10.1093/gerona/52a.5.m275. [DOI] [PubMed] [Google Scholar]
- 32.Visser M, Goodpaster BH, Kritchewsky SB, et al. Muscle Mass, Muscle Strength, and Muscle Fat Infiltration as Predictors of Incident Mobility Limitations in Well-Functioning Older Persons. Journal of Gerontology. 2005;60(A):324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 33.Kjølhede T, Vissing K, Langeskov-Christensen D, Stenager E, Petersen T, Dalgas U. Relationship between muscle strength parameters and functional capacity in persons with mild to moderate degree multiple sclerosis. Multiple sclerosis and related disorders. 2015;4:151–158. doi: 10.1016/j.msard.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Kluding P, Gajewski B. Lower-Extremity Strength Differences Predict Activity Limitations in People With Chronic Stroke. American Physical Therapy Association. 2009;83:73–81. doi: 10.2522/ptj.20070234. [DOI] [PubMed] [Google Scholar]
- 35.Falvo MJ, Schilling BK, Earhart GM. Parkinson's disease and resistive exercise:rationale, review, and recommendations. Mov Disord. 2008;23:1–11. doi: 10.1002/mds.21690. [DOI] [PubMed] [Google Scholar]
- 36.Menz HB, Morris ME, Lord SR. Foot and ankle characteristics associated with impaired balance and functional ability in older people. J Gerontol A Biol Sci Med Sci. 2005;60:1546–1552. doi: 10.1093/gerona/60.12.1546. [DOI] [PubMed] [Google Scholar]
- 37.Ramari C, Hvid LG, David AC, Dalgas U. The importance of lower-extremity muscle strength for lower-limb functional capacity in multiple sclerosis:Systematic review. Ann Phys Rehabil Med. 2020;63:123–137. doi: 10.1016/j.rehab.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Maffiuletti NA, Aagaard P, Blazevich AJ, Folland J, Tillin N, Duchateau J. Rate of force development:physiological and methodological considerations. European Journal of Applied Physiology. 2016;116:1091–1116. doi: 10.1007/s00421-016-3346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osawa Y, Studenski SA, Ferrucci L. Knee extension rate of torque development and peak torque:associations with lower extremity function. Journal of Cachexia, Sarcopenia and Muscle. 2018;9:530–539. doi: 10.1002/jcsm.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Angelozzi M, Madama M, Corsica C, et al. Rate of Force Development as an Adjunctive Outcome Measure for Return-to-Sport Decisions After Anterior Cruciate Ligament Reconstruction. Journal of Orthopaedic &Sport Physical Therapy. 2012;42:772–780. doi: 10.2519/jospt.2012.3780. [DOI] [PubMed] [Google Scholar]
- 41.Penailillo L, Blazevich A, Numazawa H, Nosaka K. Rate of force development as a measure of muscle damage. Scand J Med Sci Sports. 2014;25:417–427. doi: 10.1111/sms.12241. [DOI] [PubMed] [Google Scholar]
- 42.Del Vecchio A, Negro F, Holobar A, et al. You are as fast as your motor neurons:speed of recruitment and maximal discharge of motor neurons determine the maximal rate of force development in humans. J Physiol. 2019;597:2445–2456. doi: 10.1113/JP277396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holviala J, Hakkinen A, Alen M, Sallinen J, Kraemer W, Häkkinen K. Effects of prolonged and maintenance strength training on force production, walking, and balance in aging women and men. Scand J Med Sci Sports. 2014;24:224–233. doi: 10.1111/j.1600-0838.2012.01470.x. [DOI] [PubMed] [Google Scholar]
- 44.Noorvee K, Uueni D, Ereline J, Gapeyeva H, Taba P, Pääsuke M. Motor performance characteristics in patients with mild-to-moderate Parkinson's Disease and healthy controls. Acta Kinesiologiae Universitatis Tartuensis. 2006;11:53–63. [Google Scholar]
- 45.Pohl PS, Duncan P, Perera S, et al. Rate of isometric knee extension strength development and walking speed after stroke. J Rehabil Res Dev. 2002;39:651–657. [PubMed] [Google Scholar]
- 46.Moher D, Liberati A, Tetzlaff J, Altman DG Group. TP. Preferred Reporting Items for Systematic Reviews and Meta-Analyses:The PRISMA Statement. PLoS medicine. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 47.Schober P, Boer C, Schwarte LA. Correlation Coefficients:Appropriate Use and Interpretation. 2018;126:1763–1768. doi: 10.1213/ANE.0000000000002864. [DOI] [PubMed] [Google Scholar]
- 48.Altubasi IM. Is quadriceps muscle strength a determinant of the physical function of the elderly? J Phys Ther Sci. 2015;27:3035–3038. doi: 10.1589/jpts.27.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bento PC, Pereira G, Ugrinowitsch C, Rodacki AL. Peak torque and rate of torque development in elderly with and without fall history. Clin Biomech (Bristol, Avon) 2010;25:450–454. doi: 10.1016/j.clinbiomech.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Clark DJ, Manini TM, Fielding RA, Patten C. Neuromuscular determinants of maximum walking speed in well-functioning older adults. Exp Gerontol. 2013;48:358–363. doi: 10.1016/j.exger.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crockett K, Ardell K, Hermanson M, et al. The Relationship of Knee-Extensor Strength and Rate of Torque Development to Sit-to-Stand Performance in Older Adults. Physiotherapy Canada. 2013;65:229–235. doi: 10.3138/ptc.2012-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crozara LF, Morcelli MH, Marques NR, et al. Motor readiness and joint torque production in lower limbs of older women fallers and non-fallers. J Electromyogr Kinesiol. 2013;23:1131–1138. doi: 10.1016/j.jelekin.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 53.Hester GM, Ha PL, Dalton BE, et al. Rate of Force Development as a Predictor of Mobility in Older Adults. J Geriatr Phys Ther. 2020 doi: 10.1519/JPT.0000000000000258. [DOI] [PubMed] [Google Scholar]
- 54.Inacio M, Creath R, Rogers MW. Effects of aging on hip abductor-adductor neuromuscular and mechanical performance during the weight transfer phase of lateral protective stepping. J Biomech. 2019;82:244–250. doi: 10.1016/j.jbiomech.2018.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Izquierdo M, Ibanez J, Gorostiaga E, et al. Maximal strength and power characteristics in isometric and dynamic actions of the upper and lower extremities in middle-aged and older men. Acta Physiol Scand. 1999;167:57–68. doi: 10.1046/j.1365-201x.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- 56.Kamo T, Asahi R, Azami M, et al. Rate of torque development and the risk of falls among community dwelling older adults in Japan. Gait Posture. 2019;72:28–33. doi: 10.1016/j.gaitpost.2019.05.019. [DOI] [PubMed] [Google Scholar]
- 57.LaRoche DP, Cremin KA, Greenleaf B, Croce RV. Rapid torque development in older female fallers and nonfallers:a comparison across lower-extremity muscles. J Electromyogr Kinesiol. 2010;20:482–488. doi: 10.1016/j.jelekin.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 58.LaRoche DP, Millett ED, Kralian RJ. Low strength is related to diminished ground reaction forces and walking performance in older women. Gait Posture. 2011;33:668–672. doi: 10.1016/j.gaitpost.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mackey DC, Robinovitch SN. Mechanisms underlying age-related differences in ability to recover balance with the ankle strategy. Gait Posture. 2006;23:59–68. doi: 10.1016/j.gaitpost.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 60.Morcelli MH, Rossi DM, Karuka AH, et al. Peak torque, reaction time, and rate of torque development of hip abductors and adductors of older women. Physiother Theory Pract. 2016b;32:45–52. doi: 10.3109/09593985.2015.1091870. [DOI] [PubMed] [Google Scholar]
- 61.Morcelli MH, LaRoche DP, Crozara LF, et al. Neuromuscular performance in the hip joint of elderly fallers and non-fallers. Aging Clin Exp Res. 2016;28:443–450. doi: 10.1007/s40520-015-0448-7. [DOI] [PubMed] [Google Scholar]
- 62.Lopez P, Wilhelm EN, Rech A, Minozzo F, Radaelli R, Pinto RS. Echo intensity independently predicts functionality in sedentary older men. Muscle Nerve. 2017;55:9–15. doi: 10.1002/mus.25168. [DOI] [PubMed] [Google Scholar]
- 63.Palmer TB, Thiele RM, Thompson BJ. Age-Related Differences in Maximal and Rapid Torque Characteristics of the Hip Extensors and Dynamic Postural Balance in Healthy, Young and Old Females. J Strength Cond Res. 2017;31:480–488. doi: 10.1519/JSC.0000000000001503. [DOI] [PubMed] [Google Scholar]
- 64.Palmer TB, Thiele RM, Williams KB, et al. The identification of fall history using maximal and rapid isometric torque characteristics of the hip extensors in healthy, recreationally active elderly females:a preliminary investigation. Aging Clin Exp Res. 2015;27:431–438. doi: 10.1007/s40520-014-0305-0. [DOI] [PubMed] [Google Scholar]
- 65.Palmer TB, Thiele RM, Conchola EC, Smith DB, Thompson BJ. A Preliminary Study of the Utilization of Maximal and Rapid Strength Characteristics to Identify Chair-Rise Performance Abilities in Very Old Adults. J Geriatr Phys Ther. 2016;39:102–109. doi: 10.1519/JPT.0000000000000060. [DOI] [PubMed] [Google Scholar]
- 66.Pijnappels M, van der Burg PJ, Reeves ND, van Dieen JH. Identification of elderly fallers by muscle strength measures. Eur J Appl Physiol. 2008;102:585–592. doi: 10.1007/s00421-007-0613-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rech A, Radaelli R, Goltz FR, da Rosa LH, Schneider CD, Pinto RS. Echo intensity is negatively associated with functional capacity in older women. Age (Dordr) 2014;36:9708. doi: 10.1007/s11357-014-9708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seynnes O, Hue OA, Garrandes F, et al. Force steadiness in the lower extremities as an independent predictor of functional performance in older women. J Aging Phys Act. 2005;13:395–408. doi: 10.1123/japa.13.4.395. [DOI] [PubMed] [Google Scholar]
- 69.Sundstrup E, Jakobsen MD, Andersen JL, et al. Muscle function and postural balance in lifelong trained male footballers compared with sedentary elderly men and youngsters. Scand J Med Sci Sports. 2010;20(Suppl 1):90–97. doi: 10.1111/j.1600-0838.2010.01092.x. [DOI] [PubMed] [Google Scholar]
- 70.Thompson BJ, Whitson M, Sobolewski EJ, Stock MS. Effects of Age, Joint Angle, and Test Modality on Strength Production and Functional Outcomes. Int J Sports Med. 2018;39:124–132. doi: 10.1055/s-0043-121149. [DOI] [PubMed] [Google Scholar]
- 71.Unhjem R, van den Hoven LT, Nygård M, Hoff J, Wang E. Functional Performance With Age:The Role of Long-Term Strength Training. J Geriatr Phys Ther. 2019;42:115–122. doi: 10.1519/JPT.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 72.Pääsuke M, Mottus K, Ereline J, Gapeyeva H, Taba P. Lower limb performance in older female patients with Parkinson's disease. Aging Clin Exp Res. 2002;14:185–191. doi: 10.1007/BF03324434. [DOI] [PubMed] [Google Scholar]
- 73.Malling AS, Jensen BR. Motor intensive anti-gravity training improves performance in dynamic balance related tasks in persons with Parkinson's disease. Gait Posture. 2016;43:141–147. doi: 10.1016/j.gaitpost.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 74.Takeda K, Tanabe S, Koyama S, et al. Relationship between the rate of force development in knee extensor muscles and gait speed in patients with chronic stroke:A cross-sectional study. NeuroRehabilitation. 2018;43:425–430. doi: 10.3233/NRE-182455. [DOI] [PubMed] [Google Scholar]
- 75.Nadeau S, Gravel D, Arsenault AB, Bourbonnais D, Goyette M. Dynamometric assessment of the plantarflexors in hemiparetic subjects:relations between muscular, gait and clinical parameters. Scand J Rehabil Med. 1997;29:137–146. [PubMed] [Google Scholar]
- 76.Pijnappels M, Bobbert MF, van Dieen JH. Control of support limb muscles in recovery after tripping in young and older subjects. Exp Brain Res. 2005;160:326–333. doi: 10.1007/s00221-004-2014-y. [DOI] [PubMed] [Google Scholar]
- 77.Suetta C, Aagaard P, Rosted A, et al. Training-induced changes in muscle CSA, muscle strength, EMG, and rate of force development in elderly subjects after long-term unilateral disuse. J Appl Physiol (1985) 2004;97:1954–1961. doi: 10.1152/japplphysiol.01307.2003. [DOI] [PubMed] [Google Scholar]
- 78.Nyland J, Frost K, Quesada P, Angeli C, Swank A, Topp R. Self-reported chair-rise ability relates to stair-climbing readiness of total knee arthroplasty patients:A pilot study. Journal of Rehabilitation Research and Development. 2007;44:751–760. doi: 10.1682/jrrd.2006.11.0146. [DOI] [PubMed] [Google Scholar]
- 79.Corcos DM, Chen CM, Quinn NP, McAuley J, Rothwell JC. Strength in Parkinson's Disease:Relationship to Rate of Force Generation and Clinical Status. Annals of Neurology. 1996;39:79–88. doi: 10.1002/ana.410390112. [DOI] [PubMed] [Google Scholar]
- 80.Cruickshank T, Reyes A, Pulverenti TS, et al. Rate of torque development and striatal shape in individuals with prodromal Huntington's disease. Sci Rep. 2020;10:15103. doi: 10.1038/s41598-020-72042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yamada M, Takechi H, Mori S, Aoyama T, Arai H. Global brain atrophy is associated with physical performance and the risk of falls in older adults with cognitive impairment. Geriatrics &gerontology international. 2013;13:437–442. doi: 10.1111/j.1447-0594.2012.00927.x. [DOI] [PubMed] [Google Scholar]
- 82.Hammond KG, Pfeiffer RF, Ledoux MS, Schilling BK. Neuromuscular rate of force development deficit in Parkinson disease. Clinical Biomechanics. 2017;45:14–18. doi: 10.1016/j.clinbiomech.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 83.Erickson KI, Miller DL, Roecklein KA. The Aging Hippocampus:Interactions between Exercise, Depression, and BDNF. Neuroscientist. 2012;18:82–97. doi: 10.1177/1073858410397054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Snider J, Müller MLTM, Kotagal V, et al. Non-exercise physical activity attenuates motor symptoms in Parkinson disease independent from nigrostriatal degeneration. Parkinsonism Relat Disord. 2015;21:1227–1231. doi: 10.1016/j.parkreldis.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dalgas U, Langeskov-Christensen M, Stenager E, Riemenschneider M, Hvid LG. Exercise as Medicine in Multiple Sclerosis-Time for a Paradigm Shift:Preventive, Symptomatic, and Disease-Modifying Aspects and Perspectives. Curr Neurol Neurosci Rep. 2019;19 doi: 10.1007/s11910-019-1002-3. [DOI] [PubMed] [Google Scholar]
- 86.Svensson RB, Heinemeier KM, Couppé C, Kjaer M, Magnusson SP. Effect of aging and exercise on the tendon. J Appl Physiol (1985) 2016;121:1237–1246. doi: 10.1152/japplphysiol.00328.2016. [DOI] [PubMed] [Google Scholar]
- 87.Lim JY, Frontera WR. Single skeletal muscle fiber mechanical properties:a muscle quality biomarker of human aging. Eur J Appl Physiol. 2022 doi: 10.1007/s00421-022-04924-4. [DOI] [PubMed] [Google Scholar]
- 88.Frontera WR. Physiologic Changes of the Musculoskeletal System with Aging:A Brief Review. Phys Med Rehabil Clin N Am. 2017;28:705–711. doi: 10.1016/j.pmr.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 89.Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393:2636–2646. doi: 10.1016/S0140-6736(19)31138-9. [DOI] [PubMed] [Google Scholar]
- 90.Kara M, Özçakar L, Kaymak B, Ata AM, Frontera W. A “Neuromuscular Look”to sarcopenia:Is it a movement disorder? J Rehabil Med. 2020;52:jrm00042. doi: 10.2340/16501977-2672. [DOI] [PubMed] [Google Scholar]
- 91.Kwan P. Sarcopenia, a neurogenic syndrome? J Aging Res. 2013;2013:791679. doi: 10.1155/2013/791679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kwon YN, Yoon SS. Sarcopenia:Neurological Point of View. J Bone Metab. 2017;24:83–89. doi: 10.11005/jbm.2017.24.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gleichgerrcht E, Ibáñez A, Roca M, Torralva T, Manes F. Decision-making cognition in neurodegenerative diseases. Nat Rev Neurol. 2010;6:611–623. doi: 10.1038/nrneurol.2010.148. [DOI] [PubMed] [Google Scholar]
- 94.Sumowski JF, Benedict R, Enzinger C, et al. Cognition in multiple sclerosis:State of the field and priorities for the future. Neurology. 2018;90:278–288. doi: 10.1212/WNL.0000000000004977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fang C, Lv L, Mao S, Dong H, Liu B. Cognition Deficits in Parkinson's Disease:Mechanisms and Treatment. Parkinsons Dis. 2020;2020:2076942. doi: 10.1155/2020/2076942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mellon L, Brewer L, Hall P, Horgan F, Williams D, Hickey A. Cognitive impairment six months after ischaemic stroke:a profile from the ASPIRE-S study. BMC Neurol. 2015;15:31. doi: 10.1186/s12883-015-0288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tian Q, Osawa Y, Resnick SM, Ferrucci L, Studenski SA. Rate of Muscle Contraction Is Associated With Cognition in Women, Not in Men. J Gerontol A Biol Sci Med Sci. 2019;74:714–719. doi: 10.1093/gerona/gly115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blazevich A, Wilson CJ, Alcaraz PE, Rubio-Arias JA. Effects of Resistance Training Movement Pattern and Velocity on Isometric Muscular Rate of Force Development:A Systematic Review with Meta-analysis and Meta-regression. Sports Medicine. 2020;50:943–963. doi: 10.1007/s40279-019-01239-x. [DOI] [PubMed] [Google Scholar]
- 99.Porto JM, Freire Júnior RC, Bocarde L, et al. Contribution of hip abductor–adductor muscles on static and dynamic balance of community-dwelling older adults. Aging Clinical and Experimental Research. 2018 doi: 10.1007/s40520-018-1025-7. [DOI] [PubMed] [Google Scholar]
- 100.Califf RM. Biomarker definitions and their applications. Exp Biol Med (Maywood) 2018;243:213–221. doi: 10.1177/1535370217750088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bohannon RW. Grip Strength:An Indispensable Biomarker For Older Adults. Clin Interv Aging. 2019;14:1681–1691. doi: 10.2147/CIA.S194543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Landi F, Calvani R, Picca A, Marzetti E. Can Muscle Strength Be Considered a Composite Biomarker of Sarcopenia? J Am Med Dir Assoc. 2018;19:373–374. doi: 10.1016/j.jamda.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 103.Picca A, Calvani R, Cesari M, et al. Biomarkers of Physical Frailty and Sarcopenia:Coming up to the Place? Int J Mol Sci. 2020;21 doi: 10.3390/ijms21165635. [DOI] [PMC free article] [PubMed] [Google Scholar]


