Summary
Background
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease caused by the loss of motor neurons, and development of effective medicines is urgently required. Induced pluripotent stem cell (iPSC)-based drug repurposing identified the Src/c-Abl inhibitor bosutinib, which is approved for the treatment of chronic myelogenous leukemia (CML), as a candidate for the molecular targeted therapy of ALS.
Methods
An open-label, multicentre, dose-escalation phase 1 study using a 3 + 3 design was conducted in 4 hospitals in Japan to evaluate the safety and tolerability of bosutinib in patients with ALS. Furthermore, the exploratory efficacy was evaluated using Revised ALS Functional Rating Scale (ALSFRS-R), predictive biomarkers including plasma neurofilament light chain (NFL) were explored, and single-cell RNA sequencing of iPSC-derived motor neurons was conducted. Patients, whose total ALSFRS-R scores decreased by 1–3 points during the 12-week, received escalating doses starting from 100 mg quaque die (QD) up to 400 mg QD based on dose-limiting toxicity (DLT) occurrence, and all participants who received one dose of the study drug were included in the primary analysis. This trial is registered with ClinicalTrials.gov, NCT04744532, as Induced pluripotent stem cell-based Drug Repurposing for Amyotrophic Lateral Sclerosis Medicine (iDReAM) study.
Findings
Between March 29, 2019 and May 7, 2021, 20 patients were enrolled, 13 of whom received bosutinib treatment and 12 were included in the safety and efficacy analyses. No DLTs were observed up to 300 mg QD, but DLTs were observed in 3/3 patients of the 400 mg QD cohort. In all patients receiving 100 mg–400 mg, the prevalent adverse events (AEs) were gastrointestinal AEs in 12 patients (92.3%), liver function related AEs in 7 patients (53.8%), and rash in 3 patients (23.1%). The safety profile was consistent with that known for CML treatment, and ALS-specific AEs were not observed. A subset of patients (5/9 patients) was found to respond well to bosutinib treatment over the 12-week treatment period. It was found that the treatment-responsive patients could be distinguished by their lower levels of plasma NFL. Furthermore, single-cell RNA sequencing of iPSC-derived motor neurons revealed the pathogenesis related molecular signature in patients with ALS showing responsiveness to bosutinib.
Interpretation
This is the first trial of a Src/c-Abl inhibitor, bosutinib, for patients with ALS. The safety and tolerability of bosutinib up to 300 mg, not 400 mg, in ALS were described, and responsiveness of patients on motor function was observed. Since this was an open-label trial within a short period with a limited number of patients, further clinical trials will be required.
Funding
AMED and iPS Cell Research Fund.
Research in context.
Evidence before this study
We searched PubMed for papers published in English between database inception and May 31, 2022, using the terms “amyotrophic lateral sclerosis” AND “bosutinib” to identify studies on the effect of bosutinib in patients with ALS. Our search returned no clinical studies in which bosutinib was administered to ALS patients. In non-clinical studies, the Src/c-Abl inhibitor bosutinib, which is a drug used for the treatment of chronic myeloid leukemia, was found to inhibit the accumulation of misfolded proteins by acceleration of autophagy and to suppress motor neuron death in iPSC-derived motor neurons from ALS patients. This effect was also confirmed in ALS model mice. These findings suggest that such molecularly targeted therapies used as anticancer drugs may be effective in ALS. From the experience of the clinical use of bosutinib for CML patients, its safety has been evaluated in clinical trials and is well characterized. However, since the disease-related physical conditions of ALS patients are different from those of CML patients, in the present study evaluation of the safety and tolerability of bosutinib in ALS patients was conducted together with evaluation of its efficacy in an exploratory manner.
Added value of this study
To the best of our knowledge, this is the first clinical trial to investigate the effects of bosutinib in ALS patients. This study focuses on iPSC-based repositioning of an oncology drug in a neurological setting. It is unique in that it combines an ALS-specific study design of a 3-month observation period, which avoids the variability in the speed of disease progression that makes objective drug evaluation in ALS difficult, with a 3 + 3 dose escalation for the study design of anti-cancer drugs. This study design may provide a prototype for new study designs for future molecular-targeted drugs in neurology. Although the number of patients was limited, the speed of disease progression became stabilized in more than half of the patients in the treated group during the 3-month treatment period of the investigational drug. Furthermore, in those patients, the plasma Neurofilament L (NFL) levels, which are believed to originate from degenerated neurons, were found to be lower. The molecular pathogenesis of each patient behind the drug response biomarkers by NFL as disease indicator was further elucidated by single-cell RNA sequencing of motor neurons generated from patient iPSCs. One of the features of this study is the integrated approach from the clinical trial to reverse translational research using iPSCs.
Implications of all the available evidence
This is the first study to use a Src/c-Abl inhibitor, bosutinib, for ALS patients, together with detailed examination of each patient. The general safety and tolerability of bosutinib in ALS was confirmed, and a subset of patients was found to respond well to bosutinib by appearing to maintain clinical stability, with the treatment-responsive patients being distinguishable by plasma NFL levels, which may be a potential predictor of drug efficacy. Since the study was conducted in a short period with a limited number of patients, further longer-term studies with greater numbers of patients will be needed.
Introduction
ALS is a severe neurodegenerative disease characterized by loss of motor neuron, leading to progressive muscle weakness, loss of motor control and death from respiratory failure, with survival 3–5 years after onset.1 There are currently no fundamental treatments for ALS. Riluzole and edaravone have been approved for the treatment of ALS, although riluzole treatment2 does not provide sufficient therapeutic effect and edaravone treatment3,4 does not deliver a life-prolonging effect. On the other hand, in the fields of cancer and hematological/immune intractable diseases, therapeutic target molecules have been identified, and molecular-targeted therapies have enabled higher therapeutic efficacy compared to conventional, nonspecific anti-cancer or immunosuppressive therapies. In particular, the use of orally available tyrosine kinase inhibitor (TKI) therapies has created a paradigm shift in the treatment of these areas. Meanwhile, in ALS, although therapies such as antisense oligonucleotides (ASO) targeting mutated genes have been investigated for inherited ALS,5,6 there were no molecular-targeted drugs for the majority of patients with sporadic ALS, in which the mutated genes are not known.
The advent of induced pluripotent stem cells (iPSCs) has allowed the construction of cellular models of neurological diseases not only for inherited diseases7,8 but also for diseases with unknown causative genes9 including sporadic ALS,10 which has revealed Src/c-Abl as a candidate therapeutic target for ALS.11 Bosutinib is a selective inhibitor of Src/c-Abl tyrosine kinase, approved for the treatment of chronic myelogenous leukemia (CML). The Bcr-Abl fusion gene expressed in CML is involved in leukemia cell proliferation through its continuous activation, and bosutinib inhibits this process. In ALS, both Src and c-Abl are activated and involved in motor neuron death and abnormal protein accumulation through aberrant autophagy.11 A compound screening using iPSCs derived from patients with ALS identified bosutinib as a potential agent for ALS treatment, which inhibited death of motor neurons and the accumulation of abnormal proteins, critical pathological features of ALS, in an ALS patient iPSC model and an ALS mouse model.11 Based on these results, a therapeutic effect of bosutinib may be expected in patients with ALS.
The purpose of this study is to introduce molecular targeted therapeutics in the field of the treatment of ALS. From the experience of the clinical use of bosutinib in CML patients, its safety has been evaluated in clinical trials and is well characterized.12,13 However, since the disease-related physical conditions of patients with ALS are different from those of CML patients, such as the gastrointestinal tract dysfunction in ALS,14 evaluation of the safety and tolerability of bosutinib in patients with ALS was conducted in the present study. In addition, the efficacy of bosutinib in patients with ALS was evaluated in an exploratory manner.
Methods
Study design and patients
This study consisted of a clinical trial conducted in accordance with the guideline of Good Clinical Practice (GCP) and additional clinical research conducted in accordance with the Clinical Trials Act. This clinical trial was reported in adherence to CONSORT reporting guidelines. As a sub-study of the clinical trial, additional clinical research was conducted for those patients who participated in the clinical trial and consented to attending the ancillary study to measure biomarkers. This study was conducted in 4 sites in Japan—Kyoto University, Tokushima University, Kitasato University, and Tottori University. The protocol and informed consent form were approved by the ethics committees of Kyoto University, Tokushima University, Kitasato University, and Tottori University. All participants provided written informed consent.
The clinical trial was a phase 1, open-label, multicentre, dose-escalation study to evaluate the safety and tolerability of bosutinib in order to determine the maximum tolerated dose (MTD) and recommended phase 2 dose (RP2D) of bosutinib for the treatment of patients with ALS. Additionally, efficacy evaluation was conducted in an exploratory manner. This study included patients with ALS who met all inclusion and exclusion criteria such as those with already-reported superoxide dismutase 1 (SOD1) gene mutation and progressive muscle weakness; or sporadic patients with ALS who were categorized as “Definite ALS,” “Probable ALS,” or “Probable-laboratory supported ALS” by the Updated Awaji Criteria15 for the diagnosis of ALS, patients with Grade 1 or 2 by the Japan ALS Severity Scale of the grant-in-aid program for chronic diseases from the Japanese Ministry of Health, Labour and Welfare, with Grade 3 also being acceptable for patients with SOD1 mutation, patients with sporadic ALS that developed within 2 years of the primary registration, patients with SOD1 mutation within 5 years after disease onset, and patients with a change of −1 to −3 points in the total Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R) score during the observation period. Patients with ALS who had already been diagnosed with a mutation by genetic analysis were classified as ALS with SOD1 mutation, while patients with ALS with no family history were classified as sporadic ALS. Details of the inclusion and exclusion criteria are provided in the protocol16 and included in the supplementary file.
Besides measurements of biomarkers including neurofilament light chain (NFL) and phosphorylated neurofilament heavy chain (pNFH) in plasma in this clinical trial, additional clinical research was conducted for the patients who participated in this clinical trial and consented to the measurement of bosutinib concentration and biomarkers such as chitinase 3 like 1 (CH3L1), phosphorylated Src (p-Src), phosphorylated c-Abl (p-c-Abl), and cytokines in plasma, p75 in urine, NFL and pNFH in cerebrospinal fluid (CSF) as an ancillary study, as well as the generation of iPSCs for the single-cell RNA sequencing analysis of motor neurons derived from iPSCs.
Sample size
The planned target sample size was 12–24 patients if the study proceeded to the 400 mg/day group. The number of patients in each dose group was dependent on the results of the dose-limiting toxicity (DLT) evaluation. The definition of DLTs for this study was provided in the supplementary file. The 95% confidence interval (CI) for a DLT incidence proportion was 0–0.71 when a DLT was observed in 0 of 3 patients, and 0.004–0.64 when a DLT was observed in 1 of 6 patients. Additional patients, including those for replacement, could be enrolled for DLT evaluation when determined to be necessary by the Safety Assessment Committee.
Procedure
The study consisted of a 12-week observation period, a 1-week (acceptable window: 5–9 days) transitional period, a 12-week study treatment period, and a 4-week follow-up period.16 Patients enrolled in the 1st registration were also enrolled in the observation period. Patients who had been receiving riluzole since before enrollment were allowed to continuously receive riluzole during the 12-week observation period (with the dosage remaining unchanged), and stopped receiving riluzole at the beginning of the 1-week (acceptable window: 5–9 days) transitional period. The patients whose total ALSFRS-R score decreased by 1–3 points during the 12-week observation period were deemed eligible for the 2nd registration to enter the study treatment period. The patients received bosutinib for 12 weeks to evaluate the safety and tolerability of bosutinib in patients with ALS. All ALS drugs including riluzole were prohibited during the bosutinib treatment period because of the necessity to exclude any influence on the safety evaluation of bosutinib. Prohibited medications for safety considerations are listed in the Supplementary File.
Based on the results of non-clinical studies, 300 mg/day was considered to be the effective dose for ALS. Given no safety profile of bosutinib has been investigated in patients with ALS, the 100 mg was set as a starting dose as a conservative approach. The maximum dose was set at 400 mg which is the clinical dose for newly diagnosed CML and one dose higher than the predicted dose. In this study, 3 to 6 patients with ALS were allocated to each of the 4 bosutinib dose levels (100 mg/day [dose level 1], 200 mg/day [dose level 2], 300 mg/day [dose level 3], or 400 mg/day [dose level 4]) to evaluate the safety and tolerability of the investigational product (bosutinib) under a 3 + 3 dose escalation study design (the detail was provided in the Supplementary File). The dose was escalated by 1 dose level at a time, and no skipping of doses was allowed.
Dose escalation and MTD were determined by the Safety Assessment Committee consisting of one oncologist, two hematologists, and two ALS experts on the basis of the incidence of DLT during the first 4 weeks of treatment among 3 patients enrolled (up to 6 patients if additionally enrolled) at each dose level. RP2D was determined by the Safety Assessment Committee upon completion of the entire 12-week study treatment in all patients at all dose levels.
Outcome measurements
Primary endpoint was DLT during the first 4 weeks of treatment with bosutinib, and during the study treatment period (12 weeks). Secondary endpoint was evaluation of adverse events, laboratory test abnormal, vital signs (blood pressure, pulse rate, and body temperature), ECG, and chest X-ray findings. Adverse events were graded based on the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) v.4.03 and the incidences of the treatment-related AEs were summarized by system organ class (SOC) and preferred term (PT) of The Medical Dictionary for Regulatory Activities (MedDRA, v.24.0). Exploratory endpoints were change from baseline in total ALSFRS-R score and grade in the Japan ALS severity classification, change from baseline of % forced vital capacity (FVC) and grip power, and change in plasma NFL and pNFH during the observation period and the study treatment period. The ALSFRS-R was evaluated by trained examiners, and was also evaluated by the same examiners at each visit, as far as possible. Regarding to the grip strength and %FVC, measurements were performed by the same evaluator at the same measuring position and with the same instruments, as far as possible.
Measurement of biomarkers
Plasma NFL and plasma pNFH were quantified by Simoa assays (Quanterix, MA) with validation in the clinical trial in accordance with the guideline of GCP, and other biomarkers were evaluated by laboratory standards in the additional clinical research. Details were described in Supplementary Materials and Methods.
Generation of iPSCs and motor neuron differentiation
iPSCs were generated from peripheral blood mononuclear cells (PBMCs) and were differentiated into motor neurons as previously described. The detailed procedure was described in Supplementary Materials and Methods.
Single-cell RNA sequencing
Motor neurons were dissociated and a library preparation was conducted using the Chromium Next GEM Single Cell 3’ Reagent Kits v3.1 (10×Genomics), and the library was sequenced on NovaSeq 6000. Details of the procedure are described in Supplementary Materials and Methods.
Statistical analysis
Statistical analysis was performed after all patients completed their follow-up period according to the statistical analysis plan in the clinical trial. The frequencies of DLTs during the first 4 weeks of study treatment and during the entire study treatment period (12 weeks) were analysed for each dose group. The frequencies of adverse events (AEs) during the study treatment period were analysed for each dose group. A post-hoc analysis of change from baseline for ALSFRS-R score was performed by a linear mixed-effects model with a repeated-measures approach using the distinguished groups based on NFL levels, baseline values of ALSFRS-R score, duration after the start of treatment of bosutinib, and the interaction of the distinguished group based on NFL levels with duration after the start of treatment of bosutinib as statistical parameters. The remaining exploratory efficacy analysis and biomarker data analysis were performed in two ways: graphically and by means of descriptive statistics. Analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. K.I. and H.I. had access to the dataset and had final responsibility for the decision to submit for publication.
Results
Between March 29, 2019 and May 7, 2021, a total of 20 patients provided their consent, and 18 of them proceeded to the 1st registration, excluding 2 who failed to meet the inclusion criteria. The patients whose total ALSFRS-R score decreased by 1–3 points during the 12-week were eligible for the 2nd registration; 5 of them were then determined to be screening failures based on the eligibility criteria at the 2nd registration, 3 due to respiratory impairment and 2 to personal reasons of patients, and finally 13 were enrolled to the study treatment period for 12 weeks (Fig. 1A and B). Three patients received 100 mg, three patients received 200 mg, four patients received 300 mg, and three patients received 400 mg of bosutinib orally once daily. All patients in the bosutinib 100 mg–300 mg groups completed the 12-week study treatment, except for 1 patient in the 300 mg group; treatment was discontinued because of suicide, which was determined not to be related to bosutinib, and replacement in the section was made for the result of DLT evaluation by the Safety Assessment Committee. In the 400 mg group, all 3 patients discontinued the study treatment due to treatment-related AEs. Characteristics of patients treated with bosutinib were described in Table 1.
Fig. 1.
Profile of the clinical trial. A. CONSORT diagram for iDReAM study. B. Design of the study. The study consisted of a 12-week observation period, a 1-week transitional period, a 12-week study treatment period, and a 4-week follow-up period. The patients whose total ALSFRS-R scores decreased by 1–3 points during the 12-week observation period were eligible to enter the study treatment period. C. Dose escalation based on the 3 + 3 design. No DLTs were observed in the 100 mg–300 mg groups. There were 3 DLTs in the 400 mg group. DLT, dose-limiting toxicity; MTD, maximum tolerated dose; RP2D, recommended phase 2 dose.
Table 1.
Characteristics of patients.
| 100 mg (N = 3) | 200 mg (N = 3) | 300 mg (N = 4) | 400 mg (N = 3) | Total (N = 13) | |
|---|---|---|---|---|---|
| Sex | |||||
| Male (%) | 1 (33.3) | 3 (100.0) | 3 (75.0) | 0 (0.0) | 7 (53.8) |
| Female (%) | 2 (67.7) | 0 (0.0) | 1 (25.0) | 3 (100.0) | 6 (46.2) |
| Age Mean (SD) | 60.3 (15.95) | 52.0 (16.09) | 53.3 (11.64) | 66.0 (14.80) | 57.5 (13.77) |
| Family History of ALS (%) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 2 (15.4) |
| SOD1 Gene Mutation (%) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 2 (15.4) |
| Pre-treatment with riluzole (%) | 2 (66.7) | 3 (100.0) | 4 (100.0) | 3 (100.0) | 12 (92.3) |
As a primary endpoint, the incidence of DLT in the first 4 weeks and in the entire 12 weeks of treatment at each dose level were evaluated by the Safety Assessment Committee (Table 2). No DLTs were observed in the 100 mg–300 mg groups and dose escalation was conducted (Fig. 1C). According to the Safety Assessment Committee, 1 patient in the 400 mg group with rash papular, Grade 3 at maximum severity, was considered to have a DLT during the first 4 weeks of the study treatment, and all 3 patients in the 400 mg group, 2 patients with Grade 3 liver injury and 1 patient with Grade 3 rash papular, were judged to have DLT during the entire 12 weeks of the study treatment.
Table 2.
DLT evaluation.
| 100 mg (N = 3) | 200 mg (N = 3) | 300 mg (N = 3) | 400 mg (N = 3) | Total (N = 12) | |
|---|---|---|---|---|---|
| DLT during 4 weeks | 0 | 0 | 0 | 1 | 1 |
| DLT during 12 weeks | 0 | 0 | 0 | 3 | 3 |
AEs were evaluated as a secondary endpoint (Table 3). Their incidences of AEs in relation to the study treatment of each grade and of Grade 3 or higher are summarized in Table 4. All 13 patients experienced treatment-related AEs. The most common ones were diarrhea (9 of 13 patients [69.2%], 2 patients each in the 100 mg and 400 mg groups, 1 patient in the 200 mg group and 4 patients in the 300 mg group); constipation (3 of 13 patients [23.1%], 1 patient each in the 200 mg–400 mg groups); and liver disorder (3 of 13 patients [23.1%], 1 patient in the 200 mg group and 2 patients in the 300 mg group). Most of the AEs related to the study treatment were Grade 1 or 2 in severity. Grade 3 AEs related to the study treatment were liver injury (2 patients in the 400 mg group), increased alanine aminotransferase and aspartate aminotransferase (1 patient in the 100 mg group), and rash papular (1 patient in the 400 mg group). No Grade 4 or 5 AEs related to the study treatment were reported. A serious AE (SAE) of suicide was reported in 1 patient in the 300 mg group. This patient committed had presented anxiety before initiation of the clinical study, although this patient did not meet exclusion criteria in terms of psychiatric condition or active suicidal ideation, and it was clinically concluded that the event was not associated with the study drug, in conjunction with the results of the Safety Assessment Committee's evaluation. There were no clinically meaningful abnormalities in vital sign parameters, ECG findings, and chest X-ray findings related to the study treatment in any dose group in the study.
Table 3.
Summary of AEs.
| 100 mg (N = 3) | 200 mg (N = 3) | 300 mg (N = 4) | 400 mg (N = 3) | Total (N = 13) | |
|---|---|---|---|---|---|
| Number of AEs | 5 | 15 | 23 | 8 | 51 |
| Subject with AEs (%) | 3 (100.0) | 3 (100.0) | 4 (100.0) | 3 (100.0) | 13 (100.0) |
| AEs related to study drug (%) | 3 (100.0) | 3 (100.0) | 4 (100.0) | 3 (100.0) | 13 (100.0) |
| SAEs (%) | 0 (0.0) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 1 (7.7) |
| Maximum grade 3/4 AEs (%) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 3 (100.0) | 4 (30.8) |
Table 4.
Treatment-related AEs.
| MedDRA SOC and PT, n (%) | Total (N = 13) |
100 mg (N = 3) |
200 mg (N = 3) |
300 mg (N = 4) |
400 mg (N = 3) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Any grade | Grade 3/4 | Any grade | Grade 3/4 | Any grade | Grade 3/4 | Any grade | Grade 3/4 | Any grade | Grade 3/4 | |
| Any treatment-related AEs | 13 (100.0) | 4 (30.8) | 3 (100.0) | 1 (33.3) | 3 (100.0) | 0 (0.0) | 4 (100.0) | 0 (0.0) | 3 (100.0) | 3 (100.0) |
| Gastrointestinal | 12 (92.3) | 0 (0.0) | 2 (66.7) | 0 (0.0) | 3 (100.0) | 0 (0.0) | 4 (100.0) | 0 (0.0) | 3 (100.0) | 0 (0.0) |
| Diarrhea | 9 (69.2) | 0 (0.0) | 2 (66.7) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 4 (100.0) | 0 (0.0) | 2 (66.7) | 0 (0.0) |
| Constipation | 3 (23.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) |
| Abdominal pain upper | 2 (15.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (66.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Decreased appetite | 2 (15.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Liver function | 7 (53.8) | 3 (23.1) | 1 (33.3) | 1 (33.3) | 2 (66.7) | 0 (0.0) | 2 (50.0) | 0 (0.0) | 2 (66.7) | 2 (66.7) |
| Alanine aminotransferase increased | 2 (15.4) | 1 (7.7) | 1 (33.3) | 1 (33.3) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Aspartate aminotransferase increased | 2 (15.4) | 1 (7.7) | 1 (33.3) | 1 (33.3) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Liver disorder | 3 (23.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 2 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Liver injury | 2 (15.4) | 2 (15.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (66.7) | 2 (66.7) |
| Rash | 3 (23.1) | 1 (7.7) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 1 (33.3) | 1 (33.3) |
| Eczema | 1 (7.7) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Rash | 1 (7.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Erythema multiforme | 1 (7.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Rash papular | 1 (7.7) | 1 (7.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 1 (33.3) |
| Hematologic | 4 (30.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 3 (75.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Eosinophil count increased | 2 (15.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Anemia | 1 (7.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Eosinophilia | 2 (15.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Others | 3 (23.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (50.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) |
| Dizziness | 1 (7.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) |
| Hypokalaemia | 2 (15.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) |
| Bursitis | 1 (7.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Urine ketone body present | 1 (7.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Renal impairment | 1 (7.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
MedDRA: Medical Dictionary for Regulatory Activities, SOC: system organ class, PT: preferred term.
Among 13 patients enrolled in the study treatment period, the efficacy of bosutinib was evaluated in an exploratory manner among the 9 patients who completed 100 mg–300 mg of the study treatment, respectively. Change in total scores of ALSFRS-R during the observation and treatment periods was evaluated (Fig. 2A). Decrease from baseline in the total score of ALSFRS-R were smaller during the study treatment period than during the observation period in 2 patients each in the 100 mg and 300 mg groups, as well as in 1 patient in the 200 mg group; 5 of 9 patients were found to respond well to bosutinib, showing a change in ALSFRS-R score remained stable during 12 weeks of the treatment. The difference between change in the treatment period and change in the observation period, shown as Δ(change of ALSFRS-R), which indicates the alteration of disease progression rate, is presented in Fig. 2B.
Fig. 2.
Evaluation of exploratory efficacy of bosutinib. A. Change of ALSFRS-R of each patient in the observation and treatment periods. A patient with consistent progression shows a horizontal line. Patients whose progression slowed after treatment with bosutinib show a rise to the right. B. Differences in change of ALSFRS-R score. Δ(change of ALSFRS-R) represents the change in ALSFRS-R during the treatment period minus the change in ALSFRS-R during the observation period. Patients with a positive value indicate that the decrease in ALSFRS-R was suppressed during the treatment period compared with that of the observation period. C. Relevance of plasma NFL levels and Δ(change of ALSFRS-R). Patients with lower NFL levels indicated suppression of ALSFRS-R decline. D. Relevance of plasma pNFH levels and Δ(change of ALSFRS-R). E. Relevance of plasma NFL levels and change of ALSFRS-R during observation period. F. Least-squares mean in lower NFL group (NFL <100 pg/mL, N = 5) and higher NFL group (NFL ≥100 pg/mL, N = 4). Using a linear mixed-effects model with a repeated-measures approach, the change in ALSFRS-R score from baseline was analysed in the lower NFL group and the higher NFL group. Point estimates of group differences in change of ALSFRS-R at 6 month was 4.5 (95% confidence interval, 1.7 to 7.3). Error bars represent 95% confidence interval. ALSFRS-R, Revised Amyotrophic Lateral Sclerosis Functional Rating Scale; NFL, neurofilament light chain; pNFH, phosphorylated neurofilament heavy chain.
ALS severity remained stable during the study treatment period in 3 patients each in the 100 mg and 300 mg groups and in 1 patient in the 200 mg group. During the study treatment period, 2 of 9 patients showed no decrease in %FVC from baseline, and 5 of 9 patients showed no decreasing tendency in grip strength for both hands.
In a post-hoc analysis, we found that there was a difference in baseline plasma NFL levels between the positive Δ (change of ALSFRS-R) group which is defined as Δ (change of ALSFRS-R) >0 and the negative Δ (change of ALSFRS-R) group which is defined as Δ (change of ALSFRS-R) ≤0 in Fig. 2B. The positive Δ (change of ALSFRS-R), stable group with a low decrease in ALSFRS-R score in the treatment period, had lower plasma NFL levels in baseline (Fig. 2C). As for plasma pNFH levels, no difference was found between the two groups (Fig. 2D). The plasma NFL levels themselves were not related to the change of ALSFRS-R in the observation period (Fig. 2E). Furthermore, there was a difference in the decrease in ALSFRS-R score in the treatment period between the higher and lower plasma NFL groups after the start of treatment of bosutinib (Fig. 2F), suggesting that lower plasma NFL levels in baseline could stratify bosutinib-responsive patients, although it is required to be interpreted with caution due to the small sample size, as low baseline NFL levels are known to be associated with slower functional decline.17
As an exploratory analysis of pharmacodynamic effects of bosutinib, several biomarkers were measured in blood, CSF, and urine (Fig. 3A). At the end of the study treatment period, 6 of 9 patients showed a decrease from baseline in plasma NFL and pNFH levels (Fig. 3A). Several patients also showed a decrease in CSF NFL and pNFH levels (Fig. S1). Urine p75 levels, also measured as a biomarker reflecting neuronal damage,18 were decreased in 7 of 9 patients post-treatment. CH3L1, expressed in astrocytes and associated with inflammatory responses,19 was decreased in 6 of 9 patients in plasma, while changes in cytokines such as IL15, IL18, IL6, and monocyte chemotactic protein 1 (MCP1) were limited. An increase in serum albumin level, a marker reflecting suppression of inflammation, was observed in 7 of 9 patients. Increased creatinine/cystatin c, a biomarker reflecting muscle volume, was also found in 6 of 9 patients. Both creatinine and albumin are known to be reliable markers of the severity of the clinical status of patients with ALS.20 The plasma levels of p-c-Abl and p-Src, the targets of bosutinib, were measured by ELISA, and we confirmed that the ratio of plasma p-c-Abl levels before and after 12-week bosutinib treatment decreased in 7 out of 9 patients, and similarly, the ratio of plasma p-Src levels before and after the treatment decreased in 6 of 9 patients. We also investigated the relevance between Δ(change of ALSFRS-R) and p-Src or p-c-Abl, and found that there was a negative correlation trend between Δ(change of ALSFRS-R) and p-c-Abl in baseline (Fig. 3B). We also investigated the relevance between neurofilaments (NFL or p-NFH) and p-c-Abl or p-Src (Fig. S2), and found that there was a correlational trend between the plasma NFL levels and p-c-Abl or p-Src in baseline, which was not significant (Fig. S2), although it is required to be interpreted with caution, as the trend was largely driven by a single outlier. Plasma and CSF concentrations of bosutinib appeared to be dose-dependent at doses ranging from 100 mg to 300 mg (Fig. 3C).
Fig. 3.
Evaluation of biomarkers. A. Change of biomarkers. For each biomarker, the ratio of the value after the completion of 12 weeks of bosutinib treatment to before the start of bosutinib treatment is shown. B. Relevance between Δ(change of ALSFRS-R) and plasma p-Src/p-c-Abl at baseline. A correlation tendency between Δ(change of ALSFRS-R) and p-c-Abl was observed. C. Bosutinib levels in plasma and CSF. Mean plasma concentrations at 4, 8, and 12 weeks of bosutinib treatment and spinal fluid concentrations at 12 weeks of bosutinib treatment are shown. Bosutinib concentrations were dose-dependent in plasma and CSF. ALSFRS-R, Revised Amyotrophic Lateral Sclerosis Functional Rating Scale; CH3L1, Chitinase 3 Like 1; IL, interleukin; MCP1, monocyte chemotactic protein 1; p-Src, phosphorylated Src; p-c-Abl, phosphorylated c-Abl; OD, optical density; CSF, cerebrospinal fluid.
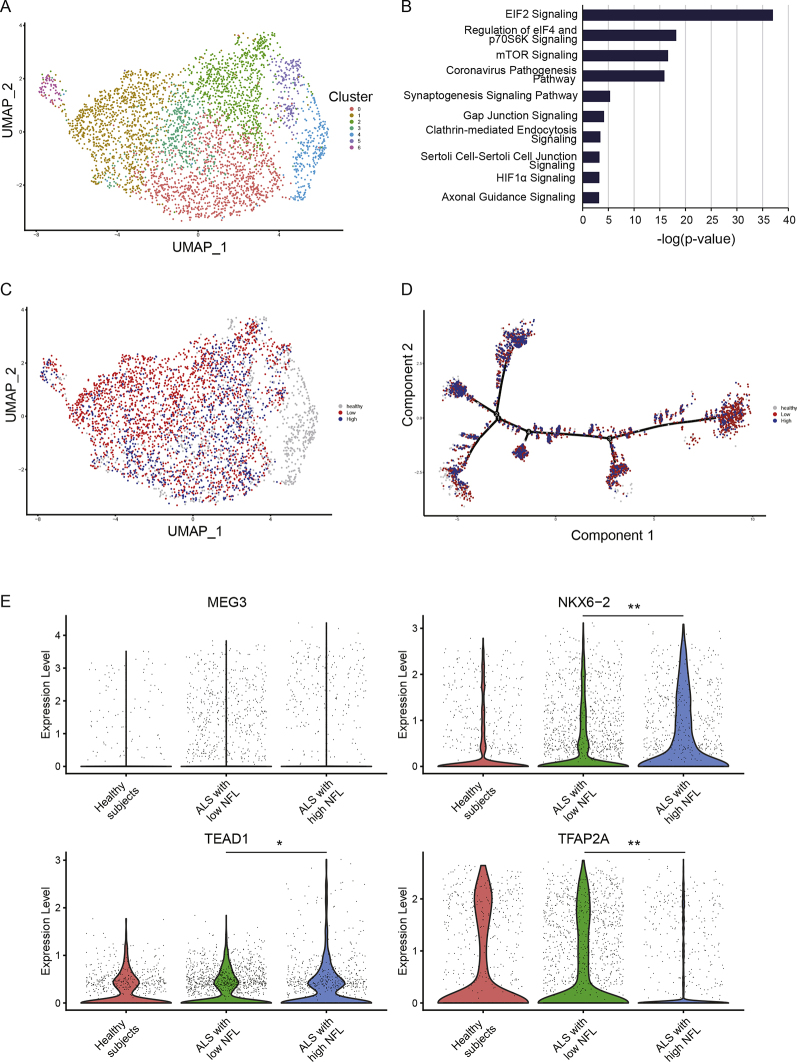
It has been reported that NFL in blood and CSF are increased in patients with ALS in association with their prognosis.21,22 NFL is considered to be released from damaged motor neurons, although the difference in disease background between patients with high and low NFL is unclear. To investigate the differences in motor neurons between patients with higher and lower NFL levels, iPSCs were generated from each patient and motor neurons were differentiated from the iPSCs. As controls, iPSCs generated from healthy individuals with no symptoms or family history of neurological disease were used.23 Gene expression analysis of the motor neurons was then conducted by single-cell RNA sequencing (Fig. S3). We confirmed that the motor neurons of patients with ALS exhibited molecular features consistent with the ALS pathogenesis (Fig. 4A and B). Then, we conducted a comparative analysis of gene expression levels in motor neurons of patients with plasma low and high plasma NFL levels, and found differences in gene expression patterns between the two groups (Fig. 4C and D). Focusing on genes that reflect differences in gene expression between healthy individuals and patients with ALS, we identified changes in the expression of four genes maternally expressed 3 (MEG3), NK6 Homeobox 2 (NKX6-2), TEA Domain Transcription Factor 1 (TEAD1), and transcription factor AP-2 alpha (TFAP2A) in motor neurons between patients with higher NFL levels and lower NFL levels (Fig. 4E).
Fig. 4.
Gene expression profiles of motor neurons based on differences in plasma NFL levels. A. Single-cell RNA sequencing of motor neurons derived from iPSCs. Motor neurons from 9 patients with ALS (N = 9) enrolled for 100 mg–300 mg of bosutinib and 6 healthy individuals (N = 6) were analysed. B. A canonical pathway shown by motor neurons in ALS. The top pathways identified by Ingenuity Pathway Analysis are shown. C. Distribution of motor neurons in patients with ALS with higher NFL levels (N = 4) and lower NFL levels (N = 5). D. Pseudotime analysis showed a cell transition trajectory to motor neurons in patients with ALS with higher NFL levels (N = 4) and lower NFL levels (N = 5). E. Among the gene expressions that differ between ALS and healthy individuals, the genes emphasizing the difference between the higher and lower NFL groups were extracted (N = 4, N = 5, respectively, ∗p = 0·0035, ∗∗p < 0·0001, Wilcoxon rank-sum test). iPSCs, induced pluripotent stem cells; NFL, neurofilament light chain; MEG3, maternally expressed 3; NKX6-2, NK6 homeobox2; TEAD1, TEA domain transcription factor 1; TFAP2A, transcription factor AP-2 alpha.
Discussion
This is the first study to use a Src/c-Abl inhibitor, bosutinib, for patients with ALS in a novel type of clinical trial design by combining the safety study with a 3 + 3 design in oncology and the efficacy study with an observation period in neurology.16 The general safety and tolerability of bosutinib in ALS was evaluated by 3 + 3 design, and the MTD and RP2D were determined to be 300 mg in this study. A subset of patients was found to respond well to bosutinib by appearing to maintain clinical stability during 12 weeks, and the treatment-responsive patients could be distinguished by plasma NFL levels, which may be a potential predictor of drug efficacy. Additionally, we identified gene expression profiles of ALS motor neurons underlying the differences in patient plasma NFL levels.
Recently, a number of ALS-causing genes, some of which encode kinases, have been identified, and it is suggested that the phosphorylation process may be associated with motor neuron degeneration.24 The efficacy of several kinase inhibitors, such as masitinib25 and dasatinib,26 in improving the pathophysiology of ALS has been reported. We previously reported that a compound screening based on a survival assay of iPSC-derived motor neurons from patients with ALS identified Src/c-Abl as a major pathway related to motor neuron degeneration.11 In addition to in vitro efficacy, Src/c-Abl inhibitor bosutinib also attenuated ALS phenotypes in an ALS mouse model with mutant SOD1.11 Although bosutinib has also shown efficacy in ALS patient iPSCs with TDP-43 mutations11 and in mutant TDP-43 mouse models,27 due to the complexity of the pathogenesis of sporadic ALS, this study set the inclusion criteria to focus specifically on the efficacy in ALS with SOD1 mutations. In this clinical trial, two patients with ALS with SOD1 mutations participated; one patient with mutant SOD1 (L126S) received 100 mg of bosutinib and presented stable ALSFRS-R, and the other patient with mutant SOD1 (L126S) received 400 mg of bosutinib and showed discontinuance based on the protocol regarding AEs.
In CML, gastrointestinal AEs (mainly diarrhea), hepatic dysfunction, hematologic AEs (mainly thrombocytopenia) and skin rash have been reported as frequent adverse events in patients treated with bosutinib. However, such adverse events were mostly manageable by supportive care or dose modifications. While patients with ALS receiving bosutinib experienced AEs with findings consistent with bosutinib's known profile, no specific safety profiles or concerns in patients with ALS were observed in this study, and adverse events observed in ALS were also manageable by supportive care or dose modifications. In ALS, unlike CML,12,13 no hematopoietic adverse events were presented. This could be due to differences in the target diseases.
In this study, one of the patients committed suicide. Although this incident was determined to be unrelated to the administration of bosutinib, it has been reported that the risk of depression and suicide is generally higher in patients with ALS than in healthy individuals.28 Thus, it is necessary to carefully assess whether the study drug may affect the psychological state of patients with ALS.
This study presents a novel type of protocol design by combining the safety study of oncology using the 3 + 3 dose escalation study and the efficacy study of neurology with a 3-month observation period for uniform subject inclusion.16 According to the 3 + 3 dose escalation, study drugs were increased up to doses higher than those estimated to be appropriate for ALS, with drop-outs due to AEs at higher doses, with the percentage of dropouts therefore appearing higher. We concluded that 100 mg–300 mg may be safe doses for ALS in this study.
Biomarkers used in drug development include genes along with gene mutations and repeat elongation, liquid biomarkers in blood and CSF, pathology of affected tissues, imaging studies, and digital biomarkers from movement and speech. Among these, liquid biomarkers in blood are the most useful due to their low invasiveness. These liquid biomarkers are expected to be used for risk/susceptibility biomarker, diagnostic biomarker, prognostic/progression biomarker, pharmacodynamic/response biomarker, predictive biomarker, safety biomarker, and surrogate biomarker.29,30 Biomarkers in ALS research are categorized as being neuronal cell death-related, including NFL and pNFH analysed in the present study, neuroinflammation-related, such as IL-6, and misfolded proteins, such as SOD1 and TDP-43. NFL and pNFH have been reported in previous studies to be prognostic/progression biomarkers predicting slow and fast progression groups of patients with sporadic ALS. In this study, clinical progression rates based on ALSFRS-R scores were classified into groups by plasma NFL levels, with the finding that NFL levels may be a predictive biomarker for the efficacy of study drugs in each patient. The results indicated two possibilities: 1) the groups with clinically similar disease severity may have different degrees of disease in situ progression, and the efficacy of the study drug may differ accordingly; 2) there may be a type of ALS with high or low NFL, and the efficacy of the study drug may differ accordingly. In addition, plasma NFL levels tended to decrease after treatment with the study drug, suggesting that it could be used as a surrogate marker. Segmenting the clinical diversity of ALS could add depth to the pipeline of future ALS therapies, potentially change the design of clinical trials, and be critical information for regulatory decisions.
Recently, several promising clinical trials have been conducted for ALS.25,31 However, the clinical course of ALS is heterogeneous due to disease-relevant factors including genetic background and epigenetic modification,32, 33, 34 and they are still largely unknown, making it difficult to evaluate the efficacy of study drugs in clinical trials. In the present study, post-hoc analysis showed that in patients with low NFL levels, motor function ceased to decline during the 3 months of bosutinib treatment. Biomarkers are urgently needed for optimisation of patient stratification for clinical trials,35,36 and some studies have explored the use of NFL as a biomarker to evaluate the effects of study drugs in clinical trials.5 We are considering the potential of plasma NFL levels being used to stratify patients with ALS for efficacy evaluation of study drugs.
NFL is considered to be released from damaged motor neurons. However, the difference in disease background between patients with high and low NFL levels is still unclear. The single-cell level signature of motor neurons produced by reprogramming patient blood cells suggested that ALS could be classified into high and low blood NFL types. Importantly, MEG3, TEAD1, NKX6-2, and TFAP2A were noted among the gene groups that changed in the order of healthy, low NLF, and high NLF groups, suggesting that the ALS pathology was well captured. These genes were previously reported to be related to the ALS pathogenesis; MEG3 is a long non-coding RNA presenting the upregulation in ALS and is related to the binding of TDP-43,37,38 TEAD1 is a transcription factor associated with the hippo pathway involving in motor neuron apoptosis,39 NKX6-2 is increased in motor neurons in the postmortem spinal cord of patients with ALS, and TFAP2A contains a SNP associated with ALS.40 The expression of these genes may contribute to the molecular background of patients stratified by NFL. Interestingly, some of them reflected the genetic signature of ALS as an abnormality of RNA metabolism, as was already revealed by previous ALS studies. The genes and pathways that distinguish these high and low groups may be the target molecules that enhance the efficacy of this study drug, as well as the ALS molecular pathophysiology underlying the NFL levels. Since they are exploratory findings in a small number of cases, further accumulation of case and laboratory data is needed.
The limitations of this study are that it is an open-label trial without placebo and that the results were obtained from only a small number of Japanese patients with limited symptoms. Furthermore, a linear mixed-effects model with a repeated-measures approach to investigate the efficacy of bosutinib was based on post-hoc analysis. It is difficult to determine the efficacy of the study drug in a short period with the small sample size of this study due to the heterogeneity of disease progression in patients with ALS. Thus, the interpretation of efficacy found in this study should be handled carefully, and further clinical trials will be required.
This was a phase 1 study with detailed examination of each patient. We succeeded in investigating the safety profiles of bosutinib in patients with ALS, and also suggesting its responsiveness and potential biomarker strategy in ALS.
Contributors
Conception and design of the study; H.I. Design of the clinical trial; H.I., K.I., Y.I., A.M., T.H., Y.F. Design of the clinical research; H.I., K.I. Acquisition of data in clinical trial; Y.I., M.N., Y.W., N.E., T.A., R.O., K.F., R.T. Statistical analysis in clinical trial; R.U., S.M. Acquisition of data in clinical research; H.I., K.I., T.Y., H.T., T.T., N.T. Statistical analysis in clinical research; K.I., T.Y. Scientific and critical discussion; H.I., K.I., Y.I., K.F., R.O., A.M., T.H., Y.F., K.N., R.H., R.T. Drafting the manuscript and preparing the figures; H.I., K.I., Y.I., K.F., R.O., A.M., T.H., Y.F. K.I. and H.I. had accessed and verified the underlying data.
Data sharing statement
The data generated in this study, including English translation of the study protocol, and the statistical analysis plan will be available as de-identified data on ClinicalTrials.gov (NCT04744532). Requests for clinical data should be emailed to the corresponding author and should include a brief description of the proposed analysis. Requests for data access will be reviewed individually, and a decision will be communicated. Data might be shared in the form of aggregate data summaries and via a data transfer agreement. Regarding additional clinical research such as iPSC research, data that support the findings of this study are available from the corresponding author upon reasonable request.
Declaration of interests
RU reports consulting fees from Eisai, Sawai Pharmaceutical, and EP Croit. NT reports speakers bureaus fees from Pfizer, Novartis Pharmaceuticals, and Otsuka Pharmaceutical. RT reports consulting fees from Kan Institute. RT and NT received research funding and honoraria for lectures from Pfizer. The other authors declare that they have no competing interests.
Acknowledgements
This research was supported by AMED under Grant Number JP18lk0201083h0003 to H.I., by iPS Cell Research Fund to H.I., and by Research Grand for Stem Cell Medicine to H.I. The study drug bosutinib and the pharmacokinetic analysis for only patient-experienced SAEs are supported by Pfizer JAPAN INC. under the Clinical Research Collaboration Agreement. We appreciate patients and families for their willingness to participate in the study. The clinical trial part of this study was conducted in collaboration with Pfizer JAPAN INC., which provided the study drug without funding. We appreciate Lucie I Bruijn for critical discussion of protocol design. We would like to express our sincere gratitude to all of our co-workers and collaborators: Kayoko Tsukita, Takako Enami, Ayako Nagahashi, Ikuyo Inoue, Mio Kabata for the generation and analysis of iPSCs, Mikie Iijima, Nozomi Kawabata, Chiho Ishida, Tomomi Urai, and Miho Nagata for their valuable administrative support, and Riko Tabuchi for the publication arrangement.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2022.101707.
Appendix A. Supplementary data
References
- 1.Maurel C., Dangoumau A., Marouillat S., et al. Causative genes in amyotrophic lateral sclerosis and protein degradation pathways: a link to neurodegeneration. Mol Neurobiol. 2018;55(8):6480–6499. doi: 10.1007/s12035-017-0856-0. [DOI] [PubMed] [Google Scholar]
- 2.Bensimon G., Lacomblez L., Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 3.Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(7):505–512. doi: 10.1016/S1474-4422(17)30115-1. [DOI] [PubMed] [Google Scholar]
- 4.Witzel S., Maier A., Steinbach R., et al. Safety and effectiveness of long-term intravenous administration of edaravone for treatment of patients with amyotrophic lateral sclerosis. JAMA Neurol. 2022;79(2):121–130. doi: 10.1001/jamaneurol.2021.4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller T., Cudkowicz M., Shaw P.J., et al. Phase 1-2 trial of antisense oligonucleotide tofersen for SOD1 ALS. N Engl J Med. 2020;383(2):109–119. doi: 10.1056/NEJMoa2003715. [DOI] [PubMed] [Google Scholar]
- 6.Tran H., Moazami M.P., Yang H., et al. Suppression of mutant C9orf72 expression by a potent mixed backbone antisense oligonucleotide. Nat Med. 2022;28(1):117–124. doi: 10.1038/s41591-021-01557-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wainger B.J., Kiskinis E., Mellin C., et al. Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient-derived motor neurons. Cell Rep. 2014;7(1):1–11. doi: 10.1016/j.celrep.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korecka J.A., Talbot S., Osborn T.M., et al. Neurite collapse and altered ER Ca(2+) control in human Parkinson disease patient iPSC-derived neurons with LRRK2 G2019S mutation. Stem Cell Rep. 2019;12(1):29–41. doi: 10.1016/j.stemcr.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vadodaria K.C., Mendes A.P.D., Mei A., et al. Altered neuronal support and inflammatory response in bipolar disorder patient-derived astrocytes. Stem Cell Rep. 2021;16(4):825–835. doi: 10.1016/j.stemcr.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okano H., Morimoto S. iPSC-based disease modeling and drug discovery in cardinal neurodegenerative disorders. Cell Stem Cell. 2022;29(2):189–208. doi: 10.1016/j.stem.2022.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Imamura K., Izumi Y., Watanabe A., et al. The Src/c-Abl pathway is a potential therapeutic target in amyotrophic lateral sclerosis. Sci Transl Med. 2017;9(391) doi: 10.1126/scitranslmed.aaf3962. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi N., Cortes J.E., Sakaida E., et al. Safety profile of bosutinib in Japanese versus non-Japanese patients with chronic myeloid leukemia: a pooled analysis. Int J Hematol. 2022;115(6):838–851. doi: 10.1007/s12185-022-03314-y. [DOI] [PubMed] [Google Scholar]
- 13.Hino M., Matsumura I., Fujisawa S., et al. Phase 2 study of bosutinib in Japanese patients with newly diagnosed chronic phase chronic myeloid leukemia. Int J Hematol. 2020;112(1):24–32. doi: 10.1007/s12185-020-02878-x. [DOI] [PubMed] [Google Scholar]
- 14.Toepfer M., Folwaczny C., Klauser A., Riepl R.L., Müller-Felber W., Pongratz D. Gastrointestinal dysfunction in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 1999;1(1):15–19. doi: 10.1080/146608299300079484. [DOI] [PubMed] [Google Scholar]
- 15.Costa J., Swash M., de Carvalho M. Awaji criteria for the diagnosis of amyotrophic lateral sclerosis: a systematic review. Arch Neurol. 2012;69(11):1410–1416. doi: 10.1001/archneurol.2012.254. [DOI] [PubMed] [Google Scholar]
- 16.Imamura K., Izumi Y., Banno H., et al. Induced pluripotent stem cell-based drug repurposing for amyotrophic lateral sclerosis medicine (iDReAM) study: protocol for a phase I dose escalation study of bosutinib for amyotrophic lateral sclerosis patients. BMJ Open. 2019;9(12) doi: 10.1136/bmjopen-2019-033131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Q., Zhao X., Li S., et al. CSF neurofilament light chain elevation predicts ALS severity and progression. Front Neurol. 2020;11:919. doi: 10.3389/fneur.2020.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shepheard S.R., Wuu J., Cardoso M., et al. Urinary p75(ECD): a prognostic, disease progression, and pharmacodynamic biomarker in ALS. Neurology. 2017;88(12):1137–1143. doi: 10.1212/WNL.0000000000003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vu L., An J., Kovalik T., Gendron T., Petrucelli L., Bowser R. Cross-sectional and longitudinal measures of chitinase proteins in amyotrophic lateral sclerosis and expression of CHI3L1 in activated astrocytes. J Neurol Neurosurg Psychiatry. 2020;91(4):350–358. doi: 10.1136/jnnp-2019-321916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiò A., Calvo A., Bovio G., et al. Amyotrophic lateral sclerosis outcome measures and the role of albumin and creatinine: a population-based study. JAMA Neurol. 2014;71(9):1134–1142. doi: 10.1001/jamaneurol.2014.1129. [DOI] [PubMed] [Google Scholar]
- 21.Lu C.H., Macdonald-Wallis C., Gray E., et al. Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology. 2015;84(22):2247–2257. doi: 10.1212/WNL.0000000000001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y.N., Chen Y.H., Dong S.Q., et al. Role of blood neurofilaments in the prognosis of amyotrophic lateral sclerosis: a meta-analysis. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.712245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imamura K., Yada Y., Izumi Y., et al. Prediction model of amyotrophic lateral sclerosis by deep learning with patient induced pluripotent stem cells. Ann Neurol. 2021;89(6):1226–1233. doi: 10.1002/ana.26047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo W., Vandoorne T., Steyaert J., Staats K.A., Van Den Bosch L. The multifaceted role of kinases in amyotrophic lateral sclerosis: genetic, pathological and therapeutic implications. Brain. 2020;143(6):1651–1673. doi: 10.1093/brain/awaa022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mora J.S., Genge A., Chio A., et al. Masitinib as an add-on therapy to riluzole in patients with amyotrophic lateral sclerosis: a randomized clinical trial. Amyotroph Lateral Scler Frontotemporal Degener. 2020;21(1-2):5–14. doi: 10.1080/21678421.2019.1632346. [DOI] [PubMed] [Google Scholar]
- 26.Katsumata R., Ishigaki S., Katsuno M., et al. c-Abl inhibition delays motor neuron degeneration in the G93A mouse, an animal model of amyotrophic lateral sclerosis. PLoS One. 2012;7(9) doi: 10.1371/journal.pone.0046185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wenqiang C., Lonskaya I., Hebron M.L., et al. Parkin-mediated reduction of nuclear and soluble TDP-43 reverses behavioral decline in symptomatic mice. Hum Mol Genet. 2014;23(18):4960–4969. doi: 10.1093/hmg/ddu211. [DOI] [PubMed] [Google Scholar]
- 28.Fang F., Valdimarsdóttir U., Fürst C.J., et al. Suicide among patients with amyotrophic lateral sclerosis. Brain. 2008;131(Pt 10):2729–2733. doi: 10.1093/brain/awn161. [DOI] [PubMed] [Google Scholar]
- 29.Vu L.T., Bowser R. Fluid-based biomarkers for amyotrophic lateral sclerosis. Neurotherapeutics. 2017;14(1):119–134. doi: 10.1007/s13311-016-0503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrews J.A., Bruijn L.I., Shefner J.M. ALS drug development guidances and trial guidelines: consensus and opportunities for alignment. Neurology. 2019;93(2):66–71. doi: 10.1212/WNL.0000000000007695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paganoni S., Macklin E.A., Hendrix S., et al. Trial of sodium phenylbutyrate-taurursodiol for amyotrophic lateral sclerosis. N Engl J Med. 2020;383(10):919–930. doi: 10.1056/NEJMoa1916945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baxi E.G., Thompson T., Li J., et al. Answer ALS, a large-scale resource for sporadic and familial ALS combining clinical and multi-omics data from induced pluripotent cell lines. Nat Neurosci. 2022;25(2):226–237. doi: 10.1038/s41593-021-01006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watanabe H., Atsuta N., Hirakawa A., et al. A rapid functional decline type of amyotrophic lateral sclerosis is linked to low expression of TTN. J Neurol Neurosurg Psychiatry. 2016;87(8):851–858. doi: 10.1136/jnnp-2015-311541. [DOI] [PubMed] [Google Scholar]
- 34.Faghri F., Brunn F., Dadu A., et al. Identifying and predicting amyotrophic lateral sclerosis clinical subgroups: a population-based machine-learning study. Lancet Digit Health. 2022;4(5):e359–e369. doi: 10.1016/S2589-7500(21)00274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petri S. Major research advances in amyotrophic lateral sclerosis in 2021. Lancet Neurol. 2022;21(1):14–15. doi: 10.1016/S1474-4422(21)00420-8. [DOI] [PubMed] [Google Scholar]
- 36.Thompson A.G., Gray E., Verber N., et al. Multicentre appraisal of amyotrophic lateral sclerosis biofluid biomarkers shows primacy of blood neurofilament light chain. Brain Commun. 2022;4(1):fcac029. doi: 10.1093/braincomms/fcac029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tollervey J.R., Curk T., Rogelj B., et al. Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nat Neurosci. 2011;14(4):452–458. doi: 10.1038/nn.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen K.W., Chen J.A. Functional roles of long non-coding RNAs in motor neuron development and disease. J Biomed Sci. 2020;27(1):38. doi: 10.1186/s12929-020-00628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto I., Azuma Y., Yamaguchi M. Cancer-related genes and ALS. Front Biosci (Landmark Ed) 2019;24:1241–1258. doi: 10.2741/4777. [DOI] [PubMed] [Google Scholar]
- 40.Xie T., Deng L., Mei P., et al. Genome-wide association study combining pathway analysis for typical sporadic amyotrophic lateral sclerosis in Chinese Han populations. Neurobiol Aging. 2014;35(7):1778.e9–1778.e23. doi: 10.1016/j.neurobiolaging.2014.01.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.