Abstract
Graves’ orbitopathy (GO) is a potentially sight-threatening and disfiguring, extrathyroidal manifestation of Graves’ disease. It often impairs patients’ quality of life, causing severe social and psychological sequelae. Intravenous glucocorticosteroids is currently the mainstay of therapy, but the efficacy is often underwhelming and recurrence rate is high. For many years, clinicians have been searching for new methods of treatment. Rituximab (RTX) is a chimeric monoclonal antibody targeted against CD20 which is a surface antigen present on B cells. It is frequently used to treat non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, or various types of vasculitis. Numerous clinical trials employing RTX in the treatment of GO have shown promising results. RTX is currently considered to be a valid second-line treatment option in patients unresponsive to previous interventions or in disease reactivation. This review summarizes the available literature on this topic, including two largest, randomized, controlled studies. Potential benefits, as well as the limitations of RTX therapy, are discussed.
Key Words: Graves’ orbitopathy, rituximab, thyroid eye disease, Graves’ disease
Epidemiology, clinical presentation and pathophysiology
Graves’ orbitopathy (GO), otherwise referred to as Graves’ eye disease or thyroid eye disease, is an autoimmune, inflammatory disorder of the retroocular tissue occurring in patients with autoimmune thyroid disease (ATD). It is mainly associated with Graves’ disease (GD) but may also be seen in patients with chronic lymphocytic thyroiditis. Most commonly, it accompanies hyperthyroidism, but it may also occur in euthyroid patients, preceding the diagnosis of ATD by months or even years. The onset of GO may also be observed many years after the diagnosis of ATD (1). Recent research suggests that as many as 70% of patients with GD have evidence of GO in MRI (2). The incidence of clinically relevant cases was 16 per 100,000 in females and 2.9 per 100,000 in males (2, 3, 4). The symptoms of GO include excessive tearing, foreign body sensation, photophobia, and/or pain in the orbit, either resting or gaze evoked. Diplopia, blurring, and desaturation of colors may also be present. Patients commonly present with swelling or redness of upper or lower eyelids and conjunctivae or proptosis. It is not rare that these symptoms are asymmetrical or even unilateral (5). Severity of the symptoms determines the prognosis and the preferred management. The natural course of the disease, described by Rundle et al., consists of an active phase when the inflammatory process is progressing lasting usually up to 24 months, a plateau phase when the inflammation is ongoing but not progressive, and lastly an inactive phase (6).
There are several known risk factors of severe course of the disease, including male sex, smoking, thyroid dysfunction, treatment with radioiodine, high thyroid-stimulating hormone (TSH)-receptor antibodies (TSHR Ab, TRAb) levels, oxidative stress, or hypercholesterolemia. Several genetic factors were also investigated, but the results are inconclusive up to date (7).
In GO, the inflammation in the orbit is believed to occur mainly due to stimulating influence of TRAb secreted by B-cells infiltrating the thyroid gland. In consequence, the fibroblasts of the periorbital tissue are stimulated to produce extracellular matrix components and proinflammatory cytokines such as interleukin-6 (IL6), IL12, or IL17 (8, 9). Specific chemokines that are also related to the pathogenesis of this disease include C-X-C motif chemokine ligand 9 (CXCL9), CXCL10, CXCL11, and their receptor chemokine receptor CXCR3. Those cytokines act by combined interferon gamma (IFNG)+ tumor necrosis factor alpha stimulation of thyroid follicular cells and take part in the T-helper 1 (Th1)-mediated immune response (10, 11). Increased serum Th1 chemokines have been linked with the active phase of GO, especially during early stages of the disease, as well as hyperthyroidism in GD but not in toxic nodular goiter (10, 12, 13, 14). It was reported that treatment with methimazole decreases the concentration of CXCL10 and that decrease was linked with a reduction of TRAb titers (13, 15).
Current management
For a very long time, the mainstay of GO treatment has been the administration of i.v. glucocorticosteroids (ivGCS), which was frequently followed by orbital decompression or squint/eyelid rehabilitative surgery. Only recently, the European Group on Graves’ Orbitopathy (EUGOGO), in its updated guidelines, proposed that mycophenolate be added to ivGCs as a first-line treatment in active, moderate-to-severe GO, considering its beneficial effect on long-term outcome and less frequent recurrences which has been shown in two large, randomized studies (16, 17, 18). Considering the complex pathogenesis of the disorder involving many molecular pathways, a number of agents are proposed as a second-line treatment in the event of poor response to ivGCs or disease relapse. Among the potential drugs are antiproliferative agents such as azathioprine and cyclosporine, biological molecules that target different pathogenetic pathways, for example, tocilizumab – a humanized monoclonal antibody against the interleukin-6 receptor (IL6R) or rituximab (RTX), a chimeric monoclonal antibody targeted against CD20 which is a surface antigen present on B cells (16). Lately, it has been suggested that cross-talk between TSHR activated by TRAbs and insulin-like growth factor 1 receptor (IGF1R), which is overexpressed in the orbital connective tissue, plays a great role in the pathogenesis of this process (19, 20). A novel therapeutic agent under investigation is teprotumumab, which is a monoclonal, blocking antibody against IGF1R. Recent studies suggest that this agent is an effective and safe medication in the treatment of active, moderate-to-severe GO (21, 22, 23).
Lastly, a second course of ivGC treatment or teleradiotherapy in combination with i.v. or oral steroids may be of benefit in selected patients as a second line of therapy. Nevertheless, studies provide only limited evidence to support any of the mentioned drugs.
Purpose of this review
In this review, we would like to focus on RTX, a molecule that is of particular interest among clinicians and investigators. RTX is a chimeric, monoclonal murine human antibody targeted against CD20 antigen expressed on the surface of B-lymphocytes in different stages of maturation (24). RTX causes the depletion of B-lymphocytes, which happens via different mechanisms, including antibody-dependent cytotoxicity, complement cytotoxicity, regulation of intracellular calcium, and apoptosis (25). Despite its main use in CD20+ B-cell lymphomas, over the years the evolving understanding of immunology led to its usage in different autoimmune diseases, for example, lupus erythematosus or rheumatoid arthritis (26, 27).
Rituximab in Graves’ disease
The use of RTX in GD was first proposed by El Fassi et al. in 2006 (28). In 2007, they published the results of a pilot study assessing the efficacy of RTX in GD. Patients were treated for GD with antithyroid drug (AT) alone (RTX–) or in combination with RTX (RTX+). Four out of ten RTX+ patients but only one patient out of ten in RTX– remained euthyroid at 12 months. The RTX+ group presented with fewer relapses compared to the RTX– group in the follow-up period (29).
In another study, 13 patients with recurrent GD were treated with RTX (2 doses of 1000 mg each). Nine of them achieved euthyroid state and were still in remission after concluding the 27-month observation period (30). Lately, a second-phase prospective study of RTX in individuals ages 12–20 with newly diagnosed GD was conducted. Twenty-seven patients received a single dose of 500 mg of RTX and were concurrently treated with titrated doses of ATs for 12 months. After 24 months of observation, almost 50% (13 of 27) patients remained in remission. This suggests that RTX might affect the natural course of disease, and as expected the remission rate was between 20% and 30% (31). To our knowledge, up to date, there were no controlled, randomized studies investigating this subject.
The concept of rituximab in Graves’ orbitopathy
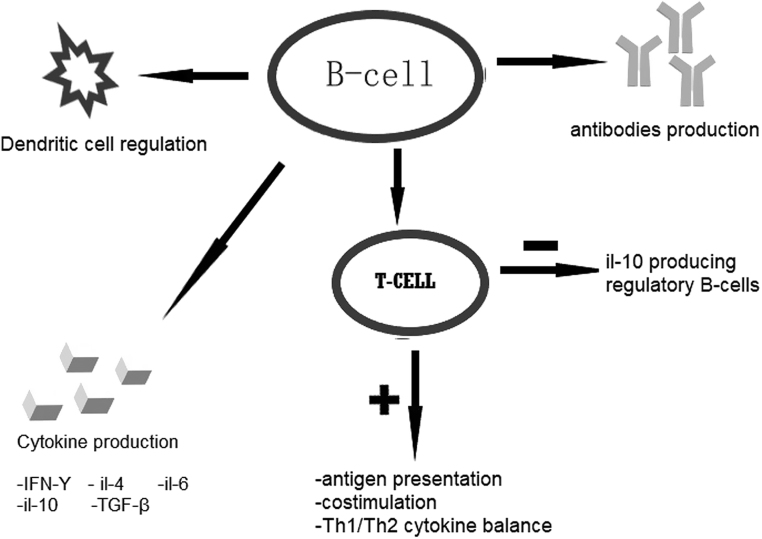
In 2003, Hasselbalch in his letter proposed the possibility of utilizing this agent in the treatment of active, moderate-to-severe GO based on the rising evidence of its efficacy in different autoimmune diseases and potential role of B-cells in their pathogenesis (32). Later in 2006, Bartalena and Tanda argue that among many different novel agents, RTX might be an interesting molecule that is worthy of further investigation (33). They quote two reported cases of successful treatment of GO with RTX (34). In 2013, Salvi et al. conducted a review in order to assess the potential utility of RTX for GO (35). The authors in their analysis point to the role of B-cells in the pathogenesis of thyroid autoimmune disease, especially B-cells’ ability to activate CD4+ T-cells and their regulatory function on human immune response through regulation of cytokines production (e.g. IL10, IL4, IL6 lymphotoxin-α, transforming growth factor-β, and IFN-γ) (Fig. 1) (for detailed information, see Supplementary Figure 1, see section on supplementary materials given at the end of this article). They also explain that the maintained levels of TRAb or thyroid-stimulating antibodies (TSAb) after treatment is due to the fact that CD20 antigen is not expressed on the long-lived antibody-producing plasma cells residing in the bone marrow.
Figure 1.
Actions of B-cells. IFN-γ, interferon gamma; IL-4, interleukin 4; IL-6, interleukin-6; IL-10, interleukin-10; TGF-β, transforming growth factor-beta; Th1/Th2, T-helper 1/T-helper 2.
Later, the authors analyze all the available reported studies of patients treated for GO with RTX. Gathered data consist of 43 patients across eight different small, uncontrolled studies between the years of 2006 and 2013. Nearly all the studies utilized 1 g dose of RTX administered twice in a 2-week interval; however, few studies differed from this pattern employing smaller doses (500 mg × 2 in a 2-week interval or a single dose of 100 mg). The results, although the authors acknowledge the limitation of the data, were very promising. The mean clinical activity score (CAS) was reduced from 4.9 at baseline to 2.2 points at 16 weeks after therapy. Ninety-one percent of patients achieved clinical improvement. Most studies also report improvement in Hertel exophtalmometry, visual acuity, and eye muscle motility. In a few patients, progression to dysthyroid optic neuropathy (DON) was observed, but it is argued that this attributes to the natural course of the disease, representing rather failure to treat than the unfavorable action of the drug itself. Side effects reported affected 13% of patients treated, but most of the adverse effects were minor and transient; one death due to cardiac incident was reported as unrelated to treatment. During the observation period, GO relapsed in only one patient.
Effect on TRAb and the possible mechanisms of action
Research carried out in the following years provided new insight into our current understanding of GO. There are numerous evidence that TSHR overexpressed in the periorbital tissue of patients with GO plays an important role in the pathogenesis of the disease (36, 37). There is evidence for correlation between TRAb titers and clinical activity, as well as severity of the disease, although not all studies confirm that (38). Karasek et al. observed a significant reduction in TRAb titers after treatment with RTX, but this change was not reflected in clinical activity and severity (39). There are numerous studies showing a reduction in TRAb titers after the infusion of RTX (39, 40, 41, 42). However, data on this subject are conflicting, as there are also studies showing no reduction in TRAb concentration after such treatment (29, 43, 44, 45). Later, El Fassi and colleagues demonstrated that the stimulatory capacity of TRAbs was reduced markedly (by 66 ± 22%), upon treatment with RTX and methimazole for 21 days, compared to an increase by 33% on average (not statistically significant) in patients receiving methimazole alone. The overall levels of TRAbs decreased by around 15% in both groups (40). This revelation was not confirmed by the study conducted by Vannucchi et al. The authors studied the TSHR-stimulating antibody (TSAb) subpopulation in patients treated with RTX and reported no significant decrease in correlation to TRAb titers (45). A recent meta-analysis of 152 patients across 12 published studies treated with RTX for GO showed no effect on TRAb titers in 1-month observation; however there was a significant decline after 6 and 12 months (46). Considering inconsistent data on the fluctuation of TRAbs and their correlation to the clinical activity and disease severity, it might be reasonable to assume that the changes in this parameter rather reflect the natural course of the disease and the treatment with RTX does not affect the production of those specific antibodies.
It was suggested that beneficial effects of RTX might occur due to inhibition of certain cytokines, mainly IL6 or its soluble receptor, which acts as an IL6R agonist. Those molecules have been found to be elevated in the serum of patients with active GO independently of the thyroid function or treatment, but after depletion of B-cells there was no significant reduction in IL6 or IL6R concentrations despite clinical improvement of the patients (47). In patients treated with GCS, there was no significant change in those mediators as well (45). The studies mentioned suggest that the beneficial effect of RTX on inflammatory process in the orbits is not mediated by changes in humoral immunity, in particular proinflammatory cytokines.
It is believed that the B-cells’ ability for antigen presentation and activation of helper CD4+ T cells plays a crucial role in the pathogenesis of GO, but our understanding of this pathway is still incomplete (35).
Clinical trials
There are several trials of RTX in the treatment of GO. Their characteristics are presented in Table 1 (for detailed information, see Supplementary Table 1).
Table 1.
Characteristics of recent studies involving RTX in the treatment of GO.
| Author | Year | Trial design | Drug | Dose | Number of patients | TRAb | CAS | CAS at 24 weeks | DON | Disease inactivation (at week 24) | Improvement in composite ophthalmic index (at week 24) | Recurrence | SAE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salvi | 2015 | Randomized and prospective | RTX | 2 × 1000 mg | 15 | 10.7 ± 9.1 | 4.4 ± 0.7 | 0.6 ± 3 | 0 | 100% | 9/15 (60%) improved | 0 | 2 |
| ivGCS | 7.5 g cumulative | 16 | 18.2 ± 21.7 | 4.7 ± 0.7 | 2.3 ± 0.5 | 1 | 68.70% | 6/16 (37.5%) improved | June 16 | 3 | |||
| Stan | 2015 | Randomized, prospective, and double-blinded | RTX | 2 × 1000 mg | 13 | 20 (9–60) | 4.9 (1.0) | 3.7 (1.9) | 2 | 4/13 at week 24 | No significant difference between groups in disease severity | No data | 5 moderate/severe |
| Placebo | n.a. | 12 | 19.5 (2.2–28.8) | 5.3 (1.0) | 3.8 (1.4) | 0 | 2/12 at week 24 | No data | 1 moderate/severe | ||||
| Karasek | 2017 | Prospective | RTX | 1 × 100 mg | 10 | 5.7 (1.1–41.0) to 1.6 (1.0–6.9) | 3.6 ± 0.9 | 0.8 ± 0.4 at month 6 | 2 | 100% | Not reported | October 1 | 0 |
| Eid | 2019 | Retrospective | RTX | 2 × 1000 mg | 15 | 4 | 3.0 (2.0–3.0) | 0 | 50% | Not reported | 5/12 (41.7%) | 0 | |
| Deltour | 2020 | Retrospective | RTX | 2 × 1000 mg | 32 | 63% reduction | 3.29 ± 1.16 | 1.59 ± 1.12 at week 24 | 0 | 20/31 | Not reported | No data | 1 (cytokine release syndrome) |
| Bennedjai | 2020 | Retrospective | Tocilizumab | 8 mg/kg on weeks 0, 4, 8, and 12 | 7 | 11 ± 4 | 5 ± 0.5 | 1.2 ± 0.9 at week 24 | 0 | July 7 | Not reported | July 1 | 0 |
| RTX | 2 × 1000 mg | 14 | 10 ± 7 | 4 ± 1.2 | 1.9 ± 1.7 at week 24 | 0 | September 14 | Not reported | April 14 | 0 | |||
| Vannucchi | 2021 | Prospective, open-label, and no control group | RTX | 1 × 100 mg | 17 | Decreased (27.6 ± 42.8 (n.v. <1.5)) | 4.56 ± 0.96 | 1.25 ± 1.14 at 24 weeks (P = 0.001) | 2 | >90% at 24 weeks | 58.30% | 0 at 72 weeks | 1 (cytokine release syndrome) |
CAS, clinical activity score; DON, dysthyroid optic neuropathy; GO, Graves’ orbitopathy; ivGCs, i.v. glucocorticosteroids; RTX, rituximab; SAE, serious adverse events; TRAb, thyroid receptor antibodies.
A study by Salvi et al. (which will be referred to as the Italian study) compared RTX to i.v. pulses of methylprednisolone in the treatment of patients with active, moderate-to-severe GO (41). Simultaneously, a study by Stan and colleagues compared RTX to placebo in the treatment of patients with active and moderate-to-severe GO and will be referred to as US trial (48).
In the US trial, out of 25 enrolled patients (12 in the placebo arm and 13 in the RTX arm), 21 completed the primary endpoint of 24 weeks and 19/21 completed the 52-week observation. Both groups were similar at baseline with regard to age, sex, smoking status, prior corticosteroid treatment, current thyroid status, TRAb level, CAS scores, as well as the quantitative ocular parameters or the disease duration. After 24 weeks, the primary endpoint, which was reduction in CAS, was similar in both groups. No significant difference in proptosis or composite clinical index was noted. Two patients developed DON. Both were treated with RTX, but no change in proptosis was observed in those patients. In the Italian study, 15 patients were treated with two infusions of 1 g RTX 2 weeks apart and 16 patients were treated with ivGCs in standard EUGOGO regimen. After 24 weeks of observation, more patients treated with RTX improved in comparison to the ivGC group. The recurrence rate after 52 and 76 weeks was lower as well. There was also an increase in proptosis (>2 mm) in three patients treated with RTX and two treated with ivGCs. In this study, patients were initially given two 1000 mg doses of RTX, but the protocol was amended to a single 500 mg dose after the observation that even a dose as low as 100 mg causes complete peripheral B-cell depletion (49). The characteristics of both groups in the Italian study were similar as well.
In the Italian study, a significant reduction of CAS was noted in both groups but was greater in the RTX arm. After 24 weeks, 100% of patients improved compared to 69% in the ivGC group. Reactivation of the disease was noted in five patients treated with ivGCs and in none treated with RTX. After 76 weeks of follow-up, rehabilitative surgery was performed in 12/16 patients in the ivGC group and in 5/15 of the RTX group. The US authors found that there was no benefit of RTX when compared to placebo in the reduction of CAS at 24 or 52 weeks. Similarly, there was no significant difference in the improvement of ophthalmic parameters. During follow-up, of the 11 patients treated with RTX that returned the survey, five needed additional surgical treatment. In the placebo group, five out of nine patients not lost to follow-up required such procedures, thus showing no additional benefit of RTX. There was a transient increase in proptosis in three RTX-treated patients from the US study and one in the Italian study, all of which returned to baseline after 24 of 52 weeks. No such change was noted in the placebo group. This raises concerns about the development of DON during treatment, which might occur or worsen due to increased intraorbital volume, most likely caused by post-infusion edema of the orbital tissues.
In order to better assess the results of those two studies, a very thorough analysis was carried out by the authors of both randomized controlled trials (RCTs) a few years later (50). The authors point out a few differences between both studies. Key discrepancy was the characteristics of the participants treated with RTX. In the Italian study, most patients were females, while in the US study the percentage of men was higher. The participants’ mean age was also markedly lower in the Italian study (51.9 vs 57.6), which might predispose to a favorable clinical outcome (51). Another factor which might contribute to a better response to treatment is a shorter disease duration (52). Again, the population treated with RTX in the US study had nearly three times longer disease duration than the Italian study. The last factor that might indicate a better response in the Italian study was the lower mean TRAb in the RTX group in comparison to the parallel group in the US trial (mean 10.7 ± 9.1 in the Italian study vs mean 28.1 ± 23.4 and median 20 (9–60) in the US trial). It is worth noting that the percentage of smokers was higher in the study by Salvi. Smoking is a known factor which predisposes to poor effect of ivGC therapy; however, it is not known if this translates to worse response in GO patients treated with RTX (53).
When comparing the protocol of both studies, the main difference of the Italian study was partial unblinding of the endocrinologists (but not ophthalmologist conducting the examination), explained by pursuing maximum safety for the patients (investigators believe that this did not cause any bias), as well as adjustment of dosage in the Italian study from 2 × 1 g initially used in both trials to a single 500 mg dose (the response to the high dose (2 × 1 g of RTX) and the low dose (1 × 500mg of RTX) was not significantly different, when analyzed statistically (ANOVA)). The authors of the review point out that the self-limiting course of the disease might influence the outcome when using the CAS as primary endpoint. It also does not take into account the additional benefits that therapeutic interventions might bring. In their comparison, the authors used a comprehensive score of disease improvement proposed by EUGOGO to analyze the data from those trials. Interestingly, the results were similar in their significance but different in magnitude when compared to when CAS was used as outcome measurement. Both RTX-treated groups remained different on the EUGOGO response, but the comparison to their respective control groups did not change.
In the years following the two RCTs, a number of clinical studies investigating RTX in active GO were published up to date. Unfortunately, these are mostly small, single-centered, uncontrolled studies without a control group. This obvious limitation requires caution when interpreting the results of said studies; nevertheless, the results of most of these studies are consistent and might serve as grounds for further research.
In 2017, a study was conducted by Karasek et al. (39) Ten euthyroid patients (six patients underwent total thyroidectomy, three patients underwent radioiodine treatment, and one patient did not undergo thyroidectomy or radioiodine treatment), comprising of eight women and two men, three smokers and seven nonsmokers, with an age range of 27–74 years and an average age of 49.3 ± 13.4 years, with active, severe-to-moderate GO (mean disease duration 8.9 ± 5.7 months) were treated with a single 100 mg dose of RTX. Four of the patients were corticoid resistant (persistent GO activity despite ivGCs), three patients had GO relapse after previous steroid treatment, and three patients had contraindications to ivGCs. Disease activity was measured with CAS and was reduced significantly from 3.6 ± 0.9 at baseline to 2.0 ± 0.8 at 1 month, 0.8 ± 0.4 at 6 months, and 0.4 ± 0.9 (P < 0.01). All patients had disease deactivation at 6 months; however, one patient experienced disease reactivation which might have been related to resumption of smoking. Proptosis was reduced from 22.3 ± 1.6 at baseline to 21.1 ± 1.8 at 12 months (P < 0.05). Furthermore , depletion of CD19+ and CD20+ B-cells positively correlated with the reduction of CAS at 6 and 12 months. The severity of the disease, except for minor decrease of proptosis, did not change significantly in the studied population. Two patients (GC resistant) developed DON requiring orbital decompression and were subsequently excluded from statistical analysis. In the observation period, one patient underwent elective decompression for exophthalmos with intermittent, spontaneous eyeball subluxation. No other side effects were noted by the authors of the study. Similar to other reports, a reduction of TRAbs was also noted.
In 2020, Eid published the results of a retrospective study of 14 patients (11 of whom were refractory to ivGCs), treated with RTX for active, moderate-to-severe GO (54). RTX was administered intravenously 1000 mg twice at a 2-week interval. Nearly all the patients received ivGCs prior to the study. Majority of them also underwent other concomitant treatments such as radiotherapy, thyroidectomy, radioiodine treatment, orbital decompression, or eyelid surgery. The median age was 60 years (range 55–69) with a mean baseline CAS of 4/10 (range 3–4.5). The mean duration of GO was 26 months (range 14–48), one patient was an active smoker , nine were ex-smokers and five never smoked. Five patients received AT combined with levothyroxine (LT), one patient received AT alone, and nine received LT alone. CAS had improved by ≥2 points in 2/14 patients at week 12 and in 5/14 patients at week 24. Disease inactivation (CAS < 3) occurred in 4/14 patients at week 12 and in 50% of patients (7/14) at week 24. Overall, the CAS improved significantly with a median improvement at 24 weeks of 1 point. Improvement in proptosis was observed in 3/9 patients at week 24 and improvement in diplopia was noted in only 1/10 patients. No effect on TRAb levels was observed. Sixty percent of patients experienced adverse events during treatment. A majority of them occurred during the first infusion and were mild and transient. One patient experienced exacerbation of arthritis and headaches that required discontinuation of RTX (225 mg/m2 was administered). None of the patients developed DON. In a longer observation, relapse occurred in 5/12 patients between 6 and 12 months after RTX therapy. This study, while still providing benefit for patients, shows a lesser efficacy of RTX when compared to other available studies. Patients in this study had a significantly longer disease duration, lower mean CAS, and a higher mean age and the group consisted of a significant portion of men.
In 2020, the results of a French retrospective study were published (55). Forty patients either not responding to ivGCs or with relapse after administration of ivGCs were treated for GO with two doses 1 g each (2-week apart infusions of RTX). Thirty-two patients received RTX in monotherapy, and one was excluded due to a severe adverse event (cytokine release syndrome). Eight patients needed concomitant optic decompression, with five of eight requiring optic decompression before RTX administration and three of eight requiring optic decompression after RTX administration. Patients were considered to have a good response if either CAS, oculomotor or visual function improved without deterioration in any of the functions. The authors emphasized that their cohort had a significant portion of patients (26%), with CAS between 1 and 3 points but with recent onset of GO (confirmed by imaging techniques), which was responsible for visual or oculomotor deterioration. The mean age of patients included in the analysis was 51.2 ± 10.7 years, with the majority being women (67%), and smokers (63%). The average duration of orbitopathy before administration of RTX was 19.1 ± 27 months. What is important is that only 32% of patients were euthyroid at the time of treatment with RTX. Proportion of patients with hypothyroidism was significantly higher in the group of patients who qualified for orbital decompression (4/7 vs 2/26, P = 0.04). At week 24, 20 out of 31 patients treated with RTX alone met the primary endpoint of improvement in either parameter without deterioration in the other. A favorable and significant effect on both inflammatory activity and visual function was noted, but only one patient improved with regard to oculomotor function. Patients treated with RTX and orbital decompression presented a more severe disease course. Hundred percent had visual dysfunction, and the mean CAS was higher. The results in these group were similar, as seven of eight patients improved in CAS and five of eight improved in visual function, but no significant effect on oculomotor function was observed. Of note, the authors also reported a 63% reduction of TRAb at week 24. Tolerance of the treatment was good, with only one serious adverse event in the aforementioned patient. This study is also limited by its nature – being retrospective, without a control group. Nevertheless, a few conclusions can be drawn. RTX was used as a second-line agent after unsuccessful ivGC treatment. In this setting, improvement in 64.5% of patients may be seen as clinically significant. Response was significantly better in patients with higher TRAb levels. Moreover, the negative correlation between smoking and clinical outcome of the treated patients was confirmed. The authors also suggest that the improvement in the oculomotor function was underestimated. They were only able to detect complete strabismus resolution due to oculomotor functions being assessed in multiple ways, preventing a reliable comparison of the outcomes.
The authors also point out that the duration of the disease is highly influential on the outcome of therapy. They refer to the two randomized studies by Stan and Salvi, which were reviewed here earlier in detail. Patients in the Italian study had significantly shorter disease duration; thus, oculomotor changes were less frequent and responded well to treatment, whereas in the study by Stan et al. as well as this study, disease duration was significantly longer and ophthalmic changes were more advanced and more resistant to treatment (41, 48).
RTX was compared to tocilizumab in the treatment of GO in a study by French authors (56). They retrospectively analyzed patients treated in their hospital for GO with either drug. Doses used were 8 mg/kg of tocilizumab on weeks 0, 4, 8, and 12 and 100 mg of RTX at days 1 and 15. All patients were refractory to ivGCs or were relapsing after previous disease inactivation. Seven patients on tocilizumab and 14 patients on RTX were included in this study. The primary endpoint of this review was reduction in CAS of at least 2, and this was achieved in all the patients treated with tocilizumab and 9 of 14 (64%) patients on RTX. Secondary outcomes (visual acuity, presence of diplopia, chemosis, eye aperture, and relapse rates) were not statistically different between those two groups. TRAb levels and proptosis decreased significantly in both groups, with mean values slightly favoring the tocilizumab group. The number of relapses was not significantly different as well. In their work, the authors suggest that the efficacy of both agents might be similar. The difference in response in favor of tocilizumab might be due to discrepancies in the treated groups, in particular higher number of male patients and higher mean age and TRAb levels in the RTX group. Both drugs, however, did not show a satisfactory effect on proptosis or diplopia.
In 2021, the results of an open-label, prospective study of RTX in GO was published (57). Seventeen patients (14 with GD and 3 with Hashimoto’s thyroiditis) were treated with a single 100 mg dose of RTX. This group consisted of 14 women and 3 men with a mean age of 51.5 ± 11.6 (range 28–72). The duration of the disease ranged between 0.8 and 10.4 months (mean ± SD, 4.2 ± 3.3). Twelve patients were previously treated with steroids, while five received RTX as a first-line treatment. Results of this study are very promising. CAS was reduced from 4.56 ± 0.96 at baseline to 1.25 ± 1.14 at 24 weeks (P = 0.001). Disease inactivation occurred within 24 weeks in >90% of patients and was unrelated to disease duration. Severity of the disease (assessed as composite ophthalmic) improved in 58.3% of patients (7 of 12), remained stable in 3 of 12 pateints, and deteriorated in two patients. No relapses were noted in the 72-week observation period. Two patients required surgical decompression due to the development of DON between 12 and 24 weeks of observation. One patient experienced a serious adverse event, which was cytokine release syndrome, and required cessation of RTX infusion. Otherwise treatment was well tolerated. One woman reported mild urticaria. which resolved with low-dose steroids. After concluding the 72-week observation period, six patients did not require any additional treatment, five patients underwent elective surgical decompression, seven patients had squint surgery, and two had eyelid surgery. TRAb concentrations were studied, and a significant reduction was noted after 24 weeks. The efficacy of RTX, contrary to other studies, was slightly better in patients with longer disease duration; however, all participants of this study had relatively short disease duration. Data regarding safety profile was similar to other studies. Moreover, no data on smoking status are available.
Discussion
RTX as a treatment of GO has been in the spotlight for many years now, and several studies, both well-designed, randomized, prospective trials and small, limited, retrospective analyses, have been published up to this date. In this review, we focused especially on efficacy and safety profile. We looked closely at the characteristics of studied populations, including risk factors, disease duration, and initial activity and severity of GO. However, it must be taken into account that results of those studies are often difficult to compare, as different primary or secondary endpoints were employed or different parameters of disease severity were measured and reported.
Safety profile
Probably the most important aspect when considering new treatments is the safety profile. According to the review by van Vollenhoven et al., in the population of patients with rheumatoid arthritis, the incidence of serious infections was no greater in patients treated with RTX in comparison to patients treated with placebo + methotrexate (58). Neither increased risk of malignancy was observed nor a higher risk of myocardial infarction or stroke than in the general rheumatoid arthritis population. Most common side effects are infusion-related symptoms caused by release of proinflammatory cytokines. The most concerning side effect reported was progressive multifocal leukoencephalopathy, which was mainly observed in patients with systemic lupus erythematosus, treated previously with other immunosuppressive agents.
In the reviewed papers, the majority of reported side effects were mild, transient, and self-limiting. These were easily preventable with premedication or managed by additional doses of paracetamol or hydrocortisone. Adverse events considered as serious were far less common. A total of six cases of DON after treatment with RTX were reported across all the above studies (39, 48, 57). Salvi et al. reported two cases of cytokine release symptom with transient edema of periorbital tissue, requiring drug cessation and additional treatment with hydrocortisone (49). Also Vannucchi and Deltour each reported one case of cytokine release syndrome (55, 57). Stan in the US randomized trial reported five adverse events classified as moderate/severe in the group treated with RTX (while only one was classified in the placebo group). Risk of DON after administration of RTX is the main safety concern in patients with GO. It is suggested that sudden release of cytokines from destroyed B-cells infiltrating the ocular tissue might contribute to the sudden swelling of tissues, thus as a result causing DON (50). Some authors believe that this happens only when there is already a subclinical form of DON present. It is also postulated that progression to DON represents failure to treat, rather than adverse event of RTX, as in some patients it is the natural course of the disease. This hypothesis is supported by RCT by Salvi, where one case of DON was reported in a patient treated with ivGCs and none in the RTX group. According to Blandford, RTX might even be of benefit in patients with DON, but the author emphasizes that more studies of this agent are necessary (59).
Efficacy
Lack of unification in reporting results of trials in GO is a major concern. The most commonly used scale for assessing disease activity is CAS. Unfortunately, it has its obvious limitations, being highly subjective and binary (yes/no) in nature. In almost all the trials, a significant reduction in CAS was observed. Results vary from 4.4 ± 0.7 points at baseline reduced to 0.6 ± 3 at week 24 in the randomized Italian study, to reduction as minor as 4 points at baseline to 3 points at week 24 in the study by Eid et al. Also disease inactivation (CAS <3) was assessed. Here also, results are different. In the studies by Salvi or Karasek, 100% of patients treated with RTX were found to be inactive at week 24. Over 90% of patients achieved remission in the prospective study published by Vannucchi. On the other hand, Stan et al. showed that less than 50% of patients had inactive GO after the observation period. The results of the other studies fall in between those two sides of the spectrum. To evaluate the severity of GO proptosis, lid aperture, eye mobility, diplopia and few other parameters should be measured. The results of above studies are difficult to compare because of different methods of reporting this data. Nevertheless, based on the provided data, RTX does not seem to significantly impact the disease severity. Nearly all the studies report no significant improvement in the evaluated ophthalmic parameters. Only recently, a unification in reporting the outcomes of ophthalmic parameters in clinical trials of GO was proposed in the form of composite ophthalmic Index, which consists of the following items: ≥2 mm reduction of lid aperture, ≥1 point reduction in five-item CAS (excluding subjective, patient-reported spontaneous or gaze-evoked pain), ≥2 mm reduction in exophthalmos, and ≥8° increase of eye muscle duction (60). Utilizing this tool might be of great use in future trials.
Data on the issue of disease recurrence do not seem to be consistent across the available studies. While some studies (e.g. Salvi et al., Karasek et al., and Vannucchi et al.) report no disease recurrence after 52- or 72-week observation period, other studies do not either provide such long follow-up or report a significantly higher percentage. Eid et al. for example reports relapse of the disease in as much as 41.7% of patients with a median time of 8 months from the initial treatment with GO. Bennedjai et al. report that 4 of 14 patients on RTX experienced disease recurrence with a median relapse-free survival of 18 ± 3.5. months. It is difficult to judge what might have influenced this outcome. In the study by Eid et al., high median age of patients was significantly higher than in other studies. Furthermore, in both aforementioned trials, disease duration at baseline was very long, with a median of 26 and 27 months, respectively. This might have contributed to poor response to treatment. Patients in the study by Deltour et al. also had long disease duration with a median of 19 months, but the rate of disease free survival was not reported.
The patient’s perception of the disease and the outcome of treatment are equally important when evaluating its efficacy, as GO might be a highly disfiguring and limiting disease, affecting physical appearance as well as visual functions. That can further lead to work absence, limitations in other activities, and withdrawal from social life. Even disease classified as mild according to ophthalmic criteria may impair the patient’s quality of life (QoL) drastically. It is recommended to assess the QoL of patients by providing them with a standardized questionnaire, which might help to monitor the subjective treatment results, as well as help make better therapeutic decisions (16). Unfortunately, evaluation of QoL was often not included in the reported data. Stan et al. report a similar improvement in QoL in both placebo and RTX groups. Salvi et al., in their RCT, report a significant improvement in QoL both in the appearance and visual function parts of the survey in the RTX-treated group, while ivGCs providing little to no benefit on QoL. Also Vannucchi in the prospective study of small-dose RTX provides results of QoL surveys. More than half of patients improved in either the 'visual function' or 'appearance' part of the questionnaire. Hopefully, future studies will be more focused around the problem of QoL in an effort to provide personalized care for patients with GO.
There are a few factors that need to be taken into consideration when analyzing the efficacy of RTX in the context of available studies. The most important aspect is the characteristics of the studied populations. The age of patients seems to be a very important prognostic factor, as in the studies showing worse results, a higher median age of patients was noted. Also, a higher percentage of women treated commonly yielded better response to treatment. The other important and known risk factor for a more severe course of GO is smoking. This, however, was not reflected in the analyzed studies. Interestingly, the opposite trend seems to be observed, as, for example, in the retrospective study by Eid et al. only 1 of 15 patients was a smoker at the time of the treatment; however, the reduction of CAS and percentage of disease inactivation was among the lowest of the analyzed studies. When looking at the studied populations, there are of course other factors in play. In the majority of studies, all, or almost all, the patients received previous steroid treatment. This represents a group of already increased risk of severe disease course, as RTX was used as a second-line treatment in the event of disease being resistant to steroids or recurrence after initial positive therapeutic outcome. Only two RCTs included a majority of patients naive to ivGCs.
Dose of medication
Initially, patients were treated with doses of RTX used in rheumatology or hematology – most commonly two doses of 1000 mg in 2-week intervals, but later Salvi et al. demonstrated that even a dose as low as a single 100 mg of RTX causes full peripheral B-cell depletion (49). Most investigators, in an effort to minimize the risk of adverse events while not giving up on efficacy, started employing lower doses of this agent, mostly single doses of 100 mg or 500 mg. This approach is also more cost-effective, which might sometimes be a very important factor in the decision-making process. Smaller dose of RTX did not seem to affect the outcome of the treatment. The authors of most recent studies also decided to use smaller doses. Recently, Campi et al. compared post-hoc different doses of RTX used in patients with GO across three different trials (61). A total of 40 patients across two open-label prospective studies and one randomized study were treated with 100 mg, 500 mg, and 2 × 1000mg of RTX, respectively. The authors investigated the efficacy of the treatment as well as the safety profile or economic aspect of therapy. They conclude that any of the employed treatment regimens is equally effective in inactivating the disease, with no significant difference with regard to its duration. There were, however, differences in disease severity. Higher doses provided more benefit on diplopia than the dose of 100 mg. The authors suggest that greater doses can be more effective in preventing fibrosis in the ocular tissues, thus reducing the severity of diplopia and potential need for surgery.
Position of RTX among other therapeutic options
We believe there are a few reasons why, in certain patients, RTX might be favorable to other second-line treatments proposed by EUGOGO. The main advantage of this agent seems to be its potential ability to modify the natural course of GD (greater percentage of patients achieving stable remission), which was suggested in the phase II trial by Cheetham et al. (31). It seems likely that a similar effect could be observed in the course of GO.
The other factor to consider is the relative simplicity of the therapy regimen. A single dose of RTX (compared to, for example, 12 consecutive, weekly doses of ivGCs) might be more easily accepted by the patients, thus ensuring compliance.
Conclusion
In our opinion, RTX is a relatively safe alternative in the treatment of GO, especially in the population of patients who could not be treated successfully with ivGCs, due to lack of satisfying response, disease recurrence, or contraindications. Current data suggest that if administered early, it can modify the natural course of GO, shortening the active phase, thus contributing to limiting the damaging influence of inflammatory processes in the orbit. While it provides limited benefit in reducing the disease severity, its beneficial influence on QoL cannot be overlooked. Furthermore, its possible effect on the course of GD is an interesting aspect, which might affect the choice of second-line therapy in some cases. However, in light of possibly high amount of adverse events reported in the trial, a number of them being severe, a careful selection of candidates for treatment is necessary. We believe that current data warrant particular caution when DON is suspected. It must be emphasized that this treatment must be carried out in multidisciplinary centers with access to emergency surgery when needed. Patients at risk of optic neuropathy should be carefully monitored throughout the treatment.
We also believe, as most authors, that there is a need for further studies of RTX in the treatment of GO before considering it a treatment of choice either in the first line or the second line. Its potential benefits when administered early in the course of disease should be the focus of investigation in the future studies.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
References
- 1.Bahn RS.Graves’ ophthalmopathy. New England Journal of Medicine 2010362726–738. ( 10.1056/NEJMra0905750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hiromatsu Y, Eguchi H, Tani J, Kasaoka M, Teshima Y. Graves’ ophthalmopathy: epidemiology and natural history. Internal Medicine 201453353–360. ( 10.2169/internalmedicine.53.1518) [DOI] [PubMed] [Google Scholar]
- 3.Tanda ML, Piantanida E, Liparulo L, Veronesi G, Lai A, Sassi L, Pariani N, Gallo D, Azzolini C, Ferrario Met al. Prevalence and natural history of Graves’ orbitopathy in a large series of patients with newly diagnosed Graves’ hyperthyroidism seen at a single center. Journal of Clinical Endocrinology and Metabolism 2013981443–1449. ( 10.1210/jc.2012-3873) [DOI] [PubMed] [Google Scholar]
- 4.Villadolid MC, Yokoyama N, Izumi M, Nishikawa T, Kimura H, Ashizawa K, Kiriyama T, Uetani M, Nagataki S. Untreated Graves’ disease patients without clinical ophthalmopathy demonstrate a high frequency of extraocular muscle (EOM) enlargement by magnetic resonance. Journal of Clinical Endocrinology and Metabolism 1995802830–2833. ( 10.1210/jcem.80.9.7673432) [DOI] [PubMed] [Google Scholar]
- 5.Soroudi AE, Goldberg RA, McCann JD. Prevalence of asymmetric exophthalmos in Graves’ orbitopathy. Ophthalmic Plastic and Reconstructive Surgery 200420224–225. ( 10.1097/01.iop.0000124675.80763.5a) [DOI] [PubMed] [Google Scholar]
- 6.Rundle FF, Wilson CW. Development and course of exophthalmos and ophthalmoplegia in Graves’ disease with special reference to the effect of thyroidectomy. Clinical Science 19455177–194. [PubMed] [Google Scholar]
- 7.Bartalena L, Piantanida E, Gallo D, Lai A, Tanda ML. Epidemiology, natural history, risk factors, and prevention of Graves’ orbitopathy. Frontiers in Endocrinology 202011615993. ( 10.3389/fendo.2020.615993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shan SJ, Douglas RS. The pathophysiology of thyroid eye disease. Journal of Neuro-Ophthalmology 201434177–185. ( 10.1097/WNO.0000000000000132) [DOI] [PubMed] [Google Scholar]
- 9.Naik VM, Naik MN, Goldberg RA, Smith TJ, Douglas RS. Immunopathogenesis of thyroid eye disease: emerging paradigms. Survey of Ophthalmology 201055215–226. ( 10.1016/j.survophthal.2009.06.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romagnani P, Rotondi M, Lazzeri E, Lasagni L, Francalanci M, Buonamano A, Milani S, Vitti P, Chiovato L, Tonacchera Met al. Expression of IP-10/CXCL10 and MIG/CXCL9 in the thyroid and increased levels of IP-10/CXCL10 in the serum of patients with recent-onset Graves’ disease. American Journal of Pathology 2002161195–206. ( 10.1016/S0002-9440(1064171-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antonelli A, Fallahi P, Elia G, Ragusa F, Paparo SR, Ruffilli I, Patrizio A, Gonnella D, Giusti C, Virili Cet al. Graves’ disease: clinical manifestations, immune pathogenesis (cytokines and chemokines) and therapy. Best Practice and Research: Clinical Endocrinology and Metabolism 202034 101388. ( 10.1016/j.beem.2020.101388) [DOI] [PubMed] [Google Scholar]
- 12.Mysliwiec J, Palyga I, Kosciuszko M, Kowalska A, Gorska M. Circulating CXCL9 and CXCL10 as markers of activity of Graves’ orbitopathy during treatment with corticosteroids and teleradiotherapy. Hormone and Metabolic Research 201244957–961. ( 10.1055/s-0032-1316352) [DOI] [PubMed] [Google Scholar]
- 13.Leite AC, Pedro AB, Romaldini JH. Influence of methimazole and radioactive iodine treatment in the serum levels of the chemokine CXCL10 in hyperthyroid patients with Graves’ disease. Hormone and Metabolic Research 201143194–199. ( 10.1055/s-0031-1271620) [DOI] [PubMed] [Google Scholar]
- 14.Antonelli A, Rotondi M, Fallahi P, Grosso M, Boni G, Ferrari SM, Romagnani P, Serio M, Mariani G, Ferrannini E. Iodine-131 given for therapeutic purposes modulates differently interferon-gamma-inducible alpha-chemokine CXCL10 serum levels in patients with active Graves’ disease or toxic nodular goiter. Journal of Clinical Endocrinology and Metabolism 2007921485–1490. ( 10.1210/jc.2006-1571) [DOI] [PubMed] [Google Scholar]
- 15.Antonelli A, Rotondi M, Fallahi P, Romagnani P, Ferrari SM, Paolicchi A, Ferrannini E, Serio M. Increase of interferon-gamma inducible alpha chemokine CXCL10 but not beta chemokine CCL2 serum levels in chronic autoimmune thyroiditis. European Journal of Endocrinology 2005152171–177. ( 10.1530/eje.1.01847) [DOI] [PubMed] [Google Scholar]
- 16.Bartalena L, Kahaly GJ, Baldeschi L, Dayan CM, Eckstein A, Marcocci C, Marino M, Vaidya B, Wiersinga WM. & EUGOGO. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. European Journal of Endocrinology 2021185G43–G67. ( 10.1530/EJE-21-0479) [DOI] [PubMed] [Google Scholar]
- 17.Kahaly GJ, Riedl M, König J, Pitz S, Ponto K, Diana T, Kampmann E, Kolbe E, Eckstein A, Moeller LCet al. Mycophenolate plus methylprednisolone versus methylprednisolone alone in active, moderate-to-severe Graves’ orbitopathy (MINGO): a randomised, observer-masked, multicentre trial. Lancet: Diabetes and Endocrinology 20186287–298. ( 10.1016/S2213-8587(1830020-2) [DOI] [PubMed] [Google Scholar]
- 18.Ye X, Bo X, Hu X, Cui H, Lu B, Shao J, Wang J. Efficacy and safety of mycophenolate mofetil in patients with active moderate-to-severe Graves’ orbitopathy. Clinical Endocrinology 201786247–255. ( 10.1111/cen.13170) [DOI] [PubMed] [Google Scholar]
- 19.Smith TJ, Hegedüs L, Douglas RS. Role of insulin-like growth factor-1 (IGF-1) pathway in the pathogenesis of Graves’ orbitopathy. Best Practice and Research: Clinical Endocrinology and Metabolism 201226291–302. ( 10.1016/j.beem.2011.10.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krieger CC, Place RF, Bevilacqua C, Marcus-Samuels B, Abel BS, Skarulis MC, Kahaly GJ, Neumann S, Gershengorn MC. TSH/IGF-1 receptor cross talk in Graves’ ophthalmopathy pathogenesis. Journal of Clinical Endocrinology and Metabolism 20161012340–2347. ( 10.1210/jc.2016-1315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith TJ, Kahaly GJ, Ezra DG, Fleming JC, Dailey RA, Tang RA, Harris GJ, Antonelli A, Salvi M, Goldberg RAet al. Teprotumumab for thyroid-associated ophthalmopathy. New England Journal of Medicine 20173761748–1761. ( 10.1056/NEJMoa1614949) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Douglas RS, Kahaly GJ, Patel A, Sile S, Thompson EHZ, Perdok R, Fleming JC, Fowler BT, Marcocci C, Marino Met al. Teprotumumab for the treatment of active thyroid eye disease. New England Journal of Medicine 2020382341–352. ( 10.1056/NEJMoa1910434) [DOI] [PubMed] [Google Scholar]
- 23.Smith TJ.Insulin-like growth factor pathway and the thyroid. Frontiers in Endocrinology 202112 653627. ( 10.3389/fendo.2021.653627) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pescovitz MD.Rituximab, an anti-CD20 monoclonal antibody: history and mechanism of action. American Journal of Transplantation 20066859–866. ( 10.1111/j.1600-6143.2006.01288.x) [DOI] [PubMed] [Google Scholar]
- 25.Eisenberg R, Looney RJ. The therapeutic potential of anti-CD20 ‘what do B-cells do?’ Clinical Immunology 2005117207–213. ( 10.1016/j.clim.2005.08.006) [DOI] [PubMed] [Google Scholar]
- 26.Mok CC.Current role of rituximab in systemic lupus erythematosus. International Journal of Rheumatic Diseases 201518154–163. ( 10.1111/1756-185X.12463) [DOI] [PubMed] [Google Scholar]
- 27.Tavakolpour S, Alesaeidi S, Darvishi M, Ghasemiadl M, Darabi-Monadi S, Akhlaghdoust M, Elikaei Behjati S, Jafarieh A. A comprehensive review of rituximab therapy in rheumatoid arthritis patients. Clinical Rheumatology 2019382977–2994. ( 10.1007/s10067-019-04699-8) [DOI] [PubMed] [Google Scholar]
- 28.El Fassi D, Nielsen CH, Hasselbalch HC, Hegedüs L. The rationale for B lymphocyte depletion in Graves’ disease. Monoclonal anti-CD20 antibody therapy as a novel treatment option. European Journal of Endocrinology 2006154623–632. ( 10.1530/eje.1.02140) [DOI] [PubMed] [Google Scholar]
- 29.El Fassi D, Nielsen CH, Bonnema SJ, Hasselbalch HC, Hegedüs L. B lymphocyte depletion with the monoclonal antibody rituximab in Graves’ disease: a controlled pilot study. Journal of Clinical Endocrinology and Metabolism 2007921769–1772. ( 10.1210/jc.2006-2388) [DOI] [PubMed] [Google Scholar]
- 30.Heemstra KA, Toes RE, Sepers J, Pereira AM, Corssmit EP, Huizinga TW, Romijn JA, Smit JW. Rituximab in relapsing Graves’ disease, a phase II study. European Journal of Endocrinology 2008159609–615. ( 10.1530/EJE-08-0084) [DOI] [PubMed] [Google Scholar]
- 31.Cheetham TD, Cole M, Abinun M, Allahabadia A, Barratt T, Davies JH, Dimitri P, Drake A, Mohamed Z, Murray RDet al. Adjuvant rituximab-exploratory trial in young people with Graves’ disease. Journal of Clinical Endocrinology and Metabolism 2022107743–754. ( 10.1210/clinem/dgab763) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasselbalch HC.B-cell depletion with rituximab-a targeted therapy for Graves’ disease and autoimmune thyroiditis. Immunology Letters 20038885–86. ( 10.1016/s0165-2478(0300032-4) [DOI] [PubMed] [Google Scholar]
- 33.Bartalena L, Tanda ML. Immunotherapy for Graves’ orbitopathy: easy enthusiasm, but let’s keep trying. Journal of Endocrinological Investigation 2006291012–1016. ( 10.1007/BF03349216) [DOI] [PubMed] [Google Scholar]
- 34.Salvi M, Vannucchi G, Campi I, Rossi SPS, Bonara P, Sbrozzi F, Guastella C, Avignone S, Pirola G, Ratiglia Ret al. Efficacy of rituximab treatment for thyroid-associated ophthalmopathy as a result of intraorbital B-cell depletion in one patient unresponsive to steroid immunosuppression. European Journal of Endocrinology 2006154511–517. ( 10.1530/eje.1.02119) [DOI] [PubMed] [Google Scholar]
- 35.Salvi M, Vannucchi G, Beck-Peccoz P. Potential utility of rituximab for Graves’ orbitopathy. Journal of Clinical Endocrinology and Metabolism 2013984291–4299. ( 10.1210/jc.2013-1804) [DOI] [PubMed] [Google Scholar]
- 36.Wall JR, Lahooti H. Pathogenesis of thyroid eye disease – does autoimmunity against the TSH receptor explain all cases? Endokrynologia Polska 201162 (Supplement 1) 1–7. [PubMed] [Google Scholar]
- 37.Mishra S, Maurya VK, Kumar S, Ankita KA, Kaur A, Saxena SK. Clinical management and therapeutic strategies for the thyroid-associated ophthalmopathy: current and future perspectives. Current Eye Research 2020451325–1341. ( 10.1080/02713683.2020.1776331) [DOI] [PubMed] [Google Scholar]
- 38.Jang SY, Shin DY, Lee EJ, Choi YJ, Lee SY, Yoon JS. Correlation between TSH receptor antibody assays and clinical manifestations of Graves’ orbitopathy. Yonsei Medical Journal 2013541033–1039. ( 10.3349/ymj.2013.54.4.1033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karasek D, Cibickova L, Karhanova M, Kalitova J, Schovanek J, Frysak Z. Clinical and immunological changes in patients with active moderate-to-severe Graves’ orbitopathy treated with very low-dose rituximab. Endokrynologia Polska 201768498–504. ( 10.5603/EP.a2017.0040) [DOI] [PubMed] [Google Scholar]
- 40.El Fassi D, Banga JP, Gilbert JA, Padoa C, Hegedus L, Nielsen CH. Treatment of Graves’ disease with rituximab specifically reduces the production of thyroid stimulating autoantibodies. Clinical Immunology 2009130252–258. ( 10.1016/j.clim.2008.09.007) [DOI] [PubMed] [Google Scholar]
- 41.Salvi M, Vannucchi G, Currò N, Campi I, Covelli D, Dazzi D, Simonetta S, Guastella C, Pignataro L, Avignone Set al. Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves’ orbitopathy: a randomized controlled study. Journal of Clinical Endocrinology and Metabolism 2015100422–431. ( 10.1210/jc.2014-3014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell AL, Gan EH, Morris M, Johnson K, Neoh C, Dickinson AJ, Perros P, Pearce SHS. The effect of B cell depletion therapy on anti-TSH receptor antibodies and clinical outcome in glucocorticoid-refractory Graves’ orbitopathy. Clinical Endocrinology 201379437–442. ( 10.1111/cen.12141) [DOI] [PubMed] [Google Scholar]
- 43.Khanna D, Chong KK-L, Afifiyan NF, Hwang CJ, Lee DK, Garneau HC, Goldberg RA, Darwin CH, Smith TJ, Douglas RS. Rituximab treatment of patients with severe, corticosteroid-resistant thyroid-associated ophthalmopathy. Ophthalmology 2010117133–139.e2. ( 10.1016/j.ophtha.2009.05.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Insull EA, Sipkova Z, David J, Turner HE, Norris JH. Early low-dose rituximab for active thyroid eye disease: an effective and well-tolerated treatment. Clinical Endocrinology 201991179–186. ( 10.1111/cen.13970) [DOI] [PubMed] [Google Scholar]
- 45.Vannucchi G, Campi I, Bonomi M, Covelli D, Dazzi D, Currò N, Simonetta S, Bonara P, Persani L, Guastella Cet al. Rituximab treatment in patients with active Graves’ orbitopathy: effects on proinflammatory and humoral immune reactions. Clinical and Experimental Immunology 2010161436–443. ( 10.1111/j.1365-2249.2010.04191.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J, Chen G, Sun H. Intravenous rituximab therapy for active Graves’ ophthalmopathy: a meta-analysis. Hormones 202120279–286. ( 10.1007/s42000-021-00282-6) [DOI] [PubMed] [Google Scholar]
- 47.Salvi M, Girasole G, Pedrazzoni M, Passeri M, Giuliani N, Minelli R, Braverman LE, Roti E. Increased serum concentrations of interleukin-6 (IL-6) and soluble IL-6 receptor in patients with Graves’ disease. Journal of Clinical Endocrinology and Metabolism 1996812976–2979. ( 10.1210/jcem.81.8.8768861) [DOI] [PubMed] [Google Scholar]
- 48.Stan MN, Garrity JA, Carranza Leon BG, Prabin T, Bradley EA, Bahn RS. Randomized controlled trial of rituximab in patients with Graves’ orbitopathy. Journal of Clinical Endocrinology and Metabolism 2015100432–441. ( 10.1210/jc.2014-2572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salvi M, Vannucchi G, Currò N, Introna M, Rossi S, Bonara P, Covelli D, Dazzi D, Guastella C, Pignataro Let al. Small dose of rituximab for Graves’ orbitopathy: new insights into the mechanism of action. Archives of Ophthalmology 2012130122–124. ( 10.1001/archopthalmol.2011.1215) [DOI] [PubMed] [Google Scholar]
- 50.Stan MN, Salvi M. MANAGEMENT OF ENDOCRINE DISEASE: Rituximab therapy for Graves’ orbitopathy – lessons from randomized control trials. European Journal of Endocrinology 2017176 R101–R109. ( 10.1530/EJE-16-0552) [DOI] [PubMed] [Google Scholar]
- 51.Perros P, Crombie AL, Matthews JN, Kendall-Taylor P. Age and gender influence the severity of thyroid-associated ophthalmopathy: a study of 101 patients attending a combined thyroid-eye clinic. Clinical Endocrinology 199338367–372. ( 10.1111/j.1365-2265.1993.tb00516.x) [DOI] [PubMed] [Google Scholar]
- 52.Terwee CB, Prummel MF, Gerding MN, Kahaly GJ, Dekker FW, Wiersinga WM. Measuring disease activity to predict therapeutic outcome in Graves’ ophthalmopathy. Clinical Endocrinology 200562145–155. ( 10.1111/j.1365-2265.2005.02186.x) [DOI] [PubMed] [Google Scholar]
- 53.Wiersinga WM.Smoking and thyroid. Clinical Endocrinology 201379145–151. ( 10.1111/cen.12222) [DOI] [PubMed] [Google Scholar]
- 54.Eid L, Coste-Verdier V, Longueville E, Ribeiro E, Nicolescu-Catargi B, Korobelnik JF. The effects of rituximab on Graves’ orbitopathy: a retrospective study of 14 patients. European Journal of Ophthalmology 2020301008–1013. ( 10.1177/1120672119845224) [DOI] [PubMed] [Google Scholar]
- 55.Deltour JB, d’Assigny Flamen M, Ladsous M, Giovansili L, Cariou B, Caron P, Drui D, Lebranchu P. Efficacy of rituximab in patients with Graves’ orbitopathy: a retrospective multicenter nationwide study. Graefe’s Archive for Clinical and Experimental Ophthalmology 20202582013–2021. ( 10.1007/s00417-020-04651-6) [DOI] [PubMed] [Google Scholar]
- 56.Bennedjaï A, Bouheraoua N, Gatfossé M, Dupasquier-Fediaevsky L, Errera MH, Tazartes M, Borderie V, Hennocq Q, Dellal A, Riviere Set al. Tocilizumab versus rituximab in patients with moderate to severe steroid-resistant Graves’ orbitopathy. Ocular Immunology and Inflammation 202230500–505. ( 10.1080/09273948.2020.1808688) [DOI] [PubMed] [Google Scholar]
- 57.Vannucchi G, Campi I, Covelli D, Currò N, Lazzaroni E, Palomba A, Soranna D, Zambon A, Fugazzola L, Muller Iet al. Efficacy profile and safety of very low-dose rituximab in patients with Graves’ orbitopathy. Thyroid 202131821–828. ( 10.1089/thy.2020.0269) [DOI] [PubMed] [Google Scholar]
- 58.van Vollenhoven RF, Emery P, Bingham 3rd CO, Keystone EC, Fleischmann RM, Furst DE, Tyson N, Collinson N, Lehane PB. Long-term safety of rituximab in rheumatoid arthritis: 9.5-year follow-up of the global clinical trial programme with a focus on adverse events of interest in RA patients. Annals of the Rheumatic Diseases 2013721496–1502. ( 10.1136/annrheumdis-2012-201956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blandford AD, Zhang D, Chundury RV, Perry JD. Dysthyroid optic neuropathy: update on pathogenesis, diagnosis, and management. Expert Review of Ophthalmology 201712111–121. ( 10.1080/17469899.2017.1276444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bartalena L, Wiersinga WM. Proposal for standardization of primary and secondary outcomes in patients with active, moderate-to-severe Graves’ orbitopathy. European Thyroid Journal 20209 (Supplement 1) 3–16. ( 10.1159/000510700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Campi I, Vannucchi G, Muller I, Lazzaroni E, Currò N, Dainese M, Montacchini B, Covelli D, Guastella C, Pignataro Let al. Therapy with different dose regimens of rituximab in patients with active moderate-to-severe Graves’ orbitopathy. Frontiers in Endocrinology 202112 790246. ( 10.3389/fendo.2021.790246) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a