Abstract
To understand more about the factors influencing the cleavage of immunoglobulin A1 (IgA1) by microbial IgA1 proteases, a recombinant human IgA2/IgA1 hybrid molecule was generated. In the hybrid, termed IgA2/A1 half hinge, a seven-amino-acid sequence corresponding to one half of the duplicated sequence making up the IgA1 hinge was incorporated into the equivalent site in IgA2. Insertion of the IgA1 half hinge into IgA2 did not affect antigen binding capacity or the functional activity of the hybrid molecule, as judged by its ability to bind to IgA Fcα receptors and trigger respiratory bursts in neutrophils. Although the IgA2/A1 hybrid contained only half of the IgA1 hinge, it was found to be cleaved by a variety of different bacterial IgA1 proteases, including representatives of those that cleave IgA1 in the different duplicated halves of the hinge, namely, those of Prevotella melaninogenica, Streptococcus pneumoniae, S. sanguis, Neisseria meningitidis types 1 and 2, N. gonorrhoeae types 1 and 2, and Haemophilus influenzae type 2. Thus, for these enzymes the recognition site for IgA1 cleavage is contained within half of the IgA1 hinge region; additional distal elements, if required, are provided by either an IgA1 or an IgA2 framework. In contrast, the IgA2/A1 hybrid appeared to be resistant to cleavage with S. oralis and some H. influenzae type 1 IgA1 proteases, suggesting these enzymes require additional determinants for efficient substrate recognition.
Secretory IgA (S-IgA) protects mucous membranes from attack by pathogenic microorganisms. It acts by neutralizing toxins, enzymes, and viruses, agglutinating bacteria, and preventing bacterial adhesion to mucous membranes by blocking receptors and, by virtue of its hydrophilic nature, causing repelling interactions with the mucosal epithelium (16, 18, 38, 40).
The ability of S-IgA to carry out its defensive effector functions is dependent on its structural integrity. The physicochemical nature of S-IgA renders it resistant to most types of proteolytic attack (20). However, a few pathogenic bacteria such as Streptococcus pneumoniae, Haemophilus influenzae, Neisseria meningitidis, N. gonorrhoeae, and Prevotella melaninogenica, which cause infections at mucous membranes leading to diseases like pneumonia, meningitis, gonorrhea, and periodontitis, produce a variety of enzymes called IgA1 proteases (for reviews, see references 15, 26, and 35). They are so named because they cleave only human IgA1 and not the IgA2 isotype. These enzymes may be important virulence factors because they are produced in vivo (3, 14), convalescing patients from infections with these bacteria have neutralizing antibodies to the enzymes (5, 9, 11), and the related but nonpathogenic species of these bacteria do not produce them (26). Moreover, some may have a role in virulence by mechanisms additional to or distinct from that arising through IgA1 cleavage (19, 30).
IgA2 is resistant to IgA1 proteases because it lacks a sequence of 16 amino acids which is present in the hinge region of IgA1 and which is the cleavage site for all IgA1 proteases. The sequence, which is rich in proline, threonine, and serine, is unusual in that it contains a repeat of two identical and contiguous sequences each of eight amino acids. Although the IgA1 proteases belong to widely different families, i.e., serine proteases, metallo proteases, and thiol proteases, they are all highly specific post-proline endopeptidases. The IgA1 proteases of the different bacteria always cleave at either Pro-Ser (type 1 enzymes) or Pro-Thr (type 2 enzymes) peptide bonds. However, they are extremely specific in that one enzyme of a given organism cleaves the specific peptide bond in only one of the duplicated eight amino acid sequences and not at the equivalent site in the other duplicated eight-amino-acid sequence (Fig. 1).
FIG. 1.
Simplified structure of monomeric serum IgA showing the amino acid sequence of the hinge region of IgA1, IgA2m(1), and the IgA2/A1 half hinge and the cleavage sites of different bacterial IgA1 proteases.
To understand more about the factors influencing the cleavage of IgA by microbial IgA1 proteases, a recombinant hybrid IgA molecule was constructed such that an amino acid sequence representing half of the duplicated hinge region of IgA1 was incorporated into the equivalent position in IgA2. The functional activity and sensitivity of the recombinant hybrid IgA2/A1 molecule to a variety of microbial IgA1 proteases were then determined.
MATERIALS AND METHODS
Primers.
Primer A1H6 (5′ GCGCGCGGATCCGGTCCAACCGCAGGCCC 3′) contained a BamHI site (italics) and annealed about 150 bp upstream of the CH1 exon of human IgA2m(1). Primer A1H2 (5′ AGATGGGGTAGGTGGAGTTGAGGGAACTGGAGTGG 3′) contained nucleotides complementary to nucleotides 675 to 688 of the coding strand for the CH1 region of human IgA2m(1) and (in italics) nucleotides complementary to nucleotides 667 to 687 of human IgA1, coding for half of the hinge region. Primer A1H3 (5′ TCAACTCCACCTACCCCATCTCCACCTCCCCCATG 3′) contained (in italics) the nucleotides coding for half the hinge region, i.e., nucleotides 667 to 687, of human IgA1, followed by nucleotides 689 to 702 of the coding strand for the CH2 region of human IgA2m(1). Primer A1H5 (5′ CCACCTCTGACTTGA 3′) was complementary to nucleotides 424 to 438 of the vector pSP73. All primers were made by the Oligonucleotide Synthesis Laboratory, Department of Biochemistry, University of Dundee.
Construction of IgA2/A1 half hinge expression vector.
A plasmid bearing the gene for the CH1, CH2, and CH3 domains of the α chain of human IgA2m(1) downstream of the mouse VNP gene (25) was cleaved with BamHI and XhoI, and the 1.4-kb fragment containing bp 1 to 737 of the α2 constant region was ligated into BamHI-XhoI-cut pSP73 (Promega, Southampton, United Kingdom). The resultant plasmid was used as a template for PCR overlap extension mutagenesis (13) using flanking primers A1H6 and A1H5 and internal primers A1H2 and A1H3. Thus, a fragment containing half of the hinge region of human IgA1 inserted between the CH1 and CH2 regions of human IgA2m(1) was engineered. Following digestion with BamHI and XhoI, the PCR product was ligated into the BamHI-XhoI-cut site of the original IgA2 expression vector, replacing the wild-type sequence in this region. Sequencing of the PCR-amplified region was performed by the dideoxy-chain termination method (7).
Preparation of recombinant hybrid IgA2/A1 half hinge immunoglobulin.
CHO-K1 cells were maintained as described previously (25). CHO-K1 cells stably transfected previously with an appropriate mouse λ light (L) chain (25) were seeded in tissue culture-grade petri dishes and transfected with the IgA2/A1 hybrid expression vector by using calcium phosphate as described previously (25). Positive transfectants were isolated by selection for the bacterial xanthine-guanine phosphoribosyltransferase selectable marker by growth in medium supplemented with hypoxanthine and thymidine (HT supplement; Life Technologies, Paisley, United Kingdom), xanthine (0.25 mg/ml), and mycophenolic acid (10 μg/ml). Several resistant colonies were picked, and cell lines producing the highest yields of IgA were identified by an enzyme-linked immunosorbent assay measuring binding to the antigen NIP (3-nitro-4-hydroxy-5-iodophenylacetate) as described previously (25) before expansion into large cultures. Recombinant antibodies were purified from supernatants of CHO-K1 transfectants by affinity chromatography on NIP-Sepharose as described previously (25). The purified antibodies were supplemented with 0.04% sodium azide and stored in small aliquots at −20°C.
Recombinant IgA1 and IgA2.
The antibodies were prepared in similar ways from previously described stable CHO-K1 transfectants (25).
Rosette assays.
Human erythrocytes were derivatized by incubation in isotonic borate buffer (pH 8.5) containing NIP-caproate-O-succinimide (100 μg/ml; Genosys, Cambridge, United Kingdom) for 1 h at room temperature. The cells were then washed three times with phosphate-buffered saline (PBS) before and after fixation for 30 min with 3% glutaraldehyde prior to sensitization with essentially saturating amounts (>250 μg/ml) of wild-type IgA1 or IgA2 or IgA2/A1 half hinge, making use of their specificity for the hapten NIP, as described previously (39). Coating levels for each antibody were found to be equivalent by reactivity with goat anti-human IgA-fluorescein isothiocyanate conjugate (Caltag; Bradsure Biologicals, Loughborough, United Kingdom) as assessed by flow cytometry. Neutrophils were isolated as previously described (27) from heparinized blood taken from healthy volunteers. Rosetting of sensitized erythrocytes to neutrophils was performed in V-bottomed microtiter plates as described previously (39). After addition of acridine orange solution (6 μg/ml, final concentration) to stain nucleated cells, the cells were resuspended and examined by UV microscopy for rosetting. A rosette was defined as a fluorescent neutrophil with three or more erythrocytes attached.
Chemiluminescence assay of respiratory bursts.
Wells of a chemiluminescence microtiter plate (Dynatech, Billinghurst, Sussex, United Kingdom) were coated with 150 μl of 10 μg of NIP-BSA/ml in coating buffer (0.1 M sodium carbonate buffer [pH 9.6]) and incubated overnight at 4°C. After three washes with PBS, 150 μl of diluted antibody (50 μg/ml) was added in triplicate to the wells and left overnight at 4°C. After three washes in PBS, 100 μl of luminol (67 μg/ml in Hanks' balanced salt solution [HBSS] containing 20 mM HEPES buffer and 0.1% [wt/vol] globulin-free BSA [HBSS-BSA]) was added to each well. Following the addition of 50 μl of neutrophils (106/ml in HBSS-BSA) to each well, the plate was transferred to a Microlumat LB96P luminometer, and chemiluminescence was measured at regular intervals for 1 h.
Microbial IgA1 proteases.
The IgA1 proteases used were from S. pneumoniae type 23 strain 3626, S. oralis NCTC 11427, S. sanguis biovar 2 strain SK4, H. influenzae HK368, R11, R12, R14, R16, R20, R25, and R27 (all type 1 enzyme), H. influenzae 110023H and R4 (both type 2 enzyme), N. meningitidis group B serotype 14 strain 3564 (type 1 enzyme), N. meningitidis group Y serotype 2c strain HF13 (type 2 enzyme), N. gonorrhoeae 3548 serogroup W1 serovar IA-6 (type 1 enzyme), N. gonorrhoeae 3547 serogroup W11/111 serovar IB-1 (type 2 enzyme), and P. melaninogenica ATCC 25845. The enzymes from S. sanguis SK4, H. influenzae HK368, N. meningitidis HF13, and P. melaninogenica ATCC 25845 were pure; the others were partially purified and either concentrated from liquid culture supernatants or prepared as previously described (34) from the bacteria grown on dialysis tubing covering appropriate culture media, blood agar, heated blood agar, or modified New York City agar for 3 days at 37°C in 5% CO2. The enzyme preparations were stored at −20°C.
Digestion of recombinant IgA preparations with microbial IgA1 proteases and immunoblotting.
Initial preliminary experiments determined the appropriate volumes of protease and antibody to use to permit assessment of cleavage by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Such volumes of recombinant IgA1 or IgA2 or hybrid IgA2/A1 and the microbial IgA1 protease preparations were added to PBS (pH 7.2) containing 0.1% sodium azide to give a total volume of 20 μl. In the case of P. melaninogenica protease, the buffer used was 0.1 M sodium phosphate (pH 5.5) containing 0.1 mM EDTA and 0.1 mM dithiothreitol. The reaction mixtures were incubated at 37°C for 72 h prior to analysis on SDS–10% polyacrylamide gels under reducing and nonreducing conditions. The proteins were then transferred to nitrocellulose membranes, which were then blocked by agitation for 30 min in 5% nonfat dried milk powder in PBS. After thorough washing in PBS, the membranes were immersed in horseradish peroxidase-labeled antibody, either sheep anti-human IgA Fc antibody (Sigma) or goat anti-human IgA (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) or sheep anti-mouse λ L-chain antibody (Nordic Immunological Laboratories, Tilburg, The Netherlands) diluted 1:1,000 in PBS containing 0.1% Tween 20 (PBST) and agitated for 2 h at room temperature.
When examination was to be made for binding of biotinylated lectins, the nitrocellulose membranes were blocked by immersion in 1% BSA in PBST and agitation for 30 min. After incubation with the biotinylated lectin (Vector Laboratories, Peterborough, United Kingdom) for 1 to 2 h at room temperature and thorough washing in PBS, the membranes were incubated with 1 μg of streptavidin-labeled horseradish peroxidase per ml in PBS for 30 min at room temperature.
In all instances, after thorough washing in PBS, the membranes were developed in 10 ml of 50 mM Tris-HCl (pH 7.6) buffer containing 0.3 mg of nickel chloride per ml, 10 mg of diaminobenzidine, and 60 μl of 30% hydrogen peroxide.
RESULTS
Expression of IgA2/A1 half hinge in CHO-K1 cells.
DNA sequence analysis of the IgA2/A1 half hinge expression vector confirmed that nucleotides 667 to 687 of α1 had been correctly incorporated between nucleotides 688 and 689 of α2 and that no misincorporations had occurred during PCR amplification.
Analysis of the IgA2/A1 half hinge antibody expressed in CHO-K1 cells showed that in the reduced form, the hybrid α2/α1 chain appeared as two glycoprotein bands of 68 and 63 kDa (Fig. 2). These differed only in the extent of N-glycosylation, for after incubation with recombinant peptide-N-glycosidase F (Glyko, Upper Heyford, Nr. Bicester, United Kingdom), which removes N-linked oligosaccharides, the hybrid α2/α1 chain no longer bound biotinylated concanavalin A and appeared as a single band of ca. 58 kDa (Fig. 2). This size was similar to or possibly a little smaller than that of the deglycosylated wild-type α1 chain and a little bigger than that of the deglycosylated wild-type α2 chain as expected. Analysis of the hybrid IgA2/A1 in its nonreduced form by SDS-PAGE and immunoblotting revealed a major band of about 50 kDa reactive with anti-L-chain antibody and another of about 120 kDa reactive with anti-IgA α-chain antibody (Fig. 3). Equivalent bands were observed with the wild-type IgA2m(1) and have been identified earlier as L-chain dimers and heavy (H)-chain dimers, respectively (25). These dimers arise under nonreducing conditions because in the majority of IgA2m(1) molecules, in contrast to most other antibody molecules, disulfide bonds do not form between H and L chains. Instead, the L chains are disulfide bonded to each other (24). The recombinant hybrid IgA2/A1 antibody appeared to share this bonding pattern, as was expected, given that it retained the elements of IgA2m(1) involved in interchain disulfide bridge formation. In wild-type IgA1 the H chains are disulfide bonded to each other and to the L chains, resulting, under nonreducing conditions, in a band of about 170 kDa which was reactive with both anti-H-chain and anti-L-chain reagents (Fig. 3). A 170-kDa band containing both H and L chains was also evident in wild-type IgA2m(1) and to a lesser extent in IgA2/A1 half hinge (Fig. 3). It is now appreciated that disulfide bonds can be formed between H and L chains in IgA2m(1) but with low frequency (8), and this obviously also applies to the IgA2/A1 half hinge molecule.
FIG. 2.
Western blot analysis of proteins separated under reducing conditions of recombinant wild-type IgA1 (lanes 1 and 4), recombinant hybrid IgA2/A1 half hinge (lanes 2 and 5), and recombinant wild-type IgA2 (lanes 3 and 6) after being either untreated (lanes 1 to 3) or incubated with N-glycosidase F (lanes 4 to 6) and probed with anti-human IgA-peroxidase conjugate. Positions of molecular mass markers in kilodaltons are shown on the left. Treatment of the two-banded hybrid IgA2/A1 half hinge glycoprotein with N-glycosidase F removed N-linked oligosaccharides to give a smaller single protein band similar in size to deglycosylated IgA1 and larger than deglycosylated IgA2.
FIG. 3.
Western blot analysis of proteins separated under nonreducing conditions of recombinant IgA1 (lanes 1 and 5), recombinant hybrid IgA2/A1 half hinge (lanes 2 and 6), and recombinant IgA2 (lanes 3 and 7) after blotting with anti-mouse λ L-chain-peroxidase conjugate (lanes 1 to 3) or anti-human IgA Fc-peroxidase conjugate (lanes 5 to 7). Positions of molecular mass markers (lane 4) in kilodaltons are indicated on the left.
The hybrid IgA2/A1 half hinge molecule was examined for the presence of O-glycosylated amino acids in the half-hinge region by determining its ability to bind the biotinylated lectin jacalin (from Artocarpus integrifolia). The results showed that like the wild-type IgA1, the hybrid IgA2/A1 half hinge molecule bound jacalin and thus contained O-glycosylated amino acids, whereas, as expected, the wild-type IgA2 molecule did not (Fig. 4).
FIG. 4.
Western blot analysis of proteins separated under reducing conditions of recombinant IgA1 (lanes 1 and 5), recombinant hybrid IgA2/A1 half hinge (lanes 2 and 6), and recombinant IgA2 (lanes 3 and 7) after probing either with anti-human IgA-peroxidase conjugate (lanes 1 to 3) or, after reaction with biotinylated jacalin, probing with streptavidin-peroxidase conjugate (lanes 5 to 7). Positions of molecular mass markers (lanes 4 and 8) in kilodaltons are shown at the right. The amounts of IgA in lanes 1 to 3 were the same as their equivalents in lanes 5 to 7. The lectin jacalin bound to recombinant wild-type IgA1 and recombinant hybrid IgA2/A1 half hinge but not to recombinant IgA2, indicating the presence in IgA2/A1 half hinge of O-glycosylated jacalin binding amino acids in the half hinge. The highly sensitive jacalin binding analysis revealed the presence in lane 6 of trace amounts of some non-IgA glycoproteins.
Ability of IgA2/A1 half hinge to bind and trigger FcαR.
Analysis of the functional activity of the hybrid IgA2/A1 molecule showed it to be comparable to wild-type IgA1 and IgA2 in the ability to mediate rosette formation between NIP-coated erythrocytes and neutrophils through interaction with Fcα receptors (FcαR) (Table 1). Moreover, chemiluminescence assays revealed that the hybrid IgA2/A1 half hinge molecule bound efficiently to FcαR on neutrophils and promoted a respiratory burst (Fig. 5). The respiratory bursts stimulated by the recombinant IgA1 and IgA2 mirror closely those reported earlier for serum-derived IgA1 and IgA2 (37). The IgA2/A1 hybrid produced a respiratory burst comparable to that produced by the wild-type antibodies. Thus, the functional activity of the recombinant IgA2/A1 hybrid in these respects was very similar to that of wild-type IgA1 and IgA2. This finding is in keeping with the localization of the FcαR binding site on IgA to the region between the CH2 and CH3 domains of the Fc, some distance from the hinge (6, 28).
TABLE 1.
Functional activity of recombinant IgA molecules by rosette assaya
| NIP-erythrocytes coated with: | % Rosettes
|
|
|---|---|---|
| Test 1 | Test 2 | |
| IgA1 | 55 | 54 |
| IgA2m(1) | 65 | 64 |
| IgA2/A1 half hinge | 50 | 52 |
| No antibody | 0 | 0 |
A rosette was defined as ≥3 erythrocytes bound to a neutrophil. In each test, 100 neutrophils were counted three times and the mean is shown. Tests 1 and 2 were performed with two different neutrophil preparations.
FIG. 5.
Stimulation of neutrophil chemiluminescence by IgA attached to NIP-BSA-coated microtiter plates. Respiratory bursts were induced by IgA1 (●), IgA2 (■), or IgA1/A2 half hinge (▴), all at 100 μg/ml. Negative controls lacking IgA, with (◊) and without (⧫) the addition of cells, are also shown. Each point shown is the mean of triplicate determinations. The experiment was performed three times each with neutrophils from a different donor. Relative light units (RLU) per second were plotted against time. The results presented are those from a typical experiment.
Activities of different microbial IgA1 proteases on IgA2/A1 half hinge.
The susceptibility of IgA2/A1 half hinge to a number of bacterial IgA1 proteases was examined. All of the enzyme preparations were shown to have IgA1 protease activity, for they all cleaved wild-type IgA1 in the hinge region to generate Fab and Fc fragments but were unable to cleave wild-type IgA2 (data not shown).
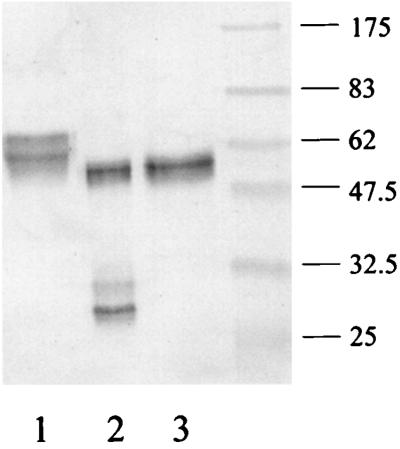
Examination of the sensitivity of wild-type IgA1 and IgA2/A1 half hinge to the different streptococcal IgA1 proteases showed that although all of the enzymes were active and cleaved IgA1 to Fab and Fc fragments, the IgA2/A1 half hinge hybrid was sensitive only to those of S. pneumoniae and S. sanguis and resistant to that of S. oralis (Fig. 6). Interpretation of the relative sizes of the cleavage products requires careful consideration of both the N-linked sugar moieties in the Fc of IgA1 (two per Fc H chain) and of IgA2/A1 (three per Fc H chain) and also the differing presence of contaminating glycosidase activity in the protease preparations. Although the proteases from S. pneumoniae and S. oralis cleaved the wild-type IgA1 to produce IgA1 fragments of the same sizes of ca. 28 and 27 kDa (Fig. 6, lanes 2 and 4), the fragments generated by cleavage with S. sanguis protease (Fig. 6, lane 3) were marginally larger. Moreover, the size of the fragments generated by cleavage of the IgA2/A1 half hinge by the protease from S. pneumoniae (Fig. 6, lane 6) differed in size from those resulting from cleavage with S. sanguis protease (Fig. 6, lane 7). The reason for this is thought to be a consequence of the S. pneumoniae (and S. oralis) protease preparations, but not that of S. sanguis, also having glycosidase activity as has been observed previously (33). Thus, when the S. pneumoniae cleavage of IgA2/A1 half hinge was repeated in the presence of 25 mM EDTA to inhibit the S. pneumoniae IgA1 metalloproteinase activity, although there was no proteolytic cleavage of IgA2/A1, the hybrid antibody was nevertheless deglycosylated to a protein of ca. 56 kDa (Fig. 7). The difference in the sizes of the IgA fragments produced by the different enzymes is more marked for the IgA2/A1 half hinge than for the wild-type IgA1, presumably because of the loss of three sugar moieties in the former, compared with just two in the latter.
FIG. 6.
Action of different streptococcal IgA1 proteases on IgA2/A1 half hinge, determined by Western blot analysis of proteins separated under reducing conditions of wild-type IgA1 (lanes 1 [control] to 4) and IgA2/A1 half hinge (lanes 5 [control] to 8) digested with IgA1 protease from S. pneumoniae (lanes 2 and 6), S. sanguis (lanes 3 and 7), and S. oralis (lanes 4 and 8) and probed with anti-human IgA-peroxidase conjugate. Positions of molecular mass markers are shown in kilodaltons on the right. The hybrid IgA2/A1 was cleaved by the proteases of S. pneumoniae and S. sanguis but was resistant to that of S. oralis.
FIG. 7.
Action of S. pneumoniae IgA1 protease preparation on IgA2/A1 half hinge in the presence and absence of EDTA. Shown is Western blot analysis of proteins separated under reducing conditions of IgA2/A1 half hinge (lanes 1 [control] to 3) digested with S. pneumoniae enzyme preparation (lanes 2 and 3) in the absence (lane 2) and presence (lane 3) of 25 mM EDTA and probed with anti-human IgA-peroxidase conjugate. Positions of molecular mass markers in kilodaltons are shown on the right. The EDTA inhibited S. pneumoniae proteolytic cleavage of the α chain but not glycosidase activity and reduction in mass of the immunoglobulin.
The action on wild-type IgA1 and the IgA2/A1 half hinge of the IgA1 proteases of S. pneumoniae, N. meningitidis type 2, and N. gonorrhoeae type 1, which cleave at different sites in the full hinge of IgA1 (Fig. 1), is shown in Fig. 8. All of these enzymes which cleaved IgA1 were also found to be able to cleave the hybrid IgA2/A1. The lower molecular weight of the fragments produced by the S. pneumoniae protease than of the fragments produced by the different Neisseria proteases is presumably due to the additional activity of glycosidases in the S. pneumoniae preparation (see above). The hybrid IgA2/A1 was also found to be sensitive to the type 1 protease of N. meningitidis and the type 2 protease of N. gonorrhoeae (results not shown), which cleave the same peptide bonds in IgA1 as N. gonorrhoeae type 1 protease and N. meningitidis type 2 protease, respectively. Moreover, the hybrid IgA2/A1 was also sensitive to P. melaninogenica protease, which cleaves IgA1 at the opposite end of the full hinge in IgA1 (Fig. 1) to that cleaved by the type 1 protease of N. meningitidis (Fig. 9).
FIG. 8.
Western blot analysis of proteins separated under reducing conditions of wild-type IgA1 (lanes 1 [control] to 4) and IgA2/A1 half hinge (lanes 5 [control] to 8) digested with IgA1 proteases from S. pneumoniae (lanes 2 and 6), N. meningitidis type 2 (lanes 3 and 7), and N. gonorrhoeae type 1 (lanes 4 and 8) and immunoblotted with anti-human IgA-peroxidase conjugate. Positions of molecular mass markers in kilodaltons are indicated on the right. Both IgA1 and the hybrid IgA2/A1 were cleaved by all of these proteases.
FIG. 9.
Western blot analysis of proteins separated under reducing conditions of wild-type IgA1 (lanes 1 [control] and 2) and IgA2/A1 half hinge (lanes 3 [control] and 4) digested with P. melaninogenica IgA1 protease and probed with anti-human IgA-peroxidase conjugate. Positions of molecular mass markers in kilodaltons are indicated on the right. The IgA1 protease of P. melaninogenica cleaved both IgA1 and the hybrid IgA2/A1.
Investigations into the sensitivity of IgA2/A1 half hinge to type 1 and type 2 IgA1 proteases of H. influenzae showed that although the hybrid immunoglobulin was cleaved by the type 2 enzyme, it was much more resistant to the type 1 enzyme of strain HK368. Further investigation with type 1 enzymes from seven different H. influenzae strains of biotypes I (R27), II (R12, R14, and R16), III (R20), IV (R11), and VII (R25) showed that the IgA2/A1 hybrid was sensitive to four of these type 1 proteases but resistant to three of them (Fig. 10).
FIG. 10.
Western blot analysis of proteins separated under reducing conditions of wild-type IgA1 (A) and IgA2/A1 half hinge (B) (lane 1, controls) digested with type 2 protease of H. influenzae 110023H and R4 (lanes 2 and 7, respectively) and type 1 protease of H. influenzae R11, R12, R14, R16, R20, R25, and R27 (lanes 3 to 6 and 8 to 10, respectively) and probed with anti-human IgA-peroxidase conjugate. The proteases of all strains were active on wild-type IgA1, but although the type 2 enzyme of H. influenzae cleaved the hybrid IgA2/A1 immunoglobulin, it was resistant to the type 1 protease of some H. influenzae strains (R12, R25, and R27).
DISCUSSION
Microbial IgA1 proteases are extremely specific. Excepting peptide bonds present in precursors of the enzymes which are cleaved in their processing, LAMP1 (the major integral membrane protein of lysosomes) (19), and some outer membrane proteins of N. gonorrhoeae (36), the only known substrate of IgA1 protease is IgA1 of humans, gorillas, chimpanzees, and orangutans (32). Little is known about what determines the specificity of IgA1 proteases. The fact that they cleave human IgA1 at specific sites in only one of the two available duplicated sites in the hinge suggests that the two duplicated half hinges have different conformations or that the enzymes recognize additional elements distant from the cleavage site.
In an attempt to gain further information about the requirements of IgA for sensitivity to IgA1 proteases and the determinants of specificity of IgA1 proteases, seven amino acids representing a half hinge region of human IgA1 were introduced into protease-resistant human IgA2 to create an artificial half hinge. The hybrid IgA2/A1 molecule was found to have an arrangement similar to that of IgA2m(1) with regard to the bonding of its H and L chains and was deemed to be functionally active in that it could form rosettes and bind efficiently to FcαR on neutrophils and trigger a respiratory burst. However, it now possessed the O-glycosylated amino acids of half the hinge of IgA1 and showed sensitivity to several diverse IgA1 proteases.
Determination of the exact site of cleavage by the enzymes in the IgA2/A1 hybrid was felt beyond the scope of this study because of the work involved in analyzing the cleavage products generated by so many different enzymes. However, because of the known extreme specificity of bacterial IgA1 proteases, it is not unreasonable to assume that the cleavage site for each enzyme in the IgA2/A1 half hinge hybrid is identical to its natural cleavage site in one of the duplicated half hinge regions of the wild-type IgA1 molecule, although conclusive proof requires amino acid sequence analysis of the cleavage products.
The IgA1 proteases active on the IgA2/A1 half hinge hybrid included representatives of those which cleave at specific sites in both of the duplicated half hinges of IgA1, namely, the IgA1 proteases of S. pneumoniae, S. sanguis, H. influenzae 2, N. gonorrhoeae 1 and 2, N. meningitidis 1 and 2, and P. melaninogenica (Fig. 1). Thus, for these enzymes it would appear that although they cleave IgA1 naturally at a specific peptide bond in only one of the duplicated half hinge areas (the preferred cleavage site), if the specific peptide is represented only once as in the IgA2/A1 half hinge hybrid, these enzymes will still cleave IgA, thereby overriding the determinants of site selectivity. The results also indicate that for these enzymes the recognition site for IgA cleavage is contained in a half hinge region or that if additional more distal elements are required, the framework of IgA2 substitutes reasonably adequately for that of IgA1. These results support the work of Pohlner et al. (29) and that of others (1) who have suggested that the consensus target sequence for serine-type IgA1 proteases of Neisseria and Haemophilus is either P-P↓S/T-P or P-X-P↓S/T/ST-P, where X is any amino acid, S/T is serine or threonine, and S/T/ST is serine or threonine or both. These sequences are provided in the IgA2/A1 half hinge molecule. A proline as the amino acid N-terminal to the cleaved peptide bond is a requirement for Haemophilus and Neisseria IgA1 proteases (1). Proline residues introduce bends into polypeptide chains, and these may expose sites for essential protein-protein interactions.
It was not very surprising, therefore, to find that the IgA2/A1 hybrid was sensitive to most of the IgA1 proteases of Haemophilus and Neiserria spp., for these are serine-type proteases that can be inhibited by short peptides. Bachovchin et al. (1) showed that tri- and tetrapeptide prolyl boronic acid analogues could block the active site of the serine-type IgA1 proteases of Haemophilus and Neisseria (but not that of the metalloproteinase IgA1 protease of S. sanguis) and that synthetic short peptides were cleaved more slowly than the IgA1 hinge. This suggests that maximum efficiency in cleavage occurs only when the substrate has the correct length and conformation. In support of this conclusion, it was repeatedly found that the cleavage of the IgA2/A1 hybrid by IgA1 proteases from some organisms was less complete than that of wild-type IgA1 after incubation for similar periods. This suggests not only that cleavage of IgA requires the presence of a cleavable peptide bond at the correct location but also that other parts of the molecule influence its sensitivity to IgA protease cleavage. Although in this study substrate-enzyme reactions were usually incubated for 72 h, this was done in order not to miss substrate cleavage by any slow-acting IgA1 protease on the IgA2/A1 hybrid. In fact, all IgA1 proteases that hydrolyzed the hybrid demonstrated cleavage within 16 h. A more detailed comparison of the kinetics of IgA1 protease cleavage of the IgA2/A1 half hinge with that of wild-type IgA1 is to be the subject of a separate investigation.
The reason for the resistance of the IgA2/A1 hybrid to some H. influenzae type 1 proteases is not clear. It is known for organisms like H. influenzae (and N. meningitidis), which produce type 1 and type 2 proteases which cleave at different sites within one of the duplicated half hinge sites, that the site of cleavage is determined by a region near the amino-terminal end of each protease known as the cleavage site determinant (CSD) (12). Comparisons between the CSDs of different organisms have shown that the CSD length varies with the enzyme and is proportional to the distance between the interchain disulfide bridge at the top of the CH2 domains prior to the hinge, i.e., Cys 241, and the specific peptide bond cleaved by the enzyme (21). It has been suggested that the CSD acts as a spacer between the catalytic site and the substrate recognition site. Consistent with this is the finding that cleavage appears to be dependent on hinge structural features C-terminal to the susceptible peptide bond because sequential incubation with different IgA1 proteases resulted in cleavage of Fc but not Fab fragments (21). As the CSD of H. influenzae type 1 protease is bigger than that of all of the other enzymes (21) and there is known to be much variation in the CSDs of type 1 proteases of H. influenzae, it is possible that for some H. influenzae type 1 proteases a single half hinge site is too small to accommodate such a large CSD spacer and the enzyme is directed to act at a site outside the half hinge where a Pro-Ser bond is not present for cleavage. Alternatively, because the IgA1 proteases of H. influenzae are the most antigenically diverse, more than 30 antigenic types having been described on the basis of antibody neutralization tests (17, 22), it may be that some are unable to cleave the IgA2/A1 hybrid because of steric hindrance due to their increased bulk and the closer approach of the Fc and Fab arms.
The inability of the protease of S. oralis alone among the streptococcal IgA1 proteases to cleave the IgA2/A1 hybrid is difficult to understand and explain, for all are metalloproteinases, the cleavage site on IgA1 is the same for all, and their amino acid sequences are highly homogeneous (31). Moreover, unlike the situation with H. influenzae type 1 proteases, the proteases of S. oralis are all of one antigenic type (33). However, the iga protease gene in S. oralis contains elements displaying subtle differences from those of S. sanguis and S. pneumoniae which may contribute to a particular cleavage site specificity. It is also possible that the S. oralis protease requires structures outside the half hinge in the hybrid antibody for which IgA2 elements are not an acceptable alternative to IgA1 elements for substrate recognition.
The susceptibility of IgA1 to IgA1 proteases can be influenced by its state of glycosylation (33), and it is possible that the carbohydrates in the hinge region contribute to the specificity of IgA1 proteases. The IgA1 hinge region contains several potential sites for O-linked glycosylation (2, 33). Classically, in studies of IgA1 myelomas, these have been considered to be the five serine residues, four of which have galactosyl-β1-3-N-acetylgalactosamine groups (and possibly sialic acid ;[33;]) whereas Ser 224 has N-acetylgalactosamine (10). More recently, however, analysis of IgA1 from serum indicates that the sugars are O-linked via both serine and threonine residues asymmetrically distributed between the two duplicated halves of the hinge (22). Thus, it could be argued that the two duplicated half hinges in wild-type IgA1 are distinguishable on the basis of differences in glycosylation.
The half hinge region of the IgA2/A1 hybrid is believed to be O-glycosylated, for it was found to bind the biotinylated lectin jacalin, which is specific for the O-linked sugars restricted to the hinge of IgA1. Although the exact state of glycosylation of the half hinge region is not known, it can at best presumably resemble only one of the half hinges of IgA. Thus, it is unlikely that the O-linked sugars act as determinants of specificity and direct the protease specifically to one of the duplicated half hinge regions in IgA1 because representatives of proteases acting in each of these different regions in IgA1 were all able to cleave IgA2/A1 half hinge. If the half hinge in the IgA2/A1 hybrid is underglycosylated or glycosylated incorrectly in other ways, it is unlikely that this is the reason for the resistance of the molecule to the S. oralis and some H. influenzae type 1 proteases because we have observed (M. R. Batten, B. W. Senior, M. Kilian, and J. M. Woof, unpublished data) that when O-linked glycosylation of the IgA1 hinge region was perturbed through substitution of its serine residues with alanine and that of threonine 225 with valine, the modified IgA1 molecules nevertheless remained sensitive to virtually all types of IgA1 proteases, including those of S. oralis and H. influenzae type 1.
A molecular model for human IgA1 based on small-angle X-ray and neutron-scattering analysis has recently been generated (4). The average conformation of the antibody is predicted to be T shaped, with the Fab arms widely separated. The hinge peptides are suggested to adopt extended, exposed structures, presumably readily accessible to IgA1 proteases. In the IgA2/A1 hybrid, a similar structural arrangement may be present but with the Fab arms held much closer to the Fc portion. A maintained exposure of the shortened hinge might then explain the continued access, recognition, and thus sensitivity of the molecule to most of the IgA1 proteases, whereas the closer proximity of the Fab and Fc regions may for some IgA1 proteases present an unfavorable arrangement for access or substrate recognition or both.
In summary, this study has shown that through the insertion into protease-resistant IgA2 of seven amino acids representing half the hinge region of IgA1, a hybrid IgA2/A1 molecule that was functionally active and sensitive to many different bacterial IgA1 proteases was formed.
ACKNOWLEDGMENTS
We thank R. Pleass for helpful discussions.
This work was supported by the Wellcome Trust.
REFERENCES
- 1.Bachovchin W W, Plaut A G, Flentke G R, Lynch M, Kettner C A. Inhibition of IgA1 proteinases from Neisseria gonorrhoeae and Haemophilus influenzae by peptide prolyl boronic acids. J Biol Chem. 1990;265:3737–3743. [PubMed] [Google Scholar]
- 2.Baenziger J, Kornfeld S. Structure of the carbohydrate units of IgA immunoglobulin. II. Structure of the O-glycosidically linked oligosaccharide units. J Biol Chem. 1974;249:7270–7281. [PubMed] [Google Scholar]
- 3.Blake M, Holmes K K, Swanson J. Studies on gonococcus infection. XVII. IgA1-cleaving protease in vaginal washings from women with gonorrhea. J Infect Dis. 1979;139:89–92. doi: 10.1093/infdis/139.1.89. [DOI] [PubMed] [Google Scholar]
- 4.Boehm M K, Woof J M, Kerr M A, Perkins S J. The Fab and Fc fragments of IgA1 exhibit a different arrangement from that in IgG: a study by X-ray and neutron scattering and homology modelling. J Mol Biol. 1999;286:1421–1447. doi: 10.1006/jmbi.1998.2556. [DOI] [PubMed] [Google Scholar]
- 5.Brooks G F, Lammel C J, Blake M S, Kusecek B, Achtman M. Antibodies against IgA protease are stimulated both by clinical disease and asymptomatic carriage of serogroup A Neisseria meningitidis. J Infect Dis. 1992;166:1316–1321. doi: 10.1093/infdis/166.6.1316. [DOI] [PubMed] [Google Scholar]
- 6.Carayannopoulos L, Hexham J M, Capra J D. Localization of the binding site for the monocyte immunoglobulin (Ig)A-Fc receptor (CD89) to the domain boundary between Cα2 and Cα3 in human IgA1. J Exp Med. 1996;183:1579–1586. doi: 10.1084/jem.183.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen E Y, Seeburg P H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985;4:165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- 8.Chintalacharuvu K R, Morrison S L. Residues critical for H-L disulfide bond formation in human IgA1 and IgA2. J Immunol. 1996;157:3443–3449. [PubMed] [Google Scholar]
- 9.Devenyi A G, Plaut A G, Grundy F J, Wright A. Post-infectious human serum antibodies inhibit IgA1 proteinases by interaction with the cleavage site specificity determinant. Mol Immunol. 1993;30:1243–1248. doi: 10.1016/0161-5890(93)90039-e. [DOI] [PubMed] [Google Scholar]
- 10.Frangione B, Wolfenstein-Todel C. Partial duplication of the hinge region of IgA1 myeloma proteins. Proc Natl Acad Sci USA. 1972;69:3673–3676. doi: 10.1073/pnas.69.12.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbert J V, Plaut A G, Longmaid B, Lamm M E. Inhibition of microbial IgA proteases by human secretory IgA and serum. Mol Immunol. 1983;20:1039–1049. doi: 10.1016/0161-5890(83)90045-7. [DOI] [PubMed] [Google Scholar]
- 12.Grundy F J, Plaut A G, Wright A. Localization of the cleavage site specificity determinant of Haemophilus influenzae immunoglobulin A1 protease genes. Infect Immun. 1990;58:320–331. doi: 10.1128/iai.58.2.320-331.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horton R M, Hunt H D, Ho S N, Pullen J K, Pease L R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- 14.Insel R A, Allen P Z, Berkowitz I D. Types and frequency of Haemophilus influenzae IgA1 proteases. Semin Infect Dis. 1982;4:225–231. [Google Scholar]
- 15.Kilian M, Reinholdt J, Lomholt H, Poulsen K, Frandsen E V G. Biological significance of IgA1 proteases in bacterial colonization and pathogenesis: critical evaluation of experimental evidence. APMIS. 1996;104:321–338. doi: 10.1111/j.1699-0463.1996.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 16.Kilian M, Russell M W. Function of mucosal immunoglobulins. In: Ogra P L, et al., editors. Handbook of mucosal immunology. London, England: Academic Press; 1994. pp. 127–137. [Google Scholar]
- 17.Kilian M, Thomsen B, Petersen T E, Bleeg H. Molecular biology of Haemophilus influenzae IgA1 proteases. Mol Immunol. 1983;20:1051–1058. doi: 10.1016/0161-5890(83)90046-9. [DOI] [PubMed] [Google Scholar]
- 18.Liljemark W F, Bloomquist C G, Ofstehage J. Aggregation and adherence of Streptococcus sanguis: role of human salivary immunoglobulin A. Infect Immun. 1979;26:1104–1110. doi: 10.1128/iai.26.3.1104-1110.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin L, Ayala P, Larson J, Mulks M, Fukuda M, Carlsson S R, Enns C, So M. The Neisseria type 2 IgA1 protease cleaves LAMP1 and promotes survival of bacteria within epithelial cells. Mol Microbiol. 1997;24:1083–1094. doi: 10.1046/j.1365-2958.1997.4191776.x. [DOI] [PubMed] [Google Scholar]
- 20.Lindh E. Increased resistance of immunoglobulin A dimers to proteolytic degradation after binding of secretory component. J Immunol. 1975;114:284–286. [PubMed] [Google Scholar]
- 21.Lomholt H, Poulsen K, Kilian M. Comparative characterization of the iga encoding IgA1 protease in Neiserria meningitidis, Neisseria gonorrhoeae, and Haemophilus influenzae. Mol Microbiol. 1995;15:495–506. doi: 10.1111/j.1365-2958.1995.tb02263.x. [DOI] [PubMed] [Google Scholar]
- 22.Lomholt H, van Alphen L, Kilian M. Antigenic variation of immunoglobulin A1 proteases among sequential isolates of Haemophilus influenzae from healthy children and patients with chronic obstructive pulmonary disease. Infect Immun. 1993;61:4575–4581. doi: 10.1128/iai.61.11.4575-4581.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattu T S, Pleass R J, Willis A C, Kilian M, Wormald M R, Lellouch A C, Rudd P M, Woof J M, Dwek R A. The glycosylation and structure of human serum IgA1, Fab and Fc regions and the role of N-glycosylation on Fcα receptor interactions. J Biol Chem. 1997;273:2260–2272. doi: 10.1074/jbc.273.4.2260. [DOI] [PubMed] [Google Scholar]
- 24.Mestecky J, Kilian M. Immunoglobulin A (IgA) Methods Enzymol. 1985;116:37–75. doi: 10.1016/s0076-6879(85)16005-2. [DOI] [PubMed] [Google Scholar]
- 25.Morton H C, Atkin J D, Owens R J, Woof J M. Purification and characterization of chimeric human IgA1 and IgA2 expressed in COS and Chinese hamster ovary cells. J Immunol. 1993;151:4743–4752. [PubMed] [Google Scholar]
- 26.Plaut A G. The IgA1 proteases of pathogenic bacteria. Annu Rev Microbiol. 1983;37:603–622. doi: 10.1146/annurev.mi.37.100183.003131. [DOI] [PubMed] [Google Scholar]
- 27.Pleass R J, Andrews P D, Kerr M A, Woof J M. Alternative splicing of the human IgA Fc receptor CD89 in neutrophils and eosinophils. Biochem J. 1996;318:771–777. doi: 10.1042/bj3180771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pleass R J, Dunlop J I, Anderson C M, Woof J M. Identification of residues in the CH2/CH3 domain interface of IgA essential for interaction with the human Fcα receptor (FcαR) CD89. J Biol Chem. 1999;274:23508–23514. doi: 10.1074/jbc.274.33.23508. [DOI] [PubMed] [Google Scholar]
- 29.Pohlner J, Halter R, Meyer T F. Neisseria gonorrhoeae IgA protease. Secretion and implications for pathogenesis. In: Poolman J T, et al., editors. Gonococci and meningococci. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1988. pp. 427–432. [Google Scholar]
- 30.Polissi A, Pontiggia A, Feger G, Altieri M, Mottl H, Ferrai L, Simon D. Large-scale identification of virulence genes from Streptococcus pneumoniae. Infect Immun. 1998;66:5620–5629. doi: 10.1128/iai.66.12.5620-5629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poulsen K, Reinholdt J, Jespersgaard C, Boye K, Brown T A, Hauge M, Kilian M. A comprehensive genetic study of streptococcal immunoglobulin A1 proteases: evidence for recombination within and between species. Infect Immun. 1998;66:181–190. doi: 10.1128/iai.66.1.181-190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu J, Brackee G P, Plaut A G. Analysis of the specificity of bacterial immunoglobulin A (IgA) proteases by a comparative study of ape serum IgAs as substrates. Infect Immun. 1996;64:933–937. doi: 10.1128/iai.64.3.933-937.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reinholdt J, Tomana M, Mortensen S B, Kilian M. Molecular aspects of immunoglobulin A1 degradation by oral streptococci. Infect Immun. 1990;58:1186–1194. doi: 10.1128/iai.58.5.1186-1194.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Senior B W, Albrechtsen M, Kerr M A. Proteus mirabilis strains of diverse type have IgA protease activity. J Med Microbiol. 1987;24:175–180. doi: 10.1099/00222615-24-2-175. [DOI] [PubMed] [Google Scholar]
- 35.Senior B W, Loomes L M, Kerr M A. Microbial IgA proteases and virulence. Rev Med Microbiol. 1991;2:200–207. [Google Scholar]
- 36.Shoberg R J, Mulks M. Proteolysis of bacterial membrane proteins by Neisseria gonorrhoeae type 2 immunoglobulin A1 protease. Infect Immun. 1991;59:2535–2541. doi: 10.1128/iai.59.8.2535-2541.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart W W, Kerr M A. The specificity of the human neutrophil IgA receptor (FcαR) determined by measurement of chemiluminescence induced by serum or secretory IgA1 or IgA2. Immunology. 1990;71:328–334. [PMC free article] [PubMed] [Google Scholar]
- 38.Svanborg-Eden C, Svennerholm A M. Secretory immunoglobulin A and G antibodies prevent adhesion of Escherichia coli to human urinary tract epithelial cells. Infect Immun. 1978;22:790–797. doi: 10.1128/iai.22.3.790-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker M R, Kumpel B M, Thompson K, Woof J M, Burton D R, Jefferis R. Immunogenic and antigenic epitopes of immunoglobulins: binding of human monoclonal anti-D antibodies to FcRI on the monocyte-like U937 cell line. Vox Sang. 1988;55:222–228. doi: 10.1111/j.1423-0410.1988.tb04701.x. [DOI] [PubMed] [Google Scholar]
- 40.Williams R C, Gibbons R J. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science. 1972;177:697–699. doi: 10.1126/science.177.4050.697. [DOI] [PubMed] [Google Scholar]