Abstract
Background: Low-carbohydrate diets may have endocrine effects, although individual studies are conflicting. Therefore, a review was conducted on the effects of low- versus high-carbohydrate diets on men's testosterone and cortisol. Methods: The review was registered on PROSPERO (CRD42021255957). The inclusion criteria were: intervention study, healthy adult males, and low-carbohydrate diet: ≤35% carbohydrate. Eight databases were searched from conception to May 2021. Cochrane's risk of bias tool was used for quality assessment. Random-effects, meta-analyses using standardized mean differences and 95% confidence intervals, were performed with Review Manager. Subgroup analyses were conducted for diet duration, protein intake, and exercise duration. Results: Twenty-seven studies were included, with a total of 309 participants. Short-term (<3 weeks), low- versus high-carbohydrate diets moderately increased resting cortisol (0.41 [0.16, 0.66], p < 0.01). Whereas, long-term (≥3 weeks), low-carbohydrate diets had no consistent effect on resting cortisol. Low- versus high-carbohydrate diets resulted in much higher post-exercise cortisol, after long-duration exercise (≥20 min): 0 h (0.78 [0.47, 1.1], p < 0.01), 1 h (0.81 [0.31, 1.31], p < 0.01), and 2 h (0.82 [0.33, 1.3], p < 0.01). Moderate-protein (<35%), low-carbohydrate diets had no consistent effect on resting total testosterone, however high-protein (≥35%), low-carbohydrate diets greatly decreased resting (−1.08 [−1.67, −0.48], p < 0.01) and post-exercise total testosterone (−1.01 [−2, −0.01] p = 0.05). Conclusions: Resting and post-exercise cortisol increase during the first 3 weeks of a low-carbohydrate diet. Afterwards, resting cortisol appears to return to baseline, whilst post-exercise cortisol remains elevated. High-protein diets cause a large decrease in resting total testosterone (∼5.23 nmol/L).
Keywords: Testosterone, cortisol, low carbohydrate diet, ketogenic diet, systematic review, meta-analysis
Introduction
Testosterone (T) is the primary male sex hormone, and vital for reproductive development and function. Moreover, low endogenous T is associated with an increased risk of chronic disease, including type 2 diabetes (Yao et al., 2018) and cardiovascular disease (Corona et al., 2018). In many respects, cortisol is biochemically opposed to T, as the administration of exogenous cortisol lowers T (Cumming et al., 1983). Also, environmental stressors such as exercise reciprocally increase cortisol and decrease total testosterone (TT) (Brownlee et al., 2005). Similarly, the reverse relationship between cortisol and chronic disease risk is observed, with higher levels being associated with an increased risk of cardiovascular disease mortality (Vogelzangs et al., 2010). The relationship between testosterone and cortisol likely stems from their respective anabolic and catabolic properties.
In recent decades, research has found low-carbohydrate (LC) diets have positive health effects including: weight loss, decreased triglycerides, and increased high-density lipoprotein cholesterol (Dong et al., 2020). Although, increased total cholesterol is a notable exception to this (Dong et al., 2020). Interestingly, a 2021 study found an 8-week LC diet significantly increased TT (+6.43 nmol/L) (Vidić et al., 2021), suggesting LC diets may have endocrine effects. However, the 5.7 kg weight loss during this study, which is well known to increase T (Corona et al., 2013), likely confounded the results. Furthermore, cortisol has a number of glucoregulatory roles (Kuo et al., 2015), and so may also be sensitive to carbohydrate intake. The impact of carbohydrate intake on post-exercise cortisol is of particular interest, as a meta-analysis showed carbohydrate supplements during exercise significantly attenuated the rise in post-exercise cortisol (−124 nmol/L) (Moreira et al., 2007). This suggests carbohydrate intake via diet, may similarly affect post-exercise cortisol. Also, a recent narrative review has suggested macronutrients may affect the anabolic response to exercise (Zamir et al., 2021), which indicates post-exercise T may be subject to dietary influence.
Studies using isocaloric LC diets have shown conflicting effects on T and cortisol (Michalczyk et al., 2019; Zajac et al., 2014), highlighting the need for systematic review. Moreover, previous reviews have only briefly covered the topics of T, cortisol, and LC diets; with none including meta-analyses or statistical investigations of heterogeneity (Kang et al., 2020). Of particular interest is the duration of LC diets, as these diets have an adaptation period of ∼3 weeks (Burke et al., 2017), or longer (Sherrier and Li, 2019). Thus, the effects on steroid hormones may differ pre- and post-adaptation. In addition, LC diets can either be high-protein (HP) or moderate-protein (MP), which may further help to differentiate effects. Therefore, we conducted a systematic review and meta-analysis, with statistical investigations of heterogeneity, on the effects of LC versus high-carbohydrate (HC) diets on men's T and cortisol.
Methods
The review was registered on PROSPERO (CRD42021255957) (Whittaker and Harris, 2021), and reported according to the PRISMA 2020 checklists (Supplementary Appendix - Tables 1, 2, and 3) (Page et al., 2021; Rethlefsen et al., 2021). The methods of this review are partly based on a previous systematic review by the lead author (Whittaker and Wu, 2021). The Cochrane Handbook for Systematic Reviews of Interventions was consulted throughout the review process (Higgins, Thomas, et al., 2021).
Eligibility criteria
Studies were eligible which met all of the following criteria.
Intervention study.
Measurement via blood sample of either resting TT; resting cortisol; 0 h, 1 h or 2 h post-exercise cortisol.
Healthy adult male participants (18 + years), to minimize variation in steroid hormone metabolism due to age, sex, and disease.
A LC diet ≤35% and HC diet >35% carbohydrate intake, measured as a percentage of total energy intake (TEI).
A difference in carbohydrate intake between LC and HC diets ≥20% of TEI, to sufficiently distinguish intervention diets on the basis of carbohydrate intake.
Duration of intervention diets ≥24 h, to minimize the influence of acute post-prandial variations in steroid hormones.
None of the following confounding variables which may affect steroid hormone metabolism: body mass change ≥3 kg (Corona et al., 2013), carbohydrate loading on LC diets (Wilson et al., 2020), exogenous hormones, phytoestrogens (Domínguez-López et al., 2020), or medications besides over-the-counter ones (Schooling et al., 2013).
In the literature, there is no consensus on what carbohydrate intake constitutes a LC diet. The UK government's dietary guidelines recommend ∼55% carbohydrate intake (Public Health England, 2016). Since, we required ≥20% difference in carbohydrate intake between intervention diets, a LC diet was defined as ≤35% carbohydrate intake. TEI rather than grams was used to measure carbohydrate intake, to account for participant differences in energy intake requirements. Randomized and non-randomized studies were included to provide a more comprehensive assessment of the evidence. <3 kg body mass change was deemed acceptable, as very LC diets incur ∼1.4 kg water weight loss (Hall et al., 2016). Studies not reporting body mass change were still eligible, provided that intervention diets were isocaloric, which was pragmatically defined as diets within 15% TEI of each other.
Search strategy
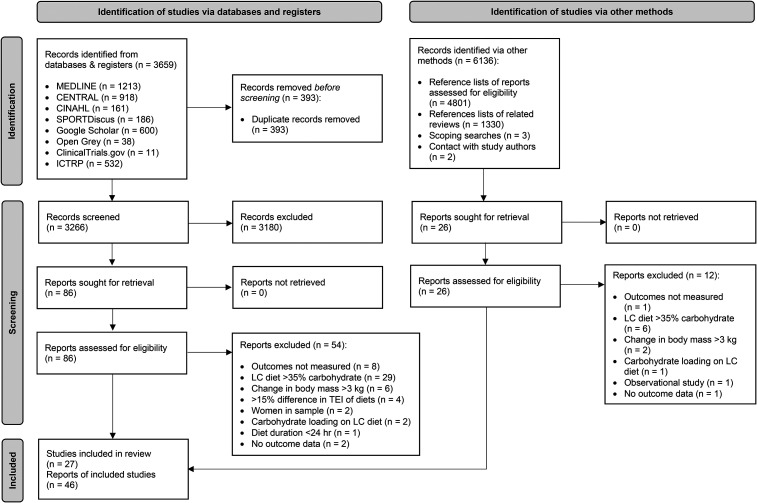
Eight databases were searched from conception to 25th May 2021: MEDLINE, CENTRAL, CINAHL, SPORTDiscus, Google Scholar, Open Grey, ClinicalTrials.gov, and ICTRP. The key search terms used were: testosterone, cortisol, low carbohydrate diet, high carbohydrate diet, and their synonyms. Where possible the search filter ‘adult male’ was used. No limits were put on date or language. For full details of the search terms and filters used see: Supplementary Appendix - Table 4. The search, and initial title and abstract screen was done by one author (J.W). Duplicate records were removed by the software: Mendeley Desktop (Mendeley, 2021). Thereafter, both authors independently screened each full text report for eligibility; differences between authors were settled by discussion. References lists of reports selected for full text screening and related reviews were also screened. The search process was documented in a PRISMA 2020 flowchart (Figure 1) (Page et al., 2021).
Figure 1.
PRISMA study selection flowchart. LC: Low-carbohydrate; TEI: Total energy intake.
Data extraction
Both authors independently extracted study characteristics and outcome data (Supplementary Appendix – Tables 6 and 7, Figure 2). When available, change from baseline data was extracted, to account for participant variation in outcomes. Afterwards, all data was double-checked and any differences resolved by reference to the original study reports. Study authors were contacted to request missing data, and graphical data was extracted using the software: WebPlotDigitizer (Rohatgi, 2021). Additional considerations regarding data extraction unique to individual studies are detailed in: Supplementary Appendix - Section 1.
Meta-analyses
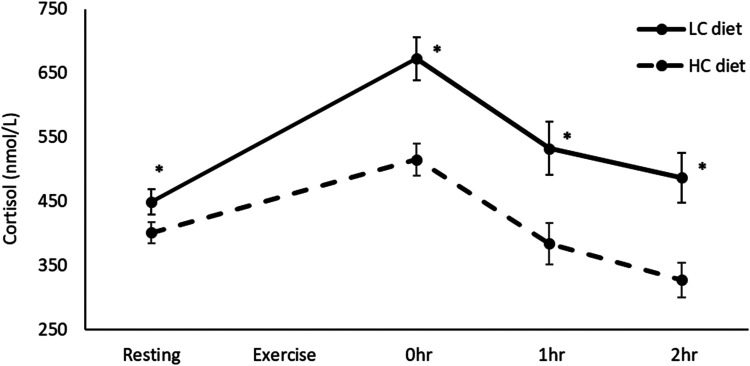
The studies contained a mix of plasma and serum samples with differing assays. Therefore, to account for possible inter-sample variation standardized mean differences (SMD) with 95% confidence intervals (CI) were used. Change score SDs were not used as this would have required variance to be calculated based on assumptions for the majority of studies (Higgins, Eldridge, et al., 2021). The results included parallel and crossover studies, thus to standardize the weighting across study designs and data types, post-intervention standard deviations (SD) were used for all studies (Deeks et al., 2021). The longest follow-up for each study was used. Standard errors (SE) were converted into SDs, subgroups within studies combined, and random-effects, meta-analyses were performed using Review Manager (The Cochrane Collaboration, 2020). Chi2 and I2 tests were used to measure heterogeneity, with I2 ≥30% and Chi2 p < 0.1 interpreted as evidence of heterogeneity (Deeks et al., 2021). SMDs were interpreted as: 0.2 = small effect, 0.5 = moderate effect, and 0.8 = large effect (Cohen, 1988). A post-hoc meta-analysis was conducted for post-exercise TT, to replicate the analysis for cortisol. Also, cortisol data for short-term LC diets and long-duration exercise was pooled and plotted onto a graph, as these results were homogeneous (Figure 4). Lastly, a post-hoc mean difference was calculated for TT: HP-LC diets using pre- and post-interventions means, to aid with clinical interpretation.
Figure 4.
Resting, 0 h, 1 h, and 2 h post-exercise cortisol after long-duration exercise, on short-term LC and HC diets. Resting (15 studies, LC: n = 131, HC: n = 134), 0 h (8 studies, LC: n = 72, HC: n = 75), 1 h (4 studies, LC: n = 41, HC: n = 40), 2 h (3 studies, LC: n = 36, HC: n = 36). Values in mean ± SE. *p < 0.001 compared to HC diet. HC: High-carbohydrate; LC: Low-carbohydrate.
Subgroup analyses
When heterogeneity was detected, two a priori subgroup analyses were conducted. Firstly, on the basis of LC diet duration: long- (≥3 weeks) versus short-term (<3 weeks) LC diets, and if heterogeneity remained unexplained, secondly on the basis of LC diet protein intake: MP (<35% protein) versus HP (≥35% protein) LC diets. In one outcome (0 h post-exercise cortisol) heterogeneity remained unexplained after both a priori subgroup analyses, therefore one post-hoc subgroup analysis was conducted on the basis of exercise duration: long- (≥20 min) versus short-duration (<20 min) exercise. Statistical differences between subgroups were investigated using Chi2 and I2 tests, with I2 ≥40% and Chi2 p < 0.05 interpreted as evidence of subgroup effects (Deeks et al., 2021).
Sensitivity analyses
Sensitivity analyses were run on all meta- and subgroup analyses, besides the three subgroup analyses that did not explain heterogeneity (Supplementary Appendix – Figures 2b, 2c, and 2g). An a priori sensitivity analysis was conducted by excluding each study in turn, and running the analyses again. Four post-hoc sensitivity analyses were conducted: exclusion of high and medium bias studies, exclusion of non-randomized studies, exclusion of studies using carbohydrate supplements during exercise on HC diets, and using alternative HC diets from studies with two eligible HC diets (Supplementary Appendix – Table 9).
Risk of bias assessment
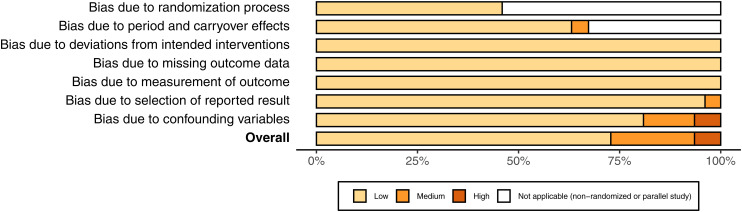
Cochrane's risk of bias tool for randomized studies was used for quality assessment (Higgins, Li, et al., 2021; Sterne et al., 2019). For non-randomized studies, the randomization questions were omitted. An additional bias domain was added for bias due to confounding variables. Both authors independently conducted the quality assessment, and differences were settled by discussion. Review and study level bias figures were produced using Cochrane's software: Robvis (Figure 2, Supplementary Appendix – Figure 1) (McGuinness and Higgins, 2021).
Figure 2.
Risk of bias of included studies.
Certainty of evidence assessment
For each of the key findings, a GRADE assessment was conducted (Table 1) (Schünemann et al., 2013). Both authors independently conducted the assessments, and differences were settled by discussion. Publication bias was assessed as part of the GRADE assessments, and was based on visual inspection of funnel plots, conflicts of interests, and number of studies excluded for missing outcome data. Statistical tests for funnel plot asymmetry were not undertaken, as the included studies had similar SEs (Sterne et al., 2011). Funnel plots were generated using Review Manager (Supplementary Appendix – Figure 3) (The Cochrane Collaboration, 2020).
Table 1.
Summary of findings.
| Outcome & subgroupa | Participants(studies) | SMD [95% CI] |
P-value (Z test) |
I2 (%) | P-value (Chi2 test) |
Interpretation | Certainty of evidence (GRADE rating) |
|---|---|---|---|---|---|---|---|
| Cortisol, resting | |||||||
| All studies | 245 (22) | 0.18 [−0.06, 0.42] | 0.15 | 32 | 0.08 | ||
| Long-term LC diets (MP-LC diets only) |
90 (7) | −0.28 [−0.7, 0.15] | 0.2 | 36 | 0.15 | Long-term, MP-LC vs HC diets neither increase nor decrease resting cortisol. |
Moderate ⊕⊕⊕⊝ Downgraded due to unexplained heterogeneity |
| Short-term LC diets | 155 (15) | 0.41 [0.16, 0.66] | <0.01 | 0 | 0.63 | Short-term LC vs HC diets moderately increase resting cortisol. | High ⊕⊕⊕⊕ |
| Test for subgroup differences | 86.7 | <0.01 | |||||
| Cortisol, 0 h post-exercise | |||||||
| All studies | 129 (12) | 0.58 [0.17, 0.99] | <0.01 | 50 | 0.02 | ||
| Long-duration exercise | 112 (10) | 0.78 [0.47, 1.1] | <0.01 | 0 | 0.51 | LC vs HC diets result in much higher 0 h post-exercise cortisol, after long-duration exercise. |
Moderate ⊕⊕⊕⊝ Downgraded due to indirectness (only includes two long-term LC diet studies)b |
| Short-duration exercise | 17 (2) | −0.67 [−1.37, 0.03] | 0.06 | 0 | 0.78 | LC vs HC diets result in much lower 0 h post-exercise cortisol, after short-duration exercise. |
Low ⊕⊕⊝⊝ Downgraded due to imprecision (small sample size and p > 0.05) |
| Test for subgroup differences | 92.8 | <0.01 | |||||
| Cortisol, 1 h post-exercise | |||||||
| All studies (long-duration exercise only) |
55 (5) | 0.81 [0.31, 1.31] | <0.01 | 24 | 0.26 | LC vs HC diets result in much higher 1 h post-exercise cortisol, after long-duration exercise. |
Moderate ⊕⊕⊕⊝ Downgraded due to indirectness (only includes one long-term LC diet study)b |
| Cortisol, 2 h post-exercise | |||||||
| All studies (short-term, MP-LC diets and long-duration exercise only) |
36 (3) | 0.82 [0.33, 1.3] | <0.01 | 0 | 0.79 | Short-term, MP-LC vs HC diets result in much higher 2 h post-exercise cortisol, after long-duration exercise. |
Moderate ⊕⊕⊕⊝ Downgraded due imprecision (small sample size) |
| Total testosterone, resting | |||||||
| All studies | 155 (13) | −0.48 [−0.87, −0.09] | 0.01 | 49 | 0.02 | ||
| MP-LC diets | 129 (10) | −0.31 [−0.74, 0.13] | 0.17 | 48 | 0.04 | MP-LC vs HC diets neither increase nor decrease resting total testosterone. |
Low ⊕⊕⊝⊝ Downgraded due to indirectness (only includes two short-term LC diet studies)b and risk of bias (randomized studies only: SMD = −0.79, p < 0.01, I2 = 0%) |
| HP-LC diets (short-term LC diets only) |
26 (3) | −1.08 [−1.67, −0.48] | <0.01 | 0 | 0.85 | Short-term HP-LC vs HC diets greatly decrease resting total testosterone. |
Moderate ⊕⊕⊕⊝ Downgraded due to imprecision (small sample size) |
| Test for subgroup differences | 76.5 | 0.04 | |||||
|
Total
testosterone, 0 h post-exercise |
|||||||
| All studies | 28 (3) | −0.03 [−0.95, 0.89] | 0.95 | 65 | 0.06 | ||
| Long-term LC diets (MP-LC diets only) |
19 (2) | 0.44 [−0.21, 1.09] | 0.18 | 0 | 0.9 | Long-term, MP-LC vs HC diets result in higher 0 h post-exercise total testosterone. |
Very low ⊕⊝⊝⊝ Downgraded due to indirectness (only includes one long- and one short-duration exercise study)c and imprecision (small sample size and p > 0.05) |
| Short-term LC diets (HP-LC diets and long-duration exercise only) |
9 (1) | −1.01 [−2, −0.01] | 0.05 | NA | NA | Short-term, HP-LC vs HC diets result in much lower 0 h post-exercise total testosterone, after long-duration exercise. |
Low ⊕⊕⊝⊝ Downgraded due to imprecision (small sample size and p = 0.05) |
| Test for subgroup differences | 82.5 | 0.02 |
Long-term (≥3 weeks), short-term (<3 weeks), long-duration exercise (≥20 min), short-duration exercise (<20 min), MP (<35% protein), HP (≥35% protein).
Indirect evidence drawn from long or short LC diets, to support a conclusion about LC diets in general.
Indirect evidence drawn from long- or short-duration exercise, to support a conclusion about exercise in general.
CI: confidence interval; HC: high-carbohydrate; HP: high-protein; LC: low-carbohydrate; MP: moderate-protein; SMD: standardized mean difference.
Results
Study selection
The initial title and abstract screen covered 9402 total records, 3,266 from databases and 6,136 from other sources (Figure 1). Next, 112 reports encompassing 78 studies were selected for full text screening, of which 27 studies encompassed within 46 reports were selected. For a list of key excluded studies see: Supplementary Appendix – Table 5.
Characteristics of included studies
There was a total of 309 participants (mean ± SD), age: 27.3 ± 4.7 (n = 309), body mass: 78.6 ± 7.1 kg (n = 303), and BMI: 24.8 ± 1.6 (n = 246) (Supplementary Appendix – Table 6). 95% of the participants were physically active during dietary interventions, and post-exercise cortisol measurements were all taken after aerobic exercise tests. Exercise tests ranged from 15 – 232 min, with intensities from ∼60 – 100% of maximal oxygen uptake. Dietary interventions ranged from 2 days – 8 weeks; and mean differences in LC versus HC diets were, body mass change: −1.4 kg (LC: −1.5; HC: −0.1; n = 222), energy intake: 8 kcal (LC: 2,873; HC: 2,866; n = 275), and carbohydrate intake: −46% of TEI (LC: 12%; HC: 58%; n = 309). Also, LC versus HC diets were higher in fat, protein, and cholesterol; whilst lower in fibre and sugar (Supplementary Appendix – Table 7).
Risk of bias
Twenty-one studies were low, five medium, and one high risk of bias; of which 12 were randomized and 15 non-randomized (Figure 2, Supplementary Appendix – Figure 1). For further details on the reasons for bias ratings see: Supplementary Appendix – Table 8. No conflicts of interests for study authors were identified, funnel plots showed no clear asymmetry (Supplementary Appendix – Figure 3), and the number of studies excluded for missing outcome data was two (Supplementary Appendix – Table 5). Thus, publication bias was unlikely to have affected the results.
Resting cortisol
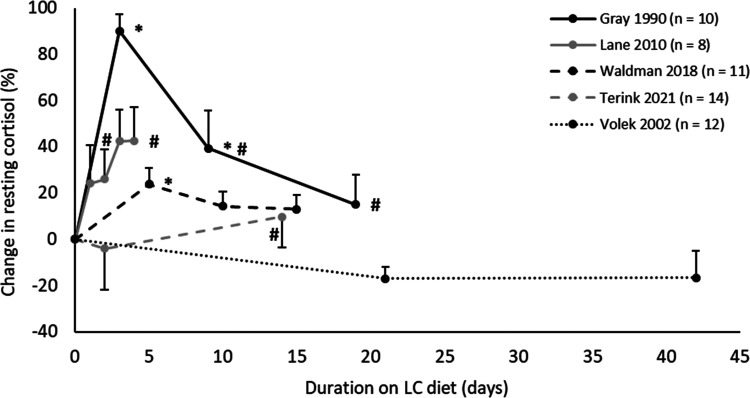
There was no overall effect of LC versus HC diets on resting cortisol (SMD = 0.18, p = 0.15) (Table 1). However, when studies were split into long- (≥3 weeks) versus short-term (<3 weeks) LC diets, there was a moderate increase in resting cortisol on short-term LC diets (SMD = 0.41, p < 0.01). Whereas, long-term LC diets showed no consistent effect on resting cortisol (SMD = −0.28, p = 0.2). The trend of LC diets increasing resting cortisol in the short-term, but returning to baseline levels in the long-term, can be seen within individual studies measuring cortisol over time, with four out five studies showing this trend (Figure 3).
Figure 3.
Percentage change in resting cortisol over time on LC diets, in studies with repeated measurements (HC diet = 100%). Values in mean ± SE. *p < 0.05 compared to HC diet. #p < 0.05 compared to first measurement on LC diet. HC: High-carbohydrate; LC: Low-carbohydrate.
Post-exercise cortisol
0 h post-exercise cortisol was much higher on LC versus HC diets (SMD = 0.58, p < 0.01) (Table 1). Subgroup analysis revealed the effect was stronger after long-duration exercise (≥20 min) (SMD = 0.78, p < 0.01), and reversed after short-duration exercise (<20 min) (SMD = −0.67, p = 0.06). 1 h and 2 h post-exercise cortisol were much higher on LC versus HC diets, after long-duration exercise (1 h: SMD = 0.81, p < 0.01; 2 h: SMD = 0.82, p < 0.01). The effects of short-term LC diets on resting and post-exercise cortisol after long-duration exercise were homogeneous, and show cortisol increasing more sharply during exercise and remaining elevated post-exercise (Figure 4).
Resting total testosterone
The overall results for resting TT showed a significant decrease on LC versus HC diets (SMD = −0.48, p = 0.01) (Table 1). However, subgroup analyses revealed this effect to be limited to HP-LC diets, which yielded a very large decrease in TT (SMD = −1.08, p < 0.01; ∼5.23 nmol/L), albeit in a small sample (n = 26). MP-LC diets had no consistent effect on TT (SMD = −0.31, p = 0.17).
Post-exercise total testosterone
There was no overall effect of LC versus HC diets on 0 h post-exercise TT (SMD = −0.03, p = 0.95) (Table 1). However, subgroup analysis showed 0 h post-exercise was non-significantly higher on long-term LC versus HC diets (SMD = 0.44, p = 0.18), and much lower on short-term LC versus HC diets (SMD = −1.01, p = 0.05); although both subgroups had small samples (n = 19, n = 9). The long- and short-term diet subgroups contained solely MP- and HP-LC diets, respectively. Meaning the observed subgroup effects could either be attributable to diet duration or protein intake.
Sensitivity analyses
The results were robust to sensitivity analyses, besides two exceptions (Supplementary Appendix – Table 9). Firstly, excluding non-randomized studies for TT: MP-LC diets, produced a stronger, more significant effect, with reduced heterogeneity (SMD = −0.79 [−1.37, −0.22], p < 0.01; I2 = 0%, p = 0.69). Secondly, excluding studies using carbohydrate supplements during exercise on HC diets, produced a weaker effect for 0 h and 1 h post-exercise cortisol (0 h, SMD = 0.41 [−0.01, 0.83]; 1 h, SMD = 0.6 [0.11, 1.09]).
Discussion
Resting cortisol
The increase in resting cortisol on short but not long-term LC diets, is likely tied to glucocorticoids’ roles in glucose homeostasis. Cortisol, glucagon, and gluconeogenesis all increase on short, but not long-term LC diets (Bisschop et al., 2000; Muller et al., 1971; Webster et al., 2016). As glucocorticoids increase gluconeogenesis (Kuo et al., 2015), the initial rise in cortisol may be partly responsible for a transient increase in gluconeogenesis, on short LC diets. Additionally, cortisol may rise to spare glucose for brain function, as the brain cannot significantly use fatty acids for fuel. Glucocorticoids inhibit glucose uptake and oxidation in adipose tissue and skeletal muscle, thereby conserving glucose for brain function (Kuo et al., 2015). In contrast, endogenous ketone production increases sharply over the first 3 weeks of a very LC diet (Vidić et al., 2021), and ketones can be used for fuel by the brain (Zhang et al., 2013). Thus, when ketones replace glucose for the majority of brain fuel, cortisol's glucose sparing effects are not needed, and thus levels may return to baseline.
Post-exercise cortisol
The results showed the increase in cortisol during exercise was greater on LC diets. Moreover, this effect appears to persist post-adaptation to a LC diet, although somewhat lessened (Terink et al., 2021). Interestingly, the rise in post-exercise cortisol was reduced in studies using carbohydrate supplements during exercise on HC diets (Supplementary Appendix – Table 9). This is supported by another meta-analysis which found post-exercise cortisol was significantly attenuated by carbohydrate supplementation during exercise (Moreira et al., 2007). Thus, it appears the rise in cortisol during exercise is increased during times of low carbohydrate availability. There are three possible, complementary explanations for this. Firstly, as glycogen stores are partially depleted on a LC diet (Webster et al., 2016), cortisol may increase more sharply on LC diets to facilitate increased gluconeogenesis during exercise. Secondly, fat oxidation is higher on LC versus HC diets during exercise (Webster et al., 2016), and thus cortisol may increase to facilitate increased fat oxidation via inducing lipolysis in adipose tissue (Djurhuus et al., 2002; Kuo et al., 2015). Thirdly, exercise upregulates skeletal muscle glucose uptake (Evans et al., 2019), thus cortisol may increase to preserve glucose for brain function.
Additionally, the results showed post-exercise cortisol was lower after short, intense exercise on LC versus HC diets. This subgroup analysis was post-hoc, and thus more likely a false-positive finding. However, the qualitative interaction observed suggests differential effects. Short, intense exercise predominantly uses glucose for fuel, whereas long-duration exercise increasingly relies on fatty acids (Purdom et al., 2018). Also, carbohydrate supplementation only attenuates cortisol during long-duration exercise (Moreira et al., 2007). Therefore, on LC diets muscle glycogen may be sufficient for short exercise (Cipryan et al., 2018), whereas for long exercise lipolysis stimulated via cortisol may be increasingly required.
Resting testosterone
MP-LC diets had no consistent effect on resting TT, however HP-LC diets caused a large decrease in resting TT. For context, mean TT for a comparably aged population (27 years) is 14 nmol/L (Kelsey et al., 2014), thus −5.23 nmol/L represents a 37% decrease. Protein intakes ≥35% may outstrip the urea cycle's capacity to convert nitrogen derived from amino acid catabolism into urea, leading to hyperammonaemia and its toxic effects (Bilsborough and Mann, 2006). T has been shown to suppress the urea cycle (Lam et al., 2017), whilst glucocorticoids upregulate the urea cycle (Okun et al., 2015). Notably, the largest decrease in resting cortisol was on the longest and best-controlled HP-LC diet study (Supplementary Appendix – Figure 2a). Thus, the decrease in T and increase in cortisol on HP diets, may serve to upregulate the urea cycle and increase nitrogen excretion, thereby limiting the adverse effects of excess protein consumption.
Post-exercise testosterone
The results showed post-exercise TT was higher on long-term MP-LC diets, and lower on short-term HP-LC diets. The finding that HP-LC diets caused a large decrease in resting TT, whilst long-term LC diets had no effect on resting TT, suggests the observed subgroup effects in post-exercise TT are explained by protein intake rather than diet duration. HP intakes may depress post-exercise TT to maintain upregulation of the urea cycle and increased nitrogen excretion, as previously discussed (Discussion: Resting testosterone). The finding that long-term LC diets increased post-exercise TT, may be explained by the increase in blood cholesterol on LC diets (Dong et al., 2020), providing greater substrate for T production, which is utilized in times of increased anabolic signalling, such as during exercise (Pasiakos, 2012).
Practical implications
The increase in cortisol during the first 3 weeks of a LC diet is likely part of the adaption process to such diets, and thus may not represent a pathological state. The results indicate cortisol returns to baseline levels after ∼3 weeks, suggesting cardiovascular disease risk is not elevated by higher cortisol on long LC diets. However, the effects of long-term LC diets on cardiovascular disease and all-cause mortality, as measured by other methods, are uncertain. Observational studies have found an increase in all-cause mortality on long-term LC diets (Noto et al., 2013), whilst interventional studies have shown improvements in cardiovascular disease biomarkers (Dong et al., 2020). Additional research on the effects of long-term LC diets is desirable, particularly as these diets have risen in popularity over recent years.
The higher increase in cortisol during exercise on LC versus HC diets appears to persist post-adaptation. Classically, cortisol is thought to have immunosuppressive effects, however in spite of elevated post-exercise cortisol, LC diets do not appear overtly immunosuppressive, according to other immune-markers (Shaw et al., 2021). The potential immunosuppressive effects of higher post-exercise cortisol may be exacerbated in athletes undergoing high volume training, and some caution may be advisable, until further research is undertaken.
The large decrease in resting and post-exercise TT on LC-HP diets may only occur on diets that outstrip the urea cycle's capacity to synthesize urea, as there were no clear adverse endocrine effects for LC diets using 30–31% protein intake (Supplementary Appendix – Table 7 and Figure 2). In practise, most free-living LC diets will fall below the urea cycle capacity threshold (≤35% protein), as population protein intakes are stable at 15–17% (Cohen et al., 2015), likely due to a protein-specific appetite mechanism (Leidy et al., 2015). However, one can find articles online advocating protein intakes ≤35%, which if followed precisely, may lead to adverse endocrine effects, particularly in individuals with lower rates of maximal urea synthesis (Bilsborough and Mann, 2006).
The higher post-exercise TT on MP-LC diets may signal an increased anabolic response to exercise, which would be advantageous, particularly in individuals with strength, power, or hypertrophy goals. Relatedly, another systematic review found that whilst absolute strength and power were unchanged by LC diets, the decrease in body fat on LC diets resulted in an improved strength/power to bodyweight ratio (Kang et al., 2020). However, the finding that LC diets increase post-exercise T should be taken with caution, as although the direction of effects was consistent, due to the small sample size, the p-value remained high. Ideally, this finding should be viewed as hypothesis generating, to be confirmed by future research.
Limitations
Firstly, there was unexplained heterogeneity in both resting cortisol and TT on long-term LC diets. The three studies which reported micronutrients showed diverse intakes on LC diets (Supplementary Appendix – Table 7), suggesting a possible source of the heterogeneity. However, due to lack of study data, this was unable to be explored as a source of heterogeneity. Secondly, the heterogeneity present in TT, long-term LC diets may be due to methodological diversity. The results for randomized studies only, showed a decrease in TT on MP-LC diets (SMD = −0.79, p < 0.01), suggesting study design may have impacted the results. Thirdly, there were differences in exercise regimes and carbohydrate intakes on LC diets (Supplementary Appendix - Tables 6 and 7). However, due to the low number of studies and risk of data dredging, these factors were not explored as possible sources of heterogeneity. This issue brings up the wider debate about ‘lumping versus splitting’ in meta-analyses (Thomas et al., 2021). Dietary studies are typically dissimilar to each other in at least one important respect, and meta-analyses attempt to estimate effects across similar, but not identical studies. As readers tend to make judgements about sets of studies regardless, it is arguably preferable to investigate such differences using statistical methods, as this is more objective. The approach of this review was to pool studies similar in major aspects (diet), but dissimilar in other aspects (duration, protein intake), and address the dissimilarities using subgroup analyses.
An issue of particular note is that the finding that HP-LC diets decreased T, may be subject to residual confounding. Per calorie, protein is more satiating than fat or carbohydrate, thus HP diets commonly cause a decrease in energy intake via increased satiety (Paddon-Jones et al., 2008); and low energy intakes are known to decrease T (Henning et al., 2014). Of the three HP diet studies, two studies provided the participants with isocaloric diets and either reported no significant changes in bodyweight (Anderson et al., 1987), or did not mention it (Langfort et al., 2001). In the third study, participants constructed their diets themselves, based on guidance from the researchers (Jaffe, 2013). Reported daily energy intakes were 23 kcal, and bodyweight 0.1 kg, lower on LC versus HC diets. Thus, it appears that energy intake was not a confounding variable in the HP diet studies, although the possibility of dietary misreporting remains in the third study.
The review had a number of strengths. Firstly, the overall sample was homogenous, being: male, young to middle-aged adults, healthy, non-obese, and physically active. However, this also means the findings are harder to generalize to other populations. Sex differences have been found in carbohydrate and lipid metabolism during exercise (Purdom et al., 2018), which may extend to post-exercise cortisol. Secondly, the majority of data was from low bias studies (73%) (Figure 1), and the results were robust to differences between randomized and non-randomized studies, besides one subgroup (TT: MP-LC) (Supplementary Appendix – Table 9). Finally, the extensive search process covering 9402 records likely identified all eligible studies (Figure 1), including studies from grey literature (Supplementary Appendix – Table 6).
Conclusions
This review found an increase in resting and post-exercise cortisol on short-term LC diets (<3 weeks). In addition, the results indicate resting cortisol levels return to baseline after ∼3 weeks on a LC diet, whilst post-exercise cortisol remains elevated. However, due to the low number of studies and unexplained heterogeneity in long-term LC diets, further research is needed to confirm the latter effects. MP-LC diets appear to have no effect on resting TT, although the decrease in resting TT on randomized MP-LC studies, highlights the need for further randomized controlled trials. Finally, HP-LC diets caused a large decrease in resting TT, suggesting individuals consuming such diets may need to be cautious about adverse endocrine effects.
Supplemental Material
Supplemental material, sj-pdf-1-nah-10.1177_02601060221083079 for Low-carbohydrate diets and men's cortisol and testosterone: Systematic review and meta-analysis by Joseph Whittaker and Miranda Harris in Nutrition and Health
Acknowledgements
The authors would like to thank the multiple study authors who provided additional information when requested. Miranda Harris would like to thank Josh Beer for his help with data extraction.
List of Abbreviations
- CI
confidence interval
- LC
low-carbohydrate
- HC
high-carbohydrate
- HP
high- protein
- MP
moderate-protein
- SD
standard deviation
- SE
standard error
- SMD
standardized mean difference
- TEI
total energy intake
- T
testosterone
- TT
total testosterone
Footnotes
Availability of data and materials: The majority of the data used in this review can be found in the Supplementary Appendix: Figure 2, Tables 6 and 7. Other data is available on request
CRediT authorship contribution statement: Joseph Whittaker: conceptualization, methodology, systematic review process (search, title & abstract screening, report screening, data extraction, quality assessment, GRADE assessment), validation, formal analysis, investigation, resources, data curation, writing – original draft, writing – review & editing, supervision, project administration. Miranda Harris: systematic review process (report screening, data extraction, quality assessment, GRADE assessment), writing - review & editing.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical statement: This research did not require ethical approval, as there was no direct involvement of human or animal subjects.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Joseph Whittaker https://orcid.org/0000-0002-4635-888X
Supplemental Material: Supplemental material for this article is available online.
References
- Anderson KE, Rosner W, Khan MS, et al. (1987) Diet-hormone interactions: Protein/carbohydrate ratio alters reciprocally the plasma levels of testosterone and cortisol and their respective binding globulins in man. Life Sciences 40(18): 1761–1768. [DOI] [PubMed] [Google Scholar]
- Bilsborough S, Mann N. (2006) A review of issues of dietary protein intake in humans. International Journal of Sport Nutrition and Exercise Metabolism 16(2): 129–152. [DOI] [PubMed] [Google Scholar]
- Bisschop PH, Pereira Arias AM, Ackermans MT, et al. (2000) The effects of carbohydrate variation in isocaloric diets on glycogenolysis and gluconeogenesis in healthy men. The Journal of Clinical Endocrinology and Metabolism 85(5): 1963–1967. [DOI] [PubMed] [Google Scholar]
- Brownlee KK, Moore AW, Hackney AC. (2005) Relationship between circulating cortisol and testosterone: Influence of physical exercise. Journal of Sports Science & Medicine 4(1): 76–83. [PMC free article] [PubMed] [Google Scholar]
- Burke LM, Ross ML, Garvican-Lewis LA, et al. (2017) Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. The Journal of Physiology 595(9): 2785–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipryan L, Plews DJ, Ferretti A, et al. (2018) Effects of a 4-week very low-carbohydrate diet on high-intensity interval training responses. Journal of Sports Science & Medicine 17(2): 259–268. [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Cragg M, DeFonseka J, et al. (2015) Statistical review of US macronutrient consumption data, 1965–2011: Americans have been following dietary guidelines, coincident with the rise in obesity. Nutrition (Burbank, Los Angeles County, Calif.) 31(5): 727–732. [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988) Statistical Power Analysis in the Behavioral Sciences. 2nd ed. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Corona G, Rastrelli G, Di Pasquale G, et al. (2018) Endogenous testosterone levels and cardiovascular risk: Meta-analysis of observational studies. The Journal of Sexual Medicine 15(9): 1260–1271. [DOI] [PubMed] [Google Scholar]
- Corona G, Rastrelli G, Monami M, et al. (2013) Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: A systematic review and meta-analysis. European Journal of Endocrinology 168(6): 829–843. [DOI] [PubMed] [Google Scholar]
- Cumming DC, Quigley ME, Yen SS. (1983) Acute suppression of circulating testosterone levels by cortisol in men. The Journal of Clinical Endocrinology and Metabolism 57(3): 671–673. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Higgins JPT, Altman DG. (2021) Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, et al. (eds) Cochrane Handbook for Systematic Reviews of Interventions. 6.2. London: Cochrane. Available at: https://training.cochrane.org/handbook/current/chapter-10 (accessed 19 August 2021). [Google Scholar]
- Djurhuus CB, Gravholt CH, Nielsen S, et al. (2002) Effects of cortisol on lipolysis and regional interstitial glycerol levels in humans. American Journal of Physiology, Endocrinology and Metabolism 283(1): E172–E177. [DOI] [PubMed] [Google Scholar]
- Domínguez-López I, Yago-Aragón M, Salas-Huetos A, et al. (2020) Effects of dietary phytoestrogens on hormones throughout a human lifespan: A review. Nutrients 12(8): 2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong T, Guo M, Zhang P, et al. (2020) The effects of low-carbohydrate diets on cardiovascular risk factors: A meta-analysis. PLOS One 15(1): e0225348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans PL, McMillin SL, Weyrauch LA, et al. (2019) Regulation of skeletal muscle glucose transport and glucose metabolism by exercise training. Nutrients 11(10): 2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall KD, Chen KY, Guo J, et al. (2016) Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. The American Journal of Clinical Nutrition 104(2): 324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning PC, Margolis LM, McClung JP, et al. (2014) High protein diets do not attenuate decrements in testosterone and IGF-I during energy deficit. Metabolism: Clinical and Experimental 63(5): 628–632. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Eldridge S, Li T. (2021) Chapter 23: Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, et al. (eds) Cochrane Handbook for Systematic Reviews of Interventions. 6.2. London: Cochrane. Available at: https://training.cochrane.org/handbook/current/chapter-23 (accessed 20 August 2021). [Google Scholar]
- Higgins JPT, Li T, Sterne J. (2021) Revised Cochrane risk of bias tool for randomized trials (RoB 2): additional considerations for crossover trials. Available at: https://www.riskofbias.info/welcome/rob-2-0-tool/rob-2-for-crossover-trials (accessed 21 August 2021).
- Higgins JPT, Thomas J, Chandler J, et al. (eds) (2021) Cochrane Handbook for Systematic Reviews of Interventions. 6.2. London: Cochrane. Available at: https://training.cochrane.org/handbook (accessed 18 August 2021). [Google Scholar]
- Jaffe DA. (2013) Effects of short-term macronutrient redistribution on endocrine and performance parameters in resistance trained males. Master’s Thesis, Springfield College, USA. [Google Scholar]
- Kang J, Ratamess NA, Faigenbaum AD, et al. (2020) Ergogenic properties of ketogenic diets in normal-weight individuals: A systematic review. Journal of the American College of Nutrition 39(7): 665–675. [DOI] [PubMed] [Google Scholar]
- Kelsey TW, Li LQ, Mitchell RT, et al. (2014) A validated age-related normative model for male total testosterone shows increasing variance but no decline after age 40 years. PLOS One 9(10): e109346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo T, McQueen A, Chen T-C, et al. (2015) Regulation of glucose homeostasis by glucocorticoids. In: Wang JC, Harris C. (eds) Advances in Experimental Medicine and Biology. New York: Springer, pp.99–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam T, Poljak A, McLean M, et al. (2017) Testosterone prevents protein loss via the hepatic urea cycle in human. European Journal of Endocrinology 176(4): 489–496. [DOI] [PubMed] [Google Scholar]
- Langfort JL, Zarzeczny R, Nazar K, et al. (2001) The effect of low-carbohydrate diet on the pattern of hormonal changes during incremental, graded exercise in young men. International Journal of Sport Nutrition and Exercise Metabolism 11(2): 248–257. [DOI] [PubMed] [Google Scholar]
- Leidy HJ, Clifton PM, Astrup A, et al. (2015) The role of protein in weight loss and maintenance. The American Journal of Clinical Nutrition 101(6): 1320S–1329S. [DOI] [PubMed] [Google Scholar]
- McGuinness LA, Higgins JPT. (2021) Risk-of-bias VISualization (robvis): An R package and shiny web app for visualizing risk-of-bias assessments. Research Synthesis Methods 12(1): 55–61. [DOI] [PubMed] [Google Scholar]
- Mendeley (2021) Mendeley Desktop. 1.19.8. London: Mendeley. [Google Scholar]
- Michalczyk MM, Chycki J, Zajac A, et al. (2019) Anaerobic performance after a low-carbohydrate diet (LCD) followed by 7 days of carbohydrate loading in male basketball players. Nutrients 11(4): 778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira A, Kekkonen RA, Delgado L, et al. (2007) Nutritional modulation of exercise-induced immunodepression in athletes: A systematic review and meta-analysis. European Journal of Clinical Nutrition 61(4): 443–460. [DOI] [PubMed] [Google Scholar]
- Muller WA, Faloona GR, Unger RH. (1971) The influence of the antecedent diet upon glucagon and insulin secretion. The New England Journal of Medicine 285(26): 1450–1454. [DOI] [PubMed] [Google Scholar]
- Noto H, Goto A, Tsujimoto T, et al. (2013) Low-carbohydrate diets and all-cause mortality: A systematic review and meta-analysis of observational studies. PLOS One 8(1): e55030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun JG, Conway S, Schmidt K V, et al. (2015) Molecular regulation of urea cycle function by the liver glucocorticoid receptor. Molecular Metabolism 4(10): 732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddon-Jones D, Westman E, Mattes RD, et al. (2008) Protein, weight management, and satiety. The American Journal of Clinical Nutrition 87(5): 1558S–1561S. [DOI] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, et al. (2021) The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clinical Research Ed.) 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasiakos SM. (2012) Exercise and amino acid anabolic cell signaling and the regulation of skeletal muscle mass. Nutrients 4(7): 740–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health England (2016) Government dietary recommendations. Report, Public Health England, UK, August
- Purdom T, Kravitz L, Dokladny K, et al. (2018) Understanding the factors that effect maximal fat oxidation. Journal of the International Society of Sports Nutrition 15: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rethlefsen ML, Kirtley S, Waffenschmidt S, et al. (2021) PRISMA-S: An extension to the PRISMA statement for reporting literature searches in systematic reviews. Systematic Reviews 10(1): 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi A. (2021) WebPlotDigitizer. Available at: https://automeris.io/WebPlotDigitizer (accessed 19 August 2021).
- Schooling CM, Au Yeung SL, Freeman G, et al. (2013) The effect of statins on testosterone in men and women, a systematic review and meta-analysis of randomized controlled trials. BMC Medicine 11: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schünemann H, Brożek J, Guyatt G, et al. (eds) (2013) GRADE Handbook. The GRADE Working Group. Available at: https://gdt.gradepro.org/app/handbook/handbook.html (accessed 19 August 2021).
- Shaw DM, Merien F, Braakhuis A, et al. (2021) Adaptation to a ketogenic diet modulates adaptive and mucosal immune markers in trained male endurance athletes. Scandinavian Journal of Medicine and Science in Sports 31(1): 140–152. [DOI] [PubMed] [Google Scholar]
- Sherrier M, Li H. (2019) The impact of keto-adaptation on exercise performance and the role of metabolic-regulating cytokines. The American Journal of Clinical Nutrition 110(3): 562–573. [DOI] [PubMed] [Google Scholar]
- Sterne JAC, Savović J, Page MJ, et al. (2019) Rob 2: A revised tool for assessing risk of bias in randomised trials. BMJ (Clinical Research Ed.) 366: l4898. [DOI] [PubMed] [Google Scholar]
- Sterne JAC, Sutton AJ, Ioannidis JPA, et al. (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ (Clinical Research Ed.) 343: d4002. [DOI] [PubMed] [Google Scholar]
- Terink R, Witkamp RF, Hopman MTE, et al. (2021) A 2 week cross-over intervention with a low carbohydrate, high fat diet compared to a high carbohydrate diet attenuates exercise-induced cortisol response, but not the reduction of exercise capacity, in recreational athletes. Nutrients 13(1): 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cochrane Collaboration (2020) Review Manager (RevMan). 5.4. London: Cochrane. [Google Scholar]
- Thomas J, Kneale D, McKenzie JE, et al. (2021) Chapter 2: Determining the scope of the review and the questions it will address. In: Higgins JPT, Thomas J, Chandler J, et al. (eds) Cochrane Handbook for Systematic Reviews of Interventions. 6.2. London: Cochrane. Available at: https://training.cochrane.org/handbook/current/chapter-02 (accessed 10 September 2021). [Google Scholar]
- Vidić V, Ilić V, Toskić L, et al. (2021) Effects of calorie restricted low carbohydrate high fat ketogenic vs. non-ketogenic diet on strength, body-composition, hormonal and lipid profile in trained middle-aged men. Clinical Nutrition (Edinburgh, Scotland) 40(4): 1495–1502. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, Beekman ATF, Milaneschi Y, et al. (2010) Urinary cortisol and six-year risk of all-cause and cardiovascular mortality. The Journal of Clinical Endocrinology and Metabolism 95(11): 4959–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster CC, Noakes TD, Chacko SK, et al. (2016) Gluconeogenesis during endurance exercise in cyclists habituated to a long-term low carbohydrate high-fat diet. The Journal of Physiology 594(15): 4389–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker J, Harris M. (2021) Low carbohydrate diets and men’s testosterone and cortisol: Systematic review and meta-analysis. Available at: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=255957 (accessed 18 August 2021).
- Whittaker J, Wu K. (2021) Low-fat diets and testosterone in men: Systematic review and meta-analysis of intervention studies. The Journal of Steroid Biochemistry and Molecular Biology 210: 105878. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Lowery RP, Roberts MD, et al. (2020) Effects of ketogenic dieting on body composition, strength, power, and hormonal profiles in resistance training men. Journal of Strength and Conditioning Research 34(12): 3463–3474. [DOI] [PubMed] [Google Scholar]
- Yao Q-M, Wang B, An X-F, et al. (2018) Testosterone level and risk of type 2 diabetes in men: A systematic review and meta-analysis. Endocrine Connections 7(1): 220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac A, Poprzecki S, Maszczyk A, et al. (2014) The effects of a ketogenic diet on exercise metabolism and physical performance in off-road cyclists. Nutrients 6(7): 2493–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir A, Ben-Zeev T, Hoffman JR. (2021) Manipulation of dietary intake on changes in circulating testosterone concentrations. Nutrients 13: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kuang Y, Xu K, et al. (2013) Ketosis proportionately spares glucose utilization in brain. Journal of Cerebral Blood Flow and Metabolism 33(8): 1307–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-nah-10.1177_02601060221083079 for Low-carbohydrate diets and men's cortisol and testosterone: Systematic review and meta-analysis by Joseph Whittaker and Miranda Harris in Nutrition and Health