Key Points
Question
Can tucatinib, trastuzumab, and capecitabine provide systemic and intracranial benefit for patients with ERBB2 (HER2)-positive metastatic breast cancer and brain metastases?
Findings
In this exploratory subgroup analysis of a randomized clinical trial of 612 patients with ERBB2-positive breast cancer, overall survival, intracranial efficacy, and new brain lesion–free survival were evaluated. Tucatinib in combination with trastuzumab and capecitabine prolonged median overall survival by 9.1 months in patients with brain metastases and reduced the risk of developing new brain lesions as sites of first progression or death by 45.1% in all patients.
Meaning
Findings suggest tucatinib in combination with trastuzumab and capecitabine provides survival benefits and delays development of new brain lesions, representing an important treatment option for patients with ERBB2-positive metastatic breast cancer, including those with brain metastases.
Abstract
Importance
It is estimated that up to 50% of patients with ERBB2 (HER2)-positive metastatic breast cancer (MBC) will develop brain metastases (BMs), which is associated with poor prognosis. Previous reports of the HER2CLIMB trial have demonstrated that tucatinib in combination with trastuzumab and capecitabine provides survival and intracranial benefits for patients with ERBB2-positive MBC and BMs.
Objective
To describe overall survival (OS) and intracranial outcomes from tucatinib in combination with trastuzumab and capecitabine in patients with ERBB2-positive MBC and BMs with an additional 15.6 months of follow-up.
Design, Setting, and Participants
HER2CLIMB is an international, multicenter, randomized, double-blind, placebo-controlled clinical trial evaluating tucatinib in combination with trastuzumab and capecitabine. The 612 patients, including those with active or stable BMs, had ERBB2-positive MBC previously treated with trastuzumab, pertuzumab, and trastuzumab emtansine. The study was conducted from February 23, 2016, to May 3, 2019. Data from February 23, 2016, to February 8, 2021, were analyzed.
Interventions
Patients were randomized 2:1 to receive tucatinib (300 mg orally twice daily) or placebo (orally twice daily), both in combination with trastuzumab (6 mg/kg intravenously or subcutaneously every 3 weeks with an initial loading dose of 8 mg/kg) and capecitabine (1000 mg/m2 orally twice daily on days 1-14 of each 3-week cycle).
Main Outcomes and Measures
Evaluations in this exploratory subgroup analysis included OS and intracranial progression-free survival (CNS-PFS) in patients with BMs, confirmed intracranial objective response rate (ORR-IC) and duration of intracranial response (DOR-IC) in patients with measurable intracranial disease at baseline, and new brain lesion–free survival in all patients. Only OS was prespecified before the primary database lock.
Results
At baseline, 291 of 612 patients (47.5%) had BMs. Median age was 52 years (range, 22-75 years), and 289 (99.3%) were women. At median follow-up of 29.6 months (range, 0.1-52.9 months), median OS was 9.1 months longer in the tucatinib-combination group (21.6 months; 95% CI, 18.1-28.5) vs the placebo-combination group (12.5 months; 95% CI, 11.2-16.9). The tucatinib-combination group showed greater clinical benefit in CNS-PFS and ORR-IC compared with the placebo-combination group. The DOR-IC was 8.6 months (95% CI, 5.5-10.3 months) in the tucatinib-combination group and 3.0 months (95% CI, 3.0-10.3 months) in the placebo-combination group. Risk of developing new brain lesions as the site of first progression or death was reduced by 45.1% in the tucatinib-combination group vs the placebo-combination group (hazard ratio, 0.55 [95% CI, 0.36-0.85]).
Conclusions and Relevance
This subgroup analysis found that tucatinib in combination with trastuzumab and capecitabine improved OS while reducing the risk of developing new brain lesions, further supporting the importance of this treatment option for patients with ERBB2-positive MBC, including those with BMs.
Trial Registration
ClinicalTrials.gov Identifier: NCT02614794
This subgroup analysis of a randomized clinical trial investigates whether tucatinib in combination with trastuzumab and capecitabine provides survival and intracranial benefits for patients with ERBB2-positive metastatic breast cancer and brain metastases.
Introduction
Approximately 15% to 20% of breast cancers overexpress human epidermal growth factor receptor 2 (ERBB2 [formerly HER2]), a subtype of breast cancer with an aggressive clinical phenotype and historically poor survival outcomes before the advent of ERBB2-targeted therapeutics.1,2,3,4 The introduction of ERBB2-directed therapies has resulted in better outcomes for patients with ERBB2-positive breast cancers.5,6,7,8,9 These approaches include using dual ERBB2 blockade, such as anti-ERBB2 antibodies with nonoverlapping target epitopes in the ERBB2 extracellular domain, anti-ERBB2 antibody plus small molecule ERBB2 tyrosine kinase inhibitors, and ERBB2-targeting antibody–drug conjugates.5,6,7,8,9,10
With improved systemic control, the incidence of brain metastases (BMs) as a sanctuary site has increased.11 It is estimated that up to 50% of patients with ERBB2-positive metastatic breast cancer (MBC) will develop BMs,12 which is associated with higher morbidity and shorter survival.13,14,15,16 Neurosurgery, radiosurgery, and whole-brain radiotherapy are often used to treat BMs; however, these techniques can lead to neurologic toxic effects and reduce patients’ quality of life.11,17 Despite the high prevalence and their poor prognosis, patients with BMs, especially those with active or untreated BMs, have been historically excluded from early- and late-stage clinical trials.18,19,20,21,22 Hence, there is a substantial need for tolerable systemic treatment options to treat established BMs and reduce the risk for progression in the central nervous system (CNS).
HER2CLIMB (NCT02614794) is a randomized, double-blind, placebo-controlled clinical trial evaluating tucatinib vs placebo, both in combination with trastuzumab and capecitabine, for ERBB2-positive MBC previously treated with trastuzumab, pertuzumab, and trastuzumab emtansine (T-DM1) in any setting (neoadjuvant, adjuvant, and metastatic).23 In contrast to other studies,11 nearly half of the enrolled population of HER2CLIMB had BMs at baseline, including active BMs. The trial’s primary analysis (median follow-up of 14.0 months; 95% CI, 12.8-14.7) demonstrated that dual ERBB2 blockade with tucatinib in combination with trastuzumab and capecitabine provided a significant benefit in overall survival (OS) and progression-free survival (PFS) for patients with ERBB2-positive MBC.23 At additional follow-up (median follow-up of 29.6 months; 95% CI, 28.2 to 31.3 months), the OS benefit associated with tucatinib was maintained.24
In the initial analysis of HER2CLIMB, tucatinib in combination with trastuzumab and capecitabine provided a PFS benefit with a risk reduction of 52% in overall disease progression or death in patients with BMs.23 In these patients, tucatinib in combination with trastuzumab and capecitabine also reduced the risk of intracranial progression or death by 68%.25 Finally, the confirmed intracranial objective response rate (ORR-IC) was higher in the tucatinib-combination group compared with the placebo-combination group (47.3% vs 20.0%).25
Our exploratory subgroup analyses report efficacy outcomes for patients with BMs, as well as time to new brain lesion(s) as the site of first progression or death in all patients enrolled in HER2CLIMB, with an additional 15.6 months of follow-up.
Methods
Study Design
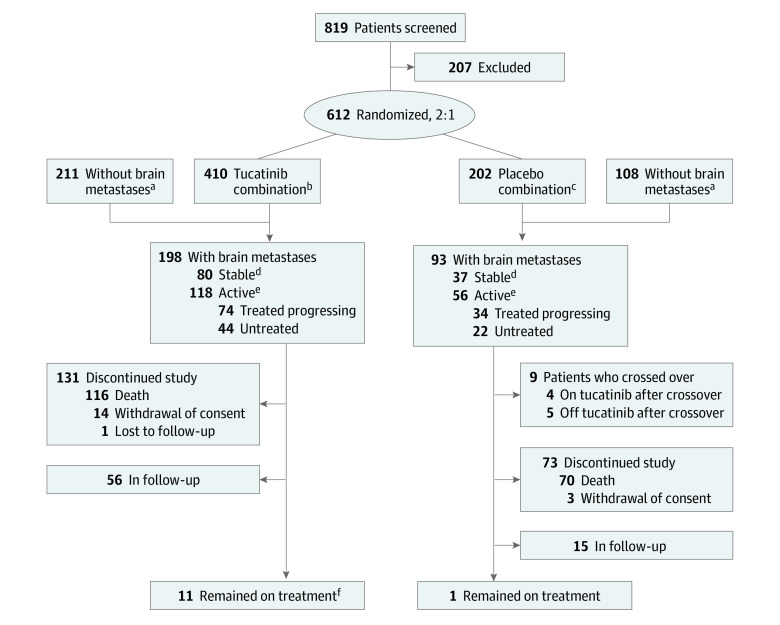
The study design for HER2CLIMB has been described previously.23 In this subgroup analysis of a randomized clinical trial, eligible patients were aged 18 years or older with centrally confirmed, locally advanced or metastatic, ERBB2-positive breast cancer previously treated with trastuzumab, pertuzumab, and T-DM1 in any setting. Patients were randomized 2:1 to receive tucatinib (300 mg orally twice daily) or placebo (orally twice daily), both in combination with trastuzumab (6 mg/kg intravenously every 3 weeks with an initial loading dose of 8 mg/kg; subcutaneous administration was allowed) and capecitabine (1000 mg/m2 orally twice daily on days 1-14 of each 3-week cycle) (Figure 1).23 Patients were stratified according to the presence of BMs (yes or no), Eastern Cooperative Oncology Group performance status score (0 or 1), and geographic region (United States, Canada, or the rest of the world). After the primary analysis, the study was unblinded, and beginning in February 2020, patients were allowed to cross over from the placebo-combination group to the tucatinib-combination group; the data cutoff date for the current analysis was February 8, 2021.24
Figure 1. CONSORT Diagram.
aTwo enrolled patients did not undergo baseline brain magnetic resonance imaging (1 in the tucatinib-combination group and 1 in the placebo-combination group).
bTucatinib, trastuzumab, and capecitabine.
cPlacebo, trastuzumab, and capecitabine.
dPatients with brain metastases that were previously treated and did not progress since most recent CNS-directed therapy.
ePatients with brain metastases that were either untreated or previously treated and progressed since most recent CNS-directed therapy.
fOriginal randomized treatment, not including crossover.
Independent ethics committees or institutional review boards at each site reviewed and approved the protocol (trial protocol and statistical analysis plan in Supplement 1). The study was conducted in accordance with the principles of the Declaration of Helsinki and the International Council for Harmonisation guidelines for Good Clinical Practice and with the study protocol. Written informed consent was provided by all patients before enrollment. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Assessment and Classification of BMs
Patients with a history or presence of BMs at baseline were eligible to participate.23 Patients with untreated brain lesions greater than 2 cm in diameter were eligible if immediate local therapy was not required according to investigator assessment. Individuals requiring immediate local therapy could still enroll after receiving surgery, radiation therapy, or both. All patients underwent brain magnetic resonance imaging at baseline, and those with BMs had imaging performed every 6 weeks for 24 weeks and then every 9 weeks until disease progression according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1 criteria. Enrolled patients with BMs were classified as having either active or stable BMs, as described previously25 and summarized in Figure 1. Active BMs were defined as those that were either untreated or previously treated and had progressed since the most recent CNS-directed therapy. Stable BMs were defined as those that were previously treated and had not progressed since the most recent CNS-directed therapy. Patients with leptomeningeal disease were not eligible.
Efficacy Assessments
Disease response and progression in the brain were evaluated according to investigator assessment with RECIST, version 1.1 to assess brain lesions independently from other organs.25 Intracranial responses were calculated according to the change in the sum of diameters of all target brain lesion measurements and evaluation of nontarget and new brain lesions, using RECIST, version 1.1. Overall survival was evaluated to assess the systemic effect of tucatinib in combination with trastuzumab and capecitabine. The following exploratory end points were evaluated to assess the intracranial responses: confirmed ORR-IC and duration of intracranial response (DOR-IC; defined as time from first intracranial objective response [complete or partial response] to intracranial disease progression or death) in patients with measurable intracranial lesions at baseline and intracranial PFS (CNS-PFS; defined as time to disease progression in the brain or death, whichever occurred first). These assessments were performed for all patients with BMs and separately for subgroups of patients with active and stable BMs.
New brain lesion–free survival was defined as the time from randomization to new lesion in the brain according to investigator assessment based on RECIST, version 1.1 or death from any cause. All patients in the intention-to-treat population were included in this analysis.
Statistical Analysis
Overall survival, CNS-PFS, and time to new brain lesion–free survival and the corresponding 95% CIs were estimated via the Kaplan-Meier method. A stratified Cox proportional hazards model was used to estimate hazard ratios (HRs) and 95% CIs. All P values reported in this exploratory subgroup analysis were 2-sided, are nominal, and were obtained from the stratified log-rank test. SAS version 9.4 was used for data analysis. Intracranial objective response rate with 95% CI was provided for patients with measurable intracranial disease at baseline by treatment group. Kaplan-Meier estimates of median DOR-IC (with corresponding 95% CIs) were provided for patients who achieved a confirmed ORR-IC. Data from February 23, 2016, to February 8, 2021, were analyzed.
For OS, patients without events were censored on the date last known to be alive. For CNS-PFS, DOR-IC, and new brain lesion–free survival, patients without events were censored at the last evaluable magnetic resonance imaging assessment. For patients who crossed over from the placebo-combination group to the tucatinib-combination group, OS analysis was based on the intention-to-treat principle (ie, according to randomization regardless of crossover). For other analyses, crossing over to tucatinib was considered as receiving a new anticancer therapy.
Results
Patient Characteristics
As described previously,25 612 patients were randomized 2:1 to receive tucatinib, trastuzumab, and capecitabine or placebo, trastuzumab, and capecitabine. Median age was 52 years (range, 22-75 years), 289 (99.3%) of the 291 patients with BMs were women, and 2 (0.7%) were men. Almost half (291 of 612 [47.5%]) of the enrolled patients had BMs at baseline (Figure 1). Nine patients with BMs crossed over from the placebo-combination group to receive tucatinib in combination with trastuzumab and capecitabine (at data cutoff, 4 patients were still receiving treatment, and 5 had discontinued treatment). Baseline demographics and disease characteristics were comparable between the 2 treatment groups and to that of the overall HER2CLIMB population.23,25 The breakdown of different BM subgroups is summarized in Figure 1.
Overall Survival
All Patients With BM at Baseline
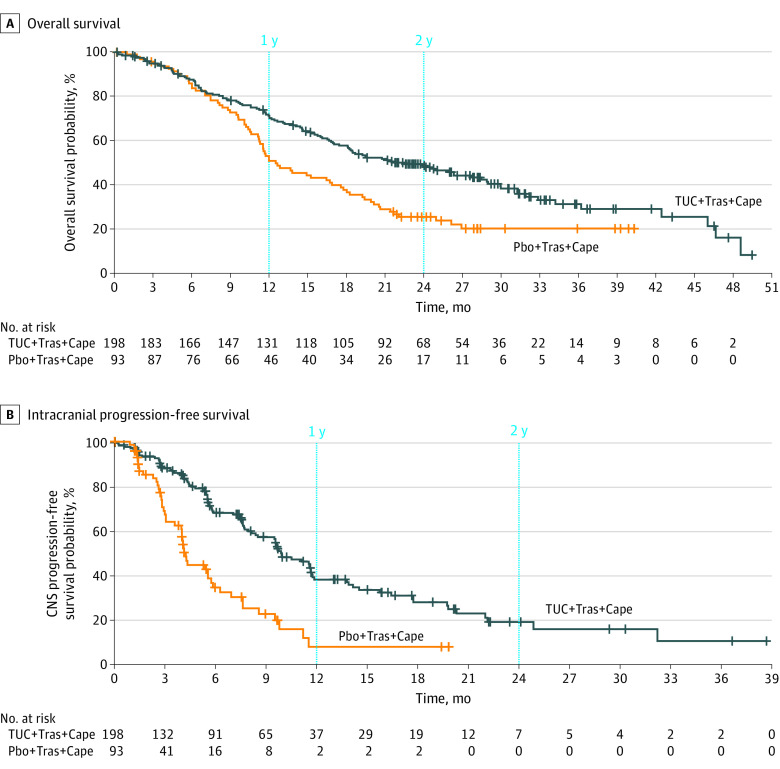
With an additional 15.6 months of follow-up (median follow-up of 29.6 months; range, 0.1-52.9 months), OS benefit with tucatinib improved compared with OS from the initial analysis.24 Median OS was 9.1 months longer—a clinically significant improvement—in the tucatinib-combination group than in the placebo-combination group for all patients with BMs (21.6 vs 12.5 months; 95% CI, 18.1-28.5 vs 11.2-16.9) (Figure 2A). The estimated 1-year OS was 70.0% (95% CI, 63.0%-76.0%) for the tucatinib-combination group and 50.6% (95% CI, 39.9%-60.3%) for the placebo-combination group; the estimated 2-year OS was 48.5% (95% CI, 41.1%-55.5%) for the tucatinib-combination group and 25.1% (95% CI, 16.8%-34.4%) for the placebo-combination group. Risk of death was reduced by 40.0% in the tucatinib-combination group vs the placebo-combination group (HR, 0.60 [95% CI, 0.44-0.81]; P < .001).
Figure 2. Efficacy of Tucatinib Combination Therapy in Patients With Brain Metastases.
A, Median overall survival was 21.6 months (95% CI, 18.1-28.5 months) for patients who received tucatinib plus trastuzumab and capecitabine (TUC + Tras + Cape) and 12.5 months (95% CI, 11.2-16.9 months) for those who received placebo plus trastuzumab and capecitabine (Pbo + Tras + Cape). B, Median progression-free survival was 9.9 months (95% CI, 8.4-11.7 months) for patients who received TUC + Tras + Cape and 4.2 months (95% CI, 3.6-5.7 months) for those who received Pbo + Tras + Cape. CNS indicates central nervous system.
Patients With Active BM at Baseline
Median OS was 9.6 months longer (95% CI, 7.6-11.1 months) in the tucatinib-combination group than in the placebo-combination group for patients with active BMs (21.4 vs 11.8 months; 95% CI, 18.1-28.9 vs 10.3-15.2 months). Estimated 1-year OS was 70.7% (95% CI, 61.5%-78.1%) for the tucatinib-combination group and 46.4% (95% CI, 33.1%-58.8%) for the placebo-combination group; estimated 2-year OS was 48.9% (95% CI, 39.4%-57.8%) for the tucatinib-combination group and 21.4% (95% CI, 11.8%-32.9%) for the placebo-combination group. Risk of death was reduced by 47.6% in the tucatinib-combination group vs the placebo-combination group (HR, 0.52 [95% CI, 0.36-0.77]; P < .001).
A total of 66 patients enrolled with untreated BMs at baseline (Figure 1). Median OS was 6.3 months longer in the tucatinib-combination group compared with the placebo-combination group (19.7 [95% CI, 13.2-28.5] vs 13.4 [95% CI, 6.0 to inestimable] months).
Patients With Stable BM at Baseline
Median OS was 5.2 months longer in the tucatinib-combination group compared with the placebo-combination group in patients with stable BMs (21.6 vs 16.4 months; 95% CI, 15.3-42.4 vs 10.6-21.6). The estimated 1-year OS was 69.1% (95% CI, 57.2%-78.3%) for the tucatinib-combination group and 57.2% (95% CI, 39.4%-71.6%) for the placebo-combination group; the estimated 2-year OS was 47.8% (95% CI, 35.9%-58.8%) for the tucatinib-combination group and 31.0% (95% CI, 16.6%-46.6%) for the placebo-combination group. Risk of death was reduced by 30.5% in the tucatinib-combination group vs the placebo-combination group (HR, 0.70 [95% CI, 0.42-1.16]; P = .16).
Intracranial Responses
CNS-PFS
The CNS-PFS benefit with tucatinib was maintained with longer follow-up for patients with BMs. Median CNS-PFS was 5.7 months longer in the tucatinib-combination group than in the placebo-combination group for all patients with BMs (9.9 vs 4.2 months; 95% CI, 8.4-11.7 vs 3.6-5.7) (Figure 2B). The estimated 1-year CNS-PFS was 38.4% (95% CI, 29.6%-47.2%) for the tucatinib-combination group and 7.9% (95% CI, 1.7%-21.0%) for the placebo-combination group; the estimated 2-year CNS-PFS was 19.3% (95% CI, 11.3%-28.9%) for the tucatinib-combination group and 0% for the placebo-combination group. Risk of progression was reduced by 61.4% in the tucatinib-combination group vs the placebo-combination group (HR, 0.39 [95% CI, 0.27-0.56]; P < .001).
Median CNS-PFS was 5.6 months longer in the tucatinib-combination group than in the placebo-combination group for patients with active BMs (9.6 vs 4.0 months; 95% CI, 7.6-11.1 vs 2.9 to 5.6). The estimated 1-year CNS-PFS was 32.1% (95% CI, 22.2%-42.5%) for the tucatinib-combination group and 0% for the placebo-combination group; the estimated 2-year CNS-PFS was 12.3% (95% CI, 4.8%-23.5%) for the tucatinib-combination group and 0% for the placebo-combination group. Risk of progression was reduced by 66.1% in the tucatinib-combination group vs the placebo-combination group (HR, 0.34 [95% CI, 0.22-0.54]; P < .001). In the subgroup of patients with untreated BMs, median CNS-PFS was 6.5 months longer in the tucatinib-combination group than in the placebo-combination group (9.6 vs 3.1 months; 95% CI, 5.5-11.6 vs 1.4-7.6).
Median CNS-PFS was 8.3 months longer in the tucatinib-combination group than in the placebo-combination group for patients with stable BMs (13.9 vs 5.6 months; 95% CI, 9.7-24.9 vs 3.0 to inestimable). The estimated 1-year CNS-PFS was 52.7% (95% CI, 35.3%-67.4%) for the tucatinib-combination group and 30.0% (95% CI, 9.4%-54.1%) for the placebo-combination group; the estimated 2-year CNS-PFS was 34.3% (95% CI, 17.1%-52.4%) for the tucatinib-combination group and 0% for the placebo-combination group. Risk of progression was reduced by 59.4% in the tucatinib-combination group vs the placebo-combination group (HR, 0.41 [95% CI, 0.20-0.85]; P = .014).
ORR-IC and DOR-IC
Patients in the tucatinib-combination group continued to show higher confirmed ORR-IC and DOR-IC than those in the placebo-combination group. Seventy-five patients had active BMs and measurable intracranial lesions at baseline (Table), with a confirmed ORR-IC of 47.3% (95% CI, 33.7%-61.2%) for the tucatinib-combination group and 20.0% (95% CI, 5.7%-43.7%) for the placebo-combination group. Median DOR-IC was 8.6 months (95% CI, 5.5-10.3 months) for the tucatinib-combination group and 3.0 months (95% CI, 3.0-10.3 months) for the placebo-combination group.
Table. Confirmed Intracranial Responses in Patients With Active Brain Metastases and Measurable Intracranial Lesions at Baseline.
| Intracranial response | Tucatinib combination (n = 55)a | Placebo combination (n = 20)b |
|---|---|---|
| Patients with objective response of confirmed complete response or partial response, No. | 26 | 4 |
| Confirmed ORR-IC, % (95% CI) | 47.3 (33.7-61.2) | 20.0 (5.7-43.7) |
| DOR-IC, median (95% CI), moc | 8.6 (5.5-10.3) | 3.0 (3.0-10.3) |
Abbreviations: DOR-IC, duration of intracranial response; ORR-IC, intracranial objective response rate.
Tucatinib, trastuzumab, and capecitabine.
Placebo, trastuzumab, and capecitabine.
Calculated with the complementary log-log transformation method.
Among the patients with untreated BMs at baseline with measurable intracranial disease (17 for the tucatinib-combination group and 6 for the placebo-combination group), the confirmed ORR-IC was 47.1% (95% CI, 23.0%-72.2%) for the tucatinib-combination group and 16.7% (95% CI, 0.4%-64.1%) for the placebo-combination group.
New Brain Lesion–Free Survival for All Patients
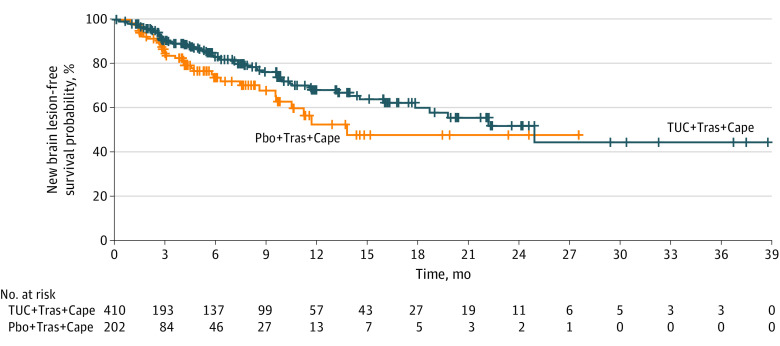
Among the entire intention-to-treat study population (N = 612), median new brain lesion–free survival was 11.1 months longer for the tucatinib-combination group than for the placebo-combination group (24.9 vs 13.8 months; 95% CI, 17.8 to inestimable vs 9.6 to inestimable) (Figure 3). Risk of developing new brain lesions as the site of first progression or death was reduced by 45.1% in the tucatinib-combination group vs the placebo-combination group (HR, 0.55 [95% CI, 0.36-0.85]; P = .006).
Figure 3. New Brain Lesion–Free Survival According to Investigator Assessment for All Patients.
Median new brain lesion–free survival was 24.9 months (95% CI, 17.8 to inestimable) for patients who received tucatinib plus trastuzumab and capecitabine (TUC + Tras + Cape) and 13.8 months (95% CI, 9.6 to inestimable) for those who received placebo plus trastuzumab and capecitabine (Pbo + Tras + Cape).
Discussion
To our knowledge, HER2CLIMB is currently the only double-blind, randomized, controlled clinical trial for patients with ERBB2-positive MBC that prospectively included individuals with both active and stable BMs; almost half of the enrolled patients had BMs at baseline. Although patients with stable BMs have been included in other clinical trials, HER2CLIMB included a substantial number of individuals with active BMs to whom systemic therapy was given instead of local CNS-directed therapy. Previous analyses of HER2CLIMB have shown that the addition of tucatinib to trastuzumab and capecitabine provided an OS benefit irrespective of the presence or absence of BMs.23,25 This exploratory analysis shows a sustained, clinically significant OS benefit for patients with BMs, regardless of whether the patient had active or stable BMs. With 15.6 months of additional follow-up, the absolute median OS benefit associated with tucatinib for all patients with BMs increased from 6.1 months25 to 9.1 months, resulting in a median OS of 21.6 months despite the previous treatment with trastuzumab, pertuzumab, and T-DM1. Median OS was longer for patients with both active and stable BMs treated with tucatinib in combination with trastuzumab and capecitabine, suggesting the intracranial benefits observed with tucatinib in combination with trastuzumab and capecitabine are irrespective of BM classification. Overall survival benefit was also observed in the exploratory subpopulation of patients who had untreated BMs.
It is estimated that up to 50% of patients with ERBB2-positive MBC will develop BMs, but current CNS-directed therapies, such as neurosurgery, radiosurgery, and whole-brain radiotherapy, can be associated with neurologic toxic effects and reduced quality of life.11,13,14,17 Although whole-brain radiotherapy has been shown to improve intracranial control compared with radiosurgery, it has not been demonstrated to improve OS compared with radiosurgery in randomized clinical trials and is associated with increased toxicity.26 Given the prevalence of BMs and the adverse effects associated with current BM treatments, the development of well-tolerated strategies to prevent the development and progression of BMs and improve survival of patients with BMs is an important clinical imperative. This analysis showed that the addition of tucatinib to trastuzumab and capecitabine resulted in a significant improvement in OS and estimated 1-year OS. Tucatinib in combination with trastuzumab and capecitabine also resulted in an approximate doubling of 2-year OS.
The subgroup of patients with untreated BMs comprised 66 individuals (23 of whom were eligible for ORR-IC analysis). Despite the small number, this analysis showed that tucatinib in combination with trastuzumab and capecitabine may lead to OS benefit for patients with untreated BMs. Given that this subgroup is unique to the current analysis and that promising clinical activity was observed, further investigation of this patient subgroup is warranted.
Tucatinib is highly selective for ERBB2 and is greater than 1000-fold more specific for ERBB2 than EGFR.27,28 In combination with trastuzumab and capecitabine, tucatinib has shown antitumor activity with generally low-grade adverse events in patients with previously treated ERBB2-positive MBC, including those with BMs.23,25 The efficacy of tucatinib for patients with BMs may be due to its ability to cross the blood-brain barrier because tucatinib and its predominant metabolite have been detected in the cerebrospinal fluid of patients treated with tucatinib.29 In HER2CLIMB, tucatinib was associated with a 61.4% reduction in the risk of CNS-PFS for patients with BMs, regardless of whether the patients had active or stable BMs. Furthermore, among all randomized patients, median new brain lesion–free survival was almost a year longer in the tucatinib-combination group than in the placebo-combination group, implying that tucatinib may delay the development of new brain lesions in patients with ERBB2-positive MBC.30 Two ongoing studies (NCT05323955 and NCT05041842) are assessing tucatinib in patients with isolated CNS progression. These findings are in contrast to T-DM1, which has not been shown to reduce the risk of intracranial relapse or progression in the adjuvant or metastatic settings,31,32 possibly because of the lack of penetration across an intact blood-brain barrier.33 The CompassHER2 RD trial (NCT04457596) is currently recruiting patients with residual disease after neoadjuvant therapy to receive T-DM1 with or without tucatinib; an important secondary end point is the incidence of BMs by treatment group.
Finally, in HER2CLIMB, tucatinib in combination with trastuzumab and capecitabine not only resulted in longer OS and PFS but also was well tolerated.23,24 A recent analysis of patient-reported outcomes demonstrated that, for patients with BMs, tucatinib in combination with trastuzumab and capecitabine had minimal effect on quality of life and reduced the risk of clinically meaningful deterioration of quality of life by almost half, further suggesting that the regimen is well tolerated among patients with BMs.34
Limitations
One limitation of this analysis was that is was exploratory; however, the HER2CLIMB trial included a total of 291 patients with ERBB2-positive MBC and BMs, which is the largest patient population to date for a randomized, placebo-controlled clinical study.11 Since the completion of HER2CLIMB, more trials on ERBB2-positive MBC have begun to include patients with active BMs (including untreated BMs), and forthcoming studies should continue to include this patient population to address the high unmet need. Recent US Food and Drug Administration guidance also recommends that clinical trials include patients with BMs, especially those with active BMs, because it will contribute to a better understanding and assessment of the efficacy and safety of investigational drugs.22
Conclusions
Developing new treatment regimens for patients with ERBB2-positive MBC, including those with BMs, remains an important medical need. This exploratory subgroup analysis showed that tucatinib in combination with trastuzumab and capecitabine provides survival benefits for patients with BMs, has a manageable safety profile, and may delay development of new brain lesions for all patients. Tucatinib in combination with trastuzumab and capecitabine is an important treatment option for patients with previously treated ERBB2-positive MBC, including those with BMs.
Trial Protocol and Statistical Analysis Plan
Data Sharing Statement
References
- 1.Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106(5):dju055. doi: 10.1093/jnci/dju055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goddard KA, Weinmann S, Richert-Boe K, Chen C, Bulkley J, Wax C. HER2 evaluation and its impact on breast cancer treatment decisions. Public Health Genomics. 2012;15(1):1-10. doi: 10.1159/000325746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lux MP, Nabieva N, Hartkopf AD, et al. Therapy landscape in patients with metastatic HER2-positive breast cancer: data from the PRAEGNANT Real-World Breast Cancer Registry. Cancers (Basel). 2018;11(1):10. doi: 10.3390/cancers11010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolff AC, Hammond MEH, Hicks DG, et al. ; American Society of Clinical Oncology; College of American Pathologists . Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997-4013. doi: 10.1200/JCO.2013.50.9984 [DOI] [PubMed] [Google Scholar]
- 5.Gradishar WJ, Anderson BO, Abraham J, et al. Breast cancer, version 3.2020: NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(4):452-478. doi: 10.6004/jnccn.2020.0016 [DOI] [PubMed] [Google Scholar]
- 6.Martínez-Sáez O, Prat A. Current and future management of HER2-positive metastatic breast cancer. JCO Oncol Pract. 2021;17(10):594-604. doi: 10.1200/OP.21.00172 [DOI] [PubMed] [Google Scholar]
- 7.Nader-Marta G, Martins-Branco D, de Azambuja E. How we treat patients with metastatic HER2-positive breast cancer. ESMO Open. 2022;7(1):100343. doi: 10.1016/j.esmoop.2021.100343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Telli ML, Gradishar WJ. Updates in HER2-positive and triple-negative breast cancers. J Natl Compr Canc Netw. 2021;19(5.5):605-609. doi: 10.6004/jnccn.2021.5005 [DOI] [Google Scholar]
- 9.Patel A, Unni N, Peng Y. The changing paradigm for the treatment of HER2-positive breast cancer. Cancers (Basel). 2020;12(8):2081. doi: 10.3390/cancers12082081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swain SM, Miles D, Kim SB, et al. ; CLEOPATRA Study Group . Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(4):519-530. doi: 10.1016/S1470-2045(19)30863-0 [DOI] [PubMed] [Google Scholar]
- 11.Stavrou E, Winer EP, Lin NU. How we treat HER2-positive brain metastases. ESMO Open. 2021;6(5):100256. doi: 10.1016/j.esmoop.2021.100256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pestalozzi BC, Holmes E, de Azambuja E, et al. CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: a retrospective substudy of the HERA trial (BIG 1-01). Lancet Oncol. 2013;14(3):244-248. doi: 10.1016/S1470-2045(13)70017-2 [DOI] [PubMed] [Google Scholar]
- 13.Leone JP, Lin NU. Systemic therapy of central nervous system metastases of breast cancer. Curr Oncol Rep. 2019;21(6):49. doi: 10.1007/s11912-019-0802-6 [DOI] [PubMed] [Google Scholar]
- 14.Bailleux C, Eberst L, Bachelot T. Treatment strategies for breast cancer brain metastases. Br J Cancer. 2021;124(1):142-155. doi: 10.1038/s41416-020-01175-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Alvarez A, Papakonstantinou A, Oliveira M. Brain metastases in HER2-positive breast cancer: current and novel treatment strategies. Cancers (Basel). 2021;13(12):2927. doi: 10.3390/cancers13122927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hurvitz SA, O’Shaughnessy J, Mason G, et al. Central nervous system metastasis in patients with HER2-positive metastatic breast cancer: patient characteristics, treatment, and survival from SystHERs. Clin Cancer Res. 2019;25(8):2433-2441. doi: 10.1158/1078-0432.CCR-18-2366 [DOI] [PubMed] [Google Scholar]
- 17.Lauko A, Rauf Y, Ahluwalia MS. Medical management of brain metastases. Neurooncol Adv. 2020;2(1):vdaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel RR, Verma V, Miller AB, et al. Exclusion of patients with brain metastases from cancer clinical trials. Neuro Oncol. 2020;22(4):577-579. doi: 10.1093/neuonc/noz246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costa R, Gill N, Rademaker AW, et al. Systematic analysis of early phase clinical studies for patients with breast cancer: inclusion of patients with brain metastasis. Cancer Treat Rev. 2017;55:10-15. doi: 10.1016/j.ctrv.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 20.Kim AE, Wang GM, Waite KA, et al. Cross-sectional survey of patients, caregivers, and physicians on diagnosis and treatment of brain metastases. Neurooncol Pract. 2021;8(6):662-673. doi: 10.1093/nop/npab042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin NU, Prowell T, Tan AR, et al. Modernizing clinical trial eligibility criteria: recommendations of the American Society of Clinical Oncology–Friends of Cancer Research Brain Metastases Working Group. J Clin Oncol. 2017;35(33):3760-3773. doi: 10.1200/JCO.2017.74.0761 [DOI] [PubMed] [Google Scholar]
- 22.US Food and Drug Administration . Cancer clinical trial eligibility criteria: brain metastases—guidance for industry. Published July 2020. Accessed October 20, 2022. https://www.fda.gov/media/121317/download
- 23.Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597-609. doi: 10.1056/NEJMoa1914609 [DOI] [PubMed] [Google Scholar]
- 24.Curigliano G, Mueller V, Borges V, et al. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): final overall survival analysis. Ann Oncol. 2022;33(3):321-329. doi: 10.1016/j.annonc.2021.12.005 [DOI] [PubMed] [Google Scholar]
- 25.Lin NU, Borges V, Anders C, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020;38(23):2610-2619. doi: 10.1200/JCO.20.00775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogelbaum MA, Brown PD, Messersmith H, et al. Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol. 2022;40(5):492-516. doi: 10.1200/JCO.21.02314 [DOI] [PubMed] [Google Scholar]
- 27.Kulukian A, Lee P, Taylor J, et al. Preclinical activity of HER2-selective tyrosine kinase inhibitor tucatinib as a single agent or in combination with trastuzumab or docetaxel in solid tumor models. Mol Cancer Ther. 2020;19(4):976-987. [DOI] [PubMed] [Google Scholar]
- 28.Moulder SL, Borges VF, Baetz T, et al. Phase I study of ONT-380, a HER2 inhibitor, in patients with HER2+-advanced solid tumors, with an expansion cohort in HER2+ metastatic breast cancer (MBC). Clin Cancer Res. 2017;23(14):3529-3536. doi: 10.1158/1078-0432.CCR-16-1496 [DOI] [PubMed] [Google Scholar]
- 29.Stringer-Reasor EM, O’Brien BJ, Topletz-Erickson A, et al. Pharmacokinetic (PK) analyses in CSF and plasma from TBCRC049, an ongoing trial to assess the safety and efficacy of the combination of tucatinib, trastuzumab and capecitabine for the treatment of leptomeningeal metastasis (LM) in HER2 positive breast cancer. J Clin Oncol. 2021;39(15)(suppl):1044. doi: 10.1200/JCO.2021.39.15_suppl.1044 [DOI] [Google Scholar]
- 30.Eberst L, Bailleux C, Bachelot T. Prevention of brain metastases in human epidermal growth factor receptor 2–positive breast cancer. Curr Opin Oncol. 2020;32(6):555-560. doi: 10.1097/CCO.0000000000000682 [DOI] [PubMed] [Google Scholar]
- 31.von Minckwitz G, Huang CS, Mano MS, et al. ; KATHERINE Investigators . Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617-628. doi: 10.1056/NEJMoa1814017 [DOI] [PubMed] [Google Scholar]
- 32.Krop IE, Lin NU, Blackwell K, et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol. 2015;26(1):113-119. doi: 10.1093/annonc/mdu486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montemurro F, Delaloge S, Barrios CH, et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial. Ann Oncol. 2020;31(10):1350-1358. doi: 10.1016/j.annonc.2020.06.020 [DOI] [PubMed] [Google Scholar]
- 34.Mueller V, Wardley A, Paplomata E, et al. Preservation of quality of life in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer treated with tucatinib or placebo when added to trastuzumab and capecitabine (HER2CLIMB trial). Eur J Cancer. 2021;153:223-233. doi: 10.1016/j.ejca.2021.05.025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
Data Sharing Statement