Abstract
Background
The menopausal transition (perimenopause) is associated with an increased risk of major depression, characterized by anxiety and anhedonia phenotypes. Greater estradiol (E2) variability predicts the development of perimenopausal depression, especially within the context of stressful life events (SLEs). While transdermal E2 (TE2) reduces perimenopausal depressive symptoms, the mechanisms underlying TE2 efficacy and predictors of TE2 treatment response remain unknown. This study aimed at determining relationships between E2 fluctuations, mood symptoms, and physiologic stress-reactivity (cortisol and interleukin-6) and whether differences in mood-sensitivity to E2 fluctuations predict mood responses to TE2 treatment.
Methods
This randomized, double-blind, placebo-controlled trial investigated medically healthy women (46–60 years) in the early or late menopause transition. Baseline E2-sensitivity strength was calculated from eight weekly individual correlations between week-to-week E2 change and index week anxiety (State-Trait Anxiety Inventory) and anhedonia (Snaith-Hamilton Pleasure Scale). Women then received eight weeks of TE2 or transdermal placebo.
Results
Analyses included 73 women (active TE2 n=35). Greater baseline E2 fluctuations predicted greater anhedonia (p=.002), particularly in women with more SLEs. Greater E2 fluctuations also predicted higher cortisol (p=.012) and blunted interleukin-6 (p=.02) stress-responses. Controlling for baseline symptoms, TE2 was associated with lower post-treatment anxiety (p<.001) and anhedonia (p<.001) versus placebo. However, the efficacy of TE2 for anxiety (p=.007) and also for somatic complaints (p=.05) was strongest in women with greater baseline E2 sensitivity strength.
Conclusions
TE2 treatment reduced perimenopausal anxiety and anhedonia. The ability of baseline mood-sensitivity to E2 fluctuations to predict greater TE2 efficacy has implications for individualized treatment of perimenopausal anxiety disorders.
Keywords: Anxiety, anhedonia, differential sensitivity, estradiol fluctuations, perimenopause, transdermal estradiol
Introduction
The menopausal transition (perimenopause) is characterized by dynamic variability in estradiol (E2), frequent stressful life events (SLEs), and increased risk of major depression (Maki et al., 2019; O’Connor et al., 2009). Perimenopausal depression typically presents with an anxious and anhedonic phenotype. Although most perimenopausal women will be exposed to erratic E2 fluctuations, only a minority (albeit sizeable) will develop impairing affective symptoms. In perimenopause, there is up to a 3-fold increase in risk to develop depressive symptoms compared to pre- and postmenopausal stages (Maki, 2019), and almost half of all women are affected by some level of anxiety symptoms (Andersen et al., 2004; Avis et al., 2001). In a national diverse sample of aging women followed longitudinally, low social support, perceived stress, and the presence of distressing life events increased the odds for having a CES-D score of 16 or higher (indicative of major depression). Ethnic differences in risk for perimenopausal depression and anxiety were also noted in that study as Hispanic women had higher odds of developing depression than White women, even after controlling for social support, perceived stress and stressful life events (Bromberger, 2004).
While the causes of perimenopausal affective illness rename unknown, a recent study (Gordon et al., 2020), showed that the within-person correlation between weekly urinary estrone-3-glucuronide (E1G) and depressive symptoms varied between women and that the strength of the correlation (i.e., hormone sensitivity strength) predicted the development of clinically significant depressive symptoms. Understanding predictors of susceptibility to hormone sensitivity and their implications for treatment will advance precision medicine for reproductive mood disorders.
Rates of distress are increased during perimenopause, when compared to pre- or postmenopausal stages (Bromberger et al., 2001). Our prior work suggests that recent SLEs amplify mood sensitivity to ovarian hormone change since we showed that greater week-to-week changes in E2 or E1G (e.g., greater variability) was most likely to predict the emergence of depressive symptomatology in perimenopausal women with more recent SLEs (Gordon et al., 2015, 2016, 2018). The possibility exists that greater sensitivity to E2 fluctuations serves as a diathesis in a stress pathway to perimenopausal psychopathology. Life stress and depression outside the perimenopause are associated with dysregulation in the hypothalamic-pituitary-adrenal (HPA) axis and a pro-inflammatory state (Burke et al., 2005; Raison et al., 2005; Slavich & Irwin, 2014). E2 modulates both the HPA axis and pro-inflammatory mediators (Klein & Flanagan, 2016; Vamvakopoulos & Chrousos, 1993), making these candidate biomarkers that may contribute to the association of E2 fluctuations with depression in the context of SLEs.
Regardless of the mechanisms linking E2 fluctuations to perimenopausal depression, prior controlled trials have shown that transdermal E2 (TE2) reduces depressive symptoms in perimenopausal women with depression (De Novaes Soares et al., 2001; Gordon & Girdler, 2014; Schmidt et al., 2000) and prevents the emergence of depressive episodes in perimenopausal women, with the prophylactic antidepressant benefit particularly evident in women with more SLEs (Gordon et al., 2018). However, the mechanisms contributing to the antidepressant effects of TE2 in perimenopausal women remain unknown, as does the predictive ability of a hormone sensitive phenotype for TE2 treatment efficacy.
The primary objectives of this research were to determine, in perimenopausal women, whether E2 fluctuations predict: 1) symptoms of anxiety and anhedonia; 2) HPA axis and pro-inflammatory cytokine stress responses; and 3) whether sensitivity to E2 fluctuations (hormone sensitivity strength) predicts the response of anxiety and anhedonia symptoms to TE2.
Methods and Materials
Trial design
This study was designed to test, in perimenopausal women, the effects of endogenous E2 fluctuations and TE2 administration on 1) anxiety and anhedonia symptoms and 2) HPA-axis stress reactivity (secondary outcome), and to examine whether recent SLEs moderated these relationships. These pre-defined study aims (NCT03003949) are extended in this report by the inclusion of pro-inflammatory mediators (cytokine and acute-phase protein) and a refinement of the E2 fluctuation aim by characterizing individual differences in the degree of sensitivity to E2 fluctuation (Gordon et al., 2020).
A randomized, double-blind, placebo-controlled design was employed, with parallel allocation to either a TE2 arm (0.1 mg E2/24 hours) or a transdermal placebo arm. The study consisted of: 1) a screening and enrollment period; 2) an 8-week baseline period, after which women were randomized using a 1:1 allocation to the active or placebo arm; and 3) an 8-week post-randomization period. Over the two adjacent 8-week intervals, subjects provided weekly blood samples for E2 and completed questionnaires assessing anxiety and anhedonia. At weeks 0, 8, and 16, women were exposed to a psychosocial stress protocol (see supplementary Figure 1).
Participants
Between January 2017 and March 2020, 73 medically healthy women aged 46–60 meeting STRAW+10 reproductive staging criteria for early (STRAW −2), late menopause transition (STRAW −1) or were within the first 12 months post of amenorrhea (STRAW +1a) (Harlow et al., 2012) were recruited through advertisements, university email announcements, and flyers. The last participant was enrolled in March 2020, after which the study was terminated prematurely due to COVID-19 restrictions on clinical research. All data were collected at the University of North Carolina (UNC) at Chapel Hill School of Medicine.
Participants were screened for inclusion and exclusion criteria via telephone (see Supplement for criteria). If eligible, enrollment commenced and participants provided written informed consent. The Structured Clinical Interview (SCID-RV) for DSM-5 disorders (First et al., 2015) and the Life Experiences Survey (LES) for SLEs in the preceding six months were administered. All participants underwent medical screening, including vitals and history, a gynecological exam, and a screening mammogram. Participants received $700 for completion of the protocol. The UNC Biomedical Institutional Review Board approved the study (IRB 16–1731). This research was conducted in accordance with the Helsinki Declaration.
Study Design
Weekly during the 16-week protocol, staff visited the participant’s home to administer questionnaires for anxiety and anhedonia symptoms and to draw a serum sample for E2 (Supplement for details).
At weeks 0, 8, and 16, women underwent a laboratory session during which the Trier Social Stress Test (TSST) was administered. Participants’ sessions were scheduled at the same time of the day to control for circadian effects. The TSST at week 0 was performed to address the novelty effect upon first exposure (Allen et al., 2014), thereby eliminating habituation to the TSST as a confound in comparing week 8 and week 16 stress reactivity. The TSST included a 5-minute simulated job interview (week 0 TSST) or talk about a specific topic like climate change (week 8 and 16 TSST) and a 5-minute serial subtraction task (Kirschbaum et al., 1993; varied at each TSST) and was performed in front of a non-responsive, two-member committee. After establishing an intravenous line in an arm vein, subjects rested quietly for 30 minutes before the TSST. Following the rest, venous blood was drawn for baseline samples, and at 10-, 20-, 30-, and 45- minutes post-TSST for stress samples. Plasma was subsequently frozen at −80°C.
Intervention
Following the 8-week baseline, women randomized to active treatment (n= 35) applied a weekly TE2 patch (Alvogen) [0.1mg E2/24 hours for 8 weeks]. After the 8-week post-randomization period, and after all research-related evaluations were completed, women took oral micronized progesterone to prevent endometrial hyperplasia (200mg/day of Prometrium for 12 days). Women randomized to placebo (n=38) followed the same regimen using placebo patches similar in appearance and took placebo capsules (Capsugel Orange, Size AA, DB Capsules). Patches were dispensed in a blinded fashion in individually wrapped packages without manufacturer branding. Pills were dispensed in blinded capsules.
To monitor adherence, study personnel confirmed patch use at each weekly visit. Participants were given written instructions with a picture showing how and where to place the patches on their lower abdomen or upper buttocks. Participants recorded dates of patch placement and removal, and returned all used and unused patches at the week 16 laboratory session. At each visit, side effects and adverse events were monitored (see Supplement).
Randomization
The study biostatistician created the randomization scheme and assigned participants to interventions. The Investigational Drug Services of UNC managed the randomization and blinded dispensing of patches and capsules. The first and second authors performed analyses and were unblinded after study discontinuation. Neither had contact with participants, nor access to identifiable information. All outcome assessors remained blinded throughout the protocol.
Measures and Outcomes
E2 fluctuation was measured using the mean absolute successive difference (MASD), calculated as the mean of the absolute E2 change (the absolute difference between weeks, absdiff) from each week to the next:
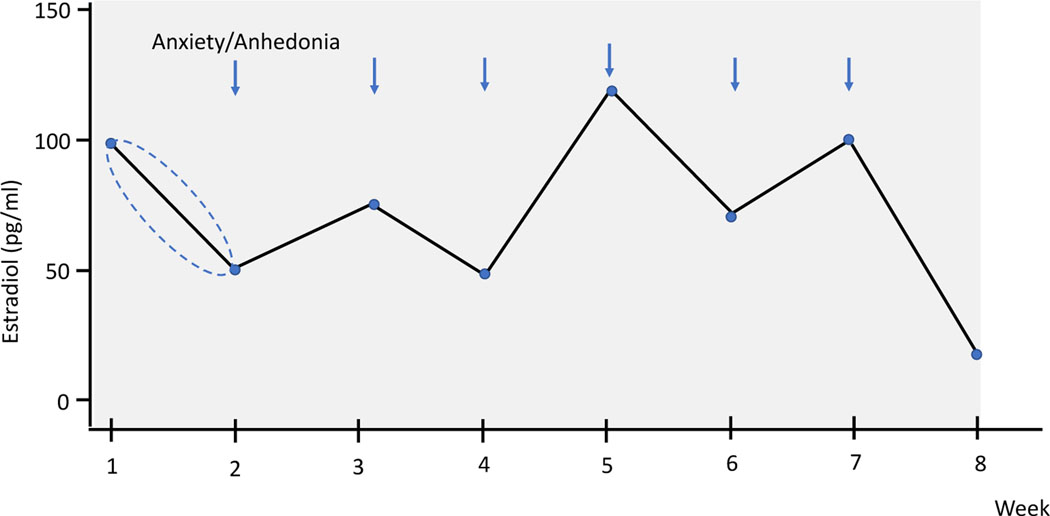
Equation for MASD: Mean of ((absdiff= week 2-week 1 E2 levels), (absdiff= week 3 - week 2 E2 levels), etc., through to (absdiff= week 8 – week 7 E2 levels)). Thus, values from the stylized Figure 1 for calculating the MASD would be: Mean ((50), (25), (25), (75), (50), (25) (75)).
Figure 1.
Stylistic figure depicting the calculation of the correlations between the change in E2 from the past week based on two methods: 1) including the direction of change and 2) using the absolute change in E2 from the past week (directionless) with mood (anxiety and anhedonia) at the index week. In the figure, there is a 50pg/ml decrease in E2 from week 1 to week 2. In one calculation: , the value −50 (with direction) is correlated with the anxiety value at week 2. In the other calculation, the value 50 (without direction) is correlated with the anxiety value at week 2. Both calculations were used for every index week, resulting in two overall correlation coefficients for anxiety for every participant (one including the direction and one without the direction). The same procedure was employed for calculating the correlations between changes in E2 and changes in anhedonia symptoms.
As such, and in comparison to the often used standard deviation (SD) to assess variability over time, the MASD takes into account the temporal order of E2 assessments and that a large change in E2 from one week to the next might have a stronger mood impact than the same magnitude increase across several weeks. This is accomplished by including week-to-week E2 differences into models, and not simply the overall SD across all weeks (Ebner-Priemer et al., 2007, 2009).
Following existing methods for measuring E2 hormone sensitivity strength (Gordon et al., 2020; Andersen et al., 2022), for each baseline week, we calculated the correlation between the change in E2 from last week (with the direction of change), and the absolute change in E2 from last week (directionless) with mood (anxiety and anhedonia) at the index week (see Figure 1).
Four correlation coefficients were examined. The correlation between 1) directional E2 change and anxiety, 2) absolute E2 change and anxiety, 3) directional E2 change and anhedonia, and 4) absolute E2 change and anhedonia. The strength of the directional E2 change-mood correlation and the absolute E2 change-mood correlation for anxiety and anhedonia were calculated separately. For each mood measure, the largest magnitude correlation was chosen. The E2-sensitivity strengths were defined as the absolute value of a person’s largest E2 change anxiety and anhedonia correlation. Thus, a participant with an E2-change anhedonia correlation of −.5 and an absolute E2-change anhedonia correlation of .7 would have an anhedonia sensitivity strength of .7. The E2-sensitivity strengths describe an individual’s maximum anxiety and anhedonia sensitivity to weekly changes in E2 (ranging from 0 to 1).
Cortisol and IL-6 reactivity were operationalized as Area Under the Curve with respect to ground (AUC), calculated using a trapezoid integration formula (Pruessner et al., 2003).
Recent stressful life events (SLEs; previous 6 months) were assessed using the Life Experiences Survey (LES) (Sarason et al., 1978), a 30-item self-report measure. Weekly self-reported anxiety symptoms (state version of the State Trait Anxiety Inventory; STAI, Spielberger et al., 1970) and anhedonia symptoms (Snaith-Hamilton Pleasure Scale; SHAPS, Snaith et al., 1995) were assessed. The Greene Climacteric scale, administered at enrollment, week 8 and week 16, assessed the degree to which respondents reported bother from vasomotor symptoms, general somatic symptoms, depression symptoms, loss of sexual interest, and anxiety symptoms (Greene, 2008). (see Supplement for scale details, calculations, and psychometrics).
For E2 concentrations, samples from home visits were immediately transported to our laboratory, where blood clotted at room temperature, was centrifuged, aliquoted, and the serum stored at −80°C. E2 was analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) (Quest Diagnostics Nichols Institute San Juan Capistrano, CA). Intra-assay coefficients of variation (CV) for E2 were < 3.41% and inter-assay CV’s < 4.06%. The lower limit of quantitation (LOD) was 2 pg/ml and levels below LOD were set at half the detection limit (Nave et al., 2018).
All laboratory-derived hormones and pro-inflammatory mediators were assessed from the baseline, pre-stress plasma sample and from the post-TSST samples capturing marker-specific stress reactivity (Brydon et al., 2004; Goodman et al., 2017; Slavish et al., 2015; Steptoe et al., 2001): cortisol (minutes 10, 20, 30, 45), pro-inflammatory cytokine Interleukin-6 (IL-6; minutes 30 and 45), and the systemic inflammatory marker, acute-phase C-reactive protein (CRP; baseline).
Plasma cortisol concentrations were analyzed using commercially available radioimmunoassay kits (MP Biomedicals, Orangeburg, NY). Cortisol assay sensitivity was at 0.07 ug/dL, with intra- and inter-assay CV’s of 4.7% and 7.6% respectively. IL-6 and CRP concentrations were determined using commercially available high-sensitivity (hs) ELISA assay kits (R&D Systems, Minneapolis and MP Biomedicals, Ohio). IL-6 assay sensitivity was .039 pg/ml with a standard range of .156 to 10 pg/ml and intra- and inter-assay variations of 7.8% and 7.2%, respectively. hsCRP assay sensitivity was 0.1 mg/l with an upper detection limit of 10 mg/l and an intra- and inter-assay variation of ≤7.5% and ≤4.1%.
Sample size
Despite the premature termination of the study, power analyses (sensitivity analyses in G*Power, Faul et al., 2007) confirmed that power was maintained to detect 1) small-to-medium effects of continuous predictors of anxiety and anhedonia and 2) medium-to-large effects of dichotomous predictors (i.e., randomization). Sensitivity analyses were based on intraclass correlation (ICC) for repeated outcome measures and a design corrected sample size (Snijders & Bosker, 2011).
Statistical analyses
Pre-Randomization Associations.
The effect of E2 variability (MASD) on continuous outcomes was assessed using repeated measures ANCOVA (proc mixed), controlling for time of day in stress-reactivity models (i.e,. Steptoe et al., 2007; Stetler & Miller, 2011), and age and BMI in models testing effects on IL-6 and hsCRP (McInnis et al., 2014; Steptoe et al., 2007). The interaction of these associations with the number of recent SLEs was tested as moderator.
Effect of E2 Treatment.
Consistent with intent-to-treat analyses, all randomized participants were included in analyses. To examine the effect of treatment on continuous outcomes, repeated measures regressions (for mixed models) were conducted with eight repeated measures of anxiety and anhedonia (post-randomization weeks 9–16) and two repeated measures for stress reactivity (week 8 and week 16) nested within women. For multi-level regression models, a first-order autoregressive covariance structure was specified for within-person error. The Kenward–Roger method computed degrees of freedom. Applied multi-level regression models did not delete missing data listwise, therefore, all available data were used. We tested whether any of these associations interacted with baseline E2-sensitivity strength. All models testing for a treatment effect controlled for baseline anxiety and depression levels and the change in vasomotor symptom bother from baseline to post-randomization using the Greene Climacteric Scale subscale scores. Data were analyzed using SAS (SAS Institute Inc; Cary, NC.). Two-sided p-values at <.05 defined statistical significance.
Results
Participant characteristics
Supplementary figure 3 depicts the CONSORT diagram. Seventy-three women were included in this RCT. Table 1 summarizes key sample characteristics. Women randomized to E2 and placebo demonstrated comparable demographics and anxiety and anhedonia symptoms at baseline. They also demonstrated comparable self-reported smoking, alcohol, caffeine and herbal supplement use, parity and number of children at home, and sleep. (See Supplemental Table 1)
Table 1.
Baseline characteristics.
| All Subjects (n=73) | Active Subjects (n=35) | Placebo Subjects (n=38) | |
|---|---|---|---|
|
| |||
| Age, mean (SD), y | 49.3 (2.8) | 48.7 (2.9) | 49.9 (2.6) |
| BMI, mean (SD) | 27.69 (6.7) | 28.17 (8.4) | 27.25 (4.5) |
| Baseline SHAPS, mean (SD)2 | 20.83 (5.78) | 19.62 (5.62) | 21.95 (5.77) |
| Baseline STAI, mean (SD)3 | 32.62 (9.03) | 30.89 (7.83) | 34.21 (9.84) |
| Baseline E2-SHAPS sensitivity strength, mean (SD) | 0.36 (0.26) | 0.41 (0.27) | 0.32 (0.24) |
| Baseline E2-STAI sensitivity strength, mean (SD) | 0.35 (0.25) | 0.36 (0.25) | 0.33 (0.26) |
| STRAW+10 Stage, n (%) | |||
| Early perimenopause (STRAW-2) | 23 (32) | 14 (40) | 9 (24) |
| Late perimenopause (STRAW-1) | 33 (45) | 12 (34) | 19 (50) |
| Within the first 12 months post of amenorrhea (STRAW +1a) | 2 (3) | 0 (0) | 2 (5) |
| Partial hysterectomy/ablation | 17 (23) | 9 (26) | 8 (21) |
| Race, No. (%) | |||
| African American/Black | 13 (18) | 8 (23) | 5 (13) |
| Caucasian/White | 57 (78) | 24 (68) | 33 (87) |
| Asian | 2 (3) | 2 (6) | 0 (0) |
| Multiracial | 1 (1) | 1 (3) | 0 (0) |
| Ethnicity, No. (%) | |||
| Hispanic/Latinx | 4 (5) | 1 (3) | 3 (8) |
| Not Hispanic/Latinx | 69 (95) | 34 (97) | 35 (92) |
| Education, No. (%) | |||
| High school diploma | 2 (3) | 2 (6) | 0 (0) |
| Some college/associate degree/trade school | 17 (23) | 7 (20) | 10 (26) |
| 4-year college degree | 30 (41) | 13 (37) | 17 (45) |
| Post-graduate degree | 24 (33) | 13 (37) | 11 (29) |
| Household Income, No. (%), USD | |||
| Below $39,999 | 3 (4) | 3 (9) | 0 (0) |
| $40,000-$79,999 | 23 (31) | 11 (31) | 12 (32) |
| $80,000-$99,999 | 10 (14) | 3 (9) | 7 (18) |
| $100,000-$159,999 | 22 (30) | 10 (28) | 12 (32) |
| Above $160,000 | 13 (18) | 6 (17) | 7 (18) |
| Income not reported | 2 (3) | 2 (6) | 0 (0) |
| Current depression No. (%) | 8 (11) | 4 (12) | 4 (11) |
| Past depression No. (%) | 18 (25) | 9 (26) | 9 (24) |
| Current anxiety disorder No. (%) | 16 (21) | 8 (22) | 8 (21) |
| Past anxiety disorder No. (%) | 6 (8) | 2 (6) | 4 (11) |
| Total # recent stressful life events, mean (SD) | 1.81 (1.53) | 1.69 (1.40) | 1.92 (1.68) |
Baseline E2 Fluctuation predicts Anxiety, Anhedonia, and Stress Reactivity
Anxiety and Anhedonia.
The magnitude of E2 fluctuations across the baseline phase predicted anhedonia symptoms (β=.019, SEM=.006, p=.002) such that greater fluctuations predicted greater symptom severity. The same direction of association was found for E2 fluctuations across the baseline phase predicting anxiety symptoms (β=.017, SEM=.01, p=.08), although this effect was not statistically significant. Moreover, E2 fluctuations interacted with the number of recent stressful life events to predict anhedonia assessed over 8 weeks, such that the relationship between E2 fluctuations and anhedonia symptom severity was greater at two or more stressful life events than at zero or one event (β= .09, SEM= .012, p<.001).
Stress Reactivity.
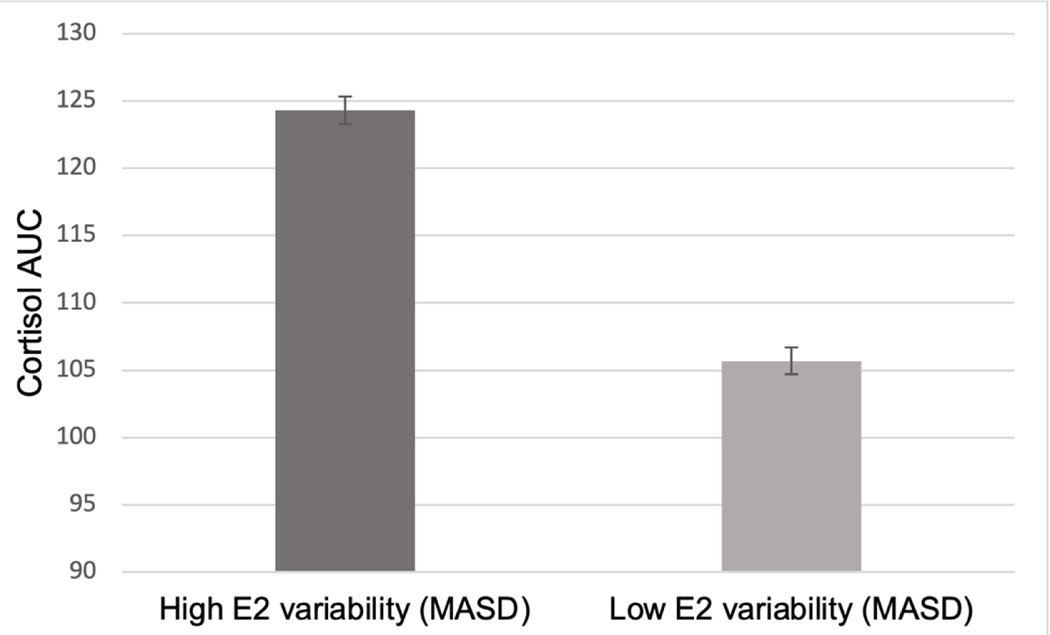
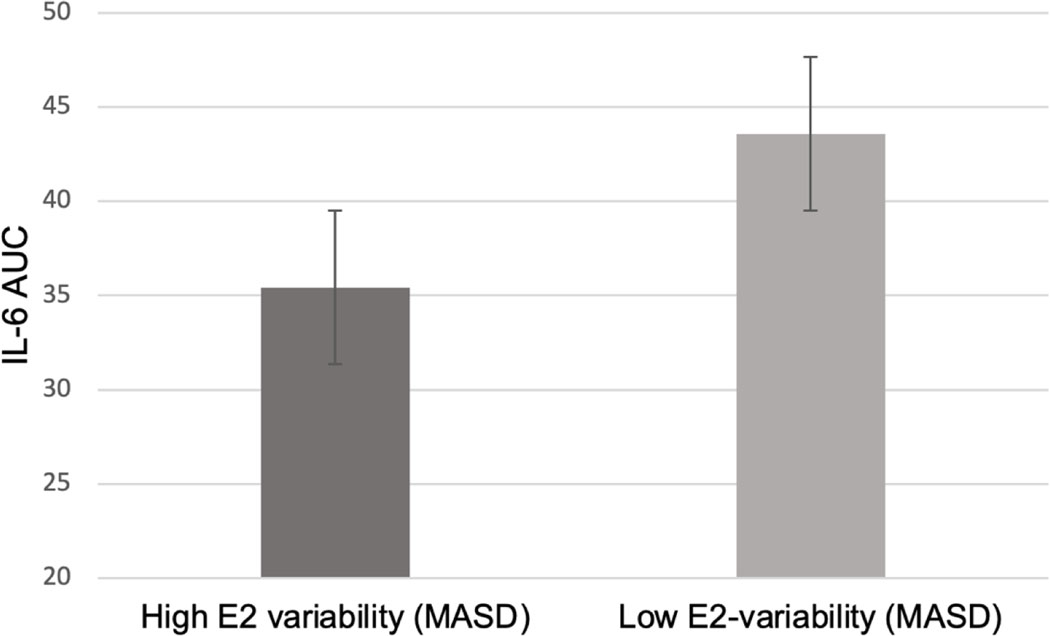
Baseline E2 fluctuations (MASD) predicted TSST cortisol AUC (β=0.17, SEM=0.067, p=.012). Specifically, greater E2 fluctuations over the baseline phase predicted a greater cortisol AUC (Figure 2). Additionally, baseline E2 fluctuations predicted TSST IL-6 AUC (β=−0.147, SEM=.06, p=.02), such that greater E2 fluctuations predicted a lower IL-6 AUC (Figure 3). E2 fluctuations did not predict resting state CRP (p>.05). Recent SLEs did not moderate any baseline stress reactivity relationship (all p>.05).
Figure 2.
Model-based estimates of the relationship between baseline E2 variability (mean absolute squared difference, MASD) and cortisol Area Under the curve (AUC) in response to the Trier Social Stress Test.
Figure 3.
Model-based estimates of the relationship between baseline E2 variability (mean absolute difference, MASD) and Interleukin 6 (IL-6) Area Under the curve (AUC) in response to the Trier Social Stress Test.
Main Effects of E2 Treatment
E2 Levels.
Mean E2 concentration (pg/ml) increased from baseline to post-randomization in those on active treatment (mean=72.39, SD=54.25 vs. mean=143.62, SD=70.12, t=7.92, p<.001), but not in those on placebo (mean=87.09, SD=64.95 vs. mean=74.15, SD=58.85, t=−1.66, p=.105; supplementary Figure 3).
Anxiety and Anhedonia.
Despite comparable baseline levels of anxiety and anhedonia, those randomized to active E2 had lower post-treatment anxiety (β= −2.61, SEM=.78, p<.001) and anhedonia (β=−2.38, SEM=.57, p<.001) symptoms than those randomized to placebo, after controlling for baseline anxiety and depression.
Stress Reactivity.
No effects of treatment were observed for CRP, cortisol or IL-6 stress reactivity (all ps>.05).
Interaction Effects Involving E2-sensitivity Strength and TE2 Treatment
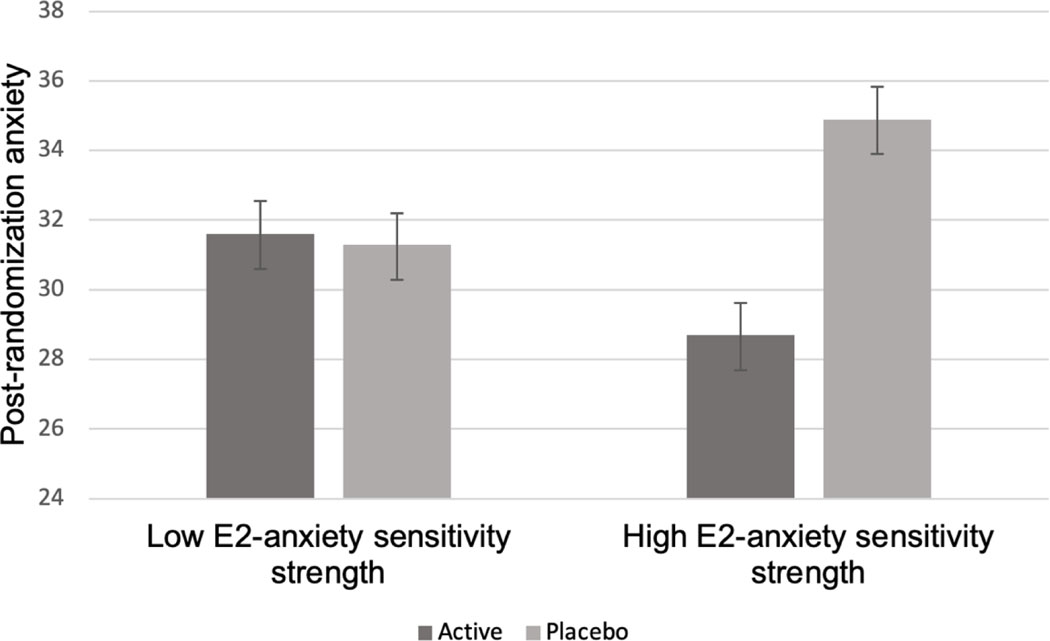
E2-Anxiety Sensitivity.
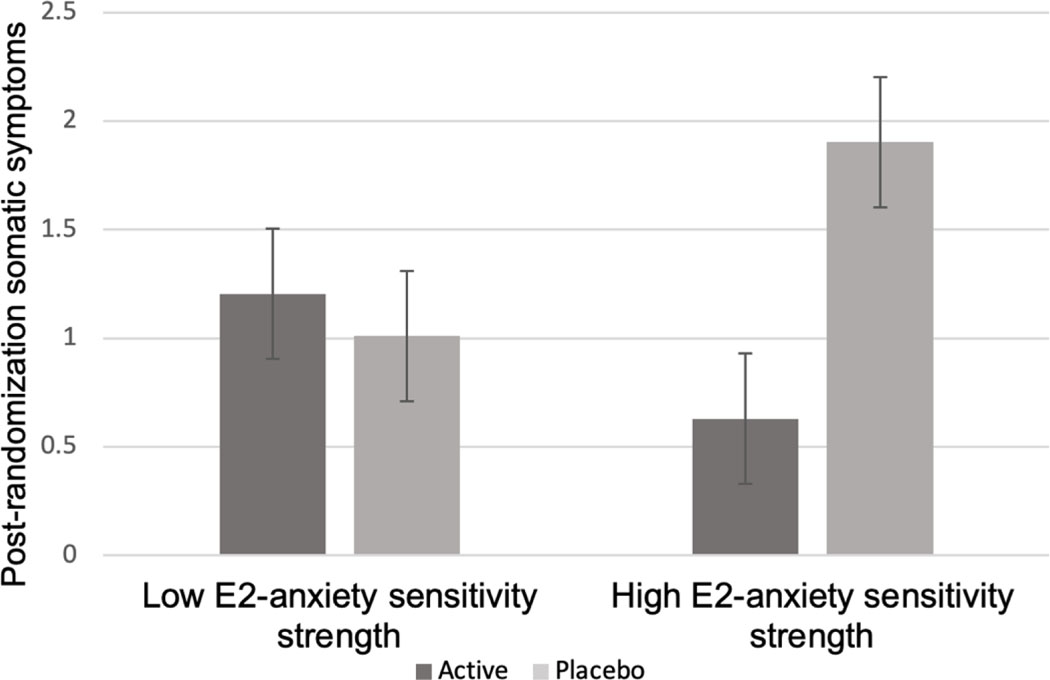
E2 treatment and baseline E2-anxiety sensitivity strength interacted to predict post-randomization anxiety (STAI: β=−18.50, SEM=3.56, p=.001; Greene: β=−3.94, SEM=1.91, p=.04). Specifically, a beneficial effect of TE2 was seen among women with high baseline E2-sensitivity, but not among women with low E2-sensitivity (Figure 4). Similarly, for somatic symptoms, post-hoc analyses showed a beneficial effect of TE2 treatment among women with high baseline E2-sensitivity but not among women with low E2-sensitivity (β=−2.76, SEM=1.44, p=.05; see Figure 5).
Figure 4.
Model-based estimates of the relationship between baseline E2-anxiety sensitivity strength and post-randomization anxiety scores (State-Trait-Anxiety Inventory) among each treatment group.
Figure 5.
Model-based estimates of the relationship between baseline E2-anxiety sensitivity strength and post-randomization somatic symptoms scores (Greene Climacteric Scale) among each treatment group.
Treatment did not interact with baseline E2-anxiety sensitivity to predict menopausal symptom bother (vasomotor symptoms, depressive symptoms, loss of sexual interest), or stress reactivity (all p>.05).
E2-Anhedonia Sensitivity.
Treatment did not interact with baseline E2-anhedonia sensitivity to predict either post-randomization anhedonia, somatic symptoms, menopausal symptom bother, or stress reactivity (all p>.05).1
Discussion
We found that perimenopausal women with greater week-to-week E2 fluctuations exhibited greater anhedonia symptom severity, particularly in those who were exposed to more recent stressful life events (SLEs). Older studies assessed E2 infrequently and found no indication for a relationship between E2 variability and mood (Avis, 2001; Bromberger, 2011; Woods, 2008). However, more recent studies, using more frequent E2 assessment with either yearly assessment over eight years (Freeman, 2006), four assessments over 13 months (Gordon 2016a), or weekly assessments over four weeks (Gordon 2016b) showed that greater variability in E2 over time (indexed by the E2 standard deviation around a woman’s mean E2 concentrations) predicted greater depression symptomatology. Our findings similarly indicate that greater weekly E2 fluctuations over 8 weeks predicts greater anhedonia symptoms.
We also documented, for the first time, that greater E2 fluctuations in perimenopausal women predicted greater cortisol reactivity along with blunted IL-6 reactivity to a laboratory psychosocial stressor. These results extend our prior research by showing that specific symptoms of anxiety and anhedonia, transdiagnostic characteristics of many psychopathologies, are sensitive to moderation by E2 fluctuation and SLEs. These findings suggest the possibility that stress system dysregulation might be one underlying pathway of risk linking E2 variability, exposure to SLEs, and perimenopausal affective symptomatology.
Estradiol influences auto-regulatory feedback mechanisms of the HPA-axis and, as an immunomodulator, regulates the production of pro-inflammatory cytokines (Ghisletti et al., 2005; Oyola & Handa, 2017). Though there is evidence for E2’s anti-inflammatory effects (Ghisletti et al., 2005), dynamic fluctuations of E2 in perimenopause may interfere with normal feedback regulatory control of both the HPA-axis and immune system stress reactivity. Exaggerated cortisol reactivity, which has been documented in depression (Burke et al., 2005; Stetler & Miller, 2011), was associated with greater E2 variability in the present study and provides a biologically plausible explanation for the blunted IL-6 reactivity (also associated with greater E2 variability). An inverse relationship between cortisol and IL-6 stress reactivity has been previously documented (Kunz-Ebrecht et al., 2003). Whether dysregulation in cortisol and/or pro-inflammatory cytokine reactivity to stress is involved in (partially mediates) the relationship between E2 variability and anxiety and anhedonia symptom severity in perimenopausal women awaits investigation in studies designed to test mediational models.
Our research is novel in its emphasis on individual differences in mood susceptibility to E2 fluctuations. Although studies have shown that women with a history of perimenopausal, postpartum, or premenstrual depression, are differentially sensitive to affective impairment associated with ovarian hormone changes (Bloch et al., 2000; Schmidt et al., 2015, 2017), there remains substantial variance within these broader diagnostic categories in terms of both affective phenotype and degree of sensitivity to hormone change. Consistent with other controlled research documenting the antidepressant effects of TE2 (De Novaes Soares et al., 2001; Gordon & Girdler, 2014b; Klein & Flanagan, 2016), we found that TE2 treatment was associated with lower post-treatment anxiety and anhedonia symptomatology, even after controlling for baseline symptoms and change in vasomotor symptom bother. However, for the first time, we demonstrate that individual differences in the degree of baseline anxiety symptom sensitivity to E2 fluctuations predicts TE2 beneficial treatment effects for anxiety. In contrast, a relationship between baseline anhedonia symptom sensitivity to E2 fluctuations was not predictive of TE2 treatment outcomes, the reason for which remains elusive.
Although the ability of baseline hormone sensitivity to predict TE2 treatment outcomes for anxiety could not be accounted for by any effect of TE2 on cortisol or IL-6 stress reactivity, this does not negate the possibility that dysregulation in HPA or inflammatory responses to stress play a role in the relationship between baseline E2 variability and affective perimenopausal symptoms (Gordon et al., 2015, 2016). Our finding that TE2 was effective at increasing E2 concentrations and in ameliorating anxiety and anhedonia symptoms, but was not effective in decreasing E2 variability (Supplemental results), suggests that TE2 beneficially modulates affective symptoms through other central and/or behavioral mechanisms that may be insensitive to E2 fluctuations. We observed that TE2 reductions in somatic symptom bother may contribute to the beneficial effects of TE2 on anxiety, particularly for women with high baseline E2-anxiety sensitivity. Symptoms such as dizziness, body aches, or tingling in extremities are associated with perimenopause, but also resemble somatic anxiety symptoms (Terauchi et al., 2013). This might explain our parallel findings that TE2, and higher baseline E2-anxiety sensitivity strength, predicted both lower anxiety symptoms and lower somatic symptom bother post-treatment. In contrast, those with higher baseline E2-anxiety sensitivity strength treated with placebo exhibited higher post-randomization anxiety symptoms and greater somatic symptom bother. However, these analyses were performed post-hoc and should be interpreted accordingly.
Our findings must be tempered in light of the study’s limitations. We did not use a global depression measure to assess changes in depressive symptom severity that could be compared to established clinical thresholds. Additionally, treatment groups differed non-significantly in their baseline STRAW stage, though when analyses testing treatment effects were repeated controlling for STRAW stage, results remained unchanged. This study did assess cortisol stress reactivity, but not cortisol daily rhythms. This limits the interpretation of the results to dysregulations under acute stress, while it remains unclear whether E2 fluctuations lead to broader HPA-axis and circadian dysregulation. The absence of measuring Sex Hormone Binding Globulin (SHBG) might be viewed as a limitation since it influences the bioavailability of E2, which is not reflected by measuring circulating blood concentrations exclusively. It is also possible that attitudes towards aging and menopause can influence a woman’s perception of mood and somatic symptoms (Ayers et al., 2010), and should be considered in future studies assessing mood and somatic symptoms in perimenopausal women. Finally, this study was originally designed to test whether stress-system dysregulation mediates the link between E2 variability and depressive symptom severity, though COVID-19-related early termination of the study prevented our ability to do so.
In conclusion, this is the first study to show that E2 fluctuations during the menopause transition predict dysregulation in cortisol and pro-inflammatory cytokine stress reactivity. This study confirms the beneficial effect of TE2 treatment, relative to placebo, for perimenopausal affective symptoms, particularly anxiety and anhedonia. This study also advances that evidence by showing that baseline individual differences in anxiety-sensitivity to E2 fluctuations predict the beneficial response of anxiety symptoms to TE2 treatment. Our results further suggest that these beneficial effects of TE2 for anxiety in hormone sensitive women may be related to the corresponding reductions in somatic symptom bother. Although these results await replication in a larger sample, this research provides the first evidence that clinical assessment of anxiety sensitivity to hormonal change, in combination with somatic symptom complaints, may guide precision medicine for the treatment or prevention of perimenopausal anxiety disorders.
Supplementary Material
Acknowledgements
Funding/Support
S.S.G. received funding from the National Institute of Mental Health (NIMH; R01 MH108690) and the North Carolina Translational and Clinical Science Institute (NC TraCS; UL1TR002489). S.F received an early postdoc mobility fellowship from the Swiss National Science Foundation (P2ZHP1_187611) and support from NC TraCS (2KR1282011) and the Foundation of Hope to analyze inflammatory markers.
Role of the Funders
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Funding
National Institute of Mental Health R01 MH108690.
Footnotes
Trial registration
clinicaltrials.gov Identifier: NCT03003949.
Due to slight, although non-significant differences in distribution of STRAW stages between treatment groups, all analyses on treatment effects were repeated controlling for STRAW stage, without resulting in any significant changes in results.
The coding was performed as suggested by Franken et al. (2007, 44), allowing an overall score ranging from 14–56. Coding according to the original publication (19, possible range of 1–14) resulted in a sample mean score of .37 (range: 0–6), with scores >2 indicating clinically significant anhedonia.
Scores >42 indicate clinically significant anxiety (Spielberger et al., 1970).
References
- Allen AP, Kennedy PJ, Cryan JF, Dinan TG, & Clarke G. (2014). Biological and psychological markers of stress in humans: Focus on the Trier Social Stress Test. Neuroscience and Biobehavioral Reviews, 38, 94–124. 10.1016/j.neubiorev.2013.11.005 [DOI] [PubMed] [Google Scholar]
- Anderson D, Yoshizawa T, Gollschewski S, Atogami F, & Courtney M. (2004). Relationship between menopausal symptoms and menopausal status in Australian and Japanese women: preliminary analysis. Nursing & health sciences, 6(3), 173–180. [DOI] [PubMed] [Google Scholar]
- Andersen E, Fiacco S, Gordon J, Kozik R, Baresich K, Rubinow D, Girdler S. (2022). Methods for characterizing ovarian and adrenal hormone variability and mood relationships in peripubertal females. Psychoneuroendocrinology, 141, 105747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avis NE, Stellato R, Crawford S, Bromberger J, Ganz P, Cain V, & Kagawa-Singer M. (2001). Is there a menopausal syndrome? Menopausal status and symptoms across racial/ethnic groups. Social science & medicine, 52(3), 345–356. [DOI] [PubMed] [Google Scholar]
- Ayers B, Forshaw M, & Hunter MS (2010). The impact of attitudes towards the menopause on women’s symptom experience: a systematic review. Maturitas, 65(1), 28–36. [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, & Rubinow DR (2000). Effects of gonadal steroids in women with a history of postpartum depression. American Journal of Psychiatry, 157(6), 924–930. 10.1176/appi.ajp.157.6.924 [DOI] [PubMed] [Google Scholar]
- Bromberger JT, Meyer PM, Kravitz HM, Sommer B, Cordal A, Powell L, Ganz PA & Sutton-Tyrrell K. (2001). Psychologic distress and natural menopause: a multiethnic community study. American journal of public health, 91(9), 1435–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberger JT, Harlow S, Avis N, Kravitz HM, & Cordal A. (2004). Racial/ethnic differences in the prevalence of depressive symptoms among middle-aged women: The Study of Women’s Health Across the Nation (SWAN). American Journal of Public Health, 94(8), 1378–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberger JT, Kravitz HM, Chang YF, Cyranowski JM, Brown C, & Matthews KA (2011). Major depression during and after the menopausal transition: Study of Women’s Health Across the Nation (SWAN). Psychological medicine, 41(9), 1879–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L, Edwards S, Mohamed-Ali V, & Steptoe A. (2004). Socioeconomic status and stress-induced increases in interleukin-6. Brain, Behavior, and Immunity, 18(3), 281–290. 10.1016/j.bbi.2003.09.011 [DOI] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, & Mohr DC (2005). Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology, 30(9), 846–856. 10.1016/j.psyneuen.2005.02.010 [DOI] [PubMed] [Google Scholar]
- De Novaes Soares C, Almeida OP, Joffe H, & Cohen LS (2001). Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: A double-blind, randomized, placebo-controlled trial. Archives of General Psychiatry. 10.1001/archpsyc.58.6.529 [DOI] [PubMed] [Google Scholar]
- Ebner-Priemer UW, Eid M, Kleindienst N, Stabenow S, & Trull TJ (2009). Analytic Strategies for Understanding Affective (In)Stability and Other Dynamic Processes in Psychopathology. Journal of Abnormal Psychology. 10.1037/a0014868 [DOI] [PubMed] [Google Scholar]
- Ebner-Priemer UW, Kuo J, Kleindienst N, Welch SS, Reisch T, Reinhard I, Lieb K, Linehan MM, & Bohus M. (2007). State affective instability in borderline personality disorder assessed by ambulatory monitoring. Psychological Medicine. 10.1017/S0033291706009706 [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, & Buchner A. (2007). GPOWER: A general power analysis program. Behavior Research Methods. [DOI] [PubMed] [Google Scholar]
- First MB, Williams JBW, Karg RS, & Spitzer RL (2015). Structured clinical interview for DSM-5 research version. American Psychiatric Association, Washington D.C. [Google Scholar]
- Freeman EW, Sammel MD, Lin H, & Nelson DB (2006). Associations of hormones and menopausal status with depressed mood in women with no history of depression. Archives of general psychiatry, 63(4), 375–382. [DOI] [PubMed] [Google Scholar]
- Franken IHA, Rassin E, & Muris P. (2007). The assessment of anhedonia in clinical and non-clinical populations: Further validation of the Snaith-Hamilton Pleasure Scale (SHAPS). Journal of Affective Disorders. 10.1016/j.jad.2006.08.020 [DOI] [PubMed] [Google Scholar]
- Ghisletti S, Meda C, Maggi A, & Vegeto E. (2005). 17β-Estradiol Inhibits Inflammatory Gene Expression by Controlling NF-κB Intracellular Localization. Molecular and Cellular Biology, 25(8), 2957–2968. 10.1128/mcb.25.8.2957-2968.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WK, Janson J, & Wolf JM (2017). Meta-analytical assessment of the effects of protocol variations on cortisol responses to the Trier Social Stress Test. Psychoneuroendocrinology, 80, 26–35. 10.1016/j.psyneuen.2017.02.030 [DOI] [PubMed] [Google Scholar]
- Gordon JL, & Girdler SS (2014). Hormone Replacement Therapy in the Treatment of Perimenopausal Depression. In Current Psychiatry Reports. 10.1007/s11920-014-0517-1 [DOI] [PubMed] [Google Scholar]
- Gordon JL, Girdler SS, Meltzer-Brody SE, Stika CS, Thurston RC, Clark CT, Prairie BA, Moses-Kolko E, Joffe H, & Wisner KL (2015). Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: A novel heuristic model. In American Journal of Psychiatry (Vol. 172, Issue 3, pp. 227–236). American Psychiatric Association. 10.1176/appi.ajp.2014.14070918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JL, Rubinow DR, Eisenlohr-Moul TA, Leserman J, & Girdler SS (2016a). Estradiol variability, stressful life events, and the emergence of depressive symptomatology during the menopausal transition. Menopause, 23(3), 257–266. 10.1097/GME.0000000000000528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JL, Eisenlohr-Moul TA, Rubinow DR, Schrubbe L, & Girdler SS (2016b). Naturally occurring changes in estradiol concentrations in the menopause transition predict morning cortisol and negative mood in perimenopausal depression. Clinical Psychological Science, 4(5), 919–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JL, Rubinow DR, Eisenlohr-Moul TA, Xia K, Schmidt PJ, & Girdler SS (2018). Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: A randomized clinical trial. JAMA Psychiatry, 75(2), 149–157. 10.1001/jamapsychiatry.2017.3998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JL, Sander B, Eisenlohr-Moul TA, & Sykes Tottenham L. (2020). Mood sensitivity to estradiol predicts depressive symptoms in the menopause transition. Psychological Medicine. 10.1017/S0033291720000483 [DOI] [PubMed] [Google Scholar]
- Greene JG (2008). Constructing a standard climacteric scale. Maturitas, 1–2(61), 78–84. 10.1016/S0378-5122(98)00025-5 [DOI] [PubMed] [Google Scholar]
- Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, & De Villiers TJ (2012). Executive summary of the Stages of Reproductive Aging Workshop +10: Addressing the unfinished agenda of staging reproductive aging. In Climacteric (Vol. 15, Issue 2, pp. 105–114). 10.3109/13697137.2011.650656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash SJ, Gillespie BL, Eisler RM, & Southard DR (1991). Sex differences in cardiovascular reactivity: Effects of the gender relevance of the stressor. Health Psychology, 10(6), 392. [DOI] [PubMed] [Google Scholar]
- Lavoie KL, Miller SB, Conway M, Fleet RP. (2001). Anger, negative emotions, and cardiovascular reactivity during interpersonal conflict in women. J Psychosom Res. 51(3):503–12. doi: 10.1016/s0022-3999(01)00217-3. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, & Hellhammer DH (1993). The “Trier social stress test” - A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 10.1159/000119004 [DOI] [PubMed] [Google Scholar]
- Klein SL, & Flanagan KL (2016). Sex differences in immune responses. In Nature Reviews Immunology. 10.1038/nri.2016.90 [DOI] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Mohamed-Ali V, Feldman PJ, Kirschbaum C, & Steptoe A. (2003). Cortisol responses to mild psychological stress are inversely associated with proinflammatory cytokines. Brain, Behavior, and Immunity, 17(5), 373–383. 10.1016/S0889-1591(03)00029-1 [DOI] [PubMed] [Google Scholar]
- Maki PM, Kornstein SG, Joffe H, Bromberger JT, Freeman EW, Athappilly G, Bobo WV, Rubin LH, Koleva HK, Cohen LS, & Soares CN (2019). Guidelines for the evaluation and treatment of perimenopausal depression: Summary and recommendations. Journal of Women’s Health. 10.1089/jwh.2018.27099.mensocrec [DOI] [PubMed] [Google Scholar]
- McInnis CM, Thoma MV, Gianferante D, Hanlin L, Chen X, Breines JG, Hong S, & Rohleder N. (2014). Measures of adiposity predict interleukin-6 responses to repeated psychosocial stress. Brain, Behavior, and Immunity, 42, 33–40. 10.1016/j.bbi.2014.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nave G, Nadler A, Dubois D, Zava D, Camerer C, & Plassmann H. (2018). Single-dose testosterone administration increases men’s preference for status goods. Nature Communications. 10.1038/s41467-018-04923-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor KA, Ferrell R, Brindle E, Trumble B, Shofer J, Holman DJ, & Weinstein M. (2009). Progesterone and ovulation across stages of the transition to menopause. Menopause, 16(6), 1178–1187. 10.1097/gme.0b013e3181aa192d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyola MG, & Handa RJ (2017). Hypothalamic–pituitary–adrenal and hypothalamic–pituitary–gonadal axes: sex differences in regulation of stress responsivity. Stress, 20(5), 476–494. 10.1080/10253890.2017.1369523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, & Hellhammer DH (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28(7), 916–931. 10.1016/S0306-4530(02)00108-7 [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. (2006). Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends in Immunology, 27(1), 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarason IG, Johnson JH, & Siegel JM (1978). Assessing the impact of life changes: Development of the Life Experiences Survey. Journal of Consulting and Clinical Psychology. 10.1037/0022-006X.46.5.932 [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Ben Dor R, Martinez PE, Guerrieri GM, Harsh VL, Thompson K, Koziol DE, Nieman LK, & Rubinow DR (2015). Effects of estradiol withdrawal on mood in women with past perimenopausal depression: A randomized clinical trial. JAMA Psychiatry, 72(7), 714–726. 10.1001/jamapsychiatry.2015.0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Martinez PE, Nieman LK, Koziol DE, Thompson KD, Schenkel L, Wakim PG, & Rubinow DR (2017). Premenstrual dysphoric disorder symptoms following ovarian suppression: Triggered by change in ovarian steroid levels but not continuous stable levels. American Journal of Psychiatry. 10.1176/appi.ajp.2017.16101113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman L, Danaceau MA, Tobin MB, Roca CA, Murphy JH, & Rubinow DR (2000). Estrogen replacement in perimenopause-related depression: A preliminary report. American Journal of Obstetrics and Gynecology, 183(2), 414–420. 10.1067/mob.2000.106004 [DOI] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR (2014). From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol Bull. 140(3):774–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavish DC, Graham-Engeland JE, Smyth JM, & Engeland CG (2015). Salivary markers of inflammation in response to acute stress. Brain, Behavior, and Immunity, 44, 253–269. 10.1016/j.bbi.2014.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, & Trigwell P. (1995). A scale for the assessment of hedonic tone. The Snaith-Hamilton Pleasure Scale. British Journal of Psychiatry. 10.1192/bjp.167.1.99 [DOI] [PubMed] [Google Scholar]
- Snijders TA, & Bosker RJ (2011). Multilevel analysis: An introduction to basic and advanced multilevel modeling (Sage; (ed.)). [Google Scholar]
- Spielberger CD, Gorsuch RL, & Lushene RE (1970). STAI manual for the state-trait anxiety inventory. Self-Evaluation Questionnaire. In Lushene Consulting Psychologists Press. [Google Scholar]
- Steptoe A, Willemsen G, Owen N, Flower L, & Mohamed-Ali V. (2001). Acute mental stress elicits delayed increases in circulating inflammatory cytokine levels. Clinical Science, 101(2), 185–192. 10.1042/CS20010038 [DOI] [PubMed] [Google Scholar]
- Steptoe Andrew, Hamer M, & Chida Y. (2007). The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain, Behavior, and Immunity, 21(7), 901–912. 10.1016/j.bbi.2007.03.011 [DOI] [PubMed] [Google Scholar]
- Stetler C, & Miller GE (2011). Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosomatic Medicine, 73(2), 114–126. 10.1097/PSY.0b013e31820ad12b [DOI] [PubMed] [Google Scholar]
- Terauchi M, Hiramitsu S, Akiyoshi M, Owa Y, Kato K, Obayashi S, Matsushima E, & Kubota T. (2013). Associations among depression, anxiety and somatic symptoms in peri-and postmenopausal women. Journal of Obstetrics and Gynaecology Research, 39(5), 1007–1013. 10.1111/j.1447-0756.2012.02064.x [DOI] [PubMed] [Google Scholar]
- Vamvakopoulos NC, & Chrousos GP (1993). Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression potential implications for the sexual dimophism of the stress response and immune/inflammatory reaction. Journal of Clinical Investigation. 10.1172/JCI116782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods NF, Smith-DiJulio K, Percival DB, Tao EY, Mariella A, & Mitchell ES (2008). Depressed mood during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Menopause, 15(2), 223–232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.