Abstract
Osteosarcopenia (OS) is a newly defined condition represented by the simultaneous presence of osteopenia/osteoporosis and sarcopenia, the main age-related diseases. The simultaneous coexistence of the two phenotypes derives from the close connection of the main target tissues involved in their pathogenesis: bone and muscle. These two actors constitute the bone–muscle unit, which communicates through a biochemical and mechanical crosstalk which involves multiple factors. Altered pattern of molecular pathways leads to an impairment of both the functionality of the tissue itself and the communication with the complementary tissue, composing the OS pathogenesis. Recent advances in the genetics field have provided the opportunity to delve deeper into the complex biological and molecular mechanisms underlying OS. Unfortunately, there are still many gaps in our understanding of these pathways, but it has proven essential to apply strategies such as exercise and nutritional intervention to counteract OS. New therapeutic strategies that simultaneously target bone and muscle tissue are limited, but recently new targets for the development of dual-action drug therapies have been identified. This narrative review aims to provide an overview of the latest scientific evidence associated with OS, a complex disorder that will pave the way for future research aimed at understanding the bone–muscle-associated pathogenetic mechanisms.
Keywords: bone–muscle crosstalk, genetics, osteoporosis, osteosarcopenia, sarcopenia
Introduction
Bone and muscle represent a functional unit in which the two tissues are intimately connected, both anatomically and mechanically.1 In fact, pathologies characterized by alterations in the physiology and function of muscle tissue also lead to consequences for the bone structure, and vice versa.2 The relationship between the functionality of the two tissues is explained by Frost’s mechanostat theory, according to which the mechanical load exerted by the muscle is what determines the resistance of the bone, exerting effects on the tissue remodelling process.3 In fact, the adaptive response of bone to mechanical stimuli results in an improvement of the microarchitecture of the bone with a consequent increase in the mass and strength of the tissue itself.4 Over the years, these observations have led to the formulation of the concept of ‘bone–muscle unit’, given also the common embryogenesis of the two tissues and the linear association existing between the body’s bone mineral content (BMC) and the amount of lean mass.5 Furthermore, recent studies in the literature have shown that the interaction between bone and muscle tissue is not only mechanical; in fact, communication between the two tissues can also occur through the secretion of biochemical factors, with paracrine and endocrine action.6 The existence of the close association between bone and muscle is further supported by the fact that with ageing, the inevitable decrease in bone mass is accompanied by the progressive loss of muscle mass and function, known collectively as sarcopenia, which can result in an increased risk of falls and fragility fractures.7 Indeed, the term osteosarcopenia (OS), which identifies the concomitant presence of sarcopenia and osteoporosis, has recently been introduced in the scientific community as further confirmation that low quality of muscle tissue is reflected in low quality of bone tissue.8 Epidemiological data on the incidence of OS are limited due to its recent coining. However, recent studies show that OS increases proportionally with ageing, physical inactivity, low body mass index (BMI), higher fat mass and comorbidities.9 A study of 680 elderly patients with a previous history of falls identifies an OS condition in 37% of the subjects that correlates with a higher frequency of comorbidities, impaired mobility and depression.10 To strengthen these data, Yoo and colleagues identify 28.7% of patients with OS in a cohort of 324 hip fracture patients. Interestingly, this condition reflects a mortality rate of 15.1% at 1-year follow-up, 1.8 times higher than in healthy, osteoporotic and sarcopenic-only subjects.11 As OS represents an emerging geriatric problem, the aim of this article is to provide an overview of the latest scientific evidence concerning this pathological condition. Bone–muscle interactions, potential factors involved in the progressive tissue changes observed during ageing and new potential therapeutic strategies to counteract the decline of the musculoskeletal system will be described.
Sarcopenia and bone health: the bone–muscle unit
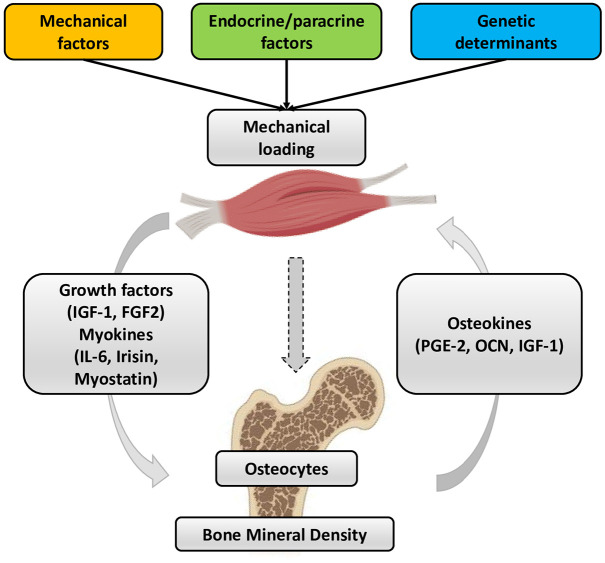
Several literature data agree on the existence of a close functional relationship between bone and muscle tissues, from embryogenesis through growth and development to ageing. This phenomenon is the basis of the biomechanical interaction theory, according to which bone provides attachment sites for muscle and skeletal muscle imparts a force on bone to facilitate body locomotion. Indeed, bones can adjust their mass and structure according to the changes in the mechanical load applied by the muscle.12,13 Consequently, a decline in muscle function causes a reduction in bone load, which results in bone loss. Unfortunately, the onset of sarcopenia cannot be completely explained by a reduction in bone mass, just as the development of osteoporosis cannot be totally related to muscle atrophy, although these musculoskeletal disorders often develop in parallel with a significant impact on the quality of life and functional status of many elderly patients.14 Interestingly, the bidirectional communication between bone and muscle tissue is known to be influenced by various factors, including mechanical factors, endocrine/paracrine factors and genetic determinants (Figure 1). Furthermore, the simultaneous dependence of bone and muscle tissue has been amply demonstrated by the effects of physical activity, disuse and age-related musculoskeletal pathologies, suggesting the understanding of the mechanisms underlying bone–muscle crosstalk as a key objective for identifying potential new therapies for osteoporosis and sarcopenia.15,16
Figure 1.
Crosstalk between bone and muscle tissue. There are numerous factors that enable communication between bone and muscle, including biomechanical factors, paracrine/endocrine factors and genetic determinants. The mechanical load exerted by muscle tissue is received by osteocytes, which are the main mechanoreceptors of bone tissue, exerting an effect on bone mineral density. The paracrine and endocrine factors released by muscle, however, which enable the biochemical interaction between the two tissues, include insulin-like growth factor 1 (IGF-1) and fibroblast growth factor 2 (FGF-2), while among the myokines are interleukin-6 (IL-6), irisin and myostatin. Osteokines released instead by bone tissue are prostaglandin E 2 (PGE-2), osteocalcin and IGF-1.
Mechanical crosstalk
Mechanical crosstalk between bones and muscles has been extensively documented to ensure the normal maintenance of an upright body position.17 Indeed, the skeleton is known to provide the muscles with rigid levers on which they apply forces of varying intensity, representing the primary source of mechanical load that generates structural deformations. This communication depends on a mechanotransduction process through which the mechanical forces of the entire body are transduced at organ level, at tissue level and, finally, at cellular level.18 In bone tissue, the main cell type responding to mechanical stimulation is osteocytes, the most abundant and long-lived cells in bone, and the main regulators of bone remodelling. Mechanotransduction in osteocytes is a complex regulatory process that occurs not only between cells and their environment but also between adjacent cells and between different functional mechanosensors in individual cells. In this regard, attention has been focused in recent years on the search for mechanosensors in osteocytes, identifying through in vitro and in vivo studies dendritic processes, the cytoskeleton, cilia, the extracellular matrix and connexin-based intercellular junctions among the main parties responsible for the transmission of extracellular mechanical signals in osteocytes.19 Integrins, which act as transmembrane receptors and facilitate the perception of near microenvironments and nanoenvironments, have also been suggested to contribute to mechanotransduction in osteocytes by promoting the activation of ion channels following various mechanical stimuli, including changes in tension and elongation and flow-related shear stress.20 The perception of mechanical stimulus by osteocytes activates a cascade of events that culminates in the regulation of mechano-sensitive genes. Among these, a key role is played by the SOST gene encoding for sclerostin, a protein that has recently emerged as an important therapeutic target for several diseases, including osteoporosis and osteopenia. The mechanisms by which mechanical signals regulate the SOST expression have not yet been elucidated. However, several trials have found increased serum levels of sclerostin in humans after immobilization and in animals subjected to limb unloading, while inhibition of the protein has been correlated with increased mechanical stimuli and increased bone mass.21,22 In addition, a correlation has been shown between mechanical stimuli and muscle function, suggesting an increased mechanical load among those responsible for muscle hypertrophy through the mechanistic target of rapamycin (mTOR), which would induce changes in muscle mass and an increase in protein synthesis.23 Noteworthy, research has also recently focused on the study of the biological effects of microgravity on bone–muscle crosstalk, highlighting how certain changes found in astronauts exposed to spaceflight, including bone loss and muscle atrophy, depend on the strong association between mechanical stress and musculoskeletal function.24 Particularly, prolonged exposure to random positioning machine (RPM) has been shown to have a strong impact on the bone mineralization process, as evidenced by the reduced presence of calcifying nodules, calcium deposits and pentraxin 3 (PTX3) expression in the SAOS-2 cell line.25 Furthermore, the expression pattern of myostatin has been proposed to play a key role in load-free muscle damage, as treatment with anti-myostatin antibodies was able to counteract the negative effect induced by RPM exposure in primary cultures of human satellite cells, restoring muscle morphology and function.26
Biochemical crosstalk
In recent decades, bone–muscle crosstalk has been shown to go beyond mechanics and occur through the secretion of biochemical factors. The first evidence dates to 2003 when Utvåg et al. found in a mouse open tibial fracture model a significant improvement in bone repair in the fracture area surrounded by muscle flaps. In contrast, a significant delay in fracture healing was observed when the muscle tissue was severely damaged.27 A few years later, Shen and colleagues detected a partial recovery of some defective muscle phenotypes in osteoblast/connexin43-deficient (Cx43) mice following subcutaneous injection of the bone-specific factor osteocalcin undercarboxylate (glu-OC).28 The idea that musculoskeletal communication is also biochemical in nature has been further confirmed by the discovery that bone and muscle perform an important endocrine function, producing signal molecules with hormonal function that influence bone and muscle metabolism both locally and systemically. However, while more than 600 factors, commonly known as myokines, have been identified for skeletal muscle, bone has only been recognized as an endocrine organ since 2007, making the identification of such molecules, called osteokines, still rather scarce.29 The myokines currently identified are cytokines synthesized and secreted by myocytes in response to muscle contraction, which regulate muscle metabolism and act on distant tissues and organs, with autocrine/paracrine action. The first myokine identified was myostatin, a protein belonging to the transforming growth factor-β (TGF-β) superfamily that acts as a negative regulator of muscle growth, inhibiting the proliferation and differentiation of satellite cells, as demonstrated by several in vivo studies in which myostatin-deficient mice showed massive muscle hypertrophy and a significant increase in bone mineral density (BMD). Conversely, myostatin acts as a positive regulator of osteoclast formation induced by receptor activator of nuclear factor κB receptor ligand (RANKL), reducing bone formation and increasing bone resorption.30,31 Among the myokines, interleukins also play a key role in bone–muscle crosstalk. For example, IL-6, which is released by muscles in response to exercise and contraction, has been suggested to have stimulatory effects on both bone formation and resorption. Interleukin-7 (IL-7), abundantly secreted by muscles, is widely considered to be an osteoclastogenic cytokine, as its overexpression has been correlated with reduced bone formation. Finally, interleukin-15 (IL-15) has been proposed to act directly on bone remodelling by stimulating pre-osteoclast differentiation.32,33 IGF-1 and FGF-2 are also growth factors involved in biochemical communication between bone and muscle. Particularly, muscle-derived IGF-1 has been suggested to intervene during bone repair, signalling osteoprogenitor cells in the periosteum expressing IGF-1 receptor (IGF-1R) to increase bone formation; while FGF-2 is known to be involved in fracture repair, bone formation and cartilage regeneration after injury or intense exercise.34 Finally, recently discovered is irisin, a myokine secreted in abundance by skeletal muscle in response to exercise, known not only to increase osteoblast differentiation and activity in vitro but also to act on bone remodelling by promoting the production of sclerostin and osteopontin.35 Conversely, several osteokines have been proposed to exert effects on muscle tissue, including osteocalcin (OCN), which is known to influence muscle contractility and mitochondrial biogenesis in the myofibers of young adult mice during exercise, as well as to reverse age-related muscle decline.36 The action of fibroblast growth factor 23 (FGF-23), a glycoprotein synthesized mainly by osteocytes, on skeletal muscle is still poorly understood. However, Li et al.37 recently demonstrated that treatment with recombinant FGF-23 in C57BL/6J mice exposed to different forms of exercise induced increased endurance and significantly reduced reactive oxygen species (ROS) levels in skeletal muscle, suggesting the existence of a correlation between increased muscle activity and FGF-23 production. Among the osteokines, a key role is played by sclerostin, which is secreted mainly by mature osteocytes and acts as a suppressor of bone formation via the canonical Wnt/β-catenin pathway. Recently, a negative correlation was found between serum sclerostin levels and skeletal muscle mass, independent of confounding factors such as age, gender, BMD, and total fat mass, suggesting a possible role of this osteokine as a marker of low muscle mass.38 Finally, a role of osteokine has also been attributed to prostaglandin E2 (PGE2), which is known to be related to multiple physiological processes such as inflammation, muscle regeneration and cancer development. Several evidences have suggested that PGE2 may also promote osteocyte survival and bone formation, as well as accelerate myoblast proliferation and differentiation in the C2C12 cell line, suggesting a role of this osteokine in muscle myogenesis.39
Bone–muscle connection in OS
There is now a growing realization that there are complex mechanical and biochemical interactions between bone and muscle tissue that, with ageing, show a decline in both structure and function.40 Indeed, there are an increasing number of studies in the literature reporting that subjects characterized by a reduction in BMD present a concomitant decrease in muscle mass.41 These two conditions in fact identify osteoporosis and sarcopenia, two pathologies of the geriatric age.8 Osteoporosis is a systemic bone disease that involves a reduction in BMD, resulting in altered bone tissue microarchitecture and an increased risk of fragility fractures, a condition that afflicts 30% of postmenopausal women above 50 years of age.42,43 Sarcopenia, however, represents a condition characterized by a reduction in muscle mass and function, resulting in impaired performance of daily activities and leading to an increased risk of falls. This condition afflicts 10–40% of postmenopausal women and, in general, results in increased disability, hospitalization rates and death.42–44 We often refer to osteoporosis and sarcopenia as a ‘hazardous duet’, two conditions whose coexistence has recently been identified under the term OS, in which the musculoskeletal system tends towards a decrease in both bone and muscle tissue quality, which is also further compromised by the concomitant increase in the amount of ectopic adipose tissue.42,45 There are numerous factors involved in the pathophysiology of OS: indeed, sarcopenia and osteoporosis share some risk factors and the same biological molecular pathways underlying the onset of the disease state.45 Genetic factors play a preponderant role in the determination of both diseases, and indeed it has been shown that muscle strength is partially genetically modulated and that genetic factors are critical in achieving peak bone mass.46 As already mentioned, there is not only a biochemical but also a biomechanical relationship between bone and muscle: according to Frost’s mechanostat theory, the forces exerted on bone by muscle tissue led to an increase in the strength of the bone tissue itself, as happens during childhood and young age. The decrease in the application of these forces that occurs in elderly individuals forced into a sedentary lifestyle leads to a decrease in the rate of apposition of new bone matrix by osteoblasts and consequently, an increase in bone fragility.47 This concept makes mechanical loading another key factor in maintaining the quality of bone and muscle, the alteration of which may be responsible for the onset of osteoporosis and sarcopenia. According to reports by Maghbooli and colleagues, basal metabolic rate in obese women above the age of 50 years appears to be correlated with both reduced muscle mass and decreased BMD at the hip and lumbar spine. With ageing, there is indeed an alteration in energy metabolism and body composition: indeed, the decrease in basal metabolic rate reflects the decreased metabolic activity of tissues and reduced energy consumption, making this a potential predictor of the onset of OS in postmenopausal age.48 This investigation confirms what has already been reported by some studies on the infiltration of adipose tissue that occurs with advancing age: a high amount of medullary adipose tissue is in fact associated with the loss of bone mass, while myosteatosis is correlated with a loss of myofiber function and subsequent decrease in muscle mass. According to recent evidence in the literature, adipose tissue undergoes an alteration in its function in ageing, producing a high amount of inflammatory peptides and resulting in an increased infiltrate of inflammatory cells. It therefore plays a predominant role in the establishment of the now well-known condition of inflammageing, a chronic low-grade inflammation involving continuous stimulation of the immune system.49 It has been reported how this condition has important effects on bone and muscle tissue, affecting the differentiation and activity of the cells that make up both tissues. According to the study by Kirkwood and colleagues, chronic activation of the immune system results in the expansion of myeloid-derived suppressor cells (MDSCs), a heterogeneous population of myeloid cells that have the ability to differentiate directly into osteoclasts, consequently increasing the rate of bone resorption and compromising the integrity of bone tissue.50 Adipose tissue is also characterized by altered lipid storage capacity, which accumulates ectopically in skeletal muscle. Lipid accumulation indicates a mitochondrial dysfunction of muscle cells, which leads to an altered β-oxidation process, resulting in increased levels of ROS produced. The resulting lipotoxic microenvironment causes the muscle to produce pro-inflammatory myokines, which through autocrine and paracrine mechanisms, induce muscle tissue dysfunction, and through endocrine pathways, act on the adipose tissue itself by exacerbating the inflammatory process.51 Taken together, these observations strongly suggest how adipose tissue plays a predominant role in the onset of OS, exerting an effect on several physiological processes that, directly or indirectly, are involved in maintaining the quality and homeostasis of bone and muscle tissue. Ageing is also characterized by a decrease in the concentration and activity of sex hormones, which exert numerous effects on bone and muscle. Indeed, hormones have been identified as key factors in the onset of OS, particularly growth hormone (GH) and IGF-1 play an important role. Thus, the GH/IGF-1 axis represents a pathway involved in the pathogenesis of the disease: it is activated as a result of GH release from the hypothalamus, which in turn results in the release by the liver of IGF-1, which acts on bone and muscle tissue by causing the release of numerous myokines (irisin, myostatin, IL-6, osteonectin) and osteokines [FGF-2, FGF-23, sclerostin, RANKL, osteoprotegerin (OPG)] affecting both tissues. The dysregulation of this axis and GH decreased secretion leads to a decrease in muscle and bone mass and to an increase in adipose tissue accumulation.52 Both muscle and bone cells express hormone receptors, making it clear how critical it is in postmenopausal women, where oestrogen decline occurs, to set up hormone replacement treatment to preserve bone and muscle mass and prevent future fragility fractures.52 In addition, it has been shown that the decrease in circulating levels of estradiol in postmenopausal women has important effects on the state of bone and muscle tissue, leading to their decline. In the male sex, hormones also appear to be involved in the pathogenesis of OS, as reduced levels of testosterone result in a decrease in protein synthesis and consequently in the reduction of muscle mass.53 Those described are some of the most important endogenous factors involved in the pathogenesis of osteoporosis and sarcopenia, but further studies will be needed to shed further light on the pathophysiology of OS, this being a newly defined syndrome whose molecular mechanisms still need to be clarified.
Genetics of bone and muscle interactions
Single-nucleotide polymorphisms analysis in target genes
Bone and muscle are both originated from the paraxial mesoderm during embryonic development and are influenced by similar genetic modulators (Table 1). Osteoporosis and sarcopenia share common risk factors with a heritability in the range of 60–70%. Genetic polymorphisms of several genes, such as androgen receptor (AR), oestrogen receptor (ER), IGF-I and vitamin D receptor (VDR), play a pivotal role in modulating bone or muscle metabolism, contributing to the pathogenesis of OS. Interestingly, literature data suggest an association between long alleles of the AR polymorphism (CAG)n and increased muscle mass and strength in athletes,54 but also with increased BMD in healthy adult humans.55 Oestrogens represent a second class of hormone modulators of bone and muscle homeostasis and polymorphisms in their receptors, ESR1 and ESR2, respectively, have been linked to altered mechanisms. It is now known that oestrogens regulate bone metabolism and their deficiency during menopause is an established cause of postmenopausal osteoporosis. The AIuI polymorphism of the ESR2 gene has been associated with spinal and femoral BMD, while RsaI only with spinal BMD in postmenopausal women.56 In muscle tissue, the C allele of ESR1 rs2234693 provides protection against muscle injury by reducing muscle stiffness.57 The contribution of genetic variability associated with IGF-I in bone metabolism has also been investigated. An interesting study identifies a significant association between the rs35767 polymorphism within IGF-I and reduced BMD with increased risk of osteoporosis in the postmenopausal female population, suggesting its role as a potential marker.58 These results are subsequently confirmed in a meta-analysis identifying an association between rs35767 polymorphism and risk of osteoporosis in Chinese postmenopausal women.59 The main function of IGF-I on skeletal muscle is to promote or inhibit protein synthesis or degradation, regulating skeletal muscle growth or recession. Previous data have demonstrated the polymorphisms of IGF-I and its binding protein-3 (IGFBP3) genes could impact serum IGF-I level, which correlate with muscle size. Yang and colleagues show that GG genotype of rs6214 in IGF-I and AC or CC genotypes of rs2854744 near IGFBP3 had a higher risk of having low appendicular skeletal muscle mass index (ASMI) compared with those with the AA and AA genotype in Taiwanese population, highlighting the pivotal role of these two single-nucleotide polymorphisms in muscle metabolism.60 Another extensively studied locus is VDR gene, in which several polymorphisms have been identified that appear to play a functional role in modulating expression levels. A recent meta-analysis from 42 studies suggests that VDR BsmI genotype is associated with an increased risk of postmenopausal osteoporosis in Caucasians.61 Genetic variability associated with this factor also plays pivotal roles in muscle metabolism, as seen for G allele of Cdx2 polymorphism and the combination of GG/CT genotypes of FokI in VDR gene that associated with a higher percentage of the atrophic type II fibres.62
Table 1.
Genetic factors with pleiotropic effects on bone and muscle involved in OS pathogenesis.
| Study design | Gene | Analysis outcome | Reference |
|---|---|---|---|
| SNPs analysis | AR | (CAG)n polymorphism is associated with greater muscle mass and strength | 54 |
| (CAG)n and (CGG)n polymorphisms are associated with increased bone mass | 55 | ||
| ESR2 | AIuI and RsaI polymorphisms are associated with spinal and femoral BMD | 56 | |
| ESR1 | rs2234693C allele polymorphism protects against muscle injury | 57 | |
| IGF-I | rs35767 polymorphism is associated with lower BMD and risk of osteoporosis | 58,59 | |
| rs6214 GG gentoype polymorphism is associated with low ASMI | 60 | ||
| VDR | BsmI genotype is associated with increased risk of postmenopausal osteoporosis in Caucasians | 61 | |
| G allele of Cdx2 polymorphism and the combination of GG/CT genotypes of FokI results associated with higher atrophic type II fibres | 62 | ||
| GWAS | METTL21C | METTL21C is a pleiotropic gene for osteoporosis and sarcopenia acting through the modulation of the NF-κB signalling pathway | 63 |
| RNA-sequencing profile | RUNX1, NGFR, CH3L1, BCL3, PLA2G2A, MYBPH, TEP1, SEMA6B, CSPG4, ACSL5, SLC25A3, NDUFB5, CYC1, ACAT1 and TCAP | Identification of differentially expressed genes in osteoporotic patients with sarcopenia | 64 |
Genome-wide association studies
Despite these interesting and novel data, no single gene or SNPs have been associated with the loss of bone mass, muscle strength or mass. The latest investigations are encompassing large populations of genes analysed simultaneously based on genomic studies such as genome-wide association studies (GWAS). GWAS have successfully identified multiple loci overlapping between the bone and muscle parameters and associated with tissues wasting. Several genes, such as myostatin, growth and differentiation factor-8 (GDF-8), myocyte enhancer factor-2C (MEF-2C), proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), and methyltransferase-like 21C (METTL21C), have been detected as being linked to muscle loss and osteoporosis concurrently.63,65 METTL21C represents a novel gene with pleiotropic effects on bone and muscle mass. Analysis performed in two murine cell lines, a C2C12 myogenic and an MLO-Y4 osteocyte-like one, shows a reduced expression level of Mettl21c followed by a siRNA modulation. An array of genes involved in different signalling pathways was analysed to understand the mechanism induced by silencing Mettl21c. Among them, NFκB was found to be modulated, suggesting an important role of this METTL21c-interacting factor in muscle and bone homeostasis.63 The pleiotropic factors influencing bone and muscle tissue are not yet fully known to refine the diagnosis and monitoring of OS. The advantages of combining related phenotypes make pleiotropic GWAS studies an excellent investigative tool. However, although our knowledge on the identification and genetic-molecular interaction of factors targeting bone and muscle has increased enormously in recent years, the genetic landscape of OS has yet to be unravelled.
Gene expression profiling
Emerging data demonstrate the importance of identifying differentially expressed genes (DEGs) for osteoporosis and sarcopenia. An analysis of four gene expression datasets GSE56814 and GSE56815 for osteoporosis and GSE1428 and GSE8479 for sarcopenia led to the identification of 133 co-expressed DEGs. The tissues in the osteoporosis datasets are blood monocytes, while the tissues in the sarcopenia datasets are the vastus lateralis muscle.66 In parallel, an interesting RNA-sequencing study in elderly osteoporotic hip fracture sarcopenic and non-sarcopenic subjects was performed. A total of 11 samples from both groups were sequenced, and 15 DEGs were identified (RUNX1, NGFR, CH3L1, BCL3, PLA2G2A, MYBPH, TEP1, SEMA6B, CSPG4, ACSL5, SLC25A3, NDUFB5, CYC1, ACAT1 and TCAP). Specifically, the expression levels of the SLC25A3 and TCAP genes in the OS group were significantly lower than in the non-OS groups, whereas an increased mRNA level of RUNX1 was observed in the OS samples.64 However, an integrated analysis will contribute to the understanding of the full molecular changes in OS and the development of new targeted therapies.
How to counteract OS?
OS is a growing global health problem due to the high morbidity and mortality rates. Its complex and multifactorial nature requires targeted treatment and prevention strategies. To date, a healthy lifestyle and regular exercise are the first-line choices for the prevention and treatment of OS.8 Particularly, consistent and well-designed training is known to prevent and delay the onset of several age-related diseases, including OS, given its beneficial effects on the health of the musculoskeletal system and on the exchange of mechanical and biochemical signals that is essential for adequate tissue communication. Several evidences agree that dynamic resistance exercise (DRT) supported by adequate dietary supplementation could be the most promising strategy to improve all physiological and functional outcomes related to OS, including metabolism, cardiovascular endpoints and brain function.67 In this regard, Lichtenberg et al. recently evaluated the effects of 28 weeks of high-intensity resistance training (HI-RT) in 43 men with OS aged ⩾72 years. A significant effect on the sarcopenia Z-score was observed in the intervention group, in association with a significant increase in the skeletal muscle index (SMI), suggesting HI-RT as a time- and cost-efficient training modality to reduce the progression and burden of sarcopenia.68 In agreement, Kemmler and colleagues proposed high-intensity dynamic resistance training (HIT-DRT), combined with the administration of milk protein, calcium and vitamin D, as a safe and effective strategy to counteract OS in older men. Indeed, evaluation of the HIT-DRT effects showed significant improvements on BMD of the lumbar spine and proximal femur in men with OS, as well as on lean body mass and muscle strength.69 Multimodal exercise, combining resistance, load impact or balance training, has also been suggested as an effective therapy to improve the aspects of OS, such as BMD, muscle mass and strength, and physical performance.9,70 In this regard, Lopez et al. systematically examined the effects of resistance training, alone or combined, on neuromuscular function, muscle morphology and functional outcomes in physically frail elderly subjects. Interestingly, the authors found that multimodal training was associated with increases in 6.6–37% in maximal strength, 3.4–7.5% in muscle mass, 8.2% in muscle power and 4.7–58.1% in functional capacity and fall risk, suggesting this type of training as an effective strategy to prevent loss of functional capacity, dependency and incidence of falls in the frail individual.71 In agreement, Daly and colleagues evaluated the effects of a 12-month multimodal exercise programme on 148 adults aged ⩾60 years with osteopenia or increased risk of falls, finding significant improvements on BMD of the lumbar spine and femoral neck, muscle strength and physical function even 6 months after the end of the training programme.72 In addition to physical activity, diet is undoubtedly an important modulator of musculoskeletal integrity, with a significant impact on the OS development. In fact, an adequate intake of protein, calcium and vitamin D has been suggested to have a dual effect on bones and muscles, contributing significantly to reducing the risk of falls and fractures in old age.73 Specifically, vitamin D and calcium supplementation is known to improve BMD and muscle strength, and to reduce falls and fractures in adults deficient in these nutrients.74 Furthermore, several observational studies have found a correlation between higher protein intake and higher retention of lean muscle mass and BMD in the elderly.75,76 Creatine supplementation has also been observed to increase the benefits of endurance exercise in healthy individuals, although the effect in clinical populations with compromised muscle and bone health has yet to be determined.77 In this context, Atlihan et al.78 have recently evaluated the effectiveness of exercise or nutritional interventions with protein, calcium, vitamin D and creatine on musculoskeletal health in elderly patients with OS, finding significant and longer-lasting improvements in muscle mass, strength and quality. Overall, most evidence agrees on the elimination of amendable risk factors, such as sedentariness and malnutrition, as the first line of defence to combat OS and achieve long-term benefits on musculoskeletal health in old age.
New advances in therapeutic strategies
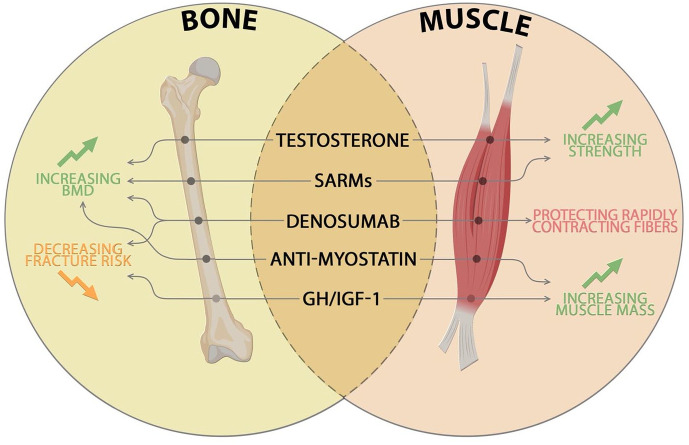
Currently, many drugs have been authorized to reduce or prevent osteoporosis, but no drug treatment has yet been approved to counteract OS. Some of these pharmacological agents also appear to be effective in treating sarcopenia and consequently OS (Figure 2). Denosumab (Dmab), an antibody directed against RANKL, acts by preventing the interaction with its receptor and leads to a decrease in osteoclastic activity. Its positive role in preserving bone health makes Dmab an excellent candidate in decreasing the risk of osteoporotic fractures.79 Several studies have hypothesized that Dmab could improve muscle mass and strength through the RANK/RANKL/OPG pathway. Specifically, RANK is a receptor expressed on the membranes of fast- and slow-twitch myofibers and interaction with its ligand RANKL inhibits myogenic differentiation leading to tissue degeneration.80,81 A prospective longitudinal multicenter controlled study confirmed Dmab as a drug potential for OS given its role in counteracting bone resorption and protecting rapidly contracting fibres. Study results showed how Dmab improved BMD, reduced the risk of falls and had a positive influence on physical performance and muscle strength, but further studies are needed to confirm its effects on muscle mass and function.80 Other important regulators in maintenance musculoskeletal system are represented by androgens, including testosterone, and their decrease in ageing compromises the quality of both target tissues. Given the promising potential of testosterone, it could represent a candidate to counteract OS development since it contributes to increased mineralization of bone tissue, along with strength and mass of muscle tissue.82 Recent data also show the potential of testosterone in preventing bone mass loss and sarcopenia in subjects affected by hypogonadism, reporting an improvement in BMD and muscle strength.83,84 Furthermore, testosterone can bind the receptor belonging to the steroid hormone receptor family, performing physiological and pathological functions in different tissues. The ubiquitous AR responds to androgenic therapies in a non-specific manner, highlighting the necessity to develop selective androgen modulators (SARMs) that promote only beneficial effects in target tissues.85 Dalton et al. in their 12-week double-blind placebo-controlled phase II clinical trial evaluated the safety and efficacy of the SARM Enobosarm, in both elderly men and postmenopausal women. The selective anabolic effects on muscle and bone led to a significant improvement in patients’ physical function and lean body mass.86 Given the potential of SARMs, these factors could represent an aspiring future treatment for OS.53 Myostatin is another targeting agent that negatively influences both bone and muscle. The protein belongs to the TGF-β family and is produced by skeletal muscle; interacting with its receptor activin IIB (ActRIIB) leads to a decrease in myoblast proliferation and an increase in muscle degradation.26 The ActRIIB receptor is also found with osteoblasts and chondroblasts and its activation appears to be involved in bone remodelling. Its role has led researchers in inhibiting myostatin pathways as drug therapy for OS.87,88 These data highlight the dual role of myostatin in musculoskeletal system and how inhibition of this axis could lead to effective therapy for associated diseases. Other molecular pathways that control bone–muscle crosstalk, such as GH and IGF-I are targeted, as they are crucial for skeletal and muscle development and health. Although studies show a close correlation between decreased GH/IGF-1 and increased risk of fractures89 and sarcopenia in the elderly,90 there are still many side effects, including fluid retention, gynaecomastia and orthostatic hypotension, reported after treatment with recombinant human growth hormone (rhGH) and recombinant human insulin-like growth factor I (rhIGF-I).91 However, no drug therapies have yet been approved and more extensive studies are needed to apply these data in the management of OS disease. The aetiology of OS is multifactorial, with complex and overlapping molecular pathways: deep investigations of the bone–muscle crosstalk will help to develop pharmacological therapies to counter and prevent the burden of this musculoskeletal disease.
Figure 2.
New advances in potential therapeutic strategies for OS. Schematic representation of pharmacological agents acting on bone and muscle tissues, which lead to increased BMD, decreased fracture risk, increased strength and muscle mass and protection of rapid contractile fibres, respectively.
Conclusion
OS is a complex recently defined syndrome in which osteoporosis and sarcopenia coexist. Given the progressive increase in the average age of the population, OS represents a public health problem of considerable importance, given the associated socioeconomic burdens. Literature data suggest that underlying the onset of this pathology, there is not only an alteration in the molecular mechanisms between bone and muscle but also a strong genetic component associated with inter- and intra-individual variability. Furthermore, the role played by risk factors in contributing to the pathogenesis of this musculoskeletal disorder is crucial. However, there are many aspects still to be clarified associated with both the pathophysiological mechanisms involved and the criteria used to diagnose this disorder. To date, there is still no standardized protocol that allows for a quick and accurate diagnosis of this pathology and that includes the simultaneous clinical evaluation of bone and muscle tissue. Further efforts must be made in this direction; as according to some literature data, there is a significant association between the qualitative assessment parameters of BMD (t-score) and the quality and functionality of muscle tissue (handgrip strength test).92 Together with clinical-instrumental investigations, it is desirable to correlate these assessments with haematochemical parameters reflecting markers of bone and muscle metabolism. Therefore, the integration of clinical evaluation of sarcopenia and osteoporosis is essential to intercept subjects at the risk of developing OS at an early stage. Given the implications that this disease has on the quality of life of those affected, it is necessary to develop prevention strategies aimed at lifestyle improvements that can be reflected in musculoskeletal health.
Acknowledgments
The authors thank the Italian Study Group in Orthopaedics of Severe Osteoporosis (GISOOS) for supporting this work.
Footnotes
ORCID iDs: Virginia Veronica Visconti  https://orcid.org/0000-0002-4543-7770
https://orcid.org/0000-0002-4543-7770
Ida Cariati  https://orcid.org/0000-0002-2102-5034
https://orcid.org/0000-0002-2102-5034
Contributor Information
Umberto Tarantino, Department of Clinical Sciences and Translational Medicine, University of Rome ‘Tor Vergata’, Rome, Italy; Department of Orthopedics and Traumatology, PTV Foundation, Rome, Italy.
Chiara Greggi, Department of Clinical Sciences and Translational Medicine, University of Rome ‘Tor Vergata’, Rome, Italy.
Virginia Veronica Visconti, Department of Clinical Sciences and Translational Medicine, University of Rome ‘Tor Vergata’, Via Montpellier 1, 00133 Rome, Italy.
Ida Cariati, Department of Biomedicine and Prevention, University of Rome ‘Tor Vergata’, Rome, Italy.
Roberto Bonanni, Department of Biomedicine and Prevention, University of Rome ‘Tor Vergata’, Rome, Italy.
Beatrice Gasperini, Department of Biomedicine and Prevention, University of Rome ‘Tor Vergata’, Rome, Italy.
Italo Nardone, Department of Orthopedics and Traumatology, PTV Foundation, Rome, Italy.
Elena Gasbarra, Department of Orthopedics and Traumatology, PTV Foundation, Rome, Italy.
Riccardo Iundusi, Department of Orthopedics and Traumatology, PTV Foundation, Rome, Italy.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Umberto Tarantino: Conceptualization; Investigation; Methodology; Resources; Supervision; Writing – original draft.
Chiara Greggi: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft.
Virginia Veronica Visconti: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft.
Ida Cariati: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft.
Roberto Bonanni: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft.
Beatrice Gasperini: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft.
Italo Nardone: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft.
Elena Gasbarra: Conceptualization; Data curation; Supervision; Visualization.
Riccardo Iundusi: Data curation; Visualization; Writing – original draft.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: Not applicable.
References
- 1. Buckingham M, Bajard L, Chang T, et al. The formation of skeletal muscle: from somite to limb. J Anat 2003; 202: 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Battafarano G, Rossi M, Marampon F, et al. Bone control of muscle function. Int J Mol Sci 2020; 21: 1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tagliaferri C, Wittrant Y, Davicco MJ, et al. Muscle and bone, two interconnected tissues. Ageing Res Rev 2015; 21: 55–70. [DOI] [PubMed] [Google Scholar]
- 4. Žofková I. Hormonal aspects of the muscle-bone unit. Physiol Res 2008; 57(Suppl. 1): S159–S169. [DOI] [PubMed] [Google Scholar]
- 5. Ferretti JL, Capozza RF, Cointry GR, et al. Gender-related differences in the relationship between densitometric values of whole-body bone mineral content and lean body mass in humans between 2 and 87 years of age. Bone 1998; 22: 683–690. [DOI] [PubMed] [Google Scholar]
- 6. Li G, Zhang L, Wang D, et al. Muscle-bone crosstalk and potential therapies for sarco-osteoporosis. J Cell Biochem 2019; 120: 14262–14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tagliafico AS, Bignotti B, Torri L, et al. Sarcopenia: how to measure, when and why. Radiol Med 2022; 127: 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Inoue T, Maeda K, Satake S, et al. Osteosarcopenia, the co-existence of osteoporosis and sarcopenia, is associated with social frailty in older adults. Aging Clin Exp Res 2022; 34: 535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kirk B, Zanker J, Duque G. Osteosarcopenia: epidemiology, diagnosis, and treatment-facts and numbers. J Cachexia Sarcopenia Muscle 2020; 11: 609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huo YR, Suriyaarachchi P, Gomez F, et al. Phenotype of osteosarcopenia in older individuals with a history of falling. J Am Med Dir Assoc 2015; 16: 290–295. [DOI] [PubMed] [Google Scholar]
- 11. Yoo J, Il Kim H, Ha YC, et al. Osteosarcopenia in patients with hip fracture is related with high mortality. J Korean Med Sci 2018; 33: e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berger JM, Singh P, Khrimian L, et al. Mediation of the acute stress response by the skeleton. Cell Metab 2019; 30: 890e8–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He C, He W, Hou J, et al. Bone and muscle crosstalk in aging. Front Cell Dev Biol 2020; 8: 585644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonanni R, Cariati I, Tancredi V, et al. Chronic pain in musculoskeletal diseases: do you know your enemy? J Clin Med 2022; 11: 2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reginster J-Y, Beaudart C, Buckinx F, et al. Osteoporosis and sarcopenia: two diseases or one? Curr Opin Clin Nutr Metab Care 2016; 19: 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tarantino U, Cariati I, Marini M, et al. Effects of simulated microgravity on muscle stem cells activity. Cell Physiol Biochem 2020; 54: 736–747. [DOI] [PubMed] [Google Scholar]
- 17. Cariati I, Bonanni R, Onorato F, et al. Role of physical activity in bone-muscle crosstalk: biological aspects and clinical implications. J Funct Morphol Kinesiol 2021; 6: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brotto M, Bonewald L. Bone and muscle: interactions beyond mechanical. Bone 2015; 80: 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qin L, Liu W, Cao H, et al. Molecular mechanosensors in osteocytes. Bone Res 2020; 8: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yavropoulou MP, Yovos JG. The molecular basis of bone mechanotransduction. J Musculoskelet Neuronal Interact 2016; 16: 221–236. [PMC free article] [PubMed] [Google Scholar]
- 21. Sapir-Koren R, Livshits G. Osteocyte control of bone remodeling: is sclerostin a key molecular coordinator of the balanced bone resorption-formation cycles. Osteoporos Int 2014; 25: 2685–2700. [DOI] [PubMed] [Google Scholar]
- 22. Uda Y, Azab E, Sun N, et al. Osteocyte mechanobiology. Curr Osteoporos Rep 2017; 15: 318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goodman CA, Hornberger TA, Robling AG. Bone and skeletal muscle: key players in mechanotransduction and potential overlapping mechanisms. Bone 2015; 80: 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bettis T, Kim BJ, Hamrick MW. Impact of muscle atrophy on bone metabolism and bone strength: implications for muscle-bone crosstalk with aging and disuse. Osteoporos Int 2018; 29: 1713–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cariati I, Bonanni R, Scimeca M, et al. Exposure to random positioning machine alters the mineralization process and PTX3 expression in the SAOS-2 cell line. Life (Basel, Switzerland) 2022; 12: 610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cariati I, Scimeca M, Bonanni R, et al. Role of myostatin in muscle degeneration by random positioning machine exposure: an in vitro study for the treatment of sarcopenia. Front Physiol 2022; 13: 782000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Utvåg SE, Grundnes O, Rindal DB, et al. Influence of extensive muscle injury on fracture healing in rat tibia. J Orthop Trauma 2003; 17: 430–435. [DOI] [PubMed] [Google Scholar]
- 28. Shen H, Grimston S, Civitelli R, et al. Deletion of connexin43 in osteoblasts/osteocytes leads to impaired muscle formation in mice. J Bone Miner Res 2015; 30: 596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mera P, Ferron M, Mosialou I. Regulation of energy metabolism by bone-derived hormones. Cold Spring Harb Perspect Med 2018; 8: a031666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Elkasrawy MN, Hamrick MW. Myostatin (GDF-8) as a key factor linking muscle mass and bone structure. J Musculoskelet Neuronal Interact 2010; 10: 56–63. [PMC free article] [PubMed] [Google Scholar]
- 31. Dankbar B, Fennen M, Brunert D, et al. Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice. Nat Med 2015; 21: 1085–1090. [DOI] [PubMed] [Google Scholar]
- 32. Chowdhury S, Schulz L, Palmisano B, et al. Muscle-derived interleukin 6 increases exercise capacity by signaling in osteoblasts. J Clin Invest 2020; 130: 2888–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Djaafar S, Pierroz DD, Chicheportiche R, et al. Inhibition of T cell-dependent and RANKL-dependent osteoclastogenic processes associated with high levels of bone mass in interleukin-15 receptor-deficient mice. Arthritis Rheum 2010; 62: 3300–3310. [DOI] [PubMed] [Google Scholar]
- 34. Hamrick MW. The skeletal muscle secretome: an emerging player in muscle-bone crosstalk. Bonekey Rep 2012; 1: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Colaianni G, Cuscito C, Mongelli T, et al. Irisin enhances osteoblast differentiation in vitro. Int J Endocrinol 2014; 2014: 902186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mera P, Laue K, Wei J, et al. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol Metab 2016; 5: 1042–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li DJ, Fu H, Zhao T, et al. Exercise-stimulated FGF23 promotes exercise performance via controlling the excess reactive oxygen species production and enhancing mitochondrial function in skeletal muscle. Metabolism 2016; 65: 747–756. [DOI] [PubMed] [Google Scholar]
- 38. Kim JA, Roh E, Hong SH, et al. Association of serum sclerostin levels with low skeletalmuscle mass: The Korean Sarcopenic Obesity Study (KSOS). Bone 2019; 128: 115053. [DOI] [PubMed] [Google Scholar]
- 39. Mo C, Zhao R, Vallejo J, et al. Prostaglandin E2 promotes proliferation of skeletal muscle myoblasts via EP4 receptor activation. Cell Cycle 2015; 14: 1507–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Locquet M, Beaudart C, Durieux N, et al. Relationship between the changes over time of bone mass and muscle health in children and adults: a systematic review and meta-analysis. BMC Musculoskelet Disord 2019; 20: 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Laskou F, Patel HP, Cooper C, et al. A pas de deux of osteoporosis and sarcopenia: osteosarcopenia. Climacteric 2022; 25: 88–95. [DOI] [PubMed] [Google Scholar]
- 42. Hirschfeld HP, Kinsella R, Duque G. Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos Int 2017; 28: 2781–2790. [DOI] [PubMed] [Google Scholar]
- 43. Sjöblom S, Suuronen J, Rikkonen T, et al. Relationship between postmenopausal osteoporosis and the components of clinical sarcopenia. Maturitas 2013; 75: 175–180. [DOI] [PubMed] [Google Scholar]
- 44. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age and Ageing 2019; 48: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hamad B, Basaran S, Coskun Benlidayi I. Osteosarcopenia among postmenopausal women and handgrip strength as a practical method for predicting the risk. Aging Clin Exp Res 2020; 32: 1923–1930. [DOI] [PubMed] [Google Scholar]
- 46. Sambrook PN, Kelly PJ, Morrison NA, et al. Genetics of osteoporosis. Br J Rheumatol 1994; 33: 1007–1011. [DOI] [PubMed] [Google Scholar]
- 47. Frost HM. On our age-related bone loss: insights from a new paradigm. J Bone Miner Res 1997; 12: 1539–1546. [DOI] [PubMed] [Google Scholar]
- 48. Maghbooli Z, Mozaffari S, Dehghani Y, et al. The lower basal metabolic rate is associated with increased risk of osteosarcopenia in postmenopausal women. BMC Womens Health 2022; 22: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zamboni M, Nori N, Brunelli A, et al. How does adipose tissue contribute to inflammageing? Exp Gerontol 2021; 143: 111162. [DOI] [PubMed] [Google Scholar]
- 50. Kirkwood KL, Zhang L, Thiyagarajan R, et al. Myeloid-derived suppressor cells at the intersection of inflammaging and bone fragility. Immunol Invest 2018; 47: 844–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kalinkovich A, Livshits G. Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev 2017; 35: 200–221. [DOI] [PubMed] [Google Scholar]
- 52. Polito A, Barnaba L, Ciarapica D, et al. Osteosarcopenia: a narrative review on clinical studies. Int J Mol Sci 2022; 23: 5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Clynes MA, Gregson CL, Bruyère O, et al. Osteosarcopenia: where osteoporosis and sarcopenia collide. Rheumatology (Oxford) 2021; 60: 529–537. [DOI] [PubMed] [Google Scholar]
- 54. Guilherme JPLF, Shikhova V, Dondukovskaya R, et al. Androgen receptor gene microsatellite polymorphism is associated with muscle mass and strength in bodybuilders and power athlete status. Ann Hum Biol 2021; 48: 142–149. [DOI] [PubMed] [Google Scholar]
- 55. Rodríguez-Garcia L, Ponce-González JG, Gonzalez-Henriquez JJ, et al. Androgen receptor CAG and GGN repeat polymorphisms and bone mass in boys and girls. Nutr Hosp 2015; 32: 2633–2639. [DOI] [PubMed] [Google Scholar]
- 56. Saoji R, Desai M, Das RS, et al. Estrogen receptor α and β gene polymorphism in relation to bone mineral density and lipid profile in Northeast Indian women. Gene 2019; 710: 202–209. [DOI] [PubMed] [Google Scholar]
- 57. Kumagai H, Miyamoto-Mikami E, Hirata K, et al. ESR1 rs2234693 polymorphism is associated with muscle injury and muscle stiffness. Med Sci Sports Exerc 2019; 51: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yun-Kai L, Hui W, Xin-Wei Z, et al. The polymorphism of Insulin-like growth factor-I (IGF-I) is related to osteoporosis and bone mineral density in postmenopausal population. Pak J Med Sci 2014; 30: 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gao ST, Lv ZT, Zhou CK, et al. Association between IGF-1 polymorphisms and risk of osteoporosis in Chinese population: a meta-analysis. BMC Musculoskelet Disord 2018; 19: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang CW, Li TC, Li CI, et al. Insulinlike growth factor-1 and its binding protein-3 polymorphisms predict circulating IGF-1 level and appendicular skeletal muscle mass in Chinese elderly. J Am Med Dir Assoc 2015; 16: 365–370. [DOI] [PubMed] [Google Scholar]
- 61. Liao JL, Qin Q, Zhou YS, et al. Vitamin D receptor Bsm I polymorphism and osteoporosis risk in postmenopausal women: a meta-analysis from 42 studies. Genes Nutr 2020; 15: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Scimeca M, Centofanti F, Celi M, et al. Vitamin D receptor in muscle atrophy of elderly patients: a key element of osteoporosis-sarcopenia connection. Aging Dis 2018; 9: 952–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Huang J, Hsu YH, Mo C, et al. METTL21C is a potential pleiotropic gene for osteoporosis and sarcopenia acting through the modulation of the NF-κB signaling pathway. J Bone Miner Res 2014; 29: 1531–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kang YJ, Yoo JI, Baek KW. Differential gene expression profile by RNA sequencing study of elderly osteoporotic hip fracture patients with sarcopenia. J Orthop Translat 2021; 29: 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Trajanoska K, Rivadeneira F, Kiel DP, et al. Genetics of bone and muscle interactions in humans. Curr Osteoporos Rep 2019; 17: 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li Y, Sun J, Wang G. Integrative analyses of biomarkers and pathways for osteosarcopenia, 2021, https://assets.researchsquare.com/files/rs-283082/v1/bd5d877a-cf00-4e81-bf46-4ee3b2f3d844.pdf?c=1631878042
- 67. Pagnotti GM, Styner M, Uzer G, et al. Combating osteoporosis and obesity with exercise: leveraging cell mechanosensitivity. Nat Rev Endocrinol 2019; 15: 339–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lichtenberg T, von Stengel S, Sieber C, et al. The favorable effects of a high-intensity resistance training on sarcopenia in older community-dwelling men with osteosarcopenia: the randomized controlled FrOST study. Clin Interv Aging 2019; 14: 2173–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kemmler W, Kohl M, Jakob F, et al. Effects of high intensity dynamic resistance exercise and whey protein supplements on osteosarcopenia in older men with low bone and muscle mass. Final results of the randomized controlled frost study. Nutrients 2020; 12: 2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Daly RM. Exercise and nutritional approaches to prevent frail bones, falls and fractures: an update. Climacteric 2017; 20: 119–124. [DOI] [PubMed] [Google Scholar]
- 71. Lopez P, Pinto RS, Radaelli R, et al. Benefits of resistance training in physically frail elderly: a systematic review. Aging Clin Exp Res 2018; 30: 889–899. [DOI] [PubMed] [Google Scholar]
- 72. Daly RM, Gianoudis J, Kersh ME, et al. Effects of a 12-month supervised, community-based, multimodal exercise program followed by a 6-month research-to-practice transition on bone mineral density, trabecular microarchitecture, and physical function in older adults: a randomized controlled tri. J Bone Miner Res off J Am Soc Bone Miner Res 2020; 35: 419–429. [DOI] [PubMed] [Google Scholar]
- 73. De Rui M, Inelmen EM, Pigozzo S, et al. Dietary strategies for mitigating osteosarcopenia in older adults: a narrative review. Aging Clin Exp Res 2019; 31: 897–903. [DOI] [PubMed] [Google Scholar]
- 74. Chevalley T, Brandi ML, Cavalier E, et al. How can the orthopedic surgeon ensure optimal vitamin D status in patients operated for an osteoporotic fracture. Osteoporos Int 2021; 32: 1921–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rogers-Soeder TS, Peters KE, Lane NE, et al. Dietary intake, D3Cr muscle mass, and appendicular lean mass in a cohort of older men. J Gerontol A Biol Sci Med Sci 2020; 75: 1353–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hruby A, Sahni S, Bolster D, et al. Protein intake and functional integrity in aging: the Framingham heart study offspring. J Gerontol A Biol Sci Med Sci 2020; 75: 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Candow DG, Forbes SC, Chilibeck PD, et al. Effectiveness of creatine supplementation on aging muscle and bone: focus on falls prevention and inflammation. J Clin Med 2019; 8: 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Atlihan R, Kirk B, Duque G. Non-pharmacological interventions in osteosarcopenia: a systematic review. J Nutr Health Aging 2021; 25: 25–32. [DOI] [PubMed] [Google Scholar]
- 79. McCloskey EV, Johansson H, Oden A, et al. Denosumab reduces the risk of osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX. J Bone Miner Res 2012; 27: 1480–1486. [DOI] [PubMed] [Google Scholar]
- 80. Miedany YE, Gaafary ME, Toth M, et al. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia. Clin Rheumatol 2021; 40: 4225–4232. [DOI] [PubMed] [Google Scholar]
- 81. Bonnet N, Bourgoin L, Biver E, et al. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J Clin Invest 2019; 129: 3214–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fatima M, Brennan-Olsen SL, Duque G. Therapeutic approaches to osteosarcopenia: insights for the clinician. Ther Adv Musculoskelet Dis 2019; 11: 1759720X19867009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rhee H, Navaratnam A, Oleinikova I, et al. A novel liver-targeted testosterone therapy for sarcopenia in androgen deprived men with prostate cancer. J Endocr Soc 2021; 5: bvab116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Dos Santos MR, Storer TW. Testosterone treatment as a function-promoting therapy in sarcopenia associated with aging and chronic disease. Endocrinol Metab Clin North Am 2022; 51: 187–204. [DOI] [PubMed] [Google Scholar]
- 85. Narayanan R, Coss CC, Dalton JT. Development of selective androgen receptor modulators (SARMs). Mol Cell Endocrinol 2018; 465: 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dalton JT, Barnette KG, Bohl CE, et al. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J Cachexia Sarcopenia Muscle 2011; 2: 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Buehring B, Binkley N. Myostatin–the holy grail for muscle, bone, and fat. Curr Osteoporos Rep 2013; 11: 407–414. [DOI] [PubMed] [Google Scholar]
- 88. Attie KM, Borgstein NG, Yang Y, et al. A single ascending-dose study of muscle regulator ACE-031 in healthy volunteers. Muscle Nerve 2013; 47: 416–423. [DOI] [PubMed] [Google Scholar]
- 89. Locatelli V, Bianchi VE. Effect of GH/IGF-1 on bone metabolism and osteoporsosis. Int J Endocrinol 2014; 2014: 235060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bian A, Ma Y, Zhou X, et al. Association between sarcopenia and levels of growth hormone and insulin-like growth factor-1 in the elderly. BMC Musculoskelet Disord 2020; 21: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sullivan DH, Carter WJ, Warr WR, et al. Side effects resulting from the use of growth hormone and insulin-like growth factor-I as combined therapy to frail elderly patients. J Gerontol A Biol Sci Med Sci 1998; 53: M183–M187. [DOI] [PubMed] [Google Scholar]
- 92. Tarantino U, Greggi C, Visconti VV, et al. T-score and handgrip strength association for the diagnosis of osteosarcopenia: a systematic review and meta-analysis. J Clin Med 2021; 10: 2597. [DOI] [PMC free article] [PubMed] [Google Scholar]