Abstract
Aim
A testing method for early diagnosis of Mild cognitive dementia (MCI) that can be easily applied in clinical practice was investigated in this study. We examined whether MCI risk can be determined through finger movements.
Methods
Between 2013 and 2020, 1097 individuals were screened. After applying propensity-score matching to adjust for variability between the groups, 173 individuals each in the mild cognitive impairment and control groups were selected. Thereafter, differences between groups in mean values of parameters extracted from finger tap movements were determined using unpaired t-test and effect size. Furthermore, area under the curve, sensitivity, and specificity were calculated from the receiver operating characteristic curve for parameters with significant difference.
Results
A significant difference was observed, especially in the number of taps in the MCI group compared with that in the control group (p < .001; 95% CI, −12.7 to −8.8; r = 0.51). A cut-off value of 30 taps was applied (sensitivity, 0.77; specificity, 0.67; AUC, 0.79). Significant differences were also observed in rhythm-related parameters.
Conclusions
These parameters might be useful for capturing MCI risk. Finger taps are easily measured and may be suitable for screening large populations. This tool might be used as a supplemental method to increase the sensitivity of traditional cognitive tests.
Keywords: mild cognitive impairment, dementia, screening, finger function, dexterity
Background
Over 55 million people worldwide were reported to have dementia in 2020, this number is expected to nearly double every 20 years, reaching 78 million in 2030 and 139 million in 2050 (Alzheimer’s Disease International, 2021). The Lancet International Commission on Dementia Prevention, Intervention and Care highlighted the importance of dementia prevention, reporting that proper assistance may delay progression in approximately one-third of dementia cases (Livingston et al., 2017). In particular, increased childhood education and exercise; maintaining social engagement; reducing or stopping smoking; and managing hearing loss, depression, diabetes, hypertension, and obesity have all been reported to potentially contribute to preventing or delaying dementia onset (Livingston et al., 2017). Although no fundamental methods of treatment or prevention of dementia have been established currently, early detection of dementia risks is important for delaying its progression and reducing its prevalence.
Various studies on early diagnosis of dementia have been conducted. However, specific or highly sensitive tests that can be easily performed in clinical settings are yet to be determined. Research aimed at the early detection of Alzheimer’s disease (AD), which accounts for about half of dementia cases, has enabled early diagnosis, to a certain extent, by using amyloid beta (Aβ), total tau (T-tau), and phosphorylated tau (P-tau) levels in cerebrospinal fluid (CSF) (Park et al., 2019) or by assessing the abnormal distribution of glucose metabolism in the brain using fluorodeoxyglucose-positron emission tomography (FDG-PET) (Miller, 2009). However, CSF and FDG-PET tests are invasive and costly, impose financial and physical burdens on patients, and require time for measurements and analyses. On the other hand, the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA) are the most widely used cognitive assessments, with test times ranging from 5 to 15 minutes (Folstein et al., 1975; Nasreddine et al., 2005). These scales can assess a broader range of cognitive abilities and, in some cases, can detect more subtle abnormalities such as those observed in MCI. In recent years, reports have started to emerge regarding motor impairments that occur prior to the onset of dementia or during the MCI phase. Buracchio et al. (2010) reported a decrease in walking speed approximately 12 years before the diagnosis of MCI. Additionally, Sabia et al. (2017) reported that physical activity begins to decrease in dementia patients approximately 9 years before the diagnosis is confirmed.
Therefore, we hypothesized that finger movements may exhibit subtle abnormalities associated with pathological changes in the brain before the appearance of the initial symptoms of dementia, focusing on finger-tap movements. Previous preliminary studies have explored the possibility of identifying speed decline of finger movement from the MCI phase to prove the hypothesis (Suzumura et al., 2016, 2018).
The objectives of the present study were to compare finger tapping functions between MCI patients and healthy elderly individuals, and to identify highly detectable parameters that capture decline in finger tapping.
Methods
Study Design and Participants
This cross-sectional study was conducted at the National Center for Geriatrics and Gerontology (Aichi, Japan). The participants were all first-time patients at the Outpatient Center for Comprehensive Care and Research on Memory Disorder of the National Center for Geriatrics and Gerontology, and the caregivers of the patients. In total, 1097 individuals were screened between February 28, 2013 and January 9, 2020. Selection criteria were as follows: older adults aged ≥65 years who had been diagnosed with MCI, were right-handed, and had the ability to thoroughly understand the study methods. The diagnostic criteria for MCI were based on the definition by Petersen et al. (Petersen et al., 1999). The main steps in the diagnosis of MCI are as follows; (1) medical examination by a dementia specialist, (2) comprehensive geriatric assessment (CGA), and psychological testing (Dubois et al 2000, Raven, 1965, Rosen 1984) (Frontal Assessment Battery [FAB], ’s Colored Progressive Matrices [RCPM], Alzheimer’s Disease Assessment Scale [ADAS]) by a clinical psychologist, and (3) magnetic resonance imaging (MRI) and single-photon emission computed tomography by a clinical laboratory technician, and (4) electrocardiogram, blood test, etc. Finally, a dementia specialist made a comprehensive evaluation of the results and diagnosed MCI. The CGA assessment includes MMSE, Barthel Index, Dementia Behavior Disturbance Scale, Geriatric Depression Scale, (Brink et al., 1982, Mahoney et al., 1965, Baumgarten et al., 1990, Toba et al., 2002, Podsiadlo & Richardson, 1991) Vitality Index, Mini Nutritional Assessment, Physical Measurement, Grip strength, gait speed, and Time Up & Go Test.
The selection criteria for the control groups were as follows: a right-handed caregiver who accompanied an MCI patient to the hospital; led a socially independent life; had no history of attending the Outpatient Center for Comprehensive Care and Research on Memory Disorder; had no problems with cognitive function; and had a MMSE score ≥28. Exclusion criteria were as follows: patients with impaired consciousness, tremor, parkinsonism, higher brain dysfunction such as aphasia or apraxia, epilepsy, paralysis, sensory disturbance, or finger dexterity impairment. The reasons for selecting only right-handed individuals for this study are as follows. (1) Including the non-dominant hand would allow differences in tapping ability due to differences in dominance, and (2) Previous studies have reported that the dominance of the dominant hand is reduced (Doody et al., 1999). Therefore, we selected right-handed individuals, who had the largest population. In addition, before the finger taps measurement, all participants were asked to fill out a medical interview sheet to confirm their medical history, daily living conditions, and degree of independence in daily living (see Supporting Information Table S1).
Ethical considerations
All participants or their family members were given detailed verbal and written descriptions of the present experiment and consent was obtained from each participant prior to enrolment. Approval for the present study was obtained from the Ethics and Conflict of Interest Committee of the National Center for Geriatrics and Gerontology in Japan (approval no. 623–9).
Procedures
The UB-2 magnetic sensing finger-tapping device (Maxell, Tokyo, Japan) was used as the measuring device (see Supporting Information Figure S1). The UB-2 is a non-medical device that measures the distance between magnetic sensors attached to the thumb and index fingers, with high measurement precision and safety for the human body. The reliability of finger tapping devices has been evaluated for three types of reproducibility: when measurements were taken at different times, when using different devices, and during inter-rater testing, and the reproducibility has been reported to be high (Sano et al., 2011). The device samples voltage output from the sensors at 100 Hz. This voltage is converted to distance based on a conversion curve calculated before every measurement from the calibration voltages obtained when the participant closes the two fingers and holds a 6-cm block. Magnetic sensors were placed on the thumb and index finger of both hands using an elastic band. The bilateral finger-tapping test was carried out using the index fingers and thumbs. The finger-tapping task consists of four types of movements: tapping of a single hand (left or right hand), tapping simultaneously with both hands (left and right tapping at the same time), and tapping with alternate hands (alternate left- and right-hand tapping) (see Supporting Information Figure S2). The finger-tapping task was assessed by occupational therapists with at least 5 years of experience. Measurements were taken individually, with the participant sitting in a chair in a quiet environment. When we examined the participants, we ensured that they kept the following positions: (1) the elbow joint was off the desk, (2) the forearms were in the intermediate position between pronation-supination, and the upper arms were kept close to the body, (3) the wrist joints were in slight dorsiflexion, and (4) the third to fifth fingers were held lightly (see Supporting Information Figure S3). In addition, measurements were taken with open eyes. Before measuring each movement, participants were instructed to practice once for approximately 5 s to confirm the degree of understanding of the tapping task. We asked the participants to tap as fast as possible. After practice, the participants performed each task in the following order: left hand, right hand, simultaneously, and alternate hands, and movements were measured for 15 s (Total time for the four movements: 60 s). When measurements were completed, the UB-2 yielded 44 measurement parameters (Suzumuara et al., 2016).
Statistical analysis
Propensity score matching was performed to adjust for variability between the MCI and control groups. The explanatory variables were age and sex, and the caliper was set at 0.25 × standard deviation (Rosenbaum & Rubin, 1985). After matching by propensity scores, differences in mean values of parameters extracted from finger-tap movements were analyzed using an unpaired t-test and effect size. Effect size was calculated as follows: where t is t-value and df is degrees of freedom. Effect size was calculated from mean values and standard deviations for each of the MCI and control groups, and Cohen’s r was used (Cohen, 1988). The criteria for the effect size “r” are r = 0.1 (small), r = 0.3 (medium), and r = 0.5 (large). Data are presented as mean ± SD, p values were two-tailed, and statistical significance was considered at p < .001. In addition, area under the curve (AUC) as well as sensitivity and specificity were calculated from the receiver operating characteristic (ROC) curve for items that indicated significance. The sensitivity/specificity cutoff was determined using Youden’s index. Youden’s index is calculated as maximum {sensitivity + specificity −1} (Akobeng, 2007). Statistical analyses were performed using SPSS and R (https://www.r-project.org/).
Results
Participant characteristics
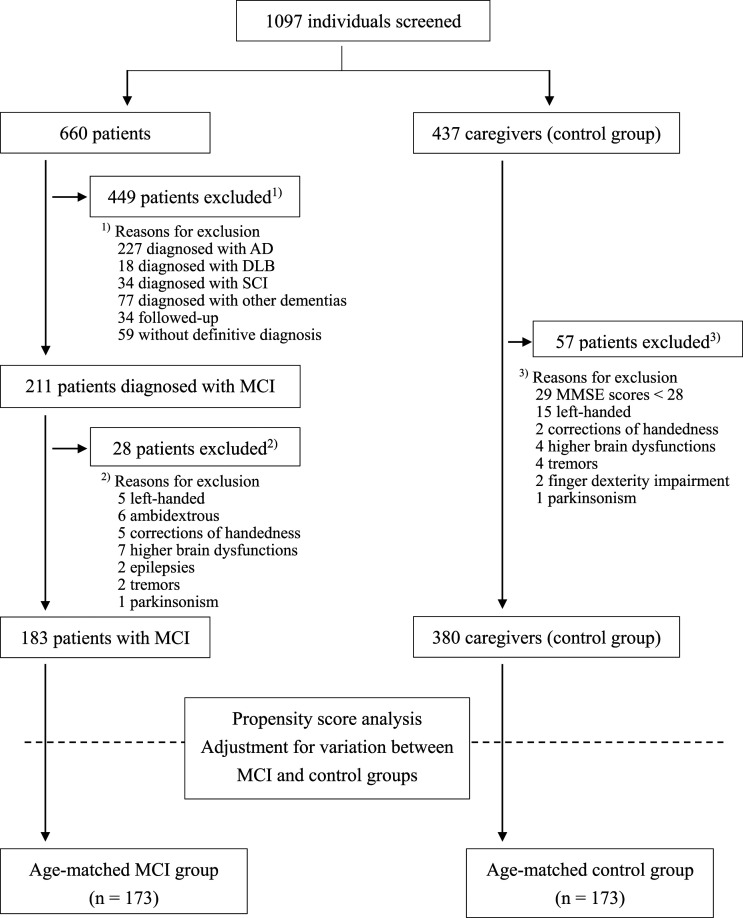
Figure 1 shows the flowchart for this study. In total, 1097 individuals were screened between February 28, 2013 and January 9, 2020, and finger taps were measured in 660 older patients and 437 caregivers (control group). According to the inclusion and exclusion criteria, propensity score analysis was conducted for 563 individuals (183 MCI group and 380 control group) to adjust for variability between the two groups. Subsequently, 346 participants (173 each in the MCI and control groups) were included in this study. Table 1 shows the baseline characteristics of the participants, while Table 2 shows the participant characteristics after propensity score matching.
Figure 1.
Trial profile.
Table 1.
Baseline characteristics of the study participants.
| Characteristic | Older Patients’ Group | Control Group |
|---|---|---|
| Number of participants | n = 660 | n = 437 |
| Age (years) | 77.0 (8.2) | 69.6 (12.1) |
| Sex (%) | ||
| Men | 269 (41%) | 156 (36%) |
| Women | 391 (59%) | 281 (64%) |
| Handedness (%) | ||
| Right | 614 (93%) | 420 (96.1%) |
| Left | 15 (2.3%) | 15 (3.4%) |
| Ambidextrous | 16 (2.4%) | 0 (0%) |
| Correction of handedness | 15 (2.3%) | 2 (0.5%) |
| Grip (kg) | ||
| Right | 21.1 (7.8) | NC |
| Left | 20.1 (7.4) | NC |
| MMSE (/30 points) | 21.9 (5.6) | 29.1 (1.3) |
| Barthel index (/100 points) | 96.0 (10.0) | NC |
Note 1: Characteristics are given as mean (SD) or n (%).
Note 2: NC, data not collected for the Control group.
Abbreviations: NC, not collected; SD, standard deviation; MMSE, Mini-Mental State Examination.
Table 2.
Participant characteristics after propensity score matching.
| Characteristic | MCI Group | Control Group |
|---|---|---|
| Number of participants | 173 | 173 |
| Age (years) | 77.2 (6.8) | 77.1 (6.8) |
| Sex (%) | ||
| Men | 74 (43%) | 78 (45%) |
| Women | 99 (57%) | 95 (55%) |
| Grip (kg) | ||
| Right | 21.7 (6.5) | NC |
| Left | 20.9 (6.5) | NC |
| MMSE (/30 points) | 24.7 (3.1) | 29.3 (0.8) |
| Barthel index (/100 points) | 98.6 (4.3) | NC |
Note 1: Characteristics are mean (SD) or n (%).
Note 2: NC, data not collected for the Control group.
Abbreviations: MNC, not collected; CI, mild cognitive impairment; SD, standard deviation; MMSE, Mini-Mental State Examination.
Comparison of parameter values between the MCI and control groups
The comparison of parameters between the MCI and control groups showed a large effect size (p <.001; 95% CI, −12.7 to −8.8; r = 0.51) for the number of taps in alternate tapping with both hands. In addition, effect sizes for parameters such as the average of tapping interval (p <.001; 95% CI, 0.17 to 0.26; r = 0.45), standard deviation of inter-tapping interval (p <.001; 95% CI, 0.06 to 0.09; r = 0.43), and number of freezing calculated from acceleration (p <.001; 95% CI, 19.8 to 32.1; r = 0.41) were moderately high at r ≥ 0.4, and effect size was greater for the left hand than for the right hand. Figure 2 shows the main results of alternate tapping with both hands (left hand), which tended to display the highest effect size (see Supporting Information Table S2–S5).
Figure 2.
Comparison between MCI and healthy control groups (left-hand tapping in the anti-phase task).
Calculation of cut-off values to differentiate between the MCI and control groups
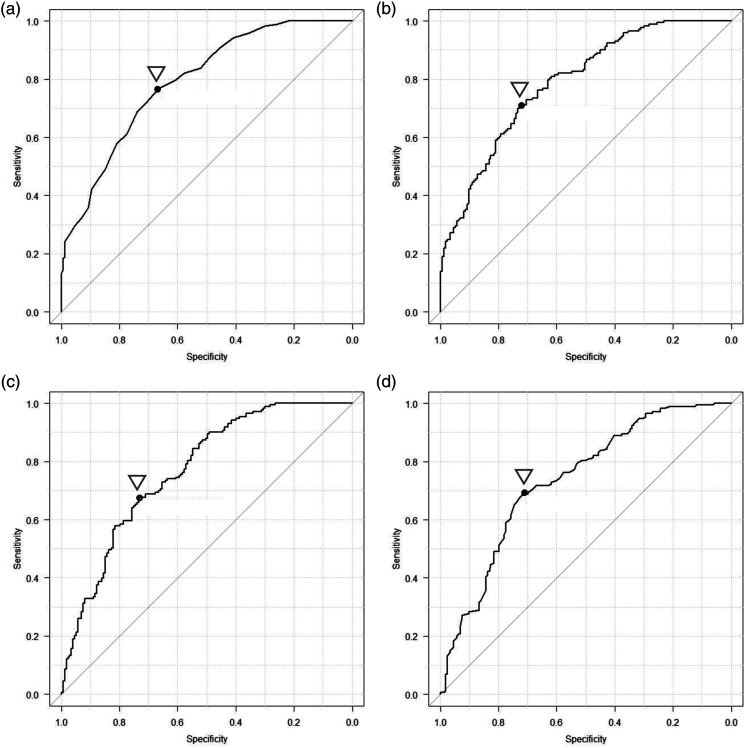
Using the above-mentioned parameters, the ROC analysis showed that the cut-off value for number of taps was 30, yielding a sensitivity of 0.77, specificity of 0.67, and an AUC of 0.79. The cut-off value for the average of tapping interval was 0.47 second, with a sensitivity of 0.71, specificity of 0.72, and an AUC of 0.79. The cut-off value for the standard deviation of inter-tapping interval was 0.07 second, with a sensitivity of 0.68, specificity of 0.73, and an AUC of 0.77. The cut-off value for the number of freezing calculated from acceleration was 32.5, with a sensitivity of 0.69, specificity of 0.72, and an AUC of 0.74 (Figure 3).
Figure 3.
ROC analysis comparing the MCI and control groups.
Discussion
In the present study, finger-tap movements were performed using a magnetic sensing finger-tapping device. Finger functions were then compared between the MCI and control groups and cut-off values were calculated to differentiate between both groups. The results showed a significant difference in finger functions for parameters such as number of taps, average of tapping interval, standard deviation of inter-tapping interval, and number of freezing calculated from acceleration during alternate tapping tasks with both hands, with a particularly large effect size of r = 0.51 in the number of taps. ROC analysis also showed an AUC ≥ 0.7 for each parameter, indicating moderate differentiating accuracy.
Declines in motor functions such as finger function and walking ability are related to the progression of cognitive decline (Abe et al., 2017). Previous studies on finger functions of MCI patients, compared with healthy elderly individuals, have reported impairment of fine motor functions of the fingers (Yan et al., 2008), declines in finger dexterity (de Paula et al., 2016), and decreases in number of taps (Roalf et al., 2018). However, no detailed studies have determined cut-off values to differentiate between healthy elderly individuals and MCI patients. The present study found significant differences in number of taps, rhythm-related parameters (Average of tapping interval and SD of inter-tapping interval), and number of freezing calculated from acceleration, suggesting that these parameters may be useful in capturing the finger functions characteristic of MCI. In particular, the number of taps showed a large effect size (r = 0.51) and moderate discrimination accuracy (AUC 0.79), suggesting that it may be the most appropriate parameter for discriminating between MCI patients and healthy older individuals. However, the MMSE, MoCA, and other screening tests for dementia showed a sensitivity of 81–92% and specificity of 81–91% (Tsoi et al., 2015). The discrimination ability of this study was lower than those of previous studies. Therefore, further research is required before finger tap movement can be adopted as a standard test for MCI.
Although various criteria have been followed to diagnose MCI, such as the diagnostic criteria by Petersen et al. (2010) and Portet et al. (2006), no international consensus has yet been reached. A large-scale cohort study of elderly individuals in the community targeting 757 community-dwelling elderly individuals found that approximately 40% of the participants had MCI when a combination of cognitive function tests including complaints of memory loss and assessments of memory, attention, and executive functions were administered to determine MCI (Sachdev et al., 2012). A large-scale population-based study targeting 5104 community-dwelling elderly individuals found that approximately 18.8% had MCI when memory, attention, and executing functions, as well as information-processing and visual-spatial cognitive function, were assessed using the National Center for Geriatrics and Gerontology – Functional Assessment Tool (Shimada et al., 2013). Other large-scale, population-based studies have been conducted to determine the prevalence of MCI among community-dwelling elderly individuals (Ding et al., 2015; Petersen et al., 2010). However, the problem is that testing and analyses take a long time. In comparison, finger-tap movements can be measured in 15 s (total time for four types: 60 s) and are considered suitable for screening tests.
Much of the information in the literature is not yet know about finger movements, as many areas of the brain are intricately involved. Previously, no studies have been reported on the analysis of finger coordination and rhythm in both hands. The present study showed significant differences, particularly in tasks involving alternate tapping with both hands, similar to our previous study (Suzumura et al., 2016). Bimanual coordination and adjustment of rhythm involve the cerebellum and dorsal premotor cortex (Debaere et al., 2004) as well as the basal ganglia (Mink, 1996), and the corpus callosum function (Kara et al., 2015) is needed for the smooth transmission of information between hemispheres. In addition, atrophy of the basal ganglia (Pievani et al., 2013) and corpus callosum (Elahi et al., 2015) have been reported as early changes in dementia; we believe that participants with cognitive decline may show a decline in movements requiring bimanual coordination and in rhythm-related parameters (Average of tapping interval and SD of inter-tapping interval). Furthermore, this study found significant differences in the number of freezing calculated from acceleration, despite excluding participants with tremors and Parkinson’s disease. Therefore, the results of this study suggest that participants with cognitive decline are more likely to exhibit fine finger freezing during finger tapping. The fact that we found significant differences in finger functions between healthy elderly individuals and the MCI group for various parameters in the present study indicate that subtle changes in the brain may affect finger function from the stages of MCI.
The present study had some limitations. First, this was a single-center study. Second, the results of propensity score matching in the present study were not adjusted for variables that were not included among the explanatory variables (variables other than age and sex), and cases omitted from matching were not reflected in the results. Third, the involvement of latent extrapyramidal disorders (Tosto et al., 2015) as a factor influencing finger function cannot be ruled out. Fourth, as this was a cross-sectional study, subsequent progress has not been followed. In the future, we plan to conduct multi-center studies, take measurements of elderly individuals in large population-based settings such as at health workshops in the community, and follow-up with the progress longitudinally. In addition to age and sex, we would also like to investigate the relationship between educational history and various test scores, which are thought to influence the finger function characteristics of MCI.
The primary benefit of the present study is the finding that finger-tap movements have the potential to be used as a screening tool for assessing MCI risk. In particular, the number of taps measured from finger tapping movements may be a useful parameter for capturing the decline in motor function during the MCI phase. This tool may be used as a supplemental method to increase the sensitivity of traditional cognitive tests for dementia. In the future, we also would like to verify the relationship between finger function and brain function in MCI patients by using neuroimaging techniques such as MRI and PET.
Supplemental Material
Supplemental Material for Finger Tapping Test for Assessing the Risk of Mild Cognitive Impairment by Shota Suzumura, Aiko Osawa, Yoshikiyo Kanada, Keisuke Maeda, Eiko Takano, Junpei Sugioka, Natsumi Maeda, Taishi Nagahama, Kenta Shiramoto, Katsumi Kuno, Shiori Kizuka, Kenji Satoh, Hiroaki Sakurai, Yuko Sano, Tomohiko Mizuguchi, Akihiko Kandori and Izumi Kondo in Hong Kong Journal of Occupational Therapy.
Acknowledgements
We would like to thank the study participants for their contribution to this study. Additionally, we would like to thank Editage (www.editage.com) for English language editing.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the JSPS Grants-in-Aid for Scientific Research under Grant number JP19K11339.
Disclosure Statement: Coauthors Yuko Sano, and Akihiko Kandori are employees of Hitachi Ltd Tomohiko Mizuguchi is an employee of Maxell Ltd The National Center for Geriatrics and Gerontology conducts joint studies with Hitachi Ltd and Maxell Ltd The device used in this study was loaned to the National Center for Geriatrics and Gerontology by Maxell Ltd in Japan. The other authors report no relevant disclosures.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Shota Suzumura https://orcid.org/0000-0001-7346-9326
References
- Abe T., Soma Y., Kitano N., Jindo T., Sato A., Tsunoda K., Tsuji T., Okura T. (2017). Change in hand dexterity and habitual gait speed reflects cognitive decline over time in healthy older adults: A longitudinal study. Journal of Physical Therapy Science, 29(10), 1737–1741. 10.1589/jpts.29.1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akobeng A. K. (2007). Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatrica, 96(5), 644–647. 10.1111/j.1651-2227.2006.00178.x [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Disease International (2021). Dementia statistics. https://www.alzint.org/about/dementia-facts-figures/dementia-statistics/. [Google Scholar]
- Brink, TL., Yesavage, JA., Owen, L., Heersema, PH., Adey, M., Rose, TL. (1982). Screening tests dor Geriatric De-pression. Clin Geron. 1(1), 37–43. 10.1589/jpts.29.1737 [DOI] [Google Scholar]
- Buracchio T., Dodge H. H., Howieson D., Wasserman D., Kaye J. (2010). The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol, 67(8), 980–986. 10.1001/archneurol.2010.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Lawrence Erlbaum. 10.4324/9780203771587 [DOI] [Google Scholar]
- Debaere F., Wenderoth N., Sunaert S., Van Hecke P., Swinnen S. P. (2004). Cerebellar and premotor function in bimanual coordination: Parametric neural responses to spatiotemporal complexity and cycling frequency. Neuroimage, 21(4), 1416–1427. 10.1016/j.neuroimage.2003.12.011 [DOI] [PubMed] [Google Scholar]
- de Paula J. J., Albuquerque M. R., Lage G. M., Bicalho M. A., Romano-Silva M. A., Malloy-Diniz L. F. (2016). Impairment of fine motor dexterity in mild cognitive impairment and Alzheimer's disease dementia: Association with activities of daily living. Revista brasileira de psiquiatria, 38(3), 235–238. 10.1590/1516-4446-2015-1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D., Zhao Q., Guo Q., Meng H., Wang B., Luo J., Mortimer J. A., Borenstein A. R., Hong Z. (2015). Prevalence of mild cognitive impairment in an urban community in China: A cross-sectional analysis of the shanghai aging study. Alzheimers Dementia, 11(3), 300–309. 10.1016/j.jalz.2013.11.002 [DOI] [PubMed] [Google Scholar]
- Doody R. S., Vacca J. L., Massman P. J., Liao T. Y. (1999). The influence of handedness on the clinical presentation and neuropsychology of Alzheimer disease. Arch Neurol, 56(9), 1133–1177. 10.1001/archneur.56.9.1133 [DOI] [PubMed] [Google Scholar]
- Dubois B., Slachevsky A., Litvan I., Pillon B. (2000). The FAB: a Frontal Assessment Battery at bedside. Neurology, 55(11), 1621–1626. 10.1212/wnl.55.11.1621 [DOI] [PubMed] [Google Scholar]
- Elahi S., Bachman A. H., Lee S. H., Sidtis J. J., Ardekani B. A., Alzheimer's Disease Neuroimaging I. (2015). Corpus callosum atrophy rate in mild cognitive impairment and prodromal Alzheimer's disease. Journal of Alzheimer's Disease, 45(3), 921–931. 10.3233/JAD-142631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. (1975). Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Kara F., Hofling C., Rossner S., Schliebs R., Van der Linden A., Groot H. J., Alia A. (2015). In vivo longitudinal monitoring of changes in the corpus callosum integrity during disease progression in a mouse model of Alzheimer's disease. Current Alzheimer Research, 12(10), 941–950. 10.2174/1567205012666151027123728 [DOI] [PubMed] [Google Scholar]
- Livingston G., Sommerlad A., Orgeta V., Costafreda S. G., Huntley J., Ames D., Ballard C., Banerjee S., Burns A., Cohen-Mansfield J., Cooper C., Fox N., Gitlin L. N., Howard R., Kales H. C., Larson E. B., Ritchie K., Rockwood K., Sampson E. L., Mukadam N. (2017). Dementia prevention, intervention, and care. Lancet, 390(10113), 2673–2734. 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- Miller G. (2009). Alzheimer's biomarker initiative hits its stride. Science, 326(5951), 386–389. 10.1126/science.326_386 [DOI] [PubMed] [Google Scholar]
- Mink J. W. (1996). The basal ganglia: Focused selection and inhibition of competing motor programs. Progress in Neurobiology, 50(4), 381–425. 10.1016/s0301-0082(96)00042-1 [DOI] [PubMed] [Google Scholar]
- Nasreddine Z. S., Phillips N. A., Bedirian V., Charbonneau S., Whitehead V., Collin I., Cummings J. L., Chertkow H. (2005). The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53(4), 695–699. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Park J. E., Choi K. Y., Kim B. C., Choi S. M., Song M. K., Lee J. J., Kim J., Song H. C., Kim H. W., Ha J. M., Seo E., Song W., Park S. G., Lee K. H. (2019). Cerebrospinal fluid biomarkers for the diagnosis of prodromal Alzheimer’s disease in Amnestic mild cognitive impairment. Dementia and Geriatric Cognitive Disorders Extra, 9(1), 100–113. 10.1159/000496920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R. C., Roberts R. O., Knopman D. S., Geda Y. E., Cha R. H., Pankratz V. S., Boeve B. F., Tangalos E. G., Ivnik R. J., Rocca W. A. (2010). Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology, 75(10), 889–897. 10.1212/WNL.0b013e3181f11d85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen R. C., Smith G. E., Waring S. C., Ivnik R. J., Tangalos E. G., Kokmen E. (1999). Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology, 56(3), 303–308. 10.1001/archneur.56.3.303 [DOI] [PubMed] [Google Scholar]
- Pievani M., Bocchetta M., Boccardi M., Cavedo E., Bonetti M., Thompson P. M., Frisoni G. B. (2013). Striatal morphology in early-onset and late-onset Alzheimer's disease: A preliminary study. Neurobiology of Aging, 34(7), 1728–1739. 10.1016/j.neurobiolaging.2013.01.016 [DOI] [PubMed] [Google Scholar]
- Portet F., Ousset P. J., Visser P. J., Frisoni G. B., Nobili F., Scheltens P., Vellas B., Touchon J., MCI Working Group of the European Consortium on Alzheimer's Disease (EADC) . (2006). Mild cognitive impairment (MCI) in medical practice: A critical review of the concept and new diagnostic procedure. Report of the MCI working group of the European consortium on Alzheimer's disease. Journal of Neurology, Neurosurgery, and Psychiatry, 77(6), 714–718. 10.1136/jnnp.2005.085332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- RCPM: Raven JC. (1965). Guide to using the Colored Progressive Matrices, Sets A, Ab, B. HK Lewis. [Google Scholar]
- Roalf D. R., Rupert P., Mechanic-Hamilton D., Brennan L., Duda J. E., Weintraub D., Trojanowski J. Q., Wolk D., Moberg P. J. (2018). Quantitative assessment of finger tapping characteristics in mild cognitive impairment, Alzheimer's disease, and Parkinson's disease. Journal of Neurology, 265(6), 1365–1375. 10.1007/s00415-018-8841-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen WG., Mohs RC., Davis KL. (1984). A new rating scale for Alzheimer's disease. Am J Psychiatry, 141(11), 1356–1364. 10.1176/ajp.141.11.1356 [DOI] [PubMed] [Google Scholar]
- Rosenbaum P. R., Rubin D. B. (1985). Constructing a control group using multivariate matched sampling methords that incorporate the propensity score. The American Statistician, 39(1), 33–38. 10.2307/2683903 [DOI] [Google Scholar]
- Sabia S., Dugravot A., Dartigues J. F., Abell J., Elbaz A., Kivimaki M., Singh-Manoux A. (2017). Physical activity, cognitive decline, and risk of dementia: 28 year follow-up of whitehall II cohort study. Bmj: British Medical Journal, 357(1), j2709. 10.1136/bmj.j2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev P. S., Lipnicki D. M., Crawford J., Reppermund S., Kochan N. A., Trollor J. N., Draper B., Slavin M. J., Kang K., Lux O., Mather K. A., Brodaty H. (2012). Ageing study, TRisk profiles of subtypes of mild cognitive impairment: The sydney memory and ageing study. Journal of the American Geriatrics Society, 60(1), 24–33. 10.1111/j.1532-5415.2011.03774.x [DOI] [PubMed] [Google Scholar]
- Sano Y., Kandori A., Shima K., Tamura Y., Takagi H., Tsuji T., Noda M., Higashikawa F., Yokoe M., Sakoda S. (2011). Repeatability evaluation of finger tapping device with magnetic sensors. Toxicological Sciences: an Official Journal of the Society of Toxicology, 47(6), 272–281. 10.9746/sicetr.47.272 [DOI] [Google Scholar]
- Shimada H., Makizako H., Doi T., Yoshida D., Tsutsumimoto K., Anan Y., Uemura K., Ito T., Lee S., Park H., Suzuki T. (2013). Combined prevalence of frailty and mild cognitive impairment in a population of elderly Japanese people. Journal of the American Medical Directors Association, 14(7), 518–524. 10.1016/j.jamda.2013.03.010 [DOI] [PubMed] [Google Scholar]
- Suzumura S., Osawa A., Maeda N., Sano Y., Kandori A., Mizuguchi T., Yin Y., Kondo I. (2018). Differences among patients with Alzheimer's disease, older adults with mild cognitive impairment and healthy older adults in finger dexterity. Geriatrics & Gerontology International, 18(6), 907–914. 10.1111/ggi.13277 [DOI] [PubMed] [Google Scholar]
- Suzumura S., Osawa A., Nagahama T., Kondo I., Sano Y., Kandori A. (2016). Assessment of finger motor skills in individuals with mili cognitive impairment and patients with Alzheimer’s disease: Relationship between finger-to-thumb tapping and cognitive function. Japanese Journal of Comprehensive Rehabilitation Science, 7(1), 19–28. 10.11336/jjcrs.7.19 [DOI] [Google Scholar]
- Tosto G., Monsell S. F., Hawes S. E., Mayeux R. (2015). Pattern of extrapyramidal signs in Alzheimer's disease. Journal of Neurology, 262(11), 2548–2556. 10.1007/s00415-015-7886-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi K. K., Chan J. Y., Hirai H. W., Wong S. Y., Kwok T. C. (2015). Cognitive tests to detect dementia: A systematic review and meta-analysis. JAMA Intern Med, 175(9), 1450–1458. 10.1001/jamainternmed.2015.2152 [DOI] [PubMed] [Google Scholar]
- Yan J. H., Rountree S., Massman P., Doody R. S., Li H. (2008). 's disease and mild cognitive impairment deteriorate fine movement control. Journal of Psychiatric Research, 42(14), 1203–1212. 10.1016/j.jpsychires.2008.01.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Finger Tapping Test for Assessing the Risk of Mild Cognitive Impairment by Shota Suzumura, Aiko Osawa, Yoshikiyo Kanada, Keisuke Maeda, Eiko Takano, Junpei Sugioka, Natsumi Maeda, Taishi Nagahama, Kenta Shiramoto, Katsumi Kuno, Shiori Kizuka, Kenji Satoh, Hiroaki Sakurai, Yuko Sano, Tomohiko Mizuguchi, Akihiko Kandori and Izumi Kondo in Hong Kong Journal of Occupational Therapy.