Abstract
Recent studies have shown that the use of mesenchymal stem/stromal cells (MSCs) may be a promising strategy for treating spinal cord injury (SCI). This study aimed to explore the effectiveness of human umbilical cord-derived MSCs (hUC-MSCs) with different administration routes and dosages on SCI rats. Following T10-spinal cord contusion in Sprague-Dawley rats (N = 60), three different dosages of hUC-MSCs were intrathecally injected into rats (SCI-ITH) after 24 h. Intravenous injection of hUC-MSCs (SCI-i.v.) and methylprednisolone reagent (SCI-PC) were used as positive controls (N = 10/group). A SCI control group without treatment and a sham operation group were injected with Multiple Electrolyte Injection solution. The locomotor function was assessed by Basso Beattie Bresnahan (BBB) rating score, magnetic resonance imaging (MRI), histopathology, and immunofluorescence. ELISA was conducted to further analyze the nerve injury and inflammation in the rat SCI model. Following SCI, BBB scores were significantly lower in the SCI groups compared with the sham operation group, but all the treated groups showed the recovery of hind-limb motor function, and rats receiving the high-dose intrathecal injection of hUC-MSCs (SCI-ITH-H) showed improved outcomes compared with rats in hUC-MSCs i.v. and positive control groups. Magnetic resonance imaging revealed significant edema and spinal cord lesion in the SCI groups, and significant recovery was observed in the medium and high-dose hUC-MSCs ITH groups. Histopathological staining showed that the necrotic area in spinal cord tissue was significantly reduced in the hUC-MSCs ITH-H group, and the immunofluorescence staining confirmed the neuroprotection effect of hUC-MSCs infused on SCI rats. The increase of inflammatory cytokines was repressed in hUC-MSCs ITH-H group. Our results confirmed that hUC-MSC administered via intrathecal injection has dose-dependent neuroprotection effect in SCI rats.
Keywords: spinal cord injury, mesenchymal stem cells (MSCs), intrathecal injection, neuro-protection, hind limb locomotor function
Introduction
Spinal cord injury (SCI) is one of the most common major injuries to the central nervous system, resulting in severe motor dysfunction or even lifelong paralysis, thus presenting an enormous public health problem1. More than one million patients suffer spine and spinal cord injuries, and SCI in China is reported to be growing at an annual rate of 120,0002. Treating SCI is still clinically difficult due to the low neuro-regenerative ability and microenvironment imbalance, including pro-inflammatory cytokines that increase following SCI, which further hinder neuron regeneration and functional recovery3,4.
Recent studies have demonstrated that mesenchymal stem cell (MSC) transplantation therapy provides an attractive treatment to promote neuro-regeneration, axonal growth, and neuron survival5–10. MSCs are adult stem cells with multi-differentiation and self-renewal ability. They also have the potential for immunomodulatory and extensive tissue regeneration via secretion of various growth factors and anti-inflammatory cytokines and by activating signal pathways through specific receptors on target cells11. A recent clinical study confirmed that intravenous administration of MSCs was safe and feasible for SCI patients, and the evaluation scores using neurological scales demonstrated improved function after MSC infusion, compared with the scores before MSC infusion in all patients12.
MSCs can be directly transplanted into the damaged spinal cord and can also be homed to the damaged tissue by intravenous injection13. Despite reports showing that various delivery methods of MSCs transplantation, including intravenous, intraspinal, and intrathecal injection in the SCI treatments, are effective14, the optimal method for MSC administration to SCI patients has not yet been established. A previous study showed that intrathecal application provides a better accumulation of the effector cells than intravenous injection15. However, those researches did not assess the behavioral, systematic, pathological mechanisms, or dose levels.
The present study aimed to determine the optimal delivery method and dosage of transplanted hUC-MSCs into the injured rat spinal cord. Fresh preparation of hUC-MSCs injection was intrathecally implanted, and the impact on functional motor recovery and spinal cord tissue repair in SCI rats was described. As intrathecal administration can eliminate the risk of direct surgical implantation while ensuring the spread of cells through the subarachnoid space and around the lesion site, we selected intrathecal administration (ITH) as the main delivery method in our experiments and compared the effect of hUC-MSCs ITH with a different number of cells (2.5, 5, 10 million/kg body weight) in SCI rats, and used the intravenous (i.v.) administration (20 million/kg body weight) as control. We also used methylprednisolone, which is a clinical first-line medication for SCI, as the positive control to compare the effects of MSCs on SCI.
Materials and Methods
Spinal Cord Injury Model
Eighty male Sprague-Dawley rats (about 220 g) were purchased from SiBeiFu Biotechnology Co, Ltd (Beijing, China). All rats were housed in specific pathogen-free animal rooms at 20°C to 26°C with 40% to 70% humidity, with free access to water and food. All procedures performed on rats were approved using the IACUC of Tianjin Institute of Pharmaceutical Research (IACUC ID is 2020123002).
The construction of the rat spinal cord injury model was established according to the previous reports16. Briefly, the rats were anesthetized by intraperitoneal injection of 3% pentobarbital sodium at a dose of 60 mg/kg. They were fixed in a prone position on the operating table, after which their skin was preserved and disinfected. A longitudinal incision was made over the thoracic-lumbar region of the back. Skin and subcutaneous fascia were cut to display transverse processes, laminae and spinous processes. The skin and muscle tissue covering the surface of vertebral body and articular process were slightly pulled and cut to fully present the joint, vertebral body, and lateral wall. T10 spinal cord was exposed and then contused using a 10-g weight falling 50 mm from the JK052 impactor device. The wound was then washed with 4U/ml penicillin saline and sutured. Those SCI rats had the characteristics of T10 segment congestion and edema, spastic tail swinging, hind-limb dysfunction, trunk tremor, etc. The Basso Beattie Bresnahan (BBB) score was performed on the second day after surgery, and the rats with BBB score of 0 were selected for the SCI model group.
Preparation and Transplantation of hUC-MSCs Injection
The hUC-MSCs injection was manufactured according to our previous study17. Briefly, female hUC-MSCs (Karyotype, Fig. S1) were isolated and cultured, after which master cell banks (MCB, passage 2) and working cell banks (WCB, passage 5) were established and tested for sterility, mycoplasma, virus, viability, surface markers, biological efficacy, and differentiation ability, etc, and only those that passed the quality criteria were used to prepare the hUC-MSCs injection. Before being used, an appropriate amount of hUC-MSCs in the WCB was thawed and cultured, after which the cells were harvested and resuspended in Multiple Electrolyte Injection solution (Shijiazhuang NO.4 Pharmaceutical, Lot No. 1707071404) for product formulation that was prepared and tested within 24 h.
Successfully modeled rats (N = 60) were divided into 6 groups: (1) control group that received Multiple Electrolyte Injection solution (SCI-CON, N = 10) as negative control; (2) 2.5 × 106 cells/kg hUC-MSCs intrathecal injection group (SCI-ITH-L, N = 10); (3) 5 × 106 cells/kg hUC-MSCs intrathecal injection group (SCI-ITH-M, N = 10)15; (4) 10 × 106 cells/kg hUC-MSCs intrathecal injection group (SCI-ITH-H, N = 10); (5) 20 × 106 cells/kg hUC-MSCs intravenous injection group (SCI-i.v., N = 10); (6) control group that received methylprednisolone reagent i.v. as positive control (SCI-PC, N = 10). Methylprednisolone reagent was made by dissolving 1 g methylprednisolone into 250 ml 20% mannitol solution. The sham operation group received Multiple Electrolyte Injection solution (SO-CON, N = 10) as healthy control. Overall, 70 rats were used in our study. Treatment of hUC-MSCs or injection of methylprednisolone was given at 24 h after the SCI. Sham-operated rats and SCI-CON rats were given 20 μl cell Multiple Electrolyte Injection solution. SCI-ITH groups were given 20 μl hUC-MSC injection intrathecal administration via a 25G needle by a lumbar puncture between L5-6 under short-time general anesthesia according to Urdzíková et al.18 Direct injection of hUC-MSCs at the injury site was not performed in order to avoid further injury and exacerbate the inflammatory environment at the injured cord, which would be unsuitable for cell transplantation according to previous reports15,19. SCI-i.v. group was given a tail vein injection of 400 μl hUC-MSCs. SCI-PC group was given 1.5 ml methylprednisolone reagent (6 mg methylprednisolone included) via tail vein.
Neurobehavioral Evaluation Using the BBB Score
The recovery of hind limb locomotor function in rats was evaluated using the BBB scoring system, which uses a scale with 0–21 points20. The BBB motor scale was performed to analyze the behavior of the rats at −1, 1 (before hUC-MSCs injection), 4 (3 days after hUC-MSCs injection), 8, 15, and 29 days after surgery. The BBB score of all rats with normal locomotor function was 21, while the rats with a complete lack of locomotor function had a BBB score of 0. The tests were performed three times to obtain an average value for each rat, and the relevant score was obtained by averaging the values of both limbs.
Magnetic Resonance Imaging
The spinal cord structure and injury location of the SCI rats were observed with magnetic resonance imaging (MRI) through coronal scanning.
MRI is a non-invasive imaging technique that can produce three-dimensional detailed anatomical images. It has become the preferred examination for SCI patients to evaluate the injury location and severity of the spinal cord and nerve roots by showing displaced fragments of damaged disks and ligaments in the spinal canal and edema and/or bleeding2,21,22. Evaluation of MRI was performed on day 4 after surgery. MR imaging data acquisition was obtained at the Tianjin Medical University General Hospital using a Signa3.0T research MRI scanner (Signa, GE Healthcare, Milwaukee, WI, USA) and animal phased array coils, where the imaging took T10 center as the core and scanned four layers back and forth. Each layer was 1 mm in thickness, and a total of nine layers were scanned. The imaging range covered the spinal cord T9-11. Sagittal and horizontal spin-spin relaxation time T2-WI image scanning was also carried out. Sagittal spin-spin relaxation time T2-WI image scanning parameters: TR/TE = 3338/74 ms; FOV = 80 mm × 80 mm; matrix = 256 × 256; NEX = 3; slice thickness = 1 mm; slice gap = 0.5 mm. Horizontal spin-spin relaxation time T2-WI image scanning parameters: TR/TE =2967/83 ms; FOV = 60 mm × 60 mm; matrix = 256 × 256; NEX = 3; slice thickness = 1 mm; slice gap = 0.5 mm.
Evaluation criteria for spinal cord lesions were as follows: the high signal area on T2-WI that can be distinguished by the naked eye. Dcm2niigui and Mricron (ver.12122012) software was used to determine the total lesion volume, spinal cord volume, and the percentage of total lesion volume to spinal cord volume.
Histology and Immunofluorescence
Hematoxylin and eosin (H&E) staining and immunohistochemistry was used to observe the tissue damage and neuron regeneration of spinal cord tissue. Luminex multiplex immunoassay was used to evaluate the expression of serum inflammatory cytokines.
Four weeks after hUC-MSCs injection, all rats were anesthetized and then killed. Spinal cord tissues were dissected and fixed with 10% neutral formaldehyde solution over 24 h, embedded in paraffin, and sectioned for H&E staining to analyze the extent of spinal tissue degradation, glial scars, and hollow tissues after spinal cord injury. Assessment of white/gray matter tissue sparing after SCI in cross-section of spinal cord tissue was performed with ImageJ software according to the previous report18. Basically, H&E images were inverted and the regions of interest were manually selected and added to the Tool-ROI manager. The selected area and mean grayscale were then measured in the ROI manager. The mean area of each spinal section was compared with the control group.
For immunofluorescence staining, the paraffin sections of spinal cord tissue were deparaffinized with xylene, rehydrated with gradient alcohol, antigen retrieval, and then blocked with 5% normal goat serum for 1 h at RT. Next, the sections were incubated with diluted primary antibodies (Table 1) overnight at 4 °C, after which they were incubated with corresponding fluorescent secondary antibodies at room temperature for 30 min. After washing in PBS, the sections were covered using the mounting medium with DAPI (Vectorlabs, Burlingame, CA, USA). Imaging was performed utilizing Synergy LX Multi-Mode Microplate Reader (Biotek Inc, Layton, UT, USA), and immunofluorescence digital analysis was evaluated with ImageJ software.
Table 1.
Primary and Secondary Antibody Information for Immunofluorescence.
| Primary ab. | Supplier | S.N. | Source | Dil. | Secondary ab. | Supplier | S.N. | Dil. |
|---|---|---|---|---|---|---|---|---|
| Anti-NF-200 | CST | 55453s | Rabbit | 1:100 | Goat anti-rabbit IgG (Alexa Fluor® 488) | CST | 4412s | 1:2000 |
| Anti-SYP (7H12) | CST | 9020s | Mouse | 1:200 | Goat anti-mouse IgG (Alexa Fluor® 594) | CST | 8890s | 1:2000 |
| Anti-CDH | Abcam | ab16505 | Rabbit | 1:500 | Goat anti-rabbit IgG (Alexa Fluor® 488) | CST | 4412s | 1:2000 |
| Anti-ICAM1 | Abcam | ab171123 | Mouse | 1:500 | Goat anti-mouse IgG (Alexa Fluor® 594) | CST | 8890s | 1:2000 |
Ab.: antibody; S.N.: serial number; Dil.: dilution; NF-200: neurofilament-H; IgG: Immunoglobulin G; SYP: synaptophysin; CDH: pan-Cadherin; ICAM-1: intercellular adhesion molecule-1.
The information of primary antibodies includes Neurofilament-H antibody (NF-200, CST Inc, Boston, MA, USA), Synaptophysin (SYP, CST Inc), pan-Cadherin (CDH, Abcam Inc, Cambridge, MA, USA), and intercellular adhesion molecule-1 (ICAM-1, Abcam Inc). The corresponding secondary bodies are described in Table 1.
Cytokine Assay
The serum cytokines of rats were analyzed at 1, 3, and 7 days after hUC-MSCs injection. About 200 μl blood was collected through intraocular canthus from each rat, and then centrifuged at 7500 RPM (Thermo Scientific Legend Microro 17R, Rotor.75003424) for 15 min to get serum. The serum was prepared with coagulant tubes centrifugation. All the serum was stored in −80°C and was performed with immunoassay to measure cytokine levels later. The serum multiplex immunoassays were performed to measure the pro-inflammatory factors (tumor necrosis factor alpha [TNF-α], interferon gamma [IFN-γ], interleukin [IL]-1β, IL-6) in the serum of each group at 1, 3, and 7 days after UC-MSCs injection, using MILLIPLEX MAP Rat Cytokine/Chemokine Magnetic Bead Panel kit # RECYTMAG-65K-04 (Millipore, Saint Louis, MO, USA), based on the xMAP Luminex technology. ELISA assays were performed to measure the anti-inflammatory factors (IL-10 and IL-13) using the rat IL-10 (ab214566, Abcam, Inc) and IL-13 ELISA kit (ab269547, Abcam, Inc), according to the manufacturer’s instructions.
Statistical Analysis
Experiments were blinded to the group identity of specimens for all quantification procedures, including BBB, MRI, cytokine assay, immunofluorescence, and pathological quantitative analysis. The software Graphpad prism 9.0 was used for statistical analysis and making graphs. The data were expressed as mean ± standard error of the mean (SEM). The Shapiro–Wilk test was used to check the normal distribution of data (P > 0.05 was considered as conforming a normal distribution). MRI, histopathological quantitation, and immunofluorescence data were analyzed by one-way analysis of variance (ANOVA) with post hoc Dunnett’s test (P < 0.05 was considered a significant difference) in which Brown-Forsythe test was used to check variance homogeneity. BBB score was analyzed by two-way ANOVA (P < 0.05 was considered a significant difference). Dunnett’s test was performed to compare treatment difference. Cytokine levels were analyzed by mixed-effects model with post hoc Dunnett’s test (P < 0.05 was considered a significant difference).
Results
Infusion of hUC-MSCs Improved Locomotor Function
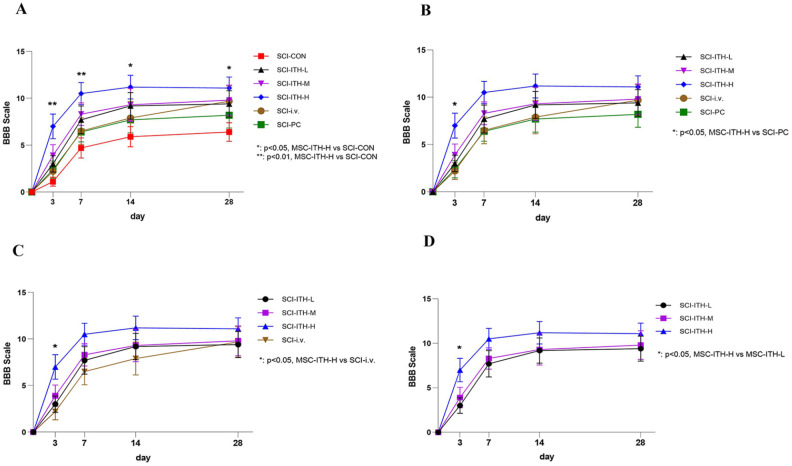
The hind limb locomotor function was assessed using the BBB behavioral score immediately prior to and 1, 3, 7, 14, and 28 days following MSCs or vehicle infusion for confirmation of injury equivalency in SCI rats and to determine whether infused MSCs influenced overall locomotor ability. Normal locomotor function in rats of the sham operation group had a BBB score of 21, while the rats after SCI surgery had a complete lack of motor function with a BBB score of 0, which indicated that the model was successful. Among three doses of hUC-MSC through intrathecal injection, only the high-dose hUC-MSC (10×106 cells/Kg) could significantly improve the BBB score on days 3, 7, 14, and 28, compared with negative control (P < 0.05–0.01) (Fig. 1A, D, Table S1). The high dose of hUC-MSCs delivered through ITH increased BBB scores beyond methylprednisolone reagent or hUC-MSC (20 × 106 cells/kg) delivered through intravenous injection (P > 0.05) (Fig. 1B, C, Table S1). The exact mean ± SEM at all timepoints is shown in Table 2.
Figure 1.
BBB scoring of rats in each SCI group. Comparison of BBB scoring of rats in each SCI group, including UC-MSCs treatment, methylprednisolone treatment, or non-treatment (n = 10, mean ± SEM) through two-way analysis of variance. P < 0.05 was considered significantly different. (A) BBB comparison of SCI treatment and non-treatment. Dunnett’s post hoc comparison. *P < 0.05, MSC-ITH-H vs SCI-CON. **P < 0.01, MSC-ITH-H vs SCI-CON. (B) BBB comparison of MSCs treatment and methylprednisolone. Dunnett’s post hoc comparison. *P < 0.05, MSC-ITH-H vs SCI-PC. (C) BBB comparison of MSC ITH and MSC i.v. Dunnett’s post hoc comparison. *P < 0.05, MSC-ITH-H vs SCI-i.v. (D) BBB comparison of different-dose MSC ITH. Dunnett’s post hoc comparison. *P < 0.05, MSC-ITH-H vs MSC-ITH-L. BBB: Basso Beattie Bresnahan; SCI: spinal cord injury; UC-MSCs: human umbilical cord-derived mesenchymal stem/stromal cells; SEM: standard error of the mean; MSC: mesenchymal stem/stromal cell; SCI-CON: spinal cord injury rat without treatment; SCI-PC: spinal cord injury rat with methylprednisolone treatment; MSC-i.v. or SCI-i.v.: spinal cord injury rat with UC-MSCs intravenous injection; MSC-ITH-L/M/H or SCI-ITH-L/M/H: spinal cord injury rat with low, medium, or high dose of UC-MSCs intrathecal injection.
Table 2.
Influence of hUC-MSCs Treatments on BBB Scoring (M ± SD, n = 10).
| Groups | −1 day (prior to SCI) | 1 day (prior to MSC) | 4 days (3 days after MSC) | 8 days (7 days after MSC) | 15 days (14 days after MSC) | 29 days (28 days after MSC) |
|---|---|---|---|---|---|---|
| SO-CON | 20.8 ± 0.1 | 20.8 ± 0.1 | 20.7 ± 0.2 | 20.9 ± 0.1 | 21.0 ± 0.0 | 21.0 ± 0.0 |
| SCI-CON | 20.9 ± 0.1 | 0.0 ± 0.0 | 1.1 ± 0.5 | 4.7 ± 1.1 | 5.9 ± 1.1 | 6.4 ± 1.0 |
| SCI-ITH-L | 21.0 ± 0.0 | 0.0 ± 0.0 | 3 ± 0.9 | 7.7 ± 1.5 | 9.2 ± 1.4 | 9.4 ± 1.4 |
| SCI-ITH-M | 21.0 ± 0.0 | 0.0 ± 0.0 | 3.9 ± 1.2 | 8.3 ± 1.2 | 9.3 ± 1.7 | 9.8 ± 1.6 |
| SCI-ITH-H | 21.0 ± 0.0 | 0.0 ± 0.0 | 7.0 ± 1.3 | 10.5 ± 1.2 | 11.2 ± 1.3 | 11.1 ± 1.2 |
| SCI-i.v. | 20.9 ± 0.1 | 0.0 ± 0.0 | 2.2 ± 0.9 | 6.5 ± 1.4 | 7.9 ± 1.8 | 9.7 ± 1.6 |
| SCI-PC | 21.0 ± 0.0 | 0.0 ± 0.0 | 2.4 ± 0.9 | 6.4 ± 1.1 | 7.7 ± 1.4 | 8.2 ± 1.4 |
hUC-MSCs: human umbilical cord-derived mesenchymal stem/stromal cells; BBB: Basso Beattie Bresnahan; SCI: spinal cord injury; MSC: mesenchymal stem/stromal cell; SO-CON: sham operation control; SCI-CON: spinal cord injury rat without treatment; SCI-ITH-L/M/H: spinal cord injury rat with low, medium, or high dose of UC-MSCs intrathecal injection; SCI-i.v.: spinal cord injury rat with UC-MSCs intravenous injection; SCI-PC: spinal cord injury rat with methylprednisolone treatment.
Infusion of hUC-MSCs Reduced the Extent of Spinal Cord Lesions
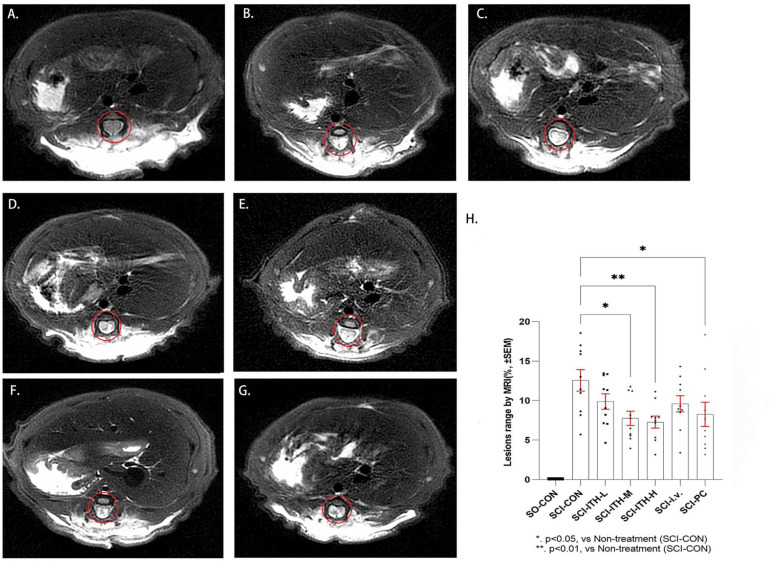
MR imaging showed obvious spinal cord injury in the SCI rats 4 days after surgery (3 days after UC-MSC infusion). After ITH delivery of 5 × 106 cells/kg or 10 × 106 cells/kg hUC-MSCs for 3 days, the extent of spinal cord lesions was significantly reduced, and the improvement rates were 38.3% (P < 0.01) and 42.1% (P < 0.01), respectively (Fig. 2H, Tables 3 and S2). A total of 20 × 106cells/kg hUC-MSCs through i.v. delivery reduced the extent of spinal cord lesions, achieving an improvement rate of 23.6% (Table 3). The positive control (methylprednisolone reagent) also significantly reduced the extent of spinal cord lesions by 34.3% (P < 0.05) [Fig. 2H, Tables 3 and S2). These results are consistent with the observed BBB scores.
Figure 2.
MRI of rats in each SCI group. Comparison of spinal cord lesions by MRI in each SCI group, including hUC-MSCs treatment, methylprednisolone treatment, or non-treatment (n = 10, mean ± SEM). All the lesions were imaged at T10. The red circle represents the lesion. (A) Sham operation control group (SO-CON). (B) Spinal cord injury rat without treatment (SCI-CON). (C-E) Spinal cord injury rat with a low, medium, or high dose of hUC-MSCs intrathecal injection (SCI-ITH-L/M/H). (F) Spinal cord injury rat with hUC-MSCs intravenous injection (SCI-i.v.). (G) Spinal cord injury rat with methylprednisolone treatment (SCI-PC). (H) Comparison of MRI between the treatment groups and the control group through one-way analysis of variance and post hoc Dunnett’s test. *P < 0.05, vs SCI-CON. **P < 0.01, vs SCI-CON. MRI: magnetic resonance imaging; SCI: spinal cord injury; hUC-MSCs: human umbilical cord-derived mesenchymal stem/stromal cells; SEM: standard error of the mean.
Table 3.
Influence of hUC-MSCs Infused on the Spinal Cord Lesions (M ± SD, n = 10).
| Groups | Extent of spinal cord lesions (%) |
Recovery rat (%) |
|---|---|---|
| SO-CON | 0.00 ± 0.00 | — |
| SCI-CON | 12.57 ± 1.40 | — |
| SCI-ITH-L | 9.89 ± 1.00 | 21.3 |
| SCI-ITH-M | 7.76 ± 0.90 | 38.3 |
| SCI-ITH-H | 7.28 ± 0.80 | 42.1 |
| SCI-i.v. | 9.60 ± 1.00 | 23.6 |
| SCI-PC | 8.26 ± 1.50 | 34.3 |
hUC-MSCs: human umbilical cord-derived mesenchymal stem/stromal cells; SO-CON: sham operation control; SCI-CON: spinal cord injury rat without treatment; SCI-ITH-L/M/H: spinal cord injury rat with low, medium or high dose of hUC-MSCs intrathecal injection; SCI-i.v.: spinal cord injury rat with hUC-MSCs intravenous injection; SCI-PC: spinal cord injury rat with methylprednisolone treatment.
Histopathological Changes After SCI
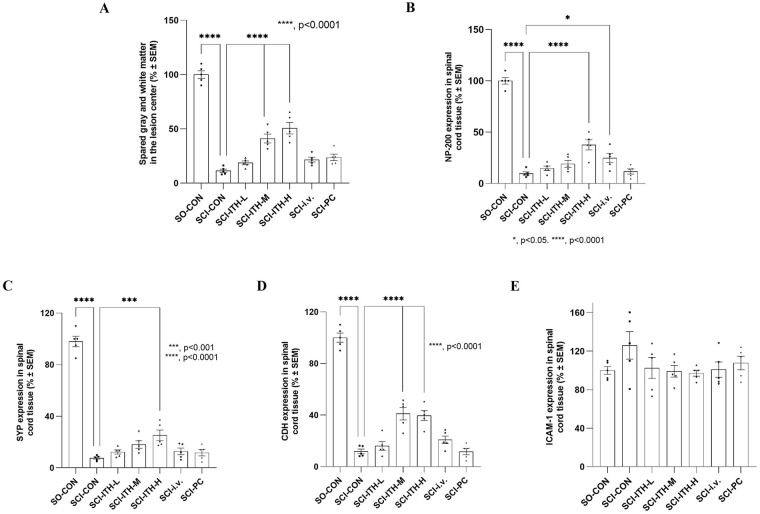
Histopathological staining of spinal cord tissues revealed irregular spinal cord tissue, destruction of dorsal white matter and central gray matter tissue, more necrosis, and obvious hyperplasia of collagen scars in the SCI group after 29 days of surgery (Figs. 3 and 4). Compared with the SCI group, hUC-MSCs ITH-M and ITH-H group showed a protective effect with significantly increased gray matter area of the spinal cord (P < 0.0001), and reduced cavity area at the main lesion site, with less necrosis and fewer infiltrated polymorphonuclear leukocytes (Figs. 3A and 4, Table S3).
Figure 3.
Histopathological and immunofluorescence analyses of the spinal cord in each group. The histopathological and immunofluorescence quantitative analysis of spinal cord tissues with ImageJ software in each group, including SO-CON, SCI control, hUC-MSCs treatment, and methylprednisolone treatment. The arithmetical mean of each spinal section and mean of quantified immunofluorescence were compared with the SCI control group through one-way analysis of variance and post hoc Dunnett’s test (100%). (N = 5, mean ± SEM). (A) Morphometry measurement of the spinal cord lesion of spared white and gray matter in the lesion center by H&E staining. (B-E) Expression of NF-200 (B), the synapse markers SYP (C), pan-Cadherin (D), and ICAM-1 (E) in spinal cord tissue by immunofluorescence staining. *P < 0.05, vs SCI-CON; ***P < 0.001, vs SCI-CON; ****P < 0.0001, vs SCI-CON. SO: sham operation; SO-CON: sham operation control; SCI: spinal cord injury; hUC-MSCs: human umbilical cord-derived mesenchymal stem/stromal cells; H&E: hematoxylin and eosin; NF-200: neurofilament-H; SYP: synaptophysin; ICAM-1: intercellular adhesion molecule-1; SEM: standard error of the mean; SCI-CON: spinal cord injury rat without treatment; SCI-ITH-L/M/H: spinal cord injury rat with low, medium or high dose of hUC-MSCs intrathecal injection; SCI-i.v.: spinal cord injury rat with UC-MSCs intravenous injection; SCI-PC: spinal cord injury rat with methylprednisolone treatment.
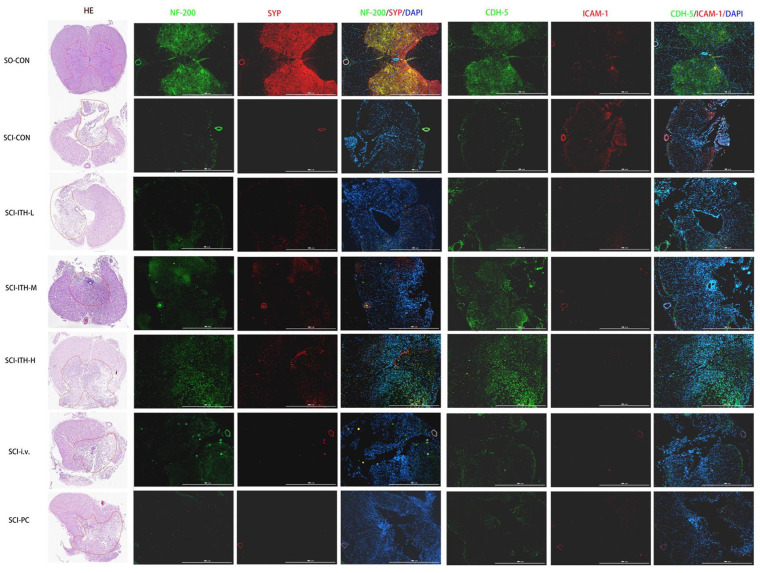
Figure 4.
Histopathologic and immunofluorescence staining of the spinal cord in each group. H&E and immunofluorescence staining of spinal cord tissues in each group, including SO-CON, SCI control, hUC-MSCs treatment, and methylprednisolone treatment. For immunofluorescence staining, the paraffin sections of spinal cord tissue were labeled with Neurofilament-H antibody (NF-200, green), Synaptophysin antibody (SYP, red), pan-Cadherin antibody (CDH, green), and intercellular adhesion molecule-1antibody (ICAM-1 red). H&E: hematoxylin and eosin; SO-CON: sham operation control; SCI: spinal cord injury; hUC-MSCs: human umbilical cord-derived mesenchymal stem/stromal cells; NF-200: neurofilament-H; SYP: synaptophysin; CDH: pan-Cadherin; ICAM-1: intercellular adhesion molecule-1; SCI-CON: spinal cord injury rat without treatment; SCI-ITH-L/M/H: spinal cord injury rat with low, medium, or high dose of hUC-MSCs intrathecal injection; SCI-i.v.: spinal cord injury rat with UC-MSCs intravenous injection; SCI-PC: spinal cord injury rat with methylprednisolone treatment.
To confirm the neuroregeneration and neuroprotective effect of hUC-MSCs infused in SCI rats, the immunofluorescence staining of mature neuron marker NF-200, the synapse markers, synaptophysin (SYP), and pan-Cadherin in the spinal cord were also detected after treatment. The results revealed that compared with the SO group, the expression level of the NF-200, SYP, and cadherin in the spinal gray matter of the SCI rats were significantly reduced (P < 0.01) (Figs. 3B–D and 4, Table S4). hUC-MSCs ITH-M, hUC-MSCs ITH-H, and hUC-MSCs i.v. treatments promoted these protein levels in SCI rats (Figs. 3B–D and 4, Table S4). ICAM-1 expression was upregulated in SCI models. A downregulation of ICAM-1 was observed after MSCs treatments or methylprednisolone treatment, but there was no significant difference (Figs. 3E and 4, Table S4).
Effect of hUC-MSCs on Serum Cytokines in SCI Rats
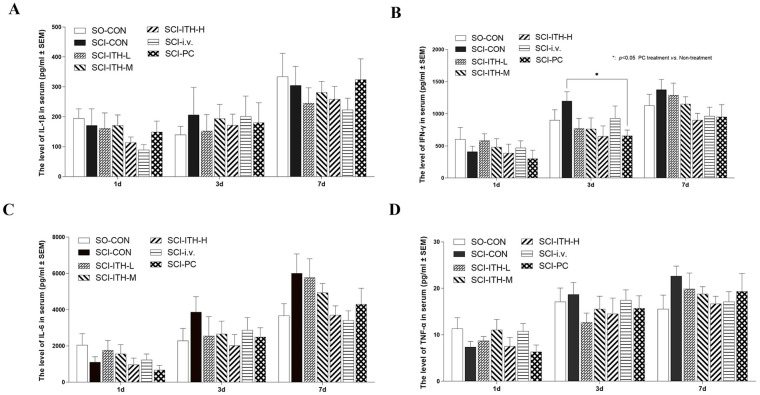
Microglia and macrophages are recruited and release pro-inflammatory cytokines in the early stage of SCI, which, in turn, cause cell death and tissue degeneration following injury. The results of multiplex immunoassays showed that after surgery, the serum levels of the inflammatory cytokines IFN-γ, TNF-α, IL-6, and IL-1β increased over time, whereas administration of hUC-MSCs or methylprednisolone repressed the increasing, although there was no significant difference (Fig. 5A–D, Table S5). Before and after surgery, IL-10 and IL-13 levels were not affected. Neither of them was influenced by the administration of hUC-MSCs or methylprednisolone (P > 0.05, Fig. S4, Table S2).
Figure 5.
Serum cytokines in each SCI group. The serum cytokines including (A) IL-1β, (B) IFN-γ, (C) IL-6, (D) TNF-α, in each SCI group including hUC-MSCs treatment, methylprednisolone treatment, or non-treatment (n = 10, mean ± SEM). As for missing values, data were analyzed through a mixed-effects model, instead of two-way analysis of variance, with a post hoc Dunnett’s test. *P < 0.05, vs SCI-CON. SCI: spinal cord injury; IL: interleukin; IFN-γ: interferon gamma; TNF-α: tumor necrosis factor alpha; hUC-MSCs: human umbilical cord-derived mesenchymal stem/stromal cells; SEM: standard error of the mean; SCI-CON: spinal cord injury rat without treatment; SO-CON: sham operation control; SCI-ITH-L/M/H: spinal cord injury rat with low, medium, or high dose of UC-MSCs intrathecal injection; SCI-i.v.: spinal cord injury rat with UC-MSCs intravenous injection; SCI-PC: spinal cord injury rat with methylprednisolone treatment.
Discussion
Mesenchymal stem cell transplantation strategies offer great promise for functional recovery after spinal cord injury23–25. Existing studies have shown that the efficacy of MSCs is mainly based on anti-inflammatory and neuroprotective paracrine secretion functions in the absence of significant side effects, such as secreting immunomodulation factors, neurotropic growth, and neuroprotective factors, which support neuron survival, neuroregeneration, axonal regeneration, and reduction of glial scarring, rather than differentiation into specialized neuronal and glial cell lineages13,26–28. The clinical translation strategies of MSCs in SCI treatment require a safe and efficient means of cellular delivery and optimal dosage. While systemic and local administration have been investigated as the routes of MSC delivery, intrathecal delivery of MSCs was identified as an alternative to direct injection and led to accumulation of stem cells15,29. The optimal route of delivery and dosage have not yet been determined. This study aimed to estimate the dose of the applied hUC-MSCs (intrathecal injection, ITH groups) and compare the intravenous (i.v.) and intrathecal (ITH) administration on functional recovery and tissue repair in SCI rats.
The hind-limb locomotor function was assessed using the behavioral BBB, the evaluation method most commonly used to assess the recovery of hind-limb motor function after SCI, and the average score of the hind limbs was calculated30,31. Before SCI modeling, all rats had a BBB score of 21, while all the spinal injured rats lost their motor function immediately after SCI modeling (BBB score = 0) and displayed dosage-dependent neuronal functional restoration following UC-MSC treatment. Interestingly, compared with positive drugs and hUC-MSC i.v., the SCI rats with a high dose of MSC treatment through ITH delivery revealed a more significant improvement.
The MRI of spinal cord lesions showed similar results. MRI is a commonly used method for the diagnosis of SCI and relevant disease32. The necrotic spinal cavity shows an abnormally increased signal, which can be seen in both acquisition planes with cell destruction and necrosis33. MR imaging showed obvious spinal cord injury in the SCI rats 4 days after surgery (3 days after hUC-MSC infusion) and also displayed dosage-dependent spinal cord lesions reduction after ITH delivery hUC-MSCs, with the improvement rates of 42.1% in the high-dose group. In contrast, the intravenous infusion group did not show a significant therapeutic effect.
Analyses of histopathologic staining and immunofluorescence showed that ITH transplantation of hUC-MSCs was beneficial in promoting nerve tissue repair, especially at high dose, facilitating axonal sprouting, supporting the increased gray matter area, and decreasing the cavity area of the main lesion and necrosis. All these findings were consistent with behavioral and MRI analysis. A significant effect of dorsal white matter and central gray matter was observed in animals treated by ITH delivery of medium and high dosages of hUC-MSCs, which revealed a strong neuroprotective effect in spinal cord tissues. At the end of the experiment, we dissected rats and found the spinal cord in rats with hUC-MSCs ITH-H treatment looked healthier than SCI, low dosage of ITH and i.v. treatment (Fig. S3). The same trend was observed in NF-200 and SYP expression. The immunofluorescence staining of NF-200 and SYP represented the presence of neurons in the original site of trauma. Compared with the SCI-CON group, the NF-200 and SYP in the hUC-MSCs ITH delivery groups revealed a dosage-dependent increase, suggesting more neuronal activities happening and consistent with the previous studies9,34. The effect of hUC-MSCs is partially due to increased cadherin expression and a decrease of ICAM-1 in the injured spinal cord tissue. As a calcium-dependent cell adhesion molecule, cadherin has an important role in cell growth and development by controlling tissue architecture and maintaining tissue integrity. Also, cadherin activity is essential for axon-dendritic spine contact, synaptic plasticity, and rearrangement35,36. Neuronal cells in the gray matter of the spinal cord exhibited significant positive results of pan-cadherin in the medium and high dosage of hUC-MSCs (ITH delivered) treated rats, which was consistent with quantitation results. Mechanical contusion of the spinal cord causes the endothelial upregulation of ICAM-1 that facilitates adhesion and extravasation of leukocytes37, which is consistent with our findings.
Neuroinflammation, which is considered a therapeutic target of SCI, occurs almost immediately after spinal cord injury. It is the major pathogenesis of the SCI secondary injury phase that causes cell death and tissue degeneration38. Mesenchymal stem cells exert immunomodulatory and anti-apoptotic effects and secrete anti-inflammatory factors and growth factors to inhibit the inflammatory reaction and improve self-repair of lesion microenvironment after SCI39. Our results displayed that the pro-inflammatory cytokines IL-1β, IFN-γ, IL-6, and TNF-α in serum increased after SCI surgery (Fig. 5). After MSC-ITH-H treatment for 3 and 7 days, increase of IFN-γ, IL-6, and TNF-α tended to decrease (P > 0.05), but only methylprednisolone showed significant repression of IFN-γ after 3 days of treatment, compared with SCI-CON (P < 0.05).
The presence of injury and dose of cells affect cell migration. When we intrathecally injected cells, we could see cell signals in the lesion of the SCI group, instead of the SO group, suggesting injury-induced cell homing. Furthermore, the homing was dosage-dependent, because migration could only be seen in high dose of cell injection (Fig. S4). The method of injection also affected homing efficiency. When we intravenously injected cells in SCI rats, although more cells were injected (20 × 106 cells/kg vs 10 × 106 cells/kg, i.v. vs ITH-H), no cell signal was found in the lesion (Fig. S3). Quantitative polymerase chain reaction was done to measure if any human COX-1 gene in rat liver, spleen, lung, and thymus after killing, yet no signal could be detected in all groups, suggesting no integration or survival of hUC-MSCs (data not shown).
The method of cell administration showed differences in treatment efficiency. Compared with i.v., the ITH-H group obtained better performance in BBB rating, MRI, H&E staining, and spinal cord dissection, suggesting improved recovery. The autocrine and paracrine effects of MSCs for inflammation regulation might not be influenced by ITH or i.v. Yet, given that ITH-H was more effective at mitigating injury than i.v., this could be because ITH-delivered hUC-MSCs migrated to SCI and relieved local inflammation or regulated nerve cells near the injury. More research should be performed to compare hUC-MSCs migration after different infusion methods. Based on our observation, we think that ITH infusion of cells would be a more suitable means to treat central nervous system diseases that require control of inflammatory cytokines or tissue repair. ITH infusion also reduced the amount of cells required for transplantation. Of course, the effect of distance between infusion site and locus of injury requires further exploration.
Conclusion
These results demonstrate that hUC-MSC can mitigate SCI in rats, and the therapeutic effect was dose-dependent for hUC-MSCs intrathecal treatment.
Supplemental Material
Supplemental material, sj-docx-1-cll-10.1177_09636897221139734 for Therapeutic Efficacy of Human Mesenchymal Stem Cells With Different Delivery Route and Dosages in Rat Models of Spinal Cord Injury by Guangyang Liu, Zhiling Zhao, Herui Wang, Chunhua Hao, Weiting Wang, Chenliang Zhang, Tiehua Wang, Xin Li, Jingjing Xi, Shaoyun Li, Haomiao Long, Yi Mi, Li Miao, Yaoyao Chen, Liqiang Xu, Libo Zheng, Hao Wang, Ning Ding, Fengmei Zhu, Qinggang Ge and Yongjun Liu in Cell Transplantation
Acknowledgments
The authors are grateful to other members of the Tianjin Institute of Pharmaceutical Research for their technical help and support.
Footnotes
Ethical Approval: This research was approved by Hospital Ethics Committee and the IACUC of Tianjin Institute of Pharmaceutical Research (IACUC ID is 2020123002).
Statement of Human and Animal Rights: All procedures for animal experiments were approved by the IACUC of Tianjin Institute of Pharmaceutical Research (IACUC ID is 2020123002).
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Guangyang Liu  https://orcid.org/0000-0002-8474-7106
https://orcid.org/0000-0002-8474-7106
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Luo L, Albashari AA, Wang X, Jin L, Zhang Y, Zheng L, Xia J, Xu H, Zhao Y, Xiao J, He Y, et al. Effects of transplanted heparin-poloxamer hydrogel combining dental pulp stem cells and bFGF on spinal cord injury repair. Stem Cells Int. 2018;2018:2398521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang H, Young W, Skaper S, Chen L, Moviglia G, Saberi H, Al-Zoubi Z, Sharma HS, Muresanu D, Sharma A, El Masry W, et al. Clinical neurorestorative therapeutic guidelines for spinal cord injury (IANR/CANR version 2019). J Orthop Translat. 2020;20:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shu J, Cheng F, Gong Z, Ying L, Wang C, Yu C, Zhou X, Xiao M, Wang J, Xia K, Huang X, et al. Transplantation strategies for spinal cord injury based on microenvironment modulation. Curr Stem Cell Res Ther. 2020;15(6):522–30. [DOI] [PubMed] [Google Scholar]
- 4. Fan B, Wei Z, Yao X, Shi G, Cheng X, Zhou X, Zhou H, Ning G, Kong X, Feng S. Microenvironment imbalance of spinal cord injury. Cell Transplant. 2018;27(6):853–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moinuddin FM, Yolcu YU, Wahood W, Siddiqui AM, Chen BK, Alvi MA, Goyal A, Nesbitt JJ, Windebank AJ, Yeh JC, Petrucci K, et al. Early and sustained improvements in motor function in rats after infusion of allogeneic umbilical cord-derived mesenchymal stem cells following spinal cord injury. Spinal Cord. 2021;59(3):319–27. [DOI] [PubMed] [Google Scholar]
- 6. Zhou H-L, Fang H, Luo H-T, Ye M-H, Yu G-Y, Zhang Y, Mao G-H, Gao Z-Y, Cheng Z-J, Zhu X-G. Intravenous administration of human amniotic mesenchymal stem cells improves outcomes in rats with acute traumatic spinal cord injury. Neuroreport. 2020;31(10):730–36. [DOI] [PubMed] [Google Scholar]
- 7. Ruzicka J, Urdzikova LM, Kloudova A, Amin AG, Vallova J, Kubinova S, Schmidt MH, Jhanwar-Uniyal M, Jendelova P. Anti-inflammatory compound curcumin and mesenchymal stem cells in the treatment of spinal cord injury in rats. Acta Neurobiol Exp. 2018;78:358–74. [PubMed] [Google Scholar]
- 8. Lin L, Lin H, Bai S, Zheng L, Zhang X. Bone marrow mesenchymal stem cells (BMSCs) improved functional recovery of spinal cord injury partly by promoting axonal regeneration. Neurochem Int. 2018;115:80–84. [DOI] [PubMed] [Google Scholar]
- 9. Krupa P, Vackova I, Ruzicka J, Zaviskova KA-O, Dubisova J, Koci Z, Turnovcova K, Urdzikova LM, Kubinova S, Rehak S, Jendelova P. The effect of human mesenchymal stem cells derived from Wharton’s jelly in spinal cord injury treatment is dose-dependent and can be facilitated by repeated application. Int J Mol Sci. 2018;19:1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yin F, Meng C, Lu R, Li L, Zhang Y, Chen H, Qin Y, Guo L. Bone marrow mesenchymal stem cells repair spinal cord ischemia/reperfusion injury by promoting axonal growth and anti-autophagy. Neural Regen Res. 2014;9:1665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Samsonraj RM, Raghunath M, Nurcombe V, Hui JH, van Wijnen AJ, Cool SM. Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl Med. 2017;6(12):2173–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Honmou O, Yamashita T, Morita T, Oshigiri T, Hirota R, Iyama S, Kato J, Sasaki Y, Ishiai S, Ito YM, Namioka A, et al. Intravenous infusion of auto serum-expanded autologous mesenchymal stem cells in spinal cord injury patients: 13 case series. Clin Neurol Neurosurg. 2021;203:106565. [DOI] [PubMed] [Google Scholar]
- 13. Cofano F, Boido M, Monticelli M, Zenga F, Ducati A, Vercelli A, Garbossa D. Mesenchymal stem cells for spinal cord injury: current options, limitations, and future of cell therapy. Int J Mol Sci. 2019;20(11):2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oliveri RS, Bello S, Biering-Sørensen F. Mesenchymal stem cells improve locomotor recovery in traumatic spinal cord injury: systematic review with meta-analyses of rat models. Neurobiol Dis. 2014;62:338–53. [DOI] [PubMed] [Google Scholar]
- 15. Paul C, Samdani AF, Betz RR, Fischer I, Neuhuber B. Grafting of human bone marrow stromal cells into spinal cord injury: a comparison of delivery methods. Spine. 2009;34(4):328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li S, Zhou J, Zhang J, Wang D, Ma J. Construction of rat spinal cord injury model based on Allen’s animal model. Saudi J Biol Sci. 2019;26(8):2122–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu G, Di Z, Hao C, Wang W, Pei T, Zheng L, Long H, Wang H, Liao W, Wang W, Zhang C, et al. Effects of different concentrations of mesenchymal stem cells treatment on LPS-induced acute respiratory distress syndrome rat model. Exp Lung Res. 2021;47(5):226–38. [DOI] [PubMed] [Google Scholar]
- 18. Urdzíková LM, Růžička J, LaBagnara M, Kárová K, Kubinová Š, Jiráková K, Murali R, Syková E, Jhanwar-Uniyal M, Jendelová P. Human mesenchymal stem cells modulate inflammatory cytokines after spinal cord injury in rat. Int J Mol Sci. 2014;15(7):11275–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bakshi A, Hunter C, Swanger S, Lepore A, Fischer I. Minimally invasive delivery of stem cells for spinal cord injury: advantages of the lumbar puncture technique. J Neurosurg Spine. 2004;1(3):330–37. [DOI] [PubMed] [Google Scholar]
- 20. Basso DM, Beattie MS, Bresnahan JC. Descending systems contributing to locomotor recovery after mild or moderate spinal cord injury in rats: experimental evidence and a review of literature. Restor Neurol Neurosci. 2002;20(5):189–218. [PubMed] [Google Scholar]
- 21. Freund P, Seif M, Weiskopf N, Friston K, Fehlings MG, Thompson AJ, Curt A. MRI in traumatic spinal cord injury: from clinical assessment to neuroimaging biomarkers. Lancet Neurol. 2019;18(12):1123–35. [DOI] [PubMed] [Google Scholar]
- 22. Lammertse D, Dungan D, Dreisbach J, Falci S, Flanders A, Marino R, Schwartz E. Neuroimaging in traumatic spinal cord injury: an evidence-based review for clinical practice and research. J Spinal Cord Med. 2007;30(3):205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D, Smithson LJ, Kawaja MD, et al. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. 2011;28(8):1611–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schultz SS. Adult stem cell application in spinal cord injury. Curr Drug Targets. 2005;6(1):63–73. [DOI] [PubMed] [Google Scholar]
- 25. Melo FR, Bressan RB, Forner S, Martini AC, Rode M, Delben PB, Rae GA, Figueiredo CP, Trentin AG. Transplantation of human skin-derived mesenchymal stromal cells improves locomotor recovery after spinal cord injury in rats. Cell Mol Neurobiol. 2017;37(5):941–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qu J, Zhang H. Roles of mesenchymal stem cells in spinal cord injury. Stem Cells Int. 2017;2017:5251313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chung HJ, Chung WH, Lee JH, Chung DJ, Yang WJ, Lee AJ, Choi CB, Chang HS, Kim DH, Suh HJ, Lee DH, et al. Expression of neurotrophic factors in injured spinal cord after transplantation of human-umbilical cord blood stem cells in rats. J Vet Sci. 2016;17(1):97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spejo AB, Carvalho JL, Goes AM, Oliveira AL. Neuroprotective effects of mesenchymal stem cells on spinal motoneurons following ventral root axotomy: synapse stability and axonal regeneration. Neuroscience. 2013;250:715–32. [DOI] [PubMed] [Google Scholar]
- 29. Shin DA, Kim JM, Kim HI, Yi S, Ha Y, Yoon DH, Kim KN. Comparison of functional and histological outcomes after intralesional, intracisternal, and intravenous transplantation of human bone marrow-derived mesenchymal stromal cells in a rat model of spinal cord injury. Acta Neurochir (Wien). 2013;155(10):1943–50. [DOI] [PubMed] [Google Scholar]
- 30. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12(1):1–21. [DOI] [PubMed] [Google Scholar]
- 31. Song RB, Basso DM, da Costa RC, Fisher LC, Mo X, Moore SA. Adaptation of the Basso-Beattie-Bresnahan locomotor rating scale for use in a clinical model of spinal cord injury in dogs. J Neurosci Methods. 2016;268:117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boldin C, Raith J, Fankhauser F, Haunschmid C, Schwantzer G, Schweighofer F. Predicting neurologic recovery in cervical spinal cord injury with postoperative MR imaging. Spine. 2006;31(5):554–59. [DOI] [PubMed] [Google Scholar]
- 33. Schwartz ED, Yezierski RP, Pattany PM, Quencer RM, Weaver RG. Diffusion-weighted MR imaging in a rat model of syringomyelia after excitotoxic spinal cord injury. AJNR Am J Neuroradiol. 1999;20(8):1422–28. [PMC free article] [PubMed] [Google Scholar]
- 34. Li C, Chen X, Qiao S, Liu X, Liu C, Zhu D, Su J, Wang Z. Effects of Wharton’s jelly cells of the human umbilical cord on acute spinal cord injury in rats, and expression of interleukin-1β and nerve growth factor in spinal cord tissues. Artif Cells Nanomed Biotechnol. 2016;44(5):1254–58. [DOI] [PubMed] [Google Scholar]
- 35. Erdogan B, Bavbek M, Sahin IF, Caner H, Ozen O, Denkbas EB, Altinors MN. Fetal allogeneic umbilical cord cell transplantation improves motor function in spinal cord-injured rats. Turk Neurosurg. 2010;20(3):286–94. [DOI] [PubMed] [Google Scholar]
- 36. Atalay B, Bavbek M, Cekinmez M, Ozen O, Nacar A, Karabay G, Gulsen S. Antibodies neutralizing Nogo-A increase pan-cadherin expression and motor recovery following spinal cord injury in rats. Spinal Cord. 2007;45(12):780–86. [DOI] [PubMed] [Google Scholar]
- 37. Isaksson J, Farooque M, Holtz A, Hillered L, Olsson Y. Expression of ICAM-1 and CD11b after experimental spinal cord injury in rats. J Neurotrauma. 1999;16(2):165–73. [DOI] [PubMed] [Google Scholar]
- 38. Wang C, Wang Q, Lou Y, Xu J, Feng Z, Chen Y, Tang Q, Zheng G, Zhang Z, Wu Y, Tian N, et al. Salidroside attenuates neuroinflammation and improves functional recovery after spinal cord injury through microglia polarization regulation. J Cell Mol Med. 2018;22(2):1148–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dasari VR, Veeravalli KK, Dinh DH. Mesenchymal stem cells in the treatment of spinal cord injuries: a review. World J Stem Cells. 2014;6(2):120–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cll-10.1177_09636897221139734 for Therapeutic Efficacy of Human Mesenchymal Stem Cells With Different Delivery Route and Dosages in Rat Models of Spinal Cord Injury by Guangyang Liu, Zhiling Zhao, Herui Wang, Chunhua Hao, Weiting Wang, Chenliang Zhang, Tiehua Wang, Xin Li, Jingjing Xi, Shaoyun Li, Haomiao Long, Yi Mi, Li Miao, Yaoyao Chen, Liqiang Xu, Libo Zheng, Hao Wang, Ning Ding, Fengmei Zhu, Qinggang Ge and Yongjun Liu in Cell Transplantation