Abstract
BACKGROUND
Vitamin D supplements are widely recommended for bone health in the general population, but data on whether they prevent fractures have been inconsistent.
METHODS
In an ancillary study of the Vitamin D and Omega-3 Trial (VITAL), we tested whether supplemental vitamin D3 would result in a lower risk of fractures than placebo. VITAL was a two-by-two factorial, randomized, controlled trial that investigated whether supplemental vitamin D3 (2000 IU per day), n–3 fatty acids (1 g per day), or both would prevent cancer and cardiovascular disease in men 50 years of age or older and women 55 years of age or older in the United States. Participants were not recruited on the basis of vitamin D deficiency, low bone mass, or osteoporosis. Incident fractures were reported by participants on annual questionnaires and adjudicated by centralized medical-record review. The primary end points were incident total, nonvertebral, and hip fractures. Proportional-hazards models were used to estimate the treatment effect in intention-to-treat analyses.
RESULTS
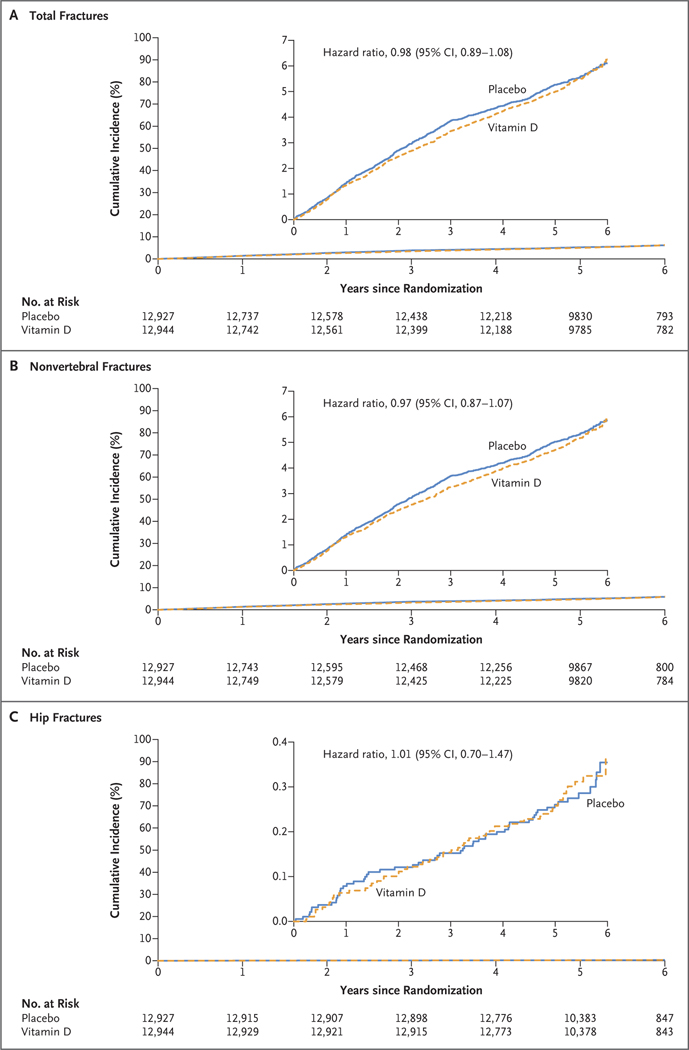
Among 25,871 participants (50.6% women [13,085 of 25,871] and 20.2% Black [5106 of 25,304]), we confirmed 1991 incident fractures in 1551 participants over a median follow-up of 5.3 years. Supplemental vitamin D3, as compared with placebo, did not have a significant effect on total fractures (which occurred in 769 of 12,927 participants in the vitamin D group and in 782 of 12,944 participants in the placebo group; hazard ratio, 0.98; 95% confidence interval [CI], 0.89 to 1.08; P = 0.70), nonvertebral fractures (hazard ratio, 0.97; 95% CI, 0.87 to 1.07; P = 0.50), or hip fractures (hazard ratio, 1.01; 95% CI, 0.70 to 1.47; P = 0.96). There was no modification of the treatment effect according to baseline characteristics, including age, sex, race or ethnic group, body-mass index, or serum 25-hydroxyvitamin D levels. There were no substantial between-group differences in adverse events as assessed in the parent trial.
CONCLUSIONS
Vitamin D3 supplementation did not result in a significantly lower risk of fractures than placebo among generally healthy midlife and older adults who were not selected for vitamin D deficiency, low bone mass, or osteoporosis. (Funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases; VITAL ClinicalTrials.gov number, NCT01704859.)
Fractures are major public health problems, especially among older adults. An estimated 53.6 million Americans have osteoporosis, low bone mass, or both,1 and in the United States, 2 million osteoporotic fractures occur annually, a number projected to exceed 3 million fractures per year by 2040, with related costs of more than $95 billion per year.2–4 Vitamin D supplements are widely recommended to the general population as a means to promote bone health. Between 1999 and 2012, use of vitamin D supplements increased from 5.1% to 19% among U.S. adults.5 However, data on whether these supplements prevent fractures are conflicting.6–10
Vitamin D may support skeletal health and improve bone mineralization by increasing intestinal calcium absorption, reducing secondary hyperparathyroidism, and decreasing bone turnover.11 Furthermore, in bone, vitamin D receptors and extrarenal activation of 1,25-dihydroxyvitamin D have been identified and linked to the formation of osteoblast progenitors, which suggests a role in bone formation.12,13
In 2011, the Institute of Medicine (IOM) established recommended dietary allowances for vitamin D of 600 to 800 IU per day, corresponding to a total 25-hydroxyvitamin D level of at least 20 ng per milliliter, in order to meet the bone health needs for 97.5% of the population.8 Other societies or foundations recommend vitamin D intakes of at least 800 to 2000 IU per day for adults 50 years of age or older to attain 25-hydroxyvitamin D levels of at least 30 ng per milliliter.8,14
Results from randomized, controlled trials investigating the effects of supplemental vitamin D on fracture outcomes have been inconsistent, with trials finding evidence for benefit, no effect, or harm.4,15–17 Differences to explain the divergent results across these trials include the use of bolus dosing,17,18 coadministration of vitamin D with calcium,19,20 and small sample sizes.15 No large randomized, controlled trials have tested the effects of daily supplemental vitamin D alone (without coadministered calcium) in preventing fractures in the U.S. population.
Systematic reviews and meta-analyses of randomized, controlled trials have raised questions about whether supplemental vitamin D has beneficial effects for primary prevention of fractures.21,22 In 2021, the U.S. Preventive Services Task Force found no effect of supplemental vitamin D on fracture incidence among community-dwelling adults with low 25-hydroxyvitamin D levels.10 The IOM identified an increased risk of fractures at both low and high 25-hydroxyvitamin D levels and emphasized the need for more research from large randomized, controlled trials.8 To address these knowledge gaps, we investigated whether supplemental vitamin D3 would result in a lower risk of incident fractures than placebo among generally healthy U.S. adults in an ancillary study of the large Vitamin D and Omega-3 Trial (VITAL).23
METHODS
TRIAL DESIGN AND OVERSIGHT
VITAL was a randomized, controlled trial that investigated the effects of supplemental vitamin D3 (cholecalciferol, 2000 IU per day), n–3 fatty acids (Omacor [Pronova BioPharma and BASF], 1 g per day), or both on the primary prevention of cancer and cardiovascular disease in a two-by-two factorial design. The rationale for this vitamin D3 dose was that it would achieve a mean 25-hydroxyvitamin D level of approximately 40 ng per milliliter in the vitamin D group and a favorable balance of safety and efficacy.23
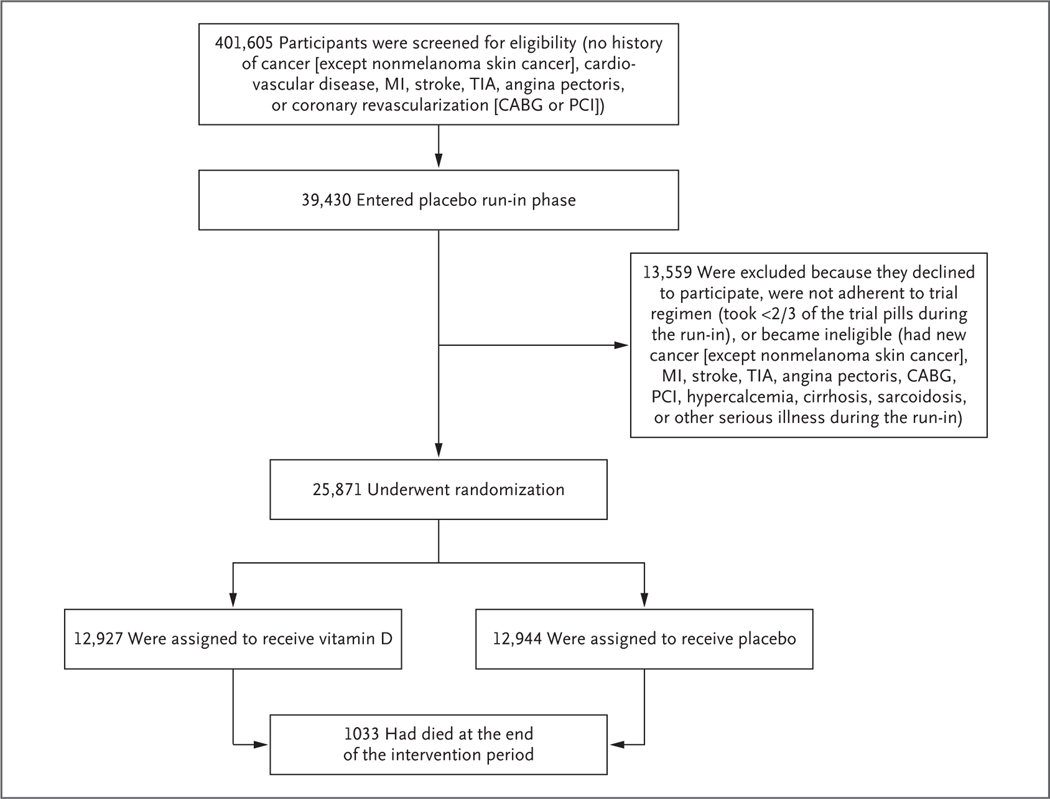
The trial protocol has been described previously23,24 and is available with the full text of this article at NEJM.org. Among the exclusion criteria was a history of cancer, cardiovascular disease, or hypercalcemia (Fig. 1). Participants were not recruited on the basis of vitamin D deficiency, low bone mineral density, or fracture history. After a 3-month placebo run-in phase, participants who took at least two thirds of the trial pills underwent randomization. Participants agreed to limit any nontrial supplements of vitamin D to 800 IU per day and of calcium to 1200 mg per day.8,23
Figure 1. Screening, Randomization, and Follow-up of the Participants.
CABG denotes coronary-artery bypass grafting, MI myocardial infarction, PCI percutaneous coronary intervention, and TIA transient ischemic attack.
In this ancillary study, we examined the effects of supplemental vitamin D3 as compared with placebo on incident fractures in 25,871 U.S. men (age, ≥50 years) and women (age, ≥55 years), including 5106 Black participants, who were enrolled from all 50 states and followed for a median of 5.3 years. During the run-in phase, participants completed detailed questionnaires to assess baseline demographic characteristics, medical history, medication use, and supplement use (e.g., calcium, vitamin D, and fish oil). Annual questionnaires assessed adherence to trial regimens, side effects, use of nontrial supplements (e.g., calcium and vitamin D) and medications, the development of major illnesses, osteoporosis or related risk factors,19,25 physical activity, falls, and fractures.24
Baseline and follow-up blood samples were provided by 16,956 and approximately 6,000 participants, respectively. Quest Diagnostics measured total 25-hydroxyvitamin D levels using liquid chromatography–tandem mass spectrometry; assays were calibrated to Centers for Disease Control and Prevention (CDC) standards.23 This protocol was approved by the Mass General Brigham institutional review board, and participants gave written informed consent.
TRIAL END POINTS
The primary end points were first incident total, nonvertebral, and hip fractures. Fractures were initially reported by the participants on annual questionnaires. Participants who reported a fracture were sent an authorization form to obtain their medical records and a fracture questionnaire, in which they recorded date, fracture location, any association with cancer or prosthesis (e.g., joint replacement), and circumstances of the fracture. Requests for medical records were then sent to health care professionals or medical facilities that provided fracture care, including radiologic reports, orthopedic notes, other hospital records, and operative or procedure reports. For participants who reported hip or femur fractures, radiologic images were also requested. All incident fractures were centrally adjudicated by investigators and study staff who were unaware of the trial-group assignments. Fractures were coded according to anatomical location and level of trauma. We included fractures regardless of the level of trauma for all end-point analyses, because even high-trauma fractures are associated with an increased risk of subsequent fractures.26,27
Secondary end points were incident total, nonvertebral, and hip fractures, with the exclusion of toe, finger, skull, periprosthetic, and pathologic fractures. We identified 42 periprosthetic and 29 pathologic fractures (e.g., tumors and Paget’s disease). For hip or femur fractures, a musculoskeletal radiologist (the fifth author) reviewed radiographs and determined whether fractures were periprosthetic or atypical femur fractures using criteria from an American Society for Bone and Mineral Research task force.28 Three female participants had a total of 4 atypical femur fractures; two were taking alendronate and had pathologic fractures. In exploratory analyses, we examined major osteoporotic fractures (defined as hip, wrist, humerus, or clinical spine fractures), pelvic fractures, and wrist fractures.
STATISTICAL ANALYSIS
The primary aim was to assess the main effects of vitamin D supplementation as compared with placebo on first incident total, nonvertebral, and hip fractures in intention-to-treat analyses; secondary end points excluded toe, finger, skull, periprosthetic, and pathologic fractures. To ensure balance, we compared baseline characteristics according to randomized trial-group assignment. We used t-tests and analysis of variance to compare continuous variables across randomized groups and used chi-square tests to compare proportions. We used Cox proportional-hazards models to allow for variable follow-up lengths and estimated the cause-specific hazard ratio for fracture incidence for each intervention using indicators for treatment exposure, controlling for n–3 fatty acid randomization group, age, sex, and race or ethnic group. In a post hoc analysis, we applied the Andersen–Gill model allowing for multiple events per person with different time between events. We also assessed for effects of adherence by censoring data from participants who were taking fewer than two thirds of the assigned trial pills and for latency by excluding the first 1 and 2 years of follow-up.
We examined modification of the treatment effect in prespecified subgroups, defined by sex, race or ethnic group, body-mass index (BMI; the weight in kilograms divided by the square of the height in meters), baseline use of calcium or vitamin D supplements, baseline serum total 25-hydroxyvitamin D levels (above or below the median and according to quartiles), and trial group. In addition, we explored whether treatment effect varied according to history of fragility fractures or baseline use of osteoporosis medications. The primary aims were to examine the effects of vitamin D and n–3 fatty acids in this two-by-two factorial trial. Here, we present the main effects of vitamin D as compared with placebo, because there was no interaction between vitamin D and n–3 fatty acids in the analysis of fracture outcomes. There was no control for multiple hypothesis testing, and no adjustment was made to the P values or confidence intervals. Thus, results regarding secondary and exploratory end points, as well as those regarding subgroups, should be interpreted with caution.
This study was designed by the first author in conjunction with the last author. The first four authors had full access to trial data and vouch for the completeness and accuracy of the data and for the fidelity of the trial to the protocol. The manuscript was written by the first author with contributions from all the authors.
Results
TRIAL PARTICIPANTS
Participants were randomly assigned to one of four groups: vitamin D plus n–3 fatty acids, vitamin D plus placebo, n–3 fatty acids plus placebo, or double placebo. Baseline characteristics (Table 1, and Table S2 in the Supplementary Appendix, available at NEJM.org) were balanced between the trial groups. The mean (±SD) age of the participants was 67.1±7.1 years, 50.6% (13,085 of 25,871) were women, and 20.2% (5106 of 25,304) were Black. The mean BMI was 28.1±5.7. Only 4.8% of the participants (1240 of 25,690) were taking osteoporosis medications at baseline, yet 10.3% (2578 of 25,023) had a history of fragility fracture. At baseline, a total of 42.6% (11,030 of 25,871) took nontrial vitamin D supplements limited to 800 IU per day, and 20.0% (5166 of 25,871) took calcium supplements limited to 1200 mg per day. Table S1 shows the representativeness of VITAL participants to the general U.S. population.
Table 1.
Characteristics of the Participants at Baseline, According to Randomized Assignment to Vitamin D or Placebo.*
| Characteristic | Total (N = 25,871) | Vitamin D Group (N = 12,927) | Placebo Group (N = 12,944) |
|---|---|---|---|
| Female sex — no. (%) | 13,085 (50.6) | 6,547 (50.6) | 6,538 (50.5) |
| Age — yr | 67.1±7.1 | 67.1±7.0 | 67.1±7.1 |
| Race or ethnic group — no./total no. (%)† | |||
| Non-Hispanic White | 18,046/25,304 (71.3) | 9,013/12,647 (71.3) | 9,033/12,657 (71.4) |
| Black | 5,106/25,304 (20.2) | 2,553/12,647 (20.2) | 2,553/12,657 (20.2) |
| Non-Black Hispanic | 1,013/25,304 (4.0) | 516/12,647 (4.1) | 497/12,657 (3.9) |
| Asian or Pacific Islander | 388/25,304 (1.5) | 188/12,647 (1.5) | 200/12,657 (1.6) |
| American Indian or Alaskan Native | 228/25,304 (0.9) | 118/12,647 (0.9) | 110/12,657 (0.9) |
| Other or unknown | 523/25,304 (2.1) | 259/12,647 (2.0) | 264/12,657 (2.1) |
| Body-mass index‡ | 28.1±5.7 | 28.1±5.7 | 28.1±5.8 |
| Diabetes — no./total no. (%) | 3,537/25,824 (13.7) | 1,804/12,900 (14.0) | 1,733/12,924 (13.4) |
| Parental history of hip fracture — no./total no. (%) | 3,704/23,979 (15.4) | 1,809/11,970 (15.1) | 1,895/12,009 (15.8) |
| Rheumatoid arthritis — no./total no. (%) | 1,118/25,512 (4.4) | 556/12,749 (4.4) | 562/12,763 (4.4) |
| History of fragility fracture — no./total no. (%) | 2,578/25,023 (10.3) | 1,287/12,513 (10.3) | 1,291/12,510 (10.3) |
| Unintentional fall in the past year — no./total no. (%) | 6,921/25,715 (26.9) | 3,521/12,848 (27.4) | 3,400/12,867 (26.4) |
| Current use of osteoporosis medication — no./total no. (%)§ | 1,240/25,690 (4.8) | 609/12,835 (4.7) | 631/12,855 (4.9) |
| Current smoker — no./total no. (%) | 1,835/25,488 (7.2) | 921/12,732 (7.2) | 914/12,756 ( 7.2) |
| Current use of supplemental vitamin D — no. (%)¶ | 11,030 (42.6) | 5,497 (42.5) | 5,533 (42.7) |
| Current use of glucocorticoids — no./total no. (%) | 461/25,427 (1.8) | 239/12,705 (1.9) | 222/12,722 (1.7) |
| Servings of milk per day | 0.71±0.91 | 0.71±0.89 | 0.72±0.92 |
| Baseline 25-hydroxyvitamin D level — ng/ml‖ | 30.7±10.0 | 30.7±10.0 | 30.7±10.0 |
| Baseline calcium level — mg/dl** | 9.00±1.61 | 9.00±1.61 | 9.00±1.61 |
Plus–minus values are means ±SD. Percentages may not total 100 because of rounding.
Race and ethnic group were reported by the participants.
A total of 30.1% of the participants had a normal body-mass index (18.5 to <25), 40.1% were overweight (25 to <30), and 28.9% were obese (≥30).
Osteoporosis medications included alendronate (Fosamax), raloxifene (Evista), risedronate (Actonel), zoledronate (Reclast), denosumab (Prolia), teriparatide injection (Forteo), salmon calcitonin (Miacalcin or Fortical), and other osteoporosis medications not listed above.
The mean 25-hydroxyvitamin D level at baseline was 34.9 ng per milliliter for participants taking vitamin D supplements (total, <800 IU per day) and 27.4 ng per milliliter for participants not taking vitamin D supplements.
Data were available for 16,757 participants.
Data were available for 15,884 participants.
The mean baseline 25-hydroxyvitamin D level (16,757 participants) was 30.7±10.0 ng per milliliter. At baseline, 401 (2.4%) had 25-hydroxyvitamin D levels of less than 12 ng per milliliter and 2161 (12.9%) had levels of less than 20 ng per milliliter. Among participants who provided 2-year blood samples, mean 25-hydroxyvitamin D levels increased from 29.2 ng per milliliter to 41.2 ng per milliliter in the vitamin D group (P<0.001, 1347 participants) and decreased slightly from 30.0 ng per milliliter to 29.4 ng per milliliter in the placebo group (P = 0.01, 1308 participants). Mean parathyroid hormone levels decreased in the vitamin D group from 40.8 ng per milliliter to 37.2 ng per milliliter (P<0.001, 1396 participants), with no changes in the placebo group. There were no 2-year changes in calcium levels in either group. Adherence to trial pills was 87.3% at 2 years and 85.4% at 5 years.
FRACTURE INCIDENCE
A total of 87.0% of the participants who reported a fracture consented to medical-record review, and documentation was obtained for 91.3% of these participants. We adjudicated 2133 total fractures and confirmed 1991 fractures (93.3%) in 1551 participants during the intervention period, with a median follow-up of 5.3 years. Supplemental vitamin D3, as compared with placebo, did not have a significant effect on first incident total fractures (which occurred in 769 of 12,927 participants in the vitamin D group and in 782 of 12,944 participants in the placebo group; hazard ratio, 0.98; 95% confidence interval [CI], 0.89 to 1.08; P = 0.70), nonvertebral fractures (in 721 participants in the vitamin D group and in 744 in the placebo group; hazard ratio, 0.97; 95% CI, 0.87 to 1.07; P = 0.50), or hip fractures (in 57 participants in the vitamin D group and in 56 in the placebo group; hazard ratio, 1.01; 95% CI, 0.70 to 1.47; P = 0.96), with adjustment for age, sex, race or ethnic group, and n–3 fatty acid randomization group (Table 2 and Fig. 2).
Table 2.
Hazard Ratios for the Primary, Secondary, and Exploratory End Points, According to Randomized Assignment to Vitamin D or Placebo, in Intention-to-Treat Analyses.*
| End Point | Vitamin D Group (N = 12,927) | Placebo Group (N = 12,944) | Hazard Ratio (95% CI) |
|---|---|---|---|
| no. of participants with event | |||
| Primary end points: confirmed incident fractures | |||
| Total | 769 | 782 | 0.98 (0.89–1.08) |
| Nonvertebral | 721 | 744 | 0.97 (0.87–1.07) |
| Hip | 57 | 56 | 1.01 (0.70–1.47) |
| Secondary end points: confirmed incident fractures excluding toe, finger, skull, periprosthetic, and pathologic fractures | |||
| Total | 678 | 685 | 0.99 (0.89–1.10) |
| Nonvertebral | 630 | 649 | 0.97 (0.87–1.08) |
| Hip | 54 | 52 | 1.03 (0.70–1.52) |
| Exploratory end points: confirmed incident fractures excluding periprosthetic and pathologic fractures | |||
| Major osteoporotic fractures: hip, wrist, humerus, or clinical spine fractures | 276 | 278 | 0.99 (0.83–1.17) |
| Pelvic | 32 | 29 | 1.08 (0.64–1.80) |
| Wrist | 118 | 132 | 0.89 (0.69–1.15) |
Analyses were performed with the use of Cox proportional-hazards models that were adjusted for age, sex, race or ethnic group, and n–3 fatty acid randomization group. Confidence intervals were not adjusted for multiple comparisons, and inferences drawn from them may not be reproducible.
Figure 2. Cumulative Incident Fractures in the Vitamin D and Placebo Groups.
The median follow-up was 5.3 years. Analyses were conducted with the use of Cox regression models that were controlled for age, sex, race or ethnic group, and n–3 fatty acid randomization group (intention-to-treat analyses). The insets show the same data on an enlarged y axis.
There was no effect modification according to baseline age, sex, race or ethnic group, BMI, or personal use of supplemental calcium or vitamin D (Tables 3, S3, and S4). Baseline 25-hydroxyvitamin D levels, when stratified above or below the median (31 ng per milliliter) or in quartiles (≤24.0, 24.1 to 30.0, 30.1 to 36.9, or ≥37.0 ng per milliliter), as prespecified, did not modify the effects of supplemental vitamin D3 as compared with placebo on incident total, nonvertebral, or hip fractures. In exploratory analyses, there was no significant effect modification on fracture incidence between the vitamin D and placebo groups according to baseline clinically relevant 25-hydroxyvitamin D thresholds (<12, <20, <30, or ≥50 ng per milliliter), serum calcium levels (15,884 participants), or parathyroid hormone levels (16,803 participants). There were no significant differences in fracture incidence among participants using osteoporosis medications or among those with a history of fragility fracture. In sensitivity analyses, results did not change among participants adherent to trial pills, and no latency effect was found. Supplemental vitamin D3 also did not result in a lower risk of recurrent fractures than placebo.
Table 3.
Hazard Ratios for Total Fractures with Vitamin D as Compared with Placebo, According to Subgroup.*
| Subgroup | No. of Participants | Vitamin D Group (N = 12,927) | Placebo Group (N = 12,944) | Hazard Ratio (95% CI) |
|---|---|---|---|---|
| no. of participants with event | ||||
| Age | ||||
| <Median of 66.7 yr | 12,859 | 282 | 285 | 0.99 (0.84–1.18) |
| ≥Median of 66.7 yr | 13,012 | 487 | 497 | 0.97 (0.86–1.10) |
| Sex | ||||
| Male | 12,786 | 265 | 250 | 1.07 (0.90–1.28) |
| Female | 13,085 | 504 | 532 | 0.94 (0.83–1.06) |
| Race or ethnic group | ||||
| Non-Hispanic White | 18,046 | 655 | 658 | 0.99 (0.89–1.11) |
| Black | 5,106 | 53 | 59 | 0.89 (0.62–1.30) |
| Other | 2,152 | 46 | 51 | 0.90 (0.61–1.35) |
| BMI category | ||||
| <25 | 7,849 | 300 | 327 | 0.93 (0.79–1.09) |
| 25 to <30 | 10,127 | 254 | 271 | 0.93 (0.78–1.11) |
| ≥30 | 7,294 | 198 | 165 | 1.17 (0.95–1.44) |
| BMI | ||||
| <Median of 27.1 | 12,589 | 435 | 456 | 0.95 (0.83–1.09) |
| ≥Median of 27.1 | 12,681 | 317 | 307 | 1.03 (0.88–1.20) |
| Osteoporosis medication† | ||||
| Yes | 1,240 | 62 | 79 | 0.74 (0.53–1.03) |
| No | 24,450 | 704 | 697 | 1.01 (0.91–1.12) |
| History of fragility fractures† | ||||
| Yes | 2,578 | 146 | 161 | 0.87 (0.69–1.09) |
| No | 22,445 | 598 | 595 | 1.01 (0.90–1.14) |
| Baseline use of supplemental vitamin D | ||||
| Yes | 11,030 | 393 | 399 | 0.97 (0.84–1.12) |
| No | 14,841 | 376 | 383 | 0.99 (0.86–1.14) |
| Supplemental calcium | ||||
| ≤1200 mg/day | 5,166 | 228 | 232 | 0.92 (0.77–1.11) |
| None | 20,705 | 541 | 550 | 1.00 (0.89–1.13) |
| Baseline 25-hydroxyvitamin D level, according to median | ||||
| <Median of 31 ng/ml | 8,430 | 239 | 241 | 1.02 (0.85–1.22) |
| ≥Median of 31 ng/ml | 8,327 | 329 | 344 | 0.93 (0.80–1.08) |
| Baseline 25-hydroxyvitamin D level in quartiles | ||||
| Quartile 1: ≤24.0 ng/ml | 4,270 | 115 | 112 | 1.04 (0.80–1.36) |
| Quartile 2: 24.1–30.0 ng/ml | 4,104 | 122 | 128 | 0.98 (0.77–1.26) |
| Quartile 3: 30.1–36.9 ng/ml | 4,097 | 151 | 154 | 0.98 (0.78–1.23) |
| Quartile 4: ≥37.0 ng/ml | 4,286 | 180 | 191 | 0.89 (0.73–1.10) |
| Baseline 25-hydroxyvitamin D level, according to threshold of 12 ng/ml† | ||||
| <12 ng/ml | 401 | 7 | 8 | 1.03 (0.36–2.95) |
| ≥12 ng/ml | 16,356 | 561 | 577 | 0.97 (0.86–1.09) |
| Randomization in the n–3 fatty acids portion of the trial | ||||
| Placebo group | 12,938 | 374 | 392 | 0.95 (0.82–1.09) |
| Active-agent group | 12,933 | 395 | 390 | 1.02 (0.88–1.17) |
Analyses were conducted with the use of Cox proportional-hazards models that were adjusted for age, sex, race or ethnic group, and n–3 fatty acid randomization group (intention-to-treat analyses). Confidence intervals were not adjusted for multiple comparisons, and inferences drawn from them may not be reproducible.
These subgroups were not prespecified, but a subgroup defined by a baseline 25-hydroxyvitamin D level of less than 10 ng per milliliter (201 participants) was prespecified.
Findings for vitamin D as compared with placebo were similar with respect to secondary end points, excluding toe, finger, skull, periprosthetic, and pathologic fractures: total fractures (hazard ratio, 0.99; 95% CI, 0.89 to 1.10), nonvertebral fractures (hazard ratio, 0.97; 95% CI, 0.87 to 1.08), and hip fractures (hazard ratio, 1.03; 95% CI, 0.70 to 1.52) (Tables 2 and S5). Findings were also similar for exploratory end points: major osteoporotic fractures (hazard ratio, 0.99; 95% CI, 0.83 to 1.17), pelvic fractures (hazard ratio, 1.08; 95% CI, 0.64 to 1.80), and wrist fractures (hazard ratio, 0.89; 95% CI, 0.69 to 1.15), excluding periprosthetic and pathologic fractures (Tables 2 and S6).
ADVERSE EVENTS
There were no substantial differences in the incidence of hypercalcemia and kidney stones between the vitamin D and placebo groups as assessed by the VITAL data and safety monitoring board.23
DISCUSSION
In this large randomized, controlled trial, supplemental vitamin D3 (2000 IU per day) without coadministered calcium did not result in a lower risk of fractures than placebo (with adjustment for age, sex, race or ethnic group, and n–3 fatty acid randomization group) among U.S. adults who were not selected on the basis of vitamin D deficiency, low bone mass, or osteoporosis. Findings were similar for secondary end points, which excluded toe, finger, skull, periprosthetic, and pathologic fractures. We also found no effect of supplemental vitamin D3 as compared with placebo on major osteoporotic fractures and other exploratory end points, including pelvic and wrist fractures. No latency effect was found. Data are presented as supplemental vitamin D3 as compared with placebo because there was no interaction between vitamin D and n–3 fatty acids in the analysis of fracture outcomes.
It has been suggested that the effects of supplemental vitamin D3 might be limited to those with low 25-hydroxyvitamin D levels. Higher prevalences of vitamin D deficiency have been reported among Black adults (from reduced cutaneous vitamin D synthesis and other mechanisms),29 persons with obesity30 (from vitamin D sequestration and increased volume in fat), post-menopausal women, older men, and older persons with hip fractures.31–33 However, our results did not suggest any differences in the effects of supplemental vitamin D3 on fracture outcomes according to race or ethnic group, BMI, or age. Although most participants in our cohort may have already reached the 25-hydroxyvitamin D level needed for bone health (mean baseline 25-hydroxyvitamin D level, 30.7±10.0 ng per milliliter), VITAL was large enough to stratify participants according to baseline 25-hydroxyvitamin D levels. We found no significant differences in fracture incidence between trial groups according to various 25-hydroxyvitamin D thresholds. In post hoc analyses, we found no benefit for supplementation in the relatively small number of participants with baseline 25-hydroxyvitamin D levels of less than 12 ng per milliliter (401 participants). Similarly, a study conducted in the Netherlands showed that supplemental vitamin D (400 IU per day) had no effect on hip or peripheral fractures in elderly adults with very low vitamin D levels.34 In post hoc analyses, we also found no substantial between-group differences in fracture incidence among participants who were at high fracture risk (i.e., those taking osteoporosis medications [1240 participants] or with a history of fragility fractures [2578 participants]). Other randomized, controlled trials19,20 and meta-analyses35 have shown that vitamin D and calcium coadministration reduce fracture risk modestly. However, in post hoc analyses, we found no substantial differences in the 20.0% of the participants who took supplemental calcium.
Previous randomized, controlled trials from around the world that investigated the effects of supplemental vitamin D on fracture outcomes have shown conflicting results. In a British trial, supplemental vitamin D3 (100,000 IU every 4 months) as compared with placebo for 5 years resulted in a marginal reduction in the relative risk of first hip, wrist, forearm, or spine fracture in men and women.18 A trial in Australia, however, showed that very high oral doses of vitamin D (500,000 IU per year) resulted in an increased fracture risk among older women, who were found to have reached a 25-hydroxyvitamin D level of 48 ng per milliliter 1 month after bolus dosing.16 However, we did not find any evidence of increased fracture risk among participants with baseline 25-hydroxyvitamin D levels of 50 ng or more per milliliter in exploratory analyses. The Vitamin D Assessment Study involving 5110 older men and women in New Zealand investigated supplemental vitamin D (100,000 IU per month) as compared with placebo and showed no effect on incident nonvertebral fractures over a period of 3.3 years.17 Bolus dosing, however, has been shown to result in nonphysiologic variability in blood 25-hydroxyvitamin D levels.36 The DO-HEALTH trial examining the effect of supplemental vitamin D (2000 IU per day) on nonvertebral fractures in 2157 older European adults also had null findings of vitamin D supplementation on fracture risk.15
In VITAL, we previously found that vitamin D supplementation did not affect incident fall risk or changes in bone mineral density or structure.37,38 We did find that in participants with baseline free 25-hydroxyvitamin D levels below the median, supplemental vitamin D3 had a slight benefit on spine and total hip bone mineral density.38 Ongoing studies in VITAL are assessing whether baseline measured free 25-hydroxyvitamin D levels or genetic variation in vitamin D absorption, metabolism, or receptor function may identify a subgroup of patients who may benefit from vitamin D supplementation with respect to fracture outcomes.
Strengths of this study include the large, diverse sample size and high adherence. Incident fractures were adjudicated and confirmed. Levels of 25-hydroxyvitamin D were measured and calibrated according to standards set by the CDC. This study also has limitations. We evaluated only one vitamin D dose, and the trial was not designed to test the effects of vitamin D supplementation in those who are vitamin D deficient. Only a small percentage of participants (2.4%) had vitamin D levels of less than 12 ng per milliliter. It would not have been feasible or ethical to study the effects of vitamin D as compared with placebo on incident fractures in a population preselected for vitamin D deficiency. No adjustment was made for multiplicity for secondary, exploratory, or parent trial end points. In addition, results may not be generalizable to adults with osteoporosis or osteomalacia or older institutionalized persons.
In this randomized, controlled trial, supplemental vitamin D3 did not result in a lower risk of incident total, nonvertebral, or hip fractures than placebo among generally healthy midlife and older adults who were not selected for vitamin D deficiency, low bone mass, or osteoporosis.
Supplementary Material
Acknowledgments
Supported by grants (R01 AR060574, R01 AR070854, and R01 AR059775, to Dr. LeBoff) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The VITAL parent trial is supported by grants (U01 CA138962, R01 CA138962, and R01AT011729, to Drs. Manson and Buring) from the National Cancer Institute, the National Heart, Lung, and Blood Institute, the Office of Dietary Supplements, the National Institute of Neurological Disorders and Stroke, and the National Center for Complementary and Integrative Health.
Footnotes
REFERENCES
- 1.Wright NC, Looker AC, Saag KG, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res 2014;29:2520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Office of the Surgeon General. Bone health and osteoporosis: a report of the Surgeon General. Rockville, MD: Department of Health and Human Services, 2004. [PubMed] [Google Scholar]
- 3.Lewiecki EM, Ortendahl JD, Vanderpuye-Orgle J, et al. Healthcare policy changes in osteoporosis can improve outcomes and reduce costs in the United States. JBMR Plus 2019;3(9):e10192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LeBoff MS, Greenspan SL, Insogna KL, et al. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 2022. April 28 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in dietary supplement use among US adults from 1999–2012. JAMA 2016;316:1464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev 2014;4:CD000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newberry SJ, Chung M, Shekelle PG, et al. Vitamin D and calcium: a systematic review of health outcomes (update). Evid Rep Technol Assess (Full Rep) 2014;217:1–929. [DOI] [PubMed] [Google Scholar]
- 8.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011;96:53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao P, Bennett D, Mafham M, et al. Vitamin D and calcium for the prevention of fracture: a systematic review and meta-analysis. JAMA Netw Open 2019;2(12):e1917789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2021;325:1443–63. [DOI] [PubMed] [Google Scholar]
- 11.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 2004; 80: Suppl: 1689S–1696S. [DOI] [PubMed] [Google Scholar]
- 12.Hewison M, Burke F, Evans KN, et al. Extra-renal 25-hydroxyvitamin D3–1alphahydroxylase in human health and disease. J Steroid Biochem Mol Biol 2007;103:316–21. [DOI] [PubMed] [Google Scholar]
- 13.Zhou S, Glowacki J, Kim SW, et al. Clinical characteristics influence in vitro action of 1,25-dihydroxyvitamin D(3) in human marrow stromal cells. J Bone Miner Res 2012;27:1992–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–30. [DOI] [PubMed] [Google Scholar]
- 15.Bischoff-Ferrari HA, Vellas B, Rizzoli R, et al. Effect of vitamin D supplementation, omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: the DO-HEALTH randomized clinical trial. JAMA 2020;324:1855–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 2010;303:1815–22. [DOI] [PubMed] [Google Scholar]
- 17.Khaw KT, Stewart AW, Waayer D, et al. Effect of monthly high-dose vitamin D supplementation on falls and non-vertebral fractures: secondary and post-hoc outcomes from the randomised, double-blind, placebo-controlled ViDA trial. Lancet Diabetes Endocrinol 2017;5:438–47. [DOI] [PubMed] [Google Scholar]
- 18.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 2003;326:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 2006;354:669–83. [DOI] [PubMed] [Google Scholar]
- 20.Chapuy MC, Arlot ME, Duboeuf F, et al. Vitamin D3 and calcium to prevent hip fractures in elderly women. N Engl J Med 1992;327:1637–42. [DOI] [PubMed] [Google Scholar]
- 21.Bouillon R, Marcocci C, Carmeliet G, et al. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr Rev 2019;40:1109–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lips P, van Schoor NM. The effect of vitamin D on bone and osteoporosis. Best Pract Res Clin Endocrinol Metab 2011;25:585–91. [DOI] [PubMed] [Google Scholar]
- 23.Manson JE, Cook NR, Lee I-M, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med 2019;380:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeBoff MS, Yue AY, Copeland T, Cook NR, Buring JE, Manson JE. VITAL-Bone Health: rationale and design of two ancillary studies evaluating the effects of vitamin D and/or omega-3 fatty acid supplements on incident fractures and bone health outcomes in the VITamin D and OmegA-3 TriaL (VITAL). Contemp Clin Trials 2015;41:259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cummings SR, Cawthon PM, Ensrud KE, et al. BMD and risk of hip and nonvertebral fractures in older men: a prospective study and comparison with older women. J Bone Miner Res 2006;2 1: 1550–6. [DOI] [PubMed] [Google Scholar]
- 26.Crandall CJ, Larson JC, LaCroix AZ, et al. Risk of subsequent fractures in post-menopausal women after nontraumatic vs traumatic fractures. JAMA Intern Med 2021;181:1055–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ensrud KE, Blackwell TL, Cawthon PM, et al. Degree of trauma differs for major osteoporotic fracture events in older men versus older women. J Bone Miner Res 2016;31:204–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shane E, Burr D, Abrahamsen B, et al. Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 2014;29:1–23. [DOI] [PubMed] [Google Scholar]
- 29.Harris SS. Vitamin D and African Americans. J Nutr 2006;136:1126–9. [DOI] [PubMed] [Google Scholar]
- 30.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 2000;72:690–3. [DOI] [PubMed] [Google Scholar]
- 31.LeBoff MS, Kohlmeier L, Hurwitz S, Franklin J, Wright J, Glowacki J. Occult vitamin D deficiency in postmenopausal US women with acute hip fracture. JAMA 1999;281:1505–11. [DOI] [PubMed] [Google Scholar]
- 32.Cauley JA, Lacroix AZ, Wu L, et al. Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med 2008;149:242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orwoll E, Nielson CM, Marshall LM, et al. Vitamin D deficiency in older men. J Clin Endocrinol Metab 2009;94:1214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lips P, Graafmans WC, Ooms ME, Bezemer PD, Bouter LM. Vitamin D supplementation and fracture incidence in elderly persons: a randomized, placebo-controlled clinical trial. Ann Intern Med 1996;124:400–6. [DOI] [PubMed] [Google Scholar]
- 35.Weaver CM, Alexander DD, Boushey CJ, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int 2016;27:367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hollis BW, Wagner CL. Clinical review: the role of the parent compound vitamin D with respect to metabolism and function: why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab 2013;98:4619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LeBoff MS, Murata EM, Cook NR, et al. VITamin D and OmegA-3 TriaL (VITAL): effects of vitamin D supplements on risk of falls in the US population. J Clin Endocrinol Metab 2020;105:2929–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.LeBoff MS, Chou SH, Murata EM, et al. Effects of supplemental vitamin D on bone health outcomes in women and men in the VITamin D and OmegA-3 TriaL (VITAL). J Bone Miner Res 2020;35:883–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.