Abstract
Intranasal administration of live attenuated Bordetella pertussis, from which the pertussis toxin gene has been deleted, has previously been shown to give rise to high levels of serum immunoglobulin G (IgG) antibodies against both the protective antigen filamentous hemagglutinin (FHA) and heterologous antigens genetically fused to FHA. Here, we extend these results by demonstrating that anti-FHA IgA and IgG antibodies are also produced in the genital tract of mice, both in the vagina and in the uterus, after a single intranasal administration of B. pertussis. By comparing the immune responses induced after infection with wild-type virulent B. pertussis with that induced by infection with an attenuated pertussis toxin-deficient strain, we conclude that pertussis toxin produced by the virulent bacteria does not modify antibody production to FHA in the genital tract of B. pertussis-infected mice. The intranasal infection with either the attenuated or the virulent B. pertussis strain also led to the development of immunologic memory that could be efficiently boosted with purified FHA administered either intranasally or intravaginally to give rise to a significant increase in the levels of specific IgA and IgG produced locally in the genital tract, as well as of specific antibodies in the serum. These observations suggest that attenuated B. pertussis could be a promising vector for intranasal administration to induce antibody responses against antigens from sexually transmitted pathogens fused to FHA.
Sexually transmitted viral and bacterial infections of the genital tract are common worldwide and cause significant morbidity. To date, no vaccine is available against such infections as caused, for example, by herpes simplex virus (HSV), human immunodeficiency virus, Chlamydia trachomatis, Neisseria gonorrhoeae, group B streptococcus (GBS), and Haemophilus ducreyi. Protective immunity against many of these sexually transmitted pathogens has been associated with local production of specific immunoglobulin G (IgG) and secretory IgA (5, 12, 20, 22, 29). In addition, the levels of specific circulating antibodies also appear to be important, since passive transfer of specific maternal IgG to the fetus can block neonatal infection with GBS (9). Moreover, systemic antibodies against HIV and HSV-2, in addition to local immunity, could potentially help to control the dissemination of such genital infection. Based on these notions, a single-dose vaccine that could be administered by a mucosal route and was able to induce local production of specific IgA and IgG antibodies in the genital tract, as well as high levels of specific antibodies in the serum, would appear to be very promising for preventing sexually transmitted diseases. However, the production of specific local antibodies in the genital tract is generally difficult to achieve. Recently, several studies with a replicating live-virus vector (6, 11) have demonstrated the efficiency of intranasal delivery for inducing genital immunity.
A live, genetically engineered attenuated form of Bordetella pertussis, the etiologic agent of whooping cough, has been recently described as a new promising bacterial vector for intranasal vaccination by a single dose (18). The novel finding that the deletion of the pertussis toxin (PT) gene results in increased serum antibody production against filamentous hemagglutinin (FHA), a major protective antigen (10), and against heterologous antigens fused to FHA makes attenuated B. pertussis particularly attractive for the development of live vaccines to protect against both whooping cough and other diseases (18). However, nothing is known about the possible induction of immune responses in the genital tract induced by B. pertussis administrations.
The aim of the present study was therefore to investigate whether intranasal administration of PT-deficient or of wild-type B. pertussis could give rise to specific anti-FHA antibody production in the genital tract of mice and to assess the influence of PT, which possesses well-known immunomodulatory properties (19), on the induction of this mucosal response. In addition, we investigated whether this immune response can subsequently be boosted with purified FHA either by the intranasal or by the intravaginal route. The kinetics of mucosal antibody production in the vagina and uterus were analyzed and compared with the corresponding antibody responses in serum and in the respiratory tract.
MATERIALS AND METHODS
Mice.
BALB/c female mice, 6 to 8 weeks old, were obtained from B&K Universal (Stockholm, Sweden, and Bomholtsgård, Denmark). The mice were maintained at the Department of Medical Microbiology and Immunology (Göteborg, Sweden) in animal facilities under pathogen-free conditions by using microisolator cages and sterile workbenches.
Bacterial strains, growth conditions, and intranasal infection of mice.
Wild-type B. pertussis BPSM (15) and attenuated B. pertussis BPRA, a strain in which the pertussis toxin gene had been deleted (2), were previously described. They were grown on Bordet-Gengou agar (Difco, Detroit, Mich.) supplemented with 5% glycerol and 20% defibrinated sheep blood and containing 100 μg of streptomycin (Sigma, St. Louis, Mo.) per ml. Mice were intranasally infected with approximately 5 × 106 B. pertussis microorganisms as described before (17). Three mice from each group were sacrificed 3 h after infection to determine the initial number of viable B. pertussis in the lungs. The lungs were removed aseptically and homogenized in 5 ml of phosphate-buffered saline (PBS). Serially diluted homogenates from individual lungs were plated onto Bordet-Gengou agar, and the number of CFU was determined after 3 to 4 days of incubation at 36°C.
Antigens and booster immunization.
FHA was purified as described elsewhere (14) from culture supernatants of B. pertussis BPRA. At 14 weeks after infection with B. pertussis, the mice were boosted intranasally or intravaginally with 6 μg of FHA in PBS (twice 25 μl at 15-min intervals). All mice were treated subcutaneously with 10 mg (70 μl) medroxyprogesteroneacetate (Depo-Provera; The Upjohn Co., Kalamazoo, Mich.), 10 and 3 days prior to the boost.
Antibody determination.
Serum and bronchoalveolar lavage (BAL) fluids were collected from mice as previously described (17). To extract antibodies from the genital tissue, we used the PERFEXT method (8). Briefly, after extensive perfusion of the animals with 0.1% heparin-PBS to remove the blood, antibodies were extracted in vitro by freezing and thawing the tissues in a solution containing 2% (wt/vol) of saponin (Sigma). After overnight incubation at 4°C, the organs were spun down at 14,000 × g, and the supernatant was analyzed for antibody content. Antibody titers were estimated by enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well microtiter plates (Nunc, Roskilde, Denmark) were coated with 50 μl of FHA (10 μg/ml) in PBS for 2 h 30 min at 37°C. Samples were added and incubated overnight at 4°C. Anti-mouse IgG and IgA horseradish peroxidase-conjugated antibodies (Southern Biotechnology Associates, Inc., Birmingham, Ala.) were then added for 1 h 30 min at 37°C. The ELISA were then developed by using o-phenylenediamine (Sigma) and H2O2. The reaction was stopped by the addition of 50 μl of 2 N HCl. Results are expressed in titers, defined as the reciprocal of the dilution giving an optical density at 492 nm three times that of the conjugate control for IgG or twice that of the conjugate control for IgA.
Cell preparation.
Before the genital tissue (both vagina and uterus) was removed, mice were perfused into the heart with at least 20 ml of 0.1% heparin-PBS (Lövens Kemiske Fabrik, Ballerup, Denmark) to maximally remove blood from the tissues. The cells were prepared by cutting the organs into small pieces. The tissue pieces were incubated in Hank's medium (Gibco, Paisley, Scotland) supplemented with collagenase-dispase (1 mg/ml; Boehringer Mannheim GmbH, Mannheim, Germany) and DNase (0.1 mg/ml; Sigma) for 30 min on a magnetic stirrer at 37°C. The supernatant was saved, and the treatment was repeated once with fresh medium. Finally, the remaining tissue fragments were passed through a nylon screen. Erythrocytes were lysed by osmotic shock with ammonium chloride, and the remaining single cell suspensions were washed three times with PBS and diluted in Iscove's medium (Gibco) containing 10% heat-inactivated fetal calf serum (Gibco), 5 × 10−5 M 2-mercaptoethanol (Sigma), 1 mM l-glutamine (Gibco), 50 μg of gentamicin (Gibco) per ml, and 1.25 μg of fungizone (Gibco) per ml.
Cell ELISA.
Cell suspensions from genital tissue, from both uterus and vagina, were analyzed for FHA-specific antibody-secreting cells by using a cell ELISA derived from a technique developed by Beech et al. (3). Briefly, under sterile conditions, 96-well plates (Greiner-Labtechnik, Frickenhausen, Germany) were coated overnight with FHA (0.5 μg/ml) and were then blocked with PBS containing 1% bovine serum albumin. The cell suspensions were added in duplicate to the antigen-coated wells and incubated for 36 h at 37°C with 5% CO2. After three washes with PBS containing 0.1% Tween, anti-mouse IgG or IgA horseradish peroxidase-conjugated antibodies (Southern Biotechnology Associates) were added for 1 h at 37°C. The ELISA was then developed by using o-phenylenediamine (Sigma) and H2O2. The reaction was stopped by the addition of 50 μl of 2 N HCl. The concentrations of specific IgA and IgG antibodies were estimated by comparison to standard curves established with known amounts of antibodies. For this purpose, in a parallel assay wells were coated overnight with a goat anti-mouse IgA or IgG (Southern Biotechnology Associates) before the addition of known amounts of recombinant mouse IgA or IgG. The results are expressed as the concentration of anti-FHA antibodies secreted per million of cells.
Statistical analysis.
Two-tailed Student's t test for unmatched data was used for analysis of the significance.
RESULTS
Induction of anti-FHA antibody responses in the genital tract after intranasal infection with B. pertussis.
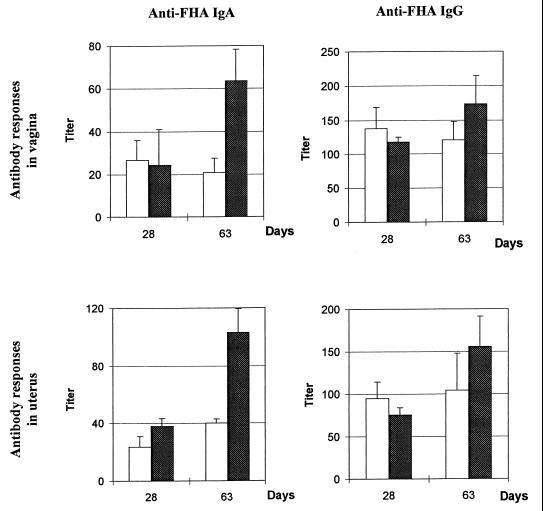
We have previously shown that the PT-deficient B. pertussis BPRA strain is an efficient live attenuated vector for inducing systemic antibody responses after a single intranasal administration (18). To determine whether it could be used to also induce antibody responses in the genital tract, mice were intranasally infected with BPRA, and anti-FHA antibody production in the genital tissues was monitored by using the PERFEXT method (8). Mice infected with the virulent B. pertussis BPSM were used for comparison to determine whether the production of PT by the bacteria may modulate the anti-FHA response in the genital tract. No antibody response could be detected in the genital tract 2 weeks after B. pertussis infection. However, as shown in Fig. 1, 28 days after intranasal infection with PT-deficient BPRA or with virulent BPSM, anti-FHA IgA and IgG were detected both in the vagina and in the uterus. Moreover, these anti-FHA antibody titers remained at a constant level for at least 2 months after infection with the virulent BPSM strain, whereas they increased after infection with the PT-deficient BPRA. Finally, whereas no difference could be observed for IgG titers between the two tissues of the genital tract, titers of anti-FHA IgA tend to reach higher levels in the uterus compared to the vagina.
FIG. 1.
Anti-FHA antibody titers in the genital tract after intranasal infection with B. pertussis. Mice were infected with BPSM (open bars) or BPRA (solid bars). After 28 and 63 days, the anti-FHA IgA and IgG titers were measured in the vagina and uterus. The control IgA and IgG titers in the genital tract of naive mice were less than 4. The results are expressed as the mean ± the standard error of the mean (SEM) of four to five mice per group and are representative of two experiments.
Local production of anti-FHA IgA and IgG in the genital tract after intranasal B. pertussis infection.
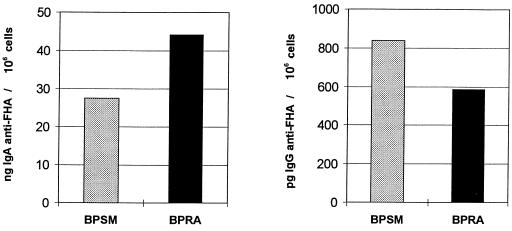
To investigate whether the anti-FHA IgA and IgG are produced locally in the genital tract, we used a cell ELISA to measure the amount of anti-FHA IgA and IgG produced by cells isolated from genital tissue. This technique has been described to detect cytokines secreted from isolated cells and has been shown to efficiently combine the sensitivity of ELISPOT with the quantification abilities of conventional sandwich ELISA (3). We adopted this technique to measure the amount of specific antibodies produced by cells isolated from the genital tissue. As shown in Fig. 2, intranasal infection with either BPRA or BPSM induced FHA-specific IgA and IgG production by cells isolated from the genital tissue of infected animals as measured 1 month after infection, indicating that these antibodies are produced locally. However, local specific IgG production seemed relatively modest as compared to IgA production.
FIG. 2.
Local production of anti-FHA IgA and IgG antibodies by cells isolated from the genital tissue of B. pertussis-infected mice. Mice were infected with BPSM or BPRA or left uninfected. After 28 days, the amounts of specific IgA (left panel) and IgG (right panel) produced by cells isolated from the genital tissue were measured by cell ELISA. Each group consisted of a pool of three mice, and values are given as the amounts of anti-FHA antibodies produced per million cells isolated from the genital tissue. The values obtained for the noninfected mice were substracted as background values. These results are representative of two experiments.
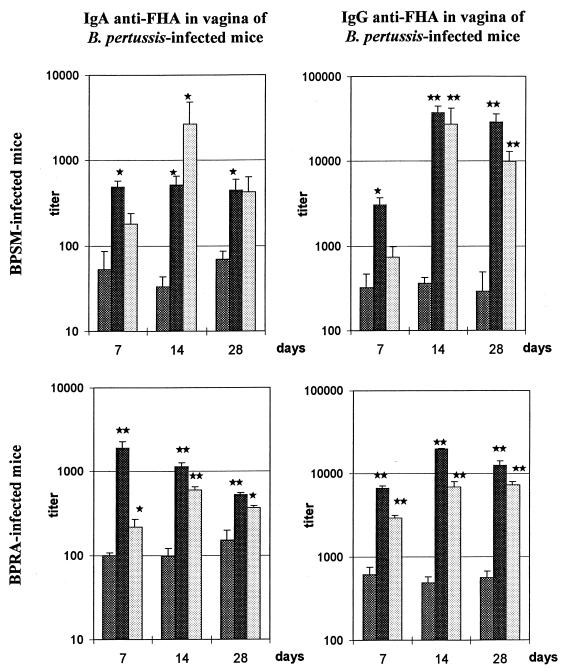
Intranasal and intravaginal boosting of anti-FHA antibody production in genital tract of B. pertussis-infected mice.
To investigate whether intranasal infection with BPSM or BPRA can induce immunologic memory in the genital tract, at 14 weeks after intranasal infection with BPRA or BPSM the mice were boosted with 6 μg of purified FHA either intranasally or intravaginally. The levels of anti-FHA IgA and IgG in the genital tract were then compared by using the PERFEXT method both to a nonboosted group of infected mice and to a noninfected, age-matched group of mice which only received purified FHA intranasally or intravaginally.
No anti-FHA IgA was detected in the genital tract of these latter noninfected but boosted animals at any time point, whereas anti-FHA IgG titers were measured 1 month after FHA administration to previously uninfected mice both in the vagina (24 ± 4 after intranasal immunization and 11 ± 5 after intravaginal immunization) and in the uterus (44 ± 7 after intranasal immunization and 20 ± 8 after intravaginal immunization). In contrast, in mice previously infected with either BPRA or BPSM, both the anti-FHA IgA and IgG responses could be boosted by purified FHA. BPRA-infected and BPSM-infected mice showed similar kinetics of antibody responses after boosting both in the vagina (Fig. 3) and in the uterus (data not shown). Already 1 week after an intranasal boost with FHA, significant increases in both anti-FHA IgA and IgG titers were observed in the vagina and the uterus of infected animals. This increase lasted for at least 1 month (Fig. 3 and data not shown). After the intravaginal boost with FHA, we observed a delay in the onset of the secondary immune response, and the highest titers of both anti-FHA IgA and IgG were found in genital tissues 2 weeks after the boost. Furthermore, an intranasal boost with purified FHA tends to induce higher levels of anti-FHA IgA and IgG in genital tissues of B. pertussis-infected mice than does an intravaginal boost.
FIG. 3.
Anti-FHA antibody titers in the vagina of mice infected with B. pertussis and boosted with purified FHA. Mice were infected with BPSM (upper panels) or BPRA (lower panels) and boosted 14 weeks later with 6 μg of purified FHA by the intranasal (solid bars) or by the intravaginal (open bars) route. The control mice (gray bars) were not boosted. Anti-FHA IgA (left panels) and IgG (right panels) were measured at three different time points (7, 14, and 28 days) after the boost. Results are expressed as mean ± the SEM of four to five mice per group and per time point. Significant differences with control mice, as determined by Student's t test, are indicated: ★, P ≤ 0.05; ★★, P ≤ 0.001.
Stimulation of anti-FHA antibody production in the respiratory tract of B. pertussis-infected mice following intranasal or intravaginal boost with purified FHA.
We also compared the effect of a boost with purified FHA by the intranasal or intravaginal route on the antibody production in the respiratory tract of mice previously infected with B. pertussis. As shown in Table 1, an increase in anti-FHA IgA production in the BAL fluids was found only after an intranasal boost with FHA. This effect was detectable 1 week after the boost and lasted for at least 1 month in mice previously infected with either BPRA or BPSM. A significant increase in anti-FHA IgG titers was measured in the BAL fluids of B. pertussis-infected animals 1 week after the intranasal boost with FHA and was followed by a slow decrease. Furthermore, a slight increase in anti-FHA IgG titers was detected in the BAL fluids after intravaginal boost with the purified FHA.
TABLE 1.
Anti-FHA antibody responses in the BAL fluids of mice infected with B. pertussis and boosted 14 weeks later with FHAa
| Day and FHA boost | Anti-FHA IgA titer
|
Anti-FHA IgG titer
|
||||
|---|---|---|---|---|---|---|
| Noninfected | BPSM infected | BPRA infected | Noninfected | BPSM infected | BPRA infected | |
| Day 7 | ||||||
| None | <4 | 9 ± 2 | 58 ± 33 | <4 | 33 ± 14 | 89 ± 19 |
| Intranasal | <4 | 258 ± 26 | 340 ± 19 | <4 | 1,685 ± 819 | 933 ± 113 |
| Vaginal | <4 | 11 ± 2 | 9 ± 3 | <4 | 104 ± 20 | 141 ± 18 |
| Day 14 | ||||||
| None | <4 | 5 ± 1 | <4 | <4 | 17 ± 6 | 47 ± 5 |
| Intranasal | <4 | 503 ± 146 | 378 ± 146 | 13 ± 2 | 984 ± 272 | 758 ± 58 |
| Vaginal | <4 | 12 ± 6 | 25 ± 7 | 11 ± 5 | 100 ± 34 | 112 ± 22 |
| Day 28 | ||||||
| None | <4 | <4 | 29 ± 8 | <4 | 12 ± 7 | 77 ± 9 |
| Intranasal | <4 | 559 ± 217 | 574 ± 89 | 16 ± 2 | 319 ± 46 | 773 ± 269 |
| Vaginal | <4 | 4 | 12 ± 8 | 7 ± 3 | 69 ± 19 | 117 ± 10 |
BALB/c mice were infected with BPSM or BPRA and either boosted or not boosted 14 weeks later with 6 μg of FHA by the intranasal or the vaginal route. Results are expressed as the means ± the SEM of four to five mice per group. Significant differences (P ≤ 0.05) with control mice, as determined by Student's t test, are indicated in boldface.
Increased serum anti-FHA titers in B. pertussis-infected mice after intranasal or intravaginal boost with purified FHA.
We have previously shown that infection with BPRA induces higher levels of serum anti-FHA IgG than does infection with BPSM (18). We therefore determined whether this difference is still apparent after boosting with FHA. As shown in Table 2, an intranasal boost with FHA in mice previously infected with BPSM or with BPRA resulted in an increase in anti-FHA IgA titers measured in the serum 1, 2, and 4 weeks after the boost. This booster effect was somewhat higher in mice infected with BPSM than in mice infected with BPRA. In contrast to the intranasal boost, no significant variation in serum IgA titers was detected after the intravaginal boost in B. pertussis-infected mice. On the other hand, both the intranasal and the intravaginal boost induced an increase in serum IgG against FHA, although the intranasal boost seemed to give rise to higher titers than the intravaginal boost. The peak of the anti-FHA IgG response in the serum of infected animals was seen 2 weeks after the intravaginal boost, whereas the increase in anti-FHA IgG already began 1 week after the intranasal boost and remained stable thereafter. No significant difference was observed between BPRA-infected mice and BPSM-infected mice, indicating that production of PT by the B. pertussis strain used for the priming of mice does not significantly influence the magnitude and kinetics of the secondary IgG response against FHA in the serum.
TABLE 2.
Anti-FHA antibody responses in the sera of mice infected with B. pertussis and boosted 14 weeks later with FHAa
| Day and FHA boost | Anti-FHA IgA titer
|
Anti-FHA IgG titer (103)
|
||||
|---|---|---|---|---|---|---|
| Noninfected | BPSM infected | BPRA infected | Noninfected | BPSM infected | BPRA infected | |
| Day 7 | ||||||
| None | <10 | 108 ± 83 | 267 ± 49 | <0.05 | 6.49 ± 3.77 | 6.9 ± 0.7 |
| Intranasal | 60 | 1,313 ± 57 | 413 ± 37 | 0.07 | 69.64 ± 22.35 | 196.14 ± 9.39 |
| Vaginal | 15 | 131 ± 57 | 148 ± 20 | 0.11 | 17.34 ± 5.64 | 20.15 ± 6.4 |
| Day 14 | ||||||
| None | ND | 68 ± 8 | 102 ± 17 | ND | 2.14 ± 0.88 | 5.94 ± 0.84 |
| Intranasal | ND | 592 ± 238 | 368 ± 44 | ND | 46.04 ± 10.76 | 41.95 ± 11.38 |
| Vaginal | ND | 295 ± 156 | 111 ± 6 | ND | 36.76 ± 14.47 | 42.85 ± 16.3 |
| Day 28 | ||||||
| None | <10 | 39 ± 13 | 95 ± 7 | 0.08 | 2.88 ± 2.18 | 8.65 ± 1.15 |
| Intranasal | 13 | 667 ± 209 | 471 ± 52 | 1.21 | 76.35 ± 20.47 | 65.55 ± 10.36 |
| Vaginal | <10 | 90 ± 28 | 95 ± 4 | 0.52 | 16.69 ± 4.99 | 26.8 ± 4.8 |
BALB/c mice were infected with BPSM or BPRA and either boosted or not boosted 14 weeks later with 6 μg of FHA by the intranasal or the vaginal route. For noninfected animals, the sera of five mice per time point was pooled before the anti-FHA antibody determination. For infected mice, the results are expressed as the means ± the SEM of four to five mice per group. ND, not determined. Significant differences (P ≤ 0.05) with control mice, as determined by Student's t test, are indicated in boldface.
DISCUSSION
In agreement with the concept of the common mucosal immune system (13, 16), several studies have demonstrated that intranasal immunization can give rise to mucosal antibody responses both in the respiratory and in the genital tracts (4, 6–8, 26, 27). Therefore, we compared the ability of the virulent BPSM and the PT-deficient BPRA B. pertussis strains to induce mucosal antibody responses against FHA after intranasal administration of the bacteria. Kinetic studies showed that the onset of antibody responses in the genital tract was similar for both B. pertussis strains and was apparent 1 month after infection with the production of specific IgA and IgG. The levels of anti-FHA IgA tended to be higher in the uterus than in the vagina. These results are consistent with the notion that the uterus is the main genital tissue involved in IgA secretion in mice (21) and the finding that hysterectomy reduces the levels of specific IgA in vaginal secretions of immunized mice to 5% of normal levels. Cell ELISA demonstrated that cells isolated from the genital tissue of B. pertussis-infected animals were able to produce anti-FHA IgG and IgA, supporting previous observations suggesting a local IgA and IgG production in the genital tract after intranasal immunization (6, 8). One month after intranasal infection, the amounts of anti-FHA antibodies produced by cells isolated from genital tissues were similar in mice previously infected either with the virulent BPSM or with the PT-deficient BPRA strain, indicating that PT production by the bacteria does not have a marked immunomodulating effect on the local antibody response in the genital tract of intranasally infected animals. Although PT has been reported to have adjuvant properties (19, 25, 28), the levels of PT released by the virulent bacteria present in the lungs may perhaps not be sufficient to exert its immunoadjuvant property in the genital tract. Robbinson et al. (24) have shown that the effects of PT on immune responses depend on the concentration. For example, at 100 ng/ml or more PT is strongly mitogenic, whereas at 1 ng/ml it inhibits cell proliferation.
Remarkably, substantial levels of anti-FHA IgA and IgG could still be detected in genital tissue 14 weeks after a single intranasal administration of the bacteria even without boost, demonstrating that a single intranasal immunization with B. pertussis, independently of PT production, induces a long-lasting local response in the genital tract of mice. Anti-FHA IgG titers were even significantly higher 14 weeks after immunization compared to 63 days after immunization, but we cannot rule out a possible contamination of the samples with the serum. This long-lasting immune response in the genital tissue is in agreement with the findings of Amsbaugh and coworkers (1), who described a long-lived systemic and respiratory B-cell-mediated immune response to FHA after respiratory infection of mice with a virulent strain of B. pertussis.
We also investigated the influence of the priming with virulent or PT-deficient B. pertussis on the ability of mice to mount a secondary immune response against FHA in the genital tract. We compared the efficiency of intranasal and intravaginal boosts with purified FHA by measuring anti-FHA IgA and IgG titers in the vaginas and the uteri of infected animals. Since the ability to respond to an antigen varies greatly with the estrous cycle (30), all mice were treated with progesterone before the boost to reduce variations in the results. This hormonal treatment has also been shown to increase the numbers of specific IgA- and IgG-secreting cells after intranasal immunization, and an even more pronounced increase was observed after intravaginal immunization (8). When mice were first infected with B. pertussis and then boosted with FHA, a 1-week delay in the onset of the secondary immune response in the genital tract was found after an intravaginal boost compared to after an intranasal boost, implying a rapid migration of FHA-activated lymphocytes from the respiratory tract to the genital tract followed by local accumulation and differentiation of specific antibody-secreting cells. This migration of immune cells was apparently not influenced by the production of PT by B. pertussis, since the kinetics of the secondary antibody responses were similar after priming with BPSM or with BPRA. Interestingly, boosting at the site of priming, i.e., the respiratory tract, appeared to be more efficient for the induction of a secondary antibody response in the genital tract than boosting at the expression site. Moreover, the vigorous secondary immune responses obtained in mice previously infected with either B. pertussis strain and subsequently boosted intranasally or intravaginally with FHA implies the induction of highly efficient immune memory by intranasal infection with B. pertussis, regardless of the production of PT.
In summary, we have demonstrated that a single intranasal administration of live attenuated B. pertussis is able to induce long-lasting specific antibody responses against FHA in the genital tract of female mice. These responses can be significantly boosted with purified FHA either by the intranasal or by the intravaginal route. Both routes give rise to high levels of IgA and IgG against FHA in the genital tissue as well as in serum of mice previously infected with B. pertussis. In addition to the ease of administration compared to the intravaginal route, the intranasal boost has the advantage to also induce mucosal IgA and a high level of IgG against the antigen in the respiratory tract. B. pertussis, especially PT-deficient attenuated strains, have been proposed as live vaccine carriers for the delivery of heterologous protective antigens to the respiratory tract (18, 23). This present study is to our knowledge the first time that a bacterial vector specifically designed for the respiratory tract, was described as a candidate vector strain to induce specific genital immunity.
In the light of this study, it would therefore be interesting to test whether the intranasal administration of attenuated recombinant B. pertussis producing antigens from sexually transmitted microorganisms may result in protective immunity against these pathogens in the genital tract.
ACKNOWLEDGMENTS
We thank E. Fort for help with purification of FHA and E.-L. Johansson for helpful discussion. We are grateful to R. Antoine for providing B. pertussis BPRA.
This work was supported by the European Community (Marie Curie fellowship contract ERB4001GT972206 and project contract BMH4-CT97-2345) and by the Swedish Medical Research Council (project 16X-3382).
REFERENCES
- 1.Amsbaugh D F, Li Z-M, Shahin R D. Long-lived respiratory immune response to filamentous hemagglutinin following Bordetella pertussis infection. Infect Immun. 1993;61:1447–1452. doi: 10.1128/iai.61.4.1447-1452.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoine R, Locht C. Roles of the disulfide bond and the carboxy-terminal region of the S1 subunit in the assembly and biosynthesis of pertussis toxin. Infect Immun. 1990;58:1518–1526. doi: 10.1128/iai.58.6.1518-1526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beech J T, Bainbridge T, Thompson S J. Incorporation of cells into an ELISA system enhances antigen-driven lymphokine detection. J Immunol Methods. 1997;205:163–168. doi: 10.1016/s0022-1759(97)00072-0. [DOI] [PubMed] [Google Scholar]
- 4.Bergquist C, Johansson E-L, Lagergård T, Holmgren J, Rudin A. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect Immun. 1997;65:2676–2684. doi: 10.1128/iai.65.7.2676-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui Z-D, Tristram D, Lascolea L J, Kwiatkowski T, Jr, Kopti S, Ogra P L. Induction of antibody response to Chlamydia trachomatis in the genital tract by oral immunization. Infect Immun. 1991;59:1465–1469. doi: 10.1128/iai.59.4.1465-1469.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallichan W S, Rosenthal K L. Specific secretory immune responses in the female genital tract following intranasal immunization with a recombinant adenovirus expressing glycoprotein B of herpes simplex virus. Vaccine. 1995;13:1589–1595. doi: 10.1016/0264-410x(95)00100-f. [DOI] [PubMed] [Google Scholar]
- 7.Hordnes K, Tynning T, Brown T A, Haneberg B, Jonsson R. Nasal immunization with group B streptococci can induce high levels of specific IgA antibodies in cervicovaginal secretions of mice. Vaccine. 1997;15:1244–1251. doi: 10.1016/s0264-410x(97)00021-2. [DOI] [PubMed] [Google Scholar]
- 8.Johansson E-V, Rask C, Fredriksson M, Eriksson K, Czerkinsky C, Holmgren J. Antibodies and antibody-secreting cells in the female genital tract after vaginal or intranasal immunization with cholera toxin B subunit or conjugates. Infect Immun. 1998;66:514–520. doi: 10.1128/iai.66.2.514-520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasper D L. Designer vaccines to prevent infections due to group B Streptococcus. Proc Assoc Am Physicians. 1995;107:369–373. [PubMed] [Google Scholar]
- 10.Locht C, Bertin P, Menozzi F D, Renauld G. The filamentous hemagglutinin, a multifaceted adhesin produced by virulent Bordetella spp. Mol Microbiol. 1993;9:653–660. doi: 10.1111/j.1365-2958.1993.tb01725.x. [DOI] [PubMed] [Google Scholar]
- 11.Lubeck M D, Natuk R J, Chengalvala M, Chanda P K, Murthy K K, Murthy S, Mizutani S, Lee S G, Wade M S, Bhat B M, Bhat R, Dheer S K, Eichberg J W, Davis A R, Hung P P. Immunogenicity of recombinant adenovirus-human immunodeficiency virus vaccines in chimpanzees following intranasal administration. AIDS Res Hum Retroviruses. 1994;10:1443–1449. doi: 10.1089/aid.1994.10.1443. [DOI] [PubMed] [Google Scholar]
- 12.Mazzoli S, Trabbatoni D, Caputo S L, Piconi S, Ble C, Meacci F, Ruzzante S, Salvi A, Semplici F, Longhi R, Fusi M L, Tofani N, Biasin M, Villa M L, Mazzotta F, Clerici M. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat Med. 1997;11:1250–1257. doi: 10.1038/nm1197-1250. [DOI] [PubMed] [Google Scholar]
- 13.McDermott M R, Bienenstock J. Evidence for a common mucosal immune system I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979;122:1892–1898. [PubMed] [Google Scholar]
- 14.Menozzi F D, Gantiez C, Locht C. Interaction of Bordetella pertussis filamentous hemagglutinin with heparin. FEMS Microbiol Lett. 1991;78:59–64. doi: 10.1111/j.1574-6968.1991.tb04417.x. [DOI] [PubMed] [Google Scholar]
- 15.Menozzi F D, Mutombo R, Renauld G, Gantiez C, Hannah J H, Leininger E, Brennan M J, Locht C. Heparin-inhibitable lectin activity of the filamentous hemagglutinin adhesin of Bordetella pertussis. Infect Immun. 1994;62:769–778. doi: 10.1128/iai.62.3.769-778.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7:265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 17.Mielcarek N, Cornette J, Schacht A-M, Pierce R J, Locht C, Capron A, Riveau G. Intranasal priming with recombinant Bordetella pertussis for the induction of a systemic immune responses against a heterologous antigen. Infect Immun. 1997;65:544–550. doi: 10.1128/iai.65.2.544-550.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mielcarek N, Riveau G, Remoué F, Antoine R, Capron A, Locht C. Homologous and heterologous protection after single intranasal administration of live attenuated recombinant Bordetella pertussis. Nat Biotechnol. 1998;16:454–457. doi: 10.1038/nbt0598-454. [DOI] [PubMed] [Google Scholar]
- 19.Munoz J J. Action of pertussigen (pertussis toxin) on the host immune system. In: Wardlaw A C, Parton R, editors. Pathogenesis and immunity in pertussis. New York, N.Y: John Wiley & Sons, Ltd.; 1988. pp. 173–192. [Google Scholar]
- 20.Pal S, The I, Peterson E M, de la Maza L M. Monoclonal immunoglobulin A antibody to the major outer membrane protein of the Chlamydia trachomatis mouse pneumonitis biovar protects mice against chlamydial challenge. Vaccine. 1997;15:575–582. doi: 10.1016/s0264-410x(97)00206-5. [DOI] [PubMed] [Google Scholar]
- 21.Parr E L, Parr M B. A comparison of antibody titres in mouse uterine fluid after immunization by several routes, and the effect of the uterus on antibody titres in vaginal fluid. J Reprod Fertil. 1990;89:619–625. doi: 10.1530/jrf.0.0890619. [DOI] [PubMed] [Google Scholar]
- 22.Parr E L, Parr M B. Immunoglobulin G is the main protective antibody in mouse vaginal secretions after vaginal immunization with attenuated herpes simplex virus type 2. J Virol. 1997;71:8109–8115. doi: 10.1128/jvi.71.11.8109-8115.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renauld-Mongénie G, Mielcarek N, Cornette J, Schacht A M, Capron A, Riveau G, Locht C. Induction of mucosal immune responses against a heterologous antigen fused to filamentous hemagglutinin after intranasal immunization with recombinant Bordetella pertussis. Proc Natl Acad Sci USA. 1996;93:7944–7949. doi: 10.1073/pnas.93.15.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robbinson D, Cockle S, Singh B, Strejan G H. Native, but not genetically inactivated, pertussis toxin protects mice against experimental allergic encephalomyelitis. Cell Immunol. 1996;168:165–173. doi: 10.1006/cimm.1996.0063. [DOI] [PubMed] [Google Scholar]
- 25.Roberts M, Bacon A, Rappuoli R, Pizza M, Cropley I, Douce G, Dougan G, Marinaro M, McGhee J, Chatfield S. A mutant pertussis toxin molecule that lacks ADP-ribosyltransferase activity, PT-9K/129G, is an effective mucosal adjuvant for intranasally delivered proteins. Infect Immun. 1995;63:2100–2108. doi: 10.1128/iai.63.6.2100-2108.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudin A, Riise G C, Holmgren J. Antibody responses in the lower respiratory tract and male urogenital tract in humans after nasal and oral vaccination with cholera toxin B subunit. Infect Immun. 1999;67:2884–2890. doi: 10.1128/iai.67.6.2884-2890.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell M W, Moldoveanu Z, White P L, Sibert G J, Mestecky J, Michalek S. Salivary, nasal, genital, and systemic antibody responses in monkeys immunized intranasally with a bacterial protein antigen and the cholera toxin B subunit. Infect Immun. 1996;64:1272–1283. doi: 10.1128/iai.64.4.1272-1283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryan R, McCarthy L, Rappuoli R, Mahon B P, Mills K H G. Pertussis toxin potentiates Th1 and Th2 responses to co-injected antigen: adjuvant action is associated with enhanced regulatory cytokine production and expression of the co-stimulatory molecules B7-1, B7-2 and CD28. Int Immunol. 1998;10:651–662. doi: 10.1093/intimm/10.5.651. [DOI] [PubMed] [Google Scholar]
- 29.Whaley K J, Zeitlin L, Barratt R A, Hoen T E, Cone R A. Passive immunization of the vagina protects mice against vaginal transmission of genital herpes infections. J Infect Dis. 1994;169:647–649. doi: 10.1093/infdis/169.3.647. [DOI] [PubMed] [Google Scholar]
- 30.Wira C R, Rossoll R M. Antigen presenting cells in the female reproductive tract: influence of sex hormones on antigen presentation in the vagina. Immunology. 1995;84:505–508. [PMC free article] [PubMed] [Google Scholar]