Abstract
Purpose
To evaluate the safety and efficacy of selective internal radiation therapy (SIRT) in combination with a PD-1 inhibitor in patients with unresectable hepatocellular carcinoma (uHCC) and liver-only disease ineligible for chemoembolization.
Patients and methods
NASIR-HCC is a single-arm, multicenter, open-label, phase 2 trial that recruited from 2017 to 2019 patients who were naïve to immunotherapy and had tumors in the BCLC B2 substage (single or multiple tumors beyond the up-to-7 rule), or unilobar tumors with segmental or lobar portal vein invasion (PVI); no extrahepatic spread; and preserved liver function. Patients received SIRT followed 3 weeks later by nivolumab (240 mg every 2 weeks) for up to 24 doses or until disease progression or unacceptable toxicity. Safety was the primary endpoint. Secondary objectives included objective response rate (ORR), time to progression (TTP), and overall survival (OS).
Results
42 patients received SIRT (31 BCLC-B2, 11 with PVI) and were followed for a median of 22.2 months. 27 patients discontinued and 1 never received Nivolumab. 41 patients had any-grade adverse events (AE) and 21 had serious AEs (SAE). Treatment-related AEs and SAEs grade 3–4 occurred in 8 and 5 patients, respectively. Using RECIST 1.1 criteria, ORR reported by investigators was 41.5% (95% CI 26.3% to 57.9%). Four patients were downstaged to partial hepatectomy. Median TTP was 8.8 months (95% CI 7.0 to 10.5) and median OS was 20.9 months (95% CI 17.7 to 24.1).
Conclusions
The combination of SIRT and nivolumab has shown an acceptable safety profile and signs of antitumor activity in the treatment of patients with uHCC that were fit for SIRT.
Trial registration number
Keywords: immunotherapy; liver neoplasms; radioimmunotherapy; programmed cell death 1 receptor; cytotoxicity, immunologic
WHAT IS ALREADY KNOWN ON THIS TOPIC
Selective internal radiation therapy (SIRT) using yttrium-90 microspheres and PD1 inhibitors are used to treat patients with liver cancer but there is very limited information about the safety and efficacy of the combination of both therapies.
WHAT THIS STUDY ADDS
In patients with hepatocellular carcinoma (HCC) who are not good candidates for TACE despite being free from extrahepatic metastasis, SIRT using SIR-Spheres resin microspheres followed by nivolumab produced no new signs of enhanced toxicity, with most patients receiving nivolumab as planned, and the observed time to progression and overall survival were encouraging.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The efficacy of the combination of SIRT and nivolumab deserves to be studied in prospective randomized clinical trials in this population of patients with HCC and large or multiple tumors or those with segmental or lobar portal vein invasion. The outcomes observed in this study provide the benchmark for the design of such trials.
Introduction
Liver cancer is the third-leading cause of cancer-related deaths worldwide, and hepatocellular carcinoma (HCC) accounts for more than 80% of cases.1 Unresectable HCC patients are typically in the intermediate and advanced stages.2 Intermediate means asymptomatic, multinodular liver-only disease while advanced means mild impairment of performance status, vascular invasion or extrahepatic spread. Intraarterial therapies are the mainstay of the treatment of the former while systemic therapy is mostly used for the latter. Immunotherapy with the combination of atezolizumab plus bevacizumab is widely recommended as first-line systemic therapy for advanced HCC.3 4 Transarterial chemoembolization (TACE) is the most common intra-arterial therapy and ideal candidates for TACE are those with limited burden of disease that can be targeted by superselective embolization.2
Selective internal radiation therapy (SIRT) has been proposed as an alternative intra-arterial therapy for patients with a higher burden of disease including those with segmental or lobar portal vein invasion (PVI).5 SIR-Spheres are resin microspheres containing yttrium-90, a pure beta-emitting isotope. Patients treated by SIRT using SIR-Spheres reach a median survival of 17 months if they are in the intermediate Barcelona Clinic Liver Cancer (BCLC) stage B and 10 months if they are in the advanced BCLC stage C with limited PVI.6 Phase 3 clinical trials have not shown improved survival when SIRT alone7 8 or in combination with sorafenib9 were compared with sorafenib alone. The most common pattern of progression after SIRT is the onset of new tumor lesions inside or outside the liver,10 an event that carries a poor prognosis. Therefore, the combination of SIRT with an effective, well-tolerated systemic therapy could result in improved efficacy and preserved quality of life.
Nivolumab is a fully human immunoglobulin G4 that selectively blocks the interaction between programmed death 1 (PD-1) expressed on activated T cells, with its ligands PD-L1/PD-L2 thus preventing T cells from being inactivated.11 Nivolumab has demonstrated durable tumor responses with good tolerability in naïve and sorafenib-treated patients with advanced HCC.12 13 SIRT increases the presence of activated CD8+T cells in the tumor microenvironment14 and may therefore provide synergistic efficacy with Nivolumab. NASIR-HCC has assessed the combination of SIRT and immunotherapy in HCC patients with liver-only disease.
Methods
Study design and population
NASIR-HCC (CA209-992) is a phase 2, multicenter, open-label, single-arm study of the safety and efficacy of Nivolumab in combination with SIRT using SIR-Spheres for the treatment of patients with HCC that are candidates for locoregional therapies. The study was conducted in nine academic centers in Spain (online supplemental file).
jitc-2022-005457supp001.pdf (349.5KB, pdf)
Eligible patients had unresectable HCC and were considered ineligible for TACE because either (i) they were in the BCLC-B2 substage,15 which includes single tumors (BCLC-A stage) if they are >5 cm or multiple tumors (BCLC-B stage) if they fall beyond the up-to-7 rule (number of tumors plus size of the largest lesion in cm >7); or (ii) they were in BCLC-C stage due to predominantly unilobar tumors with segmental or lobar PVI. Additional eligibility criteria are provided in online supplemental file.
All SIRT evaluations and treatments were centrally performed at Clinica Universidad de Navarra as a single-day procedure. A detailed SIRT protocol is provided in online supplemental file. SIRT was performed selectively, eventually through multiple microspheres injections, to preserve the largest possible liver volume from receiving any amount of radiation. Activity calculation took into account the cirrhotic status of the liver and the amount of liver volume spared from irradiation, with the aim to maximize tumor absorbed dose when deemed safe.16 Such individualized dosimetry was used whenever two liver segments were spared from radiation. Nivolumab (240 mg IV every 14 days) was started 3 weeks after SIRT visit and maintained until tumor progression, unacceptable toxicity or a maximum of 24 doses. Tumor response was assessed by investigators using Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 criteria17 every 6 weeks for the first year, and then every 12 weeks thereafter until progression. Treatment with Nivolumab beyond progression was allowed under protocol-defined circumstances detailed in online supplemental file.
Outcomes
The primary endpoints were the rate and type of adverse events (AEs), serious AEs (SAEs), events of liver decompensation, and transient and permanent drug discontinuations due to toxicity. Immune-mediated adverse events (IMAE) related to nivolumab that were treated with corticosteroids were specifically recorded. Hepatic AEs (HAEs) were defined as those AE that have the liver as the target organ or represent usual complications of cirrhosis, including hepatobiliary events, liver-related investigations, thrombocytopenia, ascites, encephalopathy, spontaneous bacterial peritonitis and GI hemorrhage. Toxicity was graded according to Common Terminology Criteria for Adverse Events (CTCAE) V.4.0. Secondary endpoints are defined in detail in supplemental data and included overall response rate (ORR), disease control rate (DCR), duration of response (DoR), time to progression (TTP), progression-free survival (PFS), and pattern of progression. Exploratory objectives were overall survival (OS); efficacy based on tumor cell programmed death ligand 1 (PD-L1) expression and other tissue and blood biomarker; impact of the albumin-bilirubin (ALBI) score on safety and efficacy; and health related quality of life (HRQoL).
Statistical analyses
The primary objective was safety, but the study was considered key to explore the clinical benefit of combining nivolumab with SIRT. A sample size of 40 patients was determined adequate to provide safety information based on a 90% probability of observing at least one occurrence of any AE that might occur with a 5% incidence. At the time of study design, the estimated TTP after SIRT alone in a similar population was 3 months18 and sample size of 40 patients receiving SIRT plus at least 3 doses of Nivolumab would therefore allow to detect a relevant signal of incremental efficacy as detailed in online supplemental file.
Safety analysis included all patients who received SIRT while efficacy analysis included those who received SIRT and one or more doses of nivolumab. All AEs were summarized and reported by organ system, preferred term, and coded per the current version of the Medical Dictionary for Regulatory Activities. ORR and the corresponding 95% CI were calculated using the Clopper-Pearson method. The Kaplan-Meier method was used to analyze and plot time to events (TTP, DoR, PFS and OS) and median values were reported with 95% CI. The analysis of HRQoL will be reported separately.
Results
Population and baseline characteristics
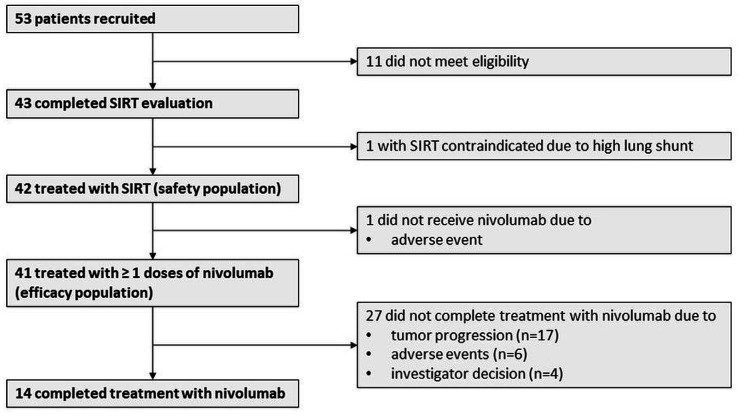
Forty-three patients were enrolled between January 2018 and April 2019 (figure 1). SIRT was contraindicated in one patient (2.3%) due to a hepatopulmonary shunt fraction >20%. The remaining 42 patients received SIRT and comprised the safety population. One patient with an incompetent ampulla of Vater developed liver abscesses after SIRT and never received Nivolumab, 27 discontinued Nivolumab during the study period mostly due to disease progression (n=17), and 14 patients received 24 doses of nivolumab as planned. Baseline demographic and clinical characteristics are listed in table 1. Six patients had received sorafenib, with a median of 10 weeks between the last dose of sorafenib and study entry.
Figure 1.
Flow chart. SIRT, selective internal radiation therapy.
Table 1.
Patient demographics and baseline characteristics
| All patients | BCLC-B2 substage | Unilobar tumors with portal vein invasion | |
| Patients (n, %) | 42 (100) | 31 (73.8) | 11 (26.2) |
| Males (n, %) | 36 (85.7) | 27 (87.1) | 9 (81.1) |
| Age in years (median, IQR) | 65 (49–79) | 65 (49–79) | 65 (55–79) |
| Vascular invasion (n, %) | 11 (26.2) | 0 | 11 (100) |
| BCLC stage (n, %) | |||
| A | 3 (7.1) | 3 (9.7) | 0 |
| B | 25 (59.6) | 25 (80.6) | 0 |
| C | 14 (33.3) | 3 (9.7) | 11 (100) |
| Etiology (n, %) | |||
| Uninfected | 32 (76.2) | 25 (80.6) | 7 (63.6) |
| Hepatitis C | 9 (21.4) | 5 (16.1) | 4 (36.4) |
| Hepatitis B | 1 (2.4) | 1 (3.2) | 0 |
| Alcohol consumption (n, %) | 5 (11.9) | 3 (9.6) | 2 (18.1) |
| Arterial hypertension (n, %) | 20 (47.6) | 16 (51.6) | 4 (36.4) |
| Diabetes (n, %) | 10 (23.8) | 7 (22.6) | 3 (27.3) |
| Dyslipidemia (n, %) | 9 (21.4) | 8 (25.8) | 1 (9.1) |
| ECOG performance status (n, %) | |||
| 0 | 38 (90.5) | 28 (90.3) | 10 (90.9) |
| 1 | 4 (9.5) | 3 (9.7) | 1 (9.1) |
| Child-Pugh class (n, %) | |||
| A5 | 36 (85.7) | 27 (87.1) | 9 (81.8) |
| A6 | 6 (14.3) | 4 (12.9) | 2 (18.2) |
| ALBI grade (n, %) | |||
| 1 | 21 (50) | 17 (54.9) | 4 (36.4) |
| 2 | 21 (50) | 14 (45.1) | 7 (63.6) |
| Previous treatment (n, %) | |||
| Liver resection | 7 (16.7) | 5 (16.1) | 2 (18.2) |
| Percutaneous ablation | 6 (14.3) | 5 (16.1) | 1 (9.1) |
| TACE | 11 (26.2) | 11 (35.5) | 0 |
| Sorafenib | 6 (14.3) | 5 (16.1) | 1 (9.1) |
| Alpha-fetoprotein >400 ng/mL (n, %) | 12 (29.3) | 7 (23.3) | 5 (45.5) |
| Platelet count, /pL (median, IQR) | 141 (46–512) | 139 (46–512) | 145 (59–288) |
| Total bilirubin, mg/dL (median, IQR) | 0.76 (0.20–1.89) | 0.72 (0.20–1.89) | 0.90 (0.40–1.40) |
| Albumin, g/dL (median, IQR) | 3.95 (3.00–4.80) | 4.00 (3.00–4.80) | 3.83 (3.20–4.80) |
| Neutrophil to lymphocyte ratio (median, IQR) | 2.69 (1.86–4.25) | 2.61 (1.83–4.15) | 3.38 (1.87–4.34) |
ALBI, Albumin-Bilirubin; BCLC, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group; TACE, Transarterial chemoembolization.
Treatment
SIRT details are summarized in online supplemental table S1. The median time from informed consent to SIRT was 22 days (IQR 12 days). An effort was made to perform SIRT highly selectively. According to the volume of liver receiving any amount of radiation, SIRT was sublobar in 17%, lobar in 55%, and extended lobar or whole-liver in 28%, with multiple SIR-Spheres injections performed in 62% of patients. Activity was calculated using the partition model to maximize the dose delivered to the tumor compartment at >120 Gy in 25 patients (tumor-targeted dose group) while in the remaining 17 patients (liver-targeted dose group) either the partition model was used to restrict the dose delivered to the non-tumoral compartment to 40 Gy (n=9) or a modified BSA method was used to calculate the activity (n=8).
At database lock in February 2021, the median minimum follow-up was 22.2 months (range 2.7–35.6). The median time from SIRT to first dose of nivolumab was 3.1 weeks and 3 patients started nivolumab 4 weeks or more after SIRT (4.5, 4.8, and 6.1 weeks) due to AEs. Twenty-eight patients (66.6%) discontinued or never received nivolumab. The reason for treatment discontinuation was as per investigator’s decision in 4 patients. In three of these cases tumors previously considered unresectable turned resectable after tumor regression and/or contralateral hypertrophy. One additional patient who interrupted nivolumab due to diarrhea was also considered resectable. Complete tumor resection was achieved in these 4 patients 26, 27, 37, and 46 weeks after SIRT, with no postoperative deaths recorded. Allfour patients were alive and recurrence-free 11, 16, 17 and 29 months after resection (22, 23, 24 and 35 months after SIRT).
Patients were on nivolumab for a median of 32.9 weeks (range 2.1–48.8 weeks). Fourteen patients (33.3%) completed nivolumab treatment as planned. Seven patients who reached the end of the treatment period with stable disease (n=4) or showing partial tumor response (n=3) were maintained on Nivolumab off-study based on local availability and investigator decision. Nine patients (21.9%) received tyrosine kinase inhibitors poststudy.
Safety
A summary of AEs is presented in table 2. AEs and SAEs grade 3–4 were observed in 19% and 26% of patients, respectively. No treatment-related deaths were reported. The incidence and type of SAEs was not different in patients in the BCLC-B2 substage versus those with lobar PVI. Treatment-related AE related to nivolumab (IMAE) or SIRT are detailed in table 3.
Table 2.
Summary of AEs
| Patients with adverse events, no (%)* | ||
| Any grade | Grade 3–4 | |
| All causality AEs | 41 (98) | 8 (19) |
| Treatment-related AEs | 33 (79) | 8 (19) |
| Related to SIRT | 21 (50) | 2 (5) |
| Related to nivolumab (IMAE) | 27 (64) | 6 (14) |
| All causality SAEs | 21 (50) | 11 (26) |
| Treatment-related SAEs | 5 (12) | 5 (12) |
| Related to SIRT | 1 (2) | 1 (2) |
| Related to Nivolumab (IMAE) | 4 (9) | 4 (9) |
| AEs of special interest with incidence >10% | ||
| Hepatic | 30 (71) | 8 (19) |
| Blood | 16 (38) | 0 |
| Gastrointestinal | 16 (38) | 3 (7) |
| Skin | 12 (29) | 0 |
| Endocrine | 10 (24) | 2 (5) |
*AEs and SAEs are reported separately.
AE, adverse event; SAEs, serious AEs; SIRT, selective internal radiation therapy.
Table 3.
Treatment-related adverse events related to SIRT or nivolumab
| System organ class, preferred term | Patients with treatment-related adverse events, no (%) | |
| Event | Any grade | Grade 3-5 |
| Related to SIRT | ||
| Hepatobiliary disorders | ||
| Hyperbilirubinemia | 8 (19) | 1 (2) |
| ALT increased | 1 (2) | 0 |
| AST Increased | 1 (2) | 0 |
| Blood and lymphatic system disorders | ||
| Thrombocytopenia | 7 (17) | 0 |
| Lymphopenia | 1 (2) | 0 |
| Infections and infestations | ||
| Liver abscess | 1 (2) | 1 (2) |
| Gastrointestinal disorders | ||
| Ascites | 1 (2) | 0 |
| General disorders | ||
| Fever | 1 (2) | 0 |
| Vascular disorders | ||
| Hematoma | 1 (2) | 0 |
| Related to nivolumab (IMAEs) | ||
| Hepatobiliary disorders | ||
| ALT increased | 5 (12) | 1 (2) |
| AST increased | 6 (14) | 1 (2) |
| Hyperbilirubinemia | 2 (5) | 0 |
| Immune hepatitis | 2 (5) | 1 (2) |
| Endocrine disorders | ||
| Hypothyroidism | 4 (10) | 0 |
| Thyroiditis | 2 (5) | 0 |
| Hyperthyroidism | 1 (2) | 0 |
| Skin and subcutaneous tissue disorders | ||
| Pruritus | 4 (10) | 0 |
| Rash | 3 (7) | 0 |
| Dermatitis | 2 (5) | 0 |
| Gastrointestinal disorders | ||
| Diarrhea | 2 (5) | 1 (2) |
| Blood and lymphatic system disorders | ||
| Anemia | 2 (5) | |
| Metabolism and nutrition disorders | ||
| Diabetes mellitus | 1 (2) | 1 (2) |
| Hyperosmolar nonketotic syndrome | 1 (2) | 1 (2) |
| Renal and urinary disorders | ||
| Renal impairment | 1 (2) | 1 (2) |
| Tubulointerstitial nephritis | 1 (2) | 1 (2) |
| Blood creatinine increased | 1 (2) | 0 |
| Musculoskeletal and connective tissue disorders | ||
| Back pain | 1 (2) | 0 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; SIRT, selective internal radiation therapy.
Eighteen patients (43.9%) had at least one nivolumab dose delay due to AEs (online supplemental table S2) and three patients (7.2%) had three or more dose delays. HAEs resulted in dose delays in 9 (21.4%) patients. Delays occurred less frequently after sublobar SIRT (14.2%) compared with lobar (52.1%) or lobar extended/whole-liver SIRT (25%). Nivolumab was discontinued due to AEs in six patients (online supplemental table S3). Two such AEs were considered related to SIRT (liver abscesses in a patient with incompetent ampulla of Vater despite antibiotic prophylaxis; and hyperbilirubinemia) and one was related to nivolumab (grade 3 diarrhea). Events of liver decompensation occurred in 18 (42.9%) patients during follow-up and were more frequent among patients receiving a liver-targeted dose (n=11, 61.1%) than a tumor-targeted dose (n=7, 38.9%), and correspondingly among patients receiving SIRT with a whole-liver or lobar extended design (n=8, 44.4%) vs a sublobar design (n=4, 16.6%).
Nine IMAEs requiring steroids were reported in eight patients and are listed in online supplemental table S4. One patient permanently discontinued Nivolumab due to diarrhea while the other patients with IMAEs were able to resume it. No patient required treatment with immunosuppressors other than corticosteroids.
HAEs that (A) were grade 3 or 4, (B) resulted in nivolumab dose delays or discontinuation, (C) were related to SIRT or to nivolumab, or (D) consisted in increased bilirubin or complications of cirrhosis, were observed more frequently in patients with ALBI grade 2 at baseline (table 4). As the volume of SIRT-targeted liver increased from sublobar to lobar or whole-liver SIRT, the proportion of patients with HAEs related to SIRT also increased, but the incidence of HAEs resulting in nivolumab dose delays or discontinuation was similar between subgroups. When treatment-related AEs of any class resulting in nivolumab dose delays were considered (and not only HAEs), a similar proportion of patients had baseline ALBI grades 1 and 2 (62% and 47%, respectively).
Table 4.
Patients with hepatic adverse events of all causality, including hepatobiliary events, liver-related investigations, liver-related infections, thrombocytopenia, ascites, encephalopathy, bacterial peritonitis and GI hemorrhage
| All patients (n=42) | ALBI grade at baseline | SIRT design | Tumor burden | |||||||||||||
| 1 (n=21) | 2 (n=21) | Sublobar (n=7) | Lobar (n=23) | Whole-liver (n=12) | BCLC-B2 (n=31) | Unilobar PVI (n=11) | ||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Any grade | 32 | 76.2 | 16 | 76.2 | 16 | 76.2 | 5 | 71.4 | 15 | 65.2 | 12 | 100.0 | 25 | 80.6 | 7 | 63.6 |
| Grade 3–4 | 9 | 21.4 | 2 | 9.5 | 7 | 33.3 | 2 | 28.6 | 4 | 17.4 | 3 | 25.0 | 7 | 22.6 | 2 | 18.2 |
| Resulting in nivolumab dose delay | 11 | 26.2 | 1 | 4.8 | 10 | 47.6 | 2 | 28.6 | 5 | 21.7 | 4 | 33.3 | 8 | 25.8 | 3 | 27.3 |
| Related to SIRT | 14 | 33.3 | 6 | 28.6 | 8 | 38.1 | 1 | 14.3 | 6 | 26.1 | 7 | 58.3 | 13 | 41.9 | 1 | 9.1 |
| Related to nivolumab | 7 | 16.7 | 2 | 9.5 | 5 | 23.8 | 1 | 14.3 | 3 | 13.0 | 3 | 25.0 | 6 | 19.4 | 1 | 9.1 |
| Type of event | ||||||||||||||||
| Increased AST/ALT* | 18 | 42.9 | 9 | 42.9 | 9 | 42.9 | 4 | 57.1 | 6 | 26.1 | 8 | 66.7 | 16 | 51.6 | 2 | 18.2 |
| Increased bilirubin† | 16 | 38.1 | 4 | 19.0 | 12 | 57.1 | 0 | 0.0 | 8 | 34.8 | 8 | 66.7 | 14 | 45.2 | 2 | 18.2 |
| Thrombocytopenia | 12 | 28.6 | 7 | 33.3 | 5 | 23.8 | 1 | 14.3 | 7 | 30.4 | 4 | 33.3 | 11 | 35.5 | 1 | 9.1 |
| Ascites | 4 | 9.5 | 0 | 0.0 | 4 | 19.0 | 0 | 0.0 | 3 | 13.0 | 1 | 8.3 | 2 | 6.5 | 2 | 18.2 |
| Bacterial peritonitis | 3 | 7.1 | 0 | 0.0 | 3 | 14.3 | 0 | 0.0 | 3 | 13.0 | 0 | 0.0 | 1 | 3.2 | 2 | 18.2 |
| Encephalopathy | 3 | 7.1 | 0 | 0.0 | 3 | 14.3 | 0 | 0.0 | 2 | 8.7 | 1 | 8.3 | 3 | 9.7 | 0 | 0.0 |
| Hepatic function abnormal | 2 | 4.8 | 0 | 0.0 | 2 | 9.5 | 0 | 0.0 | 1 | 4.3 | 1 | 8.3 | 0 | 0.0 | 2 | 18.2 |
| Liver abscess | 2 | 4.8 | 1 | 4.8 | 1 | 4.8 | 1 | 14.3 | 1 | 4.3 | 0 | 0.0 | 2 | 6.5 | 0 | 0.0 |
| Hematemesis | 1 | 2.4 | 0 | 0.0 | 1 | 4.8 | 0 | 0.0 | 0 | 0.0 | 1 | 8.3 | 1 | 3.2 | 0 | 0.0 |
| Hepatitis immune | 1 | 2.4 | 1 | 4.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 8.3 | 1 | 3.2 | 0 | 0.0 |
| Increased GGT | 1 | 2.4 | 1 | 4.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 8.3 | 1 | 3.2 | 0 | 0.0 |
| Non-tumoral portal vein thrombosis | 1 | 2.4 | 1 | 4.8 | 0 | 0.0 | 0 | 0.0 | 1 | 4.3 | 0 | 0.0 | 1 | 3.2 | 0 | 0.0 |
*Includes events of ‘increased AST’, ‘increased ALT’ or ‘hypertransaminasemia’.
†Includes events of ‘increased bilirubin’ or ‘hyperbilirubinemia’.
ALBI, Albumin-Bilirubin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BCLC, Barcelona Clinic Liver Cancer; GGT, Gamma-glutamyl transpeptidase; GI, gastrointestinal; PVI, portal vein invasion; SIRT, selective internal radiation therapy.
Efficacy
As per investigator assessment, complete and partial responses were observed in 5 and 12 patients, respectively, accounting for an ORR of 41.5% (95% CI 26.3% to 57.9%). Stable disease was the best overall response in 21 patients accounting for a DCR of 92.7% (95% CI 80.1% to 98.5%). No patient or tumor baseline characteristic, including prior TACE or Sorafenib, was associated with relevant differences in ORR, although responses were more frequent when Y90 activity was calculated based on a tumor-targeted dose (online supplemental table S5). Median time to response was 9 weeks (range 1–50 weeks) and median DoR was 31 weeks (range 6–109 weeks). Eleven (26.8%) patients had ongoing responses at the time of analysis. The waterfall plot of changes in target lesions is shown in online supplemental figure S1.
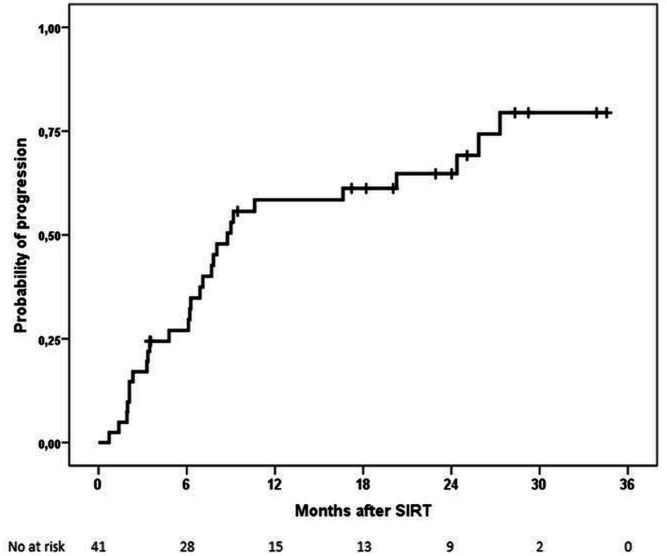
During the follow-up, 28 patients experienced disease progression, and 27 patients died. First progression was in form of growth of pre-existing lesions in 9 patients, new intrahepatic lesions in 10, and new extrahepatic lesions in 9. Median TTP was 8.8 months (95% CI 7.0 to 10.5) (figure 2). There was tendency to a shorter TTP among patients with vascular invasion, alpha-fetoprotein (AFP)>400 ng/mL or liver-targeted dose that was statistically significant only for AFP >400 ng/mL (online supplemental table S6 and figures S2–S4). Median PFS was 9.0 months (95% CI 7.0 to 10.9) (online supplemental figure S5).
Figure 2.
Kaplan-Meier plot of time to progression (TTP) per Investigator assessment. TTP rates at 1 and 2 years were 58% and 65%, respectively. SIRT, selective internal radiation therapy.
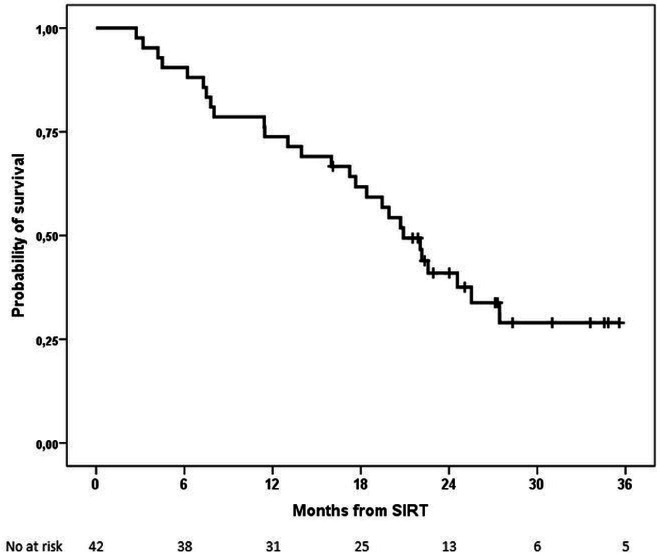
Median OS was 20.9 months (95% CI 17.7 to 24.1) (figure 3). A trend was observed toward shorter OS among patients with AFP >400 ng/mL or liver-targeted dose that was significant for the former (online supplemental table S6 and figure S6 and S7).
Figure 3.
Kaplan-Meier plot of overall survival (OS). OS rates at 1 and 2 years were 74% and 41%, respectively. SIRT, selective internal radiation therapy.
Discussion
To our knowledge, this is the first full report of a prospective evaluation of the combination of SIRT and nivolumab in a cohort of patients with HCC free from extrahepatic metastasis. The combination showed a tolerable safety profile with no signs of synergistic toxicity, and promising ORR, TTP, and OS. SIRT has shown a favorable safety profile and antitumor activity in retrospective and prospective cohorts of patients with intermediate through advanced stage HCC including those with too many or too large tumors, a wide range of patterns of PVI, or in progression to TACE.6 19 20 A recent publication has described the effects of this same combination in a more heterogenous and advanced group of HCC patients including a substantial number of patients with extrahepatic disease.21 Indeed, authors concluded that the strategy should be further evaluated in patients with HCC ineligible for TACE and patients with advanced stage but without extrahepatic spread. In NASIR-HCC, we established such stringent patient selection criteria to help define the safety and potential efficacy of SIRT and nivolumab in a homogeneous population that could be the target for future controlled clinical trials, excluding those patients with limited tumor burden where SIRT would be a radical therapy, and also those with extrahepatic metastasis where a locoregional therapy will unlikely have any benefit. The similar safety profile and OS in the two subgroups of patients in the BCLC-B2 substage and limited PVI supports our choice as a reasonable target population.
Nivolumab has demonstrated a good safety profile and relevant activity in patients that were mostly in the advanced stage.12 22 Yet, when tested against sorafenib as first-line therapy in advanced HCC a superior OS was not shown.23 The safety of the combination with SIRT was acceptable and there were no signs of new or synergistic liver or lung toxicity, the main organs with overlapping AEs. This is in line with the finding that administration of an ICI within 90 days following external irradiation was not associated with an increased risk of SAEs.24 The most frequent AEs were those expected from SIRT (thrombocytopenia, asthenia, and increased bilirubin) or nivolumab (diarrhea, asthenia, increased transaminases, or pruritus). SIRT-related AEs caused nivolumab discontinuation in only two patients. Patients with worse liver functional reserve in ALBI grade 2 at baseline had higher rates of HAEs but not AEs of any class resulting in Nivolumab delays or discontinuation.
Regarding efficacy, data from prospective trials using SIRT in HCC can provide a reasonable perspective to assess the outcomes observed in this trial. Reported median PFS and OS in trials including patients considered unsuitable for TACE were 4.1 and 8.0 months, respectively, in the SARAH trial,25 and 5.8 and 8.8 months SIRVENIB trial.8 In randomized trials comparing SIRT versus TACE among patients suited for TACE, median PFS and OS ranged from 3.6 and <12 months in the SIRTACE trial18 to 6 and 19.7 months in a German trial.26 Median PFS at 9 months and median OS at 20.9 months in NASIR-HCC are consistently higher and suggest enhanced activity of the combination of SIRT with nivolumab. When considering only the BCLC-B2 substage, again the 10.6 months median PFS observed in this trial compares well with the 6.2 months reported in a multicenter retrospective series of SIRT-treated patients.27 Response to SIRT is usually delayed for several months28 and the median time to response of 9 weeks observed in this study is certainly shorter than what would be expected from SIRT alone. The high DCR at 93% was strongly influenced by the first evaluation of tumor response 3 weeks after SIRT, an early time point when most tumors are expected to remain stable.
Several studies have demonstrated that delivering a high dose of radiation to the tumor compartment is key to obtain a good long-term outcome after SIRT.20 29 The data from NASIR-HCC point in the same direction and highlight the importance of treatment design and activity calculation in maximizing the effectiveness of SIRT.
Immune checkpoint inhibitors in combination with other therapies may provide a clinical benefit for advanced HCC patients naïve to systemic therapy. Atezolizumab plus bevacizumab has become a standard of care3 after proving superior OS and PFS compared with sorafenib.30 Improved OS and PFS benefits with the anti-PD-1 Sintilimab plus a Bevacizumab biosimilar was also shown in HBV-associated HCC.31 More recently, tremelimumab plus durvalumab has shown superior OS and PFS32 versus sorafenib. However, combinations come with more strict inclusion criteria compared with anti-PD-1/PD-L1 monotherapies, particularly for patients with cardiovascular comorbidities. SIRT plus nivolumab could be a valuable alternative for this subgroup of patients lacking an evidence-based recommended therapy.
Limitations
The single-arm design of the study should prompt caution in the interpretation of results compared with other prospective and retrospective cohorts, in particular with those large randomized trials that included patients in more advanced stages like SARAH, SIRveNIB and SORAMIC.8 25 33 Performing all SIRT procedures in a single center minimizes the effect of different levels of expertize across centers but may impact the reproducibility of the results.
Conclusions
The NASIR-HCC trial has shown that the combination of SIRT with SIR-Spheres resin microspheres, followed by nivolumab was safe and active as first-line therapy of patients with locally advanced HCC ineligible for TACE, where SIRT alone has failed to prove superiority over the standard of care. The high DCR, prolonged TTP, and encouraging OS suggest that the combination could be an option for this population and should be tested in a phase 3 controlled trial.
Footnotes
Twitter: @Maria_Var_Cal
Correction notice: This article has been corrected since it was first published online. Please see the linked correction notice for further details.
Contributors: BS and MdlTA are responsible for the overall content as guarantors and as such they accept full responsibility for the finished work and the conduct of the study, had access to the data, and controlled the decision to publish. Concept and design: MdlT-A, JIB, BS. Acquisition, analysis or interpretation of data: MdlTA, AM, MV, MI, MR, JLL, JIA, SL, MT, LM, LDF, JIA, CG-M, MR-F, JIB and BS. Drafting of manuscript: MdlTA, AM, MV, MI, MR, JLL, JIA, SL, MT, LM, LDF, JA, CG-M, MR-F, JIB and BS. Critical revision of the manuscript for important intellectual content: MdlTA, AM, AV, MI, MR, JLL, JIA, SL, MT, LM, LDF, JA, CG-M, MR-F, JIB and BS. Statistical analysis: MdlTA and BS.
Funding: This study was supported by Bristol Myers Squibb (Princeton, New Jersey, USA) and Sirtex Medical (Woburn, Massachusetts, USA).
Disclaimer: The funding organizations did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation or approval of the manuscript; and decision to submit the manuscript for publication. They have provided comments to the final draft.
Competing interests: MdlTA: travel grants from ESAI, Bayern and Pfizer. AM: consultancy fees from Astra-Zeneca, Bayer, Eisai-MSD, Roche and Sirtex Medical; and travel expenses from Astra-Zeneca, Bayer and Boston Scientific. MV: consultancy fees from Astra-Zeneca, Bayer, Eisai-MSD, BMS and Roche; honoraria from Bayer, Boston, Gilead, Eisai-MSD and Abbvie; and travel expenses from Astra-Zeneca and Bayer. MI: lecture fees and travel support from BMS. MR: consultancy fees from Astra Zeneca, Bayer, BMS, Boston Science, Ipsen, Lilly and Roche; lecture fees from Bayer, BMS, Gilead, Lilly, Roche and UniversalDX; travel support from Astra-Zeneca, Bayer, BMS and Lilly; research funding (to institution) from Bayer and Ipsen. JLL: speaker fees from Bayer and EISAI-MSD; consultancy fees from Bayer, EISAI-MSD and Roche. JIA: none. Sara Lorente: none. Milagros Testillano: travel expenses from Abbvie. LM: speaker fees from Bayer, Eisai and Gilead; advisory fees from Eisai and MSD. LDF: speaker fees from Bayer, BMS, Ipsen and Roche. JA: none. CG-M: consultancy or advisory fees from Amgen, Astra-Zeneca, BMS, EISAI, Hengrui Therapeutics, MERCK, and Roche-Spain; speaker fees from EISAI and Eli-Lilly. MR-F: consultancy and speaker fees from Sirtex Medical. JIB: consultancy fees from Boston Scientific, MSD, Sirtex Medical and Terumo; speaker fees from Sirtex; research grants from Sirtex and Terumo. BS: consultancy fees from Adaptimmune, Astra Zeneca, Bayer, BMS, Boston Scientific, BTG, Eisai, Eli Lilly, H3 Biomedicine, Ipsen, Novartis, Merck, Roche, Sirtex Medical and Terumo; speaker fees from Astra Zeneca, Bayer, BMS, BTG, Eli Lilly, Ipsen, Novartis, Merck, Roche, Sirtex Medical and Terumo; research grants (to Institution) from BMS and Sirtex Medical.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
The study protocol was approved by the ethics committee (Comite Etico de Investigacion Clinica de Navarra, EudraCT: 2017-000232-34, internal code 20/17) and the study was done in accordance with Good Clinical Practice guidelines. All patients provided written informed consent before study participation based on the principles of the Declaration of Helsinki.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin 2021;71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol 2022;76:681–93. 10.1016/j.jhep.2021.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruix J, Chan SL, Galle PR, et al. Systemic treatment of hepatocellular carcinoma: an EASL position paper. J Hepatol 2021;75:960–74. 10.1016/j.jhep.2021.07.004 [DOI] [PubMed] [Google Scholar]
- 4.Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv238–55. 10.1093/annonc/mdy308 [DOI] [PubMed] [Google Scholar]
- 5.Sangro B, Iñarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol 2012;56:464–73. 10.1016/j.jhep.2011.07.012 [DOI] [PubMed] [Google Scholar]
- 6.Sangro B, Carpanese L, Cianni R, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology 2011;54:868–78. 10.1002/hep.24451 [DOI] [PubMed] [Google Scholar]
- 7.Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol 2017;18:1624–36. 10.1016/S1470-2045(17)30683-6 [DOI] [PubMed] [Google Scholar]
- 8.Chow PKH, Gandhi M, Tan S-B, et al. SIRveNIB: selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol 2018;36:1913–21. 10.1200/JCO.2017.76.0892 [DOI] [PubMed] [Google Scholar]
- 9.Ricke J, Klümpen HJ, Amthauer H, et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol 2019;71:1164–74. 10.1016/j.jhep.2019.08.006 [DOI] [PubMed] [Google Scholar]
- 10.de la Torre-Aláez M, Jordán-Iborra C, Casadei-Gardini A, et al. The pattern of progression defines post-progression survival in patients with hepatocellular carcinoma treated with SIRT. Cardiovasc Intervent Radiol 2020;43:1165–72. 10.1007/s00270-020-02444-2 [DOI] [PubMed] [Google Scholar]
- 11.Sangro B, Sarobe P, Hervás-Stubbs S, et al. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2021;18:525–43. 10.1038/s41575-021-00438-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492–502. 10.1016/S0140-6736(17)31046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sangro B, Park J, Finn R, et al. LBA-3 CheckMate 459: long-term (minimum follow-up 33.6 months) survival outcomes with nivolumab versus sorafenib as first-line treatment in patients with advanced hepatocellular carcinoma. Ann Oncol 2020;31:S241–2. 10.1016/j.annonc.2020.04.078 [DOI] [Google Scholar]
- 14.Chew V, Lee YH, Pan L, et al. Immune activation underlies a sustained clinical response to yttrium-90 radioembolisation in hepatocellular carcinoma. Gut 2019;68:335–46. 10.1136/gutjnl-2017-315485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolondi L, Burroughs A, Dufour J-F, et al. Heterogeneity of patients with intermediate (BCLC B) hepatocellular carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis 2012;32:348–59. 10.1055/s-0032-1329906 [DOI] [PubMed] [Google Scholar]
- 16.Gil-Alzugaray B, Chopitea A, Iñarrairaegui M, et al. Prognostic factors and prevention of radioembolization-induced liver disease. Hepatology 2013;57:1078–87. 10.1002/hep.26191 [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 18.Kolligs FT, Bilbao JI, Jakobs T, et al. Pilot randomized trial of selective internal radiation therapy vs. chemoembolization in unresectable hepatocellular carcinoma. Liver Int 2015;35:1715–21. 10.1111/liv.12750 [DOI] [PubMed] [Google Scholar]
- 19.Salem R, Gabr A, Riaz A, et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000-patient 15-year experience. Hepatology 2018;68:1429–40. 10.1002/hep.29691 [DOI] [PubMed] [Google Scholar]
- 20.Garin E, Tselikas L, Guiu B, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol 2021;6:17–29. 10.1016/S2468-1253(20)30290-9 [DOI] [PubMed] [Google Scholar]
- 21.Tai D, Loke K, Gogna A, et al. Radioembolisation with Y90-resin microspheres followed by nivolumab for advanced hepatocellular carcinoma (Ca 209-678): a single arm, single centre, phase 2 trial. Lancet Gastroenterol Hepatol 2021;6:1025–35. 10.1016/S2468-1253(21)00305-8 [DOI] [PubMed] [Google Scholar]
- 22.Fessas P, Kaseb A, Wang Y, et al. Post-registration experience of nivolumab in advanced hepatocellular carcinoma: an international study. J Immunother Cancer 2020;8:e001033. 10.1136/jitc-2020-001033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yau T, Park JW, Finn RS, et al. CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (PTS) with advanced hepatocellular carcinoma (aHCC). Ann Oncol 2019;30:v874–5. 10.1093/annonc/mdz394.029 [DOI] [Google Scholar]
- 24.Anscher MS, Arora S, Weinstock C, et al. Association of radiation therapy with risk of adverse events in patients receiving immunotherapy: a pooled analysis of trials in the US food and drug administration database. JAMA Oncol 2022;8:232–40. 10.1001/jamaoncol.2021.6439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol 2017;18:1624–36. 10.1016/S1470-2045(17)30683-6 [DOI] [PubMed] [Google Scholar]
- 26.Pitton MB, Kloeckner R, Ruckes C, et al. Randomized comparison of selective internal radiotherapy (SIRT) versus drug-eluting bead transarterial chemoembolization (DEB-TACE) for the treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol 2015;38:352–60. 10.1007/s00270-014-1012-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cappelli A, Sangro P, Mosconi C, et al. Transarterial radioembolization in patients with hepatocellular carcinoma of intermediate B2 substage. Eur J Nucl Med Mol Imaging 2019;46:661–8. 10.1007/s00259-018-4152-7 [DOI] [PubMed] [Google Scholar]
- 28.Riaz A, Gates VL, Atassi B, et al. Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol Biol Phys 2011;79:163–71. 10.1016/j.ijrobp.2009.10.062 [DOI] [PubMed] [Google Scholar]
- 29.Hermann A-L, Dieudonné A, Ronot M, et al. Relationship of Tumor Radiation-absorbed Dose to Survival and Response in Hepatocellular Carcinoma Treated with Transarterial Radioembolization with 90Y in the SARAH Study. Radiology 2020;296:673–84. 10.1148/radiol.2020191606 [DOI] [PubMed] [Google Scholar]
- 30.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med Overseas Ed 2020;382:1894–905. 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- 31.Ren Z, Fan J, Xu J, et al. LBA2 Sintilimab plus bevacizumab biosimilar vs sorafenib as first-line treatment for advanced hepatocellular carcinoma (ORIENT-32)2. Ann Oncol 2020;31:S1287. 10.1016/j.annonc.2020.10.134 [DOI] [Google Scholar]
- 32.Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (PTS) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. JCO 2022;40:379. 10.1200/JCO.2022.40.4_suppl.379 [DOI] [Google Scholar]
- 33.Ricke J, Klümpen HJ, Amthauer H, et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol 2019;71:1164–74. 10.1016/j.jhep.2019.08.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-005457supp001.pdf (349.5KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.